Bioimpacts. 11(2):111-117.
doi: 10.34172/bi.2021.18
Original Research
Role of cellulose family in fibril organization of collagen for forming 3D cancer spheroids: In vitro and in silico approach
Elaheh Dalir Abdolahinia 1, 2, Behzad Jafari 3, Sepideh Parvizpour 2, Jaleh Barar 2, 4  , Samad Nadri 5, *, Yadollah Omidi 6, *
, Samad Nadri 5, *, Yadollah Omidi 6, * 
Author information:
1Department of Medical Biotechnology, Zanjan University of Medical Sciences, Zanjan, Iran
2Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Medicinal Chemistry, Faculty of Pharmacy, Urmia University of Medical Sciences, Urmia, Iran
4Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Medical Nanotechnology, Zanjan University of Medical Sciences, Zanjan, Iran
6Department of Pharmaceutical Sciences, College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL, USA
Abstract
Introduction:
Cell aggregation of three-dimensional (3D) culture systems (the so-called spheroids) are designed as in vitro platform to represent more accurately the in vivo environment for drug discovery by using semi-solid media. The uniform multicellular tumor spheroids can be generated based on the interaction of cells with extracellular matrix (ECM) macromolecules such as collagen and integrin. This study aimed to investigate the possible interactions between the cellulose family and collagen using both in vitro and in silico approaches.
Methods:
The 3D microtissue of JIMT-1 cells was generated using hanging drop method to study the effects of charge and viscosity of the medium containing cellulose family. To determine the mode of interaction between cellulose derivatives (CDs) and collagen-integrin, docking analysis and molecular simulation were further performed using open source web servers and chemical simulations (GROMACS), respectively.
Results:
The results confirmed that the addition of CDs into the 3D medium can promote the formation of solid spheroids, where methylcellulose (MC) yielded uniform spheroids compared to carboxymethyl cellulose (CMC). Moreover, the computational analysis showed that MC interacted with both integrin and collagen, while sodium carboxymethyl cellulose (NaCMC) only interacted with collagen residues. The stated different behaviors in the 3D culture formation and collagen interaction were found in the physicochemical properties of CDs.
Conclusion:
Based on in vitro and in silico findings, MC is suggested as an important ECM-mimicking entity that can support the semi-solid medium and promote the formation of the uniform spheroid in the 3D culture.
Keywords: Spheroid, Methylcellulose, Sodium carboxymethylcellulose, Collagen, Integrin, 3D cell culture, Tumoroid
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Multicellular tumor spheroids are considered as suitable in vitro system that closely simulate avascular solid tumors condition such as cell to cell and cell to extracellular matrix (ECM) interactions, as well as gradients of oxygen, nutrients, and the cancer stem cell niche.
1,2
Collagen (Col) as a main constituent of ECM, provides a tensile strength for cell-cell interaction and migration. The cells interaction with Col family members as the major structural component in ECM is moderated by integrins (Ints).
3
Further, Ints are a superfamily of α/β heterodimeric transmembrane receptors and their four subtypes are known as α1β1, α2β1, α10β1 and α11β1 .They can recognize the six-residue sequence GFOGER of the triple-helical interstitial Cols and regulate their interaction.
4-6
The signaling of α2β1 Int on cancer cells, organizes the ECM of tumor stroma and hence can modulate drug resistance,
7
metastasis,
8
tumor angiogenesis,
9
and tumor stemness
10
through promoting or inhibiting the diffusion process of cancer cells.
11
However, the exact mechanism by which the α2β1 Int-Col moderates cancer pathogenesis is yet to be fully understood.
Various methods have been developed for the formation of the tumor spheroid.
12,13
These spheroids display the diverse morphologies which are dependent on the innate nature of the cells and the culture condition that can be classified as compact aggregates, loose aggregates and singular cell suspension.
14,15
Seemingly, Col fibrils produced by some cancer cells display weak mechanical characterization and thermal stabilities that influence the solidity of spheroids. Recent findings have reported that the viscosity of cellulose (Cel) has a significant role in modulating the morphology of cancer cells adhered to Col.
16,17
Besides, the increase viscosity of the cellulose family in the cell culture can moderate the extrinsic vibration, and decrease the number of forces given to the spheroid for improved stability.
18
This characteristic appears to be in favor of the rigidity of tumor mass because tumor tissue is stiffer in comparison with the normal tissue.
19
These aggregated mass allow improved cell to cell contacts, and more realistic adherence and tight junction formation and cell growth rates. Furthermore, in such condition, the existence of diffusing gradients resembles similar features of in vivo physiological conditions.
20,21
Cellulose is the most abundant polysaccharide in the nature that has a regular and linear polymer of D-glucose units linked by β-1, 4-glycosidic bonds. The hydroxyl groups in β-D-glucopyranosyl units create intra- and intermolecular hydrogen bonds that provides a stiff structure and form crystalline regions as a result of low accessibility to reactants.
22
The hydroxyl group of Cel could form hydrogen bonds with carboxyl and hydroxyl groups of Col and affect the fibril formation of Col in vitro.
23,24
Therefore, cellulose and its derivations such as sodium carboxymethyl cellulose (NaCMC) and methylcellulose (MC) could be added as a semi-solidification agents in the three-dimensional (3D) culture.
Understanding the impact of different cellulose derivatives (CDs) on the interaction of Col-α2β1 Int in a 3D culture system is very important. To determine the best Cel subtype, the computational modeling strategy can provide valuable information. The main goal of this study was to produce a firm spheroid and examine the possible interactions between the combination of the α2β1 Int-Col with Cel, NaCMC, and MC to determine the most suitable compound for the 3D culture formation.
Materials and Methods
Materials
MC and NaCMC were purchased from Sigma-Aldrich (St. Louis, MO). Human breast cancer cell line Jimt-1 was obtained from NCBI -National Cell Bank of Iran-Pasteur Institute of Iran (Tehran, Iran). The fetal bovine serum (FBS) and the Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM-F12) were purchased from Gibco, Thermo Fisher Scientific (Waltham, USA).
Cell culture of JIMT-1 cell-line
Human breast cancer Jimt-1 cells was cultured in DMEM-F12 supplemented with 10% FBS and 1% Penicillin-Streptomycin. The cultivated cells were kept in a humidified atmosphere at 37°C in with 5% CO2. At 90% confluency, the cells were harvested from cell culture flasks using 0.25% Trypsin-EDTA solution.
