Bioimpacts. 11(4):253-261.
doi: 10.34172/bi.2021.36
Original Research
Melatonin increases 5-flurouracil-mediated apoptosis of colorectal cancer cells through enhancing oxidative stress and downregulating survivin and XIAP
Ainaz Mihanfar 1, Bahman Yousefi 2, Saber Ghazizadeh Darband 3, Shirin Sadighparvar 4, Mojtaba Kaviani 5, Maryam Majidinia 6, * 
Author information:
1Student Research Community, Urmia University of Medical Sciences, Urmia, Iran
2Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Exercise Physiology, Faculty of Sport Sciences, Urmia University, Urmia, Iran
4Neurophysiology Research Center, Urmia University of Medical Sciences, Urmia, Iran
5School of Nutrition and Dietetics, Acadia University, Wolfville, Nova Scotia, Canada
6Solid Tumor Research Center, Urmia University of Medical Sciences, Urmia, Iran
Abstract
Introduction:
Colorectal cancer (CRC) is one of the most lethal human malignancies with a global alarming rate of incidence. The development of resistance against common chemotherapeutics such as 5-fluorouracil (5-FU) remains a big burden for CRC therapy. Therefore, we investigated the effects of melatonin on the increasing 5-FU- mediated apoptosis and its underlying mechanism in SW-480 CRC cell line.
Methods:
The effects of melatonin and 5- FU, alone or in combination, on cell proliferation were evaluated using an MTT assay. Further, Annexin-V Flow cytometry was used for determining the effects of melatonin and 5-FU on the apoptosis of SW-480 cell lines. The expression levels of Bax, Bcl-2, pro-caspase-3/activated caspase 3, X-linked inhibitor of apoptosis proteins (XIAP), and survivin were measured after 48 hours incubation with drugs. Cellular levels of reactive oxygen species (ROS), catalase, superoxide dismutase and glutathione peroxidase were also evaluated.
Results:
Melatonin and 5-FU significantly decreased the cell proliferation of SW-480 cells. Combination of 5-FU with melatonin significantly decreased the IC50 value of 5-FU from 100 μM to 50 μM. Moreover, combination therapy increased intracellular levels of ROS and suppressed antioxidant enzymatic activities (P < 0.05). Treatment with either melatonin or 5-FU resulted in the induction of apoptosis in comparison to control (P > 0.05). XIAP and survivin expression levels potently decreased after combination treatment with melatonin and 5-FU (P < 0.05).
Conclusion:
We demonstrated that melatonin exerts a reversing effect on the resistance to apoptosis by targeting oxidative stress, XIAP and survivin in CRC cells. Therefore, more studies need for better understanding of underlying mechanisms for beneficial effects of combination of melatonin and 5-FU.
Keywords: Melatonin, 5-FU, XIAP, Survivin, Oxidative stress, Colorectal cancer
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Colorectal cancer (CRC), as the most common strain of gastrointestinal malignancy, is considered as one of the important leading causes of cancer-related mortality worldwide.
1
Alarmingly, the development of CRC due to lifestyle-related factors such as obesity, physical inactivity, smoking and alcohol use and inherited gene changes, has been becoming a serious health problem across the world.
2,3
Despite extensive efforts conducted to combat CRC including surgery, radiotherapy, and chemotherapy, differences in the sensitivity of certain cancer cells to chemotherapy defined as multidrug resistance (MDR) is a major burden against successful treatment and importantly responsible for the low survival rates of patients, such that nearly half of colorectal patients are resistant to 5-fluorouracil (5-FU)-based chemotherapies.
4-6
Combination of 5-FU with Oxaliplatin and irinotecan is a standard chemotherapeutic regimen for primary and metastasized CRC.
7
Hence, recent studies have focused on the combination therapy of main chemotherapeutics such as 5-FU with naturally occurring compounds, which reduces the effective dose and hence adverse side effects.
6
In this light, the application of melatonin gains more attention as an adjuvant agent for cancer treatment.
8,9
In addition to physiological functions such as regulation of circadian rhythm, oxidative stress, and immune response, melatonin also exerts oncostatic activity via anti-proliferative and pro-apoptotic effects.
10,11
Melatonin is able to increase the sensitization of antineoplastic agents, which leads to a greater response to treatment.
12
Besides various well-known mechanisms introduced for drug resistance, dysregulation in apoptosis, as a hallmark of cancer, is strongly demonstrated to be linked with failure of clinical cancer therapy.
13,14
Inhibitor of apoptosis proteins (IAPs) are involved in the inhibition of the processing of caspase-9 by cytochrome c, hence prevention of apoptosis.
15
This family including X-linked IAP (XIAP) and survivin, is consisted of important proteins with increased expression pattern in CRC.
16,17
In addition, XIAP and survivin are reported to play a critical function in the resistance to apoptosis, subsequent in antitumor resistance in various human malignancies.
18
Therefore, identifying potential agents that are able to inhibit apoptosis resistance by targeting XIAP and survivin or other IAPs is of importance in cancer treatment. In this study, we aimed to study, for the first time, the effect of melatonin on apoptosis resistance through targeting of oxidative stress and two important molecules, survivin, and XIAP, in SW-480 CRC cell lines.
Material and methods
Materials
Melatonin and 5-FU were purchased from Cayman Chemical (Ann Arbor, MI, USA). RPMI 1640, fetal bovine serum (FBS) and penicillin/streptomycin were provided from Gibco®, (Invitrogen, Grand Island, NY, USA), reactive oxygen species (ROS), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) assay kits were provided from the Elabscience Biotechnology (Wuhan, China). EZ-10 Total RNA Minipreps Kit was provided from Bio Basic Inc., (Canada). AccuPowerTM cycle script RT premix kit and Maxima Syber Green/ROX qPCR Master Mix were purchased from Bioneer, Daejeon, Korea and ThermoFisher Scientific, Bremen, Germany, respectively. Primers were also provided from Pishgam Biotech, Tehran, Iran. Primary antibody against XIAP, survivin, Bax, Bcl-2, pro-caspase-3/cleaved caspase-3 and β-actin, and the secondary antibody, was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Annexin V-FITC and propidium iodide were obtained from EXBIO (Vestec, Czech Republic).
Cell culture
Human colorectal cancer SW-480 cell lines (Pasteur Institute Cell Culture Collection, Tehran, Iran) were grown in RPMI-1640 supplemented with 10% FBS and 100 unit/mL penicillin/streptomycin at 37°C in humidified 95% and 5% CO2 incubator. The cells were harvested using trypsin–EDTA and passaged every 2–3 days to maintain exponential growth.
MTT assay
MTT assay was performed to determine cell viability following various treatments. Initially, cells (1 × 104 cells per well) were seeded at 96-well plates and subsequently were treated with different concentrations of 5-FU (up to 300 µM) and melatonin (up to 500 µM) in monotreatment or co-treatment manner. After 48 hours, media containing drugs were removed and MTT containing medium was added. After incubation for 4 hours at 37°C, the formed formazan crystals were solubilized using dimethylsuloxide (DMSO). Absorbance of each well was measured at 570 nm with a micro plate reader, ELx 800 (Biotek, Winooski, USA).
Calculation of combination index (CI)
The cytotoxicity of 5-FU and melatonin combinations were calculated using the combination index (CI) given by the formula below:
CI = (D)1/((DX)1) + (D)2/(DX)2 + α ((D)1 (D)2)/((DX)1 (DX)2)
Where (DX)1 and (DX)2 are the concentrations of drug 1or 2 alone giving a 50% reduction in cell viability compared to a control. (D)1 and (D)2 are the concentration of drugs 1 and 2 in combination. α is 0 when 1 and 2 are mutually exclusive or 1 when they are mutually non-exclusive. CI < 1, CI = 1, and CI > 1 was considered synergistic, additive and an antagonistic interaction, respectively.
ROS measurement
The effect of 5-FU, melatonin and their combination on the levels of intracellular ROS was evaluated using ROS Assay Kit. After incubation of SW-480 cells with the aforementioned drugs, washing with PBS (pH= 7.4) and incubating with 10 µM 2, 7-dichlorofluorescein diacetate (DCFH-DA) at 37°C for 45 minutes in the dark, dichlorofluorescein (DCF) was produced as a reaction product of ROS and DCFH-DA, which is highly fluorescent compound. Changes in the DCF fluorescence intensity were measured using fluorometry with excitation wavelength at 485 nm and emission wavelength at 525 nm.
