Bioimpacts. 11(3):209-217.
doi: 10.34172/bi.2021.28
Original Research
Repair of rat cranial bone defect by using amniotic fluid-derived mesenchymal stem cells in polycaprolactone fibrous scaffolds and platelet-rich plasma
Zeinab Ghaffarinovin 1  , Omid Soltaninia 2, Yousef Mortazavi 1, 3, Abdolreza Esmaeilzadeh 3, 4, Samad Nadri 3, 5, 6, 7, *
, Omid Soltaninia 2, Yousef Mortazavi 1, 3, Abdolreza Esmaeilzadeh 3, 4, Samad Nadri 3, 5, 6, 7, * 
Author information:
1Department of Medical Biotechnology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
2Department of Oral & Maxillofacial Surgery, Hamadan University of Medical Sciences, Hamadan, Iran
3Cancer Gene therapy Research Center, Zanjan University of Medical Sciences, Zanjan, Iran
4Department of Immunology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
5Department of Medical Nanotechnology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
6Zanjan Metabolic Diseases Research Center, Zanjan University of Medical Sciences, Zanjan, Iran
7Zanjan Pharmaceutical Nanotechnology Research Center, Zanjan University of Medical Sciences, Zanjan, Iran
Abstract
Introduction:
Tissue regenerative medicine strategies, as a promising alternative has become of major interest to the reconstruction of critical size bone defects. This study evaluated the effects of the simultaneous application of polycaprolactone (PCL), amniotic fluid mesenchymal stem cells (AF-MSCs) and platelet-rich plasma (PRP) on the repair of rat cranial bone defects.
Methods:
The AF-MSCs were isolated at the end of the second week of pregnancy in rats. PRP obtained from rat blood and the random PCL fibrous scaffolds were prepared using the electrospinning method. Circular full thickness (5 mm) bone defects were developed on both sides of the parietal bones (animal number=24) and the scaffolds containing AF-MSCs and PRP were implanted in the right lesions. Thereafter, after eight weeks the histological and immunohistochemistry studies were performed to evaluate the bone formation and collagen type I expression.
Results:
The spindle-shaped mesenchymal stem cells were isolated and the electron microscope images indicated the preparation of a random PCL scaffold. Immunohistochemical findings showed that collagen type I was expressed by AF-MSCs cultured on the scaffold. The results of hematoxylin and eosin (H&E) staining indicated the formation of blood vessels in the presence of PRP. Additionally, immunofluorescence findings suggested that PRP had a positive effect on collagen type I expression.
Conclusion:
The simultaneous application of fibrous scaffold + AF-MSCs + PRP has positive effects on bone regeneration. This study showed that PRP can affect the formation of new blood vessels in the scaffold transplanted in the bone defect.
Keywords: Platelet rich plasma, Amniotic fluid-derived mesenchymal stem cells, Polycaprolacton, Fibrous scaffold, Rat cranial bone defect
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Unlike small bone defects that can improve spontaneously or with minimal treatment, a critical-size bone defect requires extensive interventions.
1
The treatment of these lesions is still a challenge for surgeons. Annually over half a million cases of patients need bone procedures to correct such defects in the USA, which is expected to be double by 2020 in the United States and worldwide because of the growing needs of the baby-boomer population and increased life expectancy.
2
Annually more than 2 million bone grafts are performed worldwide and the annual treatment cost in the US only is estimated to be $5 billion. This makes bone the most frequent tissue transplantation after blood transfusion.
3-6
Traditional treatments including autografts, allografts and biocompatible prosthetic devices, have a unique set of drawbacks.
7,8
Although autologous bone grafts is a gold standard treatment for these lesions,
8-11
the growing demand and limitation of autografts such as the restricted amount of bone supply, graft failure, infection, donor-site pain and morbidity have made this field to require new techniques with appropriate features.
7,8,10
Tissue regenerative medicine strategies, as a promising alternative, have become of major interest to the reconstruction of critical size bone defects.
4,6,12,13
The first step in bone tissue engineering is understanding the bone structure, bone mechanics, and tissue formation. A bone tissue engineering system includes four main players: the scaffold, cells, morphogenic signals and vascularization.
2
The extracellular matrix (ECM) is composed of both structural and functional proteins that play many critical roles in the cell and tissue.
14-17
The scaffold can be considered as an artificial ECM that provides matrix biological functions and mechanical strength for each tissue and organ.
16
Features of a scaffold vary according to the type of tissue.
A perfect scaffold should possess all the qualities of a native ECM. Biocompatibility, biodegradability, adequate mechanical strength, nontoxicity, nonimmunogenicity and porosity with interconnected pores are some of the features of an appropriate scaffold.
18,19
Polycaprolactone (PCL) is a biodegradable polymer with good thermal stability, good mechanical strength, miscibility with a wide range of other polymers and long degradation time.
20-23
In the last decade, many studies have been conducted on the placenta, the fetal and amniotic fluid stem cells.
24
For the first time, Kaviani et al introducedamniotic fluid stem cells as a new source for tissue engineering.
25
These cells can be collected during amniocentesis, a safe procedure for the prenatal diagnosis without harm to the fetus. Therefore, AF-MSCs are ready accessible.
26,27
Unlike embryonic stem cells, the amniotic fluid stem cells are not tumorigenic potential after in vivo transplantation and do not have an ethical problem related to these cells.
28
In tissue engineering, growth factors are routinely used to accelerate the regeneration process.
29,30
Platelets play a major role in the healing process by having multiple growth factors.
31
Platelet-rich plasma (PRP) is by definition, a high concentration of platelets in a small volume of plasma which is a rich source of autologous growth factors.