Three-dimensional (3D) cultures of human breast cancer cell -line Jimt-1
To generate spheroids, the hanging drop method was employed. In brief, 25 µL of 1000 cells suspension in culture media with or without 5% of MC was placed under the lid of the cell culture plate. For NaCMC containing cell culture, the polysaccharide (%5) was added to the media of the cell aggregate on day 5. Then, lids were inverted and incubated under a standard condition for 7 days. The density of 1000 cells was mixed with 25 µL of medium.
Cellulose family model generation
To investigate the mode of interaction between the CDs and Int/Col complex, the energy minimized conformations of Cel, MC, and NaCMC were generated. In this regard, four subunits of Cel and fully substituted forms of MC and NaCMC were used as the representatives of the corresponding polysaccharides. Sugar builder module from HyperChem® software (version 8.0.8) was used to generate the four-unit saccharides attached with 1 to 4 β links followed by the manual substitution of the carboxy and methyl groups into the glucose structure to form the fully substituted NaCMC and MC, respectively. The 3D structures of the compounds were generated using the Built Optimum option of the HyperChem followed by the energy minimization approach using the AMBER force field based on the Polak-Ribiere algorithm.
The CDs and integrin/collagen complex docking study
In order to establish the docking study of the CDs and Int/Col complex, the crystal structure of Int α2I domain/Col complex (Int/Col) was retrieved from literature (PDB ID: 1DZI). The structures of the energy minimized compounds were docked with Int/Col using DockThor, a free web server for protein-ligand docking. The grid center and grid size were set in a way to cover the whole protein for blind docking. Moreover, the number of evaluations, population size, initial seed, and the number of runs were set to the default values for the standard docking procedure. The best ranks for each docking procedure were used for further analysis. DS visualizer was used for generating 2D interaction diagrams and PyMOL software was used to visualize the mode of interactions.
Molecular dynamic (MD) simulation
The stability of the CDs and Col complexes was studied by subjecting the constructed complexes to energy minimization using the GROMACS 5.1.4 software package. The intermolecular interactions during the MD simulation process were determined using GROMOS 96 43a1 as a suitable force field. To identify the corresponding pKa values, the pH of the molecular environment was defined to be 7. The three complexes of protein-ligand were first positioned into three separate properly sized simulation cubic boxes and solvated within water molecules. Furthermore, the charge of complexes was neutralized by adding an appropriate number of counterions. The steepest descent of 400 steps was used to minimize the entire system. The simulations were done for 20 ns at 300 K, and the particle mesh Ewald method was used for the electrostatic interaction analysis. The actual frame was recorded for each 1.0 ps. The quality of the protein geometry and the structure folding reliability were determined by taking the stabilized structure from the trajectory of the system. Consequently, the structural changes of the protein-ligand complexes were investigated by computing the root mean square deviation (RMSD).
Results
The uniform of firm spheroids produced by adding CDs in 3D media culture
After reaching cell to 90% confluency in 2D, the cells were cultured in the 3D method. Without viscosity inducing agent in the culture medium, no cell aggregates were observed with any spheroids in the hanging drop (data not shown). Fig. 1A represents the typical microscopic image of the cells in hanging drop using normal media on day1. Multi-spheroids were formed in the medium that contained NaCMC. After the addition of NaCMC to cell suspension on the 5th day (Fig. 1B). On day 7, the spheroids were more aggregated (Fig. 1C) in comparison with the produced spheroid by adding NaCMC on the first day. Upon the addition of NaCMC on the first day of culture, no spheroid was formed even after 7 days (Fig. 1D). However, when MC is added to the 3D culture media, the cells interacted efficiently and the spheroid started to from after 1 day, and reached to a desired size and rigidity (Fig. 2). The 7 day old spheroid, from MC containing media exhibited a diameter of about 500 µm with suitable integrity and rigidity enough to be handled for in vitro evaluation (Fig. 2D)
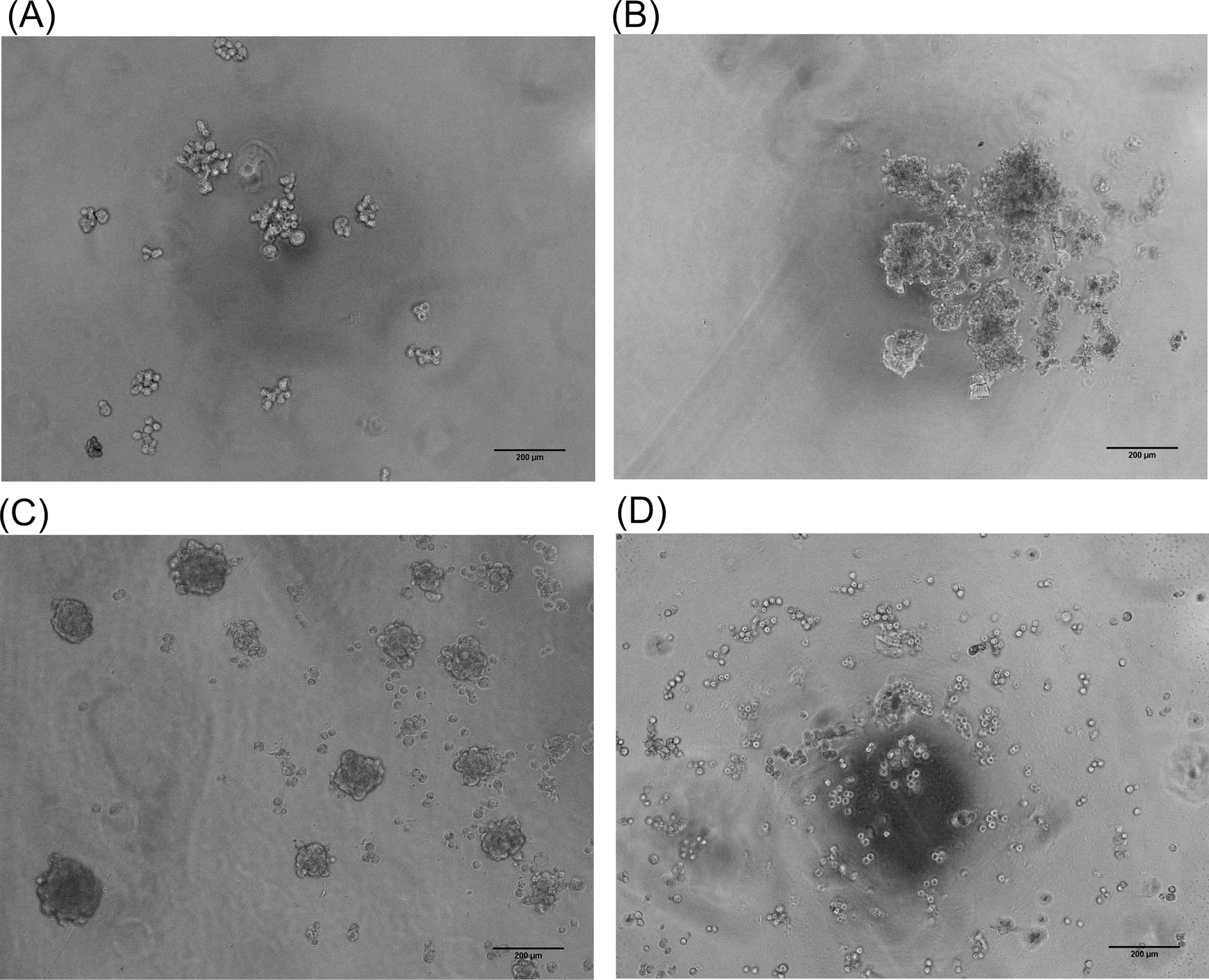
Fig. 1.
