Bioimpacts. 11(3):219-226.
doi: 10.34172/bi.2021.31
Original Research
Therapeutic effects of bone marrow mesenchymal stem cells via modulation of TLR2 and TLR4 on renal ischemia-reperfusion injury in male Sprague-Dawley rats
Zeinab Karimi 1  , Sahar Janfeshan 1, Elias Kargar Abarghouei 2, Seyedeh-Sara Hashemi 3, *
, Sahar Janfeshan 1, Elias Kargar Abarghouei 2, Seyedeh-Sara Hashemi 3, * 
Author information:
1Shiraz Nephro-Urology Research Center (SNURC), Shiraz University of Medical Sciences, Shiraz, Iran
2Department of Anatomical Sciences, Faculty of Medicine, Hormozgan University of Medical Sciences, Hormozgan, Iran
3Burn and Wound Healing Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Abstract
Introduction:
Acute kidney injury (AKI) induced by renal ischemia-reperfusion (I/R) injury is a pro-inflammatory process that activates toll-like receptors (TLRs). Stem cell therapy holds a great promise for kidney repair. Therefore, we investigated the immunomodulatory role of bone marrow stromal cells (BMSCs) on TLR2 and TLR4 expression in AKI in male Sprague-Dawley rats.
Methods:
BMSCs were isolated from the bone marrow of male rats, cultured in DMEM, and characterized using appropriate markers before transplantation. Renal I/R was induced by 45 minutes bilateral ischemia followed by 24 hours of reperfusion. Rats received intraperitoneal injections of BMSCs (1.5 × 106 cells, i.p, per rat) immediately after termination of renal ischemia. Serum samples were collected pre-and post-stem cells injection for assessment of blood urea nitrogen (BUN) and creatinine (Cr) levels. The kidneys were harvested after 24 hours of reperfusion for structural and molecular analysis.
Results:
Renal I/R caused severe tissue injuries and increased the level of BUN (166.5 ± 12.9 vs. 18.25 ± 1.75) and Cr (3.7 ± 0.22 vs. 0.87 ± 0.06) compared to the sham group. In addition, mRNA expression of TLR2 and TLR4 elevated in the renal I/R group. Administration of BMSCs improved the functional and structural state of the kidney induced by I/R and down-regulated TLR2 and TLR4 gene expression.
Conclusion:
The results showed a highly significant renoprotection by BMSCs that indicates their therapeutic potential in I/R injures. These effects are most likely associated with the TLR2/4 signaling pathway via modulation of the inflammatory response cascades.
Keywords: Bone marrow, Mesenchymal stem cells, Toll-like receptors, Ischemia- reperfusion, Acute kidney injury, Inflammation
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Renal ischemia-reperfusion (I/R) injury is one of the most common causes of acute kidney injury (AKI) that can increase acute rejection rates in kidney transplantation.
1-3
Many studies have shown that strong inflammatory responses are mediated by both innate and adaptive immune systems in post-ischemic kidneys.
1,2,4
In ischemic time, hypoxia directly damages the epithelial cell, and releases dangerous molecules that act as a ligand for toll-like receptors (TLRs), and activates signaling pathways for release of inflammatory chemokines and cytokines.
5
Therefore, inflammation begins during ischemia and accelerates upon reperfusion with activation of the complement system, endothelial activation, leukocytes recruitment, and upregulation of TLRs on the epithelial cells that result in renal tubular, endothelial, and interstitial damages.
6
In fact, TLRs are a family of pattern recognition receptors that are evolutionarily conserved from the worm Caenorhabditis elegans to mammals.
5
Expression of TLRs increased on the tubular epithelial cells in renal I/R, detected specific endogenous danger molecules, and activated pro-inflammatory pathways in the cells.
7
The recent studies demonstrated that TLR2 and TLR4 are crucial regulators of inflammation in renal I/R, so that TLR2 and TLR4 deficient mice were preserved from renal dysfunction in the I/R model.
8,9
In this respect, the immune system has a significant effect on the pathogenesis of renal I/R; therefore, many medical researchers used treatments which regulate the immune response.
10
The bone marrow has two types of stem cells, hematopoietic stem cells (HSCs) and mesenchymal stromal cells (MSCs). HSCs generate blood cells and MSCs differentiate into chondrocytes, osteocytes, and adipocytes.
11
MSCs are known as multipotent, non-hematopoietic cells that can be found in approximately all tissues such as the skeletal muscle, adipose tissue, umbilical cord, amniotic fluid, peripheral blood, dental pulp, lung, liver, and bone marrow.
12-14
Moreover, MSCs have differentiation and self-renewal potential and can be utilized in tissue engineering and clinical trial.
15
In clinical trials, bone marrow stem cells (BMSCs) have been administrated for regenerative therapies because they are easily harvested and are readily available.
16
The massive results of researchers suggest that the mechanism of action of MSCs include fusion or trans-differentiation, paracrine mechanism, and immunomodulation that lead to repair of the injured tissues and organs.
17,18
Therapeutic effects of MSCs has been investigated in kidney disease of animal models and patients.
19-21
Since other studies suggested that MSCs have an immunomodulatory effect. Moreover, TLRs are expressed on tubular epithelial cells and regulate inflammatory responses. Therefore, the present study was designed to investigate the healing effect of allogenic BMSCs on renal I/R through the effect on TLR2 and TLR4 expression in an experimental rat model.
Materials and Methods
Isolation and characterization of BMSCs
BMSCs were provided from the rat’s femur and tibia bone marrow. After three times washing with Hanks’ balanced salt solution (Invitrogen, USA), the mixture was centrifuged and the viable cells were plated at a density of 1 million cells/cm2 in 100 mm cell culture dishes adding 88% Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen) supplemented with 10% FBS (Gibco, USA), 1% L-glutamine (Invitrogen), and 1% penicillin/streptomycin (Invitrogen). After 5 days, the medium was replaced and subsequent culture media were changed every three days. The cells were kept in a 5% CO2 incubator at 37°C and saturated humidity.
