Bioimpacts. 11(1):5-14.
doi: 10.34172/bi.2021.02
Original Research
Impact of RAS/RAF mutations on clinical and prognostic outcomes in metastatic colorectal cancer
Roya Dolatkhah 1  , Saeed Dastgiri 2
, Saeed Dastgiri 2  , Amir Taher Eftekhar Sadat 3, Faris Farassati 4, Marzieh Nezamdoust 5, Mohammad Hossein Somi 3, *
, Amir Taher Eftekhar Sadat 3, Faris Farassati 4, Marzieh Nezamdoust 5, Mohammad Hossein Somi 3, * 
Author information:
1Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Tabriz Health Services Management Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Midwest Biomedical Research Foundation, Kansas City, MO, USA
5Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Early-activated RAS/RAF mutation status is a key molecular finding in colorectal cancer (CRC), while these mutations have been proposed as predictive and prognostic biomarkers. The present study has been designed as a longitudinal study to evaluate and summarize the different genotypes of metastatic CRC (mCRC), and assessing any association with the disease prognosis and clinicopathological characteristics. This study was performed in two main referral hospitals of Tabriz University of Medical Sciences, over three years (2016-2018).
Methods:
Mutations were detected by Idylla tests of KRAS/NRAS/BRAF among a total of 173 mCRCs, using surgically-resected specimens or biopsied samples. To evaluate the factors associated with overall survival (OS) and prognosis, the Cox proportional hazards model was used in two steps to estimate the outcome measures (hazard ratio, or HR) with a 95% confidence interval (CI).
Results:
The nominal 1 to 5-year OS rates were 78%, 65%, 55%, 46%, and 42%, respectively. KRAS mutations in codon 12 was an independent significant prognostic factor, as the patients with codon 12 mutations had a significantly lower OS (P Log-rank=0.049) and a higher hazard of mortality (HR=2.30; 95% CI: 0.95-5.58; P =0.066). Also, the mCRC patients with liver metastasis (HR=2.49; 95% CI: 1.49-12.52; P =0.002) and tumors of the distal colon (HR=3.36; 95% CI: 1.07-10.49; P =0.037) had a significantly worse prognosis.
Conclusion: KRAS
mutation in codon 12 was an independent significant poor prognostic factor, and patients with liver metastasis had a significantly worse prognosis. Routinely performing specific oncogenic tests may help improve the patients’ prognosis and life expectancy.
Keywords: Colorectal cancer, Overall survival, Oncogene, KRAS, Hazard ratio
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/
). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Colorectal cancer (CRC) is one of the main leading causes of cancer-related death worldwide that ranks as the second fatal cancer and accounted for around one in ten cancer deaths in 2018.
1
CRC ranked as the third most common cancer in Iran, which accounts for 9% of all cancers in the country. However, it is second most common cancer in East Azerbaijan.
2
Despite the long-standing screening and early detection programs as well as improvements in cancer treatment and management using targeted therapies in most developed countries, its incidence and mortality have shown an increasing trend in some regions of the world, especially Asia and developing countries.
1,3,4
CRC represents a heterogeneous genetic and epigenetic alteration that could lead to different phenotypes. Chromosomal instability pathway, including the mutational activation of specific proto-oncogenes, is the most common type of mutagenic pathway in CRC.
5,6
Early-activated RAS/RAF mutation status is a key molecular finding, especially in metastatic CRC (mCRC), and tests are therefore recommended in stage IV of CRC at the time of diagnosis for targeted therapy approaches.
7
These mutations have been proposed as predictive and prognostic biomarkers in mCRC.
8
Besides, KRAS gene mutation, with a 90% occurrence rate, is localized in codons 12 and 13. Herein, this point mutation can be used as a predictive biomarker, to improve the efficacy of anti-EGFR targeted therapies.
9,10
Research has also suggested that the pattern of specific KRAS gene mutation, and so the impact of specific amino acid changes, are associated with different prognostic values and responses to anti-EGFR therapies.
9,11
Although some researchers have suggested that the presence of specific mutations in CRC is associated with the metastasis and outcome potential,
4,9
but there is still an inconsistency in the available data about.
4,12,13
As very few studies have analyzed the pattern of different point mutations in Iranian mCRC, the present study was conducted to evaluate and summarize the different genotypes of mCRC and its association with the disease prognosis and OS as well as clinicopathological characteristics. This research also seeks to find any possible associations between KRAS, NRAS, and BRAF mutations in mCRC and primary tumor location and metastatic site.
Materials and Methods
Study design
This longitudinal study is performed in three phases:
Cross-sectional phase:Based on our inclusion criteria, we collected data for 264 mCRCs, with histo-pathologically-confirmed CRC and imaging- and/or surgery-confirmed metastasis. Standard demographic characteristics, clinicopathological data, and mutation subtypes at the time of diagnosis were recorded for each patient using the hospital database or through the patients' follow-up. These variables included data on somatic mutations (KRAS, NRAS, and BRAF) and type of mutation, age at diagnosis, gender, primary tumor anatomic site (proximal and distal colon and rectum), metastatic site (lymph node, brain, lung, bone, omentum, liver, etc.), morphologic type (adenocarcinoma, etc.), and tumor grade (well, moderately, and poorly differentiated). The ethics committee of Tabriz University of Medical Sciences has been approved this project (IR.TBZMED.REC.1395.18), and all patient's information and records are confidential.
Follow-up phase:For OS analysis, we contacted the patients or their family, by following exact ethical rules for following, and getting their consent.
Analytical case only phase:The relationship between clinicopathological variables and evaluated mutations has been assessed in mCRC patients. Structured questionnaires were used to collect more information about the main risk factors of the disease, including smoking, alcohol intake, marital status, body mass index, positive family history of CRC, occupation, education, and immobility. These questionnaires had been formally validated in a previous study by the same researchers.
14
Alcohol intake was evaluated in terms of frequency, quantity, and type of alcohol consumed (beer, wine and/or spirits), and the level of intake was rated as ‘never’, ‘low’ (less than once a week, one glass), moderate (two-three times a week, one to two glasses) and ‘high’ (more than three times per week, more than two glass). To achieve better regression analysis results, the patients were divided into two subgroups, including ‘no drinking’ (never or low consumption) and ‘drinkers’ (moderate or high consumption) groups. As for the smoking status, the patients were asked about their number of cigarettes smoked per day (less or more than one box) and the duration of smoking (over the span of one year). To achieve better regression analysis results, the patients were divided into subgroups, including ‘no smoking’ (never smoked during the last 20 years) and ‘smokers’ (smoking at least one cigarette per day, at least for one year over the last 20 years). As for the other lifestyle factors, the responses were designed based on a Likert scale. The occupation was categorized as ‘employed’ (full- or part-time) and ‘retired or unemployed’ (student or without a job). The subjects’ education was categorized as ‘illiterate’, ‘primary/high school education’, and ‘university education’. As for the place of residence, the patients were asked if they were owners or otherwise (including renting, non-fixed status, homeless). The subjects were categorized under three subgroups in terms of their body mass index (BMI), including the ‘BMI≤25’ group, the ‘25-30 BMI’ group, and the ‘higher than 30 BMI’ group. Also, age was categorized to <50 and ≥50, and age 50 years was taken as a reasonable cutoff point.