Morphology of the cultured JIMT-1 cells as three-dimensional (3D) model in the medium containing NaCMC. (A) The 3D culture of JIMT-1 cells via hanging drop method without NaCMC on day1. (B) The addition of CMC on day 5. (C) The multi-spheroids were produced on day 7. (D) The JIMT-1 cells with the initial NaCMC on day 7 (Scale bar: 200µm). NaCMC: sodium carboxymethylcellulose. MC: methylcellulose.
.
Morphology of the cultured JIMT-1 cells as three-dimensional (3D) model in the medium containing NaCMC. (A) The 3D culture of JIMT-1 cells via hanging drop method without NaCMC on day1. (B) The addition of CMC on day 5. (C) The multi-spheroids were produced on day 7. (D) The JIMT-1 cells with the initial NaCMC on day 7 (Scale bar: 200µm). NaCMC: sodium carboxymethylcellulose. MC: methylcellulose.
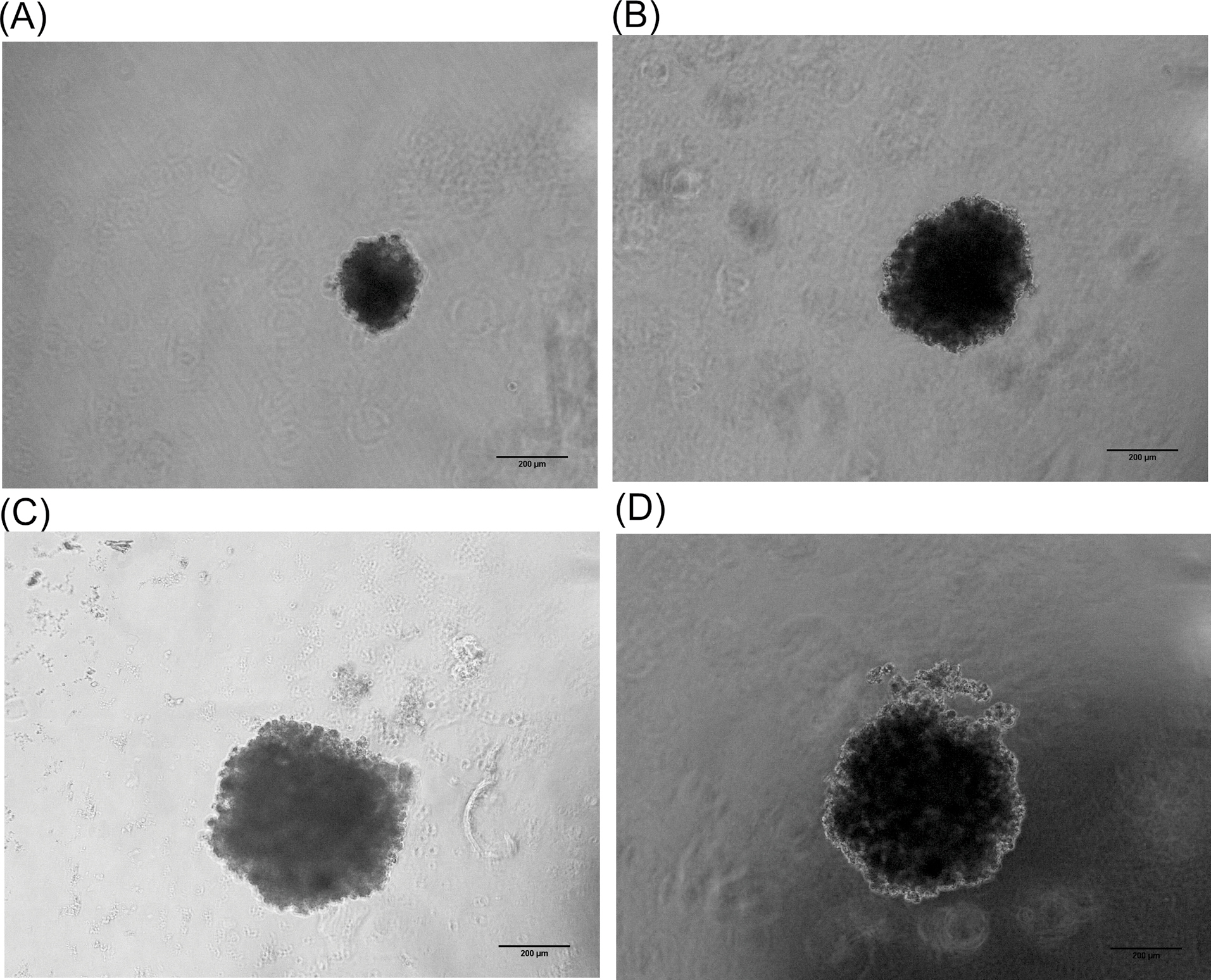
Fig. 2.
The formation of JIMT-1 spheroid. Panels A, B, C, and D show the spheroid growth on day 1, 3, 5, and 7, respectively. To generate the spheroid, about 1000 cells were seeded into a drop containing 0.5% methylcellulose diluted in culture medium.
.
The formation of JIMT-1 spheroid. Panels A, B, C, and D show the spheroid growth on day 1, 3, 5, and 7, respectively. To generate the spheroid, about 1000 cells were seeded into a drop containing 0.5% methylcellulose diluted in culture medium.
