BioImpacts. 6(1):49-67.
doi: 10.15171/bi.2016.07
Review Article
Advanced drug delivery and targeting technologies for the ocular diseases
Jaleh Barar , Ayuob Aghanejad , Marziyeh Fathi , Yadollah Omidi *
Author information:
Research Centre for Pharmaceutical Nanotechnology, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Ocular targeted therapy has enormously been advanced by implementation of new methods of drug delivery and targeting using implantable drug delivery systems (DDSs) or devices (DDDs), stimuli-responsive advanced biomaterials, multimodal nanomedicines, cell therapy modalities and medical bioMEMs. These technologies tackle several ocular diseases such as inflammation-based diseases (e.g., scleritis, keratitis, uveitis, iritis, conjunctivitis, chorioretinitis, choroiditis, retinitis, retinochoroiditis), ocular hypertension and neuropathy, age-related macular degeneration and mucopolysaccharidosis (MPS) due to accumulation of glycosaminoglycans (GAGs). Such therapies appear to provide ultimate treatments, even though much more effective, yet biocompatible, noninvasive therapies are needed to control some disabling ocular diseases/disorders.
Methods:
In the current study, we have reviewed and discussed recent advancements on ocular targeted therapies.
Results:
On the ground that the pharmacokinetic and pharmacodynamic analyses of ophthalmic drugs need special techniques, most of ocular DDSs/devices developments have been designed to localized therapy within the eye. Application of advanced DDSs such as Subconjunctival insert/implants (e.g., latanoprost implant, Gamunex-C), episcleral implant (e.g., LX201), cationic emulsions (e.g., Cationorm™, Vekacia™, Cyclokat™), intac/punctal plug DDSs (latanoprost punctal plug delivery system, L-PPDS), and intravitreal implants (I-vitaion™, NT-501, NT- 503, MicroPump, Thethadur, IB-20089 Verisome™, Cortiject, DE-102, Retisert™, Iluvein™ and Ozurdex™) have significantly improved the treatment of ocular diseases. However, most of these DDSs/devices are applied invasively and even need surgical procedures. Of these, use of de novo technologies such as advanced stimuli-responsive nanomaterials, multimodal nanosystems (NSs)/nanoconjugates (NCs), biomacromolecualr scaffolds, and bioengineered cell therapies need to be further advanced to get better compliance and higher clinical impacts.
Conclusion:
Despite mankind successful battle on ocular diseases, our challenge will continue to battle the ocular disease that happen with aging. Yet, we need to understand the molecular aspects of eye diseases in a holistic way and develop ultimate treatment protocols preferably as non-invasive systems.
Keywords: Eye diseases, Intraocular drug delivery, Ocular barriers, Ocular pharmacotherapy, Ophthalmic implants, Ocular drug targeting, Ophthalmology, Targeted therapy
Copyright and License Information
© 2016 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons
Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
In the human eye, like all other mammals, non-image-forming photosensitive ganglion cells within the retina function to receive the light signals and react accordingly towards translation of the signals and visualization. The human eye functions perfectly by harmonized co-operation of the related bio-micro-machineries of the eye (e.g., reflection of the pupil, function of eye muscles and lacrimal gland secretory processes), with responsiveness of the related neural centers of the brain (i.e., cortical and subcortical brain regions), functions of the hormonal system (e.g., regulation and suppression of melatonin) and even the regulation of body clock.
Anatomically, the eye globe is divided into anterior and posterior segments, respectively occupying one-third and two-third of ocular tissues. The anterior segment contains the cornea, conjunctiva, iris, ciliary body, tear film, and aqueous humor, and the posterior segment encompasses the sclera, choroid, Bruch’s membrane, retina and vitreous humor.
1
Impeccable functionality of the visual cells is largely dependent upon integrity of the cells/tissues in posterior and anterior segments of the eye, where selective restrictiveness of the ocular tissues membranes and barriers control the shuttling of solutes to maintain the ocular homeostasis through perfect functions of ocular biological barriers. These impediments include (a) corneal epithelial barrier, (b) iris blood vessel endothelium, (c) ciliary body epithelium (CEB), (d) inner barrier of retina formed by retinal capillary endothelial cells, and (e) retinal barrier formed by retinal-pigmented epithelial cells. Up until now, different cell models have been used to examine the functions of various barriers of the eye and to address their impacts on topical and/or intraocular delivery of ophthalmic drugs.
2
As a general principle in the ocular pharmacotherapy, treatment of the ophthalmic diseases often necessitate advanced drug delivery systems (DDSs) and devices (DDDs) ideally for programmed/long-term liberation of drug into the anterior or posterior segments of the eye to enhance the patient adherence to the treatment regimen and hence success of the therapy. However, the currently used conventional therapies often associate with low bioavailability because of physiobiologic barriers of the eye while the systemic DDSs must cross the retinal barriers.
The dry eye syndrome (DES), inflammation, allergies and infections of the eye, macular degeneration, cataracts, diabetic retinopathy and glaucoma are primarily largely age-and/or lifestyle-related diseases.
3
To address the clinical relevance of ocular barriers in targeted therapy of the ophthalmic diseases, in the current article, we will discuss the recent advancements in crossing and/or circumventing ocular barriers through implementing noninvasive sustained-/controlled release injectable or implantable DDSs.
Ocular pharmacokinetics
Drug pharmacokinetic (PK) analyses in human subjects are prerequisite for any new pharmaceutical. For the ocular drug products, however, the PK studies in human subjects are waved because the serial sampling from the aqueous humor or the vitreous humor is not applicable for PK assessments. As a substitute, animal models (e.g., rabbit, dog, monkey and pig) whose eye sizes are similar to the human eye are used to conduct ophthalmic experiments, even though there exist some differences between human and these models. Despite all these shortcomings, still the rabbits are most commonly used for PK studies.
4
It should be stated that the serial sampling of target tissues of the eye is extremely challenging in terms of sampling procedure and volume, as a result the ocular PK experiments demand a large number of animals to attain reliable PK data such as area under the curve (AUC), time to maximum tissue concentration (Tmax) and peak tissue concentration (Cmax) – necessary for the approval of any new drug application. Taken all, ocular PK studies are very laborious, time consuming and expensive, which require implementation of different techniques such as microdialysis assessment that is based on a capillary dialysis probe for continuous sampling of aqueous and/or vitreous humors from the same eye.
5
In the case of sustained-release DDSs when drug liberation needs to be assessed for a long-period of time, the microdialysis technique cannot be applied and periodic sampling with a designated number of animals can provide adequate data. Further, this approach is not suitable for the continuous drug level assessment in tissues such as the iris-ciliary body and the retina.
Using suitable animal models and drug analyses techniques, drug liberation and distribution can be assessed. PK parameters such as AUC, Tmax and Cmax are normally the ones that are used for the relative bioavailability (the so-called “relative amount of absorption”) comparison among formulations, while the absolute bioavailability (the so-called “actual fraction of the dose absorbed”) can only be calculated upon direct drug dosing in the target tissue.
In 2004, Tojo developed a PK model for the ocular drug delivery based on Fick’s second law of diffusion, assuming a modified cylindrical eye with three routes of drug transportation including the anterior aqueous chamber, the posterior aqueous chamber and the retina/choroids/scleral membrane. In this model, parameters such as the diffusion coefficient (DC) and the partition coefficient (PC) were assessed from the in vitro membrane penetration experiments by means of a side-by-side diffusion cell system for various eye tissues, and the DC for a drug can be estimated through the effect of the molecular weight of the model compound. This PK model was proposed to predict the biodistribution in the various fluids or tissues of the eye. The model was claimed to be able to simulate the effects of binding and metabolism in the eye.
6
Based on a homogeneous biodistribution of drugs within the ocular tissue, as one of the best experiments, Jones and Maurice proposed a method for determining the rate of loss of fluorescein from the aqueous humor in human eye by introducing dye into the cornea using iontophoresis and following the distribution of the dye in the eye.
7
This model was further developed by Maurice and Mishima who capitalized on an assumption that the PK of ophthalmic drugs can be assessed by compartmental models,
8
even though the compartmental analyses may associate with some limitations such as weakness in providing accurate PK data because of lack of detailed information upon the local drug distribution in the eye. Further, drug elimination via various routes of the eye as discussed previously may affect the local tissue concentration, hence the concentration of administered drugs in the aqueous chamber and in the vitreous body will explicitly be inhomogeneous showing a complex distribution pattern. As a result, in vivo data obtained by means of simple compartmental analysis may fail to fully correlate the pharmacological response that might directly be related with the local target concentration and distribution of drug. Taken all, as shown in Fig. 1, two-compartment model can be used to analyze the drug exchanges that can occur in the eye after the topical administration. In this model, the primary assumptions are (i) negligible loss of drug via tears, (ii) insignificant entry into the aqueous humor from the tears other than the cornea, (iii) trivial exchanges between the cornea and blood at the limbus, and (iv) negligible exchanges of the aqueous humor with the posterior reservoir.
8
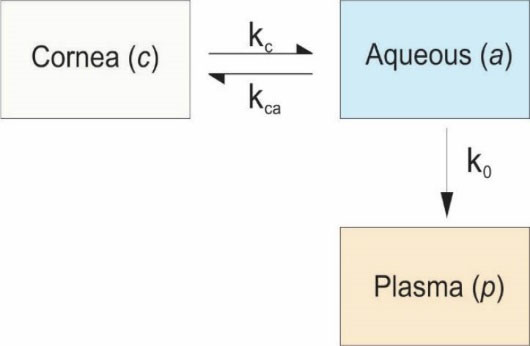
Fig. 1
.
Schematic representation of two-compartment model for topical use of ocular drugs. The kc and kca represents transfer coefficients between the cornea (c) and aqueous humor (a), and k0 is the loss coefficient from the aqueous to the plasma (p). The model was adapted from previously published works.
8
.
Schematic representation of two-compartment model for topical use of ocular drugs. The kc and kca represents transfer coefficients between the cornea (c) and aqueous humor (a), and k0 is the loss coefficient from the aqueous to the plasma (p). The model was adapted from previously published works.
8
Based on a two-compartment open system of conventional pharmacokinetics,
7,8
one may consider following kinetic equation (Eq.) 1 for the mass transfer aqueous humor to the blood after topical application:
Where, k0 is the transfer coefficient from the aqueous humor to the blood; Ca and Cp are the concentrations in the aqueous humor and blood plasma, respectively; and rap is the value of the ratio Ca/Cp at steady state.
The administered drug to the cornea can penetrate to the anterior segment, hence the transfer coefficient kc may define the exchange of the used drug between the cornea and the aqueous humor and is referred to the volume of the cornea, which can be defined by Eq. 2.
Where, Cc is the corneal concentration defined as the mass of drug in the entire cornea divided by the total volume of the tissue, Vc.
It should be noted that the transfer coefficient kca shows the drug exchange between the cornea and aqueous humor and is referred to the volume of the aqueous humor, which can be defined by Eq. 3:
Where, Va represents the volume of the anterior chamber. The equilibrium ratio rca is the value of the ratio Cc/Ca when dCc/dt is zero. The expression Vcrca can be termed the apparent volume of the cornea. It should be highlighted that since Vc and Va are determined, only two of the parameters kca, kc, and rca are independent.
Based on Fick’s second law, Tojo hypothesized that the concentration of a designated drug in the eye can be given by the following pharmacokinetic model shown as Eq. 4:
Eq. 4
Where, D is the diffusion coefficient in the eye, B(x, y, t) is the binding term, R(x, y, t) is the metabolism and degradation rate and S(x, y, t) is the release rate of drug from the DDS implanted or injected into the target site. It should be noted that in this equation D is not considered as constant and varies in the ocular tissues.
6
All together, the simple compartmental analyses seem to provide satisfactory data for the kinetics of hydrophilic substances. However, such models may not provide accurate outcome because drugs can penetrate into different tissues rather than the assumed compartments and show complex distribution patterns in large part due to solubility tendency of drugs in aqueous humor and lipid membranes of different segments of the eye such as the corneal epithelium, the lens, and the uveal tissue that are neglected. Besides, there may be nonlinear relationships between the magnitude of the reservoirs and barriers formed by these tissues and the concentration of the drug.
8
It seems that, in addition to fluorescein used for elucidating the kinetics of hydrophilic drugs, we need to capitalize on some other lipid-soluble fluorescent tracers to be able to interpret the complex pattern of drug distribution in the eye. The PK studies in the eye need further advancements using not only the experimental models but also computer-based simulation and modeling.
Ocular barriers
All the biological membranes and barriers selectively regulate traverse of locally and/or systemically administered drugs and blood-borne molecules to the anterior and posterior compartments of the eye.
9
Fig. 2 schematically represents the perfect function of the ocular biological membranes and barriers. The cornea as an avascular transparent multilayered epithelial cells represent a primary sensitive tissue that can block the translocation of topically administered pharmaceuticals (e.g., eye drops, ointments, gels, or emulsions) into the cul-de-sac. The ocular biological membranes and barriers are considered as the most robust controlling machineries of harmonized group of cells and tissue in an organ.
10
In fact, the consistency of such harmonized functions of the eye is utterly dependent upon the (a) static barriers (e.g., different layers of cornea, sclera, iris/ciliary body through blood-aqueous barrier and retina through blood-retinal barriers), (b) dynamic barriers (e.g., tear dilution, choroidal and conjunctival blood flow, lymphatic clearance), and (c) efflux pumps such as multidrug resistance (MDR) known as P-glycoprotein (P-gp) and multidrug resistance proteins (MRPs). Most of these functions within the ocular capillary are similar to the blood-brain barrier (BBB) functions that controls the inward and/or outward traverse of molecules into brain.
10-13
It should be noted that, similar to any other biological barriers,
10
the blockade function of the ocular barriers vary significantly. The bio-physiologic nature and barrier functions of the ocular barriers such as blood aqueous barrier (BAB) formed by the endothelial cells in the iris and the blood-retinal barrier (BRB) formed by the retinal inner capillary endothelial cells show different pattern and degree of impediments. This assumption can be proven by the systemic administration of ocular drugs, after which the concentration of drug in the aqueous humor is significantly higher than the vitreous humor. This clearly indicates that the BRB represents much more restrictive barrier functions to drug penetration in comparison with the BAB. While the endothelia of choroid is largely fenestrated, the retinal capillary endothelia (RCE) represent an inner tight barrier which together with the outer barrier formed by the retinal pigmented epithelia (RPE) control the traverse of blood-borne molecules into the posterior segment of the eye.
