Bioimpacts. 10(3):195-203.
doi: 10.34172/bi.2020.24
Review
Engineered nanoparticle bio-conjugates toxicity screening: The xCELLigence cells viability impact
Clarence S Yah 1, 2, *  , Geoffrey S. Simate 3
, Geoffrey S. Simate 3
Author information:
1Implementation Science Unit, Wits Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, South Africa
2School of Health Systems and Public Health, Faculty of Health Sciences, University of Pretoria, South Africa
3School of Chemical and Metallurgical Engineering, University of the Witwatersrand, Johannesburg, South Africa
Abstract
Introduction:
The vast diverse products and applications of engineered nanoparticle bio-conjugates (ENPBCs) are increasing, and thus flooding the-markets. However, the data to support risk estimates of ENPBC are limited. While it is important to assess the potential benefits, acceptability and uptake, it is equally important to understand where ENPBCs safety is and how to expand and affirm consumer security concerns.
Methods:
Online articles were extracted from 2013 to 2016 that pragmatically used xCELLigence real-time cell analysis (RTCA) technology to describe the in-vitro toxicity of ENPBCs. The xCELLigence is a +noninvasive in vitro toxicity monitoring process that mimics exact continuous cellular bio-responses in real-time settings. On the other hand, articles were also extracted from 2008 to 2016 describing the in vivo animal models toxicity of ENPBCs with regards to safety outcomes.
Results:
Out of 32 of the 121 (26.4%) articles identified from the literature, 23 (71.9%) met the in-vitro xCELLigence and 9(28.1%) complied with the in vivo animal model toxicity inclusion criteria. Of the 23 articles, 4 of them (17.4%) had no size estimation of ENPBCs. The xCELLigence technology provided information on cell interactions, viability, and proliferation process. Eighty-three (19/23) of the in vitro xCELLigence technology studies described ENPBCs as nontoxic or partially nontoxic materials. The in vivo animal model provided further toxicity information where 1(1/9) of the in vivo animal model studies indicated potential animal toxicity while the remaining results recommended ENPPCs as potential candidates for drug therapy though with limited information on toxicity.
Conclusion:
The results showed that the bioimpacts of ENPBCs either at the in vitro or at in vivo animal model levels are still limited due to insufficient information and data. To keep pace with ENPBCs biomedical products and applications, in vitro, in vivo assays, clinical trials and long-term impacts are needed to validate their usability and uptake. Besides, more real-time ENPBCs-cell impact analyses using xCELLigence are needed to provide significant data and information for further in vivo testing.
Keywords: Bionanomaterials, xCELLigence, In vitro, In vivo, Toxicity, Impact
Copyright and License Information
© 2020 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The evolution of engineered nanoparticle bio-conjugates (ENPBC) has led to a wide spectrum of biomedical and environmental products and applications.
1-5
Ideally, ENPBCs are the formulation of any pharmacological active nanomaterial (e.g., nano-bio-conjugated, recombinant proteins, vaccines, nucleic acids, or bio-products)’ that enable site-specific-targets of organs/cells with controlled release or to treat and prevent diseases (e.g., cancers, infections, bone tissue engineering, etc) in human.
4,5
In this context, nanoparticles (NPs) including those of gold, zinc, copper, platinum, carbon nanotubes and other metal oxides are widely used with varying distinctive therapeutic functions/properties.
1,6-8
The array of applications is based on NPs vast physiochemical broad multi-faceted properties derived from their nanosize scales and interactions.
1,4
However, the understanding of the diverse behavior of ENPBCs and characteristics within human cells is still limited. Therefore, simple, fast, and reliable in vitro screening techniques that detect cellular toxicity effects are vital before they reach the environment and the market.
The ENPBCs in vitro or in vivo demonstrations have shown varying biological effects in animal cell development and reproduction.
9
However, the biological effects of ENPBCs concerning the human health have not fully been established as is the case with other commercially available pharmacologic active non-nanomaterials and antibiotics.
3,10-13
For example, commercially available drugs have written mechanisms of action, toxicity, and side effects whereas such information to date are limited for ENPBCs. This is because most nanodrugs are still at the developmental phase with limited safety trials. Addressing the undesirable effects of ENPBC require clear regulations that govern their toxicity in human and environment. Specifically, this requires a considerable amount of data and evidence-based information to support ENPBCs toxicity effect/impact, safety, tolerability, adverse reactions, and others. It may also include information and background on the toxicity profile and testing tools that could be used in the standardization and expansion of local, national, regional, and international regulatory frameworks for ENPBCs. Unfortunately, the non-availability of tools for estimating nanomaterials toxicity for standardization is one of the major setbacks regarding their regulations.
It must be noted that ENPBCs have a large surface area and volume ratio leading to a high diverse-level of interaction with assays, binding and bonding to chemicals, and materials thus giving false toxicity results.
11,14
For example, graphene families of nanomaterials can interact with assays by binding to lactate dehydrogenase (LDH) thus giving false negative toxicity results.
11
The use of xCELLigence real-time cell analysis (RTCA) technology system, in this case, is vital because it has some promising characteristics that limit ENPBCs interactions with various dye, enzymes and chemicals used in estimating cytotoxicity thereby reducing false-negative and false-positive outcome. In other words, xCELLigence in vitro toxicity testing provides real-time tissue cells’ observation, cell growth, reproduction, and morphological effects without the interferences of non-external cells, chemicals and dyes interactions as in other conventional cytotoxicity testings.
14-17
This study, therefore, describes ENPBC toxicity using the xCELLigence technology system and the role of regulatory bodies regarding ENPBCs and safety guidelines.
According to reports by Ke et al in vitro toxicity studies determined via xCELLigence instrument do not use external reagents that may confound the screening. In principle, xCELLigence toxicity testing is a noninvasive electrical impedance technique that continuously monitors and quantifies cell proliferation/viability, morphological changes and attachment.
12-14
The following steps describe a simple synopsis of the xCELLigence ENPBCs toxicity screening according to Hamidi et al (Fig. 1).