Cellulose and MC have interaction with both integrin and collagen but NaCMC not
The time-consuming procedure of data management and calculations in bioinformatic analyses can be addressed using some available bioinformatic tools as free web-based interfaces and servers. In the current study, we used an on-line free docking server to inspect the mode of interaction between the CDs and the Int/Col complex. The docking results are listed in Table 1, including the best ranks, scores, total energies (i.e., intermolecular ligand-protein + intramolecular ligand energies), and the interaction energies (i.e., only intermolecular ligand-protein energy). Fig. 3 shows the binding sites of CDs generated by PyMol software. As shown in Fig. 3, MC and Cel have interaction with both Int and Col while NaCMC has only interaction with Col residues. To investigate the nature of binding between complex pairs, the 2D interaction diagrams were produced by DS visualizer software. The 2D diagrams for the best poses are shown in Fig. 4. The hydrogen bond and van der Waals interactions are the most dominant interaction. Table 2 lists the interacting groups for the CDs best poses. By taking into account the docking results, it seems that NaCMC has the best binding among the CDs; and the interaction energy for NaCMC has the value of -54.564 kcal/mol that has the lowest value comparing with -21.715 and -31.207 kcal/mol for MC and Cel, respectively.
Table 1.
The results of DockThor and MTiOpenScreen analyses for CDs in complex with Int/Col
|
Compound
|
Best rank
(run/model)
|
Score
|
Total energy
(kcal/mol)
|
Interaction energy (kcal/mol)
|
Binding energy (kcal/mol)
(MTiOpenScreen)
|
| Cellulose |
13/1 |
-6.465 |
98.079 |
-31.207 |
-10.67 |
| Methylcellulose |
20/1 |
-7.493 |
188.640 |
-21.715 |
-3.21 |
| Sodium carboxymethylcellulose |
19/1 |
-6.277 |
194.641 |
-54.564 |
-9.15 |
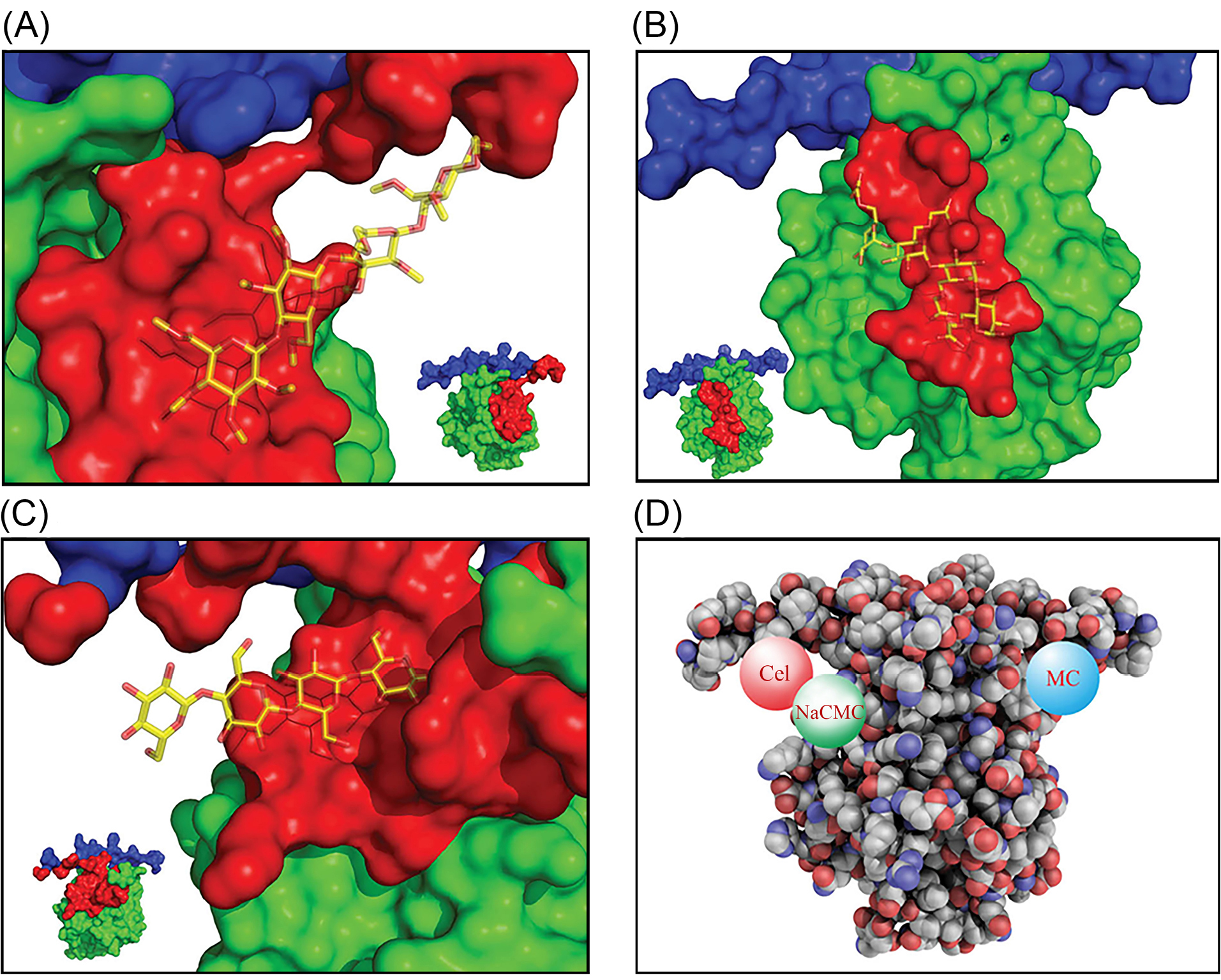
Fig. 3.
Binding and interaction modes of different CDs. (A) Methyl cellulose (MC) interaction with integrin (Int) and collagen (Col) residues. (B) NaCMC interaction with collagen. (C) Cel interaction with integrin and collagen. (D) Cel, MC, and NaCMC interacting with Int/Col complex. Data were generated by PyMol software. The protein is shown as surface view and the CDs as sticks view (panels A, B and C). The gray and blue colors show collagen and integrin sections, respectively. The red color section indicates the residues in distance of 7 Å to CDs. Image shown in panel D is QuteMol like image generated with freely available scripts by PyMol. CDs: cellulose derivatives. NaCMC: sodium carboxymethylcellulose. MC: methylcellulose. Cel: cellulose.
.
Binding and interaction modes of different CDs. (A) Methyl cellulose (MC) interaction with integrin (Int) and collagen (Col) residues. (B) NaCMC interaction with collagen. (C) Cel interaction with integrin and collagen. (D) Cel, MC, and NaCMC interacting with Int/Col complex. Data were generated by PyMol software. The protein is shown as surface view and the CDs as sticks view (panels A, B and C). The gray and blue colors show collagen and integrin sections, respectively. The red color section indicates the residues in distance of 7 Å to CDs. Image shown in panel D is QuteMol like image generated with freely available scripts by PyMol. CDs: cellulose derivatives. NaCMC: sodium carboxymethylcellulose. MC: methylcellulose. Cel: cellulose.
