Bioimpacts. 11(4):289-300.
doi: 10.34172/bi.2021.33
Review
Relationship between endocrine disruptors and obesity with a focus on bisphenol A: a narrative review
Sofiane Boudalia 1, 2, *  , Aissam Bousbia 1, 2
, Aissam Bousbia 1, 2  , Boualem Boumaaza 2, 3, Malha Oudir 4, Marie Chantal Canivenc Lavier 5
, Boualem Boumaaza 2, 3, Malha Oudir 4, Marie Chantal Canivenc Lavier 5
Author information:
1Faculté des Sciences de la Nature et de la Vie et Sciences de la Terre et de l’Univers, Université 8 Mai 1945 Guelma BP 4010 Guelma 24000, Algérie
2Laboratoire de Biologie, Eau et Environnement, Université 8 Mai 1945 Guelma BP 4010 Guelma 24000, Algérie
3Département des Sciences Agronomiques, Faculté des Sciences de la Nature et de la Vie, Université Ibn Khaldoun, Tiaret 14000, Algérie
4Laboratoire de Génie Chimique, Département de Génie des Procédés, Faculté de Technologie, Université Saâd Dahlab, USDB. BP 270, Route de Soumâa, 09000 Blida, Algérie
5Centre des Sciences du Goût et de l'Alimentation, INRA, CNRS, Université de Bourgogne - Franche-Comté, Dijon, 21000, France
Abstract
Introduction:
Scientific data suggest that early exposure to endocrine-disrupting chemicals (EDCs) affect -repro, -neuro, -metabolic systems, to which are added other notions such as mixtures, window and duration of exposure, trans-generational effects, and epigenetic mechanisms.
Methods:
In the present narrative review, we studied the relationship between exposure to EDCs with the appearance and development of obesity.
Results:
Exposure to EDCs like Bisphenol A during the early stages of development has been shown to lead to weight gain and obesity. EDCs can interfere with endocrine signaling, affect adipocytes differentiation and endocrine function and disrupt metabolic processes, especially if exposure occurs at very low doses, in the mixture, during early development stages for several generations.
Conclusion:
Exposure to EDCs is positively associated with obesity development. Moreover, the use of integrative approaches which mimicking environmental conditions are necessary and recommended to evaluate EDCs' effects in future studies.
Keywords: Adipocyte, Bisphenol A, Fat tissues, Metabolic disorders, obesity, Peroxisome proliferator-activated receptor-γ
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The distribution of adipose tissues and the food intake are different between males and females (fat mass is mainly located in the abdomen in males or the thighs in females), and the reproductive hormones appear to be important in these differences.
1-3
Moreover, at birth, the number and the functional characteristics of adipocyte cells depend on endocrine and nutritional conditions during in utero development.
4
Several human studies suggested a high association between endocrine-disrupting chemicals (EDCs) and obesity, the National Health and Nutritional Examination (NHANES) 2003-2006 study showed that urinary Bisphenol A (BPA) was associated with obesity
5
in a gender-dependent manner.
6
A direct correlation of urinary BPA level and both body mass index (BMI) and waist circumference has been found in a cross-sectional study based on NHANES 2003–2008 cycle data.
7
Another epidemiological study aimed to quantify BPA in mother and child was conducted in six European countries (Belgium, Denmark, Luxembourg, Slovenia, Spain, and Sweden) showed that BPA was found in 91.1% and 90.5% of children and mothers respectively.
8
From the Environment and Childhood (INMA) cohort in Granada (Spain), BPA concentrations were quantified in spot urine samples collected from 298 peripubertal boys aged between 9 and 11 years old. The results have shown that BPA could exert an obesogenic effect by increasing the risk of “overweight/obesity” and abdominal obesity.
9
In vivo studies endorse these epidemiological observations and suggest the influence of the EDCs on fatty mass development, mainly when exposure occurred in the prenatal phase.
10-12
Early exposure demonstrated that EDCs can indirectly act through changes in maternal endocrine status (i.e. plasma hormone levels)
13
and/or nutritional behavior. Hence, this can predispose newborns to nutritional and endocrine imbalances, adipocyte endocrine dysfunction, obesity, and related disorders.
14
Nunez et al demonstrated the influence of BPA (xenoestrogen) exposure on nutritional status: it can affect body weight in ovariectomized adult Sprague-Dawley females without diminishing food intake, contrary to estradiol-induced anorexia. These results reveal that BPA can disrupt mother nutritional habits and fetus food intake.
15
Several other studies suggest that EDCs can act on taste preferences, by increasing or decreasing in fat, salt and sugar food preference level,
16,17
indicating that EDCs do not only have an action that passes through the adipocyte cells, but they can act on other peripheral organs involved in the regulation of taste preferences, like salivary glands.
18
This can indirectly affect food intake and weight development leading to obesity.
Here, we will outline a narrative review to identify the relationship between early EDCs exposure and obesity development later in adulthood.
Endocrine disruptors chemicals
Humans are exposed daily to EDCs, which are very heterogeneous exogenous molecules. This list is not exhaustive, EDCs can be natural like phytoestrogens and mycotoxins; or synthetic like atmospheric pollutants, pesticides, detergents, plastic derivatives, varnishes, synthetic hormones, medicines and veterinary products.
19
These molecules have distinct hormonal properties that can be estrogenic like genistein and BPA,
20
or anti-androgenic like vinclozolin.
21
In 1999, the European Commission
22
proposed the following definition: "An endocrine disruptor (ED) is an exogenous substance or mixture, altering the functions of the endocrine system and thereby inducing adverse health effects in an intact organism, its descendants or subpopulations". This definition has been supplemented by the following two definitions:
"A potential endocrine disruptor is an exogenous substance or mixture with properties that can induce an endocrine disruption in an intact organism, in an offspring or a population".
23
"A known endocrine disruptor is an exogenous substance or mixture that alters the function of the endocrine system and thus induces adverse effects on the health of an intact organism, descendants or a population".
24
A 2016 European Commission defined endocrine disruptors as an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or in subpopulations".
25
The interaction between EDCs and endogenous hormones can occur at several levels: synthesis, transport, action and excretion, via their binding and their metabolism. EDCs can bind to nuclear hormone receptors such as estrogen (ER), androgen (AR), progesterone (PR), thyroid (TR), and retinoic acid receptors (RAR). They also bind the steroid hormone receptors membrane: ER, non-steroidal receptors (neurotransmitter receptors: serotonin, dopamine), orphan receptors (Aryl hydrocarbon receptor: AhR). EDCs can also affect the activity of certain enzymes involved in the metabolism and/or biosynthesis of steroids.
26
In the last decade, the number of natural or synthetic molecules that have endocrine disrupting activity has increased. In 2002, the European Commission publishes a report listing 435 endocrine disruptors.
27
To this day the number of potential EDCs listed based on TEDX (The Endocrine Disruption Exchange) reached 1428 molecules.
28
How do EDCs act?
EDCs act through an illegitimate activation of steroid receptors or via non-genomic pathways. Organochlorine pesticides endosulfan, toxaphene, o, p'dichlorodiphenyltrichloroethane (DDT) and dieldrin interact directly with the estradiol receptor and displace 17-β estradiol from its receptor. The pesticide-estradiol receptor complex can therefore trans-activate promoters containing estrogen response element, which activate estradiol sensitive genes. Other compounds such as o, p'DDT, its metabolite p, p'DDE or vinclozolin, have an anti-androgenic effect, by binding to the androgen receptor, they block its function in an identical manner of an antagonist like cyproterone acetate. These compounds induce androgen receptor binding to the androgen response element and activate the transcription of androgen-sensitive promoters.
29
Endocrine disruptors can operate on orphan receptors belonging to the superfamily of nuclear receptors. Moreover, they interfere with the metabolism of steroid hormones, by the induction of cytochromes P450 (CYPs), or by acting on the metabolism of cholesterol (precursors of steroid hormones).
30
The Trans-nonachlor is a component of the banned pesticide chlordane, it is considered an endocrine disruptor and can inhibit the activation of the orphan constitutive androstane receptor (CAR) in mice.
31
In rats, DDT increases the transcriptional activity of CAR and PXR (pregnane X receptor).
32
DTT disrupts the neuro-endocrine system via the AhR receptor and the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) glutamate receptor.
33
It is known from the literature that PXR and CAR are strongly expressed in the liver and intestine, where they induce metabolic enzymes [cytochromes P450 (eg CYP3A)], conjugation enzymes (eg UGT1A1), transporters (e.g. multidrug resistance 1, and organic anion-transporting peptide 2). Methoxychlor is an organochlorine insecticide, which has a similar structure with DDT but a relatively low half-life in the environment, can activate PXR and CAR and induce CYP3A mRNA (metabolism enzyme) in male rat liver, and therefore produce metabolites that can be reactive.
