BioImpacts. 10(2):87-95.
doi: 10.34172/bi.2020.11
Original Research
Poly(ethylene glycol)-poly(ε-caprolactone)-based micelles for solubilization and tumor-targeted delivery of silibinin
Ashkan Hassankhani Rad 1, 2, †  , Farshid Asiaee 1, 2, †
, Farshid Asiaee 1, 2, †  , Sevda Jafari 2, 3
, Sevda Jafari 2, 3  , Ali Shayanfar 2
, Ali Shayanfar 2  , Afsaneh Lavasanifar 4
, Afsaneh Lavasanifar 4  , Ommoleila Molavi 3, 5, 2, *
, Ommoleila Molavi 3, 5, 2, * 
Author information:
1Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Faculty of Pharmacy, Tabriz University of Medical Science, Tabriz, Iran
3Biotechnology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, Canada
5Molecular Medicine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
† These authors have made equal contribution to this work.
Abstract
Introduction:
Silibinin is a naturally occurring compound with known positive impacts on prevention and treatment of many types of human illnesses in general and cancer in particular. Silibinin is poorly water soluble which results in its insufficient bioavailability and lack of therapeutic efficacy in cancer. Here, we proposed to examine the potential of micelles composed of poly(ethylene glycol) (PEG) as the hydrophilic block and poly(ε-caprolactone) (PCL), poly(α-benzylcarboxylate-ε-caprolactone) (PBCL), or poly(lactide)-(PBCL) (PLA-PBCL) as hydrophobic blocks for enhancing the water solubility of silibinin and its targeted delivery to tumor.
Methods:
Co-solvent evaporation method was used to incorporate silibinin into PEG-PCL based micelles. Drug release profiles were assessed using dialysis bag method. MTT assay also was used to analyze functional activity of drug delivery in B16 melanoma cells.
Results:
Silibinin encapsulated micelles were shown to be less than 60 nm in size. Among different structures under study, the one with PEG-PBCL could incorporate silibinin with the highest encapsulation efficiency being 95.5%, on average. PEG-PBCL micelles could solubilize 1 mg silibinin in 1 mL water while the soluble amount of silibinin was found to be 0.092 mg/mL in the absence of polymeric micelles. PEG-PBCL micelles provided the sustained release of silibinin indicated with less than 30% release of silibinin within 24 hours. Silibinin encapsulated in PEG-PBCL micelles resulted in growth inhibitory effect in B16 cancer cells which was significantly higher than what observed with free drug.
Conclusion:
Our findings showed that PEG-PBCL micellar nanocarriers can be a useful vehicle for solubilization and targeted delivery of silibinin.
Keywords: Silibinin, Micelle, Copolymer, PEG-PCL, Melanoma
Copyright and License Information
© 2020 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Silibinin is a flavanone with very low toxicity and is the main substance of silymarin. Silibinin is extracted from Silybum marianum seeds.
1,2
This compound is a combination of two diastereomers: Silybin A and Silybin B (Fig. 1) in equal amounts.
3
As silibinin has poor water solubility and high permeability, it is classified as class II drug in Biopharmaceutics Classification System (BCS).
4
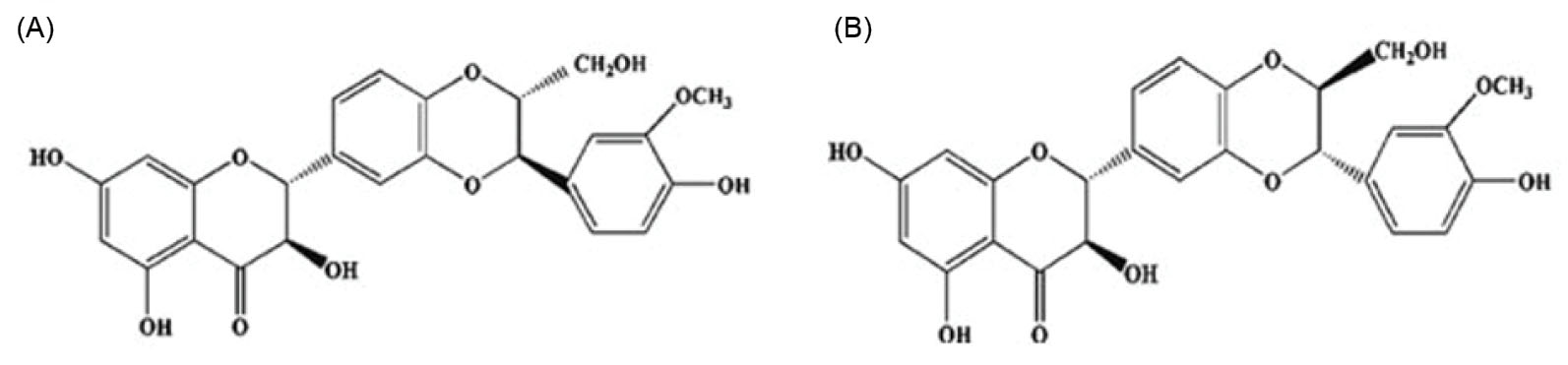
Fig. 1.
Chemical structure of silybin A and B (adopted from Lee et al
5
).
.
Chemical structure of silybin A and B (adopted from Lee et al
5
).
Silibinin is an antioxidant with significant hepatoprotective effects. Silibinin increases the activity and concentration of superoxide dismutase, peroxidase, and glutathione, which all scavenge the free radicals released by hepatocytes.
6,7
Furthermore, an increasing number of in vitro and in vivo studies have shown the remarkable anticancer effects of silibinin. The antitumor effects of silibinin have been reported in various types of cancers including prostate, kidney, bladder, colon, skin, and breast cancers.
1
Induction of apoptosis and cell cycle arrest in cancer cells are the known mechanisms for the anticancer effects of silibinin. Furthermore, studies have shown that silibinin suppresses tumor progression through inhibition of angiogenesis and metastasis.
8
Despite the well-known anticancer effects of silibinin, this poorly water soluble compound has not been moved to clinic for cancer treatment due to its low bioavailability.
9
One of the successful strategies used for solubilization and tumor-targeted delivery of poorly water soluble anticancer agents is the use of polymeric nanocarriers.
10
Nano-sized micelles are a broadly used type of vehicles for tumor-targeted delivery of antineoplastic agents. Co-polymeric micelles are made from amphiphilic polymers through self-assembling mechanism in aqueous solutions where the nonpolar tail of the amphiphilic polymer faces the core and its polar head faces out.
11
Solubilization of poorly water soluble drugs by polymeric micelles is achieved by incorporation of the drug in the hydrophobic core of micelles.
12
Several studies have previously reported that polymeric micelles can significantly enhance the water solubility of poorly water soluble drugs.
10,13,14
The size range of micelles is 20-200 nm. Polymeric micelles can provide passive targeting of the loaded drug to tumor by enhanced permeability and retention (EPR) effect.