Study setting
This research was performed in two main referral hospitals of Tabriz University of Medical Sciences, Imam Reza, and Shahid Ghazi educational and treatment centers, using the method of "gathering all eligible samples" (having our inclusion criteria) over three years, during April 2016 to April 2018. All molecular tests were performed by the co-investigate pathologist of this study, at the reference molecular laboratory, with available standard molecular tests.
Mutation status
The patients underwent RAS/RAF type specification using surgically-resected specimens or biopsied samples of their primary tumors, and molecular testing was routinely prescribed by their medical oncologists.
After confirming their mCRC, an expert pathologist undertook the histological examination of their cancer tissues. Formalin-Fixed Paraffin-Embedded (FFPE) tissue sections (5–30 µm) from the samples underwent molecular testing for KRAS, NRAS, and BRAFmutations. Mutations were detected by Idylla tests of KRAS/NRAS/BRAF (Biocartis NV, 2800 Mechelen, Belgium, BCT006631). According to the reference clinical guidelines,
15
the KRASgene mutations detected for codons 12, 13, 59, 61, 117 and 146 at the study’s reference molecular laboratory included Gln61His (c.183G>C), Gly12Ala (c.35G>C), Gly12Asp (c.35G>A), Gly12Cys (c.34G>T), Gly12Cys (c.34G>T), Gly12Ser (c.34G>A), Gly12Val (c.35G>T), Gly13Asp (c.38G>A) and Gly13Asp. The NRAS mutation detected was Q61R (c.182A>G) and the BRAF mutation detected V600E/D (c.1799T>A).
The Turnaround Time (TAT) from sample shipping to getting the mutation results were under ten days for more than 95% of the cases. Three to four FFPE tissue sections (5-30 µm) of CRC tumors were loaded in cartridges and then inserted into the system according to the manufacturer’s instructions. After the DNA extraction and purification by the system, multiplex-PCRs were performed, and finally, KRAS/NRAS/BRAF-specific software was used to automatically determine the presence of the noted mutations.
Statistical analysis
Given that only one NRAS mutation and one BRAF mutation were observed, all the statistical analyses were performed on the cases with KRAS gene mutations. All the statistical analysis was performed using STATA (MP 14.2, Stata Corp LP, College Station, Texas 77845 USA). Pearson’s Chi-Square test was used for the subgroup analysis and to test the differences in each of the studied variables in connection to the KRAS gene mutation subtypes. All the clinicopathological variables were stratified as two common types of KRAS mutations (codons 12 and 13). For the CRC-specific survival analysis, the overall survival (OS) was defined as the length of time between the pathological or surgical diagnosis until death (CRC-specific) or last follow-up time. The patients who were alive at the time of the last follow-up were considered censored in the survival analysis. The overall, one-, three- and five-year survival were estimated using the timetable survival method. The prognosis was evaluated as the OS, and subgroup analysis was performed to compare the survival function in the different mutation subtypes using Kaplan-Meier survival estimate with log rank (Mantel-Cox) test. All the other non-specific CRC mortality data were excluded.
To evaluate the factors associated with OS and prognosis, the Cox proportional hazards model was used in two steps (univariate and multivariate steps) to estimate the hazard ratio (HR) with a 95% Confidence Interval (CI). Also, the proportional hazards assumption has been tested, based on Schoenfeld residuals (ph-test). First, the unadjusted HRs were estimated for each variable and the tumor characteristics. Then, the multiple regression modeling was used based on the forward stepwise model to calculate the adjusted HRs in the presence of confounding variables (age, gender, grade, smoking status, alcohol intake, tumor subsite, metastatic site, and morphologic type). At the final step, the Poisson regression model was applied to confirm the adjusted HR results in the presence of competing risks. All the tests were two-tailed and P<0.05 was set as the level of statistical significance.
Results
Frequency of KRAS mutation types and their association with different clinicopathological characteristics
We enrolled 264 mCRC cases during three years of study. From these, we excluded 58 cases because of missing some main data, five CRCs had nonspecific deaths (other than CRC), and we lost to follow up for 28 CRC cases (because of changed contact information, or moving of patients). At the final step, a total of 173 patients were enrolled in this analytical research study (Fig. 1). Of the 106 cases with KRAS mutation data, 45 (42.5%) were positive for KRAS gene mutation and 61 cases (57.5%) had the wild-type gene. Mutations in exon 2 were identified in 44 of the cases, including 32 (71.1%) with point mutations in codon 12 and 12 (26.7%) with a single mutation in codon 13, and only one case (2.2%) had a point mutation in exon 61 of the KRAS gene. NRAS mutations were tested in 75 mCRC samples, and only one case was positive for NRAS mutation (Q61R (c.182A>G)), and of the 39 cases tested for BRAF mutation, only one case had a mutation in BRAF codon 600 (V600E). Because of missing data of NRAS and BRAF mutations in most of the cases, they were not included in the statistical analysis.