CYP3A is involved in the metabolism of steroids and is active by precursor of progesterone. The binding of the hormone-receptor complex to the hormone response element region of the DNA can induce transcription of the target gene. The recruitment of co-regulators and transcription factors on the promoter regions initiates the activation of RNA polymerase II and the synthesis of new mRNAs. Alteration of co-activators and transcription factors can then modulate gene transcription. Lonard et al, show that an increase in the level of steady-state co-activator induces an increase in ER receptor transcription in the presence of selective estrogen receptor modulators (4-hydroxytamoxifen and raloxifene).
34
In mouse uterus, BPA (0.3 mg/kg intraperitoneal injection (IP) every 24 h/5 days) activates the transcription of TRAP220 co-activator (thyroid hormone receptor activator protein 220) which increases the ER expression, suggesting that BPA can disrupt the endocrine system via an indirect action, by acting on the co-activator level.
35
Following ubiquitin addition, the degradation of the hormonal receptors is finely regulated by proteolysis-specific proteins called proteasomes. This regulation system makes it possible to avoid over-stimulation of the cells. The expression of steroidal nuclear hormone receptors is regulated by this degradation machinery.
36
EDCs can modify the hormone receptor degradation system, which results in disturbances in the endocrine system (duration of cellular response to endogenous hormones, following a malfunction in the degradation of the receptors for example).
The degree of methylation of DNA is considered as another way in which EDCs may operate. Exposure to Vinclozolin and Methoxychlor decreased spermatogenesis and increased the incidence of infertility in Fisher rats up to the fourth generation. The effects are correlated with alteration in DNA methylation.
37
EDCs can also activate the expression of PPARγ (peroxisome proliferator-activated receptor-γ) and RXRs (retinoic acid X receptors) at the adipocyte level. Peroxisome proliferator-activated receptors (PPARs) are considered ligand-activated transcription factors of nuclear hormone receptors. They are divided into three subtypes: PPARα (NR1C1), PPARβ / δ (NR1C2) and PPARγ (NR1C3). PPARs are involved in the pathways of lipid metabolism, fatty acids synthesis and metabolism, cholesterol transport,
38,39
and the regulation of energy homeostasis.
40
PPARγ is activated by the binding of natural ligands such as polyunsaturated fatty acids and prostaglandin metabolites, such as 15-deoxy-Δ
12,14
-prostaglandin J2 (15d-PGJ2). It can also be activated by synthetic ligands such as glitazones and causes insulin sensitization and enhances glucose metabolism.
41
In diabetic patients (type II diabetes with insulin resistance), PPARγ activation using synthetic ligands increases their insulin sensitivity.
42
PPARγ activators have been also used to treat dyslipidemia due to their capacity to decrease plasma triglyceride levels and increase HDL cholesterol levels.
40
Tributyltin (TBT) is another endocrine disruptor that may affect adipogenesis via activation of PPARγ and RXRs.
43
Pascal Phrakonkham et al., study of 3T3-L1 cells shows that early treatment with xeno-estrogens (BPA, Genistein and apigenin) can increase the expression of differentiation genes as well as endocrine activity of adipocyte (leptin synthesis), without modifying estrogen receptors expression.
44
Anne Riu et al, demonstrated that flame retardants, which are analogs of BPA [Tetra Bromo bisphenol A (TBBPA)] and [Tetra chloro bisphenol A (TCBPA)] can bind to PPARγ and induce adipogenesis in 3T3-L1 cells.
45
In in vitrostudy, Jedeon et al,show that rat ameloblastic HAT-7 cells treated with a combination of EDCs (BPA, Genistein and Vinclozolin) reduced klk4 and enamelin expression and caused Hypomineralization incisor.
46
Boudalia et al, showed that a mixture of Genistein and Vinclozoline administered at the early stage of 3T3-L1 development affect cells differentiation and endocrine activity (Fig. 1).
21
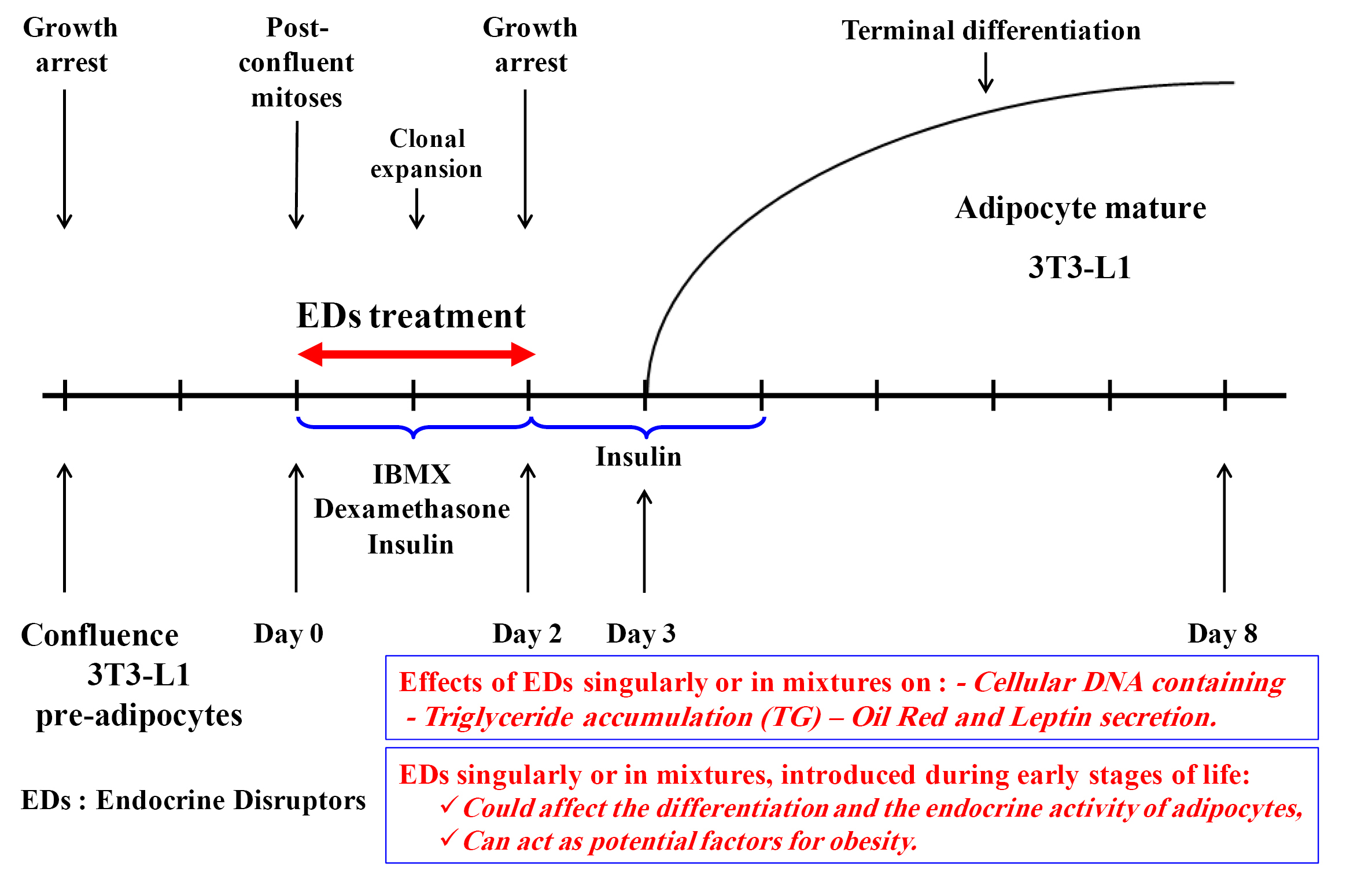
Fig. 1.
Effects of EDCs on 3T3-L1 differentiation and endocrine activity (from Boudalia et al
21
).
.
Effects of EDCs on 3T3-L1 differentiation and endocrine activity (from Boudalia et al
21
).
Endocrine Disruptors Chemicals and toxicology
Although we are exposed during our life to mixtures of contaminants and pollutants most often present in very low doses,
47
researchers continue to screen the effects of EDCs by molecule at higher doses than the NOAEL (No Observable Adverse Effect Level). Moreover, 95% of toxicological studies including “endocrine disruption” evaluate the effects of a single molecule.
48
Notions of EDCs low doses
During the last decade, research teams have been interested in the question of Low Doses, but the definitions remain imprecise, however, three notions seem to stand out:
-
Doses below NOAEL, LOAEL (lowest-observed adverse-effect level): provoke effects especially during exposure in utero.
-
Doses to which humans are exposed do not always correspond to the lowest doses used in toxicological studies.
-
Doses administered to animals at serum concentrations close to or equivalent to those found in humans (taking into account the difference between humans and animals, metabolism, bioavailability, excretion, etc).
Welshons et al, defined low doses as concentrations close to those of our endogenous hormones, and which act in most of the time according to non-monotonic curves".
49
EDCs can have more effects at low doses than that at higher ones, others have different effects at different doses.