15,16
The EPR effect is instigated in tumor because the tumor vasculature is leaky in tumor site and the lymphatic system within tumor is defective. Micelles of 20-200 nm loaded with anticancer drugs enter the tumor environment through the high permeable vessels and they get trapped inside the tumor due to the defects in tumor lymphatic system. EPR effect leads to the accumulation of drug-loaded micelles in tumor, which can provide a high concentration of anticancer drug in tumor microenvironment.
17
Despite extensive published work on the application of micellar nanocarriers for the delivery of hydrophobic drugs, to date, there are only a few micellar formulations available for clinical use.
18
The key for successful micellar formulation is to find an appropriate drug-polymer combination, which can keep its integrity in biological milieu and provide an acceptable drug release profile.
19
Poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) is a biocompatible copolymer. Several publications have reported the successful application of nanocarriers constructed from the PEG-PCL based polymers for the delivery of hydrophobic drugs.
13,20-26
Previous studies have shown that the pharmacokinetics and biodistribution of cyclosporine A is significantly improved by the encapsulation of this drug in PEG-PCL micelles
27,28
Thus, drugs delivered using PEG-PCL with different blocks have improved pharmacokinetics and pharmacological properties of encapsulated drugs including prolonged circulation, reduced distribution in normal organs, and enhanced tumor delivery.
21,25,27,29,30
PEG-PCL copolymer has also been studied for tumor-targeted delivery of several chemotherapeutic drugs. Here, we assessed the potential of micelles made with PEG-PCL copolymer with and without a benzylcarboxylate side chain for the solubilization and delivery of silibinin. The anticancer effects of the developed micelles were evaluated in B16 melanoma cells.
Materials and Methods
Materials
Methoxy polyethylene glycol (Me PEG) with the average molecular weight of 5000 g.mol-1 and di-isopropyl amine (99%) were purchased from Sigma (St. Louis, MO, USA). ε-Caprolactone (CL) was supplied from Lancaster Synthesis (UK). α-Benzylcarboxylate-ε-caprolactone (BCL) was made by Alberta Research Chemicals Inc. (ARCI, Edmonton, AB, Canada). Stannous octoate was obtained from MP Biomedicals Inc. (Germany). Tetrahydrofuran (THF) was from Caledon chemicals (Caledon, Canada). Deionized distilled water was used in all steps of experiment and it was provided from Shahid Ghazi Pharmaceutical Company (Tabriz, Iran). MTT reagent and silibinin were products of Sigma (St. Louis, MO, USA). Methanol, mannitol, dimethyl sulfoxide (DMSO) and all salts used for buffers preparation were from Merck (Germany). The murine B16 melanoma cell line was acquired from Pasteur Institute (Tehran, Iran). RPMI-1640 culture medium and fetal bovine serum (FBS) were from Gibco (Thermo Fisher Scientific, Waltham, MA, USA). The experiments were carried out using class II biological safety cabinet (JAL TAJHIZ, JTLVC2, Karaj, Iran).
Preparation and characterization of polymers
Poly(ethylene glycol)-block-poly(ε-caprolactone) (PEG5000-PCL5200), poly(ethylene glycol)-block-poly(α-benzylcarboxylate-ε-caprolactone) (PEG5000-PBCL4000), and poly(ethylene glycol)-block-poly(lactic acid)-block-poly(α-benzylcarboxylate-ε-caprolactone) (PEG-PLA3000-PBCL2500) were prepared as reported previously.
14,31,32
The synthesis of PEG-PCL and PEG-PBCL polymers was conducted by ring opening polymerization of CL and BCL, respectively. Me PEG served as an initiator and stannous octoate function as a catalyst in this reaction. Briefly, a 10 mL ampoule was loaded with Me PEG, CL or BCL and stannous octoate, then it was nitrogen purged and sealed under vacuum. The ampoule containing the reaction mixture was placed in a preheated oven (140°C) for 4 hours. To synthesize PEG-PLA-PBCL, first PEG-PLA diblock copolymer was synthesized by ring opening polymerization of lactide, using Me PEG and stannous octoate in an ampoule. The reaction mixture incubated at 160°C for 7 hours. Triblock copolymers of PEG-PLA-PBCL were synthesized by ring opening polymerization of BCL in an 8.5 hours reaction at 142°C with PEG-PLA in the presence of a catalyst (i.e. stannous octoate). The 1H NMR spectrum of the synthesized polymers in CDCl3 at 600 MHz was acquired by a Bruker Avance III spectrometer (Bruker BioSpin Corporation, Bil-lerica, MA) and used to determine the number-average molecular weight of the polymers.
Preparation and characterization of micellar formulations of silibinin
Silibinin was loaded in polymeric micelles by co-solvent evaporation method.
10
Briefly, 30 mg of polymer and 1 mg of silibinin powder were dissolved in 0.5 mL and 2 mL THF, respectively. Then, two solutions of drug and polymer were mixed. The mixture of drug and polymer in 2.5 mL THF was added to 7.5 mL of 1% mannitol solution in a drop-wise manner. Mannitol solution was used for its effects on preventing polymer aggregation during the freeze drying.
33
After 24 hours of stirring at 23 ×g in room tempreture (23-25°C) with magnetic stirrer (Heidolph, MR Hei-Tec, Schwabach, Germany), vacum was applied to remove the residual THF. The aqueous solution of micellar formulation was centrifuged (centrifuge Sigma, 2-16KL, Osterode am Harz, Germany) at 4000 ×g for 10 minutes to separate all sediments containing unassembled polymers and free silibinin. In this step, we prepared 7.5 mL of 1% mannitol solution containing micelles loaded with silibinin. This solution was then aliquoted into 1 mL microtubes (each containg 0.5 mL of the micellar solution). The aliquots of micellar solution were then freezed by dipping in liquid nitrogen for few seconds and were kept in freeze-dryer (Telstar, LYOQUEST-55, Barcelona, Spain) for 24 hours. At the end, these freezed formulations were kept for MTT assay. Size and polydispersity index (PDI) of the micelles were measured by light scattering (3000 HAS Zeta sizer Malvern Zeta-Plus TM zeta potential analyzer, Malvern Instrument Ltd., Malvern, UK).
A Leo 906E transmission electron microscope (TEM) (Zeiss, Germany) at 100 kV was used to study the morphology of micellar nanocarriers. A drop of the polymeric micelle solution was placed on a carbon-coated copper grid. To prepare the sample for observation by TEM, it was negatively stained with uranyl acetate and dried completely at room temperature.