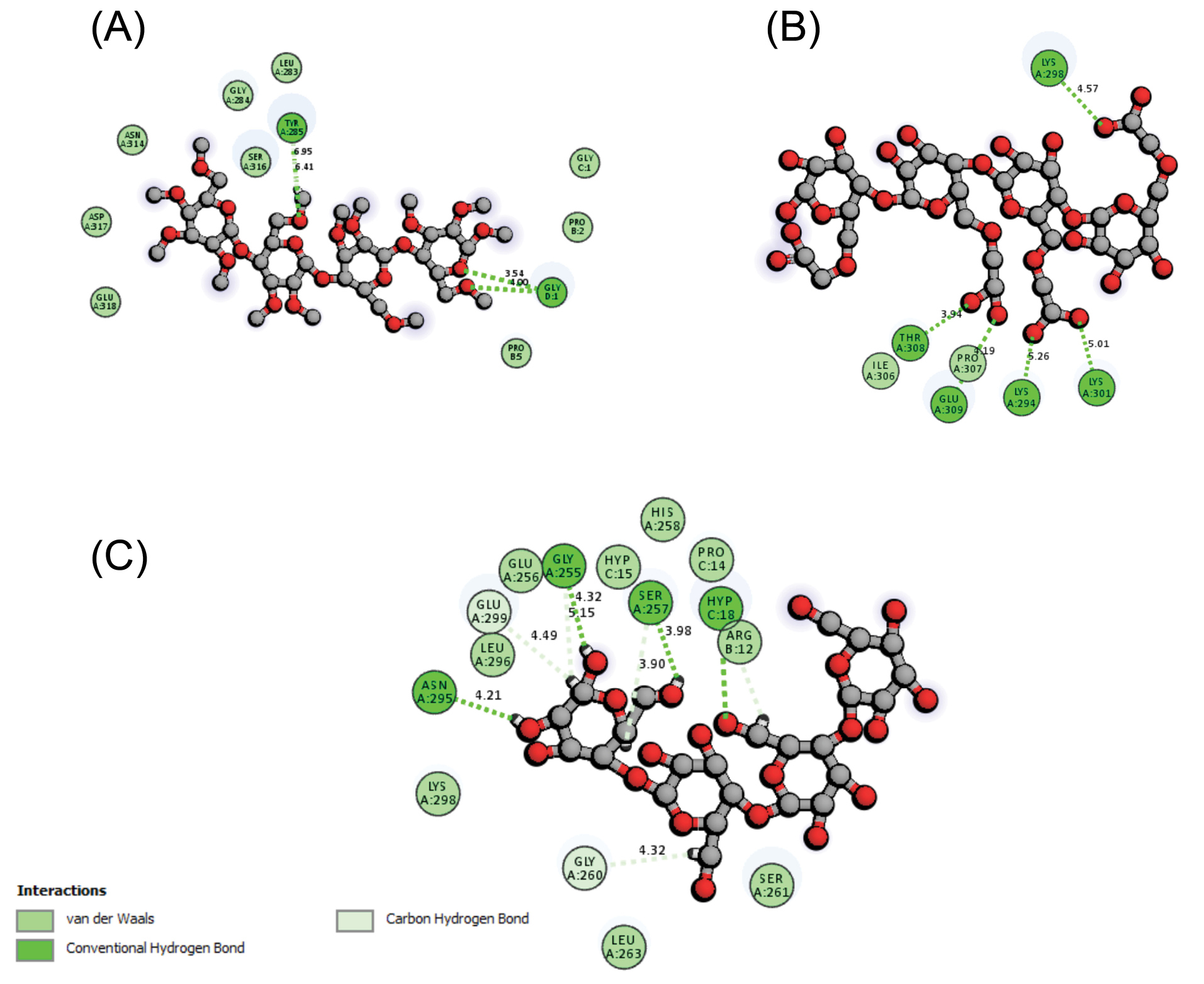
Fig. 4.
The 2D interaction diagram of CDs in complex with integrin and collagen (Int/Col). (A) MC exhibits van der Waals and hydrogen bond interactions with different residues of Int/Col represented with blue and green colored residues, respectively. (B) NaCMC shows van der Waals as well as conventional hydrogen bond interactions with Col. (C) Cel displays van der Waals and hydrogen bond interactions dominantly with Int/Col. Data were produced by DS visualizer software. CDs: cellulose derivatives. NaCMC: sodium carboxymethylcellulose. MC: methylcellulose. Cel: cellulose.
.
The 2D interaction diagram of CDs in complex with integrin and collagen (Int/Col). (A) MC exhibits van der Waals and hydrogen bond interactions with different residues of Int/Col represented with blue and green colored residues, respectively. (B) NaCMC shows van der Waals as well as conventional hydrogen bond interactions with Col. (C) Cel displays van der Waals and hydrogen bond interactions dominantly with Int/Col. Data were produced by DS visualizer software. CDs: cellulose derivatives. NaCMC: sodium carboxymethylcellulose. MC: methylcellulose. Cel: cellulose.
Table 2.
The interacting residues for CDs best poses in complex with Int/Col
|
CDs
|
Hydrogen bond interactions
|
van der Waals interactions
|
| Cellulose |
Asn295A |
Gly255A |
Ser257A |
|
|
Leu296A |
Lys298A |
Glu299A |
His258A |
Arg12B |
Ser261A |
| Methylcellulose |
Tyr285A |
|
|
|
|
Leu283A |
Gly284A |
Ser316A |
Pro2 B |
Asp317A |
Asn314A |
| Sodium carboxymethylcellulose |
Lys298A |
Thr308A |
Glu309A |
Lys294A |
Lys301A |
Ile306A |
Pro307A |
|
|
|
|
The CDs-Col complex stability study with MD simulation
The stability and mobility of the three complex structures were studied by subjecting these models to 20 ns MD simulations at 310 K. To analyze the dynamic behavior of the models, the RMSD was plotted as a crucial measure for the evaluation of the stability of the molecule during the simulation. As shown in the RMSD plot of the proteins in Fig. 5A, the lowest RMSD belongs to NaCMC. The achieved results showed that the NaCMC-Col complex is more stable than Cel-Col, and MC-Col in the optimum temperature confirming the docking analysis. Fig. 5B shows the constant energy of the system during the molecular simulation and Fig. 5C illustrates the persistent temperature of the system during the molecular simulation at 310 K.