32
Therefore, PRP can potentially improve the repair process through the release of various growth factors and cytokines in alpha granules.
30,33,34
The present study aimed to evaluate the effect of the simultaneous application of AF-MSCs, PCL scaffold with PRP in the repair of critical-sized bone defects of rats.
Material and Methods
Animals
Twenty-four Sprague-Dawley male rats (4-8-weeks old), mean weight of approximately 525 g, were used following a protocol approved by the Institutional Animal Care and Use Committee, Pasteur Institute, Karaj, Iran. They were housed one per plastic cage in a monitored environment (21°C; 12:12 light cycle). They had ad libitum access to drinking water and a standard laboratory rat food pellet diet.
AF-MSCs isolation and culture
AF-MSCs were isolated as previously described by Nadri and colleagues.
35
Amniotic fluid samples were collected from pregnant Sprague-Dawley rats at the end of the 2nd week. After anesthesia, the female rats were sacrificed by diaphragm incision and the uterus containing multiple embryos was removed. The uterus was placed in a petri dish and washed with phosphate-buffered saline (PBS). The amniotic fluid was then drawn from the bag using an insulin syringe. An amniotic fluid sample was cultured in 6-well plates in a medium consisting of Dulbecco's modified Eagle medium (DMEM; Gibco-BRL Grand Island, NY, USA) supplemented with 15% FBS (Sigma, St Louis, MO, USA), 100 U/mL penicillin, 100 U/mL streptomycin, 2 mM L-glutamine, 1.25 mg/mL amphotericin (Sigma), and incubated at 37°C with 5% humidified CO2 and 95% humidity for 2 weeks. The culture medium was changed after 2 days and subsequently every 3 days. Then, the cells were trypsinized and transferred to new 6-well plates.
Characterization of mesenchymal stem cells
In this study, we used bone, cartilage and fat differentiation test to identify the nature of the isolated cells. The confluent cells were cultured in an osteogenic (DMEM including 50 µg/mL ascorbic acid 3-phosphate (Sigma Chemical Co. St Louis, MO, USA), 10 nM dexamethasone (Sigma Chemical Co.), 10 mM β-glycerol phosphate (Sigma Chemical Co.), and adipogenic (DMEM supplemented with 50 µg/mL ascorbic acid 2-phosphate (Sigma Chemical Co), 100 nM dexamethasone (Sigma Chemical Co.), 50 µg/mL indomethacin (Sigma Chemical Co), chondrogenic (DMEM supplemented with 50 µg/mL ascorbic acid 2-phosphate (Sigma Chemical Co), 10 nM dexamethasone (Sigma Chemical Co.), transforming growth factor-ß3 (TGF-ß3; Sigma Chemical Co), bone morphogenetic protein- 6 (BMP-6), and insulin– transferrin– selenium (ITS; GIBCO- BRL) medium. At the end of the differentiation period, the cells were evaluated byalizarin red staining for osteoblasts,alcian blue staining for chondroblasts andoil red staining for adipocytes.
PRP preparation
Initially, rats were anesthetized by the injection of xylazine (6 mg/kg) and ketamine (70 mg/kg). A total of 3-mL volume of arterial blood was obtained from the heart. The blood was transferred to a 5 mL silicone tube containing 0.35 mL of 10% sodium citrate. For the preparation of PRP, two-stage centrifuges were performed on samples. In the first stage, the blood was centrifuged at 200 g for 20 minutes at 22°C to separate plasma from the red cells. In the second stage, plasma was drawn off the top and centrifuged at 22°C for an additional 8 minutes at 400 g to separate the platelets. PRP was settled at the bottom in a 5-ml layer. Next, the amount of platelet in PRP and peripheral blood was measured using a cell counter.
Fabrication of electrospun fibrous PCL scaffold
Fibrous PCL scaffolds were fabricated using an electrospinning technique. We dissolved 1.22 g PCL (MW: 80000, sigma) in 9 mL chloroform (Sigma, Steinheim, Germany) and 1 mL Dimethylformamide (DMF). The solution was transferred to a pump syringe and used a thin polyethylene tube to transfer the solution from syringe to nozzle. Random fibrous scaffolds were obtained at the voltage of 24 kV, high (2500 rpm) speed rotating disk, feed rate 0.3 mL/h and the distance between the tips of the needle 17 cm. To increase the compatibility and hydrophilicity of scaffolds, surface modification was performed using plasma treatment. We used scanning electron microscopy(SEM)and contact angle methodsto evaluate the diameter, shape and hydrophilicity of the fibrous structure, respectively. The dried–scaffolds were sputter-coated with gold and observed by an SEM (Seron technology, AIS2100).
MTT assay
The cell viability of AF-MSCs on the scaffold and tissue-culture polystyrene (TCPS) dishes was assessed by colorimetric MTT assay. The cells (3000 cells) were cultured in the TCPS and the scaffold. The samples (After 2, 4, and 6 days of cell seeding) were incubated with MTT solution (5 mg/mL in DMEM) for 3 hours at 37°C. Following that, the supernatant (remained MTT solution) was removed, the DMSO solvent was added to cultivated cells on TCPS and scaffold, and the absorbance of the final solution was read at 570 nm using a microplate reader (ELX800; BioTeK, Winooski, VT). To remove all the purple dye inside the scaffold, the scaffold was vortexed violently and several times.
