Bioimpacts. 11(2):129-133.
doi: 10.34172/bi.2021.20
Original Research
Red cell alloantibodies in beta-thalassaemia major patients’ blood referring to the regional blood transfusion center of Tehran, Iran
Parisa Ebrahimisadr 1, *  , Zahra Bakhshandeh 1, Hamidreza Majidiani 2
, Zahra Bakhshandeh 1, Hamidreza Majidiani 2
Author information:
1Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
2Departments of Parasitology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
Abstract
Introduction:
Thalassemia is associated with a genetic decline in the rate of synthesis of one or more types of natural hemoglobin polypeptide chains. One of the major complications in thalassemia patients is alloimmunization, which is antibody production by the patient against transfused red blood cells (RBCs). These RBCs are unknown by the recipient and the formed antibodies against them are called alloantibodies. This study aimed to evaluate the frequency of alloantibodies against RBCs in beta-thalassemia patients referred to Tehran Regional Blood Transfusion Center.
Methods:
In this study, antibody screening tests (Dia-cell I, II, and III) were performed on 184 thalassemia patients. An identification test by the Dia panel consisting of 11 different O RBCs groups to examine sera with Dia cells (I, II, or III) was performed.
Results:
In our study, males and females patients comprised 66 (35.87%) and 118 (64.13%), respectively, of whom 116 (63%) had alloimmunization. In addition, 68 thalassemia subjects (37%) lacked alloantibodies. Among 184 patients with beta-thalassemia major, anti-K (Kell system), anti-D, and anti-E (Rhesus system) had the most abundant alloantibody variants with an incidence of 24 (13%), 11 (5.98%), and 10 (5.4%), respectively.
Conclusion:
Before RBC transfusion, regular RBC antigen phenotypes, as well as problem-solving of alloantibody production by receiving compatible blood for Kell and RH subgroups, are suggested for all cases of transfusion-derived thalassemia.
Keywords: Thalassemia, Alloantibody, Blood transfusion
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Thalassemia is a genetically inherited disease (GID) that is caused by a deficiency in the production of globin chains, a critical constituent of red blood cells (RBCs), upon which chain is less produced, are classified such as α, β, and αβ. Thalassemia is common in the Middle East, Africa, Southeast Asia, and the Mediterranean region, also manifesting from severe (major) to mild (minor). Thalassemia has been recognized as one of the most significant complex disorders and is presumed to have some genetic interactions.
1-3
In order to deal with these conditions, long-term vigilance, regular blood transfusion, and monetary burden are introduced.
4
The inevitable complications may be due to this route of medication, including the risk of transfusion-transmitted infections, transfusion-related immunologic reactions such as pyretic, allergic, acute, and chronic haemolytic forms, as well as iron overload. However, the incidence of transfusion-transmitted infections is rare due to the advanced technologies used in blood screening.
5-8
Alloantibody production against the unknown, RBCs is called ‘alloimmunization’, which involves one of the most important complications in thalassemia patients with multiple blood transfusions. Due to obstacles to providing suitable blood for these patients, dangerous hyperhaemolysis syndrome, the extensive incidence of additional alloantibody and autoantibodies production, and delayed haemolytic blood transfusion reaction, alloimmunization may lead to complications in transfusion therapy.
9-13
In order to prevent alloimmunization, it is strongly recommended to apply enhanced methods for characterizing the phenotype of the matched red cells for transfusion. This study aimed to evaluate the prevalence of alloantibodies in thalassemia patients according to Tehran Regional Blood Transfusion Center references.