Determination of drug loading and encapsulation efficiency
To determine the level of encapsulated silibinin, first the aqueous solution of polymeric micelles were centrifuged at 4000 ×g for 10 minutes to remove free silibinin precipitates. Then, in order to separate free and micelle-incorporated drug, the aqueous solution of micellar formulations were added to centrifugal filter tubes (M. wt. cut-off = 100 000 g.mol−1) and centrifuged at 3000 ×g for 5 minutes. Upon centrifugation, 50 µL of the top layer (the micellar solution) was added to 4.5 mL methanol and vortexed (Vortex Mixer, Velp Scientifica, ZX3, Usmate, Italy) for 15 minutes to disrupt the drug loaded micelles and release the drug from the formulations. The drug concentrations in the samples were measured by UV-Spectroscopy (Shimadzu, UV-1800, Kyoto, Japan) with quartz cuvette and UV probe software. After washing the cuvette with deionized distilled water and methanol, system was calibrated by reading the methanol absorbance on 310 nm. Then, we measured UV absorbance of prepared silibinin standard concentrations in methanol prepared at 1, 5, 10, 20, 25, 50 and 100 µg/mL.
Silibinin loading and encapsulation efficiency were determined using the following equations
14
:
To evaluate the stability of micelles, the prepared silibinin micelles were aliquoted in 1.5 mL vials and kept in refrigerator at 4°C for 7 days. After determined day, the temperature was reached room temperature and silibinin micelles were examined for their stability in size, PDI, and zeta potential.
In vitro release study
Dialysis method was used to study the release of silibinin from the prepared micelles. Spectra/Por dialysis bags (M. wt. cut-off = 12000-14000 g.mol-1) were loaded with each micellar formulation (2 mL) separately, then the bags were sealed with clips and placed in a beaker with 200 mL of phosphate buffer saline (PBS). The whole system was placed on a stirrer at room temperature replacing the system with the same buffer every 2 hours. At various time points, 50 µL samples of the solution were taken from the inside of dialysis bag and diluted in methanol for quantification purpose. The samples were analyzed by UV-Spectrophotometer as described previously. The kinetics of silibinin release from the micelles was determined by applying release data to different mathematical models including Weibull, Higuchi, zero-order, first-order, and Peppas. The parameters of the models were obtained by linear regression. The model with the highest regression coefficient (R2) and the lowest mean percent error was chosen as the best fit to determine the release kinetics of silibinin from PEG-PCL based micelles.
Cell viability
Growth inhibitory effects of PEG-PBCL micellar silibinin and free silibinin were assessed in B16 melanoma cells. This cell line was grown and expanded in RPMI supplemented with 10% FBS. 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide (MTT) assay was used to examine cell viability.
34
B16 melanoma cells were acquired from the culture which was 90%–95% confluent, were seeded at a density of 4000 cells/well in 96-well plates. The plates were incubated (CO2 incubator INCO2, Memmert) overnight. After 24 hours, cells were washed three times with PBS and treated with the drug loaded micelles and free drug at different concentrations. Loaded micelles were dissolved in 1 mL sterile 20% mannitol solution to make a stock concentration of 1 mg/mL. Stock solution was diluted by culture medium to prepare 50, 75, and 100 µM concentrations of silibinin. Moreover, to make the free silibinin, the powder of silibinin was dissolved in DMSO to prepare 10 mg/mL stock solution and was diluted by culture medium to gain 50, 75, and 100 µM concentrations of silibinin in wells, while keeping DMSO concentration
35
at 2.4, 3.6, and 4.8 µL/mL, respectively. To account for potential toxicity of DMSO at the above concentrations, cells were treated with these concentrations of DMSO as a control group. Another control treatment was cells treated with empty PEG-PBCL micelles dissolved in 20% of mannitol and diluted by cell culture. Silibinin is a potent cytotoxic agent and according to dose-response studies, free silibinin at the concentration of 300 µM reduces cell viability to 9 ± 0.7% and can serve as a positive control for MTT assay. The 96-well plates were then transferred into incubator and incubated for 48 hours. Then, culture medium was completely removed from wells and 200 µL of MTT solution (0.5 mg/mL) was added to each well and 96-well plates were incubated for further 4 hours. At the final step, plates were read by ELISA reader (ELISA reader, BioTek, synergy HT) at 570 nm. The following formula was used to calculate cell viability percentages:
The MTT assay was repeated three times and the data were analyzed by GraphPad Prism software. IC50 (the half-maximal inhibitory concentration) for the cytotoxic effects of free drug and micellar formulation was calculated again by GraphPad Prism software.
Statistical analysis
In the present work, each experiment was repeated three times and all data were shown by mean ± standard deviation (SD). Difference between the mean values was analyzed by two-way ANOVA, followed by Tukey post hoc test. P value < 0.05 was accepted as the level of statistical significance.
Results
Characterization of polymers
1H NMR spectra of all three synthesized polymers are shown in Fig. 2. To determine the number- average molecular weight of PEG-PCL polymer, the peak intensity of PEG (-OCH2CH2-, δ = 3.66 ppm) was compared to the peak intensity of PCL or PBCL (-OCH2-, δ = 4.08 ppm) considering a 5000 g.mol−1 molecular weight for PEG. In the case of the triblock copolymer, to determine the molecular weight of PLA from 1H NMR spectra, the peak intensity ratio of methane protons of the PLA segment (-COCH(CH3)O-: δ = 5.18 ppm) and the methylene protons of Me PEG (-OCH2CH2-: δ = 3.67 ppm) were examined. The molecular weight of PBCL block was determined from 1H NMR spectra by examining the peak intensity ratio of methylene protons of PBCL segment (-OCH2-: δ = 4.1 ppm) and the methylene protons of Me PEG (-OCH2CH2-: δ = 3.67 ppm).