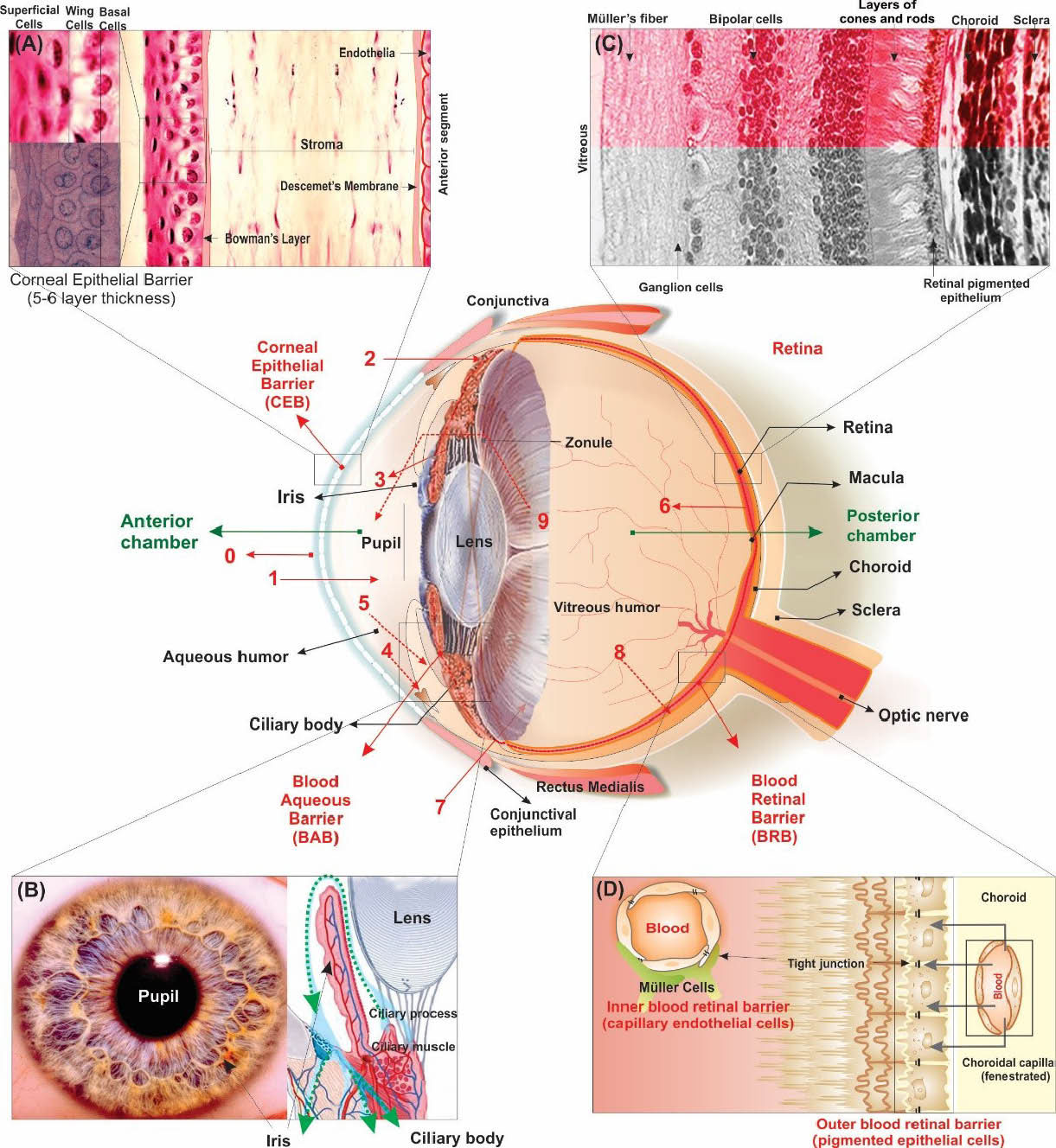

Fig. 2
.
Schematic demonstration of the anatomy and the biological membranes and barriers of the eye. Panels A, B, C and D represent the corneal epithelial barrier (CEB), the blood aqueous barrier (BAB), the biostructures of retina, and the blood-retinal barriers (BRB) both inner endothelial and outer pigmented epithelial barriers. The hemostasis of eye is performed by several static and dynamic barriers. Tear film is the first physiologic impediment against installed topical pharmaceuticals (0). The cornea forms an excellent obstacle preventing topical drugs to reach the anterior chamber of the eye (1). The conjunctival/scleral route is the most permeable path to the hydrophilic drugs and macromolecules (2). The systemically administered small compounds are able to penetrate from the iris blood vessels into the anterior chamber (3). The administered drugs reached to anterior chamber are subjected to aqueous humor outflow (4). These drugs can be carried away from anterior chamber by venous blood flow (BAB function) after diffusing across the iris surface (5). The systemically administered drugs must cross the BRBs. These drugs must cross the outer retinal barrier, “retinal pigment epithelia (RPE)” and the inner retinal barrier, “retinal capillary endothelia (RCE)” (6). For intravitreal delivery, drugs can directly be injected into the vitreous (7). Drugs can be removed from the vitreous away by the retinal blood vessels (8). Drugs within the vitreous can be diffused into the anterior chamber (9).
.
Schematic demonstration of the anatomy and the biological membranes and barriers of the eye. Panels A, B, C and D represent the corneal epithelial barrier (CEB), the blood aqueous barrier (BAB), the biostructures of retina, and the blood-retinal barriers (BRB) both inner endothelial and outer pigmented epithelial barriers. The hemostasis of eye is performed by several static and dynamic barriers. Tear film is the first physiologic impediment against installed topical pharmaceuticals (0). The cornea forms an excellent obstacle preventing topical drugs to reach the anterior chamber of the eye (1). The conjunctival/scleral route is the most permeable path to the hydrophilic drugs and macromolecules (2). The systemically administered small compounds are able to penetrate from the iris blood vessels into the anterior chamber (3). The administered drugs reached to anterior chamber are subjected to aqueous humor outflow (4). These drugs can be carried away from anterior chamber by venous blood flow (BAB function) after diffusing across the iris surface (5). The systemically administered drugs must cross the BRBs. These drugs must cross the outer retinal barrier, “retinal pigment epithelia (RPE)” and the inner retinal barrier, “retinal capillary endothelia (RCE)” (6). For intravitreal delivery, drugs can directly be injected into the vitreous (7). Drugs can be removed from the vitreous away by the retinal blood vessels (8). Drugs within the vitreous can be diffused into the anterior chamber (9).
Similar to the BBB that represents a tight barrier as coop with pericytes and astrocytes,
14,15
in the retina pericytes and Müller cells interact with the RCE and also contribute to the establishment of the BRB through production of biofactors such as angiopoietin-1 that promote a well-developed junctional complex and endothelial barrier formation. These endothelial cells secrete platelet-derived growth factor beta (PDGF-B) to harness and maintain pericytes by activating Akt, whose activity is the basis of the cell survival.
16
Perhaps, the perfect barrier functions of BRB is based on dual functions of both inner and outer barriers of retina. All these complex biological systems create the anatomical architectural hallmarks of the eye.
Technically, as the mostly used ocular pharmaceuticals, the topical dosage forms usually in the forms of solutions and semisolids are routinely locally administered. However, given that most of these medications can be easily washed away from the ocular surface, their administrations result in markedly low bioavailability failing to reach the posterior segments and hence the intended pharmacological effects do not occur. Further, the systemic delivery of ocular drugs into posterior segment of the eye often fails because of the excellent barrier function of BRB. Since the current strategies upon efficiently delivery of the ocular drugs to the site of action within the eye and treat the ocular diseases provide limited successes, intraocular drug delivery and targeted therapy of the ophthalmic diseases appear to be very challenging. At the moment, the intravitreal injection is the main treatment modality of the disabling ocular diseases such as the age-related macular degeneration (AMD) that is basically treated by anti-vascular endothelial growth factor (VEGF) therapies including pegaptanib (Macugen), ranibizumab (Lucentis) and bevacizumab (Avastin).
17-20
Nevertheless, such strategy is an invasive treatment modality that may be inevitably associated with some serious adverse consequences. Systemic administrations are also considered as suitable methods for a number of pharmaceuticals that possess deisred physicochemical and biopharmaceutical characteristics. The ocular diseases pharmacotherapy via subconjunctival and periocular (sub-Tenon’s and peribulbar) routes are deemed to provide prolonged pharmacologic impacts with low toxicity. The uses of conventional dosage forms in the ocular diseases, despite showing some benefits, have some pitfalls including (a) necessity for the repetitive use of medicament that results in a poor patient compliance, (b) difficulty of insertion in the case of ocular inserts, and (c) being considered as an invasive approach when injected/implanted that is also associated with some tissue damages too. Fig. 3 represents the currently codified routes of drug administration for the ophthalmic diseases.
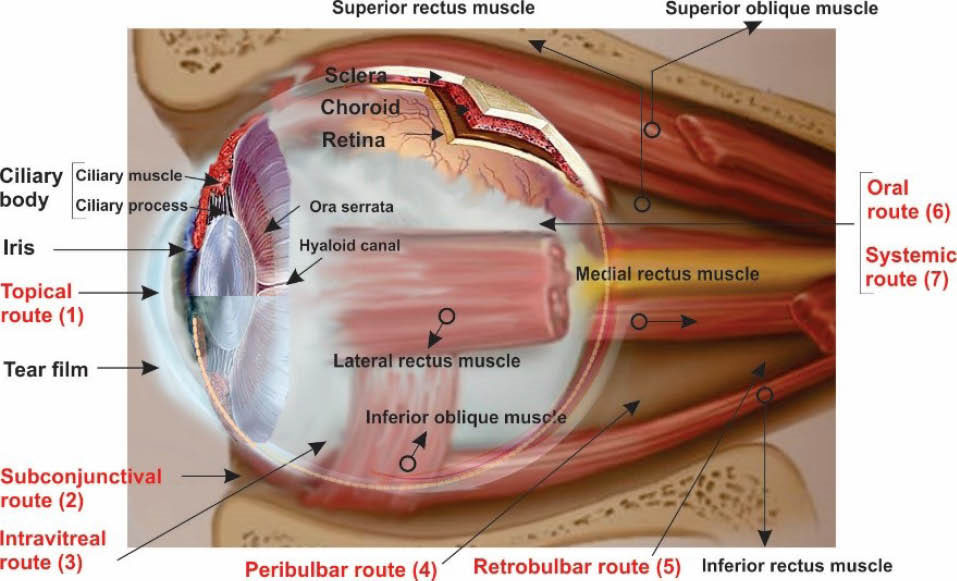
Fig. 3
.
Main routes for the administration of ophthalmologic medicaments. Administered ophthalmologic drugs face with several important physiologic and anatomic modulation and hindrance that make the eye exceedingly impermeable to exogenous substances, including the corneal epithelial barrier against topical dosage forms, non-corneal structures inability in absorption of foreign compounds, lacrimation, effective drainage through the nasolacrimal system and the excellent function of inner endothelial and outer epithelial barriers of retina. Drug administration to the eye is accomplished through non-invasive or invasive methods codified by the US FDA.
.
Main routes for the administration of ophthalmologic medicaments. Administered ophthalmologic drugs face with several important physiologic and anatomic modulation and hindrance that make the eye exceedingly impermeable to exogenous substances, including the corneal epithelial barrier against topical dosage forms, non-corneal structures inability in absorption of foreign compounds, lacrimation, effective drainage through the nasolacrimal system and the excellent function of inner endothelial and outer epithelial barriers of retina. Drug administration to the eye is accomplished through non-invasive or invasive methods codified by the US FDA.
Challenges to reach the anterior segment of the eye
To reach the desired target sites of the eye either locally or systemically, the ocular drugs must cross the ocular static, dynamic and metabolic functions. For instance, DuraSite system can be customized to deliver a wide variety of potential drug agents, which has been developed by InSitVision Inc. DuraSite™ is a drug delivery vehicle that enables stabilization of small molecules in a polymeric mucoadhesive matrix that has been used as a carrier for several ophthalmic drugs including azithromycin (AzaSite PlusTM; ISV-502), dexamethasone (DexaSiteTM; ISV-305), bromfenac (BromSiteTM; ISV-303), tetracycline (ISV-102) and prostaglandin (ISV-620; ISV-215). Likewise, Table 1 represents some of the recently developed ophthalmic medicines used for treatment of ocular diseases in the anterior segment of the eye.
Table 1
.
The recently developed ophthalmic medicines for the anterior segment of the eye
|
Drug, dosage form
|
Brand, manufacturer or stage of development
|
Clinical indication
|
Main excipient(s)
|
Ref
|
Azithromycin
Topical ophthalmic solution
|
AzaSite™, Inspire Pharmaceuticals Inc. |
Bacterial conjunctivitis |
DuraSite® drug delivery technology, Polycarbophil |
21,22
|
| Azithromycin/Dexamethasone, Topical ophthalmic solution |
AzaSite Plus™ (ISV-502), InSite Vision Inc. |
Blepharoconjunctivitis |
DuraSite® drug delivery technology, Polycarbophil |
23
|
Bromfenac
Topical ophthalmic solution
|
BromSite™ (ISV-303); InSite Vision Inc. |
Ocular inflammation; Pain; Cataract |
DuraSite® drug delivery technology, Polycarbophil |
24,25
|
Dexamethasone,
Topical ophthalmic solution
|
DexaSite™(ISV-305); InSite Vision Inc. |
Ocular inflammation such as blepharitis |
DuraSite® drug delivery technology, Polycarbophil |
24
|
Timolol maleate
Topical ophthalmic gelling vehicle
|
Rysmon™ TG; Wakamoto Pharmaceutical Co. |
Glaucoma; ocular hypertension |
Thermoresponsive gel |
26,27
|
Timolol maleate
Topical ophthalmic solution
|
Various brands and pharmaceutical companies (Glunil, Lotim Plus, Xalacom, Misopt, Latocom) |
Glaucoma |
Hydroxpropyl methylcellulose |
- |
Betaxolol
Topical ophthalmic solution
|
Betoptic S™; Alcon |
Glaucoma |
Amberlite® IRP-69 |
28-30
|
Tafluprost
Ophthalmic solution
|
DE-085; Santen Pharmaceutical Co. Ltd. |
Glaucoma, ocular hypertension |
Solution |
31
|
Lomerizine HCl
Ophthalmic solution
|
DE-090; Santen Pharmaceutical Co. Ltd. |
Glaucoma, ocular hypertension |
Solution |
- |
Tafluprost/timolol maleate
Ophthalmic solution
|
DE-111; Santen Pharmaceutical Co. Ltd. |
Glaucoma, ocular hypertension |
Solution |
- |
Adenosine A2A
Ophthalmic solution
|
DE-112; Santen Pharmaceutical Co. Ltd. |
Open-angle glaucoma, ocular hypertension |
Solution |
- |
Latanoprost
topical ophthalmic emulsion
|
Catioprost™; Santen Pharmaceutical Co. Ltd. |
Glaucoma and ocular
hypertension
|
Cationic emulsion |
32
|
Tobramycin/Dexamethasone
Topical ophthalmic solution
|
TobraDex™ ST |
Blepharitis |
Xanthan gum |
33,34
|
| Cationic ophthalmic emulsion |
Cationorm™, Santen Pharmaceutical Co. Ltd. |
Dry eye symptoms |
Cationic emulsion |
35,36
|
Cyclosporine
Cationic ophthalmic emulsion
|
Vekacia™; Santen Pharmaceutical Co. Ltd. |
Vernal keratoconjunctivitis |
Cationic emulsion |
37
|
Cyclosporine
Cationic ophthalmic emulsion
|
Cyclokat™; Santen Pharmaceutical Co. Ltd. |
Dry eye
Vernal keratoconjunctivitis
|
Cationic emulsion |
38
|
Epinastine HCl
Ophthalmic solution
|
DE-114; Nippon Boehringer Ingelheim Co., Ltd |
Allergic conjunctivitis |
Solution |
- |
Peptide combination
Ophthalmic solution
|
DE-105; Santen Pharmaceutical Co. Ltd. |
Persistent corneal epithelial defect |
Solution |
- |
Diquafosol sodium
Ophthalmic solution
|
DE-089; Santen Pharmaceutical Co. Ltd. |
Corneal/Conjunctival disease
(Dry eye)
|
Solution |
39
|
Rivoglitazone
Ophthalmic suspension
|
DE-101; Santen Pharmaceutical Co. Ltd. |
Corneal/Conjunctival disease
(Dry eye)
|
Suspension |
|
Ketotifen
Soft contact lens
|
|
Allergic conjunctivitis |
Poly(HEMA-co-AA-co-AM-co-NVP-co-PEG200DMA) soft contact lenses |
40
|
Latanoprost
Subconjunctival insert
|
|
Glaucoma, ocular hypertension |
Various polymers |
41
|
Cyclosporine
Episcleral implant
|
Lucida (LX201), Lux Biosciences, Inc. |
Aeratoconjunctivitis
|
Biosilicone
|
42
|
Impacts of corneal barrier
Topically applied drugs face various static (corneal epithelium, corneal stroma, and blood–aqueous barrier) and dynamic (conjunctival blood flow, lymph flow, and tear drainage) barrier functions of the anterior segment while the involved cells are able to control trafficking of the penetrated drugs through regulating the expression of inward and outward transport machineries and even pose metabolic functions on them.
1
Such biological structures can selectively control the transportation of substances within the eye, in which the epithelial and/or endothelial cells are sealed by the tight junctional complexes. Fig. 4 schematically illustrates the corneal barrier.