18
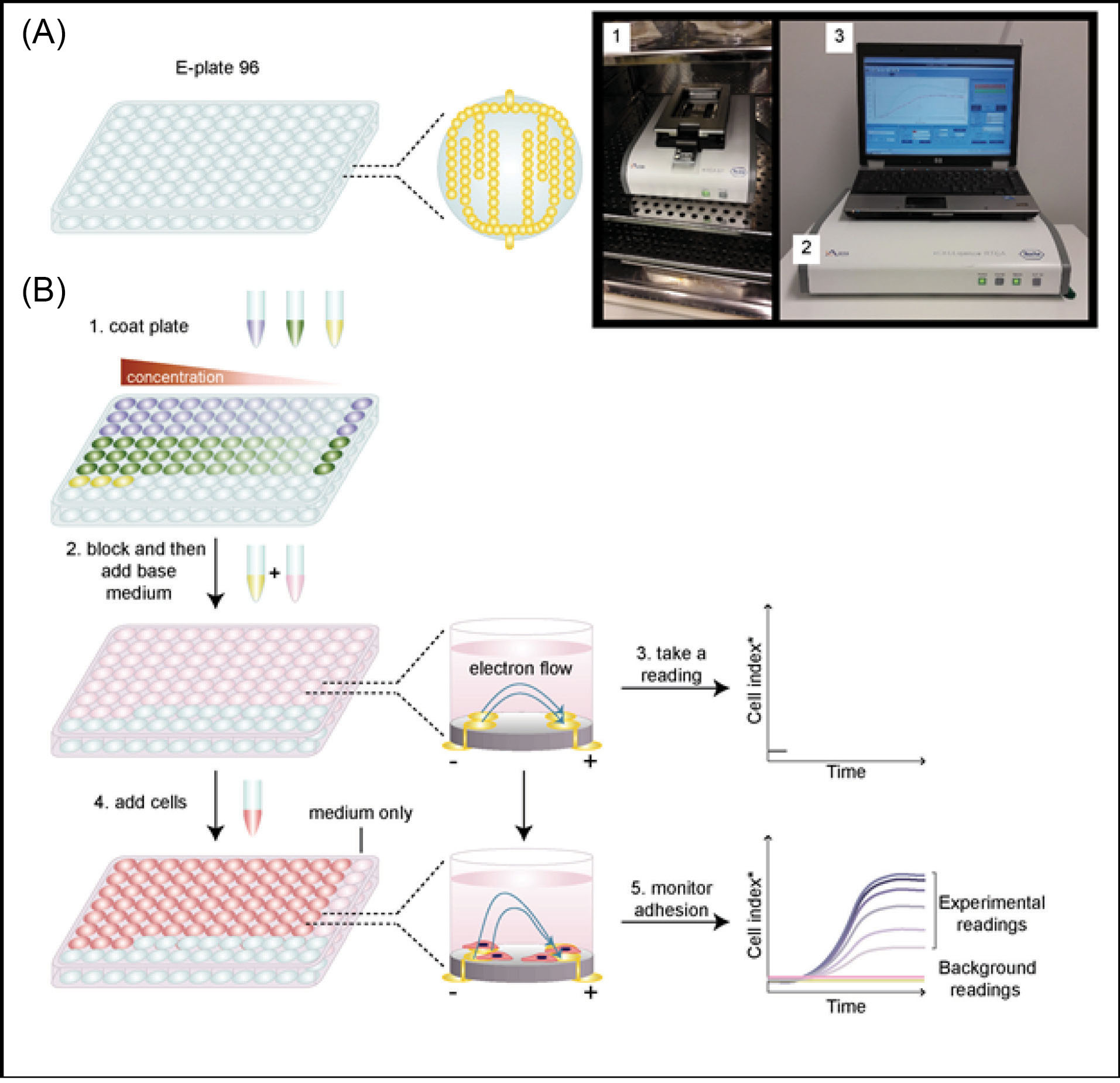
Fig. 1.
xCELLigence protocol flow system: Adapted from Hamidi et al.Using the xCELLigence RTCA system to monitor cell adhesion.
.
xCELLigence protocol flow system: Adapted from Hamidi et al.Using the xCELLigence RTCA system to monitor cell adhesion.
It must be noted that all steps are conducted under strict sterile and instructional conditions. For further details, readers are referred to Hamidi et al protocol. Step 1: Set the startup xCELLigence RTCA program as instructed for use including the experimental layout, page layout, and the schedule page layout. Step 2: Prepare the test run cell adhesion experiment using the xCELLigence RTCA background reading. Step 3: Prepared study target cells are adhered to or seeded to the microtiter 96 well plates. The wells will be placed on the gold microelectrodes that impede the flow of the electric current between electrodes. Step 4: The adhered cells are seeded with the required growth tissue culture media in question. Step 5: Addition of various sterile concentrations of the ENPBCs without any external reagents to the tissue culture media. Step 6: Monitoring of the ENPBCs and tissue culture xCELLigence real-time viability profile from a humidified CO2 incubator using an external computer system.
As for the standardization and regulation of nanomaterials, it must be noted that the toxicity levels depend on the type of ENPBC, the size and shape specific, surface chemistry, roughness, hydrophilic/hydrophobic characteristics, zeta potential, solubility and surface coatings and conjugated ligands.
15-17
As discussed already, despite the evolution of nanomedicine, coupled with other vast applications and nano-products there are no clear human safety screening techniques either in vitro levels. At the moment, the Consumer Protection Act Right requires local, government, national, regional and international regulatory organizations to protect consumers by setting/stating standards as well as published safety regulatory/instructions, protocols, and warnings that might evolve from some nano-consumable products.
19
Based on this, the National Institute of Health (NIH) and Organization for Economic Co-operation and Development (OECD) have released calls for the conceptualization of and development of safety regulations of bi-nanomaterials of biomedical devices, food, cosmetics, chemical and industrial systems.
10,19
Methodology and Data Sources
Online toxicological search themes were used to screen ENPBCs studies from March 2013 to December 2016 and conglomerate findings that used xCELLigence technology to describe in vitro toxicity. Additionally, a review of ENPBCs articles from 2008 to 2016 describing the in vivo toxicity using animal models was carried out. The ENPBCs articles included were those of significant toxicological information. The searched themes and topics covered were (1) ENPBCs xCELLigence cells’ viability, (2) chemistry of ENPBC, (3) the role of the international regulatory agencies inENPBCs toxicity, and (4) challenges in regulating ENPBCs.
Search methods
A literature search was conducted to extract studies that address the in vitro cytotoxicity of ENPBCs using xCELLigence tools as well as in vivo animal models. The articles included were those published in the English language (limitation of search) and provided a clear and comprehensive description of ENPBCs in vitro toxicity using the xCELLigence technique and in vivo animal models testing. The key search words used for the xCELLigence techniques search and screening were xCELLigence nanoparticles toxicity, xCELLigence and nanoparticles screening, and xCELLigence and nanoparticles cell viability screening. The PubMed advance mesh search used, for example, was xCELLigence[All Fields] AND ("nanoparticles"[MeSH Terms] OR "nanoparticles"[All Fields]) AND ("toxicity"[Subheading] OR "toxicity"[All Fields]). The xCELLigence in vitro toxicity testing technique was used because it reduces and limits confounding toxicity outcomes when compared to other conventional testing methods that use chemicals, dyes, and reagents.
For the in vivo ENPBCs toxicity estimation and description, only published work indicating the use of laboratory animals such as mice/rat/mouse were eligible. The In vivo nanoparticles toxicity, and In vivo nanoparticles testing/screening were the searched main keywords. For example, the PubMed advance mesh used was (("in"[All Fields] AND "vivo"[All Fields]) OR "in vivo"[All Fields]) AND ("nanoparticles"[MeSH Terms] OR "nanoparticles"[All Fields]) AND testing[All Fields]. Articles that used non-primate animal species such as fish and other aquatic animals (limitation of the study) were excluded.
The following were the main online search databases: Cochrane Library, PubMed, MeSH PsycInfo, Scopus and Google Scholar, Embase, Web of Science, BMC, Plos|One and Global Health, ScienceDirect. The Prisma proxy flow checklist methodology was used to identify, screen, and review the articles
20
as described in Fig. 2.