22
BMSCs were evaluated morphologically for being spindle shape. They were assessed for in vitro osteogenic induction seeding 5×104 BMSCs in a 12-well plate containing DMEM, supplemented with 10% FBS, and osteogenic media of 200 μM L-ascorbic acid (Sigma, USA), 10 mM glycerol phosphate (Sigma, USA), and 100 nM dexamethasone (Sigma, USA). The media were replaced for three weeks every three days. The differentiation was assessed by alizarin red staining (Sigma, USA) bound to calcium mineralized deposits and revealing a red color. Flow cytometry was undertaken on the third passage of BMSCs to assess the expression of the mesenchymal surface markers for CD105 and CD90 and hematopoietic surface markers of CD34, (Dako, Denmark).
Experimental animals
Thirty male Sprague-Dawley 260-280 g rats were purchased from Laboratory Animal Center of Shiraz University of Medical Sciences, Shiraz, Iran. During the study, the animals were kept single in cages at controlled temperature (22.0 ± 2.0˚C) and lighting (12 hours light/dark cycles) and had free access to food and water. Ketamine (50 mg/kg; Alfasan, Woerden, Netherlands) and xylazine (10 mg/kg; Alfasan) were used intraperitoneally to anesthetize the animals during all interventions.
Induction of renal ischemia-reperfusion model
The rats were randomly divided into three groups (n=10), including (i) Sham group, (ii) I/R (renal ischemia-reperfusion injury) group, and (iii) I/R+BMSCs (bone marrow mesenchymal stromal cells) group. Renal I/R injury induction has been described in our previous study.
23
Briefly, a median abdominal incision was done and kidney exposed; then, bilateral renal pedicles were clamped using a micro-aneurysm vascular clamp for 45 minutes. After termination of ischemia, the clamps were removed and verified for the adequate restoration of the blood flow to the ischemic kidney. The abdominal wound was then closed by 4/0 stitches in two layers. In the sham group, the surgical procedure was performed without pedicel clamping.
24
In the I/R+BMSCs group, rats received intraperitoneal injections of BMSCs (1.5×106cells, i.p, per rat) immediately after termination of renal ischemia.
25-27
Experimental design
All animals were re-anesthetized 24 hours after reperfusion. Blood samples were collected and immediately centrifuged; plasma was preserved at -20°C for the measurement of blood urea nitrogen (BUN) and creatinine (Cr). The animals' kidneys were subsequently harvested. The left kidney was stored at -80°C for mRNA expression analysis (TLR2 and TLR4 genes) and the right kidney was maintained in 10% formalin for hematoxylin-eosin (H & E) staining.
Plasma variables assessment
The levels of BUN and Cr were analyzed by an auto-analyzer (RA-1000 Technicon, America, Namazi Hospital Laboratory, Shiraz, Iran).
Histological analysis and injury scoring
Formalin (10%) fixed and paraffin-embedded renal tissue samples were stained with H&E. Histopathological injury scoring was performed by light microscopy in 10 non-overlapping fields (×400 magnification) at the cortex and medullary area. Renal tissue lesions including shedding of the brush border, tubular necrosis, exfoliation of the epithelial cells, cast deposition, and vascular congestion were classified from 0-5 according to the intensity of damages, 0: no damage, 1: less than 20% damage, 2: 21-40% damage, 3: 41-60% damage, 4: 61-80% damage, and 5: more than 81% damage.
24
RNA extraction and cDNA synthesis
Total RNA was extracted from each rat’s kidney tissue sample, using Trizol (YektaTajhiz, Tehran, Iran). Then, RNA purity and concentration were measured by NanoDropTM (Thermo ScientificTM, USA) at 260/280 nm. In the next step, extracted mRNAs were reversely transcribed into first-strand cDNA using a cDNA synthesis kit (BioFactTM RT-PCR PreMix, Daejeon, Korea) according to the manufacturer's instruction. Briefly, this kit is a complete system for synthesis of cDNA from RNA, which is using BioFactTM RTase, a recombinant type of reverse transcriptase with greater efficiency as inhibition of RNA's secondary structure by its thermostable characteristic at 50°C for activation. After mixture preparation, cDNA synthesis was performed using thermal cycler (Eppendorf, Germany) as follows: 30 min in 50°C and 5 min in 95°C.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The expression levels of TLR2 and TLR4 in experimental groups were determined using an in-house SYBR green real-time PCR protocol by Step One Real-Time Instrument (Applied Biosystems, Step One Plus, Foster City, USA). In this study, GAPDH gene was used as an internal control. The primer sequences were designed for amplification of GDPDH, TLR2, and TLR4 transcripts with the program of primer design on the NCBI website (Table 1).
Table 1.
The oligonucleotide sequences of primers used for the current study
|
Genes
|
Primer sequence 5´─3´
|
|
TLR2
|
Forward: 5´TGTTCCGGGCAAATGGATCA3´ |
| Reverse: 5´GCCTGAAGTGGGAGAAGTCC3´ |
|
TLR4
|
Forward: 5´TATCCAGAGCCGTTGGTGTTAT3´ |
| Reverse: 5´AATGAAGATGATGCCAGAGCG3´ |
|
GAPDH
|
Forward: 5´AGTGCCAGCCTCGTCTCATA3´ |
| Reverse: 5´GAGAAGGCAGCCCTGGTAAC3´ |
The real-time PCR mix was composed of PCR master mix (BioFactTM), forward and reverse primers (10 pmol), and template cDNA. Melting curves of the target and internal control genes were analyzed to confirm the specificity of PCR reactions. All real-time tests were performed in triplicate (Table 2).
Table 2.