Implant preparation
Before cell transplantation, AF-MSCs were labeled with lipophilic tracer CM-Dil (Invitrogen, UK) according to the manufacturer's protocol and scaffold sterilized by its immersion in alcohol 70% for 30 minutes and treated under UV irradiation for 10 minutes. Briefly, AF-MSCs were immersed in 1 µL Dil for 5 minutes at 37°C and then 15 minutes at 4°C. Then, 18 000 cells and PRP were loaded onto sterilized scaffolds in target groups. Grafting materials were maintained for 6 hours in DMEM medium supplemented with 15% FBS and antibiotic at 37°C and 5% CO2. Then, the cultured medium was changed to an osteogenic medium for 12 hours before in vivoimplantation. The cell viability and adherent on the scaffold were investigated via MTT assay and SEM imaging. The cell scaffolds were fixed in glutaraldehyde, dehydrated by increasing concentrations of ethanol (70-100%), dried, sputter-coated with gold and observed by an SEM (Seron technology, AIS2100).
Surgical procedure
The animals were anesthetized with an intraperitoneal injection of ketamine (70 mg/kg) and xylazine (6 mg/kg). The 24 rats were divided into eight equal experimental groups. The scalp was shaved and disinfected with iodine 1%. An incision of about 2 cm was made along the sagittal suture. Following the reflection of the periosteal flap, two symmetric full-thickness cranial defects (5 mm) were created by a dental bur under saline irrigation (Fig. 1). The right defect was filled with grafting materials according to Table 1 and the left one remained empty as the control. The incision was sutured with 4-0 silk (Supa, Iran). Local tetracycline was used to prevent infection.
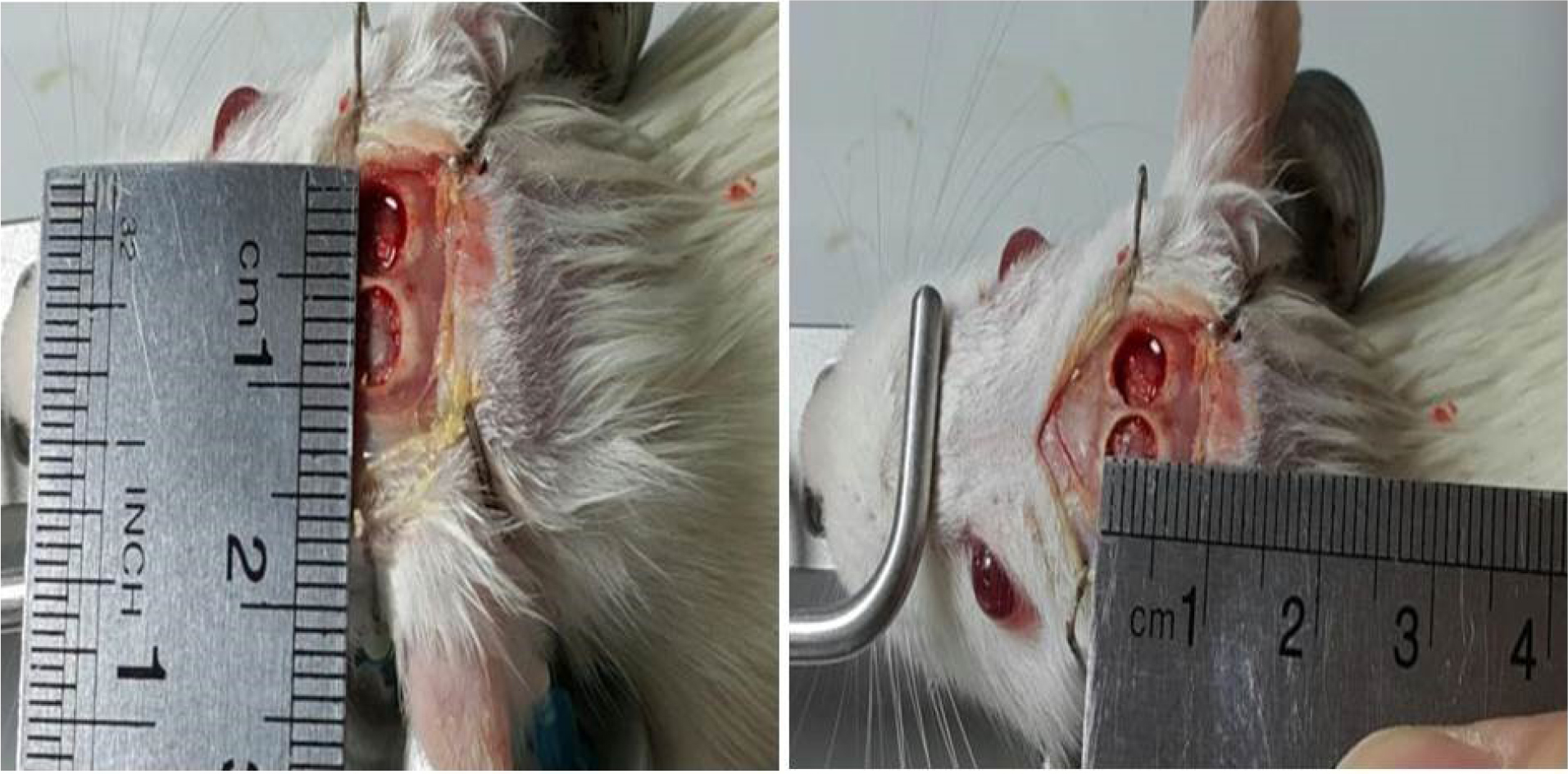
Fig. 1.
Rat cranial bone defect. Two symmetric full-thickness cranial defects (5×5 mm) were made on the skull.
.
Rat cranial bone defect. Two symmetric full-thickness cranial defects (5×5 mm) were made on the skull.
Table 1.