Materials and Methods
Antibody screening and panel test
This is a descriptive study. A total of 184 blood samples from thalassemia patients, referred to the Tehran Regional Blood Transfusion Center from different hospitals throughout Tehran and also Bahonar Hospital of Karaj (for each patient that the hospital was unable to provide suitable blood) along with data from individuals such as age, sex, blood group, history of transfusion, and any possible reaction during their last transfusion was obtained. Regular Rh D and ABO antigen tests were performed in patients before transfusion. In this study, a handheld tube method was used for screening and identification with Biovista kit (Reference Laboratory of Immunohematology, Tehran-Iran). Before each blood transfusion, serum samples were tested using a standard protocol of blood banks to detect unprecedented antibodies against RBCs. After mixing sera with RBCs suspended in saline, a short spin was performed. Subsequently, after checking the agglutination to find out the presence of cold antibodies, albumin 22% was added and then incubated at 37oC for 20 minutes. After 20 minutes, tubes were observed for the presence of agglutination. Afterward, the samples were washed three times with normal saline. Finally, the drying procedure was carried out and two drops of anti-human globulin were added. A combination of antibody screening experiments and three categories of commercial group O RBCs were assigned as regular for rare and clinically significant antigens. The three above- mentioned classes of commercial group O RBCs were specified as Dia cells I, II, and III (R1R1, R2R2, and rr). An identification test was performed by a Dia panel, consisting of 11 different groups of O RBCs, to examine sera with a Dia cell (I or II or III).
Statistical analysis
Data were analyzed using SPSS v16.0 software and the chi-square test with a confidence level of 95% and P value <0.05 was considered as a significant statistical difference.
Results