Measuring SOD activity
SOD activity was measured according to the protocol.
19
In this assay superoxide radicals generated by the xanthine and xanthine oxidase reaction system oxidize hydroxylamine to form nitrite. During measuring samples containing SOD, the SOD can specifically inhibit superoxide anion free radical; hence reduce the formation of nitrite. The absorbance was read at 550 nm using an ELISA plate reader. Enzyme-specific activities were expressed as units/mg of protein.
Measuring CAT activity
CAT activity was determined according to the protocol.
19
This kit uses the peroxidase function of CAT to determine the enzyme activity. The reaction that CAT decomposes H2O2 can be quickly stopped by ammonium molybdate. The residual H2O2 reacts with ammonium molybdate to generate a yellowish complex. CAT activity can be calculated by the production of the yellowish complex at 405 nm. Enzyme-specific activities were expressed as units/mg of protein.
Measuring GPx activity
GPx activity was evaluated in accordance with the protocol. In this assay, GPx catalyzes the reaction between H2O2 and GSH to generate H2O and oxidized GSH (GSSG). The activity of GPx can be expressed by the rate of enzymatic reaction. The activity of glutathione can be calculated by measuring the consumption of reduced glutathione. H2O2 and reduced glutathione can react without catalysis of GPx, so the portion of GSH reduction by non-enzymatic reaction should be subtracted. GSH can react with dinitrobenzoic acid to produce 5-thio-dinitrobenzoic acid anion, which showed a stable yellow color. The absorbance was read at 412 nm using an ELISA plate reader. Enzyme-specific activities were expressed as units/mg of protein.
Apoptosis assay
Cells were cultured in 6-well plates at the density of 5 × 105 per well prior to treatments with melatonin, 5-FU and their combination. Cells receiving no treatment and only fresh medium were considered as a control group. After 48 hours, cells were harvested, washed twice with cold PBS, resuspended in 500 μL of binding buffer followed by the addition of 5 μL annexin V-FITC and 5 μL propidium iodide.
20
After incubation for 15 minutes at dark and room temperature (25°C), they were analyzed by a flow cytometer (FACS Calibur; BD Biosciences; San Jose, CA, USA).
RNA isolation and qRT-PCR
Groups of our investigation were considered as following categories: blank control, melatonin treated, 5-FU treated and melatonin/5-FU combination-treated. Following harvesting the cells after 48 h incubation, total RNA was isolated by guidelines of EZ-10 Total RNA Minipreps Kit and then isolated RNA was quantified by optical density measurement (A260/A280 ratio) using NanoDrop 1000 Spectrophotometer (Wilmington, DE, USA). AccuPowerTM cycle script RT premix kit was used for RT reaction and subsequent Quantitative real-time PCR (qRT-PCR). qRT-PCR was performed using the Maxima Syber Green/ROX qPCR Master Mix in triplicate. Moreover, the primer sequences of β-actin (internal control) and other genes (are described in Table 1. Finally, in order to evaluate the relative mRNA expression levels, 2−(ΔΔCt) method was used.
Table 1.
Primer sequence for genes
|
Genes
|
Forward primer (5′–3′)
|
Reverse primer (5′–3′)
|
| Survivin |
GACCACCGCATCTCTACATTC |
TGCTTTTTATGTTCCTCTATGGG |
| XIAP |
CACTTGAGGTTCTGGTTGCAG |
TGCAAAGCTTCTCCTCTTGC |
| β-actin |
TCCCTGGAGAAGAGCTACG |
GTAGTTTCGTGGATGCCACA |
Western blot
Cultured SW-480 Cell lysate was collected using a RIPA cold buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCL and pH 8). In order to evaluate the protein concentration, Bradford reagent was used and bovine serum albumin (BSA) was applied as the standard protein (Bio-Rad, Hercules, CA, USA). The extracted proteins from samples were separated in an electrophoretic manner using a 10% polyacrylamide gel (80 volts). Then, the separated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane and subsequently, membranes were blocked using 5% skim milk in Tris-buffered saline (10 mM Tris–HCl (pH 7.5), 150 mM NaCl) with 0.02 % Tween 20 for 1 hour. Then, membranes were incubated with mouse monoclonal primary antibodies (1:500) against XIAP (cat. no. sc-55550), survivin (cat. no. sc-17779), Bcl-2 (cat. no. sc-7382), Bax (cat. no. sc-70405), Caspase-3 (cat. no. sc-136219) and β-actin (cat. no. sc-130657), and after overnight, membranes were washed with Tris-buffered saline with 0.02 % Tween 20, finally incubated with goat anti-mouse secondary antibodies conjugated to horseradish peroxidase for one hour. The signals obtained from β-actin were used as an internal control and bond images analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS software using One-way ANOVA POST HOC (Tukey and Dunnett) tests. Results were expressed as mean ± standard deviation (SD) from three independent experiments and P value < 0.05 was considered statistically significant.
Results
Anti-proliferative effects of melatonin and 5-FU on SW-480 cells
To evaluate the inhibitory effects of melatonin and 5-FU, either alone or in concomitant with each other, on the proliferation of CRC cell line, an MTT assay was applied. As shown in Figs. 1A and 1B, 5-FU and melatonin resulted in the significant suppression of cellular proliferation of SW-480 cell lines, in a dose-dependent manner. After 48 hours, the IC50 values for melatonin and 5-FU were 300 ± 0.5 µM and 100 ± 1 µM, respectively.
According to the isobologram analysis, combination therapy significantly decreased cellular proliferation more potently in comparison to monotreatment of melatonin or 5-FU. Table 2 showed the CI values for the combination of various concentrations of melatonin and 5-FU. The co-treatment of 10 µM 5-FU with various concentration of melatonin (75, 150, 200 µM) demonstrated a synergistic effect on SW-480 cells after 48 h incubation (CI < 1; Fig. 1C). After 48 hours incubation, only 50 µM 5-FU along with 150 µM melatonin induced 50% cell death in SW-480 cells synergistically (Table 2). In other words, the combination of melatonin with 5-FU, brought about a 50% decrease in the IC50 value of 5-FU.
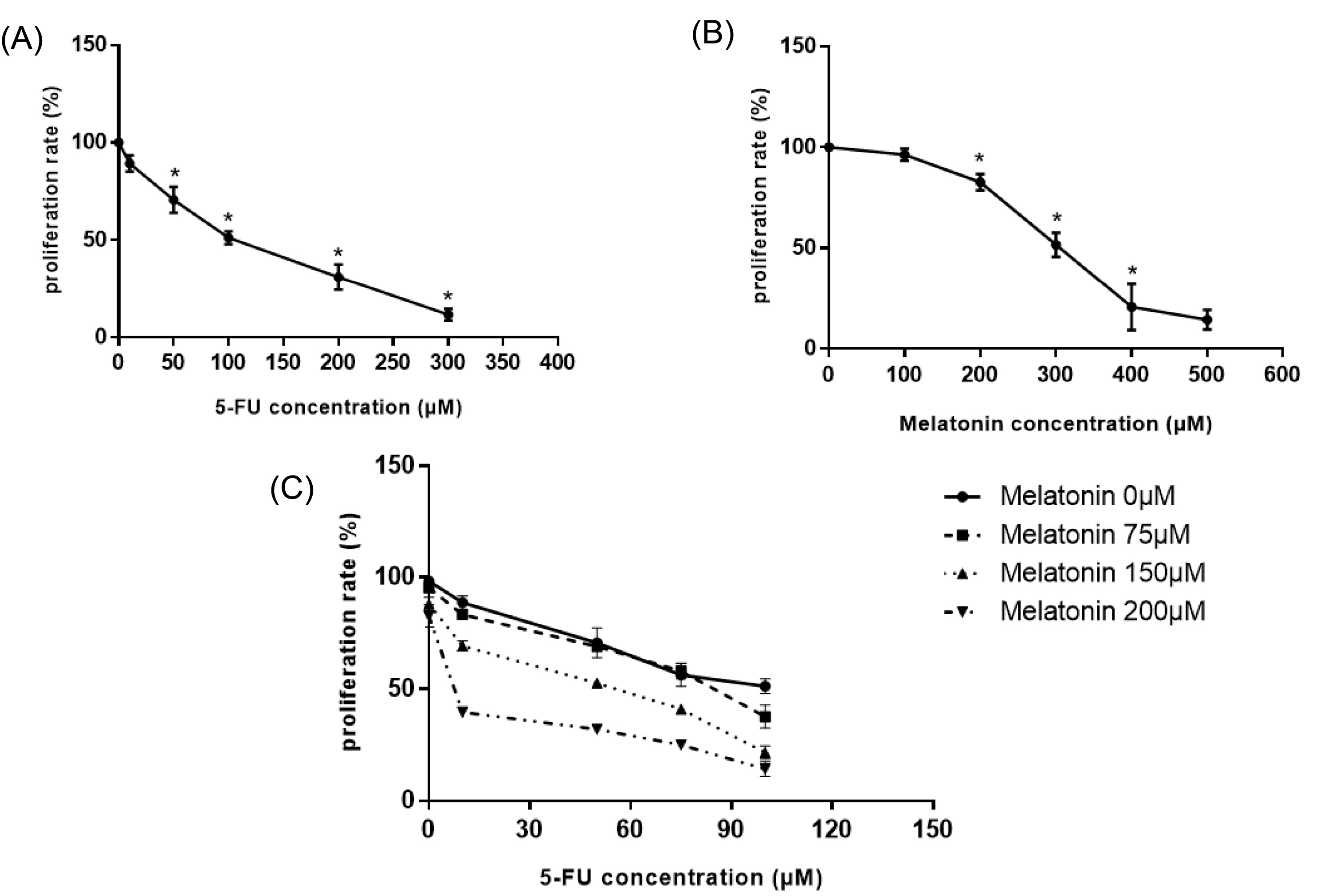
Fig. 1.