12,50-55
The term "low dose" is defined in two ways according to Owens and Chaney, (2005) reported by Bergman and Heindel:
A similar definition has been reported in the National Toxicology Program's Report of the Endocrine Disruptors Low-Dose Peer Review, NTP (2001): "low-dose effects" referred to biological changes that occur in the range of human exposures or at doses that are lower than those typically used in the US EPA's standard testing paradigm for evaluating reproductive and developmental toxicity.
Deleterious effects of BPA have been recorded at doses lower than the NOAEL, and like most hormones, this type of molecule escapes the rule of "The dose is proportional to the effects" and causes greater effects at very low doses. Another example is phthalates, known for its estrogenic and anti-androgenic activity at low doses. In Wistar rat, oral exposure to low doses of di(2-ethylhexyl) phthalate (DEHP) alters aromatase activity in the brain (aromatase: the enzyme responsible for the conversion of testosterone to estradiol, plays an important role in dimorphism at the cerebral level). This alteration varies according to the concentration, which means that the action of the molecule follows a non-monotonic curve.
56
Exposure to DEHP during pre-pubertal, pubertal and post-pubertal periods decrease sperm count and increase testosterone levels with an enhancement of the Leydig cells number and a decrease of Sertoli cells number.
55
In ovariectomized mice, exposure to methylparaben (EDC with antimicrobial properties, mainly used as a foods preservative, and cosmetics, at low doses show an alteration in the organization of uterine tissue.
57,58
TBT, used in the wood industry, has antifungal properties and is considered as an endocrine disruptor; it can bind and activate PPARγ and RXRs. In mice, in utero exposure to low doses of TBT causes an adipogenic effect, and stimulates the differentiation of multipotent cells into adipocytes.
59,60
Furthermore, neonatal exposure to low doses of BPA (20 or 40 mg/kg body weight (BW)) from post natal day (PND) 15-PND 30 can affect the thyroid gland and induced hypothyroid, it also disturbs the neonatal thyroid-brain axis via pro-oxidant and antioxidant system disturbance, leading to free radicals production.
61
Impact of EDCs exposure window
In the early stages of development, the body is more sensitive. Exposure to EDCs, drugs, or any other chemical molecules during these stages can have effects in adulthood. Several studies suggested the influence of malnutrition that causes a delay or dysfunction in “in utero” development and the appearance of diseases in adulthood (hypothesis of "fetal programming"). Barker et al showed a negative correlation between body weight at birth and disease development in adulthood such as obesity, metabolic syndrome and heart disease.
62
This hypothesis is generalized to contain other molecules that have an agonist or antagonist activity with endogenous hormones such as BPA, phthalates and diethylstilbestrol (DES).
63,64
In the rodent model, postnatal DES exposure leads to fat mass development in adulthood.
65
In utero exposure to EDCs during the early stages of development seems to have more effect: this is due to the high plasticity during this period, an uncompleted development of the immune system, a blood-brain barrier that is not well-differentiated and an immature liver detoxification system.
26
In utero exposure to BPA at doses of 25-250 ng/kg BW/day affects mammary gland development from the embryonic stage, the alteration in morphogenesis relates to the distribution of collagen fibers which subsequently affect the exocrine activity of the gland.
66
In the same way, BPA might penetrate the placental barrier and perturb the fetal thyroid adipokine axis and influence fat metabolism. In utero exposure to low doses of BPA (20 or 40 mg/kg BW) from gestational day 1 to 20, decreased serum thyroid hormone levels and affected gland development. These dysfunctions resulted in the suppression of fetal serum growth hormone, insulin growth factor-1, adiponectin levels, the increasing of fetal serum leptin, insulin and tumor necrosis factor alpha.
67
The same results were observed with the use of other EDCs, early exposure to 3,3',4,4',5-pentachlorobiphenyl (PCB 126) or 2,3,6-2',5'-pentachlorinated biphenyl (PCB 95) or 2,3,7,8-tetrachlorodibenzo-p-dioxin can affect fat metabolism through an action on thyroid gland development.
68-70
Epigenetic and trans-generational EDCs effects
The harmful effects of endocrine disruptors can extend to several generations. After a single gestational exposure, these effects seem to be transmissible via epigenetic mechanisms.
37,71-75
This change in gene expression occurs through two paths:
DNA methylation: a methyl group consists of a carbon atom and three hydrogen atoms, the methyl groups are attached or removed from the DNA support in specific locations, it plays as a switch by turning on/off certain genes.
Histone modification: it indirectly affects DNA. Histones are proteins that wind the DNA. Indeed, at the chromatin level, the nucleosome core consists of two histones of each class (H2A, H2B, H3 and H4) which associate in an octamer around which the double-strand of DNA is wound, which makes it firmly wrapped in the chromosomes inside the nucleus of the cell. However, different chemical compounds can bind to the histones' end and modify the DNA winding, which can be tight or loose. If the coil is tight, a gene may be hidden from the machinery of protein synthesis in the cell. The gene becomes extinct. However, if the coil is loose, the gene can be turned on.
In either way, endocrine disruptors cause adverse effects transmittable to subsequent generations. Anway et al showed that in utero exposure of F1 to vinclozolin can extend to the 3rd generation.
37
The same team showed that exposure to vinclozolin affects the transcriptome of two regions of the brain (the hippocampus and amygdala), which decrease anxiety behavior in males and increase the same behavior in females at the 3rd generation.
76
EDCs mixtures effects
The traditional approach in toxicology is to study the effects of a single substance. However, over the past decade, some research teams have been interested in the question of mixtures or cocktail effects. Several chemical substances considered independently as non-toxic can have adverse effects if they act together.
77,78
Kortenkamp and Altenburger tried to find a way that can predict the effects of mixtures on the integrity of the body. Usually, the calculation is done based on addition; in other words, the effect of a mixture of two molecules is equal to the sum of the effects of every single molecule. For example, in animal experiments, a molecule X leads to 35% hypospadias in a lot, a molecule Y leads to 35% hypospadias in another batch, the mixture can cause 35% × 2 = 70% hypospadias.
79
However, in other cases, it is more complicated, and mathematical modeling to predict the effects of exposure to endocrine disruptor mixtures does not give the real effects.
80
Also, the majority of studies that deal with the issue of EDCs mixtures use the same family of molecules, which mean the same type of action (estrogenic, androgenic, or thyroid). In that case, prediction by the addition of effects is rather effective. Payne et al, studied the effects of four oestrogenic o, p'-DDT, genistein, 4-nonylphenol, and 4-n-octylphenol molecules in a mixture, using the yeast estrogen screen test, which can detect ERs receptor activation, due to a colorimetric reaction. The comparison between the effects obtained and the effects predicted by addition showed that there is no difference, suggesting that the effects of the xeno-oestrogens present in a mixture can be calculated from the dose-response curves for each compound alone.
81
Combinations of mixtures of different classes make things more complex. From literature, studies with binary mixtures [1 mg Genistein (estrogen) + 1 mg v inclozolin (anti-androgenic)] showed that these low doses caused adverse effects on the male reproductive system,
82
abnormalities in the testis of fetuses with a disturbance in the hormonal secretion (testosterone).
83
In females, the morphogenesis of the mammary gland was affected by these same mixtures at low doses.
84
Also, in submandibular salivary glands, structural abnormalities associated to change in gene expression of endocrine and exocrine functions were recorded.
85
Adipose tissue
Considered as an endocrine tissue that maintains energy homeostasis, the white adipose tissue plays a crucial role in lipid and carbohydrate metabolism. There are two types of adipose tissue. White adipose tissue (the main energy source of the body), which is closely associated with food intake control, via the release of leptin. It represents 20 to 25% of the total mass in women while in men, it varies from 15 to 20%, and the maintenance of this balance depends on a fine hormonal regulation via the sexual hormones. The second type of adipose tissue is brown adipose tissue (involved in thermoregulation).
86
Histology of adipose tissue
Adipose tissue is a connective tissue that contains pre-adipocytes, fibroblasts, macrophages, blood cells and endothelial cells; this set of cells constitutes the vascular stroma of the adipose tissue. Depending on energy balance, hormonal conditions and nutrition, the pre-adipocytes cells differentiate to form adipocytes.
87
Adipocytes are spherical cells about 100 μm in diameter, the cytoplasm contains a voluminous vacuole filled with lipids (triglycerides) that can be visualized by red oil staining. This vacuole is surrounded by a thin cytoplasmic corona containing a Golgi apparatus, granular endoplasmic reticulum, smooth endoplasmic reticulum, and mitochondria. The nucleus, flattened, is pushed against the plasma membrane. A thin membrane surrounds the plasma membrane.
87
Adipose tissue distribution and development
The white adipose tissue is localized differently according to age and sex. In women, the white adipose tissue represents 20 to 25% of the total mass while in the man; it varies from 15 to 20%. We find it especially at the level:
1) Subcutaneous, diffuse and regular in the fetus and newborn, predominating on the neck and shoulders in men, on the chest, hips, thighs and buttocks in women.