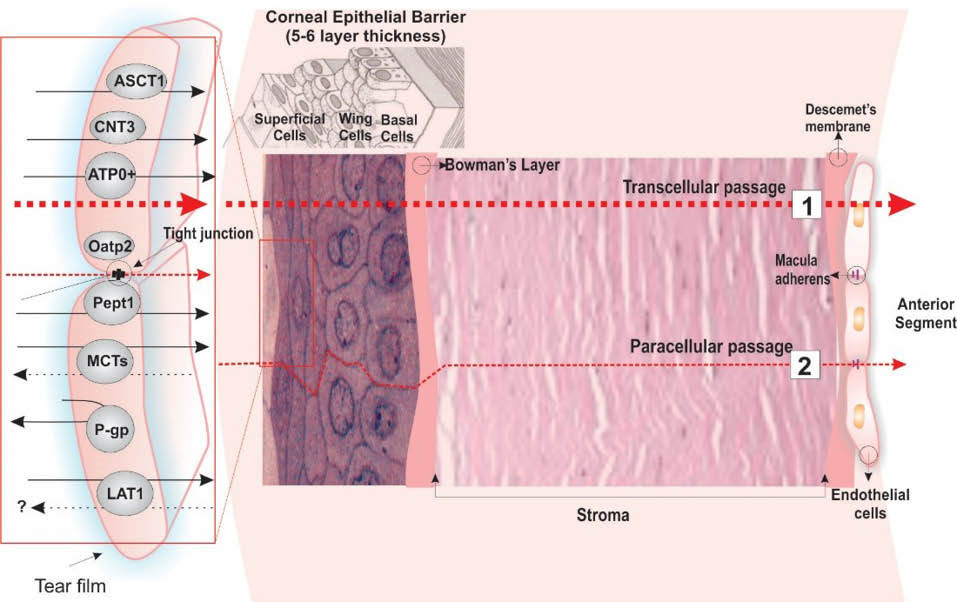
Fig. 4
.
Schematic illustration of the corneal structure. The corneal epithelial cells form 5-6 layers of cells composed of superficial, wing and basal cells creating the static barrier of the cornea. Corneal epithelial barrier (CEB) house some important transport machineries that function in favor of mechanism of CEB through selective regulation of inward and outward traverse of exogenous substances. The passive diffusion of lipophilic compounds as transcellular passage is the main drug penetration path into the anterior segment.
.
Schematic illustration of the corneal structure. The corneal epithelial cells form 5-6 layers of cells composed of superficial, wing and basal cells creating the static barrier of the cornea. Corneal epithelial barrier (CEB) house some important transport machineries that function in favor of mechanism of CEB through selective regulation of inward and outward traverse of exogenous substances. The passive diffusion of lipophilic compounds as transcellular passage is the main drug penetration path into the anterior segment.
The layers of corneal epithelial cells (i.e., superficial, wing and basal cells) forms an excellent tight barrier restrictiveness, in which functional expression of tight junctions prevent paracellular trafficking of topical drugs into the anterior segment while the transcellular trafficking of lipophilic drugs may face with selective modulations of carrier-mediated transporters such as P-gp.
Tear film and lacrimation
It should be noted that the amount and composition of the tear film (the so-called tearing or lacrimation) have profound impact on the healthiness of the ocular surface including the cornea and the conjunctiva, and is largely tightly controlled by the regulation of the orbital glands and also the secretion capacity of the ocular surface epithelia.
43
Anatomically, the organelles involved in tearing include lacrimal gland, superior and inferior lacrimal puncta, lacrimal sac, superior and inferior lacrimal canals and nasolacrimal canal.
The lightly buffered aqueous fluid forms the tear film (pH ~7.2–7.5) on the surface of the cornea. Under normal condition, when the ophthalmic medicine is used it can be washed away by the normal physiologic function of tear film that has a turnover rate of 15%-30% per min with the tear volume restoration of 2-3 min, resulting in loss of the applied medicine within the first 15–30 seconds. As a result, the bioavailability of localized therapy in ocular diseases is very low (less than 5%), in large part because of lacrimation and poor drug penetration. In addition, the penetrated drug molecules are also subjected to the absorption by the conjunctival sac and drainage through the nasal cavity and adsorption by the capillaries of the iris.
44-46
To overcome such physiologic impediments, nanoscaled medications such as nanosuspension, nanoemulsions and nanoparticles which are able to remain within the local target tissues for a longer period of time or to control the release of encapsulated/incorporated drugs are imperative.
47-53
Further, it should be noted that the drug release pattern from nanoscaled formulations is largely dependent upon the physicochemical properties of carriers and drugs. The tear film (with a pH range of 7.3–7.7) is composed of nutrients, electrolytes, proteins, lipids and mucin. It maintains the health of the cornea and conjunctiva, in which the tear proteins (e.g., lysozyme, secretory immunoglobulin IgA, lactoferrin, lipocalin, and peroxidase) provide anti-bacterial/viral potential.
54
So far, various formulations have been devised to circumvent these physiologic impediments such as gel-forming systems (e.g., Timoptol-LA and Timoptol-XE, used to treat glaucoma) and mucoadhesives polymers/liposomes and microdiscs, which are also able to prolong the desired pharmacologic activities of the incorporated drug molecules within ocular tissue.
55-64
For example, to improve the ocular bioavailability of ciprofloxacin hydrochloride (CPX), mucoadhesive chitosan (CS)-coated liposomes were formulated by means of the thin film hydration technique using L-alpha-phosphatidylcholine (PC), cholesterol (CH), stearylamine (SA) and dicetyl phosphate (DP), for which CS was used to coat the liposomes.
61
This study revealed a prolonged in vitro release of CPX from the CS-coated liposomes and a high bioavailability of ciprofloxacin. In another study, CS or Carbopol (CP) coated niosomal timolol maleate formulations as mucoadhesive ophathalmic drug delivery systems were developed and pharmacodynamically evaluated in albino rabbits through assessing the intraocular pressure (IOP) by a non-contact pneumatonometer.
65
As compared to a commercially available in situ gel-forming solution of timolol (Timolet GFS, 0.5%; Sun Pharma), it was found that CS and CP coated niosomes carrying timolol maleate can extend the drug release for up to 8 h and 6 h respectively, and decreasing the cardiovascular adverse reaction of drug. All these highlight that mucoadhesive ophthalmic formulations, in particular as nanosystems (NSs), can provide a better treatment modality for controlled and prolonged delivery of drugs into the anterior segment of the eye.
Corneal and/or noncorneal routes
The corneal and/or noncorneal are the main routes for the local drug delivery to the eye,
56,66-69
hence advanced biocompatible stimuli-responsive formulations as well as biodegradable implants
70,71
may provide much safer methods. However, it should be noted that drug delivery across the conjunctiva and sclera into the intraocular tissues is low in large part because of the functional presence of the local capillary beds that removes the drug from target sites to the general circulation. Despite such pitfall, many drugs (e.g., timolol maleate, gentamicin, and prostaglandin PGF2α) have poor corneal permeability, and hence intraocular delivery via the conjunctiva and sclera may be the best choice in particular when the subconjunctival implants are used as the drug depot.
70,72
Peng et al engineered two microfilms using poly [d,l-lactide-co-glycolide] (PLGA) and poly[d,l-lactide-co-caprolactone] (PLC) and evaluated their biocompatibility in rabits upon subconjunctival implantation. They reported that both microfilms showed degradation and surface erosion kinetics with no significant inflammation or vascularization as tested by serial slit-lamp microscopy.
Blood-aqueous barrier
In anterior segment of the eye, the endothelium of the iris/ciliary blood vessels and the non-pigmented epithelium of the ciliary body cell layers form the BAB that displays tight junctional complexes (Fig. 2). The main function of this regulating barrier is to selectively control of the traverse of solutes between the posterior and anterior segments, maintaining the transparency of the eye and the chemical composition of the ocular fluids.
73
It should be pointed out that the capillaries of the ciliary are fenestrated and leaky to macromolecules such as horse radish peroxidase (HRP) with molecular weight of 40 kDa. The microvasculature barrier of iris that controls the travers of the plasma proteins into the aqueous humor is tight,
74
while the traverse of substances from the aqueous humor into the systemic circulation through the capillary endothelia of iris is less restricted and permeated drugs into the aqueous humor can be washed away from the anterior segment by the iris blood vessels.
75
In the anterior segment, small lipophilic drugs are prone to entering into the uveal blood circulation via BAB and subsequent elimination much more rapidly than hydrophilic drugs and macromolecules whose elimination occur solely by aqueous humor turnover. In fact, because of the continuous drainage of the aqueous humor with turnover rate of 2.0–3.0 mL/min, drugs within the anterior segment are not able to enter the posterior segment. Taken all, the locally administered drugs are not able to reach beyond the anterior segment, failing to provide required pharmacological concentration in the posterior segment components such as vitreous, retina and choroid.
76
The repeated systemic and intravitreal injections appear to be the remaining options in the clinic that are also associated with some inevitable consequences.
Controlled drug delivery to anterior segment of the eye
Table 2 represents the FDA approved ophthalmologic drugs from 2000 to 2016. Several advanced medical devices and implants have been clinically examined for their clinical potentials. For example, Lacrisert, a sterile hydroxypropyl cellulose insert, has wieldy been used in the inferior cul-de-sac of the eye for lubricating, stabilizing and thickening the precorneal tear film and prolonging the tear film breakup in patients with dry eye states and keratoconjunctivitis sicca.
77-79
Accordingly, a study upon the efficacy of lacrisert in subsets of patients (418 patients including 86 contact lens wearers, 79 with cataract diagnosis, 52 with prior cataract surgery, 22 with prior laser-assisted in situ keratomileusis, and 15 with glaucoma) with dry eye syndrome has resulted in significant improvement in the quality of life.
79
Table 2
.
FDA approved drugs for ophthalmologic applications (from 2000 to 2016)
|
Drug
|
Clinical indication
|
Brand, manufacturer
|
Approved year
|
| Tasimelteon |
Treatment of non-24-hour sleep-wake disorder in the totally blind |
Hetlioz ™,Vanda Pharmaceuticals |
January 2014 |
| Phenylephrine and ketorolac injection |
For use during eye surgery to prevent intraoperative miosis and reduce post-operative pain |
Omidria™,Omeros |
June 2014 |
| Sweet Vernal, Orchard, Perennial Rye, Timothy and Kentucky Blue Grass Mixed Pollens Allergen Extract |
Treatment of grass pollen-induced allergic rhinitis with or without conjunctivitis |
Oralair™,Greer Labs |
April 2014 |
| Cysteamine hydrochloride |
Treatment of corneal cystine crystal accumulation due to cystinosis |
Cystaran™,Sigma Tau Pharmaceuticals |
October 2012 |
| Ocriplasmin |
Treatment of symptomatic vitreomacular adhesion |
Jetrea™,Thrombogenics |
October 2012 |
| Ranibizumab injection |
Treatment of diabetic macular edema |
Lucentis™, Genentech |
August 2012 |
| Tafluprost ophthalmic solution |
Treatment of elevated intraocular pressure |
Zioptan ™, Merck |
February 2012 |
| Aflibercept |
Treatment of neovascular (wet) age-related macular degeneration |
Eylea™, Regeneron Pharmaceuticals |
November 2011 |
| Gatifloxacin ophthalmic solution |
Treatment of bacterial conjunctivitis |
Zymaxid™,Allergan |
May 2010 |
| Ketorolac tromethamine |
Treatment of pain and inflammation following cataract surgery |
Acuvail ™,Allergan |
July 2009 |
| Bepotastine besilate ophthalmic solution |
Treatment of itching associated with allergic conjunctivitis |
Bepreve™,Ista Pharmaceuticals |
September 2009 |
| Besifloxacin ophthalmic suspension |
Treatment of bacterial conjunctivitis |
Besivance ™, Bausch & Lomb |
June 2009 |
| Dexamethasone |
Treatment of macular edema following branch retinal vein occlusion or central retinal vein occlusion |
Ozurdex ™, Allergan |
June 2009 |
| Ganciclovir ophthalmic gel |
Treatment of acute herpetic keratitis |
Zirgan ™, Sirion Therapeutics |
September 2009 |
| Lidocaine hydrochloride |
For anesthesia during ophthalmologic procedures |
Akten™,Akorn |
October 2008 |
| Azelastine hydrochloride nasal spray |
Treatment of seasonal and perennial allergic rhinitis |
Astepro™,Meda Pharmaceuticals Inc |
October 2008 |
| Difluprednate |
Treatment of inflammation and pain associated with ocular surgery |
Durezol™,Sirion Therapeutics |
June 2008 |
| Azithromycin |
Treatment of bacterial conjunctivitis |
AzaSite™,InSite Vision |
April 2007 |
| Ranibizumab |
Treatment of neovascular (wet) age related macular degeneration |
Lucentis™,Genentech |
June 2006 |
| Pegaptanib |
Treatment of wet age-related macular degeneration |
Macugen™,Pfizer / Eyetech Pharmaceuticals |
December 2004 |
| Cyclosporine ophthalmic emulsion |
Treatment of low tear production |
Restasis™,Allergan |
December 2002 |
| Bimatoprost ophthalmic solution |
For the reduction of intraocular pressure in patients with open-angle glaucoma or ocular hypertension |
Lumigan™, Allergan |
March 2001 |
| Travoprost ophthalmic solution |
For the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension |
Travatan™, Alcon |
March 2001 |
| Valganciclovir HCl |
For the treatment of cytomegalovirus retinitis in patients with AIDS |
Valcyte™, Roche |
March 2001 |
| Levobetaxolol hydrochloride suspension |
For lowering IOP in patients with chronic open-angle glaucoma or ocular hypertension |
Betaxon™, Alcon |
February 2000 |
| Levofloxacin |
For treatment of bacterial conjunctivitis |
Quixin™, Santen |
August 2000 |
| Unoprostone isopropyl ophthalmic solution) 0.15%; |
For the treatment of open-angle glaucoma or ocular hypertension |
Rescula™, Ciba Vision |
August 2000 |
| Verteporfin for injection |
For the treatment of wet age-related macular degeneration (wet AMD) |
Visudyne™, QLT |
April 2000 |
The glaucoma is considered as the second leading cause of blindness, several treatment modalities have been devised to control the glaucoma. Of the anti-glaucoma agents, brimonidine tartrate (BT) is currently widely used, while patient’s compliance to BT therapy is low. To tackle this issue, a BT-liberating ocular insert has been engineered using poly(lactic co-glycolic) acid (PLGA) or polyethylene glycol (PEG) with a linear BT-release profile and smooth surfaces.
80
In fact, the ocular insert has significantly advanced the treatment of various eye disease. These DDSs are sterile, thin, multilayered, drug-incorporated solid/semisolid systems that are placed into the cul-de-sac or conjuctival sac. Being composed of a polymeric scaffold, they provide increased ocular residence and sustained-release of ophthalmic drugs. The liberation of drugs from these DDSs, depending on the physicochemical properties of vehicle and drug, may occur by simple diffusion, osmosis, and bioerosion or even stimuli responsive.
81
Emergence of the bacterial infection after implanting an artificial corneal scaffold is a well-known issue that brings about serious complication, while the anti-bacterial impacts of conventional antibiotic therapy such as topical vancomycin is limited, in large part because of low bioavailability and high dosing requirement. To prevail such issues, a number of researchers have focused on development of the antibiotic-eluting corneal prosthesis with prolonged liberation of scaffold-impregnated drug molecules. In one study, an artificial corneal scaffold was produced by the incorporation of vancomycin in thick collagen hydrogel, which resulted in a sustained drug elution for up to 7 days. Once implanted intrastromally in rabbit corneas replacing the stromal tissue, the vancomcyin was detectable in the aqueous humor for up to 10 days. Upon intrastromal injection of Staphylococcus aureus inoculate on day 2 postimplantation, the implanted corneas remained clear and nonedematous on day 3 postinfection, showing a marked reduction in S. aureus in comparison with the blank hydrogel-implanted corneas that developed excessive inflammation and edematous. Such drug-eluting corneal implants appear to provide an excellent preventive scaffold on the cornea inhibiting development of bacterial infections.
82
As shown in Fig. 5A, the ocular punctal plugs (OPPs, the so-called tear duct plugs) with/without active agents are small medical device inserted into the tear duct to block the duct and possibly release desired drugs.
83
The OPPs are usually made from collagen and hence are dissolvable, which can further be advanced to become thermosensitive systems usable for the long-term treatment of the dry eye,
84
even though some side effects such as conjunctivitis may limit their applications.