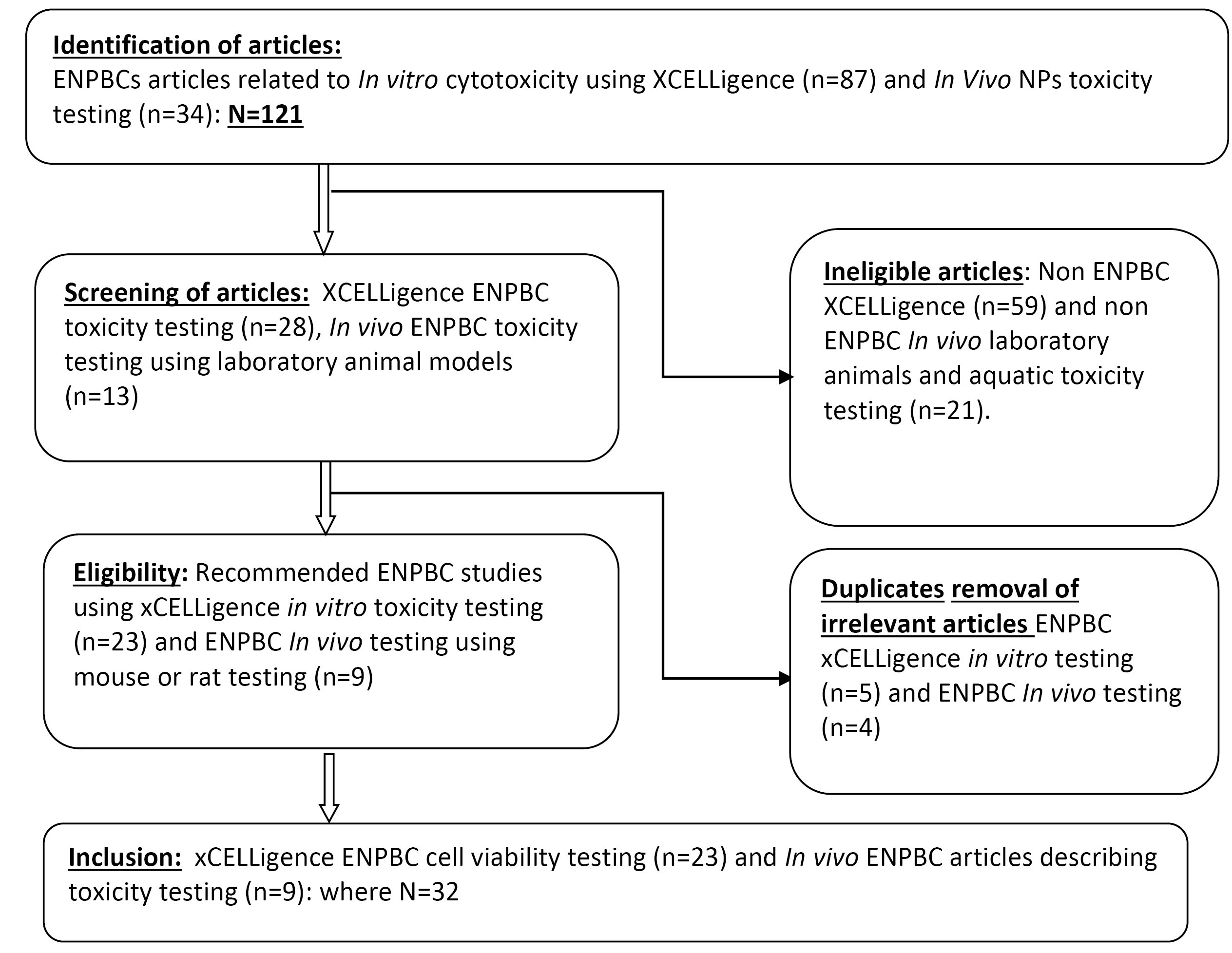
Fig. 2.
The identification, selection and screening of ENPBCs XCELLigence in vitro viability and the in vivo ENPBCs toxicity articles flowchart for dose estimation.
.
The identification, selection and screening of ENPBCs XCELLigence in vitro viability and the in vivo ENPBCs toxicity articles flowchart for dose estimation.
Selection of studies
Two independent reviewers (CSY and GSS) reviewed the relevant studies.
Selection and management of studies
Using the Microsoft Excel spreadsheet, CSY and GSS independently reviewed the extracted studies, screened the titles, and results. Thereafter, the pre-piloted checklist was applied to identify eligible studies. The eligible studies were further screened for duplicates and other irrelevant studies. To reduce discrepancies, a clear screening and selection approach was used based on the headings generated in Tables S1
14,21-43
and S2
24,28-31,38,44-51
(see Supplementary file 1). Similarly, some findings were further discussed to find a suitable outcome especially for the same studies extracted from conference abstracts and the full published articles.
Results and Discussion
The limited reliable and specific standardized protocols for determining the toxicity of nanostructures or nanosized bio-conjugate materials is still a challenge in both in vitro and in vivo bio-nanotechnology-applications.
1,16
Besides, the paucity of data has also compounded the difficulty of providing a generalized conclusion regarding the facts surrounding ENPBCs toxicities and regulatory standards. Out of the 121 articles, which were identified 23 (26.4%) of them, were used in this study are listed in Tables S1 and S2. They show 23 (71.9%) describing the xCELLigence- in vitro toxicity and 9 (28.1%) the in vivo toxicity of ENPBCs (Fig. 1). Of the 23 articles, 4 of them (17.4%) had no size estimation of ENPBCs, while the xCELLigence technology provided information on cell interactions, viability, and proliferation process. However, the xCELLigence cytotoxicity characteristics varied and were mostly due to the allotropic nature of the NP, dose, and size, as described in Table S1. For example, toxic ENPBCs were mostly those that initiated cell death, inflammation, disruption of cell membranes, DNA damage, and oxidative stress.
21-25
Non-cytotoxic ENPBCs were those that failed to initiate cell damage, had no cytotoxicity effect on the cells, were used for the diagnostic purposes/treatment, were biocompatible to cells, and induced no oxidative stress on the cells.
26-36
Partially nontoxicENPBCsindicated both toxic and nontoxic effects due to size variations or the allotropic forms (e.g., carbon allotropes such as diamond, graphite, fullerenes, etc).14,21,
27-29,37
The in vivo animal model provided further toxicity information where 3 out of 9 of the in vivo animal model studies indicated partial nontoxic animal effect, one was toxic while the remaining results recommended ENPBCs as a potential candidate for drug therapy with limited information on toxicity (Table S2). Some of the in vivo ENPBCs toxicity effects demonstrated programmed cell death, causing the release of H2S.
29
The ENPBCs that exhibited in vivo nontoxic effect were mostly those that had the therapeutic effects of antibody-drug conjugate,
27
CTAB layers of gold for diagnosing rheumatoid arthritis,
30
surface coating polymeric NPs used for inhibiting cancerous cells
31,38-47
and felodipine-loaded polymers for pathological examination of different organs of Wister albino mice
50
The ENPBCs with partial nontoxic effect were chitosan-coated polymers for drug delivery,
49
silver NPs coated polymers that were found localized in various body organs,
48
and drug delivery polymeric NPs for anti-cancer.
51
The findings indicated limited data and information regarding the regulation of ENPBCs toxicity. This may be due to limited available standardized protocols and methodologies for nanomaterials toxicities analyses. Similarly, there were no clear epidemiological studies or data from established longitudinal cohort studies describing the causal effect/impact of ENPBCs.