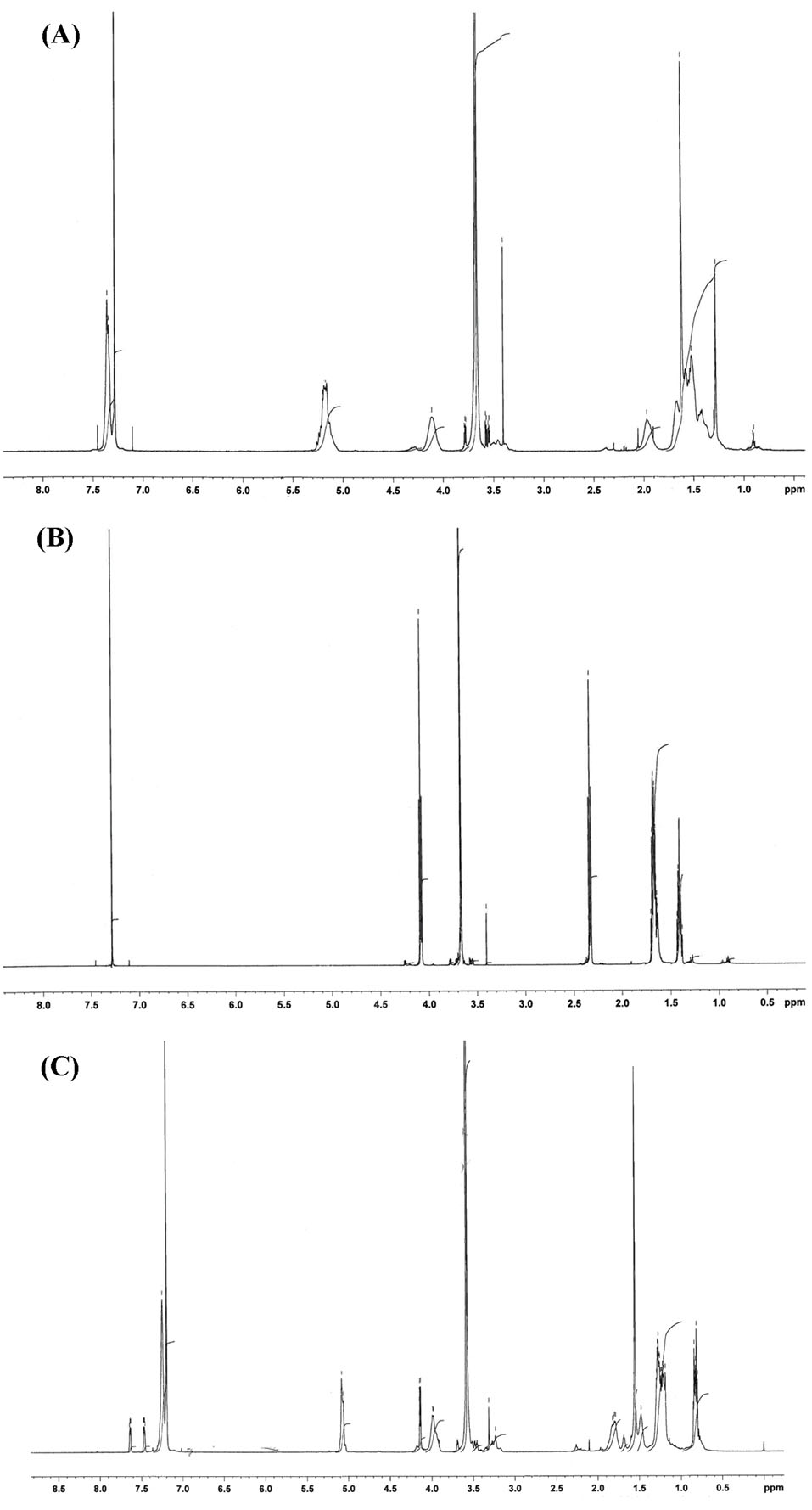
Fig. 2.
The 1H NMR spectrum of (a) PEG-PLA-PBCL, (b) PEG-PCL, and (c) PEG-PBCL.
.
The 1H NMR spectrum of (a) PEG-PLA-PBCL, (b) PEG-PCL, and (c) PEG-PBCL.
Characteristics of micellar formulation of silibinin
Physicochemical characteristics of micellar nanocarriers including size, PDI, silibinin encapsulation, and loading efficiency (%) have been summarized in Table 1. As shown in Table 1, all micellar formulation was less than 60 nm in size, on average, with acceptable PDI. The highest percentage of encapsulation efficiency, being 95.54 ± 3.47%, was obtained with PEG-PBCL co-polymer micelles. Characterization of silibinin-loaded PEG-PBCL for size and PDI following reconstitution from freeze-dried samples showed that freeze-drying did not cause significant changes in the size and PDI of micelles. The size and PDI of freeze-dried silibinin-loaded micelles were found to be 52 ± 0.01 nm and 0.384 ± 0.03 nm, respectively, on average. The study of micelles morphology by TEM revealed that the prepared micelles are spherical in shape (Fig. 3).
Table 1.
Characteristics of silibinin-loaded micelles (n = 3)
|
Compound
|
Block copolymer
|
Silibinin loading ± SD
(mg/mg)
|
Encapsulation efficiency ± SD (%)
|
Micellar size (nm) day1
|
PDI (day 1)
|
| Silibinin |
PEG-PLA-PBCL |
0.014 ± 0.006 |
43.93 ± 19.4 |
47.35 ± 0.66 |
0.26 ± 0.08 |
|
|
PEG-PCL |
0.015 ± 0.002 |
47.61 ± 5.82 |
54.73 ± 1.82 |
0.31 ± 0.06 |
|
|
PEG-PBCL |
0.033 ± 0 .001 |
95.54 ± 3.47 |
46.9 ± 0.33 |
0.33 ± 0.01 |
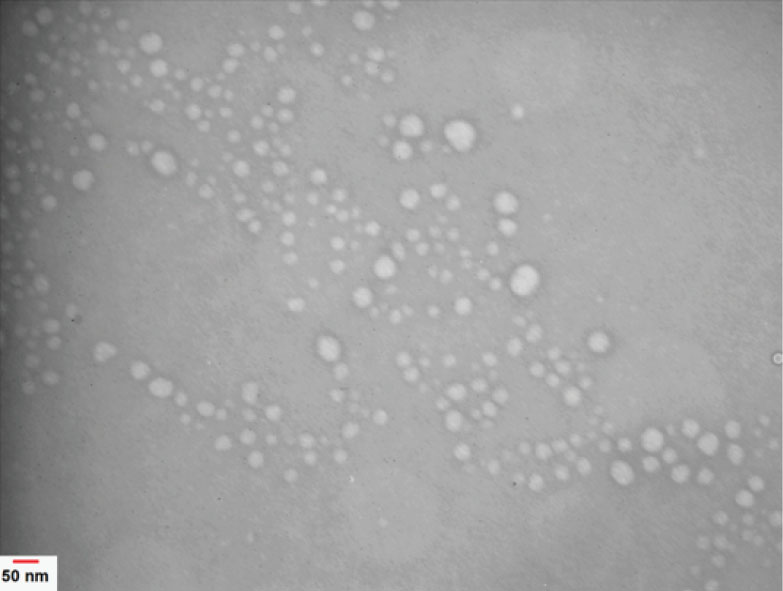
Fig. 3.
TEM image of PEG-PBCL micelles loaded with silibinin.
.
TEM image of PEG-PBCL micelles loaded with silibinin.
Micelles stability
Next, we tested the micelles stability and tried to find out whether micellar formulations of silibinin can retain their nanoscale size over a short period of time, which is usually required for performing preclinical studies in animal cancer models. As shown in Table 2, all of the polymeric micelles retained their nanoscale size with acceptable PDI, seven days after preparing.
Table 2.
