Azam Safary is Assistant Professor of Medical Biotechnology at Connective Tissue Diseases
Research Center (CTDRC) and RCPN. She is working on the enzyme replacement therapy, production and
nanoformulation of recombinant enzymes.
Jaleh Barar (PharmD, PhD) is Professor of Drug Delivery and Targeting. She currently is the chair of the
department of Pharmaceutics at the Faculty of Pharmacy, Tabriz university of Medical Sciences. Professor
Barar’s research is focused on various aspects of the pharmaceutical cell biology with the particular emphasis
on the development of novel drug delivery and targeting strategies to combat cancer.
Abstract
Summary
An important arena of the sophisticated nanosystems (NSs) is the combination of the responsive features of NSs with the biocatalytic properties of enzymes. The development of such smart drug delivery systems (DDSs) has seminal effectiveness in targeting, imaging, and monitoring of cancer. These NSs can exhibit site-specific delivery of the toxic cargo in response to the endogenous/exogenous stimuli. Enzyme responsive/targeted DDSs display enhanced accumulation of cargo molecules in the tumor microenvironment (TME) with a spatiotemporal controlled-release behavior. Based on the unique features of enzyme responsive/targeted DDSs, they offer incredible promise in overcoming some limitations of the currently used conventional DDSs. Taken all, targeting TME with the enzyme-responsive targeted DDSs may lead to versatile clinical outcomes in various malignancies.
Keywords: Enzyme-responsive, Smart drug delivery system, Tumor targeting, Nanomaterial, Stimuli-responsive
Copyright and License Information
© 2020 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
During the past decade, various advents in the nanoscaled drug delivery systems (DDSs), so-called multimodal nanosystems (NSs), have paved the path for the development of novel strategies for cancer diagnosis and treatment. These multifunctional NSs can incorporate simultaneously both therapeutic and imaging agents and deliver them to the target site with optimal efficiency and mitigated adverse effect.1-4 The tailor-made surface modification and biofunctionalization allow a significant increase in the circulation time and hence efficient tumor-targeting and accumulation.5-10 Stimuli-responsive NSs have been emerged as a promising alternative for conventional DDSs due to their responsive nature towards exogenous (e.g., temperature, magnetic field, ultrasound, and light) and endogenous (e.g., enzymes, pH, redox, and hypoxia) stimuli, which can even cross the biological barriers safely.11-20