The real-time PCR program and PCR mix content for TLR2 and TLR4 gene amplification
|
Stages
|
Temperature (C
◦
)
|
Time
|
Cycles
|
PCR Mix
|
| Holding |
95 |
30 s |
1 |
SYBR green Premix: (5 µL; 2×concentration);
Forward primers: 0.4 μL and 10 pM;
Reverse primers:0.4 μL and 10 pM
|
| Denaturation |
95 |
5 s |
40 |
| Annealing |
58 |
20 s |
| Extension |
72 |
30 s |
| Melting |
95 |
15 s |
1 |
| 58 |
1 min |
| 95 |
15 s |
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Duncan’s post-hoc test. Data were analyzedusing SPSS 24.0 software package.The Pvalue <0.05was considered statistically significant.
Results
Characterization of BMSCs
Morphologically, BMSCs were adherent to the culture flasks and were fibroblast-like cells morphology (Fig. 1A). The osteogenic differentiation was confirmed by alizarin red staining (Fig. 1B). In the flow cytometry, the cells were positive for expression of mesenchymal markers including CD105 and CD90, and were negative for expression hematopoietic marker of CD34 (Fig. 1C).
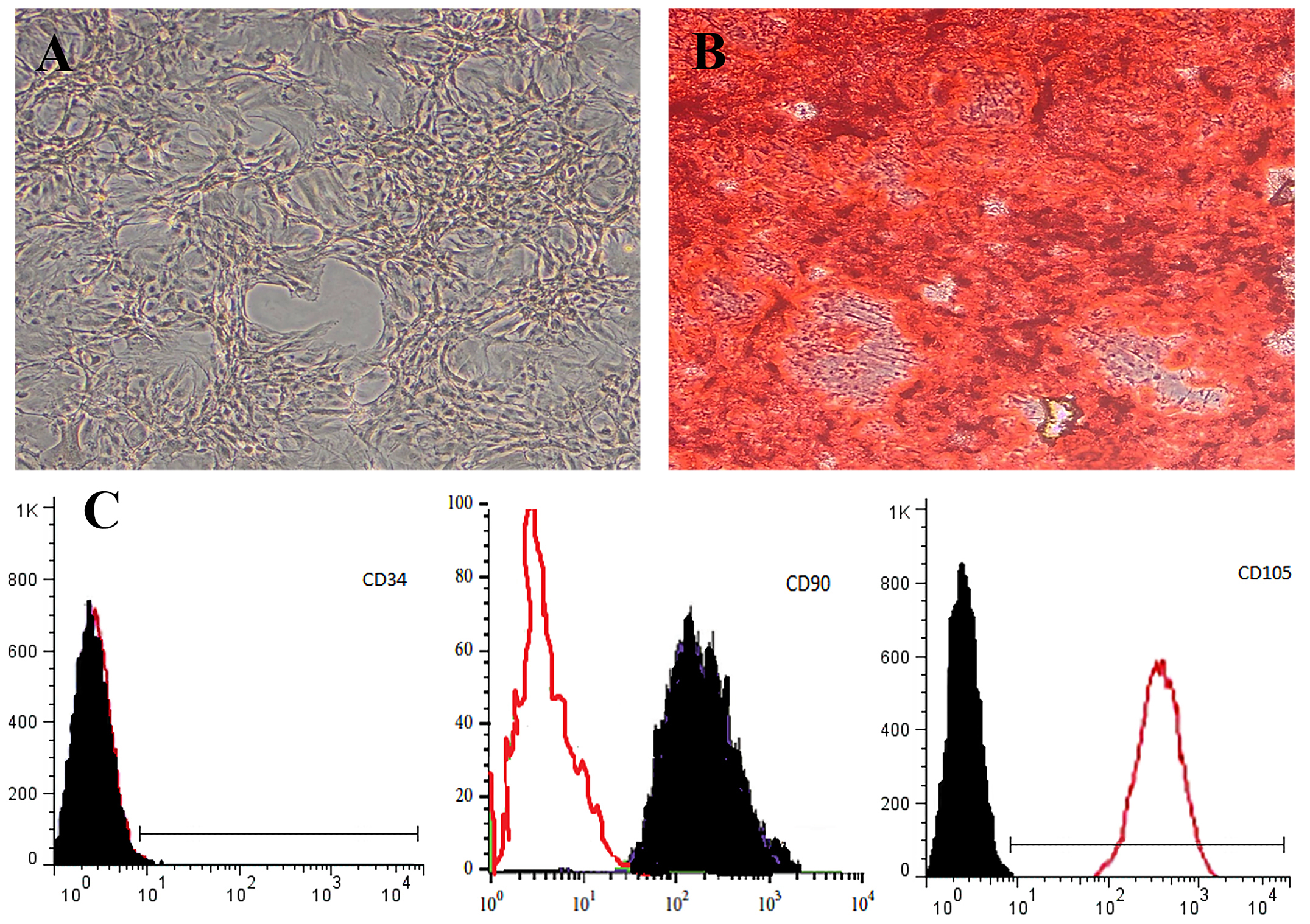
Fig. 1.
Morphology of isolated bone marrow derived stem cells (BMSCs). With time, fibroblast-like cells morphology can be seen by phase-contrast microscopy. BMSCs in Passage 1 (Magnification: ×200) (A), osteogenic differentiation (alizarin red staining) (B), and Markers of mesenchymal stem cells were isolated from BMSCs to be negative for CD34 and positive for CD105 and CD90 (C).
.
Morphology of isolated bone marrow derived stem cells (BMSCs). With time, fibroblast-like cells morphology can be seen by phase-contrast microscopy. BMSCs in Passage 1 (Magnification: ×200) (A), osteogenic differentiation (alizarin red staining) (B), and Markers of mesenchymal stem cells were isolated from BMSCs to be negative for CD34 and positive for CD105 and CD90 (C).