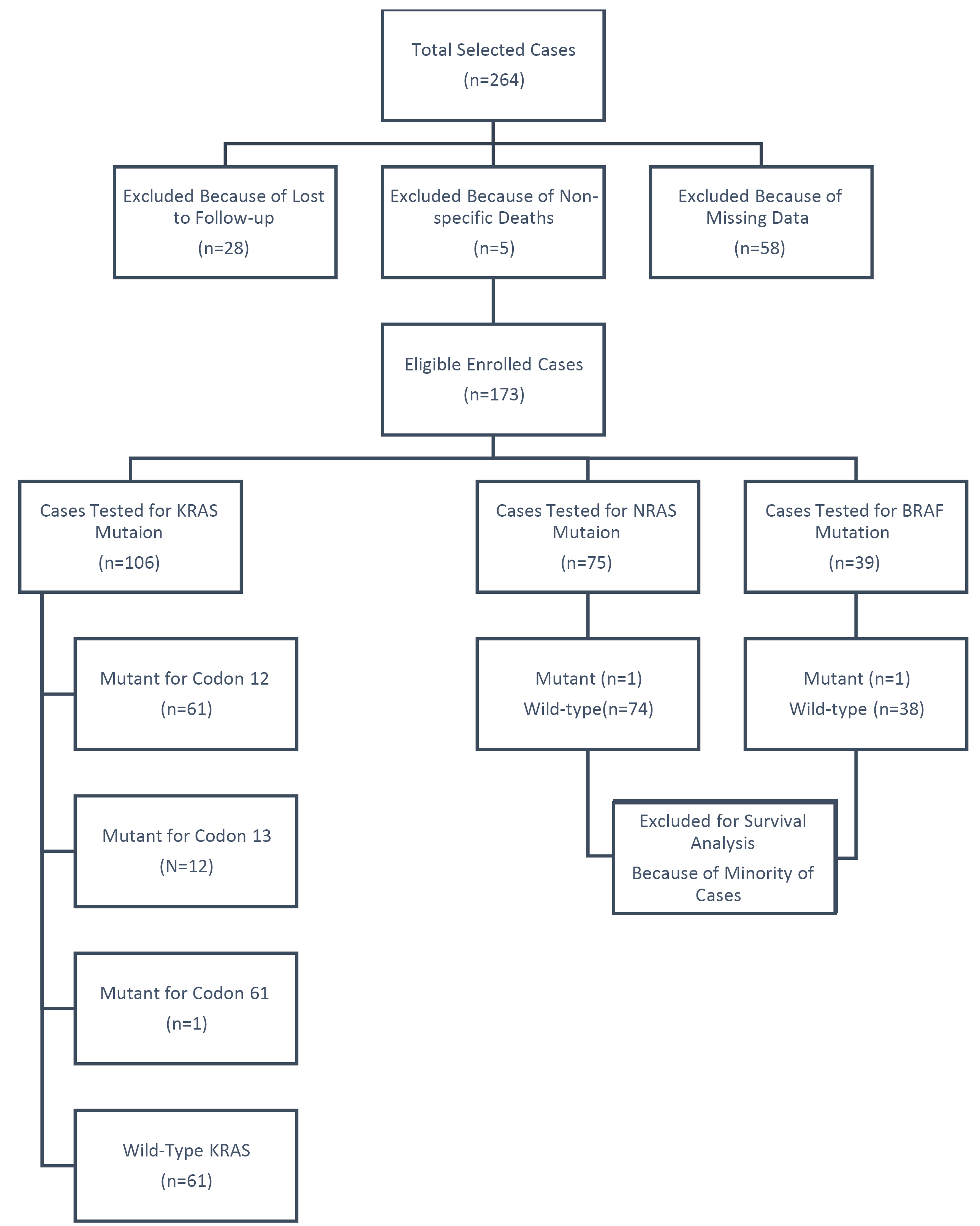
Fig. 1.
Flow diagram of study, including and excluding cases.
.
Flow diagram of study, including and excluding cases.
From the 173 enrolled mCRC samples, 97 cases (56.1%) were male and 76 cases (43.9%) were female. Their mean age was 58.91 ± 12.95 years (range: 21 to 90 years). Most patients were ≥50 years old (n=130, 75.1%) and the most common metastatic site was the liver (n=46, 26.6%), followed by the omentum (n=23, 13.3%). The most common subsite of the primary tumor was the rectum (n=99, 57.2%), with the majority of the tumors being well-differentiated adenocarcinoma (n=67, 38.7%). KRAS codon 13 mutations were more common in the male participants and the distal colon, while KRAS codon 12 was most obvious in the rectum lesions and the colorectal patients with liver metastasis. The tumors that were well-differentiated and were adenocarcinoma in terms of morphologic type had more KRAS 12 mutations. There were no significant associations between these clinicopathological characteristics and the KRAS mutation type. The presence of KRAS 12, 13, and 61 mutations was not significantly associated with any specific risk factors (smoking, alcohol intake, education, occupation, marital status, immobility, and BMI). Table 1 presents the associations between KRAS mutation and different demographic, clinicopathological, and lifestyle factors.
Table 1.
Baseline association between wild-type and mutant KRAS subtypes and clinicopathological aspects in mCRC
|
Variable
|
KRAS
WT
N=62 (57.9%)
|
KRAS
Mutant, N=45 (43.9)
|
Codon 12
N=32 (72.7%)
|
Codon 13,16
N=13 (28.2%)
|
P
value
|
|
No. (%)
|
No. (%)
|
No. (%)
|
| Sex |
Female (n=45) |
27 (25.2) |
16 (15.0) |
3 (2.8) |
0.147 |
| Male (n=61) |
35 (32.7) |
1615.0) |
10 (9.3) |
| Age |
<50 (n=32) |
12 (11.2) |
15 (14.0) |
4 (3.7) |
0.018 |
| ≥50 (n=75) |
50 (46.7) |
17 (15.9) |
8 (7.5) |
| Morphological type |
Adenocarcinoma (n=94) |
53 (49.5) |
29 (27.1) |
12 (11.2) |
0.836 |
| Others (n=13) |
9 (8.4) |
3 (2.8) |
1 (0.9) |
| Grade (differentiated) |
Well (n=45) |
22 (20.6) |
17 (15.9) |
6 (5.6) |
0.482 |
| Moderate (n=37) |
25 (23.4) |
7 (6.5) |
5 (4.7) |
| Poor (n=25) |
15 (14.0) |
8 (7.5) |
2 (1.9) |
| Metastatic Site |
Lymph node (n=10) |
4 (3.7) |
5 (4.7) |
1 (0.9) |
0.212 |
| Brain (n=12) |
5 (4.7) |
6 (5.6) |
1 (0.9) |
| Lung (n=11) |
10 (9.3) |
1 (0.9) |
0 (0.0) |
| Bone (n=17) |
10 (9.3) |
6 (5.6) |
1 (0.9) |
| Omentum (n=18) |
12 (11.2) |
2 (1.9) |
4 (3.7) |
| Liver (n=26) |
13 (12.1) |
9 (8.4) |
4 (3.7) |
| Others (n=13) |
8 (7.5) |
3 (2.8) |
2 (1.8) |
| Subsite |
Proximal (n=20) |
13 (12.1) |
6 (5.6) |
1 (0.9) |
0.118 |
| Distal (n=33) |
13 (12.1) |
12 (11.2) |
8 (7.4) |
| Rectum (n=54) |
36 (33.6) |
14 (13.1) |
4 (3.7) |
| Marital Status |
Married (n=98) |
57 (53.3) |
29 (27.1) |
12 (11.2) |
0.987 |
| Unmarried (n=9) |
5 (4.7) |
3 (2.8) |
1 (0.9) |
| Positive Family History of CRC |
Yes (n=21) |
13 (12.4) |
6 (5.7) |
2 (1.8) |
0.165 |
| No (n=84) |
48 (45.7) |
25 (23.8) |
11 (10.5) |
| Occupation |
Employed (n=25) |
9 (8.4) |
10 (9.3) |
6 (5.6) |
0.082 |
| Retired (n=31) |
22 (20.6) |
6 (5.6) |
3 (2.8) |
| Unemployed (n=51) |
31 (29.0) |
16 (15.0) |
4 (3.7) |
| Education |
University (n=16) |
9 (8.5) |
3 (2.8) |
4 (3.8) |
0.155 |
| School (n=68) |
39 (36.8) |
22 (20.8) |
5 (6.6) |
| Illiterate (n=22) |
14 (13.20 |
6 (5.7) |
2 (1.9) |
| Residence |
Private (n=86) |
52 (48.6) |
22 (20.6) |
11 (10.3) |
0.218 |
| Rental/Unstable (n=21) |
10 (9.3) |
10 (9.3) |
1 (0.9) |
| Immobility |
Yes (n=46) |
26 (24.3) |
14 (13.1) |
6 (5.6) |
0.712 |
| No (n=61) |
36 (33.6) |
18 (16.8) |
7 (6.5) |
| Smoking |
Yes (n=21) |
10 (9.3) |
7 (6.5) |
5 (4.6) |
0.339 |
| No (n=86) |
52 (48.6) |
25 (23.4) |
8 (7.5) |
| Drinking |
Yes (n=15) |
7 (6.5) |
5 (4.7) |
3 (2.8) |
0.375 |
| No (n=92) |
55 (51.4) |
27 (25.2) |
10 (9.3) |
| BMI |
≤25 (n=57) |
31 (29.0) |
19 (17.8) |
7 (6.5) |
0.369 |
| 25-30 (n=37) |
24 (22.4) |
9 (8.4) |
4 (3.7) |
| ≥30 (n=13) |
7 (6.5) |
4 (3.7) |
2 (1.9) |
CRC: colorectal cancer; BMI: body mass index.