Enzymes play a critical role in the development of many debilitating diseases and the imbalance in their expression/activity underpins the pathobiology of such ailments.21 Furthermore, enzymes as biological triggers have several features, including (i) fast catalyzing chemical reactions under mild conditions with high efficiency, (ii) unique chemo/regio/enantioselectivity and specificity in detection of target molecules and substrates, and (iii) high relevance for different tissues and diseases. All these characteristics render the enzyme responsive nanomaterials as ideal smart biomacromolecules that can be used in an extensive array of biomedical applications.5,7,22-25, Therefore, exploitation of enzyme-responsive DDSs can be an extremely valuable strategy as theranostics/diapeutics and can harness the biocatalytic property of enzyme and exceptional physicochemical properties of the nanocarrier. This strategy represents a wide range of advantages in targeting TME and diagnosing and controlling/curing cancer therapy, including (i) protection of drug molecules during circulation in the bloodstream, (ii) tumor-selective accumulation, (iii) controlled-release of anticancer drug(s), (iv) enhanced cellular uptake and intracellular delivery of drugs, and (v) improved pharmacokinetic (PK) and pharmacodynamic (PD) outcomes with much lower adverse reactions.11,12 In fact, high affinity and exceptional selectivity of the enzymes towards their targets make them a suitable choice for specific, complicated, and biologically inspired chemical reactions.5,26,27
Different types of nanoscaled enzyme-responsive delivery systems have been constructed using polymers (e.g., micelles, hydrogels, dendrimers, and inorganic polymeric hybrids), lipids, liposomes, small organic molecules or inorganic/organic hybrid materials (Fig. 1). Enzyme-induced reactions can alter the physicochemical properties of constructed nanoparticles via cleavage of the covalent bond or some non-covalent interactions.5,28,29 Several classes of enzymes including proteases, phospholipases, and oxidoreductases have been used in the development of enzyme-responsive controlled DDSs.5
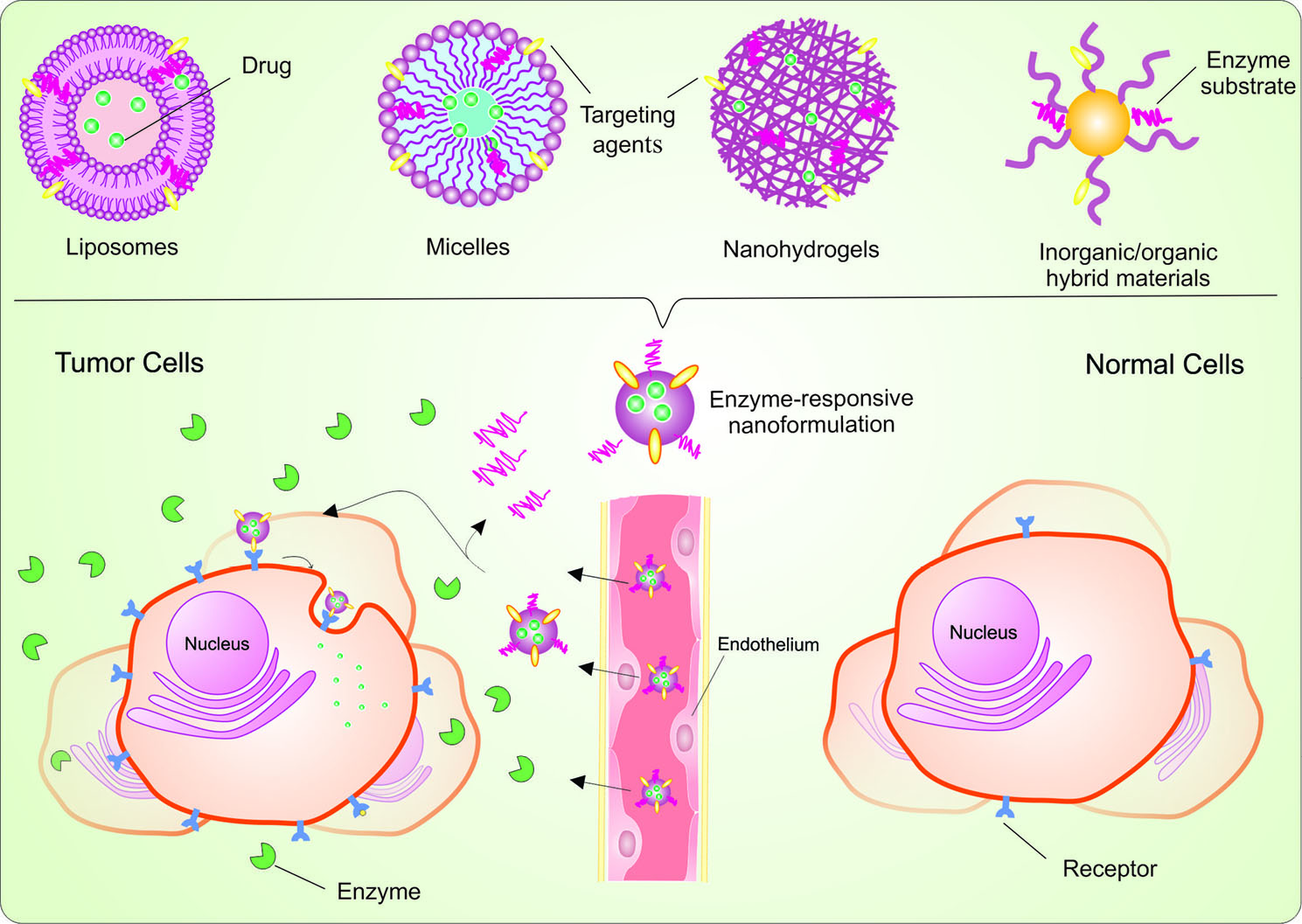
Fig. 1.