BMSCs attenuated renal dysfunction induced by I/R
Renal I/R increased significantly (both P<0.001) BUN (166.5 ± 12.9 vs. 18.25 ± 1.75) and Cr (3.7 ± 0.22 vs. 0.87 ± 0.06) as compared with the sham group. Treatment with BMSCs partially improved the renal dysfunction, so that the levels of BUN and Cr in the I/R+BMSCs group (99 ± 14.7 and 1.79 ± 0.14, respectively) were significantly lower than the I/R group, but did not return to the level of sham group (Fig. 2).

Fig. 2.
Effects of BMSCs on the renal function markers 24 h after reperfusion. The serum creatinine levels (A) and blood urea nitrogen (B). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 represent significant difference with the sham group.ɸɸP< 0.01, ɸɸɸP< 0.001 represent a significant difference between I/R and I/R+BMSCs groups.
.
Effects of BMSCs on the renal function markers 24 h after reperfusion. The serum creatinine levels (A) and blood urea nitrogen (B). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 represent significant difference with the sham group.ɸɸP< 0.01, ɸɸɸP< 0.001 represent a significant difference between I/R and I/R+BMSCs groups.
Effect of BMSCs on the gene expression of TLR2 and TLR4 in the renal tissue
Renal I/R caused up-regulation of TLR4 (Fig. 3B). However, expression levels of TLR2 (Fig. 3A) decreased in the I/R group as compared to the sham group, but this decrease was not statistically significant. Administration of BMSCs immediately after renal ischemia down-regulated the gene expression of both TLR2 and TLR4, as compared with the I/R group.
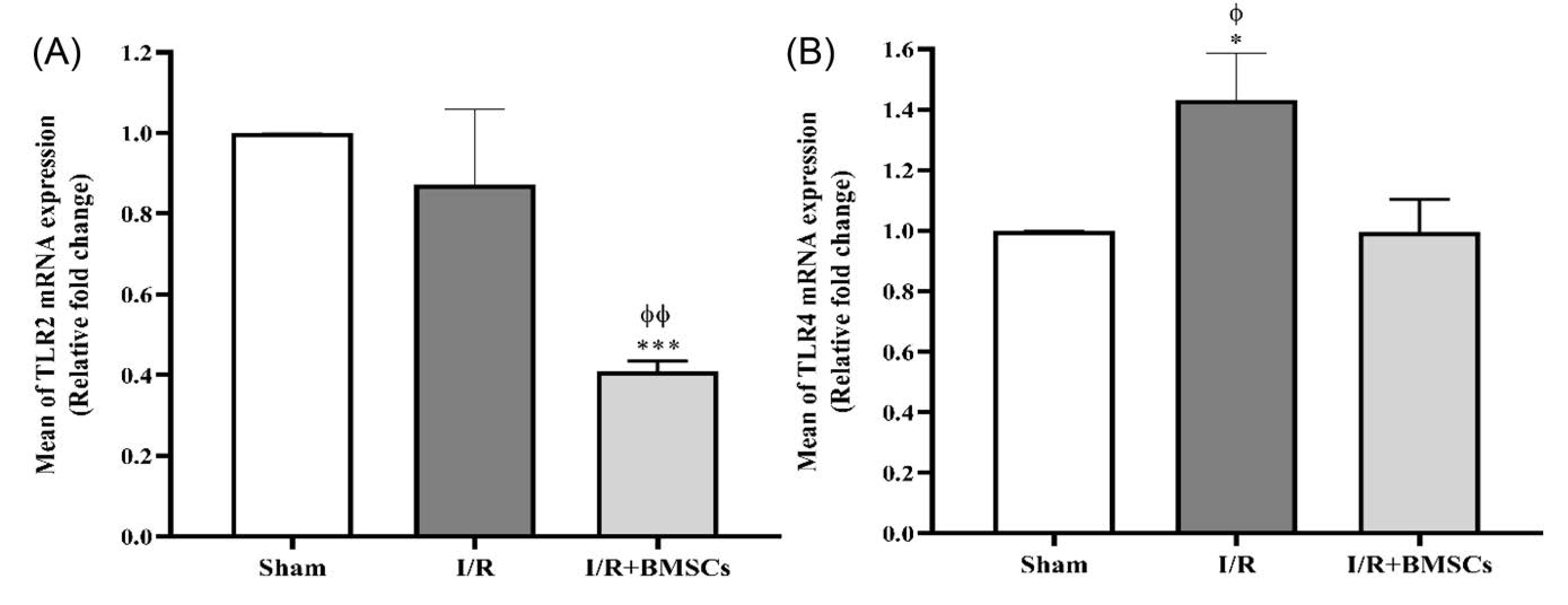
Fig. 3.
Effects of BMSCs on the gene expression of TLR2 and TLR4 24 h after reperfusion. The levels of mRNA expression of TLR2 (A) and TLR4 (B). Data are expressed as mean ± SEM. *P< 0.05, ***P< 0.001 represent a significant difference with the sham group. ɸP< 0.05, ɸɸP< 0.01 represent a significant difference between I/R and I/R+BMSCs groups.
.
Effects of BMSCs on the gene expression of TLR2 and TLR4 24 h after reperfusion. The levels of mRNA expression of TLR2 (A) and TLR4 (B). Data are expressed as mean ± SEM. *P< 0.05, ***P< 0.001 represent a significant difference with the sham group. ɸP< 0.05, ɸɸP< 0.01 represent a significant difference between I/R and I/R+BMSCs groups.
BMSCs improved the histologic damages induced by renal I/R
Histological study of the kidney tissues showed that 45 minutes of renal bilateral ischemia with 24 hours reperfusion caused serious tubular and vascular damages. Tissue injuries included the shedding of brush borders, acute necrosis, and exfoliation of the epithelial cells of both the proximal and the thick ascending limb. Since the outer medulla area is sensitive to hypoxia, these damages were more severe than in the cortex area. In addition, renal I/R caused widened urinary space as well as the formation of tubular casts and vascular congestion (Fig. 4B and 4E). Injection of BMSCs attenuated the tubular epithelial cell damages and decreased the vascular congestion (Fig. 4C and 4F).