Survival analysis
The median follow-up time was 17 months (0.33 to 71.97 months) and 65 CRC patients (37.6%) died during the follow-up. The mean and median OS were 39.44 (95% CI: 34.86-44.02) and 42.27 (95% CI: 35.56-48.97), respectively. The nominal 1-year, 2-year, 3-year, 4-year, and 5-year survival were 78%, 65%, 55%, 46%, and 42%, respectively.
The median OS was 42.27 (95% CI: 27.93-56.60) months for the colorectal patients with wild-type KRAS gene. Kaplan-Meier survival estimate didn’t show any significant difference in OS between KRAS mutant and the wild-type gene (P Log-rank = 0.214) (Fig. 2A). The median OS for the patients with mutations in codon 12 of the KRAS gene was 36.17 (95% CI: 18.18-54.15) and the log rank (Mantel-Cox) test for survival distribution of the different KRAS mutations showed that KRAS codon 12 point mutation was independently associated with a worse OS compared to the wild-type tumors (Log-rank P=0.049) (Fig. 2B). For the patients with mutations in codon 13 of the KRAS gene, the median OS was 46.35 (95% CI: 30.97-61.74) and was not associated with a worse OS compared to the wild-type tumors (Log-rank P=0.575). Kaplan-Meier survival estimate did not show any significant difference in OS between KRAS codon 12 and 13 mutations and wild-type genes (P Log-rank=0.102) (Fig. 2C). Nonetheless, the OS was 34.4%, 66.7%, and 69.4% in the KRAS codon 12 and 13 mutations and the wild-type KRAS mutations, respectively, and there was only one case of a KRAS 16 mutation, which remained alive.
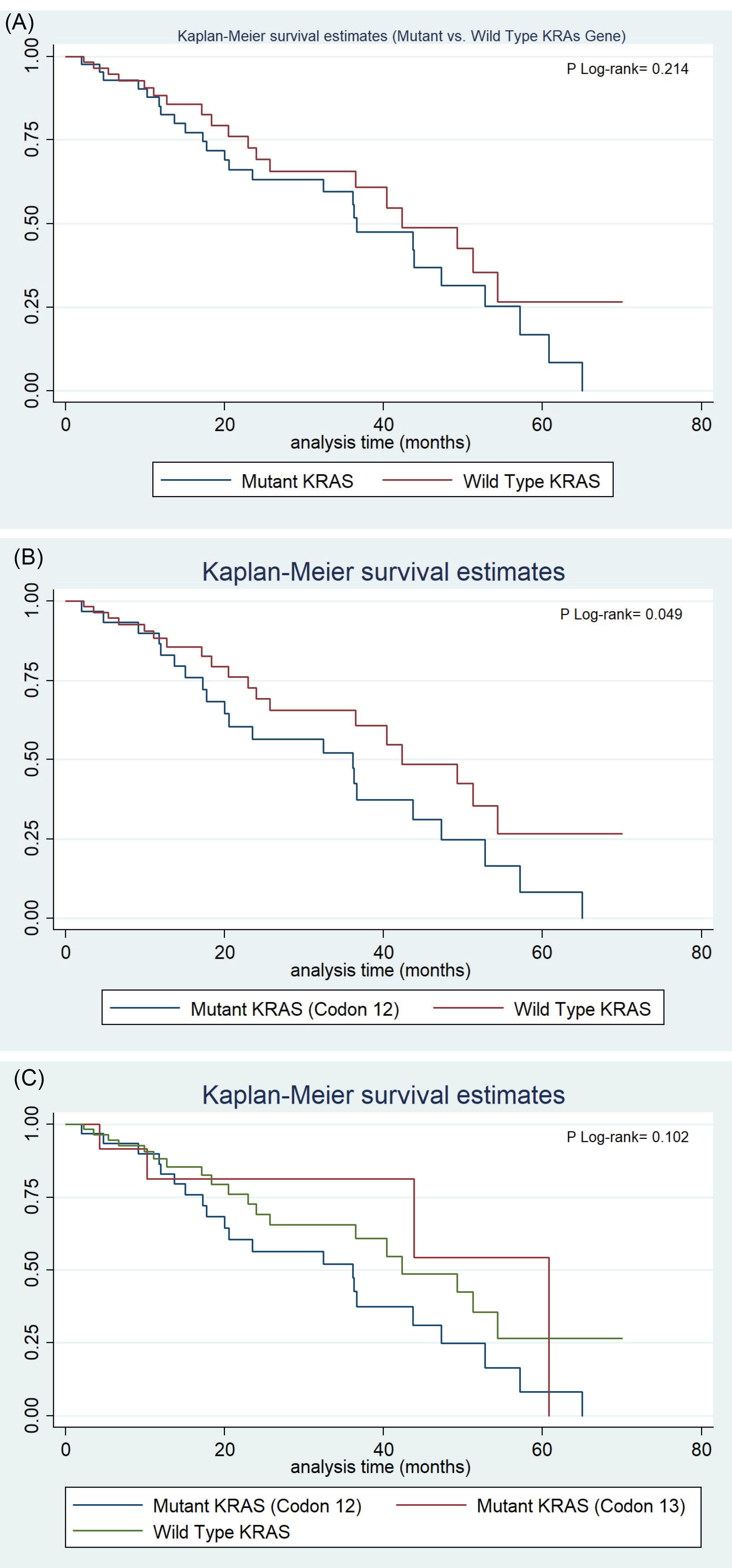
Fig. 2.