Treatment Groups
|
Group
|
Rat number
|
Graft at right defect
|
Graft at left defect
|
| 1 |
3 |
AF-MSCs + pPCL + PRP |
Empty |
| 2 |
3 |
AF-MSCs + PCL + PRP |
Empty |
| 3 |
3 |
AF-MSCs + pPCL |
Empty |
| 4 |
3 |
AF-MSCs + PCL |
Empty |
| 5 |
3 |
pPCL + PRP |
Empty |
| 6 |
3 |
PCL + PRP |
Empty |
| 7 |
3 |
pPCL |
Empty |
| 8 |
3 |
PCL |
Empty |
AF-MSCs: Amniotic fluid-mesenchymal stem cells; pPCL: plasma-treated PCL scaffold; PCL: plasma-untreated PCL scaffold; PRP: platelet-rich plasma.
Histological evaluation
Animals were sacrificed at week 8 post-implantation. Calvarial specimens were fixed in 4% paraformaldehyde for 1 week. The samples were decalcified in 10% nitric acid and then dehydrated with increasing concentrations of ethyl alcohol (70-100%) infiltrated with paraffin. A serial section was cut (5 µm) parallel to the midsagittal suture from the center of each defect using a microtome and stained with hematoxylin and eosin (H&E). The most central portion of each defect was identified and subject to histologic analysis.
Immunohistochemistry analysis
Immunohistochemical procedure was performed on 5 µm sections of calvarial specimens. The section constructs (section from microtome) were permeabilized with 0.5% Triton X-100 for 10 minutes and then stained with antibody for collagen type I (the most abundant protein in bone) as primary antibody (sc‐59772; 1:1000; Santa Cruz Biotechnology, USA). Goat anti-mouse secondary antibody-fluorescein conjugate (ab97022; 1:250; Abcam, USA) was added to enable colored presentation. Images were captured using a fluorescent microscope.
Statistical Analysis
Two-way ANOVA was done for MTT (sample number=3) and contact angle (sample number =3) analysis.
Results
In vitro differentiation of AF-MSCs
In this study, we isolated the spindle-shaped morphology cells based on the ability of adhesion to TCPS (Fig. 2). After 3 weeks of differentiation in the induction medium, specific staining confirmed the nature of the isolated cells. A few days after the treatment with adipoinduction media, fat droplets were visible. Over time, in some cells, all of the cytoplasms slowly accumulated from lipid droplets, proved withoil red staining. Alizarin red staining showed the formation of mineralized nodules. This structure presented bone differentiation of mesenchymal cells. Alcian blue staining demonstratedthechondrogenic differentiation capacity of AF-MSCs, as a result of this coloring ECM was stained dark-blue (Fig. 3).
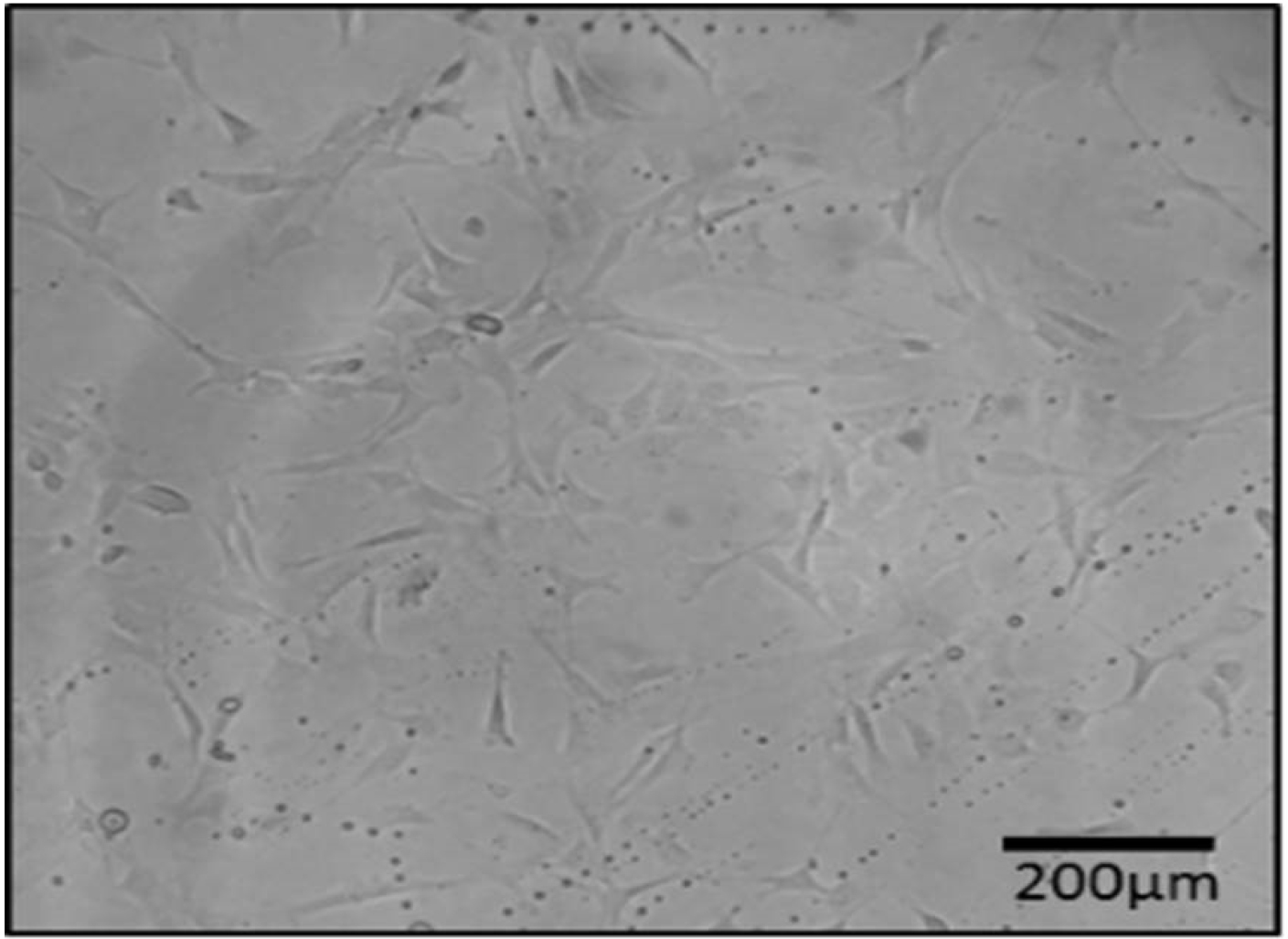
Fig. 2.