The 184 subjects enrolled in this study were suffering from beta-thalassemia major, undergoing routine transfusion procedure in 3-5 week intervals, and receiving Rh D and ABO matched homologues. In our study, males and females constituted 66 (35.87%) and 118 (64.13%) patients, respectively, among which 116 subjects (63%) were already alloimmunization. In addition, 68 thalassemia subjects (37%) lacked alloantibody. Fig. 1 Summarizes the age distribution of thalassemia patients with/without alloantibodies. Statistically, the prevalence of alloantibodies was significantly correlated with age (P = 0.018) in Table 1. We demonstrated the correlation of age group with sex and alloantibodies, as well as the distribution of alloantibodies based on the blood group system shown in Table 2. No significant relationship was found between alloantibodies with sex and blood group system (P > 0.05). In addition, the discovered alloantibodies are shown in Tables 3 and 4. Of the 184 patients with beta-thalassemia major, anti-K (Kell system), anti-D, and anti-E (Rhesus system) were the most frequent alloantibodies with an incidence of 24 (13%), 11 (5.98%), and 10 (5.4%), respectively. Sixty-four cases (55.14%) of single, 36 cases (31%) of double-antibody, 11 cases (9.5%) of triple antibody, four cases (3.5%) of quadruple antibody, and one case (0.86%) of quintuple antibody were detected among the positive individuals. In accordance with the age classification, the identification of antibodies identified is shown in Fig. 2.
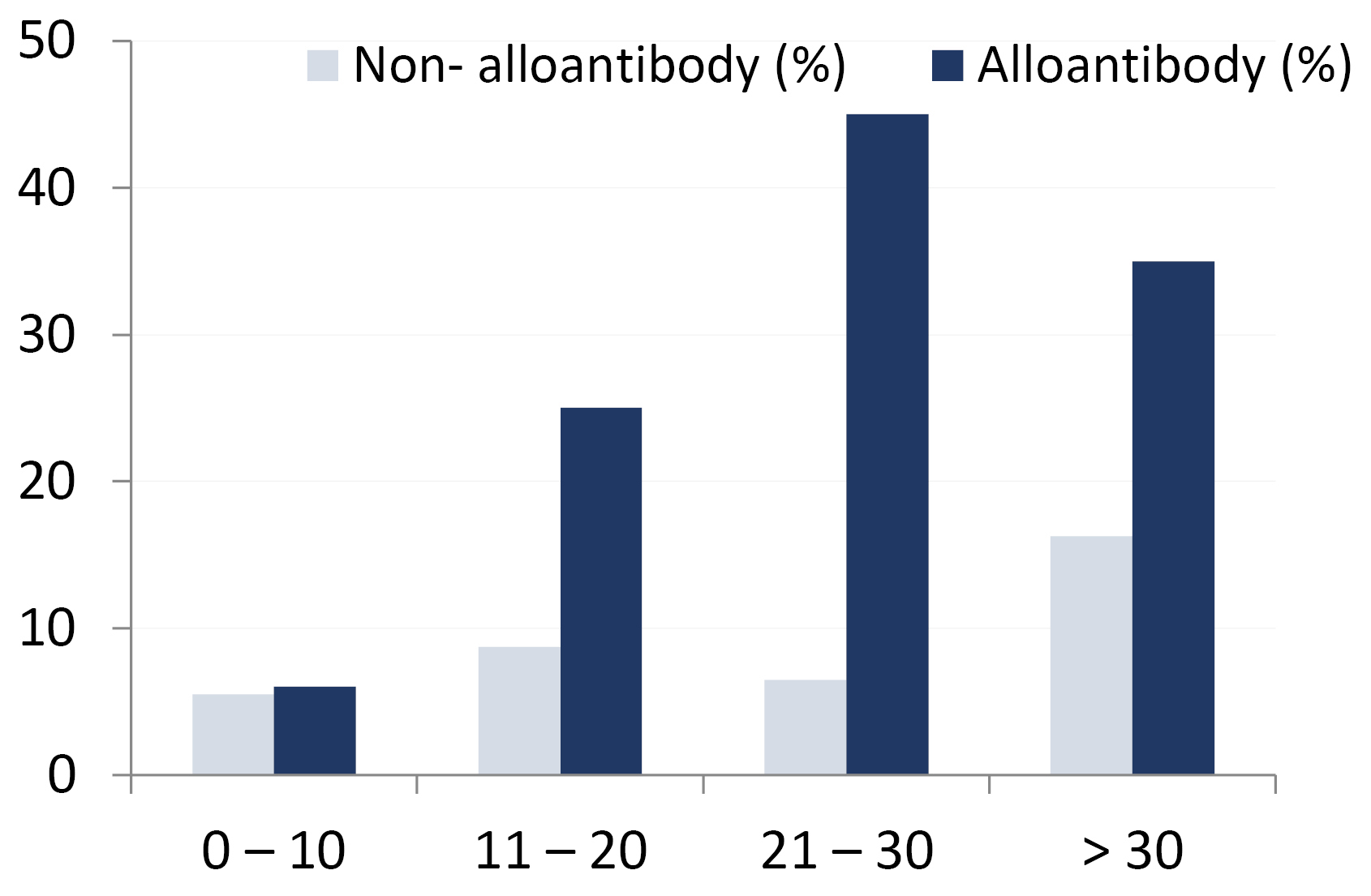
Fig. 1.
Correlation of age group with presence of alloantibody (P = 0.018).
.
Correlation of age group with presence of alloantibody (P = 0.018).
Table 1.
Correlation of age group with sex and Alloantibody (P > 0.05)
|
Age group (y)
|
Female/with Alloantibody
|
Male/with Alloantibody
|
| 0-10 |
10/6 |
11/5 |
| 11-20 |
21/14 |
20/11 |
| 21-30 |
29/22 |
28/23 |
| >30 |
35/21 |
30/14 |
| Total |
105(51.7%)/63(60%) |
79(48.3%)/53(67/08%) |
Table 2.
Distribution of alloantibodies according to the sex and blood group system (P > 0.05)
|
Age group (y)
|
A
+
(F/M)
|
A
–
(F/M)
|
B
+
(F/M)
|
B
–
(F/M)
|
AB
+
(F/M)
|
AB
–
(F/M)
|
O
+
(F/M)
|
O
–
(F/M)
|
| 0-10 |
2/1 |
0/0 |
1/2 |
0/0 |
1/1 |
0/0 |
2/1 |
0/0 |
| 11-20 |
4/2 |
1/0 |
1/2 |
1/0 |
2/2 |
1/1 |
4/3 |
0/1 |
| 21-30 |
5/8 |
2/2 |
2/2 |
2/1 |
2/1 |
0/1 |
7/5 |
2/3 |
| >30 |
6/4 |
2/2 |
3/2 |
1/0 |
2/1 |
2/0 |
3/4 |
2/1 |
Table 3.