(A) Survival Estimated Difference between KRAS mutant and the wild-type gene. (B) Survival Estimated Difference between KRAS codon 12 mutation independently and wild-type gene. (C) Survival Estimated Difference between KRAS codon 12 and 13 mutations and wild-type gene
.
(A) Survival Estimated Difference between KRAS mutant and the wild-type gene. (B) Survival Estimated Difference between KRAS codon 12 mutation independently and wild-type gene. (C) Survival Estimated Difference between KRAS codon 12 and 13 mutations and wild-type gene
Cox regression hazard function analysis for different clinicopathological characteristics
The Cox regression analysis showed that mCRC patients with mutations in codon 12 of the KRAS gene had a worse OS (HR=1.77; 95% CI: 0.95-3.30) and the female patients had a worse prognosis than the males (HR=1.3; 95% CI: 0.69-1.85). Smoking in any amount and duration increased the mortality hazard by about 1.5 times (HR=1.46; 95% CI: 0.82-2.57), but alcohol consumption did not have a significant association with the patients’ prognosis. The metastatic patients with liver metastasis had a worse OS (HR=1.59; 95% CI: 0.72-3.50) and the patients with rectal tumors had a higher hazard of mortality compared to those with tumors in the other subsites (HR=1.45; 95% CI: 0.69-3.03). Multivariate Cox regression model after adjusting the included risk factors, showed that patients with codon 12 mutations had a lower OS (HR=2.30; 95% CI: 0.95-5.58; P=0.066). In comparison, KRAS mutations in codon 13 had no significant impact on the patients’ prognosis (HR=0.52; 95% CI: 0.11-2.55; P=0.420). Smoking increased the hazard of mortality by about 7 times (HR=7.32; 95% CI: 2.29-23.28; P=001). Also, the mCRC patients with liver metastasis (HR=2.49; 95% CI: 1.49-12.52; P=0.002) and tumors of the distal colon (HR=3.36; 95% CI: 1.07-10.49; P = 0.037) had a significantly worse OS (Table 2). The Poisson regression analysis showed exactly the same results for Cox regression multivariate analysis. Also, the proportional hazards assumption test showed that all the variables contributed to each model satisfied the PH assumption of the Cox regressions globally (chi2 = 29.83, P = 0.231).
Table 2.
Results of Cox regression analysis of clinicopathologic factors with KRAS mutation types
|
Variable
|
Univariable analysis
|
Multivariable analysis
|
|
HR
†
(95% CI)
|
P
value
|
HR
*
(95% CI)
|
P
value
|
|
KRAS mutation
|
Codon 12 (n=32) |
1.77 (0.95-3.29) |
0.073 |
2.30 (0.95-5.58) |
0.066 |
| Codon 13 (n=12) |
0.76 (0.26-2.24) |
0.616 |
0.52 (0.11-2.55) |
0.420 |
| Wild-type (n=62) |
1 (Reference) |
- |
1 (Reference) |
- |
| Sex |
Female (n=45) |
1.13 (0.69-1.85) |
0.634 |
1.21 (0.45-3.29) |
0.710 |
| Male (n=61) |
1 (Reference) |
- |
1 (Reference) |
- |
| Age |
<50 (n=32) |
1 (Reference) |
- |
1 (Reference) |
- |
| ≥50 (n=75) |
1.18 (0.66-2.11) |
0.584 |
2.59 (0.92-7.28) |
0.071 |
| Morphological type |
Adenocarcinoma (n=94) |
1.15 (0.58-2.29) |
0.683 |
1.46 (0.38-5.56) |
0.580 |
| Others (n=13) |
1 (Reference) |
- |
1 (Reference) |
- |
| Grade (differentiated) |
Well (n=45) |
1.02 (0.56-1.86) |
0.949 |
0.34 (0.11-1.10) |
0.071 |
| Moderate (n=37) |
0.75 (0.40-1.39) |
0.359 |
0.35 (0.10-1.18) |
0.090 |
| Poor (n=25) |
1 (Reference) |
- |
1 (Reference) |
- |
| Metastatic site |
Lymph Node (n=10) |
1.07 (0.37-3.16) |
0.897 |
0.24 (0.05-1.22) |
0.086 |
| Brain (n=12) |
0.76 (0.30-1.95) |
0.570 |
1.20 (0.20-7.40) |
0.844 |
| Lung (n=11) |
1.09 (0.45-2.63) |
0.851 |
0.73 (0.16-3.41) |
0.686 |
| Bone (n=17) |
1.10 (0.43-2.87) |
0.839 |
0.43 (0.74-2.43) |
0.336 |
| Omentum (n=18) |
0.69 (0.22-1.89) |
0.417 |
1.09 (0.25-4.84) |
0.909 |
| Liver (n=26) |
1.59 (0.72-3.50) |
0.248 |
2.49 (1.49-12.52) |
0.002
|
| Others (n=13) |
1 (Reference) |
- |
1 (Reference) |
- |
| Subsite |
Proximal (n=20) |
1 (Reference) |
- |
1 (Reference) |
- |
| Distal (n=33) |
1.14 (0.51-2.52) |
0.750 |
3.36 (1.07-10.49) |
0.037
|
| Rectum (n=54) |
1.45 (0.69-3.03) |
0.324 |
2.52 (0.90-7.01) |
0.077 |
| Marital status |
Married (n=98) |
1.32 (0.56-3.08) |
0.524 |
1.52 (0.46-5.05) |
0.495 |
| Unmarried (n=9) |
1 (Reference) |
- |
1 (Reference) |
- |
| Positive family History of CRC |
Yes (n=21) |
1 (Reference) |
- |
1 (Reference) |
- |
| No (n=84) |
1.40 (0.69-2.83) |
0.355 |
0.85 (0.25-2.85) |
0.792 |
| Occupation |
Employed (n=25) |
1 (Reference) |
- |
1 (Reference) |
- |
| Retired (n=31) |
1.27 (0.61-2.63) |
0.528 |
N/A |
N/A |
| Unemployed (n=51) |
1.24 (0.69-2.23) |
0.482 |
N/A |
N/A |
| Education |
University (n=16) |
1.27 (0.52-3.11) |
0.597 |
N/A |
N/A |
| School (n=68) |
1.35 (0.76-2.41) |
0.313 |
N/A |
N/A |
| Illiterate (n=22) |
1 (Reference) |
- |
1 (Reference) |
- |
| Residence |
Private (n=86) |
1.04 (0.54-1.99) |
0.913 |
0.98 (0.26-3.78) |
0.981 |
| Rental/Unstable (n=21) |
1 (Reference) |
- |
1 (Reference) |
- |
| Immobility |
Yes (n=46) |
1 (Reference) |
- |
1 (Reference) |
- |
| No (n=61) |
1.22 (0.73-2.04) |
0.439 |
N/A |
N/A |
| Smoking |
Yes (n=21) |
1.46 (0.82-2.57) |
0.197 |
7.32 (2.30-23.28) |
0.001
|
| No (n=86) |
1 (Reference) |
- |
1 (Reference) |
- |
| Drinking |
Yes (n=15) |
0.99 (0.47-2.10) |
0.984 |
0.19 (0.02-0.50) |
0.005
|
| No (n=92) |
1 (Reference) |
- |
1 (Reference) |
- |
| BMI |
≤25 (n=57) |
1 (Reference) |
- |
1 (Reference) |
- |
| 25-30 (n=37) |
1.04 (0.61-1.77) |
0.899 |
0.64 (0.32-2.03) |
0.639 |
| ≥30 (n=13) |
1.11 (0.49-2.53) |
0.800 |
0.71 (0.15-3.39) |
0.672 |
CRC: Colorectal cancer; BMI: Body mass index. HR: Hazard ratio; CI: Confidence interval; N/A: Not applicable. a Unadjusted hazard ratio; b Adjusted hazard ratio
Estimated power for Cox PH regression Wald test, for log-hazard metric by alpha = 0.050 (two-sided), and 0.05 significance, the power of this survival analysis was 0.950 with a sample size of 173 cases.