Cytotoxic effects of (A) 5-FU, (B) melatonin, and (C) combination of 5-FU and melatonin in various concentrations after 48 h incubation on SW-480 colorectal cancer cell line. Results are shown as mean ± SD for at least 3 independent experiments. * P < 0.05. 5-FU: 5-fluorouracil.
.
Cytotoxic effects of (A) 5-FU, (B) melatonin, and (C) combination of 5-FU and melatonin in various concentrations after 48 h incubation on SW-480 colorectal cancer cell line. Results are shown as mean ± SD for at least 3 independent experiments. * P < 0.05. 5-FU: 5-fluorouracil.
Table 2.
CI values for combination of various concentrations of melatonin and 5-FU
|
Drug combination
|
CI
|
Drug combination
|
CI
|
Drug combination
|
CI
|
| 5-FU 10μM/ MLT 75μM |
0.4 |
5-FU 50μM/ MLT 75μM |
0.8 |
5-FU 75μM/ MLT 75μM |
1.1 |
| 5-FU 10μM/ MLT 150μM |
0.6 |
5-FU 50μM/ MLT 150μM |
0.9 |
5-FU 75μM/ MLT 150μM |
1.3 |
| 5-FU 10μM/ MLT 200μM |
0.9 |
5-FU 50μM/ MLT 200μM |
1.2 |
5-FU 75μM/ MLT 200μM |
1.6 |
5-FU: 5-fluorouracil, CI: combination index, MLT: melatonin.
The effects of melatonin and 5-FU combination on the oxidative stress status in SW-480
One of the main mechanisms involved in the anticancer effects of melatonin is its pro-oxidant functions in cancer cells, hence it is suggested that by increasing intracellular levels of ROS, as well as suppression of antioxidant activity within cells, melatonin triggers cellular death. Therefore, we measured ROS generation in all groups by a fluorescent probe, DCFH-DA. SW-480 cells treatment with 5-FU and melatonin significantly increased ROS levels by 2.2 and 1.9 fold in comparison to controls (P < 0.05; Fig. 2A). Combination treatment of cancer cells with 5-FU and melatonin resulted in a potent increase in the intracellular ROS levels by 2.6 fold in comparison to the control group (P < 0.05; Fig. 2A). In addition to ROS levels, we measured the activity of antioxidant enzymes including CAT, SOD and GPx in all groups to better elucidate possible anti-cancer effects of melatonin. We observed that melatonin and 5-FU resulted in the significant suppression of the enzymatic activities of CAT and SOD (P < 0.05; Figs. 2B and C). However, these two agents failed to significantly reduce the enzymatic activity of GPx in comparison to the control group (P > 0.05; Fig. 2D). Overall, the enzymatic activity of all three antioxidants significantly decreased in the 5-FU + Melatonin group in comparison to either 5-FU or melatonin groups. Therefore, melatonin can enhance 5-FU mediated increase in the ROS levels and by decreasing the antioxidants activities led to cellular death, as explained in the following paragraphs.
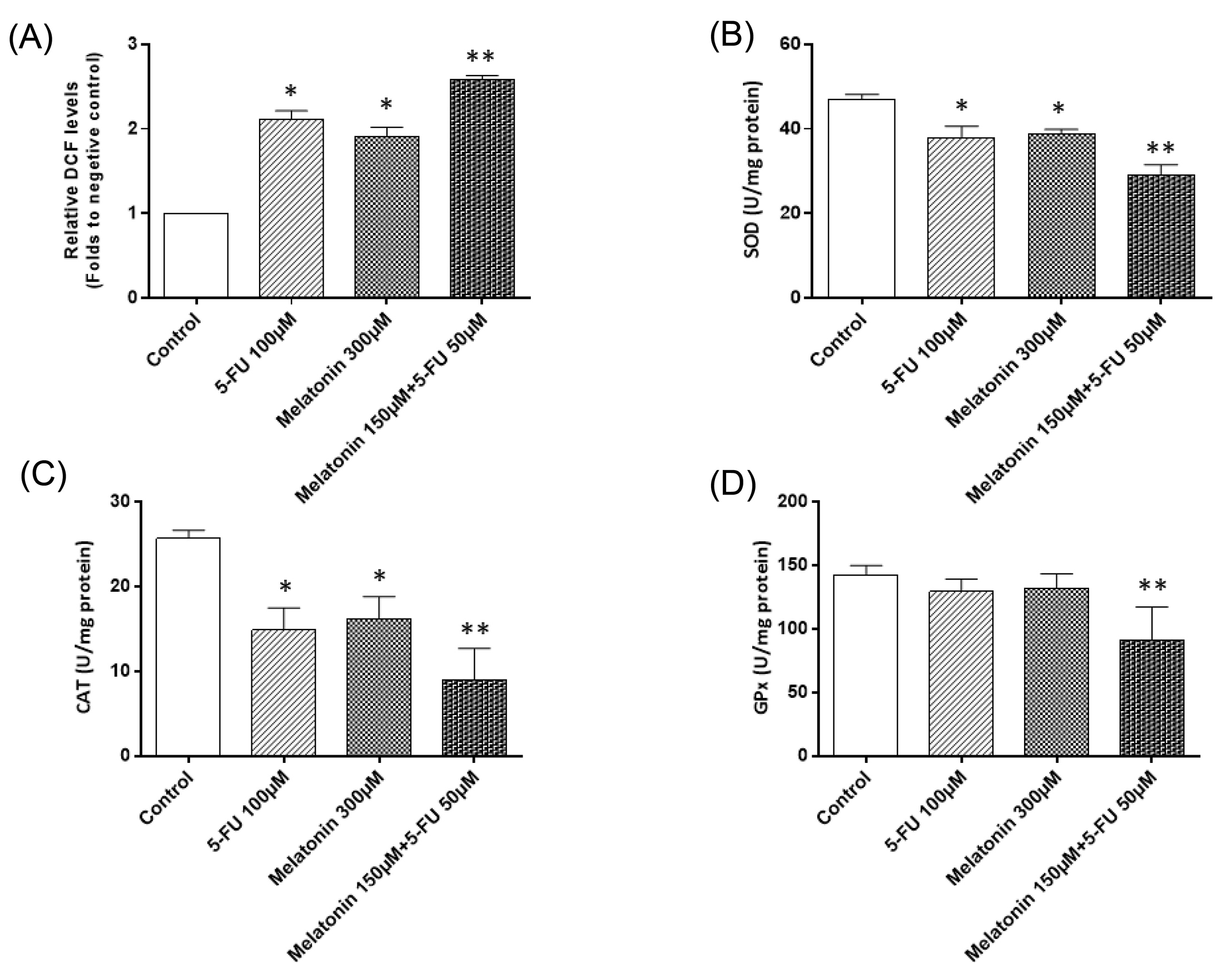
Fig. 2.