Schematic representation of enzyme-responsive nanosystems using different nanomaterials. In the tumor microenvironment, enzymatic cleavage of the covalent bond or physical encapsulation leads to cargoes releasing from nanocarriers.
.
Schematic representation of enzyme-responsive nanosystems using different nanomaterials. In the tumor microenvironment, enzymatic cleavage of the covalent bond or physical encapsulation leads to cargoes releasing from nanocarriers.
For instance, matrix metalloproteinases (MMPs), as a large family of zinc-containing endopeptidases, are overexpressed in many types of cancers and are involved in cancer initiation, progression, and metastasis via cleaving peptide substrates in the extracellular matrix (ECM).30-32 This group of enzymes can be considered as a suitable candidate to improve the efficacy of the therapeutic agents. In this line, several MMP-responsive smart NSs have been engineered which have shown to improve drug specificity and efficacy in TME.5,33,34 Besides, enzyme-responsive nanomaterials in conjugation with active targeting moieties such as antibodies (Abs), aptamers (Aps), and peptides can significantly increase drug accumulation at the target site via reducing unspecific uptake to non-targeted tissue, and site-specific controlled drug release.35-44 Despite all the advantageous of enzyme-responsive DDSs, essential criteria for designing more effective delivery systems still pose a striking challenge, which needs to be considered for their clinical applications. In fact, before clinical applications of enzyme-responsive DDSs, a wide variety of issues need to be addressed, including (i) enzyme dysregulations in diseases, (ii) spatial and temporal patterns of enzyme activity, (iii) substrates overlap for closely related enzyme families, (iv) complexity in the large-scale production of the NSs (in terms of their physicochemical properties, e.g., size, stability, and surface charge), and (v) biocompatibility of nanoformulations and their long-term biological impacts.29,45 Nonetheless, dual/multi-stimuli responsive DDSs have been designed to further enhance the targeting efficiency of the cancer therapy agent.5,19,20 In short, aberrant enzymatic system of the TME could be considered as pivotal target, through which enzyme-responsive NSs can be triggered for on-demand drug release/activation. As a result, if successfully utilized, enzyme-responsive NSs would be a step forward in overcoming the limitations of conventional delivery strategies.
Acknowledgments
Authors like to acknowledge the support of the Research Center for Pharmaceutical Nanotechnology at Biomedicine Institute, Tabriz University of Medical Sciences.
Funding sources
None to be declared.
Ethical statement
There is none to be stated.
Competing interests
No competing interests to be disclosed.
Authors’ contribution
MF, AS, and JB developed the idea, drafted the manuscript. JB finalized the submission.