85,86
Of the ocular devices, the intacs corneal inserts or implants (Fig. 5B), are considered as a minimally invasive surgical option that are primarily used for the treatment of keratoconus. They have originally been approved by the US FDA for the surgical treatment of mild myopia in 1999, and then in 2006 FDA announced it as a therapeutic device, which have been tested worldwide for the safety and efficacy.
87-89
These devices have good potential to become drug-impregnated system to ensure upon the prevention of consequences such as infection.
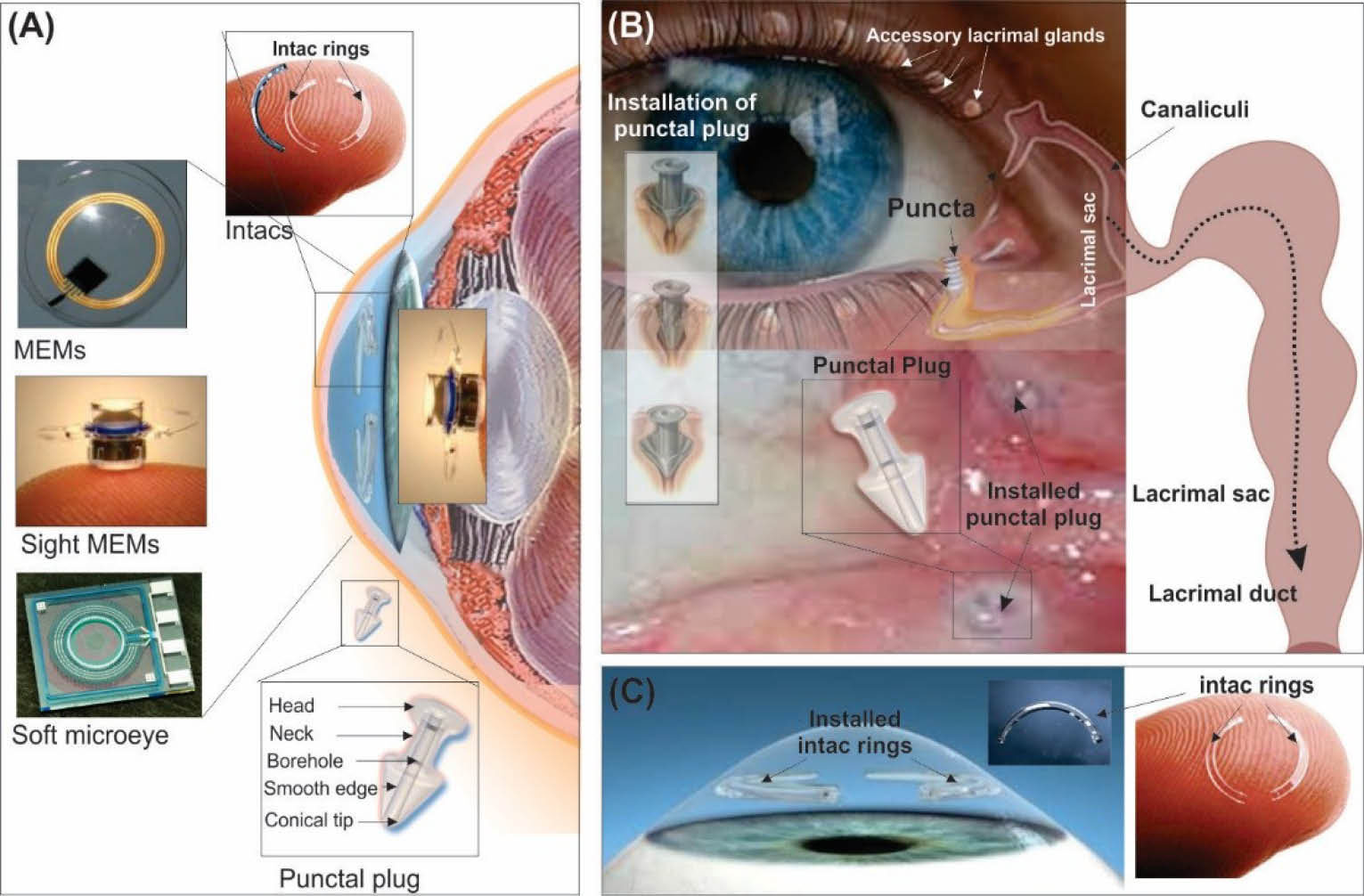
Fig. 5
.
Selected ocular medical devices. A) Different medical devices used in the anterior segment of the eye, including sight MEMs, soft microeye, intac and punctal plug. The ocular punctal plugs (panel A) and intacs (panel B) used for controlling the dry eye symptoms and Keratoconus, respectively. Punctal plug alone or as impregnated with drug molecules is installed into the punctum. Drug-impregnated punctal plugs can be devised as stimuli sensitive system. Intracorneal rings, intacs, are surgically installed between the layers of the corneal stroma – one crescent ring on each side of the pupil. Readers are directed to see excellent electronic sources in the following URLs from where images were adapted: http://www.allaboutvision.com; http://www.eyedocs.co.uk ; http://www.bouldereyesurgeons.com and http://www.iqlaservision.com as well as MEMS Journal.
.
Selected ocular medical devices. A) Different medical devices used in the anterior segment of the eye, including sight MEMs, soft microeye, intac and punctal plug. The ocular punctal plugs (panel A) and intacs (panel B) used for controlling the dry eye symptoms and Keratoconus, respectively. Punctal plug alone or as impregnated with drug molecules is installed into the punctum. Drug-impregnated punctal plugs can be devised as stimuli sensitive system. Intracorneal rings, intacs, are surgically installed between the layers of the corneal stroma – one crescent ring on each side of the pupil. Readers are directed to see excellent electronic sources in the following URLs from where images were adapted: http://www.allaboutvision.com; http://www.eyedocs.co.uk ; http://www.bouldereyesurgeons.com and http://www.iqlaservision.com as well as MEMS Journal.
Further, flexible silicon hydrogels have been devised as flexible contact lens for continuous daily uses including narafilcon A (Acuvue TruEye), lotrafilcon B (Air Optix) and balafilcon A (BAUSCH & LOMB PureVision™). These silicon based systems can be used to depot necessary ophthalmic drugs.
90-94
Table 3 represents some selected ophthalmic drug delivery systems or devices for controlled delivery of medicines to the anterior segment of the eye.
Table 3
.
Selected ophthalmic devices/systems for controlled delivery of medicines to the anterior segment of the eye
|
Drug delivery system
|
Clinical trial description
|
Clinical indication
|
Sponsor/Collaborator
|
Latanoprost punctal plug
delivery system (L-PPDS)
|
A Study of the L-PPDS With Adjunctive Xalatan® Eye Drops in Subjects With OH or OAG (ID: NCT01037036; Phase 2) |
Ocular hypertension (OH); open-angle glaucoma (OAG) |
Mati Therapeutics Inc.; QLT Inc. |
| Punctal plug |
Safety and Efficacy of Punctal Plug Insertion in Patients With Dry Eye (ID: NCT01684436; Phase 4) |
Dry Eye |
Allergan |
| Perforated Punctal Plugs |
Perforated Punctal Plugs for Treatment of Papillary Conjunctivitis in Otherwise Healthy Patients (ID: NCT02503956;) |
Epiphora; Conjunctivitis |
Rabin Medical Center; Alpha Net Co. Ltd. |
| L-PPDS |
Comparison of Latanoprost PPDS With Timolol Maleate GFS in Subjects With Ocular Hypertension or Open-Angle Glaucoma (ID: NCT02014142; Phase 2) |
OH; OAG |
Mati Therapeutics Inc. |
| L-PPDS |
A Safety Study of the Latanoprost Punctal Plug Delivery System (L-PPDS) in Subjects With Ocular Hypertension or Open Angle Glaucoma (ID: NCT00820300; Phase 2) |
Glaucoma; OH; OAG |
Mati Therapeutics Inc. |
| Sirolimus |
Subconjunctival Sirolimus for the Treatment of Autoimmune Active Anterior Uveiti (ID: NCT00876434) |
Anterior Uveitis |
National Eye Institute (NEI) |
| Gamunex-C |
Subconjunctival IVIg (Gamunex-C) Injection for Corneal Neovascularization and Inflammatory Conditions (ID: NCT02042027; Phase 1) |
Corneal Neovascularization
Corneal Graft Failure
Anterior Segment Inflammation
|
University of Utah |
Timolol; Bimatoprost
Ocular Insert
|
Dose-Ranging Study of the Bimatoprost Ocular Insert (ID: NCT02358369; Phase 2) |
Glaucoma; OH; OAG |
ForSight Vision5, Inc. |
| DiscoVisc®; Healon®; Amvisc® Plus |
To Compare the Ability of DiscoVisc® OVD to Protect the Corneal Endothelium and Maintain Anterior Chamber Space With Healon® and Amvisc® PLUS During Cataract Surgery (ID: NCT00763360; Phase 4) |
Cataract |
Alcon Research |
Therefore, de novo treatment modalities such as subconjunctival implants and polymeric depot systems and medical devices that can be injected/implanted directly into the vitreous may provide a long-term sustained-release treatment modalities that are yet to be fully examined for their impacts in a long period use.
Novel intraocular drug delivery technologies
As an axiom, any ophthalmic dosage form must provide suitable biopharmaceutical characteristics as well as an appropriate ocular tolerability in particular when used for treatment of intraocular diseases. However, the topical administration of ophthalmic solutions (nearly 90% as eye drops) fail to meet such criteria. In fact, an excellent restrictiveness of the tight corneal epithelia controls the permeation of drug molecules into the anterior segment of the eye, and the penetrated molecules are significantly washed away by the capillary bed of the iris/ciliary body and/or lacrimation. As a result, very little part of the topically administered drug molecules can reach the posterior segment of the eye. To tackle such shortcomings, we need to use systemic route for the administration of ophthalmic drugs such as anti-glaucoma agents, corticosteroids and certain antibiotics, nevertheless their use through systemic route face with restrictive barrier functionality of BRB as well as BAB. Alternatively, we must advance intravitreal DDSs or devices to be able to deliver the acquired doses of the designated drugs into the posterior segment of the eye and maintain the therapeutic concentration for a long period of time.
9
Drug delivery into the posterior segment of the eye
As a thin film light-sensitive tissue, the retina covers the entire inner wall of the eye. The retina includes two main biological barriers that control the entrance of blood-circulating substances into the posterior segment. Such biological barriers together with the functional presence of BAB make drug delivery into the posterior segment a very challenging issue.
Anatomically, in addition to the retinal endothelial cells that form the BRB, the inner part of retina encompasses several cells and tissue including neural cells and glial cells (i.e., Müller cells, astrocytes, microglial cells and oligodendroglial cells). While within the middle part of the retina the photoreceptor cells (rods and cons) are located over the epithelial cells, the outmost part of the retina includes a single layer of specialized pigmented cuboidal epithelial cells that form the excellent impediment function of RPE barrier. The impediment functions of the RPE and BRB are tightly coupled within retina, hence these two barriers of retina play imperative roles in intravitreal drug delivery and targeting. Fig. 6 shows the cellular organization of the retina.
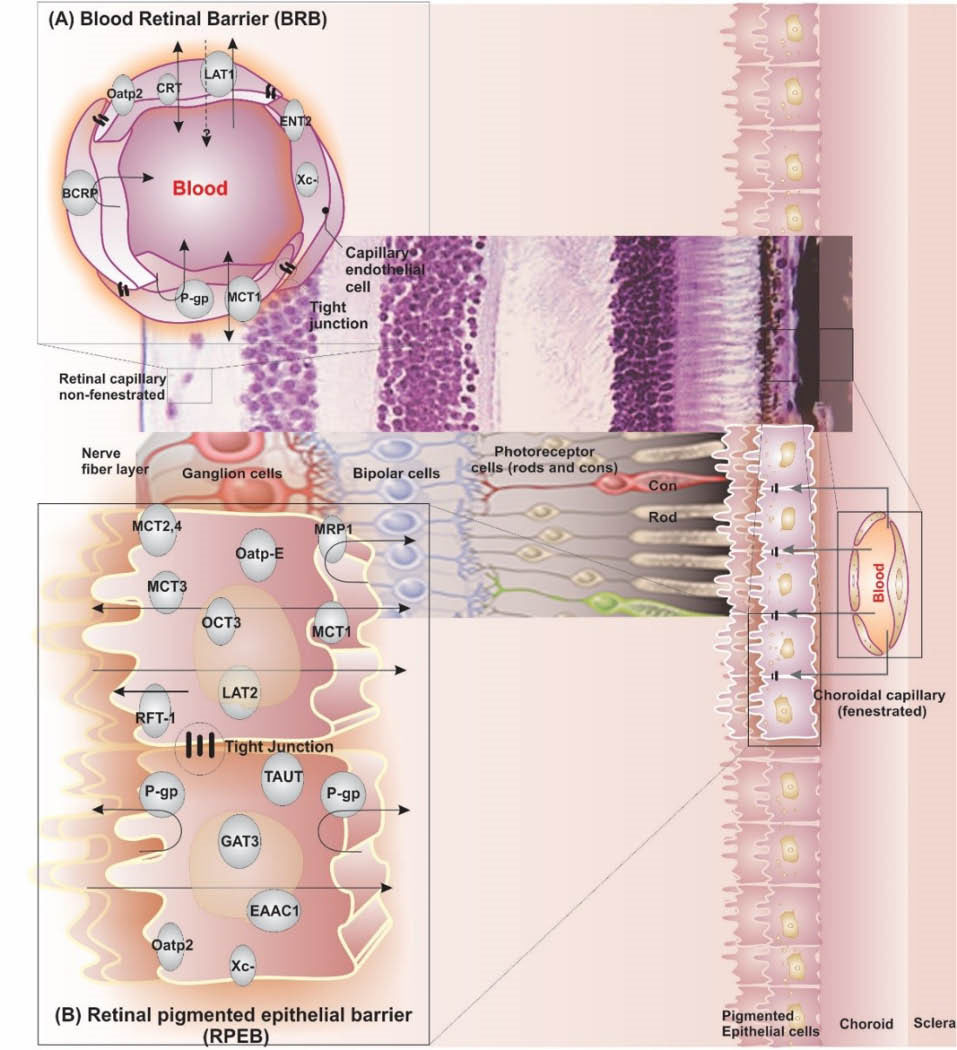
Fig. 6
.
The retinal cellular structure. A) The inner blood retinal endothelial cells (RECs) form the blood-retinal barrier (BRB). B) The outer pigmented epithelial cells (PECs) form the retinal pigmented epithelial barrier (PREB). As polarized cells, both retinal endothelial and pigmented epithelial cells possess tight junctions and traverse of nutrients are selectively controlled by transport machineries as carrier-mediated transportation and receptor-mediated transportation.
.
The retinal cellular structure. A) The inner blood retinal endothelial cells (RECs) form the blood-retinal barrier (BRB). B) The outer pigmented epithelial cells (PECs) form the retinal pigmented epithelial barrier (PREB). As polarized cells, both retinal endothelial and pigmented epithelial cells possess tight junctions and traverse of nutrients are selectively controlled by transport machineries as carrier-mediated transportation and receptor-mediated transportation.
In early 1990s, to achieve much greater compliance, some important sustained-release ophthalmologic DDSs were developed to control ocular diseases. Since then, few success story encouraged researchers to continue on developing more advanced DDSs for the targeted therapy of ocular ailments. In 1996, Vitrasert™ (ganciclovir-loaded implant) was approved for the treatment of cytomegalovirus retinitis that is associated with late-stage AIDS causing blindness. This DDS liberates the ganciclovir directly to the target site for a long period of time up to 6-8 months, which has successfully been used for the treatment of a large number of patients over the past decades. In addition to VitrasertTM, sustained DDSs can be used as an intravitreal implants including Retisert™ (fluocinolone-loaded implant) and Iluvien™ (fluocinolone-loaded implant) whose drug liberation occurs for up to 3 years, while similar DDSs seem to be necessary for the anterior segment of the eye too.
4
These developments appear as the resurgence of advanced intravitreal DDSs shown schematically in Fig. 7.