The effects of melatonin and 5-FU treatment, alone or in combination, on (A) the ROS levels, (B) superoxide dismutase, (C) catalase, and (D) glutathione peroxidase activities in SW-480 colorectal cancer cell line. The results are expressed as mean ± SD values from at least 3 independent experiments. * P < 0.05 for melatonin and 5-FU treated groups in comparison to the control group, ** P < 0.05 for the combination-treated group in comparison to melatonin and 5-FU treated groups. 5-FU: 5-fluorouracil, ROS: reactive oxygen species, CAT: catalase, SOD: superoxide dismutase, GPx: glutathione peroxidase.
.
The effects of melatonin and 5-FU treatment, alone or in combination, on (A) the ROS levels, (B) superoxide dismutase, (C) catalase, and (D) glutathione peroxidase activities in SW-480 colorectal cancer cell line. The results are expressed as mean ± SD values from at least 3 independent experiments. * P < 0.05 for melatonin and 5-FU treated groups in comparison to the control group, ** P < 0.05 for the combination-treated group in comparison to melatonin and 5-FU treated groups. 5-FU: 5-fluorouracil, ROS: reactive oxygen species, CAT: catalase, SOD: superoxide dismutase, GPx: glutathione peroxidase.
The effects of melatonin and 5-FU combination on the apoptosis in SW-480
In order to examine the effects of monotreatment of melatonin and 5-FU treatment and their concomitant treatment on the apoptosis of the SW-480 cell line, Annexin V flow cytometry was used after 48h treatment. In line with the results obtained from the MTT assay, treatment of cells with melatonin, at the doses of 300 µM and 5-FU, at the dose of 100 µM, induced apoptosis (Fig. 3). In addition, co-treatment of 150 µM melatonin with 50 µM 5-FU increased the migration of SW-480 cells to apoptotic regions as compared to melatonin and 5-FU alone, however, the results were not significant (P > 0.05).
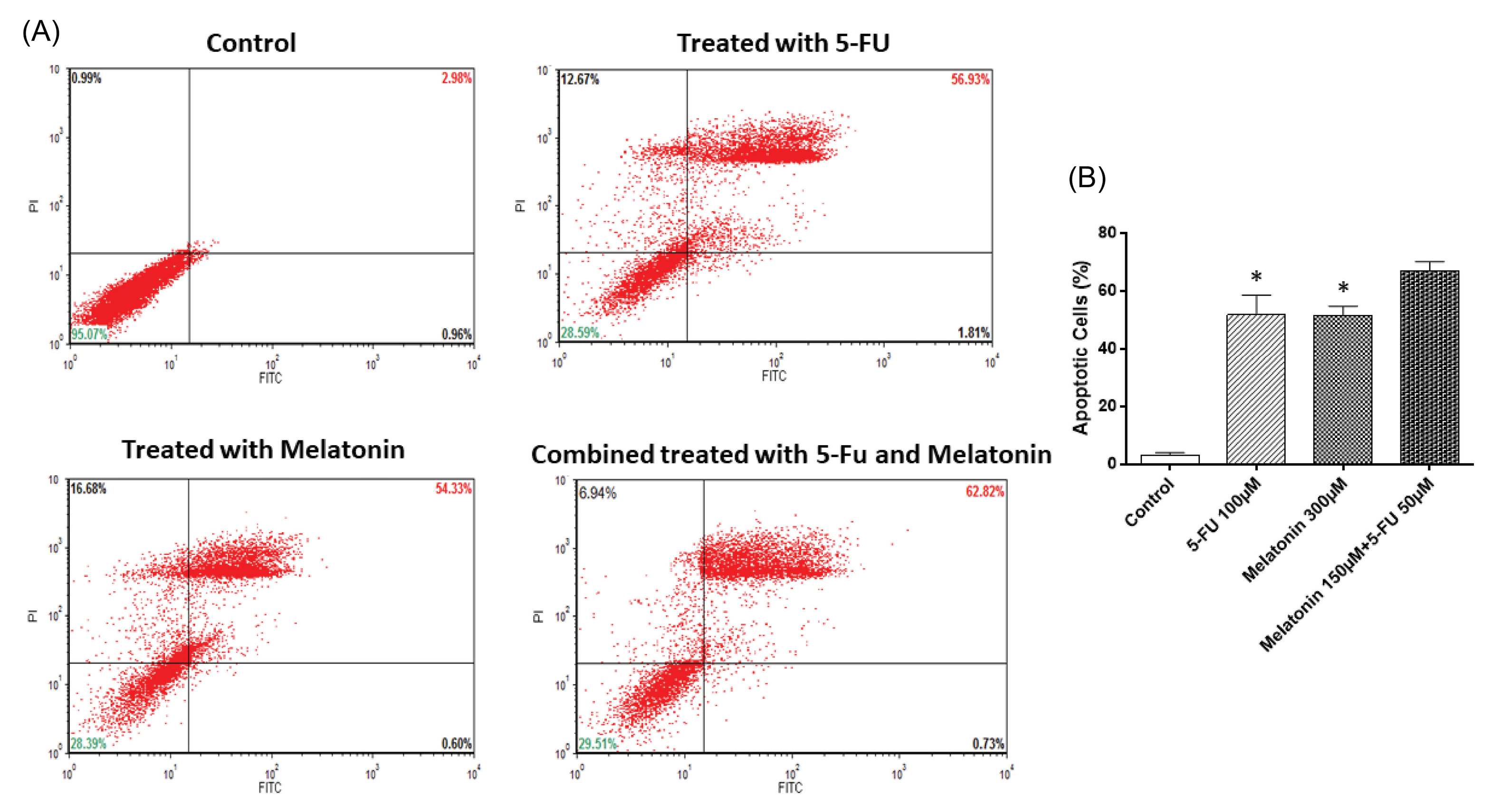
Fig. 3.
Flow cytometry analysis of apoptosis in SW-480 cell line. Cells were treated with f-FU, melatonin and their combination for 48 h and then stained with annexin V/PI. The results are expressed as mean ± SD values from at least 3 independent experiments. * P < 0.05 for melatonin and 5-FU treated groups in comparison to the control group. 5-FU: 5-fluorouracil.
.
Flow cytometry analysis of apoptosis in SW-480 cell line. Cells were treated with f-FU, melatonin and their combination for 48 h and then stained with annexin V/PI. The results are expressed as mean ± SD values from at least 3 independent experiments. * P < 0.05 for melatonin and 5-FU treated groups in comparison to the control group. 5-FU: 5-fluorouracil.
The effects of melatonin and 5-FU combination on the expression levels of apoptotic mediators in SW-480
To approve the pro-apoptotic function of the melatonin and 5-FU combination, we evaluated the protein expression levels of three important mediators of apoptosis including Bax, Bcl-2 and pro-caspase-3/actiated-caspase-3 (Fig. 4A). Western blotting analysis showed that monotreatment of melatonin or 5-FU resulted in a significant increase in the expression levels of pro-apoptotic protein, Bax, and downregulation of anti-apoptotic protein, Bcl-2 (P < 0.05; Figs. 4B and C). Combination treatment exerted a potent effect on the expression levels of Bax (P< 0.05). Additionally, Bax/Bcl-2 ratio was also significantly higher in the combination group in comparison to monotreatments (P < 0.05; Fig. 4D).
The effects of melatonin and 5-FU mono- and combination treatments were also evaluated on pro-caspase-3 and activated caspase-3. Our results demonstrated that melatonin and 5-FU alone significantly increase the activation of pro-caspase into cleaved and activated caspase-3 in comparison to the control group (P < 0.05; Fig. 4E).