References
- Khiavi MA, Safary A, Aghanejad A, Barar J, Rasta SH, Golchin A. Enzyme-conjugated gold nanoparticles for combined enzyme and photothermal therapy of colon cancer cells. Colloid Surf A 2019; 572:333-44. doi: 10.1016/j.colsurfa.2019.04.019 [Crossref] [ Google Scholar]
- Barar J, Kafil V, Majd MH, Barzegari A, Khani S, Johari-Ahar M. Multifunctional mitoxantrone-conjugated magnetic nanosystem for targeted therapy of folate receptor-overexpressing malignant cells. J Nanobiotech 2015; 13:26. doi: 10.1186/s12951-015-0083-7 [Crossref] [ Google Scholar]
- Nakhlband A, Barar J, Bidmeshkipour A, Heidari HR, Omidi Y. Bioimpacts of anti epidermal growth receptor antisense complexed with polyamidoamine dendrimers in human lung epithelial adenocarcinoma cells. J Biomed Nanotech 2010; 6:360-9. doi: 10.1166/jbn.2010.1131 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Induction of human alveolar epithelial cell growth factor receptors by dendrimeric nanostructures. Int J Toxicol 2009; 28:113-22. doi: 10.1177/1091581809335177 [Crossref] [ Google Scholar]
- Hu Q, Katti PS, Gu Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 2014; 6:12273-86. doi: 10.1039/C4NR04249B [Crossref] [ Google Scholar]
- Wu L, Lin B, Yang H, Chen J, Mao Z, Wang W. Enzyme-responsive multifunctional peptide coating of gold nanorods improves tumor targeting and photothermal therapy efficacy. Acta Biomater 2019; 86:363-72. doi: 10.1016/j.actbio.2019.01.026 [Crossref] [ Google Scholar]
- Qiao Y, Wan J, Zhou L, Ma W, Yang Y, Luo W. Stimuli‐responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2019; 11:e1527. doi: 10.1002/wnan.1527 [Crossref] [ Google Scholar]
- Zhang J, Ren X, Tian X, Zhang P, Chen Z, Hu X. GSH and enzyme responsive nanospheres based on self-assembly of green tea polyphenols and BSA used for target cancer chemotherapy. Colloids Surf B 2019; 173:654-61. doi: 10.1016/j.colsurfb.2018.10.037 [Crossref] [ Google Scholar]
- Fathi M, Zangabad PS, Aghanejad A, Barar J, Erfan-Niya H, Omidi Y. Folate-conjugated thermosensitive O-maleoyl modified chitosan micellar nanoparticles for targeted delivery of erlotinib. Carbohydr Polym 2017; 172:130-41. doi: 10.1016/j.ijbiomac.2017.08.020 [Crossref] [ Google Scholar]
- Fathi M, Zangabad PS, Barar J, Aghanejad A, Erfan-Niya H, Omidi Y. Thermo-sensitive chitosan copolymer-gold hybrid nanoparticles as a nanocarrier for delivery of erlotinib. Int J Biol Macromol 2018; 106:266-76. doi: 10.1016/j.ijbiomac.2017.08.020 [Crossref] [ Google Scholar]
- Jafari B, Pourseif MM, Barar J, Rafi MA, Omidi Y. Peptide-mediated drug delivery across the blood-brain barrier for targeting brain tumors. Expert Opin Drug Deliv 2019; 16:583-605. doi: 10.1080/17425247.2019.1614911 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Impacts of blood-brain barrier in drug delivery and targeting of brain tumors. Bioimpacts 2012; 2:5-22. doi: 10.5681/bi.2012.002 [Crossref] [ Google Scholar]
- Barar J, Aghanejad A, Fathi M, Omidi Y. Advanced drug delivery and targeting technologies for the ocular diseases. Bioimpacts 2016; 6:49-67. doi: 10.15171/bi.2016.07 [Crossref] [ Google Scholar]
- Shakoori Z, Ghanbari H, Omidi Y, Pashaiasl M, Akbarzadeh A, Jomeh Farsangi Z. Fluorescent multi-responsive cross-linked P(N-isopropylacrylamide)-based nanocomposites for cisplatin delivery. Drug Dev Ind Pharm 2017; 43:1283-91. doi: 10.1080/03639045.2017.1313859 [Crossref] [ Google Scholar]