Frequency of detected alloantibodies according to single and double antibodies in four age groups (P > 0.05)
|
Positive results
|
Age category
|
Total
|
|
0 - 10
|
11 - 20
|
21 - 30
|
> 30
|
| Anti-k |
3 (12.5%) |
8 (33%) |
9 (37.5%) |
4 (17%) |
24 |
| Anti-E |
1 (10%) |
2 (20%) |
3 (30%) |
4 (40%) |
10 |
| Anti-D |
1 (6%) |
1 (9%) |
4 (36.5%) |
5 (45.5%) |
11 |
|
Anti-C–
|
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
|
Anti-kp9
|
0 (0%) |
3 (33.5%) |
4 (44.5%) |
2 (22%) |
9 |
|
Anti-Cw
|
0 (0%) |
0 (0%) |
2 (100%) |
0 (0%) |
2 |
| Anti-M |
0 (0%) |
0 (0%) |
1 (50%) |
1 (50%) |
2 |
|
Anti-Jka
|
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
| Anti-S |
0 (0%) |
1 (50%) |
0 (0%) |
1 (50%) |
2 |
|
Anti-Lea
|
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
| Anti-e |
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
| Anti-C + S |
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
|
Anti-D + S–
|
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
| Anti-D + kell |
0 (0%) |
0 (0%) |
1 (100%) |
0 (0%) |
1 |
| Anti-D + C |
2 (28.5%) |
3 (43%) |
0 (0%) |
2 (28.5%) |
7 |
|
Anti-E + Cw
|
1 (50%) |
0 (0%) |
0 (0%) |
1 (50%) |
2 |
|
Anti-E + C–
|
0 (0%) |
1 (20%) |
4 (80%) |
0 (0%) |
5 |
| Anti-C + kell |
1 (50%) |
0 (0%) |
1 (50%) |
0 (0%) |
2 |
| Anti-D + E |
0 (0%) |
0 (0%) |
1 (100%) |
0 (0%) |
1 |
| Anti-E + kell |
1 (25%) |
1 (25%) |
1 (25%) |
1 (25%) |
4 |
|
Anti-C + e–
|
0 (0%) |
1 (33%) |
2 (67%) |
0 (0%) |
3 |
|
Anti-E + Jka
|
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
|
Anti-kell + Cw
|
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
| Anti-S + kell |
0 (0%) |
0 (0%) |
1 (100%) |