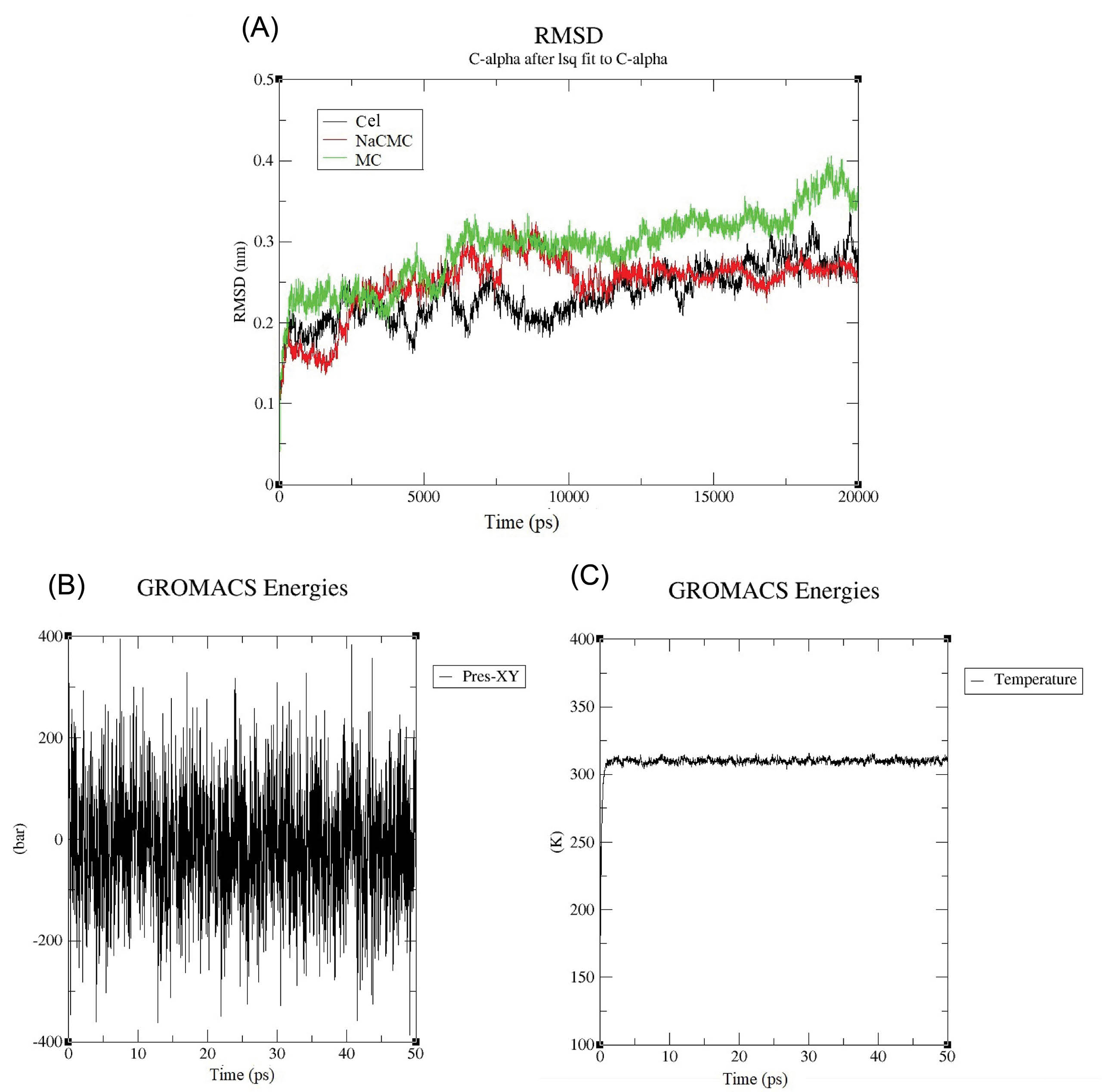
Fig. 5.
The molecular dynamic simulation results: (A) The RMSD plot of CDs-Col during MD simulation at 310°C.The result shows that the RMSD of NaCMC is more stable than two others. (B) Constant energy of system during molecular simulation. (C) Persistent temperature of system during molecular simulation at 310 K. CDs: cellulose derivatives. NaCMC: sodium carboxymethylcellulose. MC: methylcellulose. Cel: cellulose.
.
The molecular dynamic simulation results: (A) The RMSD plot of CDs-Col during MD simulation at 310°C.The result shows that the RMSD of NaCMC is more stable than two others. (B) Constant energy of system during molecular simulation. (C) Persistent temperature of system during molecular simulation at 310 K. CDs: cellulose derivatives. NaCMC: sodium carboxymethylcellulose. MC: methylcellulose. Cel: cellulose.
Discussion
Classically, in vitro drug screening and development is valuable tool using cell culture models. However, the 2D cell culture models often fail to mimic the in vivo situation. Therefore, the development of 3D cell culture models such as spheroids have attracted huge attention and is considered a much better model to resemble the in vivo condition to a greater extent. This 3D model is relatively easy to establish and can replace the traditional 2D monolayer culture.
25
Of note, the uniformly sized spheroids are a key tools for various studies, including cell-based drug delivery and drug screening in vitro.
26,27
The 3D hanging drop technique is a scaffold-free system. In this line, some matrix supporting factors such as Matrigel®, polyglycolic acid, or microporous supports can stimulate the natural features of cells to aggregate by gravitational force and finally lead to the formation of spheroid. Moreover, using this system, one can produce a uniform, high throughput, and multi-cell-type spheroid.
28,29
In the current study, we evaluated the role of MC and NaCMC as stiffening factors in the 3D culture establishment. The uniform spheroids were developed by adding MC to culture, while such uniform spheroid could not be achieved in the presence of NaCMC in the culture media (Fig. 1). Due to the fact that Col fibrils as part of ECM can be produced after 5 days of the cell seeding,
30
NaCMC was added into media on the 5th day. The production of Col fibrils appears to lower the negative charge of the cell surface on day 5, which in turn makes it possible for a better interaction with NaCMC (Fig. 1B). In addition, the addition of MC from the initial seeding might result in the formation of spheroids from day 1, and reaching the optimum size with good integrity on day 7 in the culture (Fig. 2). To investigate such discrepancy between the impact of two polymers, their physiochemical properties were further evaluated. Their net charges might be important in providing the required stability for the cell aggregation. This effect may play a substantial role in terms of the electrostatic repulsions or van der Waals interactions as well as the aggregation ability of the cells.
31,32
As listed in Table 3, some properties of MC and NaCMC are presented.
Table 3.