Stability characteristics of silibinin-loaded micelles at 4°C (n= 3)
|
Compound
|
Block copolymer
|
Micellar size (nm) day 7
|
PDI day 7
|
Zeta-potential
|
| Silibinin |
PEG-PLA-PBCL |
50.68 ± 7.98 |
0.26 ± 0.08 |
-2.15 ± 0.31 |
|
|
PEG-PCL |
81.64 ± 16.83 |
0.34 ± 0.08 |
-2.23 ± 0.14 |
|
|
PEG-PBCL |
48.15 ± 0.89 |
0.35 ± 0.01 |
-3.23 ± 0.32 |
In vitro release of silibinin from polymeric micelles
Anticancer drugs are loaded in micellar formulation for tumor-targeted delivery which is achieved through accumulation of micelles in tumor tissues due to EPR effects. For efficient accumulation in tumor, a micellar formulation should keep its integrity and retain drug inside cores following entry to the blood circulation. Therefore, we attempted to assess the release of silibinin from micellar formulation under the sink condition in vitro. As shown in Fig. 4, the presence of sink condition under experimental condition is confirmed by the fast release of non-encapsulated free silibinin through the dialysis cassette in the release media (97 ± 0.5%) in the first 4 hours. Among polymeric micelles, PEG-PBCL and PEG-PLA-PBCL micelles showed a controlled release pattern in first 24 hours. The percentage of drug release from PEG-PBCL and PEG-PLA-PBCL micelles during 24 hours were found to be 30.5 ± 1.5% and 32.3 ± 1.2 %, respectively. On the other hand, PEG-PCL micelles released 91.7 ± 0.81% of their drug content in a 24-hour period (Fig. 4). Drug release kinetics from PEG-PLA-PBCL and PEG-PBCL micelles during the first 6 hours was best fitted to the Weibull's distribution model with an R2 of 0.96 and 0.97, respectively. The release of silibinin from PEG-PCL micelles over the first 6 hours obeyed Peppas model with R2 being 0.8.
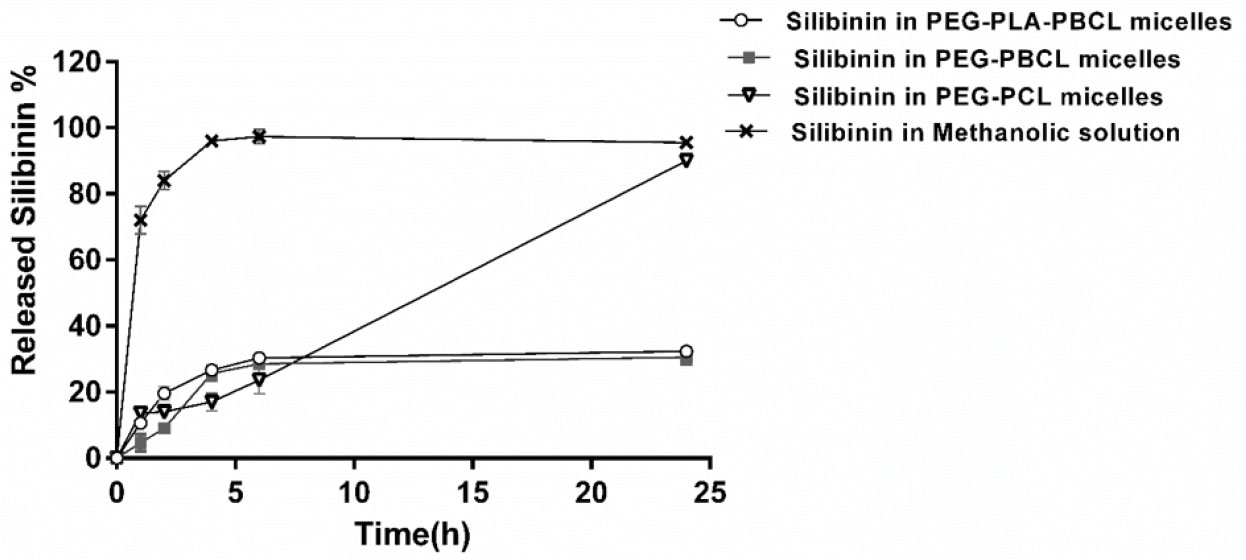
Fig. 4.
Silibinin release profile from micellar formulations in vitro. Data shown are representative of three independent experiments and the values for each time point are mean of triplicates ± SD.
.
Silibinin release profile from micellar formulations in vitro. Data shown are representative of three independent experiments and the values for each time point are mean of triplicates ± SD.
Anti-proliferative effects of silibinin-loaded micelles in B16 melanoma cell line
PEG-PBCL micelles of silibinin were selected for further functional analysis due to high encapsulation efficiency and a good release profile. MTT assay was used to evaluate the anti-proliferative effects of the developed micelles in B16 melanoma cells.
Fig. 5A shows anti-proliferative effects of silibinin as free drug, micellar formulation of silibinin, empty micelles, and DMSO in B16 melanoma cells after 48 hours incubation. As depicted in Fig. 5A, cell viability of B16 cells treated with micellar formulation at three different concentrations of 50, 75, 100 µM was significantly lower than that of B16 cells treated with free silibinin at the same concentrations (P < 0.001). Empty PEG-PBCL micelles did not significantly reduce cell viability of B16 cells as compared with control untreated cells. Fig. 5B depicts dose-response curves for both free drug and silibinin encapsulated PEG-PBCL micelles. IC50 values for cytotoxic effects of free silibinin and micelles of silibinin were found to be 115.6 ± 5.3 µM and 47.4 ± 3.2 µM, respectively. These data show that the prepared micelles can deliver functional drug to cancer cells and induce better cytotoxicity against B16 melanoma cells.