0 (0%) |
1 |
|
Anti-E + Jkb
|
0 (0%) |
1 (50%) |
1 (50%) |
0 (0%) |
2 |
|
Anti-kell + Fyb
|
0 (0%) |
1 (100%) |
0 (0%) |
0 (0%) |
1 |
|
Anti-Cw + Kpa
|
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
|
Anti-S + Jka
|
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
|
Anti-kell + Kpa
|
0 (0%) |
0 (0%) |
1 (100%) |
0 (0%) |
1 |
|
Total
|
10 (10%)
|
23 (23%)
|
36 (36%)
|
31 (31%)
|
100 (100%)
|
Table 4.
Frequency of detected alloantibodies according to multiple antibodies in four age groups (P > 0.05)
|
Positive results
|
Age category
|
Total
|
|
0-10
|
11-20
|
21-30
|
>30
|
|
Anti-kell + Cw + Kpa
|
0 (0%) |
1 (50%) |
1 (50%) |
0 (0%) |
2 |
|
Anti-E + C- + Jka
|
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
|
Anti-E + kell + Jkb
|
0 (0%) |
0 (0%) |
1 (100%) |
0 (0%) |
1 |
| Anti-E + C- + kell |
0 (0%) |
0 (0%) |
1 (50%) |
1 (50%) |
2 |
|
Anti-E + C- + Jkb
|
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
| Anti-D + C + S |
0 (0%) |
0 (0%) |
1 (100%) |
0 (0%) |
1 |
|
Anti-C- + kell + Cw
|
0 (0%) |
1 (100%) |
0 (0%) |
0 (0%) |
1 |
| Anti-D + C + kell |
0 (0%) |
0 (0%) |
1 (100%) |
0 (0%) |
1 |
|
Anti-E + S + Jkb
|
0 (0%) |
0 (0%) |
1 (100%) |
0 (0%) |
1 |
| Anti-E + C- + kell + M |
1 (100%) |
0 (0%) |
0 (0%) |
0 (0%) |
1 |
|
Anti-E + kell + Fyb + S
|
0 (0%) |
0 (0%) |
1 (100%) |
0 (0%) |
1 |
|
Anti-D + kell + S + Jkb
|
0 (0%) |
0 (0%) |
1 (100%) |
0 (0%) |
1 |
| Anti-C + D + E + kell |
0 (0%) |
0 (0%) |
1 (100%) |
0 (0%) |
1 |
|
Anti-E + C + Fyb + Jka + S
|
0 (0%) |
0 (0%) |
0 (0%) |
1 (100%) |
1 |
| Total |
1 (6%) |
2 (12.5%) |
9 (56.5%) |
4 (25%) |
16 (100%) |
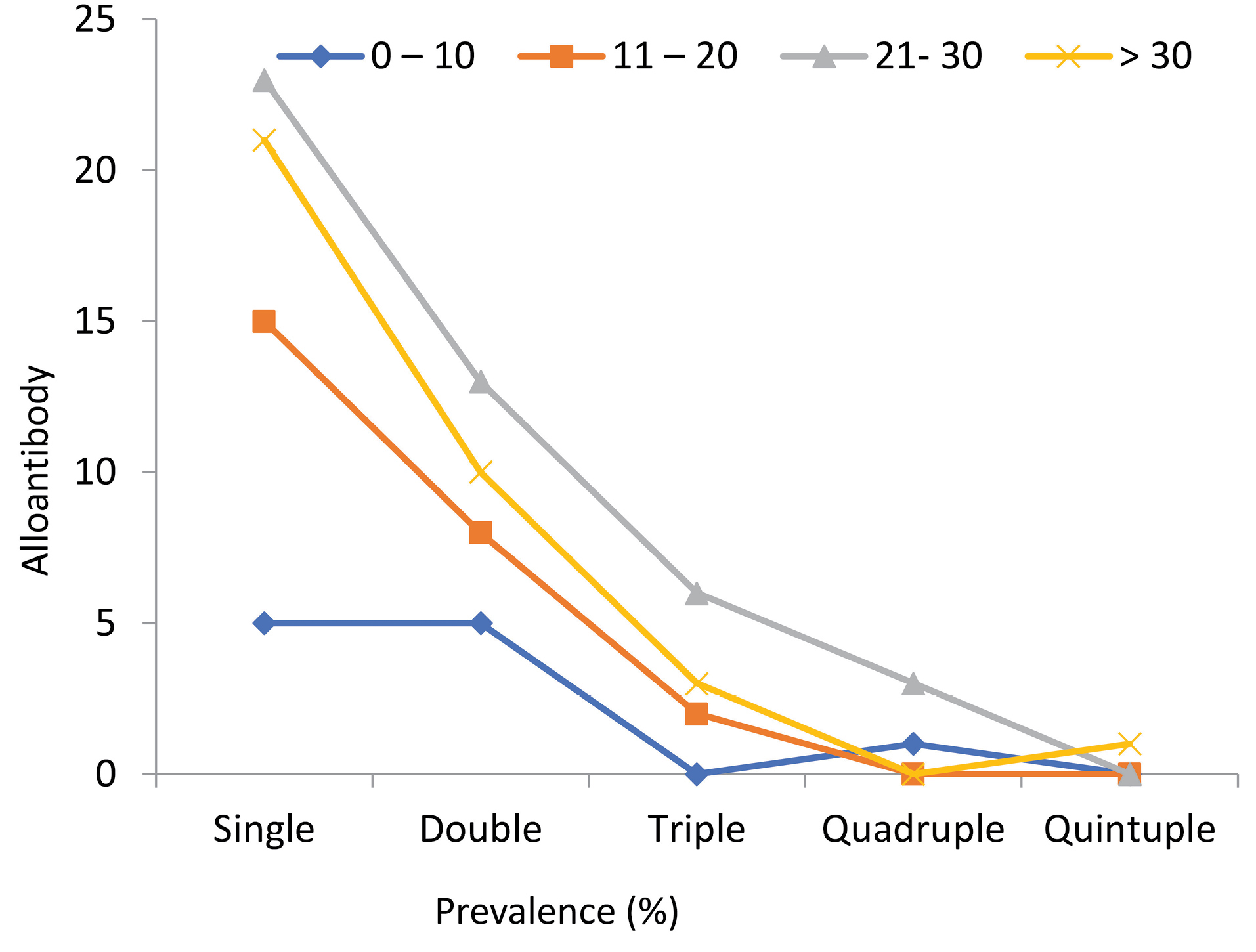
Fig. 2.