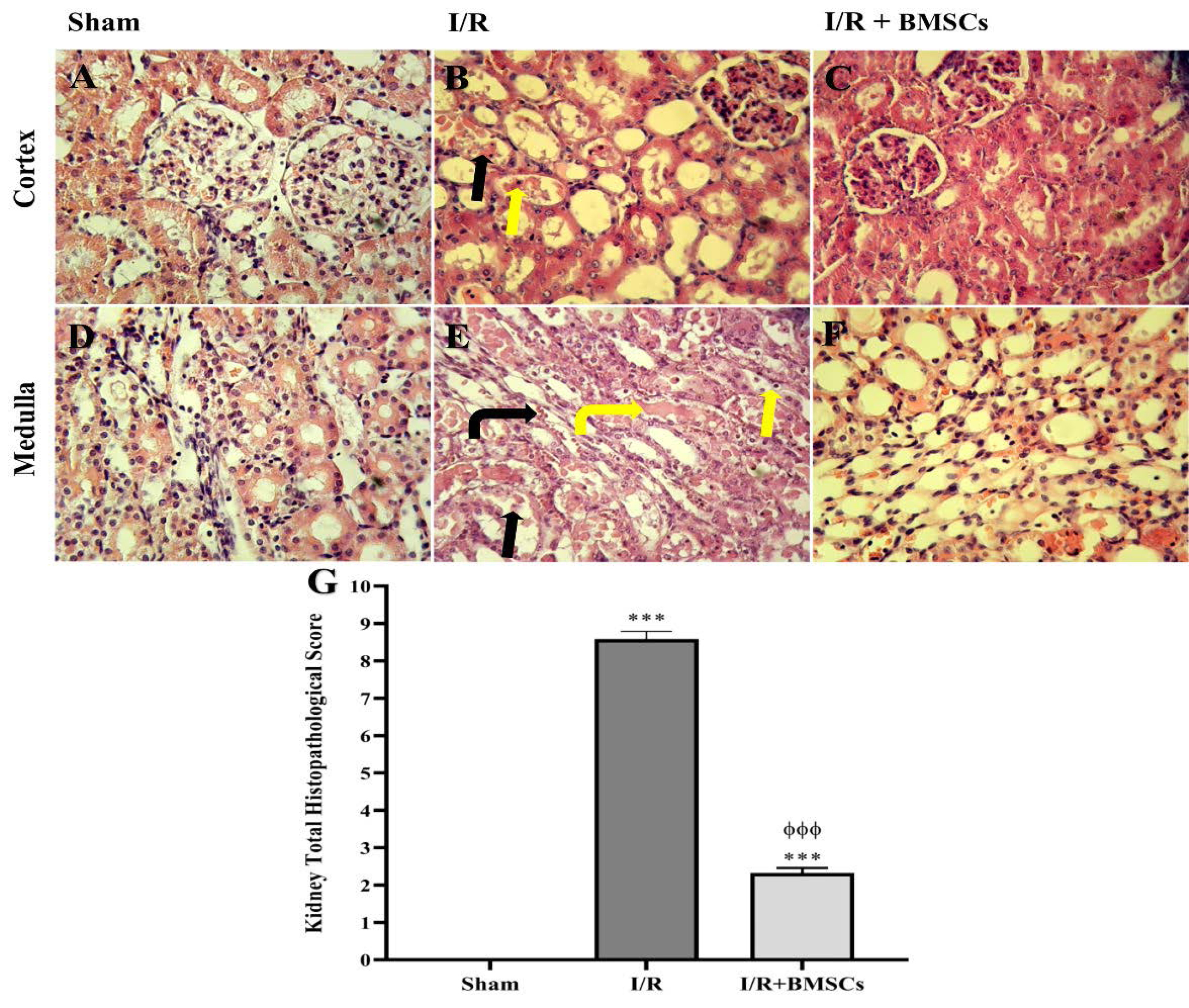
Fig. 4.
Effect of BMSCs on the renal histopathological damages 24 h after reperfusion in the Sham (A, D), IR (B, E) and IR+BMSCs (C, F) groups stained with H & E (Magnification: × 400). Administration of BMSCs (C and F) decreased the shedding of brush border (black arrow), acute necrosis and exfoliation of the epithelial cells (yellow arrow), vascular congestion (black bent arrow), and cast deposition (yellow bent arrow) in the cortex and medulla area, as compared to the I/R group (B and E). The total histopathological score in the renal tissue (G). ***P < 0.001 represents a significant difference with the sham group. ɸɸɸP< 0.001 represents a significant difference between I/R and I/R+BMSCs groups.
.
Effect of BMSCs on the renal histopathological damages 24 h after reperfusion in the Sham (A, D), IR (B, E) and IR+BMSCs (C, F) groups stained with H & E (Magnification: × 400). Administration of BMSCs (C and F) decreased the shedding of brush border (black arrow), acute necrosis and exfoliation of the epithelial cells (yellow arrow), vascular congestion (black bent arrow), and cast deposition (yellow bent arrow) in the cortex and medulla area, as compared to the I/R group (B and E). The total histopathological score in the renal tissue (G). ***P < 0.001 represents a significant difference with the sham group. ɸɸɸP< 0.001 represents a significant difference between I/R and I/R+BMSCs groups.
Discussion
Stem cells have the capacity of self-renewal and differentiation into specialized cell types; for this reason, stem cell therapy is a potential treatment for injured tissues, for example, the kidneys.
28
Mesenchymal stem cells (MSCs) are commonly defined as adherent cells, which express MHC class II antigens; therefore, they are appropriate for allogeneic applications.