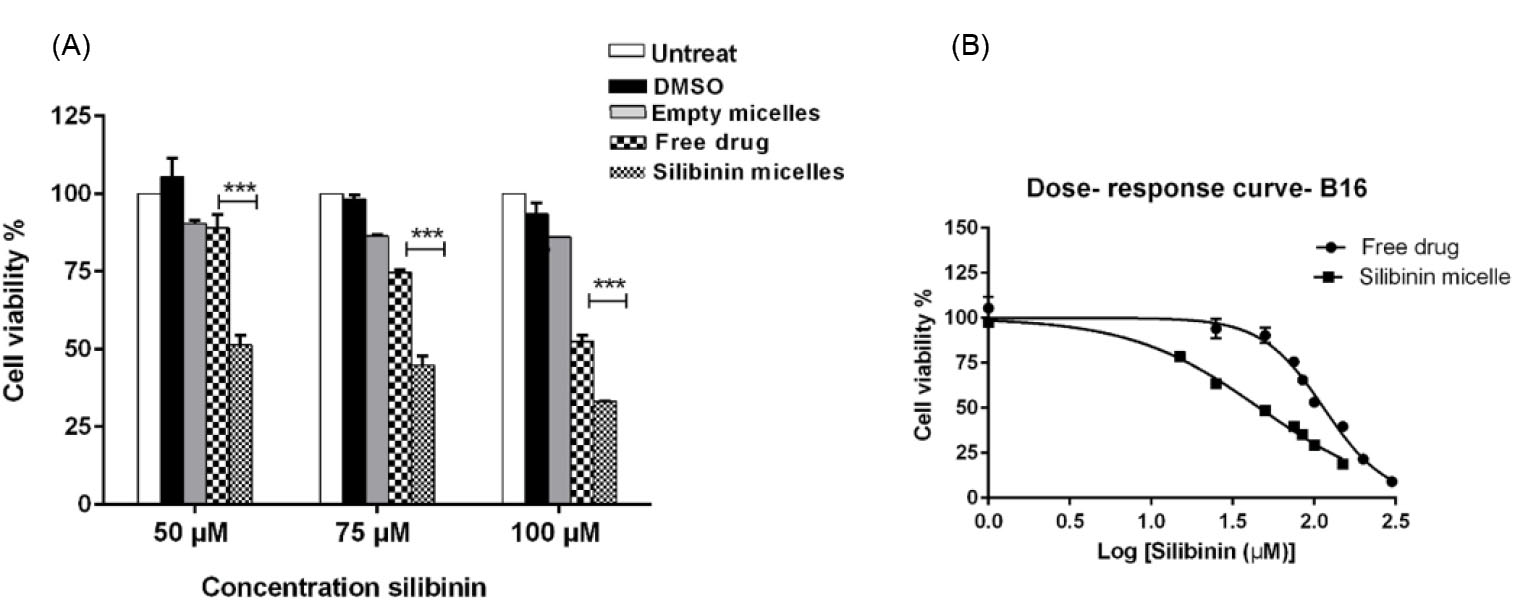
Fig. 5.
(A) Anti-proliferative effect of free silibinin and micellar formulation of silibinin in B16 melanoma cells. The cells were treated with free silibinin and silibinin loaded micelles at three different concentrations and cell viability was assessed by MTT assay. (B) Dose-response curves of free silibinin and its micellar formulation in B16 melanoma cell line. Dose-response curve was generated by GraphPad prism software. Data shown are representative of three independent experiments and the values for each time point are mean of triplicates ± S.D. *** indicating P <0.001.
.
(A) Anti-proliferative effect of free silibinin and micellar formulation of silibinin in B16 melanoma cells. The cells were treated with free silibinin and silibinin loaded micelles at three different concentrations and cell viability was assessed by MTT assay. (B) Dose-response curves of free silibinin and its micellar formulation in B16 melanoma cell line. Dose-response curve was generated by GraphPad prism software. Data shown are representative of three independent experiments and the values for each time point are mean of triplicates ± S.D. *** indicating P <0.001.
Discussion
Silibinin is considered a promising anticancer agent. The potential of silibinin as an anticancer drug is highlighted by several studies showing that this compound can lower the expression level of anti-apoptotic genes (i.e. BCl-2, Survivin) while increasing the expression level of pro-apoptotic genes (i.e. BAX).
36
Besides, silibinin is shown to have suppressive effects on key oncogenic proteins (i.e. STAT3).
8
The major advantage of silibinin for cancer therapy is its low toxicity which has been shown in previous studies.
37
The major limitation for clinical use of this compound is its poor water solubility. Silibinin is considered practically insoluble according to USP solubility criteria.
9
Poor water solubility of silibinin limits the drug absorption and lowers the ability of drug to reach tumor environment. In this research, we found that encapsulation of silibinin in PEG-PCL based polymeric nanomicelles significantly enhances the water solubility of silibinin and provides controlled release of functional drug to cancer cells in vitro.
Among the micellar formulations loaded with silibinin in this study, the micelles prepared from PEG-PBCL showed the best results in terms of encapsulation efficiency and drug release profile. These findings are in line with our previous studies demonstrating that PEG-PBCL micelles show better encapsulation efficiency and drug release for poorly water soluble anticancer drugs.
38
The reason behind the better efficiency of PBCL blcok in encapsulation and retaining of silibinin might be related to its chemical structure. In the PCL block of PEG-PBCL polymer, there is an aromatic ring which can increase the hydrophobicity of core forming block resulting in a better compatibility of the micellar core with the hydrophobic structure of silibinin. The superior efficiency of PEG-PBCL micelles over PEG-PLA-PBCL micelles for encapsulation of silibinin can be explained by the presence of PLA and the shorter length of PBCL chain in the structure of the triblock as compared with PEG-PBCL. PLA has been shown to have poor compatibility with hydrophobic drugs; therefore, it can reduce the compatibility of hydrophobic block for silibinin.
32,39
PEG-PBCL copolymers have been shown to have a very low critical micellar concentration (CMC) as compared with other PEG-PCL based copolymers of similar molecular weights.
32,40,41
The thermodynamic and kinetic stability of micelles detemine the stability of micellar structure against disassociation in blood in vivo.
41
The thermodynamic stability of a micellar structure is characterized by CMC of the applied block copolymers. In other words, the lower CMC of copolymer induces the higher stability of micellar formulation upon dilution in biological environment.
42
The amount of PEG-PBCL polymer used in the developed micellar formulation is well above its CMC
41
; therefore, the micelles are expected to retain their integrity and keep drug content upon dilution in blood concentration.
41
The results of release study indicates that PEG-PBCL micelles can retain almost 70% of their drug content during 24 hours under the sink condition in vitro.
The analysis for the activity of silibinin loaded PEG-PBCL micelles were done in B16 melanoma cells. Our findings showed that silibinin micelles induce better anti-proliferative effects as compared with free drug. These observations are consistent with a previous study reporting that encapsulation of paclitaxel, a hydrophobic drug, in micellar formulations improves its anticancer effects in breast and ovarian cancer cell lines in vitro.
43
This observation can be due to the fact that the micelles construction plays an important role in silibinin solubilization resulting in better delivery of functional drug to cancer cells. The better cytotoxicity of silibinin micelles compared to free silibinin, can be also explained by the fact that cancer cells absorb free drug mainly by diffusion, however drug loaded nanoparticles are taken up by endocytosis. The uptake of PEG-PCL based micelles has been reported in our previous publication.
44
The uptake of micelles by cancer cells can lead to a much higher concentration of anticancer drug inside cancer cells and better biological effects as compared with free drug added to cancer cell culture. Besides, when there is a high concentration of drug inside cancer cells, drug efflux pumps such as p-glycoproteins cannot efficiently pump anticancer drug molecules out of the cells resulting in better retention of drug inside the cells and more potent anticancer effects.
Conclusion
To conclude, our findings showed that among PEG-PCL based micelles used in this project, PEG-PBCL micelles efficiently encapsulated silibinin and provided controlled release of this drug. Functional analysis revealed that PEG-PBCL micelles of silibinin are capable of delivering functional drug to B16 melanoma cell line and exerting more potent growth inhibitory effects as compared with what observed in the cells treated with free drug. Altogether, our results showed that PEG-PBCL micelles have a potential for tumor-targeted delivery of silibinin. It is suggested that future studies should be conducted to evaluate the anticancer effects of the developed formulation in animal cancer models in vivo. In addition, the pharmacokinetics and biodistribution of the developed micelles need to be evaluated to show the capability of developed formulation for tumor-targeted delivery of silibinin.