Frequency of detected alloantibodies according to the number of antibodies in the four age categories (P> 0.05).
.
Frequency of detected alloantibodies according to the number of antibodies in the four age categories (P> 0.05).
Discussion
Only a few studies have investigated the multiplicity of RBC alloimmunization among thalassemia major patients with multiple transfusions in Iran. Consequently, we aimed to determine the red cell alloantibodies in referred blood samples of thalassemia patients to Tehran Regional Blood Transfusion Centre of Iran. It is noteworthy that feto-maternal transfusion, incompatible blood transfusion, in addition to rare instances such as allogeneic transfusion in transplantation cases are the major reasons for the formation of alloantibodies. In this case, considering that multiple transfusions are performed in these populations, thalassemia patients are at high-risk and are exposed to develop red cell alloantibodies with further complications.
14-16
Although there are variations in reported types of alloantibodies, most are classified as potentially haemolytic. There is a general consensus that typically developed alloantibodies are produced against the Kell system and Rh subgroups.
17-20
According to our results, this study differs from others in that the prevalence rate of alloantibodies in referred blood samples of the patients with thalassemia to Tehran Regional Blood Transfusion Center is high (63%). Maybe the reason for this high incidence is the fact that hospital blood banks are not able to provide appropriate blood for their respective thalassemia patients, due to multiple transfusions in this population. Therefore, most of these patients respond to transfused blood by producing alloantibody. In Iran, some articles have also reported the prevalence of alloantibodies, such as Azarkeivan et al with 12.1% [the most common alloantibodies were Anti-kell (33%), Anti-Rh (D) (10.9%), Anti-Rh (E) (9.9%)] Karimi et al with 5.6% [Anti-kell (33%), Anti-Rh (D) (10.9%), Anti-Rh (E) (9.9%)] and Shamsian et al with 7.4%. Similarly, studies in Turkey and Egypt showed a 6.4% and 11.3% prevalence rate, respectively, but Kuwait and Taiwan showed a higher rate with 30% and 37%, respectively.
4,17,18,20-22
Of the 184 thalassemia patients in our study, 66 were male and 118 were female. In addition, 116 (63%) patients had alloantibodies and 68 (37%) had no alloantibodies. Among the four categorized age groups (0–10, 11–20, 21–30, and above 30), the blood group of 21–30 class included the most diverse alloantibodies, with 45 (24.5%) out of 116 patients having alloantibody. In a study led by Azarkeivan et al in Iran, 22 (7.7%) out of 287 cases in a paediatric group and 79 (14.4%) out of 548 adults were alloantibody positive. In Kuwait, Ameen et al demonstrated that 66 (49.6%) out of 190 patients aged 2–10 years had alloantibodies.
17-20
Among various age groups, 64 thalassemia patients had a single antibody and most of them belonged to the 21–39-year age group with 23 cases (40%). According to our findings, the most common antibody is against Kell. The best way to manage alloimmunized patients is by identifying the type of alloantibodies and negative antigen blood transfusions. However, if alloantibodies are developed against high prevalence antigens, it will be extremely difficult to find compatible blood. In this case, using blood from relatives, especially siblings, can be helpful. Also, one may determine clinically important phenotype antigens of the blood groups in these patients and give them fully compatible blood. Due to the limitations of serological methods in determining the phenotype of patients with recurrent blood transfusions, the genotypes can be used instead. In a 3-year study by Putzulu et al. blood group genotypes were determined on 1220 regular blood donors and 10 patients with periodic blood transfusion (thalassemia and anemia cycle of tuberculosis), within 3 years the blood was completely compatible with the genotype for transfused patients. Screening for alloantibodies showed that no alloantibody was induced during a 3- year blood transfusion period, and patients who had already been alloimmunized revealed no increase in their antibody levels; so the correct determination of the blood group through the genotype can have a significant effect on the reduction of alloimmunization and the production of antibodies.