Spidle-shape morphology of isolated cells from rat amniotic fluid (AF).
.
Spidle-shape morphology of isolated cells from rat amniotic fluid (AF).
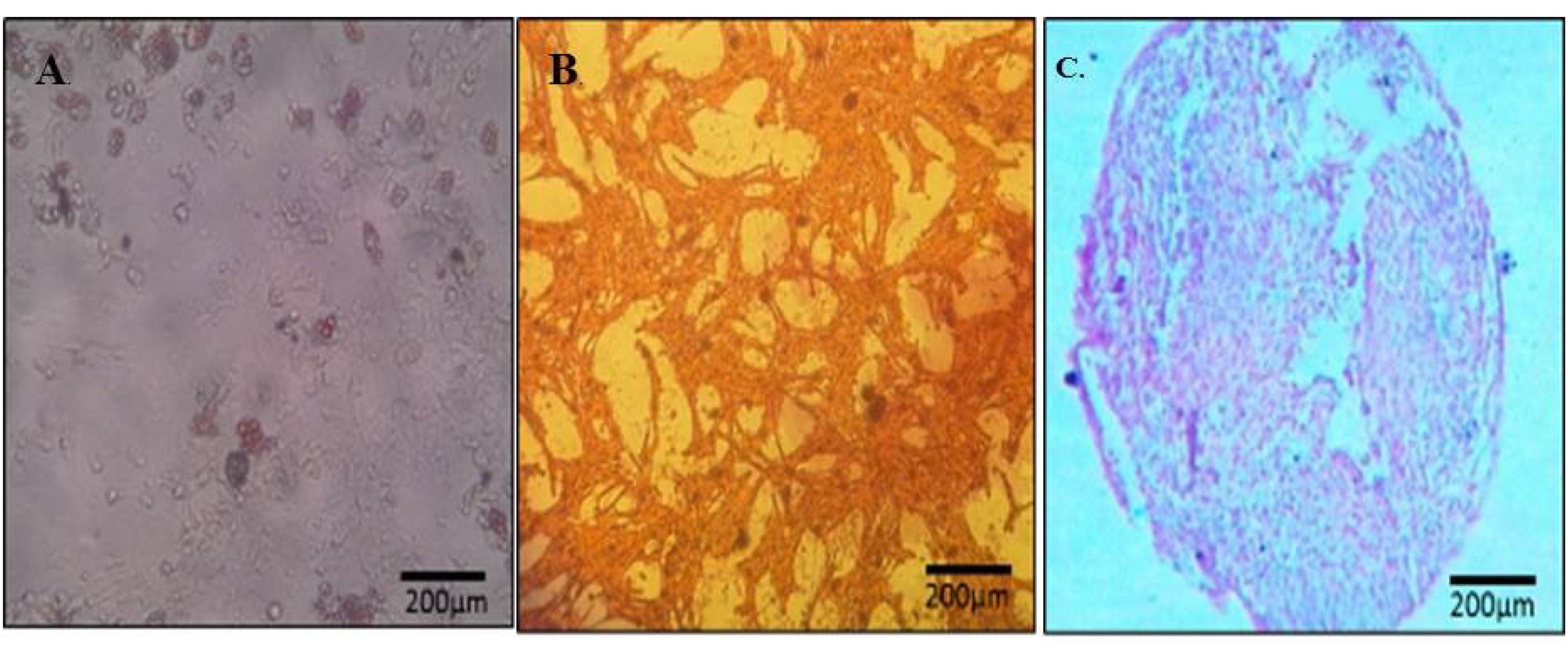
Fig. 3.
Nature of mesenchymal stem cells confirmed by oil red (A), alizarin red (B) and aclian blue staining (C).
.
Nature of mesenchymal stem cells confirmed by oil red (A), alizarin red (B) and aclian blue staining (C).
Platelet count
Platelet count was performed by an automated cell counter. The platelet number of the rat whole blood concentrates was 340 000 platelets/µL; the platelet count in PRP was 896 000 platelets/µL. Platelet number in PRP rose compared with the whole blood, which was on average a 2.6-fold increase.
Fabrication of fibrous PCL scaffold
SEM images demonstrated that PCL-based fibers resulted in a scaffold were composed of uniform fibers without any beads (Diameter Mean =1526 nm) which the cells were spread on those (Fig. 4). The contact angle gained from the untreated PCL scaffold and the plasma-treated scaffolds were 120°± 6° and zero°, respectively (Fig. 5). This established that the surface of the scaffold was hydrophilic.