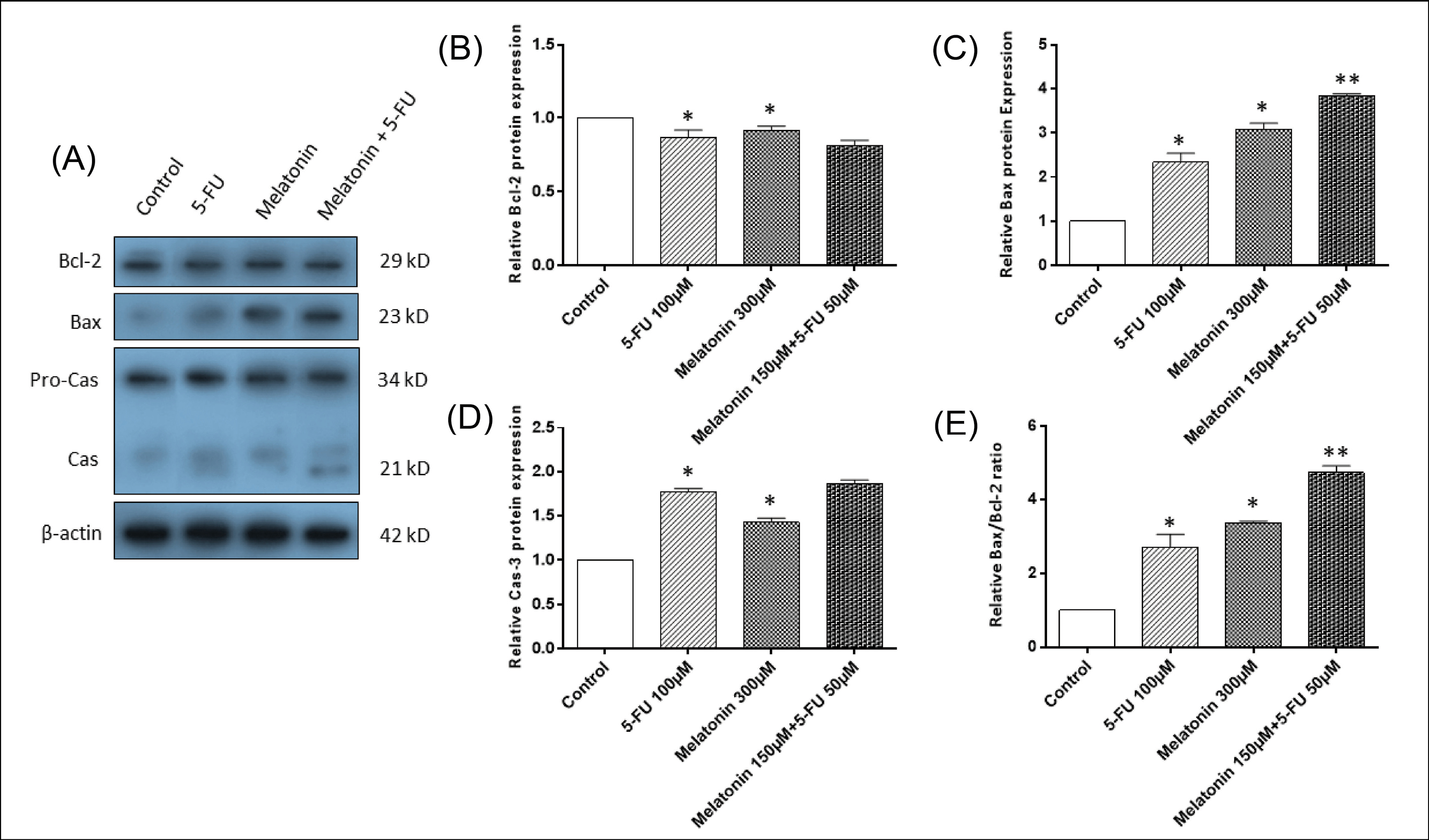
Fig. 4.
The effects of melatonin and 5-FU treatment, alone or in combination, on the expression levels of apoptotic mediators in SW-480 colorectal cancer cell line. (A) Western blotting of apoptotic proteins including Bax, Bcl-2 amd pro-caspase-3/cleaved-caspase-3. β-actin was used as the loading control. (B) Quantitative analysis of Bcl-2, (C) Bax, (D) cleaved-caspase-3, (E) Bax/Bcl-2 ratio levels. The results are expressed as mean ± SD values from at least 3 independent experiments. * P < 0.05 for melatonin and 5-FU treated groups in comparison to the control group, ** P < 0.05 for the combination-treated group in comparison to melatonin and 5-FU treated groups. 5-FU: 5-flourouracil, Cas-3: Caspase-3.
.
The effects of melatonin and 5-FU treatment, alone or in combination, on the expression levels of apoptotic mediators in SW-480 colorectal cancer cell line. (A) Western blotting of apoptotic proteins including Bax, Bcl-2 amd pro-caspase-3/cleaved-caspase-3. β-actin was used as the loading control. (B) Quantitative analysis of Bcl-2, (C) Bax, (D) cleaved-caspase-3, (E) Bax/Bcl-2 ratio levels. The results are expressed as mean ± SD values from at least 3 independent experiments. * P < 0.05 for melatonin and 5-FU treated groups in comparison to the control group, ** P < 0.05 for the combination-treated group in comparison to melatonin and 5-FU treated groups. 5-FU: 5-flourouracil, Cas-3: Caspase-3.
The effects of melatonin and 5-FU co-treatment on the expression levels of survivin and XIAP
For determining the mechanisms underlying increased sensitivity of SW-480 to apoptosis, when combination treatment with melatonin and 5-FU was applied, the expression levels of IAPs including XIAP and survivin were evaluated at the mRNA (Figs. 5A and 5B) and protein levels (Figs. 6A, B, and C). SW-480 cells treatment with 300 µM of melatonin and 100 µM 5-FU, alone, brought about a significant decrease in the mRNA and protein expression levels of both XIAP and survivin (P < 0.05). More importantly, the combination of melatonin and 5-FU downregulated their expression levels more potently in comparison with monotreatment. Collectively, these findings demonstrated that the reversing effect of melatonin on apoptosis resistance in CRC cells is at least mediated by inhibiting XIAP and survivin expression.
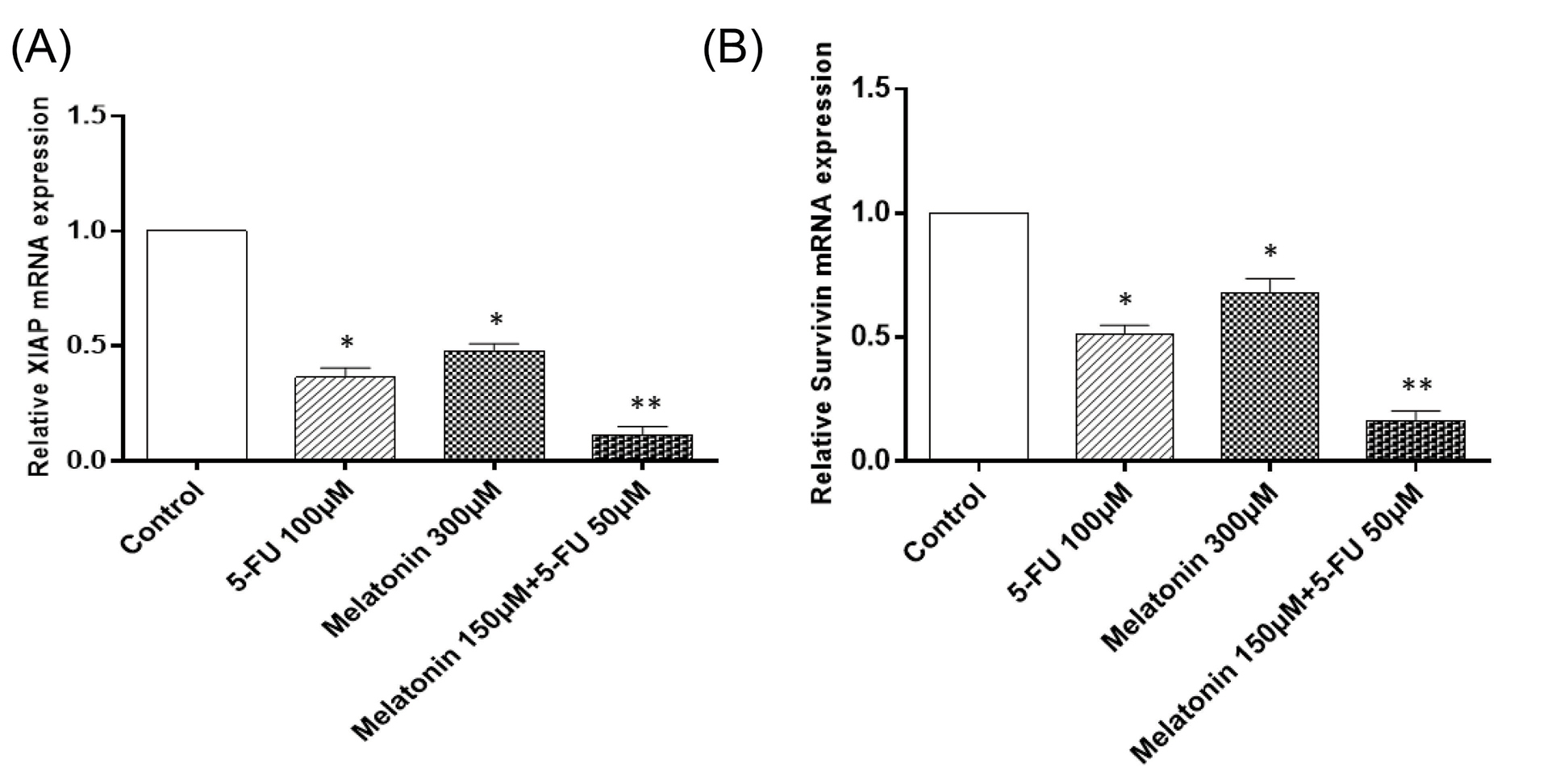
Fig. 5.