29,30
MSCs are attractive applicants for renal injuries treatment. Previous studies have revealed that administration of MSCs could enhance the improvement of AKI induced by I/R in animal models. In fact, nephrons have mesenchymal sources and MSCs are of crucial importance for signaling, which leads to the new tubular epithelial cells differentiation for replacement of lost cells in the nephrons and collecting ducts.
31-33
In this study, the induction renal I/R model elevated the levels of BUN and Cr. Moreover, renal I/R induced pathological damage such as shedding of brush border, acute tubular necrosis, and vascular congestion. Intraperitoneal (i.p) injected rats with BMSCs revealed that the kidney tissues improved in the functional and histological parameters. Several previous studies demonstrated that BMSCs have interactions with each other as well as with injured kidney cells, and this effect causes renoprotective and regenerative actions after AKI and renal I/R, and it was revealed by checking the functional and structural mechanisms in ischemic renal animals that received BMSCs.
34,35
Sadek et al showed that intravenous injection of BMSCs into renal I/R of the male rats improved the renal function both 24 hours and 72 hours after reperfusion. Periodic Acid-Schiff and H & E staining showed a reduction in the tissue damage in the treatment group.
36
This result was in accordance with that reported by Xie and colleagues.
28
The mechanisms of stem cell therapy are unclear in different damages. One prevailing opinion about a therapeutic approach to BMSCs is an improvement of tissue injury, due to replacing the lost kidney cells with physically transdifferentiated stem cells. However, paracrine mechanisms that might be primarily responsible for the organ protective actions of the administered stem cells have not been fully identified.
37-39
MSCs can interact with each of the immune systems isolated cells and probably play a role as an active component in inflammation modulation, tolerance induction, and reduction of transplantation complications. Abdel Aziz et al found that MSCs with hepatocyte growth factors can exert their effect by paracrine mechanisms through down-regulation of pro-inflammatory cytokine TNF-α and up-regulation of anti-inflammatory IL-10 and VEGF.
40
On the other hand, during ischemia, hypoxia directly damages the tubular epithelial cells through increased levels of reactive oxygen species and NO with iNOS origin, and the products released from damaged epithelial cells act as a ligand for TLRs, due to the release of cytokines and chemokines. Thus, the inflammation that begins following ischemia is exacerbated by reperfusion, all of them trigger and exaggerate inflammation response through the innate and adaptive immune systems.
24,41
Our findings demonstrated that MSCs treatment decreased gene expression in both TLRs 2 and 4. In this regard, the TLR2 and TLR4 are the innate immunity receptors on cell surfaces and the key regulator of signaling pathway inflammatory cascade after I/R. These results are further supported by the fact that improved renal function in I/R, mediated by immunomodulatory and their anti-apoptotic effects of MSCs, results in renal down-regulation of TLR2/4 activation.
42
MSCs secretes various growth factors and cytokines that result in the inhibition or modulation of the T-cell response.
39
Therefore, the finding of the current study with respect to other researches suggests that organ protective and repair mechanisms that are activated by MSCs resemble those that can be induced by individual growth factors and anti-inflammatory cytokines in experimental I/R. The expression of inflammatory cytokines was significantly increased after I/R, an increase positively related to the activation of TLR2 and TLR4 and nuclear factor-κB (NF-κB) signaling.
43
Indeed, Leemans et al already showed that renal-associated TLR2 plays a proinflammatory and subsequent detrimental role during I/R injury in the kidney of mice.
44
However, TLR4 can exert different immunological effects as demonstrated by studies showing different effects of TLR2 and TLR4 in infection
45-47
and models of tissue injury.
48,49
This could be because TLR4 detects different endogenous danger ligands compared to TLR2 and used an alternative signaling cascade. In addition, TLR4 unlike TLR2 does not hybridize with other TLRs. The specific role of TLR4 in I/R injury remains therefore unknown.
50
In the present study, it was found that the expression of TLR2 and TLR4 is regulated by different pathways during renal I/R injury. However, treatment with BMSCs down-regulated both receptors. The definition of the specific roles of various pathways in TLR signaling might offer new potentials for the selective blockade of pathways downstream of TLRs.
Conclusion
In conclusion, the present study demonstrated for the first time that the decrement of TLR2 and TLR4 gene expression is involved in the therapeutic effect of BMSCs in a rat model of renal I/R. Furthermore, our results show that TLR4 seems to be a cellular sensor for acute renal injuries that control innate immunity and tissue integrity. Hence, selective targeting of TLR4 may be more effective for the development of therapeutic strategies to prevent I/R injury. These results are advantageous in knowing the mechanism of potential therapeutic effects of cell therapy in renal ischemic states in clinics.
Acknowledgments
The authors would like to thank the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for their invaluable assistance in editing this manuscript.
Funding Sources
The Vice-Chancellery of Research and Technology of Shiraz University of Medical Sciences financially supported this study.
Ethical statement
All of the experimental methods complied with the guidelines for the care and handling of animals provided by the Ethics Committee of Shiraz University of Medical Sciences (Code: 12749).
Competing interests
The authors have declared that no competing interests exist.
Authors' contribution
ZK: experiments design, data analysis, study validation, supervision, provision of study materials and equipment, writing, and reviewing. SJ: data handling, data analysis, data presentation, and draft preparation. EKA: data handling, data analysis, data presentation, and draft preparation. SSH: experiments design, data analysis, study validation, supervision, writing, and reviewing.
Research Highlights
What is the current knowledge?
simple
-
√ Renal ischemia/reperfusion injury is one of the most common causes of AKI.
-
√ BMSCs have a potential protective effect on renal ischemia/reperfusion damages.
What is new here?
simple
-
√ BMSCs decreased the gene expression of TLR2 and TLR4 and the renoprotective effects of BMSCs may be related to the cell signaling pathway of TLR2 and TLR4.