23
Finally, we recommend that hospitals and related centers be more attentive when conducting cross-match testing.
Conclusion
It is noteworthy that one of the likely adverse consequences of blood transfusion in patients suffering from thalassemia is the process of alloimmunization obtained from foreign RBC supplies. Before RBC transfusion, regular RBC antigen phenotypes as well as resolving the problem of alloantibody production by receiving compatible blood, regarding Kell and RH subgroups, are suggested for all the transfusion-derived thalassemia cases. In addition, the high prevalence rate of alloantibodies in our study may be due to inappropriate compatibility between donor and recipient in hospital centers.
Acknowledgments
We would like to thank the head of Tehran Blood Transfusion Center (Dr. Seyyed Morteza Tabatabaei), for his assistance in conducting this research.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical statement
The sample was collected after being informed. Their consent was confirmed according to the guidelines of the Ethical Committee of Tehran Blood Transfusion Center. This study was approved by the Medical Ethics Committee in 2018-May-15, with issue number 97/D/800.
Competing interests
The authors declare no conflict of interest.
Authors’ contribution
PE: Research idea, experimental, and writing manuscript. ZB: experimental and statistical analysis. HM: writing manuscript and provision of study materials and equipment.
Research Highlights
What is the current knowledge?
simple
-
√ Alloimmunization is a major challenge in multi-transfused patients, especially in patients with thalassemia.
What is new here?
simple
-
√ Since alloimmunization is common in patients with thalassemia, extensive RBCs phenotype matching is essential for transfusion in alloimmunized patients.
References
- Weatherall DJ. The molecular basis for phenotypic diversity of genetic disease. Ann N Y Acad Sci 1995; 758:245-60. doi: 10.1111/j.1749-6632.1995.tb24832.x [Crossref] [ Google Scholar]
- Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet 2018; 391:155-67. doi: 10.1016/S0140-6736(17)31822-6 [Crossref] [ Google Scholar]
- Rund D, Rachmilewitz E. β-Thalassemia. N Engl J Med 2005; 353:1135-46. doi: 10.1056/NEJMra050436 [Crossref] [ Google Scholar]
- Ahmed AM, Hasan NS, Ragab SH, Habib SA, Emara NA, Aly AA. Red cell alloimmunization and autoantibodies in Egyptian transfusion-dependent thalassaemia patients. Arch Med Sci 2010; 6:592-8. doi: 10.5114/aoms.2010.14473 [Crossref] [ Google Scholar]
- Olivieri N. 5 Thalassaemia: clinical management. Baillière's Clinical Haematology 1998; 11(1):147-62. doi: 10.1016/S0950-3536(98)80073-5 [Crossref] [ Google Scholar]
- Wonke B. Clinical management of β-Thalassemia major. Semin Hematol 2001; 38:350-9. doi: 10.1016/S0037-1963(01)90029-0 [Crossref] [ Google Scholar]
- Dellinger EP, Anaya DA. Infectious and immunologic consequences of blood transfusion. Crit Care 2004; 8 Suppl 2:S18-23. doi: 10.1186/cc2847 [Crossref] [ Google Scholar]
- Azarkeivan A, Ahmadi M, Hajibeigy B, Gharebaghian A, Shabeh pour Z, Maghsoodlu M. Evaluation of Transfusion Reactions in Thalassemic Patients Referred to the Tehran Adult Thalssemia Clinic. J Adv Med Biomed Res 2008; 16:57-66. [ Google Scholar]
- Schonewille H, Haak HL, van Zijl AM. Alloimmunization after blood transfusion in patients with hematologic and oncologic diseases. Transfusion 1999; 39:763-71. doi: 10.1046/j.1537-2995.1999.39070763.x [Crossref] [ Google Scholar]