2) Abdominal, such as mesentery, or retroperitoneal regions. Adipose tissue appears in the fetus in the second trimester of pregnancy in the cheeks, neck, shoulders and kidneys.
The mechanisms involved in the development of adipose tissue are not yet well defined, but a localized modulation of lipolytic activity, via lipoprotein-lipase or the stimulation of the adrenergic nerve pathways, could play a role, the various fatty deposits are not regulated in the same way.
88
In humans and from the early stages of development, there is a difference in the composition and distribution of body fat and lean body mass. At birth, fat mass in girls is higher than that recorded for the boys. This difference persists from the first to the third month and disappears in the sixth month.
89
Body fat varies with BMI, and age, this variation is more pronounced in adolescent girls.
90
Adipocyte differentiation is a process involving coordinated and sequential activation of several transcription factors. Among them, peroxisome proliferator-activated receptors (PPAR), in particular, PPARγ2, and CCAT/enhancer-binding proteins (C-EBP), lead to the activation of specific genes for mature adipocytes.
91,92
Adipogenesis can be induced in vitro using a mitogenic hormone cocktail on cells of pre-adipocyte lineages such as 3T3-L1. Briefly, the expression of pre-adipocyte factor-1, a differentiation inhibitor, is first inhibited by stimulating agents (such as insulin, dexamethasone and 3-isobutyl-1-methylxanthine) while expression of C/EBPα and C/EBP increases. This induces the expression of PPARγ2 and C/EBPα which in turn, stimulate the transcription of genes specific to the mature adipocyte. The expression of steroid receptors is modulated during adipogenesis and may be involved in the control of cascade activation of transcription factors.
93
The potential role of ER and AR receptors in this process is unclear. However, the involvement of steroid hormones in the distribution of adipose mass and the pathologies associated with obesity is recognized.
Hormonal regulation of adipose tissue
The regulation, development and distribution of fat are under the control of the endocrine system, and sex hormones seem to play a determining role. In men, the fat mass is mainly located in the abdomen (android), while in women it is rather in the thighs (gynoid). This sexual dimorphism observed in humans is influenced by sex hormones.
94
At the adipose tissue level, AR receptors and both types of ER are expressed.
95,96
Experimentally, the states of estrogen deficiency, as in ovariectomized animals and ArKO (aromatase knock-out) and ERKO (estrogen receptor knock-out) mice, are accompanied by an increase in the weight of the animals mainly affecting adipose tissue.
97
ARKO (androgen receptor knock-out) mice also develop obesity.
98
In humans, the alteration of estrogen synthesis pathways such as a mutation of the aromatase gene (which allows the conversion of androgens to estrogens) leads to an early onset of overweight in android, with insulin resistance and dyslipidemia.
99
Besides, estrogens seem to play a determining role in the proliferation, the differentiation of pre-adipocytes into mature adipocytes, as well as the endocrine activity of adipocytes such as the synthesis of leptin.
44
In rodents, estrogens regulate body fat by acting on the adipocyte. Heine et al showed an increase in body weight which results in hyperplasia and hypertrophy of adipocyte in ERKO mice. This change is accompanied by insulin resistance, glucose intolerance, an increase in food intake and a decrease in energy expenditure.
100
Endocrine disruptors and obesity: the case of Bisphenol A
Chemical nature and source of exposure
Bisphenol A [(2,2- (4,4'-Dihydroxydiphenyl) propane] (BPA, Fig. 2) is synthesized for the first time in 1891, the molecule is used in the synthesis of plastic polycarbonates for the manufacture of food packaging (film, plastic bottles), internal varnish, cans, medicinal products, but also halogenated derivatives used as flame retardants,
101
poor resistance to sterilization by autoclaving lead to contamination of foodstuffs.
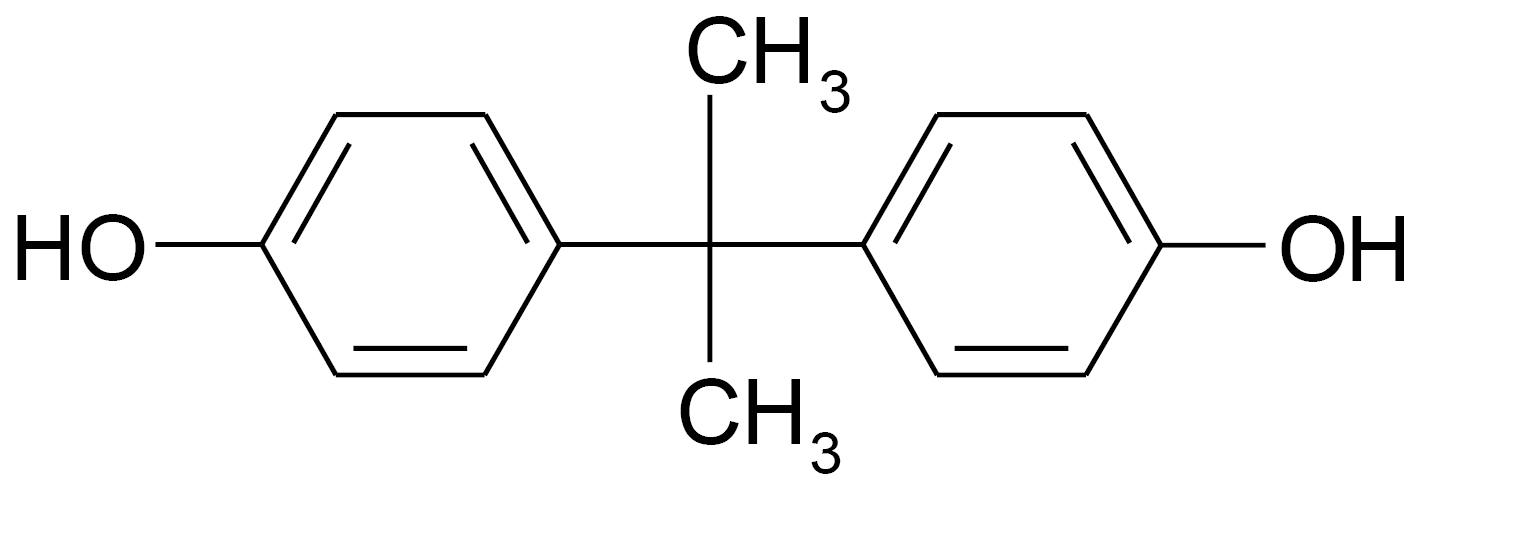
Fig. 2.
Chemical structure of bisphenol A.
.
Chemical structure of bisphenol A.
Human exposure varies with diet and age. It is estimated at less than 1 μg/kg BW/day.
102
According to the EPA (U.S. Environmental Protection Agency), the LOAEL is 50 mg/kg BW/day.
103
However, several studies show that BPA at low doses has unpredictable effects, proving that BPA escapes the rule "it is the dose that makes the poison".
104
BPA binds to both types of ER receptors, with a 10-fold higher affinity for ERβ, but remaining 1000 to 10 000 less than that of estradiol.
105
It can also bind to other receptors in a more moderate way, such as androgen receptors (AR) and progesterone (PR) and corticosteroids.
106
Several epidemiological studies showed correlations between EDCs concentrations recorded in blood and urine, and the appearance of pathologies in adulthood (obesity, diabetes, cardiovascular diseases).
107,108
Other studies in rats support the hypotheses of epidemiological studies.
12,109
Distribution of bisphenol A in adipose tissue
At the gastrointestinal level, BPA was absorbed; then metabolized to a hydrophilic form using phase II enzymes via glucuronidation in the liver and intestine; it can act on several targets such as the brain, gonads, and adipose tissue.
110
From Bioaccumulation studies; Nunez et al, showed that BPA can be detected in blood, brain and adipose tissues after repeated doses of 4 or 5 mg/d for 15 days to ovariectomized female Sprague Dawley rats. The molecule was preferentially accumulated in brown adipose tissue.
15
In human studies, BPA concentrations were evaluated at 3.16 ± 4.11 ng/g of adipose tissue in adipose tissue collected from twenty Spanish adult women in the course of surgical treatment of malignant and benign diseases.
111
BPA was also detected in several tissues collected during autopsy from eleven patients (eight males and three females) at the University Hospital of Antwerp (Belgium) with the highest concentrations found in adipose tissue (mean 3.78 ng/g adipose tissue) compared to the liver (1.48 ng/g adipose tissue) and brain (0.91 ng/g adipose tissue).
112
However, these concentrations are low compared with those found by Olea et al, who detected BPA in adipose tissues collected from eighty-six children (21 girls and 65 boys) in the course of surgical treatment of benign diseases at 17.46±14.82 µg/g of adipose tissue.