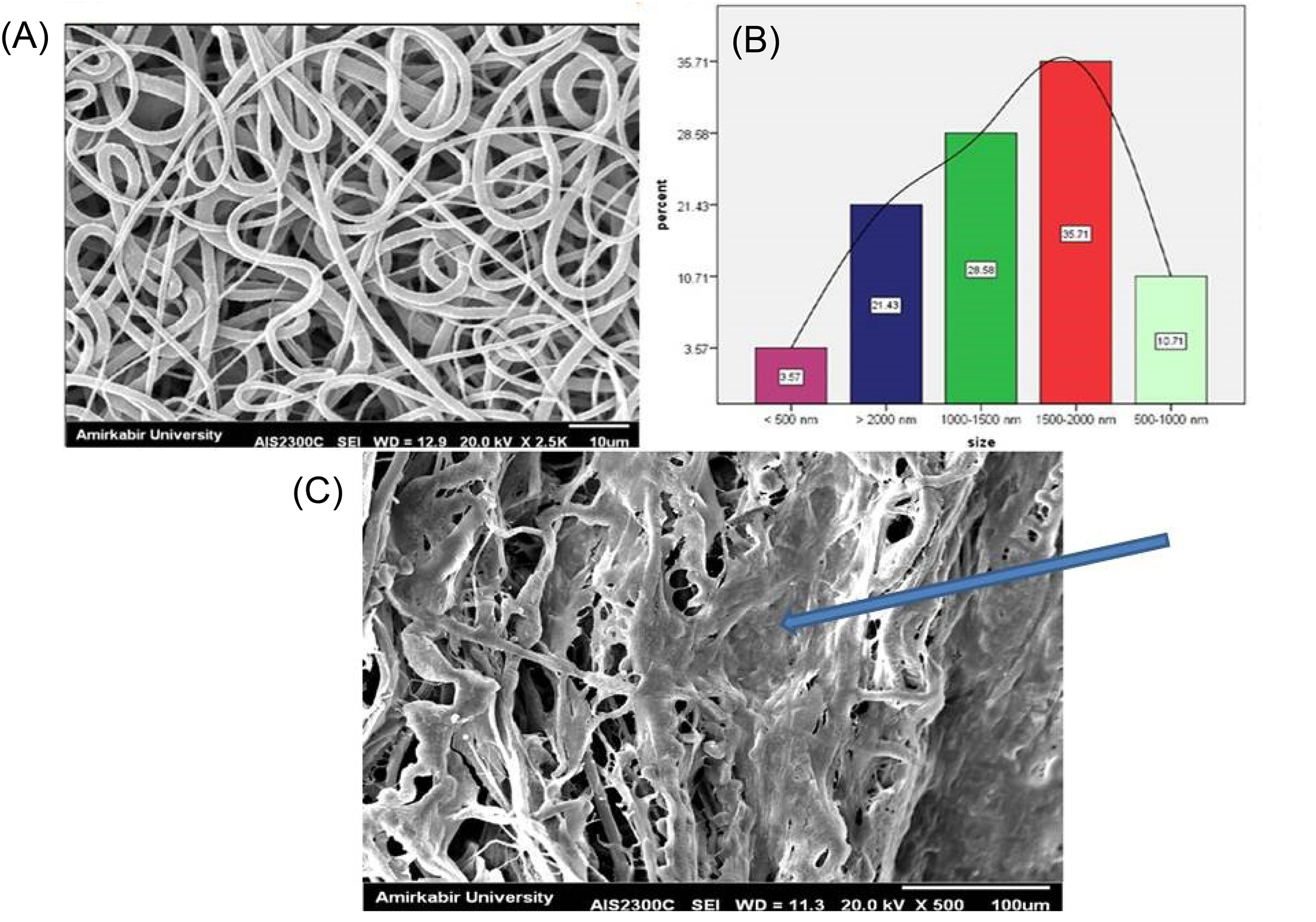
Fig. 4.
SEM images of the electrospun fibrous scaffold of PCL polymer (A), the size distribution of fiber (B) and cell-seeded on the scaffold (C). The arrow shows the seeded cells on the scaffold.
.
SEM images of the electrospun fibrous scaffold of PCL polymer (A), the size distribution of fiber (B) and cell-seeded on the scaffold (C). The arrow shows the seeded cells on the scaffold.
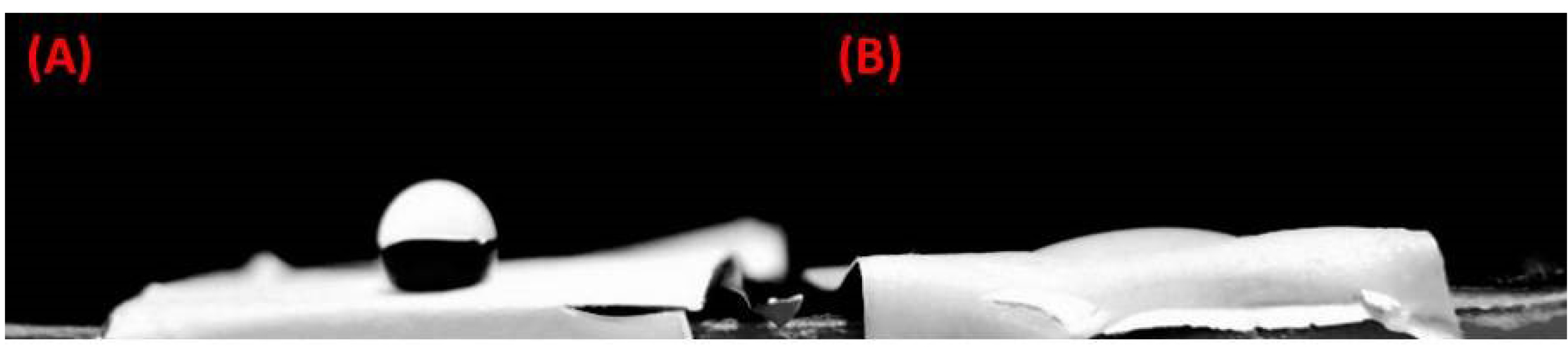
Fig. 5.