The in vitro xCELLigence cell viability test techniques were used as indicators for the first-line screening, observing cell growth responses and effects of ENPBCs on human cell cultures.9,
21-23
Similarly, the in vitro xCELLigence profiles were identified as broad sensitivity testing effects in cell cultures which were good precursors for specific toxicological testing in animal models and clinical trials in humans. Table S1 indicates and describes the diversity of the in vitro xCELLigence technique toxicity profiles of ENPBCs in cell cultures. The characteristics that were used in assessing the in vitro xCELLigence toxicity were: nature of ENPBC, shape, nanosize, animal cell lines used, dose toxicity estimation, and outcome effect on cell cultures.
52-55
The toxicological, dose concentrations and effect of ENPBCs in cell lines showed varying toxicity levels. For example, citrate stabilized gold NPs (AuNPs) at a concentration of 1nM, 2nM and 5nM were shown to be moderately toxic to BEAS-2B cell lines.
14
Similarly, using Ru(II) complex (2–4) 4 at a concentration of 0.5nM, 1nM, 2nM and 5nM: the 0.5 μM was toxic to A549R cells resulting to the death of cells after a short interval, confirming the cytotoxicity of complex 4 against A549R cells.
23
According to reports by Li et allimitations to validate of xCELLigence technique impact are due to inadequate data or denominator indexes that could be used to quantify the behavior of ENPBCs in humans as well as the guideline monitoring methods and procedures for characterizing ENPBCs toxicity levels. Moreover, the limitations are also prevalent despite a host of other instruments including UV-Vis absorption spectroscopy, TEM, SEM, HrTEM, DLS, HPLC, ICP-MS, FTIR, and AFM for characterizing ENPBCs properties,
9
as listed in Table S1.14,21-43
The changes and mechanisms that take place in living cells in response to ENPBCs delivery are useful for understanding cell impact and after effect of ENPBCs.
17
The in vitro xCELLigence impendence technique has emerged as an alternative method that challenges the limited earlier traditional standards. The technique permeates cells to have direct contact with the ENPBCs without interference with other compounds or molecules as is the case with other in vitro testing methods.
11
For example, studies have shown ENPBCs interactions with MTT formazan assay crystals such as dyes like Neutral Red or Alamar Blue have resulted in conflicting false outcomes.
44-45
Further, ENPBCs and lactate dehydrogenase (LDH) assay interaction has been found to confound outcome measurements, and thus, resulting in inconclusive endpoints results.
46
On the other hand, the in vitro xCELLigence technique incorporate an integrated sensitive impedance detection sensor chips system at the bottom of the cell culture plates that track ENPBC-cell interaction changes and their morphologies in cell and cell proliferation.
14,17
This method shows internalized ENPBCs within cells and their subsequent intracellular aggregation.
14
However, in vitro xCELLigence techniques seem to have some limitations as well. According to findings by Meindl et al the xCELLigence technique was found to be less sensitive to the CNT-induced cytotoxicity. The reasons for the less sensitive were not mentioned in the study. This indicates that xCELLigence techniques may not be a good standard for ENPBCs screening and thus, other in vitro cytotoxicity methods may be explored.
To enhance ENPBCs acceptability, the usage and sustained uptake in human drug delivery systems, the in vivo animal model toxicity testings are the next steps for assessing the activities, effect, and impact of ENBPCs in humans. Table S2 describes the in vivo toxicity effect of ENPBCs in animal models with varying effects from toxic to non-toxic ones. The varying effects occur at the absorption level, blood concentration, distribution and organ concentration, metabolic-breakdown and excretion/elimination phases.
47-48
The effects are influenced by several factors, depending on the nature of the ENPBC including size, the zeta potentials, shape, and the ENPBC general characteristics.
48
Other inherent influential characteristics are the route of administration, dose-effect, time of dose-to-cell effect interaction, ENPBC-cell–interaction, and host-immunological profiles.
48
For example, the intranasal inoculation of Fe3O4 magnetic nanoparticles (MNPs) coupled with polymer poly(lactic-co-glycolic acid) (PLGA) at a concentration of 50 μL containing one mg/mL of MNP was tolerated in vivo but inhibited lung adenocarcinoma growth in mice.
31
In another study conducted by Wang et al while performing aortic allografts with 20 nm hydrogen sulfide (H2S) mesoporous silica NPs (MSNs) at the concentrations of 3.13 μg/mL to 800 μg/mL, the study revealed that the release of H2S in a controlled fashion can result in apoptosis of graft endothelium. An experimental study by Recordati et al reported the mid-zonal hepatocellular necrosis, gall bladder and hemorrhage toxic effects when treating mice with an intravenous dose of 10 mg/kg of 10nm silver NPs (AgNPs) coupled with citrate (CT)/polyvinylpyrrolidone (PVP). These varying outcomes are described in Table S2.24,28,30-31,38,44-51
Altogether, more in vivo animal predictive data are needed to validate standard methods for determining the safe applications of ENPBCs in humans.
Chemistry of engineered nanoparticles bio-conjugates
It should be recalled that ENPBCs have multifactorial complexity that may span from structural activities and interactions with biomolecules/cellular structures including proteins, membranes, cells, DNA, and organelles.
17,48
Some of these effects include the generation of radicals/oxidative stress, modulation of inflammatory profiles, and mutations.
1,9,52
It has also been found that interference of ENPBC with detector instrument, assays, dyes use in cytotoxicity (neutral red, Alamar blue, etc) and buffers have resulted in false negative/positives outcomes and misinterpretation or inappropriate toxicity results.
44,45
ENPBCs in this context are referred to as core nanoparticle and/ outer stabilizing layers made up of ligands (bio-conjugates). The bio-conjugates may be drugs, carboxyl (-COOH), amine (-NH2), hydroxyl (-OH), methyl (-OCH3), esters (-CO- OR), PEGylated layer intended for drug delivery applications. These bio-conjugates have great cellular interaction with cells, biodegradation as well as the elimination of the core nanoparticle. Therefore, elucidating toxicity effects that occur upon the interaction of functional groups with the cell surfaces or cellular components can lead to elevated intracellular reactive oxygen species resulting in DNA, lipids, proteins damage, cytotoxicity, apoptosis, unregulated cell signaling, tumor enhancer.
48,52-54
Engineered nanoparticles bio-conjugates-structures
The nature/size of ENPBCs can be investigated using various optical systems, including TEM, SEM, ICP-MS to determine bio-distribution. Furthermore, the FT-IR can be used to determine the chemical bonding of the functionalized ligands and HR-TEM the capping image with ENPBCs.
53
The structure of ENPBCs can initiate an effect that may lead to bio-persistence, bio-durability, and long-term toxicity effect when inhaled.
55
The functional activity, stability and chemistry of ENPBCs may influence the chemical and toxicity both in vitro and in vivo. The bio-dissolution of ENPBC which is a measure of bio-durability of chemical and physical properties is a dependent function of size, surface area-shape as well as the medium and ionic strength.
48,53,54
The challenges resulting from these properties have hindered the development of standardized protocols for determining the bioavailability, absorption, distribution, bioaccumulation, metabolism, excretion, release of ENBPCs.