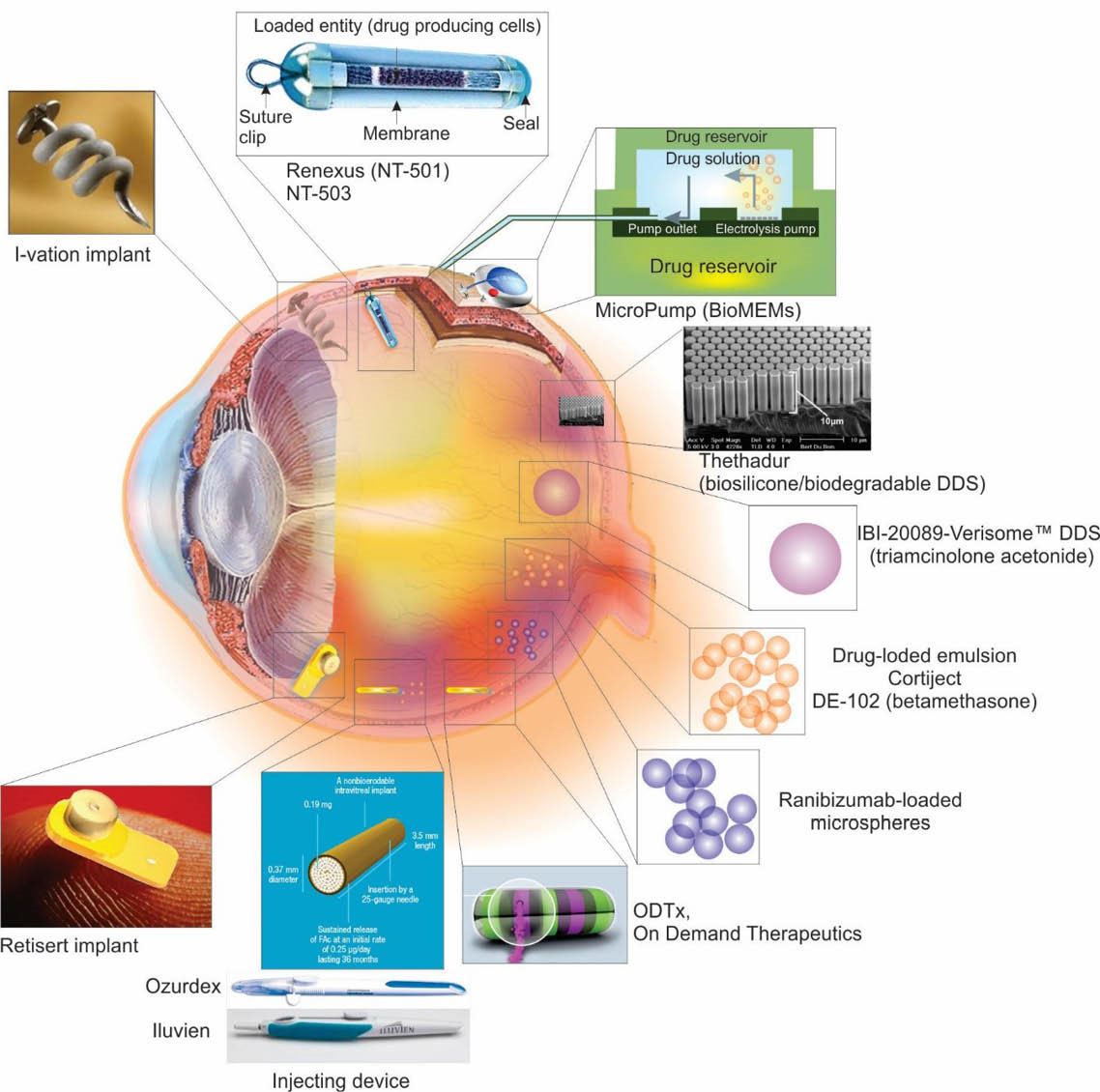
Fig. 7
.
Advanced intravitreal drug delivery systems and devices.
.
Advanced intravitreal drug delivery systems and devices.
Advanced intravitreal drug delivery systems and devices
Ideally, liberation of the ophthalmic drugs from the DDS/delivery device in the posterior segment of the eye should be performed as sustained/controlled-release or even on-demand by an external stimuli. Most of these sustained-release DDSs are injected intravitreally once without any further needs for repeated injections, while some of the intravitreal devices such as micropumps are refillable systems. Of these, the nanoscaled biodegradable DDSs and sol-gel injectable hydrogels are novel effective ophthalmologic formulations that are deemed to provide maximal clinical benefits with minimal side effects. So far a number of advanced DDSs and devices has significantly improved the intravitreal drug delivery and targeting. Among them, smart NSs were shown to be able to efficiently circumvent the related barriers, enter into the posterior segment of the eye and pose minimal adverse reactions.
95
Selected advanced DDSs and devices used to deliver ocular drugs into the posterior segment of the eye are shown in Fig. 7 and some selected forms are listed in Table 4.
Table 4
.
Selected ophthalmic devices/systems for controlled delivery of medicines to the posterior segment of the eye
|
Drug, dosage form
|
Brand, manufacturer or stage of development
|
Clinical indication
|
Main excipient/device
|
Betamethasone
Sub-Tenon injection of sustained-release microsphere
|
DE-102; Santen Pharmaceutical Co. Ltd. and Oakwood Laboratories |
Macular edema associated with diabetes and branch retinal vein occlusion |
Biodegradable microspheres |
Sirolimus
Intravitreal injections
|
DE-109; Santen Pharmaceutical Co. Ltd. and Oakwood Laboratories |
Non-infectious uveitis of the posterior eye |
A slowly dissolving depot |
| Biosilicon DDS for small molecules and macromolecules |
Thethadur; pSivida Corp. |
Intraocular diseases |
Honeycomblike nanostructured bioerodible/non-erodible porous silicon |
| Ganciclovir ophthalmic implant |
Vitrasert™, Zirgan™; pSivida Corp. |
Cytomegalovirus (CMV) ocular infection |
Sustained-release silicon implant |
| Fuocinolone-loaded ophthalmic implant |
Retisert™; pSivida Corp. |
Chronic non-infectious posterior uveitis |
Sustained-release silicon implant |
| Fuocinolone-loaded ophthalmic implant |
Iluvien™; pSivida Corp. |
Chronic non-infectious posterior uveitis |
Sustained-release silicon implant |
Lntanoprost
ophthalmic implant
|
Durasert™; pSivida Corp. |
Ocular hypertension and glaucoma |
Sustained-release biodegradable implant |
dexamethasone
Intravitreal implant
|
Ozurdex™; Allergan, Inc. |
Diabetic macular edema (DME) |
Sustained-release silicon implant |
Dexamethasone
Injectable ophthalmic emulsion
|
Cortiject™; Santen Pharmaceutical Co. Ltd. |
DME; noninfectious inflammation of the uvea; central retinal vein occlusion (CRVO) |
Injectable ophthalmic emulsion containing a corticosteroid prodrug |
| Triamcinolone ophthalmic injection |
IBI-20089 Verisome™; Icon Bioscience |
Cystoid macular edema (CME) |
Sustained-release intravitreal lipid-based DDS as verisome (translucent liquid to form gel in the eye) |
| BioSilicon-based protein delivery system |
Tethadur; pSivida Corp. |
Neuroprotective agents |
Sustained-release biodegradable (PLGA)/biosilicon implant |
Brimonidine
Intravitreal implant
|
NCT02087085; Allergan Inc. |
Geographic atrophy, Macular degeneration |
Intravitreal Implant |
Bimatoprost
Sustained-release DDS
|
NCT02250651; Allergan Inc. |
Glaucoma, Open-Angle
Ocular Hypertension
|
Sustained-release DDS |
triamcinolone acetonide
Sustained-release implant
|
I-vation™; Surmodics Inc. |
DME |
Sustained-release DDS
poly(methyl methacrylate), ethylene-vinyl acetate copolymer
|
| Laser activated injectable DDS |
ODTx |
Different diseases in posterior segment of the eye |
Controled drug delivery by activating specific reservoirs |
Ciliary neurotrophic factor (CNTF) producing cells
Semipermeable
hollow fiber membrane
|
Renexus (NT-501); Renexus Group & Noah Group |
Atrophic age-relate macular degeneration (AMD) |
Semipermeable
hollow fiber membrane with ciliary neurotrophic factor (CNTF) producing cells [retinal pigment epithelium (RPE)]
|
| VEGF receptor Fc-fusion protein (VEGFR-Fc)-releasing cells |
NT-503; Neurotech |
AMD |
Semipermeable
hollow fiber membrane with ciliary neurotrophic factor (CNTF) producing cells [retinal pigment epithelium (RPE)]
|
Triamcinolone acetonide (TA)
Injection device for DDS
|
iTrack microcatheter; iScience Interventional |
neovascular AMD |
Injection device for suprachoroidal delivery of TA |
| Refilled with drug solution to provide long-term pharmacotherapy |
|
Different diseases in posterior segment of the eye |
Microelectromechanical systems (MEMS) as refillable DDD |
Ranibizumab
Refillable port drug delivery system (PDS)
|
Genentech, ForSight Vision 4 Inc. |
ADM |
Ranibizumab controlled-release PDS |
Ocular nanomedicines
Having considered the biological impediments in the posterior segment of the eye imposed mainly by the retinal barriers, the ocular nanomedicines can locally/systemically be administered for the delivery of the drug molecules into the vitreous depending on their size and architecture. These nanoscaled DDSs are deemed to provide sustained/controlled liberation of drugs and prolonged pharmacologic impacts. Such objective can be achieved by localized retention in the cul-de-sac, where the entrapped drug can be liberated based on a simple diffusion mechanism or by the means of an external stimuli such as photodynamic therapies from conveying NS. The nanoscaled ophthalmic formulations can provide longer exposure time at the ocular surface by confronting the clearance mechanisms of the eye providing more drug concentration , reducing the dose and frequency of drug administration,
96
a number of various nanostructured DDSs have also been developed for the intravitreal applications.
97,98
Thus far, different advanced polymer-/lipid-based soft matters have been used for development of a number of nanostructures including nanoparticles (NPs), nanomicelles, nanoemulsions, nanosuspensions, nanocapsules. To attain the best utility of nanomedicines, various parameters should be considered including (a) lipophilic-hydrophilic properties of the advanced materials used, (b) the biocompatibility of the advanced soft materials in association with ocular tissues such as precorneal pocket and subconjunctival tissue, (c) longevity and durability of the NS in biological microenvironment of the eye, (d) drug release profile with or without external stimuli, and (e) the retention efficiency of the NS in the ocular tissues such as precorneal pocket.
99-103
To achieve an optimized effect in the case of topical nanoformulations, it is highly desirable to engineer bioadhesive/mucoadhesive NSs to enhance the reactivity and bioavailability of NSs in the ocular cul-de-sac.
104
Such aims seem to be achievable by using various micellar colloidal NSs, emulsion nanoformulation, biodegradable polymeric NPs, hydrogel based NSs.
105
For instance, amphiphilic molecules with hydrophilic head and hydrophobic tail termed as surfactants can be used for formulation of nanomicelles. These polarized molecules can be found in different status as dipolar/zwitterionic (e.g., dioctanoyl phosphatidyl choline), charged or anionic/cationic (e.g., sodium dodecyl sulfate as an anionic surfactant; dodecyltrimethylammonium bromide as a cationic surfactant), or neutral/non-ionic (e.g., ethylene oxide (N-dodecyl; tetra, C12E4), vitamin E TPGS [d-alpha tocopheryl polyethylene glycol (PEG) 1000 succinate], octoxynol-40). Nanosized micelles are formed when surfactants are dissolved in water at concentration above critical micelle concentration (CMC) under sonication/agitation, showing different architecture (spherical, cylindrical, or planar/discs/bilayers) depending on the aggregation driving force(s). These NSs can be used for controlled topical/intraocular drug delivery.
99
Zhang et al. formulated dexamethasone (Dex)-loaded PLGA NPs (Dex-NPs) and evaluated their pharmacokinetics and tolerability in rabbits after intravitreal injection.
106
Based on some key ophthalmic examinations (e.g., intraocular pressure measurement, and B-scan ocular ultrasonography), they claimed that the injection of Dex-NPs induce no abnormalities even after 50 days post-injection in rabbits, while the Dex-NPs were able to maintain a sustained liberation of drug for a long period of time (i.e., up to 50 days) in vitreous with a fairly constant level of drug for up to 30 days. The rabbits treated with Dex-NPs showed significantly higher bioavailability of the drug as compared the control group injected with Dex alone.
Jo et al. studied the antiangiogenic impacts of silicate nanoparticles (SiNPs) on the retinal neovascularization, and showed that the SiNPs impose no direct toxicity in the retinal tissues. The intravitreal injection of nanoparticles was able to substantially reduce the anomalous retinal angiogenesis in oxygen-induced retinopathy mice. They claimed that the SiNPs can markedly inhibit the VEGF-elicited angiogenesis through blockage of activation ERK 1/2.
107
On the basis that the intravitreal injection of DDSs/implants demands relatively large 22 gauge needle, novel dipeptide (phenylalanine-alpha,beta-dehydrophenylalanine; Phe-Phe) based nanotubes (PNTs) were designed by Panda et al.
108
The self-assembled PNTs (with a diameter of ~15-30 nm and a length of ~1500 nm) were designed for the intravitreal delivery of pazopanib with suitable loading efficiency and bioavailability. No cytotoxic impacts were found on the human retinal pigment epithelial (ARPE-19) cells by the PNTs. Once injected, after a period of 15 days in vivo, the PNTs were found to retain the drug levels in the vitreous humor, retina, and choroid-RPE respectively 4.5, 5, and 2.5-folds higher than that of the plain drug.
To treat the macular degeneration and diabetic retinopathy, intravitreal injections every 4-8 weeks are inevitable – a treatment modality that is undoubtedly considers as an invasive uncomfortable retinal damaging intervention. Hence, Huu et al developed a novel stimuli-responsive NP-based reservoir platform for the delivery of nintedanib (BIBF 1120) which is a small molecule angiogenesis inhibitor.
109
To this end, the researchers capitalized on a far ultraviolet (UV) light-degradable polymer to be able to trigger the liberation of the drug molecules on-demand. Once injected, the NSs were found to be able to keep the encapsulated drug molecules in the vitreous for up to 30 weeks without inducing significant inadvertent side effects, while the liberation of cargo drug molecules was plausible through emission of far UV. They showed that the choroidal neovascularization (CNV) in rats can be suppressed 10 weeks after injection of nintedanib carrying NPs.
Hydrogels as novel intravitreal DDSs
Hydrogels are defined as crosslinked polymeric networks capable of holding large quantities of water or other biological fluids due to the presence of hydrophilic groups or domains within their porous polymeric structure. They are synthesized from both naturally and synthetic polymers by chemical or physical crosslinking methods.
110-112
The key properties associated with hydrogels are their remarkable characteristics such as hydrophilicity, flexibility, elasticity and high water content. All these features make them suitable entities with broad application spectra in different biomedical fields such as tissue engineering, regenerative medicine, protein separation, matrices for cell encapsulation and devices for the controlled release of drugs and proteins.
In general, hydrogels show great compatibility with biological settings and their hydrophilic surface has a low interfacial free energy in contact with body fluids. Such characteristics make hydrogels to show low tendency of adhering to proteins and cells, and hence less aggregation. Moreover, the soft and rubbery nature of the hydrogels minimizes irritation to surrounding tissue.
112-115
Of the hydrogels, the stimuli-responsive or environmentally-sensitive hydrogels (the so-called smart hydrogels) offer the fantastic swelling – deswelling characteristics (as volume collapse or phase transition) in response to presented physical or chemical stimuli (Fig. 8). They have been used in diverse applications including production of artificial muscles, biomimetic biosensor/bioactuator, immobilization of enzymes and cells, bioseparation and self-regulated DDSs. In situ gel formation makes hydrogels very favorable for the delivery of ophthalmic small and macromolecular drugs as well as tissue engineering. This system provides safe applications in vivo.
116,117
Multiple stimuli-responsive hydrogels have attracted significant research interest because the most pH- and temperature-sensitive dual functional systems have a great importance in biological applications and can mimic the responsive macromolecules founds in nature.