The effects of melatonin and 5-FU treatment, alone or in combination, on mRNA levels of (A) XIAP and (B) Survivin in SW-480 colorectal cancer cell line. The results are expressed as mean ± SD values from at least 3 independent experiments. * P < 0.05 for melatonin and 5-FU treated groups in comparison to the control group, ** P < 0.05 for the combination-treated group in comparison to melatonin and 5-FU treated groups. 5-FU: 5-fluorouracil, XIAP: inhibitor of apoptosis proteins.
.
The effects of melatonin and 5-FU treatment, alone or in combination, on mRNA levels of (A) XIAP and (B) Survivin in SW-480 colorectal cancer cell line. The results are expressed as mean ± SD values from at least 3 independent experiments. * P < 0.05 for melatonin and 5-FU treated groups in comparison to the control group, ** P < 0.05 for the combination-treated group in comparison to melatonin and 5-FU treated groups. 5-FU: 5-fluorouracil, XIAP: inhibitor of apoptosis proteins.
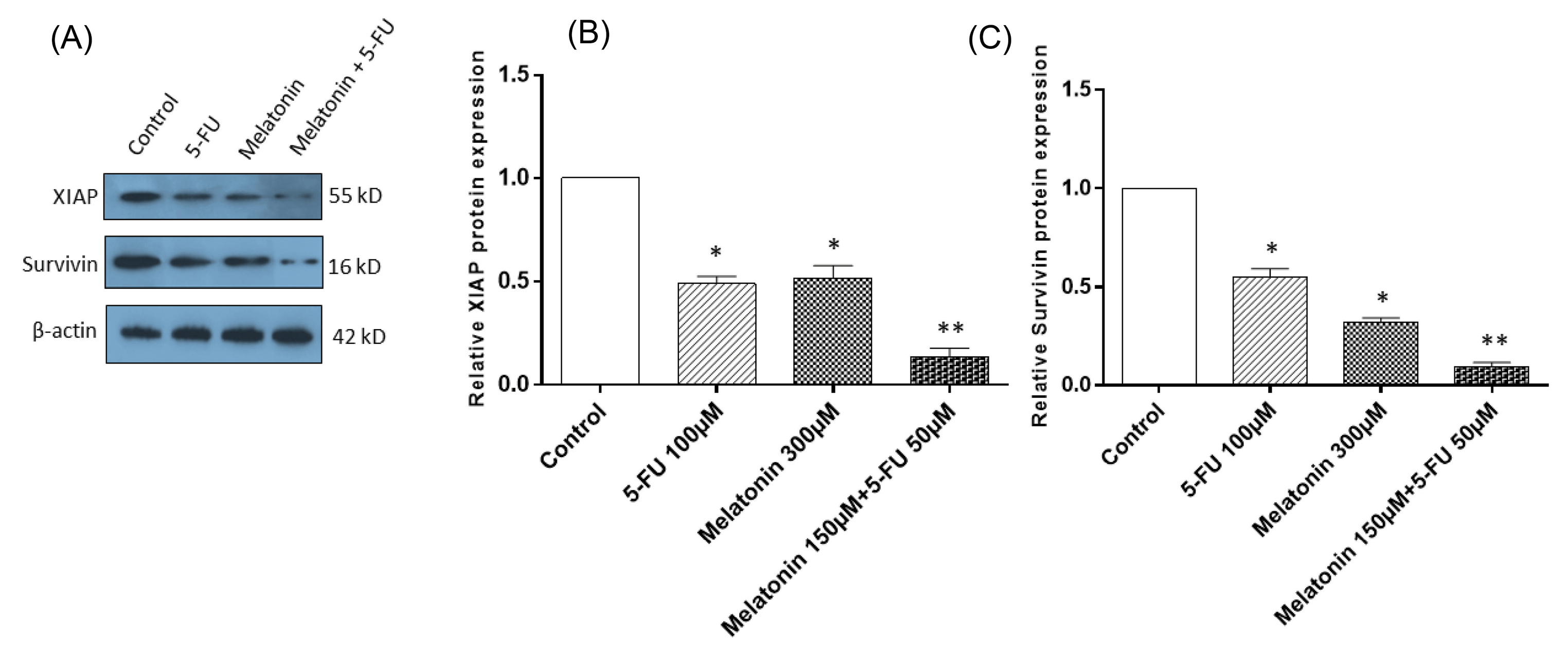
Fig. 6.
The effects of melatonin and 5-FU treatment, alone or in combination, on the expression levels of inhibitors of apoptosis in SW-480 colorectal cancer cell line. (A) Western blotting of inhibitors of apoptosis including XIAP and survivin. β-actin was used as the loading control. (B) Quantitative analysis of XIAP, and (C) survivin. The results are expressed as mean ± SD values from at least 3 independent experiments. * P < 0.05 for melatonin and 5-FU treated groups in comparison to the control group, ** P < 0.05 for the combination-treated group in comparison to melatonin and 5-FU treated groups. 5-FU: 5-fluorouracil, XIAP: inhibitor of apoptosis proteins.
.
The effects of melatonin and 5-FU treatment, alone or in combination, on the expression levels of inhibitors of apoptosis in SW-480 colorectal cancer cell line. (A) Western blotting of inhibitors of apoptosis including XIAP and survivin. β-actin was used as the loading control. (B) Quantitative analysis of XIAP, and (C) survivin. The results are expressed as mean ± SD values from at least 3 independent experiments. * P < 0.05 for melatonin and 5-FU treated groups in comparison to the control group, ** P < 0.05 for the combination-treated group in comparison to melatonin and 5-FU treated groups. 5-FU: 5-fluorouracil, XIAP: inhibitor of apoptosis proteins.
Discussion
In this study, our results showed that melatonin in combination with 5-FU reduced the IC50 value of 5-FU and increased its cytotoxic effects on CRC SW-480 cell lines. In addition, melatonin increased the sensitivity of SW-480 to apoptosis in presence of 5-FU, maybe due to increased oxidative stress and downregulating XIAP and survivin, as the main members of IAPs family. In response to chemotherapeutics, a cascade of cellular events occurs in tumor cells, which exerts significant impacts on proliferation and apoptosis.
21,22
Particularly activation of apoptosis is contributed in the cytotoxic function of various chemotherapeutic, which have extremely demonstrated in in vitro and in vivo investigations.
23-26
As one of the most used chemotherapeutic agents in the treatment of CRC, 5-FU is also demonstrated to selectively act through induction of apoptosis in CRC cells.
27,28
Accumulating recent studies have focused on the IAPs, with a critical role in oncogenesis, because of their apoptosis inhibitory potential.
29
Significant correlation between poor prognosis and expression levels of IAPs, hence the prognostic significance of IAPs has been reported in some of these studies.
30,31
It has been reported that downregulation of IAPs through enhancement of the sensitivity of certain cancer cells to chemotherapeutic agents participates in successful treatment. For example, Hehlgans et al
32
showed that double targeting of survivin and XIAP radiosensitized human colorectal tumor cells and decreased migration. This finding suggests that IAPs may exert critical effects on the initiation and progression of cancer because of regulation of apoptotic pathways. Therefore, exploring underlying mechanisms for overexpression of IAPs and their involvement in the pathogenesis of CRC may open new avenue in order to develop novel therapeutic strategies for CRC. In other words, IAPs could be an innovative and effective therapeutic target with low toxicity against CRC.