The role of international agencies in engineered nanoparticle (ENP) bio-conjugates (BC)
Toxicity determination and characterization for drug delivery
Accurate and reliable information about the characteristics, concentration, and cytotoxicity of ENPBCs are major properties that can be used to understand the safe use of ENPBC as well as their profile in drug delivery agents. Engineered NPs (ENPs) either naked or coated with bio-conjugates are governed and influenced by their physicochemical properties.1,48,
52-55
The physicochemical property profiles of any drug delivery are the function of morphological-shape, size distribution, zeta potential, subcellular localization, expression of cell markers, and chemical composition concerning cells’ activities. The toxicity of ENPBC, therefore, depends mostly on the physicochemical properties.
1,55
The physicochemical characterization of ENPBCs must be precise and fully determined before assessing their cytotoxicity. The development of regulatory standards and methods for ENPBCs in biological/biomedical applications and systems are urgent mandatory requirements. Various internationals, regionals, cross border organizations, nationals and local organizations have been constituted to provide regulatory guidelines, standards, and safety information for the safe use and discharge of ENPBCs.
The International Organization for Standardization (ISO) main body including the Organization for Economic Cooperation and Development (OECD), Food and Drug Administration (FDA or USFDA), Scientific Committee on Emerging and Newly Identified Health Risks (SCENHIHR) and other regulatory bodies functions is to develop technical safe regulatory guidelines regarding the physicochemical properties of engineered NPs and toxicological assessment
56
which can be used to shape safe usage of nanomaterials before subjecting them to human consumption and other usages. The objectives aimed at describing the inherent nano-toxicity of ENPs and nanomaterials that assure consumer security, safety, and their usage. Furthermore, the ENPs characteristics must be described to detail in terms of their physiochemical properties, impurities, and toxicological levels at in vitro and in vivo, cellular and molecular level, routes of exposure, uptake, and absorption rates.
17
Other areas of concern are the ethical, legal, and societal implications of ENPBCs and future areas of research.
57
Likewise, the generation of reliable data that supports further development, risk estimation, and potential risks of nanoproducts. However, according to a report by OECD, there are no specific detailed data, regulations available, and standard test methods for ENPBCs human exposure measurement as well as methodologies for risk assessments. Currently, several ISO committee groups are working on various aspects of nano-toxicity and risk assessments. The Nanotechnology Industries Association (NIA),
58,59
ISO-Technical Committees on nanotechnologies have been released for determining the potential biological effects of ENPBCs. More information on NIA standard catalog can be found at https://www.iso.org/ics/07.120/x/.
Due to ENPBCs sizes, ion release, and strong potential ability to functionalized to varying groups of molecules and protein, they have been found to initiate and cause diverse biological effects. Furthermore, these ENPBC-based therapeutics can catalyze properties that overcome biological barriers, and initiate intracellular effective drugs delivery in cells such as macrophages,
3
and targeted disease cells.
3,6
To be sure that ENPBCs are safe, regulatory and legislative bodies including testing and risk assessment and management bodies must be constituted to guide their usage
Challenges of engineered NPs bio-conjugates
As earlier mentioned, ENPBCs have great potential for improving biomedical research and drug delivery applications.
4
Their successes rely on the ability of surface functionality and stabilization of NPs as drug carriers and as targets to specific cells. However, most challenges facing ENPBC are size specificity and reproducibility. There is no specific equipment responsible for reproducing the exact sizes of ENPBCs and bio-conjugates attachments. The inability to produce exact specific attachments and sizes of ENPBCs have prompted regulatory bodies to examine ENPBCs on a case-by-case-base-effect. This is because different ENPBC nano-sizes have different properties that tend to affect the physicochemical properties and the functionality including pharmacological, immunological, and toxicity profiles. According to a study by Desai,
6
the complexity and nature of ENPBCs may substantially vary and thus, may be difficult to control and predict their behavior in biological systems. It is also difficult to sterilize ENPBC for biomedical applications.
60
It must also be noted that newer toxicity screening techniques such as xCELLigence always come with their limitations.
61-64
According to Kho et alhigh capital and consumables costs play a significant role. Secondly, xCELLigence technology work on cells that must adhere to the bottom of microplates and generate viable impedance signals. However, floating/ non-adherent and poorly plated cells may fail to generate signals resulting in false toxicity,
63,64
a common phenomenon of neuronal cells
61,62
and cancer cells due to lack of adherence to the gold microelectrodes. This is likely the main limitation of xCELLigence toxicity testing technology.
On the other hand, the measurement of broth suspended cells and their interactions with ENPBCs using xCELLigence technology is one of the ongoing future technological developments.
64-65
Because non-adherence cells including hematopoietic stem cells by nature have suspension and circulating characteristics
66,67
that have limited their attachment potentials and ability to attach and grow on E-plate wells.
68
However, the attachment characteristics can be enhanced by functionalizing or binding/coating the blood cell-matrix surfaces or the E-plate wells, for example, with peptide immobilization substrates such as fibronectin, laminin, and collagen types I and IV
66-68
that allow for cell adhesion.
66,67,69
Even though xCELLigence operations are designed to work with adherent cells,
8
however, by using binding protein matrices non-adherent cells including those of cancers can now be monitored on xCELLigence technology.
66-68,70
In such instances and/or procedures, the protein matrix binds to cells and enhances their attachment capability on cell well plates. For example, Martinez-Serra et al improved the attachment capacity of non-adherent cells of hematological malignancies such as leukemia/lymphoma cells with fibronectin. The results showed that the non-adherence leukemia/lymphoma cells were able to attach and grow profusely on the surface of the E-plates wells. In other studies, Abbasalipour et al
71
while testing for transduction characteristics of Lentivirus on K562 cells reported an enhanced adhesion, and multiplication of K562 cells coated with fetal bovine serum. Similarly, Hillger et al
72
while investigating the cellular properties and GPCR drug responses in lymphoblastoid cell lines coated the detector surface with fibronectin to enhance cell surface adhesion that allows for the detection of cellular responses with xCELLigence system. It must be noted that, from several studies, cell adhesion, invasion, proliferation and migration, enhances and changes the local ionic interface at the E-plate gold electrodes such that better cell index impedance is generated with real-time analytical graphical plots. Without this phenomenon of cells attachment and growth in the well plates, the cell index is close to the zero-mark
69
since only viable cells with adherence characteristics potential can benefit from the xCELLigence toxicity enumeration.
Conclusion
Several biomedical benefits accorded to ENPBCs have been applauded by several scholars. As a result, this has advanced research and development in the field of nanotechnology. However, toxicological standards are almost non-existent. The results showed that ENPBCs have different bio-impacts either at the in-vitro or in vivo animal model levels. These variations are due to differences in the ENPBCs, size compositions, adjustment, storage, route of administration, dose concentration, types of cell lines, target organs, molecular and the model animal used. It is important to generate large data on specific sizes of ENPBC health outcome effects in different settings to estimate and validate the generalizability of specific ENPBC toxicity impact. To keep pace with ENPBCs biomedical products and applications, in vitro, in vivo assays, clinical trials and long-term impacts are needed to validate their usability and uptake. In addition, more real-time ENPBCs-cell impact analysis using xCELLigence technology is needed to provide significant data for further in vivo testing and subsequent steps in the development of safety standards.
Acknowledgments
We acknowledge the postgraduate and intern students that assisted in the extraction of the data. However, the findings, interpretations, and conclusions expressed in this publication do not necessarily reflect the views of any regional or national government but, represent those of the researchers.