Funding sources
This research project was funded by Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran (grant number: 976).
Ethical statement
None to be declared.
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
AHR and FA contributed equally to this research work by designing the experimental plans, performing most of the experiments, and writing the manuscript. OM, AL, and SJ designed the experiments, performed data analysis, and wrote the manuscript. SJ also contributed to cell culture in this study. AS contributed to designing the experimental plan and data analysis in preparation and characterization of micelles.
Research Highlights
What is the current knowledge?
simple
-
√ The clinical application of silibinin is limited by its poor
water solubility. PEG-PCL based micelles are useful for
solubilization of hydrophobic drugs.
What is new here?
simple
-
√ Our recently developed PEG-PBCL copolymer efficiently
encapsulated silibinin and provided controlled delivery of
functional drug to cancer cells.
References
- Wing Ying Cheung C, Gibbons N, Wayne Johnson D, Lawrence Nicol D. Silibinin-a promising new treatment for cancer. Anticancer Agents Med Chem 2010; 10:186-95. doi: 10.2174/1871520611009030186 [Crossref] [ Google Scholar]
- Agarwal C, Tyagi A, Kaur M, Agarwal R. Silibinin inhibits constitutive activation of Stat3, and causes caspase activation and apoptotic death of human prostate carcinoma DU145 cells. Carcinogenesis 2007; 28:1463-70. doi: 10.1093/carcin/bgm042 [Crossref] [ Google Scholar]
- Davis-Searles PR, Nakanishi Y, Kim N-C, Graf TN, Oberlies NH, Wani MC. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res 2005; 65:4448-57. doi: 10.1158/0008-5472.CAN-04-4662 [Crossref] [ Google Scholar]
- Sahibzada MUK, Sadiq A, Khan S, Faidah HS. Fabrication, characterization and in vitro evaluation of silibinin nanoparticles: an attempt to enhance its oral bioavailability. Drug Des Devel Ther 2017; 11:1453. doi: 10.2147/DDDT.S133806 [Crossref] [ Google Scholar]
- Lee JI, Hsu BH, Wu D, Barrett JS. Separation and characterization of silybin, isosilybin, silydianin and silychristin in milk thistle extract by liquid chromatography–electrospray tandem mass spectrometry. J Chromatogr A 2006; 1116:57-68. doi: 10.1016/j.chroma.2006.03.053 [Crossref] [ Google Scholar]
- Polyak SJ, Ferenci P, Pawlotsky JM. Hepatoprotective and antiviral functions of silymarin components in hepatitis C virus infection. Hepatology 2013; 57:1262-71. doi: 10.1002/hep.26179 [Crossref] [ Google Scholar]
- Vargas-Mendoza N, Madrigal-Santillán E, Morales-González A, Esquivel-Soto J, Esquivel-Chirino C, García-Luna Y González-Rubio M. Hepatoprotective effect of silymarin. World J Hepatol 2014; 6:144-9. doi: 10.4254/wjh.v6.i3.144 [Crossref] [ Google Scholar]
- Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett 2008; 269:352-62. doi: 10.1016/j.canlet.2008.03.053 [Crossref] [ Google Scholar]
- Polachi N, Bai G, Li T, Chu Y, Wang X, Li S. Modulatory effects of silibinin in various cell signaling pathways against liver disorders and cancer–A comprehensive review. Eur J Med Chem 2016; 123:577-95. doi: 10.1016/j.ejmech.2016.07.070 [Crossref] [ Google Scholar]
- Molavi O, Ma Z, Mahmud A, Alshamsan A, Samuel J, Lai R. Polymeric micelles for the solubilization and delivery of STAT3 inhibitor cucurbitacins in solid tumors. Int J Pharm 2008; 347:118-27. doi: 10.1016/j.ijpharm.2007.06.032 [Crossref] [ Google Scholar]
- Reddy PD, Swarnalatha D. Recent advances in novel drug delivery systems. Int J Pharmtech Res 2010; 2:2025-7. doi: 10.2240/azojono0111 [Crossref] [ Google Scholar]
-
Mitra AK, Cholkar K, Mandal A. Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices. William Andrew; 2017.
- Shin H-C, Alani AW, Rao DA, Rockich NC, Kwon GS. Multi-drug loaded polymeric micelles for simultaneous delivery of poorly soluble anticancer drugs. J Control Release 2009; 140:294-300. doi: 10.1016/j.jconrel.2009.04.024 [Crossref] [ Google Scholar]
- Aliabadi HM, Mahmud A, Sharifabadi AD, Lavasanifar A. Micelles of methoxy poly (ethylene oxide)-b-poly (ɛ-caprolactone) as vehicles for the solubilization and controlled delivery of cyclosporine A. J Control Release 2005; 104:301-11. doi: 10.1016/j.jconrel.2005.02.015 [Crossref] [ Google Scholar]
-
Murthy RSR. Polymeric micelles in targeted drug delivery. In: Devarajan PV, Jain S, eds. Targeted Drug Delivery: Concepts and Design. Springer; 2015. p. 501-41. 10.1007/978-3-319-11355-5
- Oerlemans C, Bult W, Bos M, Storm G, Nijsen JFW, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res 2010; 27:2569-89. doi: 10.1007/s11095-010-0233-4 [Crossref] [ Google Scholar]
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 2000; 65:271-84. doi: 10.1016/S0168-3659(99)00248-5 [Crossref] [ Google Scholar]
- Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res 2016; 33:2373-87. doi: 10.1007/s11095-016-1958-5 [Crossref] [ Google Scholar]
- Shi Y, Lammers T, Storm G, Hennink WE. Physico‐chemical strategies to enhance stability and drug retention of polymeric micelles for tumor‐targeted drug delivery. Macromol Biosci 2017; 17:1600160. doi: 10.1002/mabi.201600160 [Crossref] [ Google Scholar]
- Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Wan Kim S. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release 2001; 72:191-202. doi: 10.1016/s0168-3659(01)00275-9 [Crossref] [ Google Scholar]
- Aliabadi HM, Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin Drug Deliv 2006; 3:139-62. doi: 10.1517/17425247.3.1.139 [Crossref] [ Google Scholar]
- Allen C, Yu Y, Maysinger D, Eisenberg A. Polycaprolactone-b-poly(ethylene oxide) block copolymer micelles as a novel drug delivery vehicle for neurotrophic agents FK506 and L-685,818. Bioconjug Chem 1998; 9:564-72. [ Google Scholar]