Discussion
The impact of specific oncogenic mutations concerning different clinicopathological characteristics was assessed on the prognosis and OS of patients with metastatic CRC. This analytical research study enrolled a total of 173 mCRC patients, and 65 CRC patients (37.6%) died during the follow-up time and the nominal 1-year through 5-year survival was 78%, 65%, 55%, 46%, and 42% respectively. The OS was 34.4%, 66.7%, and 69.4% in the KRAS codon 12 and 13 mutations and the wild-type KRAS mutations, respectively, and the KRAS codon 12 point mutation was independently associated with a worse OS compared to the wild-type tumors (Log-rank P=0.049). The Cox-Regression Hazard Model also showed that the mCRC patients with codon 12 mutations had a nearly 2 times higher hazard than the codon 13 and KRAS wild-type mutations. Smoking strongly increased the hazard of mortality by about 7 times and the mCRC patients with liver metastasis and tumors of the distal colon had a significantly worse OS with HRs of 2.49 and 3.36, respectively. KRAS mutations were positive in 42.5% of the cases and only one positive case was found for the NRAS mutations (1.3%), and of the 39 cases tested for BRAF mutations, only one case (2.6%) had mutations in BRAF codon 600 (V600E). These results are similar to other reports from Iran and recent surveys.
4,9
Despite the many studies on the clinical relevance of single point mutations in CRC, this study sought to present a more comprehensive and analytical survey of the prognostic impact of KRAS mutation subtypes in mCRC in the northwest of Iran as part of a larger analytical study in this region and had a median follow-up time of 17 months (0.33 to 71.97 months).
Despite the well-recognized role of KRAS mutations in the poor efficacy of targeted therapies (anti-EGFR) in metastatic CRCs, their oncogenic and prognostic effects remain controversial.
6,16,17
Several studies have shown that KRAS mutations are significantly associated with poor prognosis and OS
6
; however, some other studies have demonstrated that OS and disease-free survival do not differ significantly, not only for mutant genes compared to the wild-type but also for different KRAS mutation subtypes.
16
Although there is some evidence on the alterations of specific KRAS mutations with tumor phenotypes,
9,18,19
limited studies have shown any differences between the patients' prognosis in terms of different KRAS mutation types.
20
The present study showed that patients with KRAS codon 12 mutations have a significantly worse OS compared to those with the wild-type, but this difference was not significant for KRAS codon 13 mutations. The present findings were similar to the results of some previous studies,
4
and inconsistent with the results of some others. Summer et al reported that both codon 12 and 13 mutations had a worse OS, but the hazard function of KRAS codon 13 mutations was higher than that of codon 12 mutations (HR=1.53 vs. 1.44) while NRAS mutations showed no differences in the prognosis of CRCs and BRAF mutations were strongly associated with a worse OS.
21
A recent systematic review and meta-analysis showed that although KRAS codon 13 mutations have a worse OS compared to patients with the wild-type gene (pooled HR=1.76), there were no significant differences between KRAS codon 12 and 13 mutations in terms of OS in CRC patients.
11
The present study demonstrated that not only do KRAS codon 12 mutations have a worse OS (Log-rank P=0.049), but they also have a significantly higher hazard of mortality (HR=2.01) than KRAS codon 13 mutations (HR=0.58) and wild-type KRAS genes. These results are consistent with some previous reports,
9,10,20
and seem to infer that KRAS codon 12 mutations have a greater oncogenic capacity that leads to their poor prognosis.
9,10,18,19
There is some theories about the differences in the more aggressive cellular transformation between different point mutations of the KRAS gene, especially KRAS codon 12 mutations, which are corroborated by in-vitro studies.
9,18,19
The present findings are in line with a report from China in which Bai et al revealed similar prognostic effects for KRAS codon 12 and 13 mutations with HR values equal to 2.53, and 0.66, respectively.
10
This study included only the metastatic CRC patients and the most common metastatic site in them was the liver (n=46, 26.6%). These patients had a significantly worse OS (HR=2.49; 95% CI: 1.49-12.52; P=0.002). The present findings differed from some previous findings, as some researchers had formerly found that KRAS mutations are more associated with metastasis in the lung and less in the liver.
21,22
A large Australian mCRC database analysis revealed that lung metastasis is more frequent in mCRCs with KRAS mutations and reported significant adverse outcomes for the brain, bone, and peritoneal metastasis, while liver metastasis did not have any impact on the OS (HR=1.06) and the median OS was significantly higher in the liver-only metastatic CRCs.
4
Another notable finding of the present study was that KRAS mutations are a significantly negative prognostic factor in distal colon tumors (HR=3.36) compared to proximal colon cancers, which is in line with a few previous reports,
7,23
but different from some other studies; for instance, Jones et al did not find any significant impact for tumor location on OS.
9
Meanwhile Prasanna et al. also reported that left-side tumors have better survival compared to right-side and rectal tumors.
4
This disparity of findings may be due to certain environmental and genetic alterations in different mutation subtypes and the clinicopathological characteristics of CRC.
Alcohol consumption (any type in any amount and with any frequency) is a known risk factor for CRC, as evidence suggests that long-term alcohol consumption is associated with an approximately 50% increased risk of CRC.