Funding sources
No special funding received for the study.
Ethical statement
No ethical approval was obtained because the data needed no human subject interaction. The data were freely available online.
Competing interests
The authors declare no conflict of interest.
Authors’ contribution
CSY developed the concept, designed the study, extracted, and reviewed the data, and wrote the initial draft of the article. GSS extracted the data, reviewed the data, and the article. All authors have consented and aligned the article according to Bioimpacts submission guidelines.
Supplementary Materials
Supplementary file 1 contains Tables S1-S2.
(pdf)
Review Highlights
What is the current knowledge?
simple
-
√ Traditionally bionanomaterials cytotoxicity exhibits
complex toxicity outcomes difficult to interpret.
What is new here?
simple
-
√ The non-invasive-xCELLigence cellular-bio-response
require pooled data to validate bionanomaterials cytotoxicity
protocols and methodological development.
References
- Yah CS. The toxicity of gold nanoparticles in relation to their physiochemical properties. Biomed Res 2013; 24:400-413. [ Google Scholar]
- Phillips CL, Yah CS, Iyuke SE, Rumbold K, Pillay V. The cellular response of Saccharomyces cerevisiae to multi-walled carbon nanotubes (MWCNTs). J Saudi Chem Soc 2015; 19:147-54. doi: 10.1016/j.jscs.2012.01.005 [Crossref] [ Google Scholar]
- Yah CS, Simate GS. Nanoparticles as potential new generation broad spectrum antimicrobial agents. Daru 2015; 23:43. doi: 10.1186/s40199-015-0125-6 [Crossref] [ Google Scholar]
- Ngoy JM, Iyuke SE, Neuse WE, Yah CS. Covalent Functionalization for Multi-Walled Carbon Nanotube (f-MWCNT) -Folic Acid bound bioconjugate. J Applied Sci 2011; 11:2700-11. doi: 10.3923/jas.2011.2700.2711 [Crossref] [ Google Scholar]
- Yah CS, Simate GS, Hlangothi P, Somai BM. Nanotechnology and the future of condoms in the prevention of sexually transmitted infections. Ann Afr Med 2018; 17:49-57. doi: 10.4103/aam.aam_32_17 [Crossref] [ Google Scholar]
- Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J 2012; 14:282-95. doi: 10.1208/s12248-012-9339-4 [Crossref] [ Google Scholar]
- Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M. Toxicity of nanomaterials. Chem Soc Rev 2012; 41:2323-43. doi: 10.1039/c1cs15188f [Crossref] [ Google Scholar]
- Callaghan NI, Allen GJP, Robart TE, Dieni CA, MacCormack TJ. Zinc oxide NPs trigger cardiorespiratory stress and reduce aerobic scope in the white sucker, Catostomus commersonii. NanoImpact 2016; 2:29-37. doi: 10.1016/j.impact.2016.06.004 [Crossref] [ Google Scholar]
- Rahman Md M, Lee JK, Jeong J, Seo YR. Usage of nanoparticles with their potential toxicity assessment and regulatory guidelines. Toxicol Environ Health Sci 2013; 5:49-54. doi: 10.1007/s13530-013-0156-7 [Crossref] [ Google Scholar]
- Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int J Nanomedicine 2014; 9:4357-73. doi: 10.2147/IJN.S46900 [Crossref] [ Google Scholar]
- McCallion C, Burthem J, Rees-Unwin K, Golovanov A, Pluen A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur J Pharm Biopharm 2016; 104:235-50. doi: 10.1016/j.ejpb.2016.04.015 [Crossref] [ Google Scholar]
- Ke N, Wang X, Xu X, Abassi YA. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol Miol 2011; 740:33-43. doi: 10.1007/978-1-61779-108-6_6 [Crossref] [ Google Scholar]
- Sherwood Cl, Boitano S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res 2016; 17:57. doi: 10.1186/s12931-016-0369-9 [Crossref] [ Google Scholar]
- Vetten MA, Tlotleng N, Tanner Rascher D, Skepu A, Keter FK, Boodhia K. Label-free in vitro toxicity and uptake assessment of citrate stabilised gold NPs in three cell lines. Part Fibre Toxicol 2013; 10:50. doi: 10.1186/1743-8977-10-50 [Crossref] [ Google Scholar]
- Choi J1, Reipa V, Hitchins VM, Goering PL, Malinauskas RA. Physicochemical characterization and in vitro hemolysis evaluation of silver nanoparticles. Toxicol Sci 2011; 123:133-43. doi: 10.1093/toxsci/kfr149 [Crossref] [ Google Scholar]
- Farcal L, Torres Andón F, Di Cristo L, Rotoli BM, Bussolati O, Bergamaschi E. Comprehensive in vitro toxicity testing of a panel of representative oxide nanomaterials: first steps towards an intelligent testing strategy. PLoS One 2015; 10:e0127174. doi: 10.1371/journal.pone.0127174 [Crossref] [ Google Scholar]
- Li W, Zhou J, Xu Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed Rep 2015; 3:617-20. doi: 10.3892/br.2015.481 [Crossref] [ Google Scholar]
- Hamidi H, Lilja J, Ivaska J. Using xCELLigence RTCA instrument to measure cell adhesion. Bio Protoc 2017; 7:e2646. doi: 10.21769/BioProtoc.2646 [Crossref] [ Google Scholar]
-
Organization for Economic Co-Operation and Development (OECD). Categorization of Manufactured Nanomaterials Workshop Report Paris 2016. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)59/ADD&doclanguage=en. Accessed 15 January 2017.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009; 6:e1000097. doi: 10.1371/journal.pmed1000097 [Crossref] [ Google Scholar]
- Pisani C, Gaillard JC, Nouvel V, Odorico M, Armengaud J, Prat O. High-throughput, quantitative assessment of the effects of low-dose silica NPs on lung cells: grasping complex toxicity with a great depth of field. BMC Genomics 2015; 16:315. doi: 10.1186/s12864-015-1521-5 [Crossref] [ Google Scholar]
- Yu K-N, Chang S-H, Park SJ, Lim J, Lee J, Yoon T-J. Titanium dioxide NPs induce endoplasmic reticulum stress-mediated autophagic cell death via mitochondria-associated endoplasmic reticulum membrane disruption in normal lung cells. PLoS One 2015; 10:e0131208. doi: 10.1371/journal.pone.0131208 [Crossref] [ Google Scholar]
- Zeng L, Chen Y, Liu J, Huang H, Guan R, Ji L, Chao H. Ruthenium(II) complexes with 2-phenylimidazo[4,5-f][1,10] phenanthroline derivatives that strongly combat cisplatin-resistant tumor cells. Sci Rep 2016; 6:19449. doi: 10.1038/srep19449 [Crossref] [ Google Scholar]