113
Metabolic disturbance and obesity
From literature, the studies contradict one another on the effects of BPA, however, the majority concludes the existence of effects at very low doses on energy balance and general homeostasis. An epidemiological study conducted by the NHANES 2003-2008 in the United States shows a correlation between the increase in BPA concentrations in urine and the onset of diabetes in 3967 participants (51.7% women).
114
In the animal model, in utero exposure from day 6 of pregnancy to weaning in drinking water at low doses (0.1 mg/kg BW/day and 1.2 mg/kg BW/day) shows an increase in body weight in adulthood in both sexes with a more pronounced effect in females. The effect is also dose-dependent because the low dose has the most effect.
115
Somm et al, showed that early exposure to BPA affects adipogenesis at adulthood. These effects are more pronounced in female, which demonstrates the determining role of sex hormones.
109
In adult male mice, exposure to BPA via subcutaneous injections (for 8 days) at relatively low doses (100 μg/kg BW/day) decreases energy metabolism which results in a deficiency in insulin signaling at the peripheral level (especially at the muscular level). Bodyweight was not affected by decreased food intake, decreased body temperature, and locomotor activity, suggesting that BPA can be considered as a risk factor for the development of diabetes.
116
In ovariectomized adult Sprague-Dawley females, exposure to BPA at doses of 4 mg/d or 5 mg/d via mini pumps implanted at the dorsal level for 15 days showed a decrease in weight gain, with preferential accumulation at the level of brown adipose tissue.
15
Despite the effects of BPA on the energy balance, the mechanisms remain unclear. Xu et alshow a variation in sweetness preference (saccharin and sucrose) after in utero exposure at low doses of BPA (0.1 mg/kg BW/day, 1 mg/kg BW/day). This increase in sugar intake means an increase in energy intake that increases body weight.
17
Interactions in the neuroendocrine system that control the energy balance on the one hand and reproduction on the other can be the target of endocrine disruptors, which may explain these effects.
117
In vitro, Phrakonkham has shown that BPA affects the expression of genes for adipocyte differentiation in 3T3-L1, without altering the accumulation of triglycerides.
44
Angel Nadal's team shows that low-dose BPA (1 nM) decreases potassium channel activity, increases insulin secretion in mouse or human pancreatic cells - with a more pronounced effect on human pancreatic cells. This means that BPA at very low doses can alter glucose homeostasis.
118
This analysis suggests that BPA at low doses alone or in mixtures can have other effects.
Conclusion
The dramatic increase in obesity incidence has occurred in parallel with a dramatic increase in the use of plastic and food containing EDCs. Currently, scientific research and data on the relationship between obesity appearance and development with exposure to EDCs are increasing. Epidemiological, in vivo experimental animal, in vito and in silico studies research relating environmental chemical exposure to obesity has been increased. Findings from these studies have shown that EDCs exposure can cause an increase in cells number and size; alter endocrine regulation of adipose tissue development, alter hormone regulating appetite, satiety and food preferences; alter basic metabolic rate; alter energy balance in favor of storing calories; alter insulin sensitivity, lipid metabolism in endocrine tissues.
119
Studies showing a relationship between accelerated postnatal growth and obesity appearance due to developmental exposure to daily doses of BPA or others EDCs within the range of human exposure provide a strong argument for competent authorities and policies to review the reliance on the use of EDCs in food.
Concluding Remarks
For some years, a large number of studies have shown a strong correlation between exposure to EDCs and the development of obesity and metabolic disorders. However, and with all these data that we have until the day, makes this research area “EDCs vs obesity” even more complex and involves no longer considering the biological effects independently on just one target type (e.g., fat tissue, brain, pancreas, thyroid, etc.) but according to an integrative approach to the effect of exposure to cocktails mimicking environmental conditions and including molecular, behavioral and ultra-structural, histological analyses.
There are a number of gaps in our knowledge around “EDCs vs obesity” research area and would benefit from further research, taking into consideration the recommendations that we have developed here: (i) Research to develop integrative approaches (exposure to cocktails) mimicking environmental conditions. (ii) It would be helpful to consider the biological effects of EDCs on several target types (e.g., fat tissue, brain). (iii) It would be useful to re-evaluate acceptable levels of exposure or releases to the environment that is protective of human health and the environment according to the new data.
Acknowledgements
The authors gratefully acknowledge Dr. Bassim TAZIR (Max Planck Institute, Germany) for his pertinent comments on the manuscript.
Funding sources
The financial support from the Algerian Ministry of Higher Education and Scientific Research is gratefully acknowledged ((grant agreement no: D00L01UN240120180001).
Ethical statement
None to be declared.
Competing interests
The author declares no conflict interests.
Authors’ contribution
SB and MCCL have chosen the theme of the review, conceived the original idea, and supervised the project. SB, AB and MO collected the data and contributed to the conceptualization of the manuscript and the overall writing and editing of the manuscript. BB has contributed to manuscript revision and editing. All authors discussed the review topic, contents and contributed to the final manuscript.
Review Highlights
What is the current knowledge?
simple
-
√ Endocrine disruptors (EDCs) can predispose newborns to endocrine dysfunction of adipocytes and obesity.
-
√ EDCs mixtures, at very low doses, can induce more pronounced effects on adipose tissue.
-
√ EDCs mixtures can induce trans-generational effects on adipose tissue.
What is new here?
simple
-
√ The necessity to considering the biological effects of EDCs independently on just one target type (e.g., fat tissue, brain).
-
√ Use an integrative approach (exposure to cocktails) mimicking environmental conditions and including molecular, behavioral and ultra-structural, histological analyses.
References
- Ferguson SA, Delclos KB, Newbold RR, Flynn KM. Dietary ethinyl estradiol exposure during development causes increased voluntary sodium intake and mild maternal and offspring toxicity in rats. Neurotoxicol Teratol 2003; 25:491-501. doi: 10.1016/S0892-0362(03)00015-1 [Crossref] [ Google Scholar]
- Geary N. Estradiol, CCK and satiation. Peptides 2001; 22:1251-63. doi: 10.1016/S0196-9781(01)00449-1 [Crossref] [ Google Scholar]
- Curtis KS, Davis LM, Johnson AL, Therrien KL, Contreras RJ. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol Behav 2004; 80:657-64. doi: 10.1016/j.physbeh.2003.11.007 [Crossref] [ Google Scholar]
- Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol 2003; 179:293-9. doi: 10.1677/joe.0.1790293 [Crossref] [ Google Scholar]
- Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ Res 2011; 111:825-30. doi: 10.1016/j.envres.2011.05.014 [Crossref] [ Google Scholar]
- Li J, Lai H, Chen S, Zhu H, Lai S. Gender differences in the associations between urinary bisphenol A and body composition among American children: The National Health and Nutrition Examination Survey, 2003-2006. J Epidemiol 2017; 27:228-34. doi: 10.1016/j.je.2016.12.001 [Crossref] [ Google Scholar]
- Shankar A, Teppala S, Sabanayagam C. Urinary bisphenol a levels and measures of obesity: results from the national health and nutrition examination survey 2003-2008. ISRN Endocrinol 2012; 2012:965243. doi: 10.5402/2012/965243 [Crossref] [ Google Scholar]
- Covaci A, Hond ED, Geens T, Govarts E, Koppen G, Frederiksen H. Urinary BPA measurements in children and mothers from six European member states: Overall results and determinants of exposure. Environ Res 2015; 141:77-85. doi: 10.1016/j.envres.2014.08.008 [Crossref] [ Google Scholar]
- Mustieles V, Casas M, Ferrando-Marco P, Ocón-Hernández O, Reina-Pérez I, Rodríguez-Carrillo A. Bisphenol A and adiposity measures in peripubertal boys from the INMA-Granada cohort. Environ Res 2019; 173:443-51. doi: 10.1016/j.envres.2019.03.045 [Crossref] [ Google Scholar]
- Heindel JJ. Endocrine Disruptors and the Obesity Epidemic. Toxicol Sci 2003; 76:247-9. doi: 10.1093/toxsci/kfg255 [Crossref] [ Google Scholar]
- Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Perinatal exposure to environmental estrogens and the development of obesity. Mol Nutr Food Res 2007; 51:912-7. doi: 10.1002/mnfr.200600259 [Crossref] [ Google Scholar]
- Boudalia S, Berges R, Chabanet C, Folia M, Decocq L, Pasquis B. A multi-generational study on low-dose BPA exposure in Wistar rats: effects on maternal behavior, flavor intake and development. Neurotoxicol Teratol 2014; 41:16-26. doi: 10.1016/j.ntt.2013.11.002 [Crossref] [ Google Scholar]
- Silva BS, Bertasso IM, Pietrobon CB, Lopes BP, Santos TR, Peixoto-Silva N. Effects of maternal bisphenol A on behavior, sex steroid and thyroid hormones levels in the adult rat offspring. Life Sci 2019; 218:253-64. doi: 10.1016/j.lfs.2018.12.039 [Crossref] [ Google Scholar]
- Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology 2003; 144:3575-85. doi: 10.1210/en.2003-0320 [Crossref] [ Google Scholar]
- Nunez AA, Kannan K, Giesy JP, Fang J, Clemens LG. Effects of Bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere 2001; 42:917-22. doi: 10.1016/S0045-6535(00)00196-X [Crossref] [ Google Scholar]
- Folia M, Boudalia S, Ménétrier F, Decocq L, Pasquis B, Schneider C. Oral homeostasis disruption by medical plasticizer component bisphenol A in adult male rats. Laryngoscope 2013; 123:1405-10. doi: 10.1002/lary.23791 [Crossref] [ Google Scholar]
- Xu X, Tan L, Himi T, Sadamatsu M, Tsutsumi S, Akaike M. Changed preference for sweet taste in adulthood induced by perinatal exposure to bisphenol A—A probable link to overweight and obesity. Neurotoxico Teratol 2011; 33:458-63. doi: 10.1016/j.ntt.2011.06.002 [Crossref] [ Google Scholar]
- Kouidhi W, Berges R, Tiffon C, Desmetz C, El May M, Auger J. Perinatal xenohormone exposure impacts sweet preference and submandibular development in male rats. Oral Dis 2013; 19:812-23. doi: 10.1111/odi.12078 [Crossref] [ Google Scholar]
- Darbre PD. The history of endocrine-disrupting chemicals. Current Opinion in Endocrine and Metabolic Research 2019; 7:26-33. doi: 10.1016/j.coemr.2019.06.007 [Crossref] [ Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol 2009; 304:84-9. doi: 10.1016/j.mce.2009.02.024 [Crossref] [ Google Scholar]
- Boudalia S, Belloir C, Miller ML, Canivenc-Lavier MC. Early endocrine disruptors exposure acts on 3T3-L1 differentiation and endocrine activity. Bioimpacts 2017; 7:83-9. doi: 10.15171/bi.2017.11 [Crossref] [ Google Scholar]
-
C.C.E. Stratégie communautaire concernant les perturbateurs endocriniens: une série de substances suspectées d'influer sur le système hormonal des hommes et des animaux. Bruxelles: Commission des Communautés Européennes; 1999.