24-26
The present research study did not find any associations between alcohol intake and the prognosis of mCRC, which is in line with the results of some studies
24
; however, one reason could be that alcohol consumption is generally less prevalent in Iran due to cultural and religious restrictions. Recent surveys in Iran have shown that the prevalence of smoking is high in northwestern Iran, and in East Azerbaijan Province, 23.7% of men and 0.7% of women smoke.
27
The present study also showed that smoking is associated with a much lower OS with a hazard of 7.32 (P=0.001). Javasekara et al, however, found no associations between smoking and CRC-specific survival.
24
The association between BMI and survival in CRC was less clear and the examined BMI subgroups of this study showed no significant associations with the mCRC survival.
24,28
These results may highlight the importance of adding other anthropometric measures in line with BMI to better assess the role of obesity as a known risk factor of at least seven common cancers.
28
The main limitation of this study was the lack of mutational tests results for all included CRCs, and so lack of a statistical analysis of NRAS and BRAF mutations, owing to the low percentage of these mutations in the mCRC cases diagnosed, which could not offer a good statistical power. Another limitation of the study was the lack of cancer treatment data, because in most cases, the patients could not access properly to the targeted therapies as per the standard recommended guidelines due to the poor drug availability and the high costs of treatment. Therefore opted for the routine chemotherapy provided by the oncology center.
However, mutations were detected by Idylla RAS mutation tests (for KRAS/NRAS/BRAF), in the referral molecular lab, which is a fully automated Real-time PCR-based system, with approved reliability due to the convenience and practicality of mutation detecting. The validation of reliably detecting of RAS mutations using the Idylla system has been confirmed recently, comparing the gold standard method (Sanger sequencing).
29-32
Conclusion
Some recent research has suggested that the pattern of specific KRAS gene mutation, and so the impact of specific amino acid changes, are associated with different prognostic values and responses to anti-EGFR therapies. Routinely performing specific oncogene tests, including KRAS/NRAS/BRAF mutation testing, at all oncology centers may help oncologists make a better treatment decision and may thus improve the patients’ prognosis and life expectancy. The present findings clearly demonstrate that metastatic CRC with mutations in codon 12 of the KRAS gene has an independent and significantly worse OS. Also, smoking, liver metastasis, and distal colon tumors are associated with poor disease prognosis. Specific mutations of oncogenes involved in the tumorigenesis of CRC may suggest a different aggressiveness and response to treatment.
Acknowledgments
We would like to acknowledge the technical support of the reference molecular lab staff.
Funding sources
This work was supported by the Hematology and Oncology Research Center of Tabriz University of Medical Sciences as a confirmed research project [Grant number: 5/d/4876, 1395/2]; and Ministry of Health and Medical Education, Deputy of Research and Technology for manuscript submission (Grant number: 700/98, 1394/12/24).
Ethical statement
The ethics committee of Tabriz University of Medical Sciences has been approved this project, and all patients' information and records are confidential (IR.TBZMED.REC.1395.18).
Competing interests
The authors have no conflicts of interest.
Authors’ contribution
RD, SD, and MHS made substantial contributions to the conception and design of the work; and the acquisition, analysis, interpretation of data. MZ made data extraction and management. ATE performed the DNA extraction and genotyping in the laboratory, interpreted the results and the creation of new molecular tests used in the work. RD, SD, FF, and MHS drafted the work or revised it critically for important intellectual content. All authors approved the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Research Highlights
What is the current knowledge?
simple
-
√ CRC represents a heterogeneous genetic and epigenetic alteration that could lead to different phenotypes.
-
√ Recently some research has suggested that the pattern of specific RAS/RAF gene mutation, and so the impact of specific amino acid substitutions, are associated with different prognostic values and responses to anti-EGFR therapies.
What is new here?
simple
-
√ The impact of specific oncogenic mutations in relation to different clinicopathological characteristics was assessed on the prognosis and OS of patients with metastatic CRC.
-
√
KRAS codon 12-point mutation was independently associated with a worse OS compared to the wild-type tumors.
-
√ CRC patients with mutations in codon 12 had higher hazard than the codon 13 and KRAS wild-type mutations.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394-424. doi: 10.3322/caac.21492 [Crossref] [ Google Scholar]
- Somi MH, Dolatkhah R, Sepahi S, Belalzadeh M, Sharbafi J, Abdollahi L. Cancer incidence in the East Azerbaijan province of Iran in 2015-2016: results of a population-based cancer registry. BMC Public Health 2018; 18:1266. doi: 10.1186/s12889-018-6119-9 [Crossref] [ Google Scholar]
-
Global Burden of Disease Cancer C, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2018. 10.1001/jamaoncol.2018.2706
- Prasanna T, Karapetis CS, Roder D, Tie J, Padbury R, Price T. The survival outcome of patients with metastatic colorectal cancer based on the site of metastases and the impact of molecular markers and site of primary cancer on metastatic pattern. Acta Oncol 2018; 57:1438-44. doi: 10.1080/0284186X.2018.1487581 [Crossref] [ Google Scholar]
- Dolatkhah R, Somi MH, Bonyadi MJ, Asvadi Kermani I, Farassati F, Dastgiri S. Colorectal cancer in iran: molecular epidemiology and screening strategies. J Cancer Epidemiol 2015; 2015:643020. doi: 10.1155/2015/643020 [Crossref] [ Google Scholar]
- Yoshino T, Portnoy DC, Obermannova R, Bodoky G, Prausova J, Garcia-Carbonero R. Biomarker analysis beyond angiogenesis: RAS/RAF mutation status, tumour sidedness, and second-line ramucirumab efficacy in patients with metastatic colorectal carcinoma from RAISE-a global phase III study. Ann Oncol 2019; 30:124-31. doi: 10.1093/annonc/mdy461 [Crossref] [ Google Scholar]