Because of the wide range of biological function, and the increasing importance of melatonin, as an anticancer agent, the chemotherapeutic potential of this hormone is demonstrated in various types of human malignancies including CRC. More importantly, recent studies have demonstrated the potential of melatonin in reversing the resistance of CRC cells to various chemotherapeutic agents. For example, Fic et al
33
showed that melatonin reversed the resistance of LoVo CRC cells to doxorubicin by inhibiting the expression levels of p-glycoprotein. In another study, it was reported that melatonin played a critical function in overcoming Oxaliplatin- resistance of CRC cell lines through increasing Oxaliplatin-mediated apoptosis.
34
In consistent with these studies, our results showed that melatonin significantly revered 5-FU induced apoptosis through inhibition of cancer cells proliferation in a dose-dependent manner, and its combination with 5-FU exerted more potent effects. Therefore, melatonin increased the 5-FU mediated apoptosis in SW-480 cell lines. In a study by Fan et al
35
it was demonstrated that melatonin overcame apoptosis resistance in human hepatocellular carcinoma by targeting survivin and XIAP. Melatonin significantly inhibited the growth of HepG2 and SMMC-7721 cells and promoted apoptosis along with the downregulation of survivin and XIAP. In agreement with these findings, our study also showed that melatonin treatment of SW-480 cell lines in combination with 5-FU significantly downregulated the expression of XIAP and survivin. Furthermore, the success of the treatment agent in cancer remedy and induction of apoptosis is related to several key factors such as Bcl-2, Bax and caspase3. Bax as a member of Bcl-2 family participates in the important step of apoptosis by translocation to the mitochondria and subsequently promotion of protein release from intermembrane space into the cytoplasm.
36,37
In accordance with previous studies, our results demonstrated that melatonin in combination with 5-FU induced an increase in Bax expression as well as Bax/Bcl-2 ratio.
ROS have dual functions in cancer, it has already been proven that ROS in higher levels through induction of apoptosis acts as an anti-cancer agent. On the other hand, in moderate levels, ROS through several mechanisms including inflammation, DNA mutation and cellular damage provide an appropriate condition for cancer initiation and progression.
38
ROS overproduction results in potential damages to various macromolecules and hence induction of apoptosis.
39
Our findings showed that combination therapy results in potent ROS overproduction. Similar results have been reported, such that there is evidence that anti-cancer effects of melatonin may be in part mediated through ROS overproduction, hence results in more potent apoptosis especially in combination with other current chemotherapeutic drugs.
40,41
For instance, melatonin co-treatment with cisplatin results in ROS overproduction as well as augmentation of DNA fragmentation.
40
Pro-oxidant activity of melatonin was also found in our study. It is suggested that melatonin plays a substantial role in decreasing antioxidant levels through the reduction of SOD, CAT, GPx, glutathione reductase, and glutathione S-transferase activities. Several studies have pinpointed the fact that reduction of antioxidant levels has paramount importance in the successful treatment of cancer. Similar findings are reported by Buldak et al
41
in which anti-cancer effects of melatonin mediated through enhancement of MDA levels and reduction of antioxidant enzymes.
Conclusion
Our results showed that melatonin may exert a beneficial effect by reversing the resistance to apoptosis through increasing oxidative stress and targeting XIAP and survivin in SW-480 CRC cells. In other words, melatonin may target two key components of IAPs, as well as oxidative stress, through modulation of cellular response to apoptosis and oxidative stress, increase the cytotoxicity of 5-FU. This may open new insights into the molecular mechanisms of melatonin-mediated increasing in 5-FU induced apoptosis in CRC and propose that melatonin has the potential to consider as a promising agent for the successful treatment of CRC. Particularly, since 5-FU has various serious side effects, the combination of melatonin may be effective in decreasing 5-FU effective dose hence reduces its severe side effects in patients with CRC.
Funding sources
This study was financially supported by the Vice-Chancellor for Research of Urmia University of Medical Sciences grant (No. 348), Urmia, Iran.
Ethical Issues
Not applicable.
Competing interests
There is not any conflict of interest.
Authors’ contribution
BY contributed to data analysis, data presentation, and writing and reviewing of the manuscript. AM contributed to the cell culture, flow cytometry and fluorometry experiments and collected the data. SGD contributed to the experiments including Cell Culture, MTT assay and Western Blotting; SS involved in qRT-PCR and oxidative stress analysis; MK contributed in paper revision and English editing. MM contributed to the study consultation, conceptualization of the manuscript, and overall writing and editing of the manuscript. All authors discussed the contents and contributed to the final manuscript.
Research Highlights
What is the current knowledge?
simple
-
√ Melatonin can increase the sensitization of antineoplastic agents such as 5-FU by increased response to treatment.
-
√ Melatonin is a potent pro-apoptotic and pro-oxidant agent in various cancer types.
What is new here?
simple
-
√ Melatonin increase 5-FU-mediated apoptosis in SW-480 cancer cell lines through downregulation of XIAP and Survivin.
-
√ Melatonin enhances the sensitivity of SW-480 cells to 5-FU by increasing ROS levels and suppressing antioxidant defense.
References
- Mirza-Aghazadeh-Attari M, Darband SG, Kaviani M, Mihanfar A, Attari JA, Yousefi B. DNA damage response and repair in colorectal cancer: Defects, regulation and therapeutic implications. DNA Rep 2018; 69:34-52. doi: 10.1016/j.dnarep.2018.07.005 [Crossref] [ Google Scholar]
- Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PloS One 2013; 8:e53916. doi: 10.1371/journal.pone.0053916 [Crossref] [ Google Scholar]
-
Macrae FA. Colorectal cancer: Epidemiology, risk factors, and protective factors. 2016. https://www.uptodate.com/contents/colorectal-cancer-epidemiology-risk-factors-and-protective-factors/.
- Lech G, Słotwiński R, Słodkowski M, Krasnodębski IW. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J Gastroenterol 2016; 22:1745. doi: 10.3748/wjg.v22.i5.1745 [Crossref] [ Google Scholar]
- Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med 2015; 66:83-95. doi: 10.1146/annurev-med-051513-102539 [Crossref] [ Google Scholar]
- Linnekamp JF, Wang X, Medema JP, Vermeulen L. Colorectal cancer heterogeneity and targeted therapy: a case for molecular disease subtypes. Cancer Res 2015; 75:245-9. doi: 10.1158/0008-5472.CAN-14-2240 [Crossref] [ Google Scholar]
- Bahrami A, Hassanian SM, ShahidSales S, Farjami Z, Hasanzadeh M, Anvari K. Targeting RAS signaling pathway as a potential therapeutic target in the treatment of colorectal cancer. J Cell Physiol 2018; 233:2058-66. doi: 10.1002/jcp.25890 [Crossref] [ Google Scholar]
- Abraha AM, Ketema EB. Apoptotic pathways as a therapeutic target for colorectal cancer treatment. World J Gastrointest Oncol 2016; 8:583. doi: 10.4251/wjgo.v8.i8.583 [Crossref] [ Google Scholar]