-
WHO-IPCS. World Health Organization, International Programme on Chemical Safety (WHO-IPCS), 2002. In: Damstra T, Barlow S, Bergman A, Kavlock R, Van Der Kraak G. (Eds.). Global Assessment of the State-of-the-Science of Endocrine Disrupters. Geneva: World Health Organization; 2002.
-
WHO-UNEP.. United Nations Environment Programme (WHO-UNEP). In: Bergman A, Heindel JJ, Jobling S, Kidd KA, Zoeller RT, eds. State of the Science of Endocrine Disrupting Chemicals. World Health Organization; 2012.
- Solecki R, Kortenkamp A, Bergman A, Chahoud I, Degen GH, Dietrich D. Scientific principles for the identification of endocrine-disrupting chemicals: a consensus statement. Arch Toxicol 2017; 91:1001-6. doi: 10.1007/s00204-016-1866-9 [Crossref] [ Google Scholar]
- Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr Rev 2009; 30:293-342. doi: 10.1210/er.2009-0002 [Crossref] [ Google Scholar]
-
C.C.E. Endocrine disruptors: study on gathering information on 435 substances with insufficient data. Bruxelles: Commission des Communautés Européennes ; 2002.
-
Colborn T. TEDX list of potential endocrine disruptors, the endocrine disruptor exchange. Available from: http://www.endocrinedisruption.com/endocrine.TEDXList.overview.php. Accessed August 28, 2019].
- Massaad C, Barouki R. Xénohormones : mode d'action et effets suspectés. MS Médecine Ssciences 1999; 15:1362-9. [ Google Scholar]
- Tabb MM, Blumberg B. New Modes of Action for Endocrine-Disrupting Chemicals. Molecular Endocrinology 2006; 20:475-82. doi: 10.1210/me.2004-0513 [Crossref] [ Google Scholar]
- Moore LB, Maglich JM, McKee DD, Wisely B, Willson TM, Kliewer SA. Pregnane X Receptor (PXR), Constitutive Androstane Receptor (CAR), and Benzoate X Receptor (BXR) Define Three Pharmacologically Distinct Classes of Nuclear Receptors. Mol Endocrinol 2002; 16:977-86. doi: 10.1210/mend.16.5.0828 [Crossref] [ Google Scholar]
- Wyde ME, Bartolucci E, Ueda A, Zhang H, Yan B, Negishi M. The Environmental Pollutant 1,1-Dichloro-2,2-bis (p-chlorophenyl)ethylene Induces Rat Hepatic Cytochrome P450 2B and 3A Expression through the Constitutive Androstane Receptor and Pregnane X Receptor. Mol Pharmacol 2003; 64:474-81. doi: 10.1124/mol.64.2.474 [Crossref] [ Google Scholar]
- Rasier G, Parent A-S, Gérard A, Denooz R, Lebrethon M-C, Charlier C. Mechanisms of Interaction of Endocrine-Disrupting Chemicals with Glutamate-Evoked Secretion of Gonadotropin-Releasing Hormone. Toxicol Sci 2007; 102:33-41. doi: 10.1093/toxsci/kfm285 [Crossref] [ Google Scholar]
- Lonard DM, Tsai SY, O'Malley BW. Selective Estrogen Receptor Modulators 4-Hydroxytamoxifen and Raloxifene Impact the Stability and Function of SRC-1 and SRC-3 Coactivator Proteins. Mol Cell Biol 2004; 24:14-24. doi: 10.1128/mcb.24.1.14-24.2004 [Crossref] [ Google Scholar]
- Inoshita H, Masuyama H, Hiramatsu Y. The different effects of endocrine-disrupting chemicals on estrogen receptor-mediated transcription through interaction with coactivator TRAP220 in uterine tissue. J Mol Endocrinol 2003; 31:551-61. doi: 10.1677/jme.0.0310551 [Crossref] [ Google Scholar]
- Syvälä H, Vienonen A, Zhuang Y-H, Kivineva M, Ylikomi T, Tuohimaa P. Evidence for enhanced ubiquitin-mediated proteolysis of the chicken progesterone receptor by progesterone. Life Sci 1998; 63:1505-12. doi: 10.1016/S0024-3205(98)00417-2 [Crossref] [ Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic Transgenerational Actions of Endocrine Disruptors and Male Fertility. Science 2005; 308:1466-9. doi: 10.1126/science.1108190 [Crossref] [ Google Scholar]
- Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends in Pharmacological Sciences 2005; 26:244-51. doi: 10.1016/j.tips.2005.03.003 [Crossref] [ Google Scholar]
- Xie YI, Yang Q, DePierre JW. The Effects of Peroxisome Proliferators on Global Lipid Homeostasis and the Possible Significance of These Effects to Other Responses to These Xenobiotics. Ann N Y Acad Sci 2002; 973:17-25. doi: 10.1111/j.1749-6632.2002.tb04600.x [Crossref] [ Google Scholar]
- Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. Journal of advanced pharmaceutical Technology & Research 2011; 2:236-40. doi: 10.4103/2231-4040.90879 [Crossref] [ Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An Antidiabetic Thiazolidinedione Is a High Affinity Ligand for Peroxisome Proliferator-activated Receptor γ (PPARγ). J Biol Chem 1995; 270:12953-6. [ Google Scholar]
- Staels B, Fruchart J-C. Therapeutic Roles of Peroxisome Proliferator–Activated Receptor Agonists. Diabetes 2005; 54:2460. doi: 10.2337/diabetes.54.8.2460 [Crossref] [ Google Scholar]
- Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J-i. Organotin Compounds Promote Adipocyte Differentiation as Agonists of the Peroxisome Proliferator-Activated Receptor γ/Retinoid X Receptor Pathway. Mol Pharmacol 2005; 67:766-74. doi: 10.1124/mol.104.008409 [Crossref] [ Google Scholar]
- Phrakonkham P, Viengchareun S, Belloir C, Lombès M, Artur Y, Canivenc-Lavier M-C. Dietary xenoestrogens differentially impair 3T3-L1 preadipocyte differentiation and persistently affect leptin synthesis. J Steroid Biochem Mol Biol 2008; 110:95-103. doi: 10.1016/j.jsbmb.2008.02.006 [Crossref] [ Google Scholar]
- Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environmen Health Perspect 2011; 119:1227-32. doi: 10.1289/ehp.1003328 [Crossref] [ Google Scholar]
- Jedeon K, Marciano C, Loiodice S, Boudalia S, Canivenc Lavier MC, Berdal A. Enamel hypomineralization due to endocrine disruptors. Connect Tissue Res 2014; 55 Suppl 1:43-7. doi: 10.3109/03008207.2014.923857 [Crossref] [ Google Scholar]
- Gioiosa L, Palanza P, Parmigiani S, Vom Saal FS. Risk Evaluation of Endocrine-Disrupting Chemicals: Effects of Developmental Exposure to Low Doses of Bisphenol A on Behavior and Physiology in Mice (Mus musculus). Dose Response 2015; 13:1559325815610760. doi: 10.1177/1559325815610760 [Crossref] [ Google Scholar]
- Magos L. Magos LRSHYang (ed)Toxicology of chemical mixtures case studies, mechanisms, and novel approachesAcademic Press, San Diego, 1994; 720 pp, $1750. J Appl Toxicol 1995; 15:243. doi: 10.1002/jat.2550150317 [Crossref] [ Google Scholar]
- Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures I Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environmen Health Perspect 2003; 111:994-1006. doi: 10.1289/ehp.5494 [Crossref] [ Google Scholar]
- vom Saal FS, Welshons WV. NIH panel confirms that endocrine disrupting chemicals cause effects at very low doses (via Inside EPAcom, access date: 2 January 2005). Risk Pol Rep 2000; 7:47-50. [ Google Scholar]
-
WHO-UNEP. State of the science of endocrine disrupting chemicals. Bergman, Å. Heindel, J. Jobling, S. Kidd, K. Zoeller, R. T. (Eds.): World Health Organization (WHO), United Nations Environment Programme (UNEP) 2012 Contract No.: 978-92-807-3274-0 (UNEP) and 9789241505031 (WHO). Available at: https://www.who.int/publications/i/item/state-of-the-science-of-endocrine-disrupting-chemicals. Accessed 21.06.2021.