- Fathi M, Sahandi Zangabad P, Barar J, Aghanejad A, Erfan-Niya H, Omidi Y. Thermo-sensitive chitosan copolymer-gold hybrid nanoparticles as a nanocarrier for delivery of erlotinib. Int J Biol Macromol 2018; 106:266-76. doi: 10.1016/j.ijbiomac.2017.08.020 [Crossref] [ Google Scholar]
- Fathi M, Sahandi Zangabad P, Majidi S, Barar J, Erfan-Niya H, Omidi Y. Stimuli-responsive chitosan-based nanocarriers for cancer therapy. Bioimpacts 2017; 7:269-77. doi: 10.15171/bi.2017.32 [Crossref] [ Google Scholar]
- Akbarzadeh KM, Safary A, Barar J, Ajoolabady A, Somi M, Omidi Y. Multifunctional nanomedicines for targeting epidermal growth factor receptor in colorectal cancer. Cell Mol Life Sci 2019. doi: 10.1007/s00018-019-03305-z [Crossref]
- Fathi M, Alami-Milani M, Geranmayeh MH, Barar J, Erfan-Niya H, Omidi Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int J Biol Macromol 2019; 128:957-64. doi: 10.1016/j.ijbiomac.2019.01.122 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. BioImpacts 2014; 4:55. doi: 10.5681/bi.2014.021 [Crossref] [ Google Scholar]
- Fathi M, Zangabad PS, Majidi S, Barar J, Erfan-Niya H, Omidi Y. Stimuli-responsive chitosan-based nanocarriers for cancer therapy. BioImpacts 2017; 7:269. doi: 10.15171/bi.2017.32 [Crossref] [ Google Scholar]
- Renoux B, Raes F, Legigan T, Péraudeau E, Eddhif B, Poinot P. Targeting the tumour microenvironment with an enzyme-responsive drug delivery system for the efficient therapy of breast and pancreatic cancers. Chem Sci 2017; 8:3427-33. doi: 10.1039/C7SC00472A [Crossref] [ Google Scholar]
- Mu J, Lin J, Huang P, Chen X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem Soc Rev 2018; 47:5554-73. doi: 10.1039/C7CS00663B [Crossref] [ Google Scholar]
- Thornton PD, McConnell G, Ulijn RV. Enzyme responsive polymer hydrogel beads. Chem Commun 2005:5913-5. doi: 10.1039/B511005J [Crossref]
- Kuang T, Liu Y, Gong T, Peng X, Hu X, Yu Z. Enzyme-responsive nanoparticles for anticancer drug delivery. Curr Nanosci 2016; 12:38-46. doi: 10.2174/1573413711666150624170518 [Crossref] [ Google Scholar]
- Safary A, Moniri R, Hamzeh-Mivehroud M, Dastmalchi S. Highly efficient novel recombinant L-asparaginase with no glutaminase activity from a new halo-thermotolerant Bacillus strain. BioImpacts 2019; 9:15. doi: 10.15171/bi.2019.03 [Crossref] [ Google Scholar]
- Safary A, Khiavi MA, Mousavi R, Barar J, Rafi MA. Enzyme replacement therapies: what is the best option?. BioImpacts 2018; 8:153. doi: 10.15171/bi.2018.17 [Crossref] [ Google Scholar]
- Safary A, Moniri R, Hamzeh-Mivehroud M, Dastmalchi S. Identification and molecular characterization of genes coding pharmaceutically important enzymes from halo-thermo tolerant Bacillus. Adv Pharm Bull 2016; 6:551. doi: 10.15171/apb.2016.069 [Crossref] [ Google Scholar]
- Dong X, Yin W, Zhang X, Zhu S, He X, Yu J. Intelligent MoS2 nanotheranostic for targeted and enzyme-/pH-/NIR-responsive drug delivery to overcome cancer chemotherapy resistance guided by PET imaging. ACS Appl Mater Interfaces 2018; 10:4271-84. doi: 10.1021/acsami.7b17506 [Crossref] [ Google Scholar]
- Yao Q, Kou L, Tu Y, Zhu L. Mmp-responsive ‘smart’drug delivery and tumor targeting. Trends Pharmacol Sci 2018; 39:766-81. doi: 10.1016/j.tips.2018.06.003 [Crossref] [ Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001; 17:463-516. doi: 10.1146/annurev.cellbio.17.1.463 [Crossref] [ Google Scholar]