Some physicochemical properties of CMC and MC
|
Compound
|
Solubility
|
Viscosity
|
Electrostatic charge
|
Flocculating agent
|
Appearance
|
Hygroscopicity
|
| NaCMC |
Hot and cold water |
400-800 cPa
|
Anionic |
Yes |
White or lightly yellow powder |
80%-85% |
| MC |
Cold water |
4000 cPb
|
Neutral |
No |
White |
|
NaCMC: sodium carboxymethylcellulose; MC: methylcellulose. * Viscosity of 2%w/v NaCMC in water and 25°C; bViscosity of 0.5-1% w/v MC in water and 25°C.
At the first glance, the inverse relationship between the solubility and the viscosity of these two CDs are noticeable. With the same working concentration and temperature, NaCMC appears to be less viscous than MC, which results in the better dissolution rate and solubility profile.
33,34
It should be noted that NaCMC is soluble in both hot and cold water, while MC is only soluble in cold water even in the low temperature due to its physicochemical properties such as viscosity.
35
For the same reason, MC is capable of absorbing more water compared to NaCMC. By the same token, MC is administered as a laxative agent for the treatment of constipation.
36
On the other hand, NaCMC has the deflocculating property because of the anionic carboxyl groups.
37
In definition, a deflocculating agent or thinning agent tends to reduce the viscosity or prevent the flocculation in the medium. Having considered the physicochemical properties of NaCMC and MC, the uniformity of the spheroids formed by the addition of MC might be explained.
In order to investigate the mode of the interaction between the CDs and Int/Col, key docking analyses were performed. Interestingly, NaCMC showed better bindings with Col and Int in comparison with MC and Cel, while the opposite was expected to happen. To confirm the achieved results, a second round of docking analysis was carried out using the available online web server, MTiOpenScreen, which is based on AutoDock Vina. The results displayed the same pattern by producing the lowest binding energies for NaCMC (Table 1). The further inspection of interactions reveals that hydrogen bonds might be the foremost type of the interactions for NaCMC, which can explain the higher binding energy between the NaCMC and Int/Col complex. Overall, the physicochemical properties of MC surpass the infirm binding with Col as one of the important skeletal entities of ECM.
Conclusion
The Col-Int interaction is an important factor for the cell-cell communication. The cellulose derivatives, which might be considered as biological glue, were added in the 3D cell culture media, which promoted tight connection between Col and Ints. Based on in vitro studies, MC proved to be an important factor not only to support semi-solid medium but also to promote the formation of the uniform 3D spheroids. Although NaCMC appears to have better interactions with Col and Int according to docking results, the physicochemical properties of NaCMC might restrict its benefit in terms of the formation of spheroid in vitro.
Acknowledgment
The authors like to acknowledgethe Research Center for Pharmaceutical Nanotechnology at Tabriz University of Medical Sciences for the technical support.
Funding sources
This study is part of a Ph.D. thesis and supported financially by Zanjan University of Medical Sciences (ID: A-12-892-11, Ethical Code: ZUMS.REC. 1395.42), the Cancer Control Research Center, Cancer Control Foundation, Iran University of Medical Sciences (ID: CCF-97067).
Ethical statement
None to be declared.
Competing interests
The authors declare that they have no conflict of interest.
Authors’ contribution
EDA is the PhD student and conducted the experiments, gathered the data and drafted the manuscript. BJ and SP performed the analyses of computational results. JB was an advisor in this study. YO and SN designed the experiments and finalized the manuscript and supervised the overall study.
Research Highlights
What is the current knowledge?
simple
-
√ Cell aggregation can form three-dimensional (3D) culture (the so-called spheroids).
-
√ Uniform multicellular tumor spheroids can be generated based on the interaction of cells with extracellular matrix (ECM).
-
√ The 3D micro-tissue of JIMT-1 cells was generated using hanging drop method.
What is new here?
simple
-
√ The mode of interaction between cellulose derivatives and collagen-integrin was studied.
-
√ Methyl cellulose is suggested as an important ECM-mimicking entity for uniform spheroid formation.
References
- Langhans SA. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front Pharmacol 2018; 9. doi: 10.3389/fphar.2018.00006 [Crossref]
- Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release 2012; 164:192-204. doi: 10.1016/j.jconrel.2012.04.045 [Crossref] [ Google Scholar]
- Jokinen J, Dadu E, Nykvist P, Kapyla J, White DJ, Ivaska J. Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem 2004; 279:31956-63. doi: 10.1074/jbc.M401409200 [Crossref] [ Google Scholar]
- Zeltz C, Gullberg D. The integrin-collagen connection--a glue for tissue repair? J Cell Sci 2016; 129: 653-64. The integrin-collagen connection--a glue for tissue repair? J Cell Sci 2016; 129:653-64. doi: 10.1242/jcs.180992 [Crossref] [ Google Scholar]
- Popova SN, Lundgren-Akerlund E, Wiig H, Gullberg D. Physiology and pathology of collagen receptors. Acta Physiol 2007; 190:179-87. doi: 10.1111/j.1748-1716.2007.01718.x [Crossref] [ Google Scholar]
- Bezerra KS, Neto JL, Oliveira JIN, Albuquerque EL, Caetano EWS, Freire VN. Computational investigation of the α 2 β 1 integrin–collagen triple helix complex interaction. New J Chem 2018; 42:17115-25. doi: 10.1039/C8NJ04175J [Crossref] [ Google Scholar]
- Aoudjit F, Vuori K. Integrin signaling in cancer cell survival and chemoresistance. Chemother Res Pract 2012; 283181:11. doi: 10.1155/2012/283181 [Crossref] [ Google Scholar]
- Casal JI, Bartolome RA. RGD cadherins and alpha2beta1 integrin in cancer metastasis: A dangerous liaison. Biochim Biophys Acta Rev Cancer 2018; 2:321-32. doi: 10.1016/j.bbcan.2018.04.005 [Crossref] [ Google Scholar]