- Allen C, Han J, Yu Y, Maysinger D, Eisenberg A. Polycaprolactone-b-poly(ethylene oxide) copolymer micelles as a delivery vehicle for dihydrotestosterone. J Control Release 2000; 63:275-86. doi: 10.1016/S0168-3659(99)00200-X [Crossref] [ Google Scholar]
- Kim SY, Shin IG, Lee YM, Cho CS, Sung YK. Methoxy poly(ethylene glycol) and epsilon-caprolactone amphiphilic block copolymeric micelle containing indomethacin II Micelle formation and drug release behaviours. J Control Release 1998; 51:13-22. doi: 10.1016/S0168-3659(97)00124-7 [Crossref] [ Google Scholar]
- Aliabadi HM, Mahmud A, Sharifabadi AD, Lavasanifar A. Micelles of methoxy poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization and controlled delivery of cyclosporine A. J Control Release 2005; 104:301-11. doi: 10.1016/j.jconrel.2005.02.015 [Crossref] [ Google Scholar]
- Xiong MP, Yanez JA, Kwon GS, Davies NM, Forrest ML. A cremophor-free formulation for tanespimycin (17-AAG) using PEO-b-PDLLA micelles: characterization and pharmacokinetics in rats. J Pharm Sci 2009; 98:1577-86. doi: 10.1002/jps.21509 [Crossref] [ Google Scholar]
- Aliabadi HM, Brocks DR, Lavasanifar A. Polymeric micelles for the solubilization and delivery of cyclosporine A: pharmacokinetics and biodistribution. Biomaterials 2005; 26:7251-9. doi: 10.1016/j.biomaterials.2005.05.042 [Crossref] [ Google Scholar]
- Shi B, Fang C, You XM, Zhang Y, Fu S, PEI YY. Stealth MePEG-PCL micelles: Effects of polymer composition on micelle physicochemical characteristics, in vivo drug release, in vivo pharmacokinetics in rats and biodistribution in S180 tumor bearing mice. Coll Polym Sci 2005; 283:954-67. doi: 10.1007/s00396-004-1243-8 [Crossref] [ Google Scholar]
- Mahmud A, Patel S, Molavi O, Choi P, Samuel J, Lavasanifar A. Self-associating poly(ethylene oxide)-b-poly(alpha-cholesteryl carboxylate-epsilon-caprolactone) block copolymer for the solubilization of STAT-3 inhibitor cucurbitacin I. Biomacromolecules 2009; 10:471-8. doi: 10.1021/bm800846a [Crossref] [ Google Scholar]
- Patel SK, Lavasanifar A, Choi P. Prediction of the solubility of cucurbitacin drugs in self-associating poly(ethylene oxide)-b-poly(alpha-benzyl carboxylate varepsilon-caprolactone) block copolymer with different tacticities using molecular dynamics simulation. Biomaterials 2010; 31:345-57. doi: 10.1016/j.biomaterials.2009.09.051 [Crossref] [ Google Scholar]
- Mahmud A, Xiong X-B, Lavasanifar A. Novel self-associating poly (ethylene oxide)-b lock-poly (ε-caprolactone) block copolymers with functional side groups on the polyester block for drug delivery. Macromolecules 2006; 39:9419-28. doi: 10.1021/ma0613786 [Crossref] [ Google Scholar]
- Abyaneh HS, Vakili MR, Zhang F, Choi P, Lavasanifar A. Rational design of block copolymer micelles to control burst drug release at a nanoscale dimension. Acta Biomater 2015; 24:127-39. doi: 10.1016/j.actbio.2015.06.017 [Crossref] [ Google Scholar]
- Lee MK, Kim MY, Kim S, Lee J. Cryoprotectants for freeze drying of drug nano-suspensions: effect of freezing rate. J Pharm Sci 2009; 98:4808-17. doi: 10.1002/jps.21786 [Crossref] [ Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65:55-63. doi: 10.1016/0022-1759(83)90303-4 [Crossref] [ Google Scholar]
- Galvao J, Davis B, Tilley M, Normando E, Duchen MR, Cordeiro MF. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J 2014; 28:1317-30. doi: 10.1096/fj.13-235440 [Crossref] [ Google Scholar]
- Molavi O, Narimani F, Asiaee F, Sharifi S, Tarhriz V, Shayanfar A. Silibinin sensitizes chemo-resistant breast cancer cells to chemotherapy. Pharm Biol 2017; 55:729-39. doi: 10.1080/13880209.2016.1270972 [Crossref] [ Google Scholar]
- Tiwari P, Kumar A, Balakrishnan S, Kushwaha H, Mishra K. Silibinin-induced apoptosis in MCF7 and T47D human breast carcinoma cells involves caspase-8 activation and mitochondrial pathway. Cancer Invest 2011; 29:12-20. doi: 10.3109/07357907.2010.535053 [Crossref] [ Google Scholar]
- Soleimani AH, Garg SM, Paiva IM, Vakili MR, Alshareef A, Huang Y-H. Micellar nano-carriers for the delivery of STAT3 dimerization inhibitors to melanoma. Drug Deliv Transl Res 2017; 7:571-81. doi: 10.1007/s13346-017-0369-4 [Crossref] [ Google Scholar]
- Theerasilp M, Nasongkla N. Comparative studies of poly (ε-caprolactone) and poly (D, L-lactide) as core materials of polymeric micelles. J Microencapsul 2013; 30:390-7. doi: 10.3109/02652048.2012.746746 [Crossref] [ Google Scholar]
- Wang Q, Jiang J, Chen W, Jiang H, Zhang Z, Sun X. Targeted delivery of low-dose dexamethasone using PCL–PEG micelles for effective treatment of rheumatoid arthritis. J Control Release 2016; 230:64-72. doi: 10.1016/j.jconrel.2016.03.035 [Crossref] [ Google Scholar]
- Garg SM, Vakili MR, Lavasanifar A. Polymeric micelles based on poly (ethylene oxide) and α-carbon substituted poly (ɛ-caprolactone): An in vitro study on the effect of core forming block on polymeric micellar stability, biocompatibility, and immunogenicity. Colloids Surf B Biointerfaces 2015; 132:161-70. doi: 10.1016/j.colsurfb.2015.05.015 [Crossref] [ Google Scholar]
- Owen SC, Chan DP, Shoichet MS. Polymeric micelle stability. Nano today 2012; 7:53-65. doi: 10.1016/j.nantod.2012.01.002 [Crossref] [ Google Scholar]
- Bernabeu E, Gonzalez L, Cagel M, Gergic EP, Moretton MA, Chiappetta DA. Novel Soluplus®—TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf B Biointerfaces 2016; 140:403-11. doi: 10.1016/j.colsurfb.2016.01.003 [Crossref] [ Google Scholar]
- Mahmud A, Lavasanifar A. The effect of block copolymer structure on the internalization of polymeric micelles by human breast cancer cells. Colloids Surf B Biointerfaces 2005; 45:82-9. doi: 10.1016/j.colsurfb.2005.07.008 [Crossref] [ Google Scholar]