- Schonewille H, van de Watering LM, Brand A. Additional red blood cell alloantibodies after blood transfusions in a nonhematologic alloimmunized patient cohort: is it time to take precautionary measures?. Transfusion 2006; 46:630-5. doi: 10.1111/j.1537-2995.2006.00764.x [Crossref] [ Google Scholar]
- Eder AF, Chambers LA. Noninfectious complications of blood transfusion. Arch Pathol Lab Med 2007; 131:708-18. doi: 10.1043/1543-2165(2007)131[708:NCOBT]2.0.CO;2 [Crossref] [ Google Scholar]
- Zumberg MS, Procter JL, Lottenberg R, Kitchens CS, Klein HG. Autoantibody formation in the alloimmunized red blood cell recipient: clinical and laboratory implications. Arch Intern Med 2001; 161:285-90. doi: 10.1001/archinte.161.2.285 [Crossref] [ Google Scholar]
- Blumberg N, Heal JM, Gettings KF. WBC reduction of RBC transfusions is associated with a decreased incidence of RBC alloimmunization. Transfusion 2003; 43:945-52. doi: 10.1046/j.1537-2995.2003.00443.x [Crossref] [ Google Scholar]
- Davenport RD. Pathophysiology of hemolytic transfusion reactions. Semin Hematol 2005; 42:165-8. doi: 10.1053/j.seminhematol.2005.04.006 [Crossref] [ Google Scholar]
- Michail-Merianou V, Pamphili-Panousopoulou L, Piperi-Lowes L, Pelegrinis E, Karaklis A. Alloimmunization to red cell antigens in thalassemia: comparative study of usual versus better-match transfusion programmes. Vox Sanguinis 1987; 52:95-8. doi: 10.1111/j.1423-0410.1987.tb02999.x [Crossref] [ Google Scholar]
- Rebulla P. Blood transfusion in beta thalassaemia major. Transfus Med 1995; 5:247-58. doi: 10.1111/j.1365-3148.1995.tb00210.x [Crossref] [ Google Scholar]
- Azarkeivan A, Ansari S, Ahmadi MH, Hajibeigy B, Maghsudlu M, Nasizadeh S. Blood transfusion and alloimmunization in patients with thalassemia: multicenter study. Pediatr Hematol Oncol 2011; 28:479-85. doi: 10.3109/08880018.2011.568595 [Crossref] [ Google Scholar]
- Karimi M, Nikrooz P, Kashef S, Jamalian N, Davatolhagh Z. RBC alloimmunization in blood transfusion-dependent beta-thalassemia patients in southern Iran. Int J Lab Hematol 2007; 29:321-6. doi: 10.1111/j.1365-2257.2006.00856.x [Crossref] [ Google Scholar]
- Canatan D. The Thalassemia center of Antalya State Hospital: 15 years of experience (1994 to 2008). J Pediatr Hematol Oncol 2013; 35:24-7. doi: 10.1097/MPH.0b013e3182755f1e [Crossref] [ Google Scholar]
- Ameen R, Al-Shemmari S, Al-Humood S, Chowdhury RI, Al-Eyaadi O, Al-Bashir A. RBC alloimmunization and autoimmunization among transfusion-dependent Arab thalassemia patients. Transfusion 2003; 43:1604-10. doi: 10.1046/j.1537-2995.2003.00549.x [Crossref] [ Google Scholar]
- Wang LY, Liang DC, Liu HC, Chang FC, Wang CL, Chan YS. Alloimmunization among patients with transfusion-dependent thalassemia in Taiwan. Transfus Med 2006; 16:200-3. doi: 10.1111/j.1365-3148.2006.00656.x [Crossref] [ Google Scholar]
- Kocyigit C, Eliacik K, Kanik A, Atabay B, Turker M. Frequency of red cell allo- and autoimmunization in patients with transfusion-dependent beta thalassemia and affecting factors. Turk J Pediatr 2014; 56:487-92. [ Google Scholar]
- Putzulu R, Piccirillo N, Orlando N, Massini G, Maresca M, Scavone F. The role of molecular typing and perfect match transfusion in sickle cell disease and thalassaemia: An innovative transfusion strategy. Transfus Apher Sci 2017; 56:234-7. doi: 10.1016/j.transci.2017.01.003 [Crossref] [ Google Scholar]