118,119
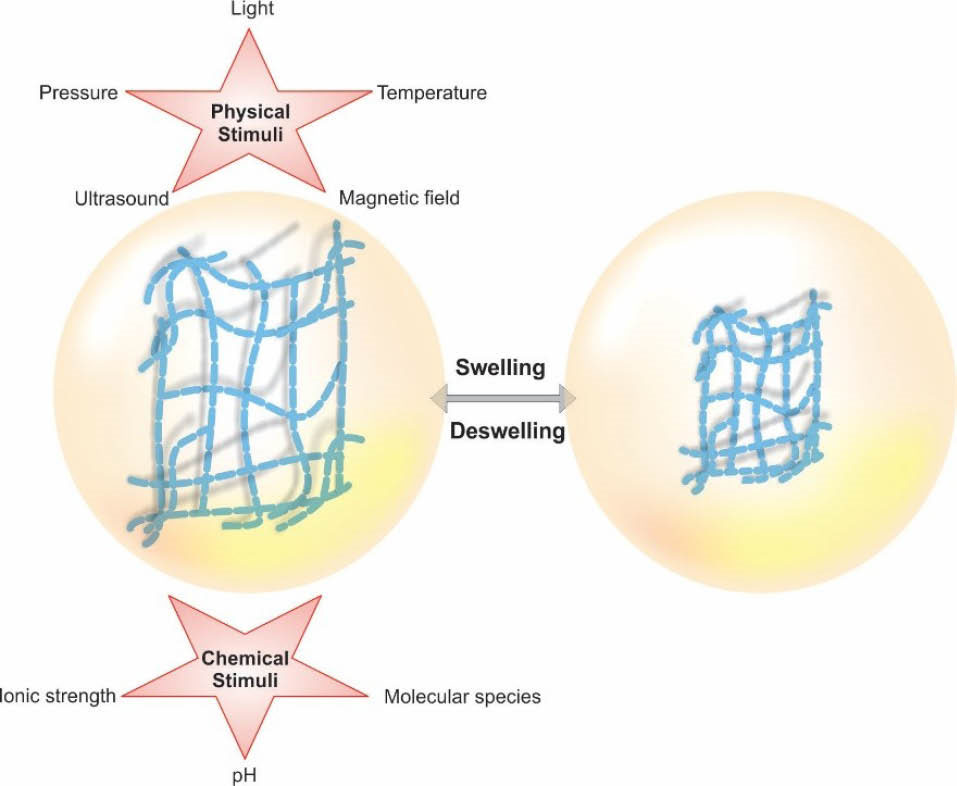
Fig. 8
.
Schematic representation of stimuli-responsive smart hydrogels.
.
Schematic representation of stimuli-responsive smart hydrogels.
As for the ocular applications, it should be noted that the stroma of the cornea is a naturally occurring hydrogel (a water-swollen polymer network), and the basic physicochemical principle of a swelling hydrogel should be applicable to this layer.
120
Further, it should be also pointed out that the vitreous humor as a clear gel fills the posterior segment of the eye. It is composed of 98%-99% water and a type of collagen called vitrosin that forms a network of collagen type II fibrils in association with glycosaminoglycan, hyaluronan, opticin (a protein belonging to a small leucine-rich repeat protein family), and a wide spectra of other soluble proteins, as a result its viscosity is 2-4 folds higher than that of water. The vitreous humor, unlike the continuously replenished aqueous humor, has a gelatinous consistency and is stagnant. Taken all, the intravitreal hydrogels should mimic such characteristics.
Among various types of polymers, for the intraocular drug delivery, the bioadhesive polymers are deemed to provide a better means. Of these, hydroxypropyl cellulose, hydroxypropyl methylcellulose, chitosan, dextran and poly(acrylic acid) derivatives (e.g., carbomer 934 and polycarbophil) have been reported as the most appropriate polymers.
In addition, the high viscosity of the carbomer hydrogels results in the prolonged retention, improving the ocular bioavailability of some drugs. In addition to the effective treatment of edema on the ocular surface
121-123
and applications in soft silicone lenses using polymers like poly (2–hydroxyethyl methacrylate), the hydrogels have been used for the intravitreal delivery of active pharmaceutical agents such as small molecules and macromolecules.
124-130
Intravitreal implants and devices
In an ideal world, the intravitreal DDSs/DDDs should be degraded imposing trivial/no detrimental impacts on the cells/tissue in the posterior segment of the eye. Despite usefulness of the nanoscaled biodegradable NSs in treatment of ocular diseases, some devices used for intravitreal drug delivery are non-biodegradable such as silicone implants/inserts and micro-pumps (Fig. 7). These devices, which contain drug molecules or drug-producing cells, can be either injected or surgically installed in the vitreous to deliver the designated drug. Here, we provide some concise information upon these implants/devices.
Encapsulated cell technology (ECT)
Renexus (NT-501) developed by Neurotech Pharmaceuticals offers an encapsulated cell technology that provides extracellular delivery of ciliary neurotrophic factor (CNTF). The system encompasses the genetically modified human retinal pigment epithelial (RPE) cells (NTC-200 cell line), which can be implanted into the vitreous. As an in-house cellular reactor, the system is able to secrete the recombinant human CNTF and maintain the drug concentration at constant desired doses for a long period of time. RPE cells are mounted on the polyethylene terephthalate yarn scaffold and the produced drug molecules can be released from the device through semipermeable hollow fiber membrane. This ingenious device can protect the mounted exogenous cells from the endogenous immune response of the host while they survive within the vitreous and produce CNTF for inhibition of photoreceptor degradation in patients with retinitis pigmentosa.
131-133
Neurotech Pharmaceuticals has also developed another cell-based DSS named NT-503 ECT that encompasses VEGF receptor Fc-fusion protein (VEGFR-Fc)-releasing cells. Having compared to ranibizumab, this cell-based treatment modality showed significantly higher (20-30 folds higher) VEGF neutralization with a drug liberation longevity for up to 1 year in the rabbit vitreous. Based on a phase I and II trial commenced in 2014, this VEGF neutralizing ECT has been being used for the treatment of recurrent CNV secondary to AMD.
131
Implants
Here, we provide a concise update on some ocular DDDs that are in clinic or under clinical trial. Of these, I-vation (Surmodics Inc.) is a sustained delivery system that is used as an intravitreal implant to deliver triamcinolone acetonide (TA). It is a titanium helical coil that is coated with poly(methyl methacrylate) and ethylene-vinyl acetate and the polymer coat has been loaded with TA
131
Further, On Demand Therapeutics has developed intravitreal non-biodegradable implant called ODTx that contains reservoir of drug molecules whose liberation can be triggered by low-energy laser during eye examination.
In addition to few biodegradable intravitreal NSs (see Table 3), pSivida has developed Thethadur which is porous nanoscaled honeycomblike silicon (biosilicon) or biodegradable polymeric device. It can be loaded with the designated drug molecules (e.g., small molecules and biologics such as antibodies) that can be released in a controlled manner once implanted into the vitreous.
Refillable devices
Of the refillable microelectromechanical systems (MEMs), MicroPumps developed by Replenish Inc. is DDD that can be used for the treatment of chronic and refractory ocular diseases such as DME.
In a study, its safety and surgical feasibility has been evaluated as the first-in-man ocular implant of a novel posterior MicroPump DDD in DME patients, proving its safety for a period of 90 days and possibility for its refiling that makes multiple programmable drug delivery feasible.
134
The effectiveness and biocompatibility of the MicroPump DDD has further been followed up in a one-year feasibility study.
135
These systems are deemed to last over a 5-year time course before further need for the replacement.
Final remarks and outlook
The eye possesses bio-micro-machineries (i.e., tear film, corneal epithelia, the ciliary epithelium and capillaries of the iris, retina endothelia and epithelia) that selectively restrictively control the entry of exogenous substances (both topical compounds and blood-borne molecules) into the anterior and posterior segments.
Although the restrictive physiological and biological barriers, functionality of the eye retain the normal function of the eye, it makes ocular drug delivery and targeting very challenging.
The locally administered drugs, mostly as topical ophthalmic solutions, must cross the tear film (with fast turnover) and the corneal epithelial barrier. Once entered into the anterior segment, the drug molecules are often subjected to the absorption by the conjunctival sac and the capillaries of the iris.
While the drug molecules in the anterior segment can scarcely enter the posterior segment of the eye, the systemically delivered drugs face with BRB and cannot reach the desired pharmacologic concentration. As a result, the novel DDSs and DDDs aim to elongate the pharmacologic presence of drug molecules in the ocular segments or tissues to perform maximal therapeutic benefits with minimal undesired side effects.
To date, a number of DDSs/DDDs have been used for increasing the bioavailability of drugs in the ocular segments based on nanoscaled colloidal systems,
136
nanobioadhesives,
137
stimuli-responsive hydrogels,
118,138
subconjunctival implants,
70,72
refillable DDDs such as MEMs/NEMs-based micropumps and episcleral exoplants
139-141
as well as encapsulated cell technology.
142
Remarkable characteristics of these systems in extended liberation of drugs in particular for the posterior segment of the eye make some of these advanced systems as the sole treatment modality option for life-devastating diseases such as AMD and DME.
Extra developments are needed to tackle some genetic defects that affect the ocular systems such as mucopolysaccharidoses (MPSs), which are a class of disorders triggered by hereditary genetic defects in lysosomal enzymes resulting in extensive intracellular and extracellular accumulation of glycosaminoglycans (GAGs).
143
In the eye, MPSs (e.g., MPS I such as IH/S (Hurler/Scheie) and MPS IH (Hurler) because of deficiency in a-L-iduronidase; MPS VI Maroteaux-Lamy due to deficiency in N-acetylgalactosamine-4-sulfatase) manifest as corneal opacification, optic nerve swelling and atrophy, ocular hypertension, and glaucoma.
143
To tackle these ocular malfunctions, one powerful, yet simple, approach seems to be the use of injectable stimuli-responsive and transforming hydrogels that can be used for delivery of gene-based nanomedicines into the eye by a single injection. Once injected into the ocular segment/tissue, it can be triggered to liberate the cargo drug molecules on-demand.
144
While the advanced treatment modalities for the systemic manifestations of MPSs is bone marrow transplant, the enzyme replacement therapies such as Aldurazyme (laronidase) for MPS I and Naglazyme (galsulfase) for MPS VI appear to be the only treatment modalities for these orphan diseases.
In fact, after proof-of-technologies and several success stories of ocular implants (e.g., Ocusert(1974) for 1 week constant release of pilocarpine from conjunctiva; Vitrasert (1996) for 6 months constant release of ganciclovir from the pars plana area of vitreous; Retisert(2005) for 2.5 years constant release of fluocinolone acetonide; intravitreal injectable biodegradable (Posurdex) and non-biodegradable (Medidur) implants, wouldn’t it be fantastic if we implement these technologies or even the encapsulated cell technology or smart hydrogels and multimodal nanomedicines to treat such diseases?
To achieve such goals, we need to empower the translational medicine researches as well as the leading researchers who tackle these issues through capitalizing on new biomimicry technologies in a holistic manner. Such endeavor needs to be advocated by both governmental and private sectors.
Ethical approval
There is none to be declared.
Competing interests
No competing interests to be disclosed.
Acknowledgments
Authors would like to acknowledge the Research Center for Pharmaceutical Nanotechnology at Tabriz University of Medical Sciences for the financial support.
Review Highlights
What is current knowledge?
simple
-
√ Lacrimation, corneal epithelial barrier (CEB) and bloodaqueous
barrier (BAB) restrictedly control entrance of
topically applied pharmaceuticals into the anterior and
posterior segments of the eye.
-
√ Retinal endothelial and epithelial barriers, together
with BAB, can selectively control traverse of blood-borne
substances and systemically administered pharmaceuticals
into the posterior segment of the eye.
-
√ Currently used ophthalmic medicines need to be repeatedly
administered to maintain the required concentrations of
drug.
-
√ Long-acting ocular drug delivery systems (DDSs)
and devices (DDDs) loaded with small molecules or
macromolecules can maintain required intravitreal
concentration for a long period
What is new here?
simple
-
√ Stimuli-responsive DDSs/DDDs with on-demand drug
liberation potential can provide maximal impacts with
minimal side effects.
-
√ Nano-scaled targeted DDSs provide much more selective
pharmacological effects.
-
√ Long-term safety of ocular implants needs to be fully
addressed.
References
- Cholkar K, Patel SP, Vadlapudi AD, Mitra AK. Novel strategies for anterior segment ocular drug delivery. J Ocul Pharmacol Ther 2013; 29:106-23. doi: 10.1089/jop.2012.0200 [Crossref] [ Google Scholar]
- Barar J, Asadi M, Mortazavi-Tabatabaei SA, Omidi Y. Ocular drug delivery: impact of in vitro cell culture models. Journal of Ophthalmic & Vision Research 2009; 4:238-52. [ Google Scholar]
- Willoughby CE, Ponzin D, Ferrari S, Lobo A, Landau K, Omidi Y. Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function–a review. Clinical & Experimental Ophthalmology 2010; 38:2-11. [ Google Scholar]
- Kompella UB, Kadam RS, Lee VH. Recent advances in ophthalmic drug delivery. Ther Deliv 2010; 1:435-56. doi: 10.4155/TDE.10.40 [Crossref] [ Google Scholar]
- Boddu SH, Gunda S, Earla R, Mitra AK. Ocular microdialysis: a continuous sampling technique to study pharmacokinetics and pharmacodynamics in the eye. Bioanalysis 2010; 2:487-507. doi: 10.4155/bio.10.2 [Crossref] [ Google Scholar]
- Tojo K. A pharmacokinetic model for ocular drug delivery. Chem Pharm Bull (Tokyo) 2004; 52:1290-4. [ Google Scholar]
- Jones RF, Maurice DM. New methods of measuring the rate of aqueous flow in man with fluorescein. Exp Eye Res 1966; 5:208-20. doi: 10.1016/S0014-4835(66)80009-X [Crossref] [ Google Scholar]
-
Maurice DM, Μισηιµα S. Ocular Pharmacokinetics. In: Sears ML, editor. Pharmacology of the Eye. New York: Springer-Verlag; 1984.
- Barar J, Javadzadeh AR, Omidi Y. Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin Drug Deliv 2008; 5:567-81. doi: 10.1517/17425247.5.5.567 [Crossref] [ Google Scholar]
-
Omidi Y, Gumbleton M. Biological Membranes and Barriers. In: Mahato RI, editor. Biomaterials for Delivery and Targeting of Proteins Nucleic Acids. New York: CRC Press; 2005. p. 232-74.