- Wang W, Sun X, Zhang H, Yang C, Liu Y, Yang W. Controlled release hydrogen sulfide delivery system based on mesoporous silica NPs protects graft endothelium from ischemia–reperfusion injury. Int J Nanomedicine 2016; 11:3255-3263. doi: 10.2147/IJN.S104604 [Crossref] [ Google Scholar]
- Meindl C, Absenger M Roblegg E, Fröhlich E. Suitability of Cell-Based Label-Free Detection for Cytotoxicity Screening of Carbon Nanotubes. Biomed Res Int 2013; 2013:564804. doi: 10.1155/2013/564804 [Crossref] [ Google Scholar]
- Zaloga J, Janko C, Nowak J, Matuszak J, Knaup S, Eberbeck D. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int J Nanomedicine 2014; 9:4847-4866. doi: 10.2147/IJN.S68539 [Crossref] [ Google Scholar]
- Zakrzewska KE, Samluk A, Wierzbicki M, Jaworski S, Kutwin M, Sawosz E. Analysis of the cytotoxicity of carbon-based NPs, diamond and graphite, in human glioblastoma and hepatoma cell lines. PLoS One 2015; 10:e0122579. doi: 10.1371/journal.pone.0122579 [Crossref] [ Google Scholar]
- Yamamoto Y, Hyodo I, Koga Y, Tsumura R, Sato R, Obonai T. Enhanced antitumor effect of anti-tissue factor antibody-conjugated epirubicin-incorporating micelles in xenograft models. Cancer Sci 2016; 106:627-34. doi: 10.1111/cas.12645 [Crossref] [ Google Scholar]
- Xu C, Liu Q, Liu H, Zhang C, Shao W, Gu A. Toxicological assessment of multi-walled carbon nanotubes in vitro: potential mitochondria effects on male reproductive cells. Oncotarget 2016; 7:26. doi: 10.18632/oncotarget.9689 [Crossref] [ Google Scholar]
- Vonnemann J, Beziere N, Böttcher C, Riese SB, Kuehne C, Dernedde J. Polyglycerolsulfate functionalized gold nanorods as optoacoustic signal nanoamplifiers for in vivo bioimaging of rheumatoid arthritis. Theranostics 2014; 4:629-41. doi: 10.7150/thno.8518 [Crossref] [ Google Scholar]
- Verma NK, Crosbie-Staunton K, Satti A, Gallagher S, Ryan KB, Doody T. Magnetic core-shell NPs for drug delivery by nebulization. J Nanobiotechnology 2013; 11:1. doi: 10.1186/1477-3155-11-1 [Crossref] [ Google Scholar]
- Unterweger H, Tietze R, Janko C, Zaloga J, Lyer S, Dürr S. Development and characterization of magnetic iron oxide NPs with a cisplatin-bearing polymer coating for targeted drug delivery. Int J Nanomedicine 2014; 9:3659-76. doi: 10.2147/IJN.S63433 [Crossref] [ Google Scholar]
- de Souza LR, Muehlmann LA, Dos Santos MS, Ganassin R, Simón-Vázquez R, Joanitti GA. PVM/MA-shelled selol nanocapsules promote cell cycle arrest in A549 lung adenocarcinoma cells. J Nanobiotechnology 2014; 12:32. doi: 10.2147/IJN.S6665 [Crossref] [ Google Scholar]
- Pan C-H, Liu W-T, Bien M-Y, Lin C, Hsiao T-C, Ma C-M. Effects of size and surface of zinc oxide and aluminum-doped zinc oxide NPs on cell viability inferred by proteomic analyses. Int J Nanomedicine 2014; 9:3631-43. doi: 10.2147/IJN.S66651 [Crossref] [ Google Scholar]
- Lindemann A, Lüdtke-Buzug K, Fräderich BM, Gräfe K, Pries R, Wollenberg B. Biological impact of superparamagnetic iron oxide NPs for magnetic particle imaging of head and neck cancer cells. Int J Nanomedicine 2014; 9:5025. doi: 10.2147/IJN.S63873 [Crossref] [ Google Scholar]
- Skopalik J, Polakova K, Havrdova M, Justan I, Magro M, Milde D. Mesenchymal stromal cell labeling by new uncoated superparamagnetic maghemite NPs in comparison with commercial Resovist--an initial in vitro study. Int J Nanomedicine 2014; 9:5355-72. doi: 10.2147/IJN.S66986 [Crossref] [ Google Scholar]
- Paget V, Dekali S, Kortulewski T, Grall R, Gamez C, Blazy K. Specific uptake and genotoxicity induced by polystyrene nanobeads with distinct surface chemistry on human lung epithelial cells and macrophages. PLoS One 2015; 10:e0123297. doi: 10.1371/journal.pone.0123297 [Crossref] [ Google Scholar]
- Mangraviti A, Tzeng SY, Kozielski KL, Wang Y, Jin Y, Gullotti D. Polymeric NPs for nonviral gene therapy extend brain tumor survival in vivo. ACS Nano 2015; 9:1236-49. doi: 10.1021/nn504905q [Crossref] [ Google Scholar]
- Lin ZC, Lee CW, Tsai MH, Ko HH, Fang JY, Chiang YC. Eupafolin NPs protect HaCaT keratinocytes from particulate matter-induced inflammation and oxidative stress. Int J Nanomedicine 2016; 11:3907-26. doi: 10.2147/IJN.S109062 [Crossref] [ Google Scholar]
- Kharin A, Syshchyk O, Geloen A, Alekseev S, Rogov A, Lysenko V. Carbon fluoroxide NPs as fluorescent labels and sonosensitizers for theranostic applications. Sci Technol Adv Mater 2015; 16:044601. doi: 10.1088/1468-6996/16/4/044601 [Crossref] [ Google Scholar]
- Jiráková K, Šeneklová M, Jirák D, Turnovcová K, Vosmanská M, Babič M. The effect of magnetic NPs on neuronal differentiation of induced pluripotent stem cell-derived neural precursors. Int J Nanomedicine 2016; 11:6267-81. doi: 10.2147/IJN.S116171 [Crossref] [ Google Scholar]
- Dzamukova MR, Naumenko EA, Lvov YM, Fakhrullin RF. Enzyme-activated intracellular drug delivery with tubule clay nanoformulation. Sci Rep 2015; 5:10560. doi: 10.1038/srep10560 [Crossref] [ Google Scholar]
- Bezuidenhout M, Liu P, Singh S, Kiely M, Ryan KM. Promoting Cell Proliferation Using Water Dispersible Germanium Nanowires. PLoS One 2014; 9:e108006. doi: 10.1371/journal.pone.0108006 [Crossref] [ Google Scholar]
- Monteiro-Riviere NA, Inman AO. Challenges for assessing carbon nanomaterial toxicity to the skin. Carbon 2006; 44:1070. doi: 10.1016/j.carbon.2005.11.004 [Crossref] [ Google Scholar]
- Herzog E, Casey A, Lyng FM, Chambers G, Byrne HJ, Davoren M. A new approach to the toxicity testing of carbon-based
nanomaterials--the clonogenic assay. Toxicol Lett 2007; 174:49. doi: 10.1016/j.toxlet.2007.08.009 [Crossref] [ Google Scholar]
- Dutta D, Sunddaram SK, Teeguarden JG, Riley BJ, Fifiels LS, Jacobs JM. Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxicol Sci 2007; 100:303. doi: 10.1093/toxsci/kfm217 [Crossref] [ Google Scholar]
- Gu L, Fang RH, MJ MJ, Park J-H. In Vivo Clearance and Toxicity of Monodisperse Iron Oxide Nanocrystals. ACS Nano 2012:6. doi: 10.1021/nn300456z [Crossref]