- Charlton ME, Kahl AR, Greenbaum AA, Karlitz JJ, Lin C, Lynch CF. KRAS Testing, Tumor Location, and Survival in Patients With Stage IV Colorectal Cancer: SEER 2010-2013. J Natl ComprCancNetw 2017; 15:1484-93. doi: 10.6004/jnccn.2017.7011 [Crossref] [ Google Scholar]
- Alwers E, Jia M, Kloor M, Blaker H, Brenner H, Hoffmeister M. Associations Between Molecular Classifications of Colorectal Cancer and Patient Survival: A Systematic Review. Clin Gastroenterol Hepatol 2019; 17:402-10 e2. doi: 10.1016/j.cgh.2017.12.038 [Crossref] [ Google Scholar]
- Jones RP, Sutton PA, Evans JP, Clifford R, McAvoy A, Lewis J. Specific mutations in KRAS codon 12 are associated with worse overall survival in patients with advanced and recurrent colorectal cancer. Br J Cancer 2017; 116:923-9. doi: 10.1038/bjc.2017.37 [Crossref] [ Google Scholar]
- Bai B, Shan L, Xie B, Huang X, Mao W, Wang X. Mutations in KRAS codon 12 predict poor survival in Chinese patients with metastatic colorectal cancer. Oncol Lett 2018; 15:3161-6. doi: 10.3892/ol.2017.7709 [Crossref] [ Google Scholar]
- Kwak MS, Cha JM, Yoon JY, Jeon JW, Shin HP, Chang HJ. Prognostic value of KRAS codon 13 gene mutation for overall survival in colorectal cancer: Direct and indirect comparison meta-analysis. Medicine (Baltimore) 2017; 96:e7882. doi: 10.1097/MD.0000000000007882 [Crossref] [ Google Scholar]
- Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016; 6:29765. doi: 10.1038/srep29765 [Crossref] [ Google Scholar]
- Augestad KM, Bakaki PM, Rose J, Crawshaw BP, Lindsetmo RO, Dorum LM. Metastatic spread pattern after curative colorectal cancer surgery A retrospective, longitudinal analysis. Cancer Epidemiol 2015; 39:734-44. doi: 10.1016/j.canep.2015.07.009 [Crossref] [ Google Scholar]
- Dolatkhah R, Somi MH, Shabanloei R, Farassati F, Fakhari A, Dastgiri S. Main Risk Factors Association with Proto-Oncogene Mutations in Colorectal Cancer. Asian Pac J Cancer Prev 2018; 19:2183-90. doi: 10.22034/APJCP.2018.19.8.2183 [Crossref] [ Google Scholar]
- Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol 2017; 35:1453-86. doi: 10.1200/JCO.2016.71.9807 [Crossref] [ Google Scholar]
- Bruera G, Pepe F, Malapelle U, Pisapia P, Mas AD, Di Giacomo D. KRAS, NRAS and BRAF mutations detected by next generation sequencing, and differential clinical outcome in metastatic colorectal cancer (MCRC) patients treated with first line FIr-B/FOx adding bevacizumab (BEV) to triplet chemotherapy. Oncotarget 2018; 9:26279-90. doi: 10.18632/oncotarget.25180 [Crossref] [ Google Scholar]
- Tessitore A, Bruera G, Mastroiaco V, Cannita K, Cortellini A, Cocciolone V. KRAS and 2 rare PI3KCA mutations coexisting in a metastatic colorectal cancer patient with aggressive and resistant disease. Hum Pathol 2018; 74:178-82. doi: 10.1016/j.humpath.2018.01.021 [Crossref] [ Google Scholar]
- Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol 2008; 9:517-31. doi: 10.1038/nrm2438 [Crossref] [ Google Scholar]
- Guerrero S, Casanova I, Farre L, Mazo A, Capella G, Mangues R. K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res 2000; 60:6750-6. [ Google Scholar]
- Pang XL, Li QX, Ma ZP, Shi Y, Ma YQ, Li XX. Association between clinicopathological features and survival in patients with primary and paired metastatic colorectal cancer and KRAS mutation. Onco Targets Ther 2017; 10:2645-54. doi: 10.2147/OTT.S133203 [Crossref] [ Google Scholar]
- Summers MG, Smith CG, Maughan TS, Kaplan R, Escott-Price V, Cheadle JP. BRAF and NRAS Locus-Specific Variants Have Different Outcomes on Survival to Colorectal Cancer. Clin Cancer Res 2017; 23:2742-9. doi: 10.1158/1078-0432.CCR-16-1541 [Crossref] [ Google Scholar]
- Christensen TD, Palshof JA, Larsen FO, Poulsen TS, Hogdall E, Pfeiffer P. Associations between primary tumor RAS, BRAF and PIK3CA mutation status and metastatic site in patients with chemo-resistant metastatic colorectal cancer. Acta Oncol 2018; 57:1057-62. doi: 10.1080/0284186X.2018.1433322 [Crossref] [ Google Scholar]
- Lee DW, Han SW, Cha Y, Bae JM, Kim HP, Lyu J. Association between mutations of critical pathway genes and survival outcomes according to the tumor location in colorectal cancer. Cancer 2017; 123:3513-23. doi: 10.1002/cncr.30760 [Crossref] [ Google Scholar]
- Jayasekara H, English DR, Haydon A, Hodge AM, Lynch BM, Rosty C. Associations of alcohol intake, smoking, physical activity and obesity with survival following colorectal cancer diagnosis by stage, anatomic site and tumor molecular subtype. Int J Cancer 2018; 142:238-50. doi: 10.1002/ijc.31049 [Crossref] [ Google Scholar]
- Jayasekara H, MacInnis RJ, Williamson EJ, Hodge AM, Clendenning M, Rosty C. Lifetime alcohol intake is associated with an increased risk of KRAS+ and BRAF-/KRAS- but not BRAF+ colorectal cancer. Int J Cancer 2017; 140:1485-93. doi: 10.1002/ijc.30568 [Crossref] [ Google Scholar]
- Jayasekara H, MacInnis RJ, Room R, English DR. Long-Term Alcohol Consumption and Breast, Upper Aero-Digestive Tract and Colorectal Cancer Risk: A Systematic Review and Meta-Analysis. Alcohol Alcohol 2016; 51:315-30. doi: 10.1093/alcalc/agv110 [Crossref] [ Google Scholar]
- Nemati S, Rafei A, Freedman ND, Fotouhi A, Asgary F, Zendehdel K. Cigarette and Water-Pipe Use in Iran: Geographical Distribution and Time Trends among the Adult Population; A Pooled Analysis of National STEPS Surveys, 2006-2009. Arch Iran Med 2017; 20:295-301. [ Google Scholar]
- Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 2006; 55:62-7. doi: 10.1136/gut.2005.068189 [Crossref] [ Google Scholar]
- Al-Turkmani MR, Schutz SN, Tsongalis GJ. Potential of STAT Somatic Mutation Testing at Resection. Clin Chem 2018; 64:865-6. doi: 10.1373/clinchem.2017.285759 [Crossref] [ Google Scholar]
- De Luca C, Rappa AG, Gragnano G, Malapelle U, Troncone G, Barberis M. Idylla assay and next generation sequencing: an integrated EGFR mutational testing algorithm. J Clin Pathol 2018; 71:745-50. doi: 10.1136/jclinpath-2018-205197 [Crossref] [ Google Scholar]
- Prieto-Potin I, Montagut C, Bellosillo B, Evans M, Smith M, Melchior L. Multicenter Evaluation of the Idylla NRAS-BRAF Mutation Test in Metastatic Colorectal Cancer. J Mol Diagn 2018; 20:664-76. doi: 10.1016/j.jmoldx.2018.05.008 [Crossref] [ Google Scholar]
- Uguen A, Troncone G. A review on the Idylla platform: towards the assessment of actionable genomic alterations in one day. J Clin Pathol 2018; 71:757-62. doi: 10.1136/jclinpath-2018-205189 [Crossref] [ Google Scholar]