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 2012; 33:378-455. doi: 10.1210/er.2011-1050 [Crossref] [ Google Scholar]
- Auxietre T-A, Dumontier M-F, Balguy I, Frapart Y, Canivenc-Lavier M-C, Berges R. Sub-NOAEL amounts of vinclozolin and xenoestrogens target rat chondrogenesis in vivo. Biochimie 2014; 99:169-77. doi: 10.1016/j.biochi.2013.12.001 [Crossref] [ Google Scholar]
- Schug TT, Johnson AF, Birnbaum LS, Colborn T, Guillette LJ Jr, Crews DP. Minireview: Endocrine Disruptors: Past Lessons and Future Directions. Mol Endocrinol 2016; 30:833-47. doi: 10.1210/me.2016-1096 [Crossref] [ Google Scholar]
- Oudir M, Chader H, Bouzid B, Bendisari K, Latreche B, Boudalia S. Male rat exposure to low dose of di(2-ethylhexyl) phthalate during pre-pubertal, pubertal and post-pubertal periods: Impact on sperm count, gonad histology and testosterone secretion. Reprod Toxicol 2018; 75:33-9. doi: 10.1016/j.reprotox.2017.11.004 [Crossref] [ Google Scholar]
- Andrade AJM, Grande SW, Talsness CE, Grote K, Chahoud I. A dose–response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): Non-monotonic dose–response and low dose effects on rat brain aromatase activity. Toxicology 2006; 227:185-92. doi: 10.1016/j.tox.2006.07.022 [Crossref] [ Google Scholar]
- Lemini C, Hernández A, Jaimez R, Franco Y, Avila ME, Castell A. Morphometric analysis of mice uteri treated with the preservatives methyl, ethyl, propyl, and butylparaben. Toxicol Ind Health 2004; 20:123-32. doi: 10.1191/0748233704th202oa [Crossref] [ Google Scholar]
-
Anton R, Barlow S, Boskou D, Castle L, Crebelli R, Dekant W, et al. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a Request from the Commission related to para hydroxybenzoates (E 214-219). 2004.
-
EPA. Tributyltin Oxide (CAS No. 56-35-9)1997.
- Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol 2010; 24:526-39. doi: 10.1210/me.2009-0261 [Crossref] [ Google Scholar]
- Ahmed RG, Walaa GH, Asmaa FS. Suppressive effects of neonatal bisphenol A on the neuroendocrine system. Toxicol Ind Health 2018; 34:397-407. doi: 10.1177/0748233718757082 [Crossref] [ Google Scholar]
- Barker D, Eriksson J, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002; 31:1235-9. doi: 10.1093/ije/31.6.1235 [Crossref] [ Google Scholar]
- Heindel JJ. The fetal basis of adult disease: Role of environmental exposures—introduction. Birth Defects Res A Clin Mol Teratol 2005; 73:131-2. doi: 10.1002/bdra.20119 [Crossref] [ Google Scholar]
- Jedeon K, De la Dure-Molla M, Brookes SJ, Loiodice S, Marciano C, Kirkham J. Enamel defects reflect perinatal exposure to bisphenol A. Am J Pathol 2013; 183:108-18. doi: 10.1016/j.ajpath.2013.04.004 [Crossref] [ Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Int J Androl 2008; 31:201-8. doi: 10.1111/j.1365-2605.2007.00858.x [Crossref] [ Google Scholar]
- Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to Environmentally Relevant Doses of the Xenoestrogen Bisphenol-A Alters Development of the Fetal Mouse Mammary Gland. Endocrinology 2007; 148:116-27. doi: 10.1210/en.2006-0561 [Crossref] [ Google Scholar]
- Ahmed RG. Maternal bisphenol A alters fetal endocrine system: Thyroid adipokine dysfunction. Food Chem Toxicol 2016; 95:168-74. doi: 10.1016/j.fct.2016.06.017 [Crossref] [ Google Scholar]
- Ahmed RG, El-Gareib AW, Shaker HM. Gestational 3,3',4,4',5-pentachlorobiphenyl (PCB 126) exposure disrupts fetoplacental unit: Fetal thyroid-cytokines dysfunction. Life Sci 2018; 192:213-20. doi: 10.1016/j.lfs.2017.11.033 [Crossref] [ Google Scholar]
- Ahmed RG. Early weaning PCB 95 exposure alters the neonatal endocrine system: thyroid adipokine dysfunction. J Endocrinol 2013; 219:205-15. doi: 10.1530/JOE-13-0302 [Crossref] [ Google Scholar]
- Ahmed RG. Perinatal TCDD exposure alters developmental neuroendocrine system. Food Chem Toxicol 2011; 49:1276-84. doi: 10.1016/j.fct.2011.03.008 [Crossref] [ Google Scholar]
-
Skinner MK. Epigenetic Transgenerational Actions of Endocrine Disruptors through the Male Germ-Line. In: Diana Anderson MHB, editor. in: Anderson D, Brinkworth MH, eds. Male-mediated Developmental Toxicity. RSC Publishing; 2007. 10.1039/9781847557643
- Singh S, Li SS. Epigenetic effects of environmental chemicals bisphenol A and phthalates. Int J Mol Sci 2012; 13:10143-53. doi: 10.3390/ijms130810143 [Crossref] [ Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One 2013; 8:e55387. doi: 10.1371/journal.pone.0055387 [Crossref] [ Google Scholar]
- Xin F, Susiarjo M, Bartolomei MS. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation?. Semin Cell Dev Biol 2015; 43:66-75. doi: 10.1016/j.semcdb.2015.05.008 [Crossref] [ Google Scholar]
-
Bowman JD, Rahman SM, Choudhury M. Endocrine Disruptors and Epigenetics In: Mandal SS, editor. Gene Regulation, Epigenetics and Hormone Signaling. 2017. p. 577-606.