The contact angle of the scaffold before (A) and after (B) plasma treatment (sample number=3).
.
The contact angle of the scaffold before (A) and after (B) plasma treatment (sample number=3).
Cell viability
We used the MTT assay to assess the cell viability of AF-MSCs on the PCL fibrous scaffold and TCPS after 2, 4 and 6 days of cell culture. The cell proliferation on TCPS was significantly higher than that on PCL fibrous scaffold (Fig. 6).
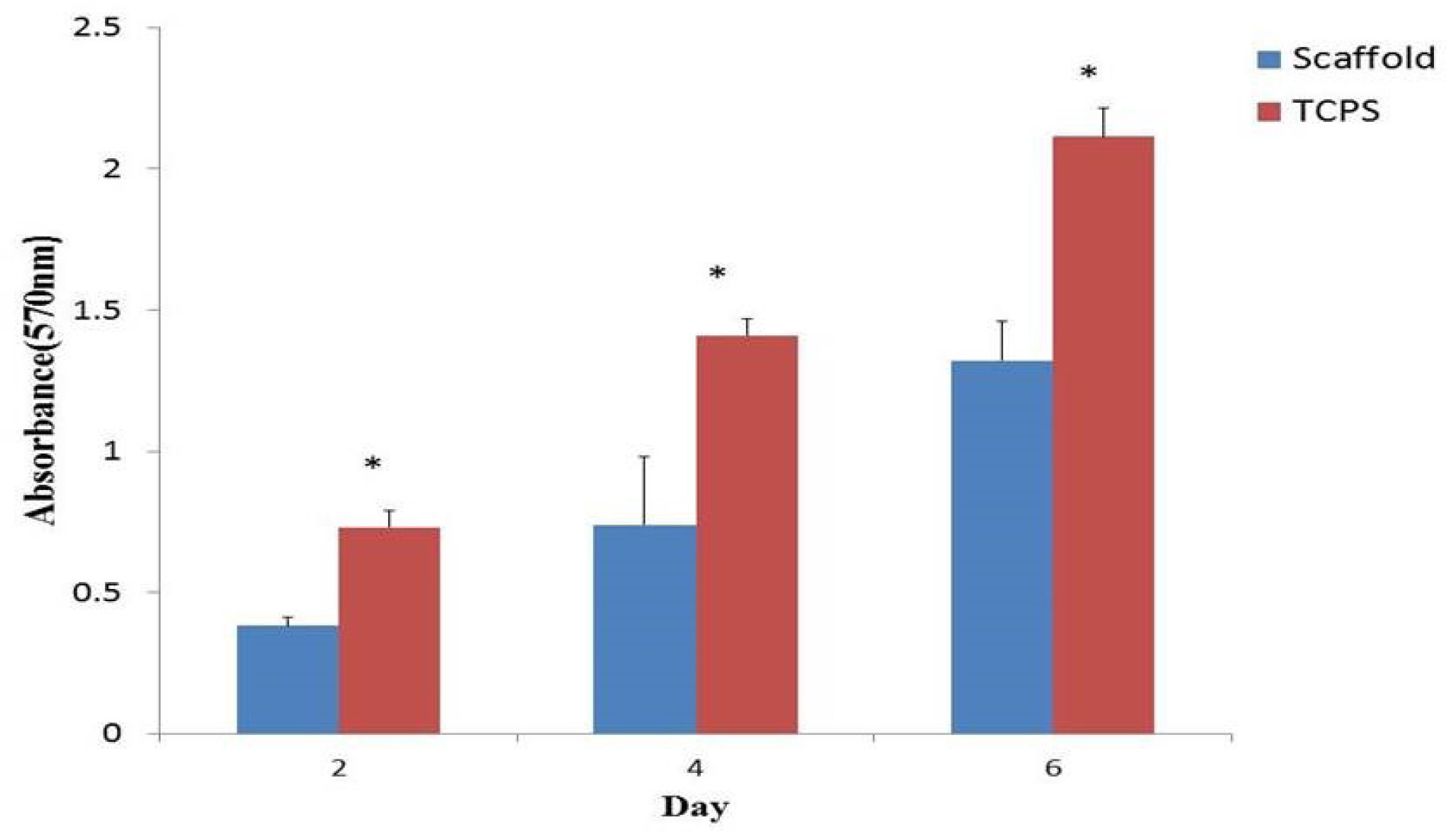
Fig. 6.
Cell viability assay of Amniotic Fluid-Mesenchymal Stem Cells (AF-MSCs) cultured on polycaprolactone (PCL) scaffold and tissue-culture polystyrene (TCPS) at days 2, 4 and 6 (sample number = 3; P≤0.05).
.
Cell viability assay of Amniotic Fluid-Mesenchymal Stem Cells (AF-MSCs) cultured on polycaprolactone (PCL) scaffold and tissue-culture polystyrene (TCPS) at days 2, 4 and 6 (sample number = 3; P≤0.05).
Histological analysis
Representative images of histologic observations for the cranial defect at 8 weeks post-implantation are described in Fig. 7. We analyzed a total of 21 experimental rats for histological and immunohistochemical evaluation of defect sites. Blood vessel formation was observed in some groups. Our findings showed that the blood vessel was formed in all groups with PRP versus groups without PRP. It can be seen from Fig. 7 that the presence of PRP had a positive effect on the blood vessel formation in the transplanted scaffold.