References
- Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia–reperfusion and immune responses in the kidney. J Mol Med 2009; 87:859-64. doi: 10.1007/s00109-009-0491-y [Crossref] [ Google Scholar]
- Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol 2009; 130:41-50. doi: 10.1016/j.clim.2008.08.016 [Crossref] [ Google Scholar]
- Rabb H, Ramirez G, Saba SR, Reynolds D, Xu J, Flavell R. Renal ischemic-reperfusion injury in L-selectin-deficient mice. Am J Physiol 1996; 271:F408-F13. doi: 10.1152/ajprenal.1996.271.2.F408 [Crossref] [ Google Scholar]
- Okusa MD. The Inflammatory Cascade in Acute Ischemic Renal Failure. Nephron 2002; 90:133-8. doi: 10.1159/000049032 [Crossref] [ Google Scholar]
- Takeuchi O, Akira S. Pattern Recognition Receptors and Inflammation. Cell 2010; 140:805-20. doi: 10.1016/j.cell.2010.01.022 [Crossref] [ Google Scholar]
- Kelly KJ, Williams WW, Jr. Jr., Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 1996; 97:1056-63. doi: 10.1172/JCI118498 [Crossref] [ Google Scholar]
- Tsan M-F, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol 2004; 76:514-9. doi: 10.1189/jlb.0304127 [Crossref] [ Google Scholar]
- Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS. TLR2 Is Constitutively Expressed within the Kidney and Participates in Ischemic Renal Injury through Both MyD88-Dependent and -Independent Pathways. J Immunol 2007; 178:6252-8. doi: 10.4049/jimmunol.178.10.6252 [Crossref] [ Google Scholar]
- Rusai K, Sollinger D, Baumann M, Wagner B, Strobl M, Schmaderer C. Toll-like receptors 2 and 4 in renal ischemia/reperfusion injury. Pediatr Nephrol 2010; 25:853-60. doi: 10.1007/s00467-009-1422-4 [Crossref] [ Google Scholar]
- Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol 2007; 123:7-13. doi: 10.1016/j.clim.2006.09.008 [Crossref] [ Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999; 284:143-7. doi: 10.1126/science.284.5411.143 [Crossref] [ Google Scholar]
- Prockop DJ. Marrow Stromal Cells as Stem Cells for Nonhematopoietic Tissues. Science 1997; 276:71-4. doi: 10.1126/science.276.5309.71 [Crossref] [ Google Scholar]
- Pourfath MR, Behzad-Behbahani A, Hashemi SS, Derakhsahnfar A, Taheri MN, Salehi S. Monitoring wound healing of burn in rat model using human Wharton's jelly mesenchymal stem cells containing cGFP integrated by lentiviral vectors. Iran J Basic Med Sci 2018; 21(1):70-76. doi: 10.22038/IJBMS.2017.19783.5212 [Crossref] [ Google Scholar]
- Joyce NC, Harris DL, Markov V, Zhang Z, Saitta B. Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol Vis 2012; 18:547-64. [ Google Scholar]
- 15 Hashemi SS, Pourfath MR, Derakhshanfar A, Behzad-Behbahani A, Moayedi J. The role of labeled cell therapy with and without scaffold in early excision burn wounds in a rat animal model. Iran J Basic Med Sci 2020; 23(5):673-679. doi: 10.22038/ijbms.2020.34324.8156 [Crossref] [ Google Scholar]
- Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: Harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci U S A 2003; 100:11917-23. doi: 10.1073/pnas.1834138100 [Crossref] [ Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006; 98:1076-84. doi: 10.1002/jcb.20886 [Crossref] [ Google Scholar]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105:1815-22. doi: 10.1182/blood-2004-04-1559 [Crossref] [ Google Scholar]
- Chung BH. Use of mesenchymal stem cells for chronic kidney disease. Kidney Res Clin Pract 2019; 38:131-4. doi: 10.23876/j.krcp.19.051 [Crossref] [ Google Scholar]
- Missoum A. Recent Updates on Mesenchymal Stem Cell Based Therapy for Acute Renal Failure. Curr Urol 2019; 13:189-99. doi: 10.1159/000499272 [Crossref] [ Google Scholar]
- Sávio-Silva C, Soinski-Sousa PE, Balby-Rocha MTA, Lira ÁdO, Rangel ÉB. Mesenchymal stem cell therapy in acute kidney injury (AKI): review and perspectives. Rev Assoc Med Bras (1992) 2020; 66:s45-s54. [ Google Scholar]
- Ahdjoudj S, Lasmoles F, Oyajobi BO, Lomri A, Delannoy P, Marie PJ. Reciprocal control of osteoblast/chondroblast and osteoblast/adipocyte differentiation of multipotential clonal human marrow stromal F/STRO-1+ cells. J Cell Biochem 2001; 81:23-38. doi: 10.1002/1097-4644(20010401)81:1<23::aid-jcb1021>3.0.co;2-h [Crossref] [ Google Scholar]
- Gholampour F, Roozbeh J, Janfeshan S, Karimi Z. Remote ischemic per-conditioning protects against renal ischemia-reperfusion injury via suppressing gene expression of TLR4 and TNF-α in rat model. Can J Physiol Pharmacol 2019; 97:112-9. doi: 10.1139/cjpp-2018-0543 [Crossref] [ Google Scholar]
- Karimi Z, Ketabchi F, Alebrahimdehkordi N, Fatemikia H, Owji SM, Moosavi SMS. Renal ischemia/reperfusion against nephrectomy for induction of acute lung injury in rats. Ren Fail 2016; 38:1503-15. doi: 10.1080/0886022X.2016.1214149 [Crossref] [ Google Scholar]
- Eliopoulos N, Zhao J, Bouchentouf M, Forner K, Birman E, Yuan S. Human marrow-derived mesenchymal stromal cells decrease cisplatin renotoxicity in vitro and in vivo and enhance survival of mice post-intraperitoneal injection. Am J Physiol Renal Physiol 2010; 299:F1288-98. doi: 10.1152/ajprenal.00671.2009 [Crossref] [ Google Scholar]