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational Epigenetic Programming of the Brain Transcriptome and Anxiety Behavior. PLoS One 2008; 3:e3745. doi: 10.1371/journal.pone.0003745 [Crossref] [ Google Scholar]
- Kortenkamp A, Faust M, Scholze M, Backhaus T. Low-level exposure to multiple chemicals: reason for human health concerns?. Environmen Health Perspect 2007; 115 Suppl 1:106-14. doi: 10.1289/ehp.9358 [Crossref] [ Google Scholar]
- Ribeiro E, Ladeira C, Viegas S. EDCs Mixtures: A Stealthy Hazard for Human Health?. Toxics 2017; 5. doi: 10.3390/toxics5010005 [Crossref]
- Kortenkamp A, Altenburger R. Synergisms with mixtures of xenoestrogens: A reevaluation using the method of isoboles. Science of The Total Environment 1998; 221:59-73. doi: 10.1016/S0048-9697(98)00261-7 [Crossref] [ Google Scholar]
- Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environmen Health Perspect 2007; 115 Suppl 1:98-105. doi: 10.1289/ehp.9357 [Crossref] [ Google Scholar]
- Payne J, Rajapakse N, Wilkins M, Kortenkamp A. Prediction and assessment of the effects of mixtures of four xenoestrogens. Environmen Health Perspect 2000; 108:983-7. doi: 10.1289/ehp.00108983 [Crossref] [ Google Scholar]
- Eustache F, Mondon F, Canivenc-Lavier MC, Lesaffre C, Fulla Y, Berges R. Chronic dietary exposure to a low-dose mixture of genistein and vinclozolin modifies the reproductive axis, testis transcriptome, and fertility. Environmen Health Perspect 2009; 117:1272-9. doi: 10.1289/ehp.0800158 [Crossref] [ Google Scholar]
- Lehraiki A, Messiaen S, Berges R, Canivenc-Lavier M-C, Auger J, Habert R. Antagonistic effects of gestational dietary exposure to low-dose vinclozolin and genistein on rat fetal germ cell development. Reprod Toxicol 2011; 31:424-30. doi: 10.1016/j.reprotox.2010.12.005 [Crossref] [ Google Scholar]
- Saad HES, Meduri G, Phrakonkham P, Bergès R, Vacher S, Djallali M. Abnormal peripubertal development of the rat mammary gland following exposure in utero and during lactation to a mixture of genistein and the food contaminant vinclozolin. Reprod Toxicol 2011; 32:15-25. doi: 10.1016/j.reprotox.2011.03.001 [Crossref] [ Google Scholar]
- Kouidhi W, Desmetz C, Nahdi A, Bergès R, Cravedi J-P, Auger J. In Utero and Lactational Exposure to Low-Dose Genistein-Vinclozolin Mixture Affects the Development and Growth Factor mRNA Expression of the Submandibular Salivary Gland in Immature Female Rats. Toxicol Pathol 2012; 40:593-604. doi: 10.1177/0192623311436183 [Crossref] [ Google Scholar]
- Shafei AE-S, Nabih ES, Shehata KA, Abd Elfatah ESM, Sanad AbA, Marey MY. Prenatal Exposure to Endocrine Disruptors and Reprogramming of Adipogenesis: An Early-Life Risk Factor for Childhood Obesity. Childhood Obesity 2017; 14:18-25. doi: 10.1089/chi.2017.0180 [Crossref] [ Google Scholar]
- Matsuzawa Y. White adipose tissue and cardiovascular disease. Best Pract Res Clin Endocrinol Metab 2005; 19:637-47. doi: 10.1016/j.beem.2005.07.001 [Crossref] [ Google Scholar]
- Monjo M, Pujol E, Roca P. α2- to β3-Adrenoceptor switch in 3T3-L1 preadipocytes and adipocytes: modulation by testosterone, 17β-estradiol, and progesterone. Am J Physiol Endocrinol Metab 2005; 289:E145-E50. doi: 10.1152/ajpendo.00563.2004 [Crossref] [ Google Scholar]
- Butte NF, Hopkinson JM, Wong WW, Smith EOB, Ellis KJ. Body Composition during the First 2 Years of Life: An Updated Reference. Pediatr Res 2000; 47:578-85. doi: 10.1203/00006450-200005000-00004 [Crossref] [ Google Scholar]
- Taylor RW, Jones IE, Williams SM, Goulding A. Body fat percentages measured by dual-energy X-ray absorptiometry corresponding to recently recommended body mass index cutoffs for overweight and obesity in children and adolescents aged 3–18 y. Am J Clin Nutr 2002; 76:1416-21. doi: 10.1093/ajcn/76.6.1416 [Crossref] [ Google Scholar]
- Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr 2000; 130:3122S-6S. [ Google Scholar]
- Tang Q-Q, Zhang J-W, Daniel Lane M. Sequential gene promoter interactions of C/EBPbeta, C/EBPalpha, and PPARgamma during adipogenesis. Biochem Biophys Res Commun 2004; 319:235-9. [ Google Scholar]
- Fu M, Sun T, Bookout AL, Downes M, Yu RT, Evans RM. A Nuclear Receptor Atlas: 3T3-L1 Adipogenesis. Mol Endocrinol 2005; 19:2437-50. doi: 10.1210/me.2004-0539 [Crossref] [ Google Scholar]
- Bouchard C, Després J-P, Mauriège P. Genetic and Nongenetic Determinants of Regional Fat Distribution. Endocr Rev 1993; 14:72-93. doi: 10.1210/edrv-14-1-72 [Crossref] [ Google Scholar]
- Dieudonné MN, Pecquery R, Boumediene A, Leneveu MC, Giudicelli Y. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am J Physiol Cell Physiol 1998; 274:C1645-C52. doi: 10.1152/ajpcell.1998.274.6.C1645 [Crossref] [ Google Scholar]
- Dieudonné MN, Leneveu MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors α and β in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol 2004; 286:C655-C61. doi: 10.1152/ajpcell.00321.2003 [Crossref] [ Google Scholar]
- Cooke PS, Naaz A. Role of Estrogens in Adipocyte Development and Function. Exp Biol Med 2004; 229:1127-35. doi: 10.1177/153537020422901107 [Crossref] [ Google Scholar]
- Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H, Kato S. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem Biophys Res Commun 2003; 300:167-71. doi: 10.1016/S0006-291X(02)02774-2 [Crossref] [ Google Scholar]
- Faustini-Fustini M, Rochira V, Carani C. Oestrogen deficiency in men: where are we today?. Eur J Endocrinol 1999; 140:111-29. [ Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America 2000; 97:12729-34. doi: 10.1073/pnas.97.23.12729 [Crossref] [ Google Scholar]
- Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environmen Health Perspect 2007; 115 Suppl 1:69-76. doi: 10.1289/ehp.9368 [Crossref] [ Google Scholar]
- Kang J-H, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology 2006; 226:79-89. doi: 10.1016/j.tox.2006.06.009 [Crossref] [ Google Scholar]
- vom Saal FS, Welshons WV. Large effects from small exposures II The importance of positive controls in low-dose research on bisphenol A. Environmental Research 2006; 100:50-76. doi: 10.1016/j.envres.2005.09.001 [Crossref] [ Google Scholar]
-
Myers P, Hessler W. Does 'the dose make the poison?'. Available from: https://endocrinedisruption.org/assets/media/documents/2007-04-30_does_the_dose_make_the_poison.pdf. 2007.
- Takemura H, Ma J, Sayama K, Terao Y, Zhu BT, Shimoi K. In vitro and in vivo estrogenic activity of chlorinated derivatives of bisphenol A. Toxicology 2005; 207:215-21. doi: 10.1016/j.tox.2004.09.015 [Crossref] [ Google Scholar]
- Stroheker T, Chagnon M-C, Pinnert M-F, Berges R, Canivenc-Lavier M-C. Estrogenic effects of food wrap packaging xenoestrogens and flavonoids in female Wistar rats: a comparative study. Reprod Toxicol 2003; 17:421-32. doi: 10.1016/S0890-6238(03)00044-3 [Crossref] [ Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB. Association of Urinary Bisphenol A Concentration With Medical Disorders and Laboratory Abnormalities in Adults. JAMA 2008; 300:1303-10. doi: 10.1001/jama.300.11.1303 [Crossref] [ Google Scholar]
- Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One 2010; 5:e8673-e. doi: 10.1371/journal.pone.0008673 [Crossref] [ Google Scholar]
- Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect 2009; 117:1549-55. doi: 10.1289/ehp.11342 [Crossref] [ Google Scholar]
- Yokota H, Iwano H, Endo M, Kobayashi T, Inoue H, Ikushiro S. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem J 1999; 340:405-9. [ Google Scholar]
- Fernandez MF, Arrebola JP, Taoufiki J, Navalón A, Ballesteros O, Pulgar R. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol 2007; 24:259-64. doi: 10.1016/j.reprotox.2007.06.007 [Crossref] [ Google Scholar]
- Geens T, Neels H, Covaci A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012; 87:796-802. doi: 10.1016/j.chemosphere.2012.01.002 [Crossref] [ Google Scholar]
- Olea N, Arrebola JP, Taoufiki J, Fernández-Valades R, Prada R, Navea N. Alkylphenols and bisphenol-A and its chlorinated derivatives in adipose tissue of children. WIT Transactions on Ecology and the Environment 2008; 110:129-138. [ Google Scholar]
- Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab 2011; 96:3822-6. doi: 10.1210/jc.2011-1682 [Crossref] [ Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environmen Health Perspect 2001; 109:675-80. doi: 10.1289/ehp.01109675 [Crossref] [ Google Scholar]
- Batista TM, Alonso-Magdalena P, Vieira E, Amaral MEC, Cederroth CR, Nef S. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS One 2012; 7:e33814-e. doi: 10.1371/journal.pone.0033814 [Crossref] [ Google Scholar]
- Bourguignon J-P, Rasier G, Lebrethon M-C, Gérard A, Naveau E, Parent A-S. Neuroendocrine disruption of pubertal timing and interactions between homeostasis of reproduction and energy balance. Mol Cell Endocrinol 2010; 324:110-20. doi: 10.1016/j.mce.2010.02.033 [Crossref] [ Google Scholar]
- Soriano S, Alonso-Magdalena P, García-Arévalo M, Novials A, Muhammed SJ, Salehi A. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor β. PLoS One 2012; 7:e31109-e. doi: 10.1371/journal.pone.0031109 [Crossref] [ Google Scholar]
- Darbre PD. Endocrine Disruptors and Obesity. Curr Obes Rep 2017; 6:18-27. doi: 10.1007/s13679-017-0240-4 [Crossref] [ Google Scholar]