- Yamada T, Oshima T, Yoshihara K, Tamura S, Kanazawa A, Inagaki D. Overexpression of MMP-13 gene in colorectal cancer with liver metastasis. Anticancer Res 2010; 30:2693-9. [ Google Scholar]
- Liu Y, Ding X, Li J, Luo Z, Hu Y, Liu J. Enzyme responsive drug delivery system based on mesoporous silica nanoparticles for tumor therapy in vivo. Nanotech 2015; 26:145102. doi: 10.1088/0957-4484/26/14/145102 [Crossref] [ Google Scholar]
- Fracasso G, Falvo E, Colotti G, Fazi F, Ingegnere T, Amalfitano A. Selective delivery of doxorubicin by novel stimuli-sensitive nano-ferritins overcomes tumor refractoriness. J Control Release 2016; 239:10-8. doi: 10.1016/j.jconrel.2016.08.010 [Crossref] [ Google Scholar]
- Falvo E, Tremante E, Arcovito A, Papi M, Elad N, Boffi A. Improved doxorubicin encapsulation and pharmacokinetics of ferritin–fusion protein nanocarriers bearing proline, serine, and alanine elements. Biomacromolecules 2015; 17:514-22. doi: 10.1021/acs.biomac.5b01446 [Crossref] [ Google Scholar]
- Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov 2014; 13:813. doi: 10.1038/nrd4333 [Crossref] [ Google Scholar]
- Safary A, Akbarzadeh Khiavi M, Omidi Y, Rafi MA. Targeted enzyme delivery systems in lysosomal disorders: an innovative form of therapy for mucopolysaccharidosis. Cell Mol Life Sci 2019. doi: 10.1007/s00018-019-03135-z [Crossref]
- Majidi J, Barar J, Baradaran B, Abdolalizadeh J, Omidi Y. Target therapy of cancer: implementation of monoclonal antibodies and nanobodies. Hum Antibodies 2009; 18:81-100. doi: 10.3233/HAB-2009-0204 [Crossref] [ Google Scholar]
- Matthaiou EI, Barar J, Sandaltzopoulos R, Li C, Coukos G, Omidi Y. Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int J Nanomedicine 2014; 9:1855-70. doi: 10.2147/IJN.S51880 [Crossref] [ Google Scholar]
- Azhdarzadeh M, Atyabi F, Saei AA, Varnamkhasti BS, Omidi Y, Fateh M. Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. Colloids Surf B Biointerfaces 2016; 143:224-32. doi: 10.1016/j.colsurfb.2016.02.058 [Crossref] [ Google Scholar]
- Khiavi MA, Safary A, Somi MH. Recent advances in targeted therapy of colorectal cancer: impacts of monoclonal antibodies nanoconjugates. BioImpacts 2019; 9:123. doi: 10.15171/bi.2019.16 [Crossref] [ Google Scholar]
- Siminzar P, Omidi Y, Golchin A, Aghanejad A, Barar J. Targeted delivery of doxorubicin by magnetic mesoporous silica nanoparticles armed with mucin-1 aptamer. J Drug Target 2019:1-10. doi: 10.1080/1061186X.2019.1616745 [Crossref]
- Vandghanooni S, Eskandani M, Barar J, Omidi Y. Aptamedicine: a new treatment modality in personalized cancer therapy. Bioimpacts 2019; 9:67-70. doi: 10.15171/bi.2019.09 [Crossref] [ Google Scholar]
- Abdolahinia ED, Nadri S, Rahbarghazi R, Barar J, Aghanejad A, Omidi Y. Enhanced penetration and cytotoxicity of metformin and collagenase conjugated gold nanoparticles in breast cancer spheroids. Life Sci 2019; 231. doi: 10.1016/j.lfs.2019.116545 [Crossref]
- Fathi M, Barar J, Erfan-Niya H, Omidi Y. Methotrexate-conjugated chitosan-grafted pH- and thermo-responsive magnetic nanoparticles for targeted therapy of ovarian cancer. Int J Biol Macromol 2019. doi: 10.1016/j.ijbiomac.2019.10.272 [Crossref]
- Gu Z, Biswas A, Zhao M, Tang Y. Tailoring nanocarriers for intracellular protein delivery. Chem Soc Rev 2011; 40:3638-55. doi: 10.1039/C0CS00227E [Crossref] [ Google Scholar]