- Semedo P, Palasio CG, Oliveira CD, Feitoza CQ, Gonçalves GM, Cenedeze MA. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol 2009; 9:677-82. doi: 10.1016/j.intimp.2008.12.008 [Crossref] [ Google Scholar]
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 2005; 289:F31-42. doi: 10.1152/ajprenal.00007.2005 [Crossref] [ Google Scholar]
- Xie L-B, Chen X, Chen B, Wang X-D, Jiang R, Lu Y-P. Protective effect of bone marrow mesenchymal stem cells modified with klotho on renal ischemia-reperfusion injury. Ren Fail 2019; 41:175-82. doi: 10.1080/0886022X.2019.1588131 [Crossref] [ Google Scholar]
- Imai E, Ito T. Can bone marrow differentiate into renal cells?. Pediatr Nephrol 2002; 17:790-4. doi: 10.1007/s00467-002-0949-4 [Crossref] [ Google Scholar]
- Hashemi SS, Mohammadi AA, Kabiri H, Hashempoor MR, Mahmoodi M, Amini M, Mehrabani D. The healing effect of Wharton's jelly stem cells seeded on biological scaffold in chronic skin ulcers: A randomized clinical trial. J Cosmet Dermatol 2019; 18(6):1961-1967. doi: 10.1111/jocd.12931 [Crossref] [ Google Scholar]
- Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int 2005; 68:1613-7. doi: 10.1111/j.1523-1755.2005.00573.x [Crossref] [ Google Scholar]
- Anonymous Anonymous. Kallikrein-Modified Mesenchymal Stem Cell Implantation Provides Enhanced Protection Against Acute Ischemic Kidney Injury by Inhibiting Apoptosis and Inflammation. Hum Gene Ther 2008; 19:807-19. doi: 10.1089/hum.2008.016 [Crossref] [ Google Scholar]
- Anglani F, Forino M, Del Prete D, Tosetto E, Torregrossa R, D'Angelo A. In search of adult renal stem cells. J Cell Mol Med 2004; 8:474-87. doi: 10.1111/j.1582-4934.2004.tb00472.x [Crossref] [ Google Scholar]
- Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 2003; 112:42-9. doi: 10.1172/JCI17856 [Crossref] [ Google Scholar]
- Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 2007; 292:F1626-F35. doi: 10.1152/ajprenal.00339.2006 [Crossref] [ Google Scholar]
- Sadek EM, Afifi NM, Elfattah LIA, Mohsen MAA-E. Histological study on effect of mesenchymal stem cell therapy on experimental renal injury induced by ischemia/reperfusion in male albino rat. Int J Stem Cells 2013; 6:55-66. doi: 10.15283/ijsc.2013.6.1.55 [Crossref] [ Google Scholar]
- Burst VR, Gillis M, Pütsch F, Herzog R, Fischer JH, Heid P. Poor Cell Survival Limits the Beneficial Impact of Mesenchymal Stem Cell Transplantation on Acute Kidney Injury. Nephron Exp Nephrol 2010; 114:e107-e16. doi: 10.1159/000262318 [Crossref] [ Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S. Local Delivery of Marrow-Derived Stromal Cells Augments Collateral Perfusion Through Paracrine Mechanisms. Circulation 2004; 109:1543-9. doi: 10.1161/01.CIR.0000124062.31102.57 [Crossref] [ Google Scholar]
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 2005; 289:F31-F42. doi: 10.1152/ajprenal.00007.2005 [Crossref] [ Google Scholar]
- Abdel Aziz M, Wassef M, Rashed L, Mhfouz S, Omar N. Mesenchymal Stem Cells Therapy in Acute Renal Failure: Possible Role of Hepatocyte Growth Factor. Journal of Stem Cell Research & Therapy 2011; 3:1-7. [ Google Scholar]
- Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement Altern Med 2014; 14:19. doi: 10.1186/1472-6882-14-19 [Crossref] [ Google Scholar]
- Anders H-J. Toll-Like Receptors and Danger Signaling in Kidney Injury. J Am Soc Nephrol 2010; 21:1270-4. doi: 10.1681/asn.2010030233 [Crossref] [ Google Scholar]
- Ghaly E, Gergis S, Aziz J, Yassa H, Hassan H. Role of mesenchymal stem cell therapy in cisplatin induced nephrotoxicity in adult albino rats: Ultrastructural & biochemical study. Acta Medica International 2014; 1:57-66. [ Google Scholar]
- Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJD, Kirschning CJ. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 2005; 115:2894-903. doi: 10.1172/JCI22832 [Crossref] [ Google Scholar]
- Arko‐Mensah J, Julian E, Singh M, Fernandez C. TLR2 but not TLR4 Signalling is Critically Involved in the Inhibition of IFN‐γ‐induced Killing of Mycobacteria by Murine Macrophages. Scand J Immunol 2007; 65:148-57. [ Google Scholar]
- Gil ML, Gozalbo D. TLR2, but not TLR4, triggers cytokine production by murine cells in response to Candida albicans yeasts and hyphae. Microbes and infection 2006; 8:2299-304. [ Google Scholar]
- Rodriguez N, Wantia N, Fend F, Dürr S, Wagner H, Miethke T. Differential involvement of TLR2 and TLR4 in host survival during pulmonary infection with Chlamydia pneumoniae. Eur J Immunol 2006; 36:1145-55. [ Google Scholar]
- Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest 2003; 111:1571-8. [ Google Scholar]
- Zhai Y, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol 2004; 173:7115-9. [ Google Scholar]
- Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, Leemans JC. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One 2008; 3(10):e3596. doi: 10.1371/journal.pone.0003596 [Crossref] [ Google Scholar]