- Gil‐Martín E, Egea J, Reiter RJ, Romero A. The emergence of melatonin in oncology: Focus on colorectal cancer. Med Res Rev 2019; 39:2239-2285. doi: 10.1002/med.21582 [Crossref] [ Google Scholar]
- Majidinia M, Reiter RJ, Shakouri SK, Yousefi B. The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Ageing Res Rev 2018; 47:198-213. doi: 10.1016/j.arr.2018.07.010 [Crossref] [ Google Scholar]
- Majidinia M, Reiter RJ, Shakouri SK, Mohebbi I, Rastegar M, Kaviani M. The multiple functions of melatonin in regenerative medicine. Ageing Res Rev 2018; 45:33-52. doi: 10.1016/j.arr.2018.04.003 [Crossref] [ Google Scholar]
- Asghari MH, Ghobadi E, Moloudizargari M, Fallah M, Abdollahi M. Does the use of melatonin overcome drug resistance in cancer chemotherapy?. Life Sci 2018; 196:143-55. doi: 10.1016/j.lfs.2018.01.024 [Crossref] [ Google Scholar]
- Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer 2015; 14:48. doi: 10.1186/s12943-015-0321-5 [Crossref] [ Google Scholar]
- Yousefi B, Azimi A, Majidinia M, Shafiei-Irannejad V, Badalzadeh R, Baradaran B. Balaglitazone reverses P-glycoprotein-mediated multidrug resistance via upregulation of PTEN in a PPARγ-dependent manner in leukemia cells. Tumour Biol 2017; 39:1010428317716501. doi: 10.1177/1010428317716501 [Crossref] [ Google Scholar]
- Jaiswal PK, Goel A, Mittal R. Survivin: A molecular biomarker in cancer. Indian J Med Res 2015; 141:389. doi: 10.4103/0971-5916.159250 [Crossref] [ Google Scholar]
- Bucur O. microRNA regulators of apoptosis in cancer. Discoveries (Craiova) 2016; 4:e57. doi: 10.15190/d.2016.4 [Crossref] [ Google Scholar]
- Knauer SK. Survivin antagonizes chemotherapy-induced cell death of colorectal cancer cells. Oncotarget 2018; 9:27835. doi: 10.18632/oncotarget.25600 [Crossref] [ Google Scholar]
- Shojaei F, Yazdani-Nafchi F, Banitalebi-Dehkordi M, Chehelgerdi M, Khorramian-Ghahfarokhi M. Trace of survivin in cancer. Eur J Cancer Prev 2019; 28:365-72. doi: 10.1097/CEJ.0000000000000453 [Crossref] [ Google Scholar]
- Mihanfar A, Darband SG, Sadighparvar S, Kaviani M, Mirza-Aghazadeh-Attari M, Yousefi B. In vitro and in vivo anticancer effects of syringic acid on colorectal cancer: Possible mechanistic view. Chem Biol Interact 2021; 337:109337. doi: 10.1016/j.cbi.2020.109337 [Crossref] [ Google Scholar]
- Mashayekhi S, Yousefi B, Tohidi E, Darband SG, Mirza‐Aghazadeh‐ Attari M, Sadighparvar S. Overexpression of tensin homolog deleted on chromosome ten (PTEN) by ciglitazone sensitizes doxorubicin‐resistance leukemia cancer cells to treatment. J Cell Biochem 2019; 120:15719-29. doi: 10.1002/jcb.28841 [Crossref] [ Google Scholar]
- Johnstone RW, Ruefli AA, Lowe S. Apoptosis: a link between cancer genetics and chemotherapy. Cell 2002; 108:153-64. doi: 10.1016/s0092-8674(02)00625-6 [Crossref] [ Google Scholar]
- Nourazarian SM, Nourazarian A, Majidinia M, Roshaniasl EJAPJoCP. Effect of root extracts of medicinal herb Glycyrrhiza glabra on HSP90 gene expression and apoptosis in the HT-29 colon cancer cell line. Asian Pac J Cancer Prev 2016; 16:8563-6. doi: 10.7314/apjcp.2015.16.18.8563 [Crossref] [ Google Scholar]
- Prabhudesai S, Rekhraj S, Roberts G, Darzi A, Ziprin P. Apoptosis and chemo‐resistance in colorectal cancer. J Surg Oncol 2007; 96:77-88. doi: 10.1002/jso.20785 [Crossref] [ Google Scholar]
- Mehdizadeh A, Bonyadi M, Darabi M, Rahbarghazi R, Montazersaheb S, Velaei K. Common chemotherapeutic agents modulate fatty acid distribution in human hepatocellular carcinoma and colorectal cancer cells. Bioimpacts 2017; 7:31-9. doi: 10.15171/bi.2017.05 [Crossref] [ Google Scholar]
- Rafi M. Gene and stem cell therapy: alone or in combination?. Bioimpacts 2011; 1:213-8. doi: 10.5681/bi.2011.030 [Crossref] [ Google Scholar]
- Khiavi MA, Safary A, Somi M. Recent advances in targeted therapy of colorectal cancer: impacts of monoclonal antibodies nanoconjugates. Bioimpacts 2019; 9:123. doi: 10.15171/bi.2019.16 [Crossref] [ Google Scholar]
- Rigas A, Dervenis C, Giannakou N, Kozoni V, Shiff SJ, Rigas B. Selective induction of colon cancer cell apoptosis by 5-fluorouracil in humans. Cancer Invest 2002; 20:657-65. doi: 10.1081/cnv-120002491 [Crossref] [ Google Scholar]
- Arango D, Wilson A, Shi Q, Corner G, Aranes M, Nicholas C. Molecular mechanisms of action and prediction of response to oxaliplatin in colorectal cancer cells. Br J Cancer 2004; 91:1931. doi: 10.1038/sj.bjc.6602215 [Crossref] [ Google Scholar]
- Ndubaku C, Cohen F, Varfolomeev E, Vucic D. Targeting inhibitor of apoptosis proteins for therapeutic intervention. Future Med Chem 2009; 1:1509-25. doi: 10.4155/fmc.09.116 [Crossref] [ Google Scholar]
- Chen L, Liang L, Yan X, Liu N, Gong L, Pan S. Survivin status affects prognosis and chemosensitivity in epithelial ovarian cancer. Int J Gynecol Cancer 2013; 23:256-63. doi: 10.1097/IGC.0b013e31827ad2b8 [Crossref] [ Google Scholar]
- Rosato A, Menin C, Boldrin D, Dalla Santa S, Bonaldi L, Scaini MC. Survivin expression impacts prognostically on NSCLC but not SCLC. Lung Cancer 2013; 79:180-6. doi: 10.1016/j.lungcan.2012.11.004 [Crossref] [ Google Scholar]
- Hehlgans S, Petraki C, Reichert S, Cordes N, Rödel C, Rödel FJR. Double targeting of Survivin and XIAP radiosensitizes 3D grown human colorectal tumor cells and decreases migration. Radiotherapy and Oncology 2013; 108:32-9. doi: 10.1016/j.radonc.2013.06.006 [Crossref] [ Google Scholar]
- Fic M, Gomulkiewicz A, Grzegrzolka J, Podhorska-Okolow M, Zabel M, Dziegiel P. The impact of melatonin on colon cancer cells’ resistance to doxorubicin in an in vitro study. Int J Mol Sci 2017; 18:1396. doi: 10.3390/ijms18071396 [Crossref] [ Google Scholar]
- Lee JH, Yoon YM, Han Y-S, Yun CW, Lee SH. Melatonin Promotes Apoptosis of Oxaliplatin-resistant Colorectal Cancer Cells Through Inhibition of Cellular Prion Protein. Anticancer Res 2018; 38:1993-2000. doi: 10.21873/anticanres.12437 [Crossref] [ Google Scholar]
- Fan L, Sun G, Ma T, Zhong F, Wei W. Melatonin overcomes apoptosis resistance in human hepatocellular carcinoma by targeting S urvivin and XIAP. J Pineal Res 2013; 55:174-83. doi: 10.1111/jpi.12060 [Crossref] [ Google Scholar]
- Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 1997; 385:637. doi: 10.1038/385637a0 [Crossref] [ Google Scholar]
- Kang DW, Choi CH, Park JY, Kang SK, Kim YK. Ciglitazone induces caspase-independent apoptosis through down-regulation of XIAP and survivin in human glioma cells. Neurochem Res 2008; 33:551-61. doi: 10.1007/s11064-007-9475-x [Crossref] [ Google Scholar]
- Lin S, Li Y, Zamyatnin Jr AA, Werner J, Bazhin AV. Reactive oxygen species and colorectal cancer. J Cell Physiol 2018; 233:5119-32. doi: 10.1002/jcp.26356 [Crossref] [ Google Scholar]
- Mangal D, Vudathala D, Park J-H, Lee SH, Penning TM, Blair IA. Analysis of 7, 8-dihydro-8-oxo-2′-deoxyguanosine in cellular DNA during oxidative stress. Chem Res Toxicol 2009; 22:788-97. doi: 10.1021/tx800343c [Crossref] [ Google Scholar]
- Pariente R, Bejarano I, Rodríguez AB, Pariente JA, Espino J. Melatonin increases the effect of 5-fluorouracil-based chemotherapy in human colorectal adenocarcinoma cells in vitro. Mol Cell Biochem 2018; 440:43-51. doi: 10.1007/s11010-017-3154-2 [Crossref] [ Google Scholar]
- Bułdak RJ, Pilc-Gumuła K, Bułdak Ł, Witkowska D, Kukla M, Polaniak R. Effects of ghrelin, leptin and melatonin on the levels of reactive oxygen species, antioxidant enzyme activity and viability of the HCT 116 human colorectal carcinoma cell line. Mol Med Rep 2015; 12:2275-82. doi: 10.3892/mmr.2015.3599 [Crossref] [ Google Scholar]