- Zhang Z, Ramirez NE, Yankeelov TE, Li Z, Ford LE, Qi Y. alpha2beta1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor cell-specific manner. Blood 2008; 111:1980-8. doi: 10.1182/blood-2007-06-094680 [Crossref] [ Google Scholar]
- Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol 2015; 25:234-40. doi: 10.1016/j.tcb.2014.12.006 [Crossref] [ Google Scholar]
- Naci D, Vuori K, Aoudjit F. Alpha2beta1 integrin in cancer development and chemoresistance. Semin Cancer Biol 2015; 35:145-53. doi: 10.1016/j.semcancer.2015.08.004 [Crossref] [ Google Scholar]
- Breslin S, O'Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 2013; 18:240-9. doi: 10.1016/j.drudis.2012.10.003 [Crossref] [ Google Scholar]
- Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol 2005; 15:365-77. doi: 10.1016/j.semcancer.2005.05.002 [Crossref] [ Google Scholar]
- Santini MT, Rainaldi G. Three-dimensional spheroid model in tumor biology. Pathobiology 1999; 67:148-57. doi: 10.1159/000028065 [Crossref] [ Google Scholar]
- Froehlich K, Haeger J-D, Heger J, Pastuschek J, Photini SM, Yan Y. Generation of multicellular breast cancer tumor spheroids: Comparison of different protocols. J Mammary Gland Biol Neoplasia 2016; 21:89-98. doi: 10.1007/s10911-016-9359-2 [Crossref] [ Google Scholar]
- Ding C, Shi R, Zheng Z, Zhang M. Effect of carboxymethylcellulose on fibril formation of collagen in vitro. Connect Tissue Res 2018; 59:66-72. doi: 10.1080/03008207.2017.1306059 [Crossref] [ Google Scholar]
- Ding C, Zhang M, Tian H, Li G. Effect of hydroxypropyl methylcellulose on collagen fibril formation in vitro. Int J Biol Macromol 2013; 52:319-26. doi: 10.1016/j.ijbiomac.2012.10.003 [Crossref] [ Google Scholar]
- Leung BM, Lesher-Perez SC, Matsuoka T, Moraes C, Takayama S. Media additives to promote spheroid circularity and compactness in hanging drop platform. Biomater Sci 2015; 3:336-44. doi: 10.1039/c4bm00319e [Crossref] [ Google Scholar]
- Lopez JI, Kang I, You WK, McDonald DM, Weaver VM. In situ force mapping of mammary gland transformation. Integr Biol 2011; 3:910-21. doi: 10.1039/c1ib00043h [Crossref] [ Google Scholar]
-
Garcia MA, Nelson WJ, Chavez N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb Perspect Biol 2018; 10. 10.1101/cshperspect.a029181
- Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta 2009; 1788:872-91. doi: 10.1016/j.bbamem.2008.11.005 [Crossref] [ Google Scholar]
- Cocinero EJ, Gamblin DP, Davis BG, Simons JP. The building blocks of cellulose: the intrinsic conformational structures of cellobiose, its epimer, lactose, and their singly hydrated complexes. J Am Chem Soc 2009; 131:11117-23. doi: 10.1021/ja903322w [Crossref] [ Google Scholar]
- Ding C, Shi R, Zheng Z, Zhang M. Effect of carboxymethylcellulose on fibril formation of collagen in vitro. Connect Tissue Res 2018; 59:66-72. doi: 10.1080/03008207.2017.1306059 [Crossref] [ Google Scholar]
- Ding C, Zhang M, Tian H, Li G. Effect of hydroxypropyl methylcellulose on collagen fibril formation in vitro. Int J Biol Macromol 2013; 52:319-26. doi: 10.1016/j.ijbiomac.2012.10.003 [Crossref] [ Google Scholar]
- Desoize B, Jardillier J-C. Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol 2000; 36: 193-207. a paradigm for clinical resistance? Crit Rev Oncol Hematol 2000; 36:a paradigm for clinical resistance? Crit Rev Oncol Hematol 2000; 36. doi: 10.1016/s1040-8428(00)00086-x [Crossref] [ Google Scholar]
- Lee JM, Yang L, Kim E-J, Ahrberg CD, Lee K-B, Chung BG. Generation of uniform-sized multicellular tumor spheroids using hydrogel microwells for advanced drug screening. Sci Rep 2018; 8:17145. doi: 10.1038/s41598-018-35216-7 [Crossref] [ Google Scholar]
- Vadivelu RK, Ooi CH, Yao R-Q, Velasquez JT, Pastrana E, Diaz-Nido J. Generation of three-dimensional multiple spheroid model of olfactory ensheathing cells using floating liquid marbles. Sci Rep 2015; 5:15083. doi: 10.1038/srep15083 [Crossref] [ Google Scholar]
- Shri M, Agrawal H, Rani P, Singh D, Onteru SK. Hanging drop, a best three-dimensional (3D) culture method for primary buffalo and sheep hepatocytes. Sci Rep 2017; 7:1203. doi: 10.1038/s41598-017-01355-6 [Crossref] [ Google Scholar]
- Ware MJ, Colbert K, Keshishian V, Ho J, Corr SJ, Curley SA. Generation of homogenous three-dimensional pancreatic cancer cell spheroids using an improved hanging drop technique. Tissue Eng Part C Methods 2016; 22:312-21. doi: 10.1089/ten.TEC.2015.0280 [Crossref] [ Google Scholar]
- Laurila P, Leivo I. Basement membrane and interstitial matrix components form separate matrices in heterokaryons of PYS-2 cells and fibroblasts. J Cell Sci 1993; 104:59-68. [ Google Scholar]
- Matak D, Brodaczewska KK, Lipiec M, Szymanski Ł, Szczylik C, Czarnecka AM. Colony, hanging drop, and methylcellulose three dimensional hypoxic growth optimization of renal cell carcinoma cell lines. Cytotechnology 2017; 69:565-78. doi: 10.1007/s10616-016-0063-2 [Crossref] [ Google Scholar]
- Nasatto PL, Pignon F, Silveira JL, Duarte MER, Noseda MD, Rinaudo M. Interfacial properties of methylcelluloses: The Influence of molar mass. Polymers 2014; 6:2961-73. doi: 10.3390/polym6122961 [Crossref] [ Google Scholar]
- Nasatto P, Pignon F, Silveira J, Duarte M, Noseda M, Rinaudo M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015; 7:777-803. [ Google Scholar]
- Yang XH, Zhu WL. Viscosity properties of sodium carboxymethylcellulose solutions. Cellulose 2007; 14:409-17. doi: 10.1007/s10570-007-9137-9 [Crossref] [ Google Scholar]
- Takahashi M, Shimazaki M, Yamamoto J. Thermoreversible gelation and phase separation in aqueous methyl cellulose solutions. J Polym Sci B 2001; 39:91-100. doi: 10.1002/1099-0488(20010101)39:1<91::AID-POLB80>3.0.CO;2-C [Crossref] [ Google Scholar]
- Wald A. Appropriate use of laxatives in the management of constipation. Curr Gastroenterol Rep 2007; 9:410-4. doi: 10.1007/s11894-007-0051-y [Crossref] [ Google Scholar]
- Cai T, Li H, Yang R, Wang Y, Li R, Yang H. Efficient flocculation of an anionic dye from aqueous solutions using a cellulose-based flocculant. Cellulose 2015; 22:1439-49. doi: 10.1007/s10570-015-0571-9Os [Crossref] [ Google Scholar]