- Barar J, Gumbleton M, Asadi M, Omidi Y. Barrier functionality and transport machineries of human ECV304 cells. Med Sci Monit 2010; 16:BR52-60. [ Google Scholar]
- Omidi Y, Campbell L, Barar J, Connell D, Akhtar S, Gumbleton M. Evaluation of the immortalised mouse brain capillary endothelial cell line, bEnd3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res 2003; 990:95-112. [ Google Scholar]
- Smith M, Omidi Y, Gumbleton M. Primary porcine brain microvascular endothelial cells: biochemical and functional characterisation as a model for drug transport and targeting. J Drug Target 2007; 15:253-68. doi: 10.1080/10611860701288539 [Crossref] [ Google Scholar]
- Omidi Y, Barar J, Ahmadian S, Heidari HR, Gumbleton M. Characterization and astrocytic modulation of system L transporters in brain microvasculature endothelial cells. Cell Biochem Funct 2008; 26:381-91. doi: 10.1002/cbf.1455 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Impacts of blood-brain barrier in drug delivery and targeting of brain tumors. Bioimpacts 2012; 2:5-22. doi: 10.5681/bi.2012.002 [Crossref] [ Google Scholar]
- Antonetti D. Eye vessels saved by rescuing their pericyte partners. Nat Med 2009; 15:1248-9. doi: 10.1038/nm1109-1248 [Crossref] [ Google Scholar]
- Ng EW, Shima DT, Calias P, Cunningham ET, Jr Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nature reviews Drug discovery 2006; 5:123-32. doi: 10.1038/nrd1955 [Crossref] [ Google Scholar]
- Spitzer MS, Yoeruek E, Sierra A, Wallenfels-Thilo B, Schraermeyer U, Spitzer B. Comparative antiproliferative and cytotoxic profile of bevacizumab (Avastin), pegaptanib (Macugen) and ranibizumab (Lucentis) on different ocular cells. Graefes Arch Clin Exp Ophthalmol 2007; 245:1837-42. doi: 10.1007/s00417-007-0568-7 [Crossref] [ Google Scholar]
- Ni Z, Hui P. Emerging pharmacologic therapies for wet age-related macular degeneration. Ophthalmologica 2009; 223:401-10. doi: 10.1159/000228926 [Crossref] [ Google Scholar]
- Algvere PV, Kvanta A, Seregard S. Shall we use Avastin or Lucentis for ocular neovascularization?. Acta Ophthalmol 2008; 86:352-5. doi: 10.1111/j.1755-3768.2008.01317.x [Crossref] [ Google Scholar]
- Si EC, Cheung PS, Bowman L, Hosseini K. Ocular pharmacokinetics of AzaSite Xtra-2% azithromycin formulated in a DuraSite delivery system. Curr Eye Res 2009; 34:485-91. [ Google Scholar]
- Opitz DL, Harthan JS. Review of Azithromycin Ophthalmic 1% Solution (AzaSite((R))) for the Treatment of Ocular Infections. Ophthalmol Eye Dis 2012; 4:1-14. doi: 10.4137/OED.S7791 [Crossref] [ Google Scholar]
- Hosseini K, Hutcheson J, Lindstrom RL. A Phase III clinical study to evaluate the efficacy of combined azithromycin and dexamethasone in the treatment of blepharoconjunctivitis. Clin Ophthalmol 2013; 7:2225-34. doi: 10.2147/OPTH.S52474 [Crossref] [ Google Scholar]
- Harris E. Industry Update: The latest developments in therapeutic delivery. Ther Del 2013; 4:659-65. [ Google Scholar]
- Hosseini K, Hutcheson J, Bowman L. Aqueous humor concentration of Bromfenac 009%(Bromday) compared with Bromfenac in DuraSite 0075%(Bromsite) in cataract patients undergoing phacoemulsification after 3 days dosing. Invest Ophthal Vis Sci 2013; 54:5061-
. [ Google Scholar]
- Ohno Y, Iga T, Yamada Y, Nagahara M, Araie M, Takayanagi R. Pharmacokinetic and pharmacodynamic analysis of systemic effect of topically applied timolol maleate ophthalmic gelling vehicle (Rysmon TG). Curr Eye Res 2005; 30:319-28. doi: 10.1080/02713680590923294 [Crossref] [ Google Scholar]
- Kawase K, Lin W, Aoyama Y, Yamamoto T, Shimazawa M, Hara H. Effects of timolol-related ophthalmic solutions on cultured human conjunctival cells. Jpn J Ophthalmol 2010; 54:615-21. doi: 10.1007/s10384-010-0881-2 [Crossref] [ Google Scholar]
- Trope GE, Liu GS, Basu PK. Toxic effects of topically administered Betagan, Betoptic, and Timoptic on regenerating corneal epithelium. J Ocul Pharmacol 1988; 4:359-66. [ Google Scholar]
- Fry LL. Comparison of the postoperative intraocular pressure with Betagan, Betoptic, Timoptic, Iopidine, Diamox, Pilopine Gel, and Miostat. J Cataract Refract Surg 1992; 18:14-9. [ Google Scholar]
- Yarangumeli A, Kural G. Are there any benefits of Betoptic S (betaxolol HCl ophthalmic suspension) over other beta-blockers in the treatment of glaucoma?. Expert Opin Pharmacother 2004; 5:1071-81. doi: 10.1517/14656566.5.5.1071 [Crossref] [ Google Scholar]
- Izumi N, Nagaoka T, Sato E, Mori F, Yoshida A. DE–085 Increases Retinal Blood Flow. Invest Ophthal Vis Sci 2004; 45:2340-
. [ Google Scholar]
- Daull P, Buggage R, Lambert G, Faure MO, Serle J, Wang RF. A comparative study of a preservative-free latanoprost cationic emulsion (Catioprost) and a BAK-preserved latanoprost solution in animal models. J Ocul Pharmacol Ther 2012; 28:515-23. doi: 10.1089/jop.2011.0245 [Crossref] [ Google Scholar]
- Scoper SV, Kabat AG, Owen GR, Stroman DW, Kabra BP, Faulkner R. Ocular distribution, bactericidal activity and settling characteristics of TobraDex ST ophthalmic suspension compared with TobraDex ophthalmic suspension. Adv Ther 2008; 25:77-88. doi: 10.1007/s12325-008-0019-9 [Crossref] [ Google Scholar]
- Potter WS, Nelson LB, Raber IM. Corneal graft rejection in a child and inadvertent substitution of Tobrex for TobraDex. Ophthalmic Surg 1990; 21:671-2. [ Google Scholar]
- Kinnunen K, Kauppinen A, Piippo N, Koistinen A, Toropainen E, Kaarniranta K. Cationorm shows good tolerability on human HCE-2 corneal epithelial cell cultures. Exp Eye Res 2014; 120:82-9. doi: 10.1016/j.exer.2014.01.006 [Crossref] [ Google Scholar]
- Pinheiro R, Panfil C, Schrage N, Dutescu RM. Comparison of the lubricant eyedrops Optive(R), Vismed Multi(R), and Cationorm(R) on the corneal healing process in an ex vivo model. Eur J Ophthalmol 2015; 25:379-84. doi: 10.5301/ejo.5000593 [Crossref] [ Google Scholar]
- Souza JG, Dias K, Pereira TA, Bernardi DS, Lopez RF. Topical delivery of ocular therapeutics: carrier systems and physical methods. J Pharm Pharmacol 2014; 66:507-30. [ Google Scholar]
- Buggage RR, Amrane M, Ismail D, Deniaud M, Lemp MA, Baudouin C. The Effect of Cyclokat(preservative-free cyclosporine 01% cationic emulsion) on Dry Eye Disease Signs and Symptoms in Sjogren and non-Sjogren Patients with Moderate to Severe DED in a Phase III Randomized Clinical Trial. Invest Ophthal Vis Sci 2012; 53:576. [ Google Scholar]
- Colligris B, Crooke A, Huete-Toral F, Pintor J. An update on dry eye disease molecular treatment: advances in drug pipelines. Expert Opin Pharmacother 2014; 15:1371-90. [ Google Scholar]
- Tieppo A, White CJ, Paine AC, Voyles ML, McBride MK, Byrne ME. Sustained in vivo release from imprinted therapeutic contact lenses. J Control Release 2012; 157:391-7. doi: 10.1016/j.jconrel.2011.09.087 [Crossref] [ Google Scholar]
- Cheng YH, Hung KH, Tsai TH, Lee CJ, Ku RY, Chiu AW. Sustained delivery of latanoprost by thermosensitive chitosan-gelatin-based hydrogel for controlling ocular hypertension. Acta Biomater 2014; 10:4360-6. doi: 10.1016/j.actbio.2014.05.031 [Crossref] [ Google Scholar]
- Bock F, Matthaei M, Reinhard T, Bohringer D, Christoph J, Ganslandt T. High-dose subconjunctival cyclosporine a implants do not affect corneal neovascularization after high-risk keratoplasty. Ophthalmology 2014; 121:1677-82. doi: 10.1016/j.ophtha.2014.03.016 [Crossref] [ Google Scholar]
-
Dartt DA, Hodges RR, Zoukhri D. Tear and Their Secretion. In: Fischbarg J, editor. The Biology of Eye. New York: Academic Press; 2006. p. 21-82.
- Leeming JP. Treatment of ocular infections with topical antibacterials. Clin Pharmacokinet 1999; 37:351-60. [ Google Scholar]
- Urtti A. Delivery of antiglaucoma drugs: ocular vs systemic absorption. J Ocul Pharmacol 1994; 10:349-57. [ Google Scholar]
- Hamalainen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci 1997; 38:627-34. [ Google Scholar]
- Souto EB, Doktorovova S, Gonzalez-Mira E, Egea MA, Garcia ML. Feasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugs. Curr Eye Res 2010; 35:537-52. doi: 10.3109/02713681003760168 [Crossref] [ Google Scholar]
- Badawi AA, El-Laithy HM, El Qidra RK, El Mofty H, El dally M. Chitosan based nanocarriers for indomethacin ocular delivery. Arch Pharm Res 2008; 31:1040-9. doi: 10.1007/s12272-001-1266-6 [Crossref] [ Google Scholar]
- Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Development of dorzolamide hydrochloride in situ gel nanoemulsion for ocular delivery. Drug Development and Industrial Pharmacy 2010; 36:1330-9. doi: 10.3109/03639041003801885 [Crossref] [ Google Scholar]
- Akhter S, Talegaonkar S, Khan ZI, Jain GK, Khar RK, Ahmad FJ. Assessment of ocular pharmacokinetics and safety of Ganciclovir loaded nanoformulations. J Biomed Nanotechnol 2011; 7:144-5. [ Google Scholar]
- Adibkia K, Siahi Shadbad MR, Nokhodchi A, Javadzedeh A, Barzegar-Jalali M, Barar J. Piroxicam nanoparticles for ocular delivery: physicochemical characterization and implementation in endotoxin-induced uveitis. J Drug Target 2007; 15:407-16. doi: 10.1080/10611860701453125 [Crossref] [ Google Scholar]
- Adibkia K, Omidi Y, Siahi MR, Javadzadeh AR, Barzegar-Jalali M, Barar J. Inhibition of endotoxin-induced uveitis by methylprednisolone acetate nanosuspension in rabbits. J Ocul Pharmacol Ther 2007; 23:421-32. doi: 10.1089/jop.2007.0039 [Crossref] [ Google Scholar]
- Barzegar-Jalali M, Adibkia K, Valizadeh H, Shadbad MR, Nokhodchi A, Omidi Y. Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci 2008; 11:167-77. [ Google Scholar]
- Fullard RJ, Tucker D. Tear protein composition and the effects of stimulus. Adv Exp Med Biol 1994; 350:309-14. [ Google Scholar]
- Cao Y, Zhang C, Shen W, Cheng Z, Yu LL, Ping Q. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J Control Release 2007; 120:186-94. [ Google Scholar]
- Patel SP, Vaishya R, Yang X, Pal D, Mitra AK. Novel thermosensitive pentablock copolymers for sustained delivery of proteins in the treatment of posterior segment diseases. Protein Pept Lett 2014; 21:1185-200. [ Google Scholar]
- Hosny KM. Preparation and evaluation of thermosensitive liposomal hydrogel for enhanced transcorneal permeation of ofloxacin. AAPS Pharm Sci Tech 2009; 10:1336-42. doi: 10.1208/s12249-009-9335-x [Crossref] [ Google Scholar]
- Bochot A, Fattal E, Gulik A, Couarraze G, Couvreur P. Liposomes dispersed within a thermosensitive gel: a new dosage form for ocular delivery of oligonucleotides. Pharm Res 1998; 15:1364-9. [ Google Scholar]
- Schenker HI, Silver LH. Long-term intraocular pressure-lowering efficacy and safety of timolol maleate gel-forming solution 05% compared with Timoptic XE 05% in a 12-month study. Am J Ophthalmol 2000; 130:145-50. [ Google Scholar]
- Yoncheva K, Vandervoort J, Ludwig A. Development of mucoadhesive poly(lactide-co-glycolide) nanoparticles for ocular application. Pharm Dev Technol 2011; 16:29-35. doi: 10.3109/10837450903479954 [Crossref] [ Google Scholar]
- Abdelbary G. Ocular ciprofloxacin hydrochloride mucoadhesive chitosan-coated liposomes. Pharm Dev Technol 2011; 16:44-56. doi: 10.3109/10837450903479988 [Crossref] [ Google Scholar]
- Mehanna MM, Elmaradny HA, Samaha MW. Mucoadhesive liposomes as ocular delivery system: physical, microbiological, and in vivo assessment. Drug Dev Ind Pharm 2009. doi: 10.3109/03639040903099751 [Crossref]
- Choy YB, Park JH, McCarey BE, Edelhauser HF, Prausnitz MR. Mucoadhesive microdiscs engineered for ophthalmic drug delivery: effect of particle geometry and formulation on preocular residence time. Invest Ophthalmol Vis Sci 2008; 49:4808-15. [ Google Scholar]
- Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Advanced Drug Delivery Reviews 2005; 57:1595-639. [ Google Scholar]
- Aggarwal D, Kaur IP. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int J Pharm 2005; 290:155-9. doi: 10.1016/j.ijpharm.2004.10.026 [Crossref] [ Google Scholar]
- Agrawal AK, Das M, Jain S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opinion on Drug Delivery 2012; 9:383-402. doi: 10.1517/17425247.2012.665367 [Crossref] [ Google Scholar]
- Gupta S, Vyas SP. Carbopol/chitosan based pH triggered in situ gelling system for ocular delivery of timolol maleate. Sci Pharm 2010; 78:959-76. doi: 10.3797/scipharm.1001-06 [Crossref] [ Google Scholar]
- Mansour M, Mansour S, Mortada ND, Abd Elhady SS. Ocular poloxamer-based ciprofloxacin hydrochloride in situ forming gels. Drug Dev Ind Pharm 2008; 34:744-52. doi: 10.1080/03639040801926030 [Crossref] [ Google Scholar]
- Gupta H, Jain S, Mathur R, Mishra P, Mishra AK, Velpandian T. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv 2007; 14:507-15. doi: 10.1080/10717540701606426 [Crossref] [ Google Scholar]
- Peng Y, Ang M, Foo S, Lee WS, Ma Z, Venkatraman SS. Biocompatibility and biodegradation studies of subconjunctival implants in rabbit eyes. PLoS One 2011; 6:e22507. doi: 10.1371/journal.pone.0022507 [Crossref] [ Google Scholar]
- Lee SS, Hughes P, Ross AD, Robinson MR. Biodegradable implants for sustained drug release in the eye. Pharm Res 2010; 27:2043-53. doi: 10.1007/s11095-010-0159-x [Crossref] [ Google Scholar]
- Paula JS, Ribeiro VR, Chahud F, Cannellini R, Monteiro TC, Gomes EC. Bevacizumab-loaded polyurethane subconjunctival implants: effects on experimental glaucoma filtration surgery. J Ocul Pharmacol Ther 2013; 29:566-73. doi: 10.1089/jop.2012.0136 [Crossref] [ Google Scholar]
- Bill A. The blood-aqueous barrier. Trans Ophthalmol Soc UK 1986; 105(Pt 2):149-55. [ Google Scholar]
- Schlingemann RO, Hofman P, Klooster J, Blaauwgeers HG, van der GR, Vrensen GF. Ciliary muscle capillaries have blood-tissue barrier characteristics. Exp Eye Res 1998; 66:747-54. [ Google Scholar]
- Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev 2006; 58:1136-63. doi: 10.1016/j.addr.2006.07.024 [Crossref] [ Google Scholar]
-
Mitra AK, Anand BS, Duvvuri S. Drug Delivery to the Eye. In: Fischbarg J, editor. The Biology of Eye. New York: Academic Press; 2006. p. 307-51.