- Recordati C, De Maglie M, Bianchessi S, Argentiere S, Cella C, Mattiello S. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects. Part Fibre Toxicol 2016; 13:12. doi: 10.1186/s12989-016-0124-x [Crossref] [ Google Scholar]
- Wang M, Zhang Y, Feng J, Gu T, Dong Q, Yang X. Preparation, characterization, and in vitro and in vivo investigation of chitosan-coated poly (d,l-lactide-co-glycolide) NPs for intestinal delivery of exendin-4. Int J Nanomedicine 2013; 8:1141-54. doi: 10.2147/IJN.S41457 [Crossref] [ Google Scholar]
- Jana U, Mohanty AK, Pal SL, Manna PK, Mohanta GP. Felodipine loaded PLGA NPs: preparation, physicochemical characterization and in vivo toxicity study. Nano Convergence 2014; 1:31. doi: 10.1186/s40580-014-0031-5 [Crossref] [ Google Scholar]
- Abbad S, Wang C, Waddad AY, Lv H, Zhou J. Preparation, in vitro and in vivo evaluation of polymeric NPs based on hyaluronic acid-poly(butyl cyanoacrylate) and D-alpha-tocopheryl polyethylene glycol 1000 succinate for tumor-targeted delivery of morin hydrate. Int J Nanomedicine 2015; 10:305-20. doi: 10.2147/IJN.S73971 [Crossref] [ Google Scholar]
- França FR1, Burke TN, Hanada ES, Marques AP. Segmental stabilization and muscular strengthening in chronic low back pain: a comparative study. Clinics (Sao Paulo) 2010; 65:1013-7. doi: 10.1590/S1807-59322010001000015 [Crossref] [ Google Scholar]
- Ngoy JM, Iyuke SE, Yah CS, Neuse WE. Kinetic Optimization of Folic Acid Polymer Conjugates for Drug Targeting. Am J Appl Sci 2011; 8:508-19. doi: 10.3844/ajassp.2011.508.519 [Crossref] [ Google Scholar]
- Fu PP, Xia Q, Hwang HM, Ray PC, Yu H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J Food Drug Anal 2014; 22:64-75. doi: 10.1016/j.jfda.2014.01.005 [Crossref] [ Google Scholar]
- Yah CS, Iyuke SE, Simate GS. Nanoparticles toxicity and their routes of exposures. Pak J Pharm Sci 2012; 25:477-91. [ Google Scholar]
-
ISO/TR 16197: Nanotechnologies - Compilation and description of toxicological screening methods for manufactured nanomaterials. 2014. https://www.iso.org/standard/55827.htm. Accessed 02/01/2017.
- Boverhof DR, Bramante CM, Butala JH, Clancy SF, Lafranconi M, West J, Gordon SC. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul Toxicol Pharmacol 2015; 73:137-50. doi: 10.1016/j.yrtph.2015.06.001 [Crossref] [ Google Scholar]
-
Nanotechnology Industries Association (NIA). ISO publishes two Nano Toxicology Standards. 2014. http://www.nanotechia.org/news/news-articles/iso-publishes-two-nano-toxicology-standards. Accessed 02/01/2017.
-
ISO/TS 16550:2014. Nanotechnologies -- Determination of silver NPs potency by release of muramic acid from Staphylococcus aureus. 2014. https://www.iso.org/standard/57084.html. Accessed 02/01/2017.
- Vetten MV, Yah CS, Singh T, Gullumian M. Challenges facing sterilization and depyrogenation of nanoparticles: Effects on structural stability and biomedical applications. Nanomedicine 2014; 10:1391-9. doi: 10.1016/j.nano.2014.03.017 [Crossref] [ Google Scholar]
- Moodley K, Angel CE, Glass M, Graham ES. Real-time profiling of NK cell killing of human astrocytes using xCELLigence technology. J Neurosci Methods 2011; 200:173-180. doi: 10.1016/j.jneumeth.2011.07.005 [Crossref] [ Google Scholar]
- MacDonald C, Unsworth CP, Graham ES. Enrichment of differentiated hNT neurons and subsequent analysis using flow-cytometry and xCELLigence sensing. J Neurosci Methods 2014; 227:47-56. doi: 10.1016/j.jneumeth.2014.02.004 [Crossref] [ Google Scholar]
- Kho D, MacDonald C, Johnson R, Unsworth CP, O’Carroll SJ, du Mez E. Application of xCELLigence RTCA biosensor technology for revealing the profile and window of drug responsiveness in real time. Biosensors 2015; 5:199-222. doi: 10.3390/bios5020199 [Crossref] [ Google Scholar]
- Yan G, Du Q, Wei X, Miozzi J, Kang C, Wang J. Application of Real-Time Cell Electronic Analysis System in Modern Pharmaceutical Evaluation and Analysis. Molecules 2018; 23:E3280. doi: 10.3390/molecules23123280 [Crossref] [ Google Scholar]
- Agbakoba VC, Yah CS, Simate GS, Hlangothi SP. A study of the flow behavior of prevulcanised natural rubber latex/singlewalled carbon nanotubes (SWCNT) blends using rotational viscometry and power law model. Appl Rheol 2018; 28:64175. doi: 10.3933/APPLRHEOL-28-64175 [Crossref] [ Google Scholar]
- Viswanathan P, Ondeck MG, Chirasatitsin S, Ngamkham K, Reilly GC, Engler AJ, Battaglia G. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015; 52:140-7. doi: 10.1016/j.biomaterials.2015.01.034 [Crossref] [ Google Scholar]
- Zhang P, Zhang C, Li J, Han J, Liu X, Yang Yang. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res Ther 2019; 10:327. doi: 10.1186/s13287-019-1422-7 [Crossref] [ Google Scholar]
- Martinez-Serra J, Gutierrez A, Muñoz-Capó S, Navarro-Palou M, Ros T, Amat JC. xCELLigence system for real-time label-free monitoring of growth and viability of cell lines from hematological malignancies. Onco Targets Ther 2014; 7:985-94. doi: 10.2147/OTT.S62887 [Crossref] [ Google Scholar]
-
ACEA. Using xCELLigence Real-Time Cell Analysis to Monitor Immune Cell-Mediated Killing of B Cells. ACEA Biosciences Inc; 2016. https://www.aceabio.com/wp-content/uploads/B-Cell-Killing-Protocol-v1.4.pdf. Accessed 2 December 2019.
- Selli C, Erac Y, Tosun M. Effects of cell seeding density on real-time monitoring of anti-proliferative effects of transient gene silencing. J Biol Res (Thessalon) 2016; 1:20. doi: 10.1186/s40709-016-0057-4 [Crossref] [ Google Scholar]
- Abbasalipour M, Khosravi MA, Zeinali S, Khanahmad H, Karimipoor M, Azadmanesh K. Improvement of K562 Cell Line Transduction by FBS Mediated Attachment to the Cell Culture Plate. BioMed Research International 2019; 2019:9540702. doi: 10.1155/2019/9540702 [Crossref] [ Google Scholar]
- Hillger JM, Schoop J, Boomsma DI, Slagboom PE, IJzerman AP, Heitman LH. Whole-cell biosensor for label-free detection of GPCR-mediated drug responses in personal cell lines. Biosens Bioelectron 2015; 74:233-42. doi: 10.1016/j.bios.2015.06.031 [Crossref] [ Google Scholar]