- Prause JU. Treatment of keratoconjunctivitis sicca with Lacrisert. Scand J Rheumatol Suppl 1986; 61:261-3. [ Google Scholar]
- McDonald M, D’Aversa G, Perry HD, Wittpenn JR, Donnenfeld ED, Nelinson DS. Hydroxypropyl cellulose ophthalmic inserts (lacrisert) reduce the signs and symptoms of dry eye syndrome and improve patient quality of life. Trans Am Ophthalmol Soc 2009; 107:214-21. [ Google Scholar]
- Luchs JI, Nelinson DS, Macy JI, Group LACS. Efficacy of hydroxypropyl cellulose ophthalmic inserts (LACRISERT) in subsets of patients with dry eye syndrome: findings from a patient registry. Cornea 2010; 29:1417-27. doi: 10.1097/ICO.0b013e3181e3f05b [Crossref] [ Google Scholar]
- Mealy JE, Fedorchak MV, Little SR. In vitro characterization of a controlled-release ocular insert for delivery of brimonidine tartrate. Acta Biomater 2014; 10:87-93. doi: 10.1016/j.actbio.2013.09.024 [Crossref] [ Google Scholar]
- Kumari A, Sharma PK, Garg VK, Garg G. Ocular inserts - Advancement in therapy of eye diseases. J Adv Pharm Technol Res 2010; 1:291-6. doi: 10.4103/0110-5558.72419 [Crossref] [ Google Scholar]
- Riau AK, Mondal D, Aung TT, Murugan E, Chen L, Lwin NC. Collagen-Based Artificial Corneal Scaffold with Anti-Infective Capability for Prevention of Perioperative Bacterial Infections. ACS Biomaterials Science & Engineering 2015; 1:1324-34. [ Google Scholar]
- Yellepeddi VK, Sheshala R, McMillan H, Gujral C, Jones D, Raghu Raj Singh T. Punctal plug: a medical device to treat dry eye syndrome and for sustained drug delivery to the eye. Drug Discov Today 2015; 20:884-9. doi: 10.1016/j.drudis.2015.01.013 [Crossref] [ Google Scholar]
- Kojima T, Matsumoto Y, Ibrahim OM, Wakamatsu TH, Dogru M, Tsubota K. Evaluation of a thermosensitive atelocollagen punctal plug treatment for dry eye disease. Am J Ophthalmol 2014; 157:311-7 e1. doi: 10.1016/j.ajo.2013.10.019 [Crossref] [ Google Scholar]
- Yokoi N, Okada K, Sugita J, Kinoshita S. Acute conjunctivitis associated with biofilm formation on a punctal plug. Jpn J Ophthalmol 2000; 44:559-60. [ Google Scholar]
- Rumelt S, Remulla H, Rubin PA. Silicone punctal plug migration resulting in dacryocystitis and canaliculitis. Cornea 1997; 16:377-9. [ Google Scholar]
- Colin J. European clinical evaluation: use of Intacs for the treatment of keratoconus. J Cataract Refract Surg 2006; 32:747-55. doi: 10.1016/j.jcrs.2006.01.064 [Crossref] [ Google Scholar]
- Kymionis GD, Siganos CS, Tsiklis NS, Anastasakis A, Yoo SH, Pallikaris AI. Long-term follow-up of Intacs in keratoconus. Am J Ophthalmol 2007; 143:236-44. doi: 10.1016/j.ajo.2006.10.041 [Crossref] [ Google Scholar]
- Rabinowitz YS. INTACS for Keratoconus. Int Ophthalmol Clin 2010; 50:63-76. doi: 10.1097/IIO.0b013e3181e21b76 [Crossref] [ Google Scholar]
- Engle AT, Laurent JM, Schallhorn SC, Toman SD, Newacheck JS, Tanzer DJ. Masked comparison of silicone hydrogel lotrafilcon A and etafilcon A extended-wear bandage contact lenses after photorefractive keratectomy. J Cataract Refract Surg 2005; 31:681-6. doi: 10.1016/j.jcrs.2004.09.022 [Crossref] [ Google Scholar]
- Edwards JD, Bower KS, Sediq DA, Burka JM, Stutzman RD, Vanroekel CR. Effects of lotrafilcon A and omafilcon A bandage contact lenses on visual outcomes after photorefractive keratectomy. J Cataract Refract Surg 2008; 34:1288-94. doi: 10.1016/j.jcrs.2008.04.024 [Crossref] [ Google Scholar]
- Long B, Schweizer H, Bleshoy H, Zeri F. Expanding your use of silicone hydrogel contact lenses: using lotrafilcon A for daily wear. Eye Contact Lens 2009; 35:59-64. doi: 10.1097/ICL.0b013e318196ade7 [Crossref] [ Google Scholar]
- Mohammadpour M, Amouzegar A, Hashemi H, Jabbarvand M, Kordbacheh H, Rahimi F. Comparison of Lotrafilcon B and Balafilcon A silicone hydrogel bandage contact lenses in reducing pain and discomfort after photorefractive keratectomy: A contralateral eye study. Cont Lens Anterior Eye 2015; 38:211-4. doi: 10.1016/j.clae.2015.01.014 [Crossref] [ Google Scholar]
- Sarac O, Gurdal C, Bostanci-Ceran B, Can I. Comparison of tear osmolarity and ocular comfort between daily disposable contact lenses: hilafilcon B hydrogel versus narafilcon A silicone hydrogel. Int Ophthalmol 2012; 32:229-33. doi: 10.1007/s10792-012-9556-y [Crossref] [ Google Scholar]
- Cai X, Conley S, Naash M. Nanoparticle applications in ocular gene therapy. Vision Res 2008; 48:319-24. doi: 10.1016/j.visres.2007.07.012 [Crossref] [ Google Scholar]
- Vandervoort J, Ludwig A. Ocular drug delivery: nanomedicine applications. Nanomedicine (Lond) 2007; 2:11-21. doi: 10.2217/17435889.2.1.11 [Crossref] [ Google Scholar]
- Nakhlband A, Barar J. Impacts of nanomedicines in ocular pharmacotherapy. Bioimpacts 2011; 1:7-22. doi: 10.5681/bi.2011.003 [Crossref] [ Google Scholar]
-
Omidi Y, Barar J, Hamzeiy H. Nanomedicines Impacts in Ocular Delivery and Targeting. Nanotechnology in Health Care 2012; 43.
- Vaishya RD, Khurana V, Patel S, Mitra AK. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2014; 6:422-37. doi: 10.1002/wnan.1272 [Crossref] [ Google Scholar]
- Prow TW. Toxicity of nanomaterials to the eye. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2010; 2:317-33. doi: 10.1002/wnan.65 [Crossref] [ Google Scholar]
- Wadhwa S, Paliwal R, Paliwal SR, Vyas SP. Nanocarriers in ocular drug delivery: an update review. Curr Pharm Des 2009; 15:2724-50. [ Google Scholar]
- Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today 2008; 13:144-51. doi: 10.1016/j.drudis.2007.10.021 [Crossref] [ Google Scholar]
-
Kothuri MK, Pinnamaneni S, Das NG, Das SK. Microparticles and Nanoparticles in Ocular Drug Delivery. In: Mitra AK, editor. Ophthalmic Drug Delivery Systems. New York: Marcel Dekker, Inc.; 2003. p. 437-66.
- Visor GC. Drug design strategies for ocular therapeutics. Advanced Drug Delivery Reviews 1994; 14:269-79. [ Google Scholar]
-
Barar J, Omidi Y. Nanoparticles for Ocular Drug Delivery. Nanomedicine in Drug Delivery 2013; 287.
- Zhang L, Li Y, Zhang C, Wang Y, Song C. Pharmacokinetics and tolerance study of intravitreal injection of dexamethasone-loaded nanoparticles in rabbits. Int J Nanomedicine 2009; 4:175-83. [ Google Scholar]
- Jo DH, Kim JH, Yu YS, Lee TG, Kim JH. Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomedicine 2012; 8:784-91. doi: 10.1016/j.nano.2011.09.003 [Crossref] [ Google Scholar]
- Panda JJ, Yandrapu S, Kadam RS, Chauhan VS, Kompella UB. Self-assembled phenylalanine-alpha,beta-dehydrophenylalanine nanotubes for sustained intravitreal delivery of a multi-targeted tyrosine kinase inhibitor. J Control Release 2013; 172:1151-60. doi: 10.1016/j.jconrel.2013.09.016 [Crossref] [ Google Scholar]
- Huu VA, Luo J, Zhu J, Zhu J, Patel S, Boone A. Light-responsive nanoparticle depot to control release of a small molecule angiogenesis inhibitor in the posterior segment of the eye. J Control Release 2015; 200:71-7. doi: 10.1016/j.jconrel.2015.01.001 [Crossref] [ Google Scholar]
- Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Advanced Drug Delivery Reviews 2008; 60:1638-49. [ Google Scholar]
- Ahmed EM. Hydrogel: preparation, characterization, and applications. J Adv Res 2015; 6:105-21. doi: 10.1016/j.jare.2013.07.006 [Crossref] [ Google Scholar]
- Hennink W, Van Nostrum CF. Novel crosslinking methods to design hydrogels. Advanced Drug Delivery Reviews 2012; 64:223-36. [ Google Scholar]
- Joglekar M, Trewyn BG. Polymer‐based stimuli‐responsive nanosystems for biomedical applications. Biotechnol J 2013; 8:931-45. [ Google Scholar]
- Deligkaris K, Tadele TS, Olthuis W, van den Berg A. Hydrogel-based devices for biomedical applications. Sensors and Actuators B: Chemical 2010; 147:765-74. [ Google Scholar]
- Radhakrishnan J, Krishnan UM, Sethuraman S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol Adv 2014; 32:449-61. [ Google Scholar]
- Jeong B, Kim SW, Bae YH. Thermosensitive sol–gel reversible hydrogels. Advanced Drug Delivery Reviews 2002; 54:37-51. [ Google Scholar]
- Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Advanced Drug Delivery Reviews 2012; 64:49-60. [ Google Scholar]
- Casolaro M, Casolaro I, Lamponi S. Stimuli-responsive hydrogels for controlled pilocarpine ocular delivery. European Journal of Pharmaceutics and Biopharmaceutics 2012; 80:553-61. [ Google Scholar]
- Fathi M, Entezami AA, Arami S, Rashidi M-R. Preparation of N-Isopropylacrylamide/Itaconic Acid Magnetic Nanohydrogels by Modified Starch as a Crosslinker for Anticancer Drug Carriers. International Journal of Polymeric Materials and Polymeric Biomaterials 2015; 64:541-9. [ Google Scholar]
- Yasuda H. Biocompatibility of Nanofilm‐Encapsulated Silicone and Silicone‐Hydrogel Contact Lenses. Macromolecular Bioscience 2006; 6:121-38. [ Google Scholar]
- Abrego G, Alvarado H, Souto EB, Guevara B, Bellowa LH, Parra A. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. Eur J Pharm Biopharm 2015; 95:261-70. doi: 10.1016/j.ejpb.2015.01.026 [Crossref] [ Google Scholar]
- Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Development and Industrial Pharmacy 2002; 28:353-69. [ Google Scholar]
- Stapleton F, Stretton S, Papas E, Skotnitsky C, Sweeney DF. Silicone hydrogel contact lenses and the ocular surface. The Ocular Surface 2006; 4:24-43. [ Google Scholar]
- Xie B, Jin L, Luo Z, Yu J, Shi S, Zhang Z. An injectable thermosensitive polymeric hydrogel for sustained release of Avastin(R) to treat posterior segment disease. Int J Pharm 2015; 490:375-83. doi: 10.1016/j.ijpharm.2015.05.071 [Crossref] [ Google Scholar]
- Kirchhof S, Goepferich AM, Brandl FP. Hydrogels in ophthalmic applications. Eur J Pharm Biopharm 2015; 95:227-38. doi: 10.1016/j.ejpb.2015.05.016 [Crossref] [ Google Scholar]
- Rauck BM, Friberg TR, Medina Mendez CA, Park D, Shah V, Bilonick RA. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Invest Ophthalmol Vis Sci 2014; 55:469-76. doi: 10.1167/iovs.13-13120 [Crossref] [ Google Scholar]
- Hu CC, Chaw JR, Chen CF, Liu HW. Controlled release bevacizumab in thermoresponsive hydrogel found to inhibit angiogenesis. Biomed Mater Eng 2014; 24:1941-50. doi: 10.3233/BME-141003 [Crossref] [ Google Scholar]
- Gilger BC, Mandal A, Shah S, Mitra AK. Episcleral, intrascleral, and suprachoroidal routes of ocular drug delivery - recent research advances and patents. Recent Pat Drug Deliv Formul 2014; 8:81-91. [ Google Scholar]
- Stanzel BV, Liu Z, Brinken R, Braun N, Holz FG, Eter N. Subretinal delivery of ultrathin rigid-elastic cell carriers using a metallic shooter instrument and biodegradable hydrogel encapsulation. Invest Ophthalmol Vis Sci 2012; 53:490-500. doi: 10.1167/iovs.11-8260 [Crossref] [ Google Scholar]
- Gao SQ, Maeda T, Okano K, Palczewski K. A microparticle/hydrogel combination drug-delivery system for sustained release of retinoids. Invest Ophthalmol Vis Sci 2012; 53:6314-23. doi: 10.1167/iovs.12-10279 [Crossref] [ Google Scholar]
- Kuno N, Fujii S. Biodegradable intraocular therapies for retinal disorders: progress to date. Drugs Aging 2010; 27:117-34. doi: 10.2165/11530970-000000000-00000 [Crossref] [ Google Scholar]
- Thanos CG, Bell WJ, O’Rourke P, Kauper K, Sherman S, Stabila P. Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Eng 2004; 10:1617-22. doi: 10.1089/ten.2004.10.1617 [Crossref] [ Google Scholar]
- Emerich DF, Thanos CG. NT-501: an ophthalmic implant of polymer-encapsulated ciliary neurotrophic factor-producing cells. Curr Opin Mol Ther 2008; 10:506-15. [ Google Scholar]
- Humayun M, Santos A, Altamirano JC, Ribeiro R, Gonzalez R, de la Rosa A. Implantable MicroPump for Drug Delivery in Patients with Diabetic Macular Edema. Transl Vis Sci Technol 2014; 3:5. doi: 10.1167/tvst.3.6.5 [Crossref] [ Google Scholar]
- Gutierrez-Hernandez JC, Caffey S, Abdallah W, Calvillo P, Gonzalez R, Shih J. One-Year Feasibility Study of Replenish MicroPump for Intravitreal Drug Delivery: A Pilot Study. Transl Vis Sci Technol 2014; 3:8. doi: 10.1167/tvst.3.3.8 [Crossref] [ Google Scholar]
- Fangueiro JF, Veiga F, Silva AM, Souto EB. Ocular Drug Delivery - New Strategies for targeting anterior and posterior segments of the eye. Curr Pharm Des 2015. doi: 10.2174/1381612822666151216145900 [Crossref]
- du Toit LC, Pillay V, Choonara YE, Govender T, Carmichael T. Ocular drug delivery - a look towards nanobioadhesives. Expert Opin Drug Deliv 2011; 8:71-94. doi: 10.1517/17425247.2011.542142 [Crossref] [ Google Scholar]
- Almeida H, Amaral MH, Lobao P, Lobo JM. In situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov Today 2014; 19:400-12. doi: 10.1016/j.drudis.2013.10.001 [Crossref] [ Google Scholar]
- Pontes de Carvalho RA, Krausse ML, Murphree AL, Schmitt EE, Campochiaro PA, Maumenee IH. Delivery from episcleral exoplants. Invest Ophthalmol Vis Sci 2006; 47:4532-9. doi: 10.1167/iovs.06-0030 [Crossref] [ Google Scholar]
- Lo R, Li PY, Saati S, Agrawal R, Humayun MS, Meng E. A refillable microfabricated drug delivery device for treatment of ocular diseases. Lab Chip 2008; 8:1027-30. doi: 10.1039/b804690e [Crossref] [ Google Scholar]
- Lo R, Li PY, Saati S, Agrawal RN, Humayun MS, Meng E. A passive MEMS drug delivery pump for treatment of ocular diseases. Biomed Microdevices 2009; 11:959-70. doi: 10.1007/s10544-009-9313-9 [Crossref] [ Google Scholar]
- Bush RA, Lei B, Tao W, Raz D, Chan CC, Cox TA. Encapsulated cell-based intraocular delivery of ciliary neurotrophic factor in normal rabbit: dose-dependent effects on ERG and retinal histology. Invest Ophthalmol Vis Sci 2004; 45:2420-30. [ Google Scholar]
- Ashworth JL, Biswas S, Wraith E, Lloyd IC. Mucopolysaccharidoses and the eye. Surv Ophthalmol 2006; 51:1-17. doi: 10.1016/j.survophthal.2005.11.007 [Crossref] [ Google Scholar]
- Fathi M, Barar J, Aghanejad A, Omidi Y. Hydrogels for ocular drug delivery and tissue engineering. Bioimpacts 2015; 5:159-164. doi: 10.15171/bi.2015.31 [Crossref] [ Google Scholar]