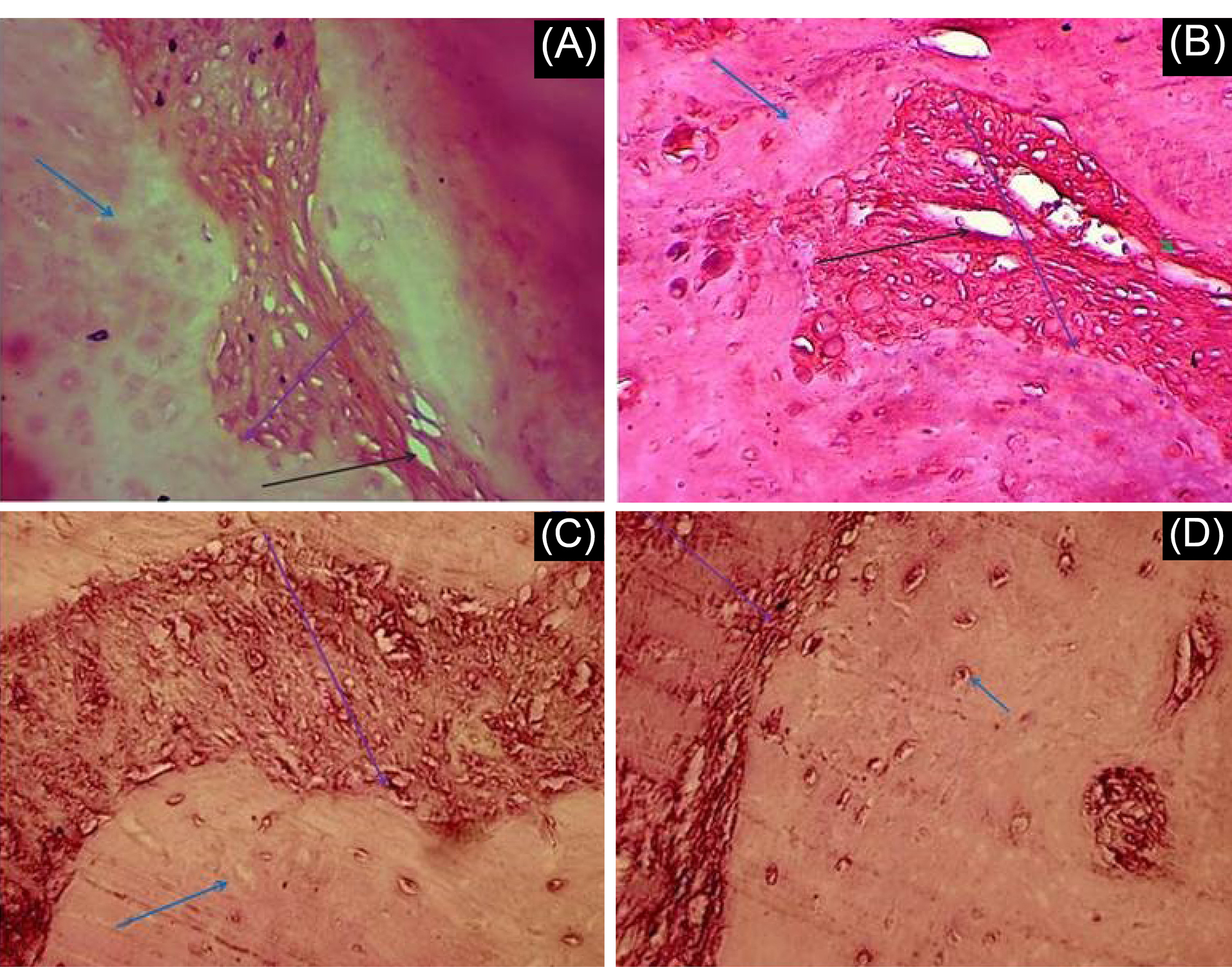
Fig. 7.
Histological results of Hematoxylin and Eosin (H&E) staining. Cranial defect filled with A) AF-MSCs+pPCL+PRP B) AF-MSCs+PCL+PRP C) AF-MSCs+pPCL D) AF-MSCs+PCL. Blue, black and purple arrows show respectively host bone, blood vessel and scaffold. Scale bar = 100 µm.
.
Histological results of Hematoxylin and Eosin (H&E) staining. Cranial defect filled with A) AF-MSCs+pPCL+PRP B) AF-MSCs+PCL+PRP C) AF-MSCs+pPCL D) AF-MSCs+PCL. Blue, black and purple arrows show respectively host bone, blood vessel and scaffold. Scale bar = 100 µm.
Immunohistochemical observation
Histological methods were used to assess the bone regeneration at the site of rat calvarial defect. Dil staining and immunostaining were employed to detect transplanted AF-MSCs and determining collagen content, respectively. Eight weeks after transplantation, AF-MSCs were recognizable in all groups embedded with cells. Positive immunoreaction for collagen type I was observed in some groups. The highest collagen type I expression (as the main marker of bone reconstruction) was documented in the rats that were cured with AF-MSCs+scaffold+PRP 8 weeks after implantation (Fig. 8). Improved collagen type I deposition was detected in animals that were given AF-MSCs+scaffold+PRP compared to animals given AF-MSCs+scaffold. Immunofluorescence results demonstrated the collagen type I expression in implanted cells. Additionally, it can be seen from Fig. 7 that the presence of PRP had a positive effect on collagen type I expression in the transplanted scaffold.
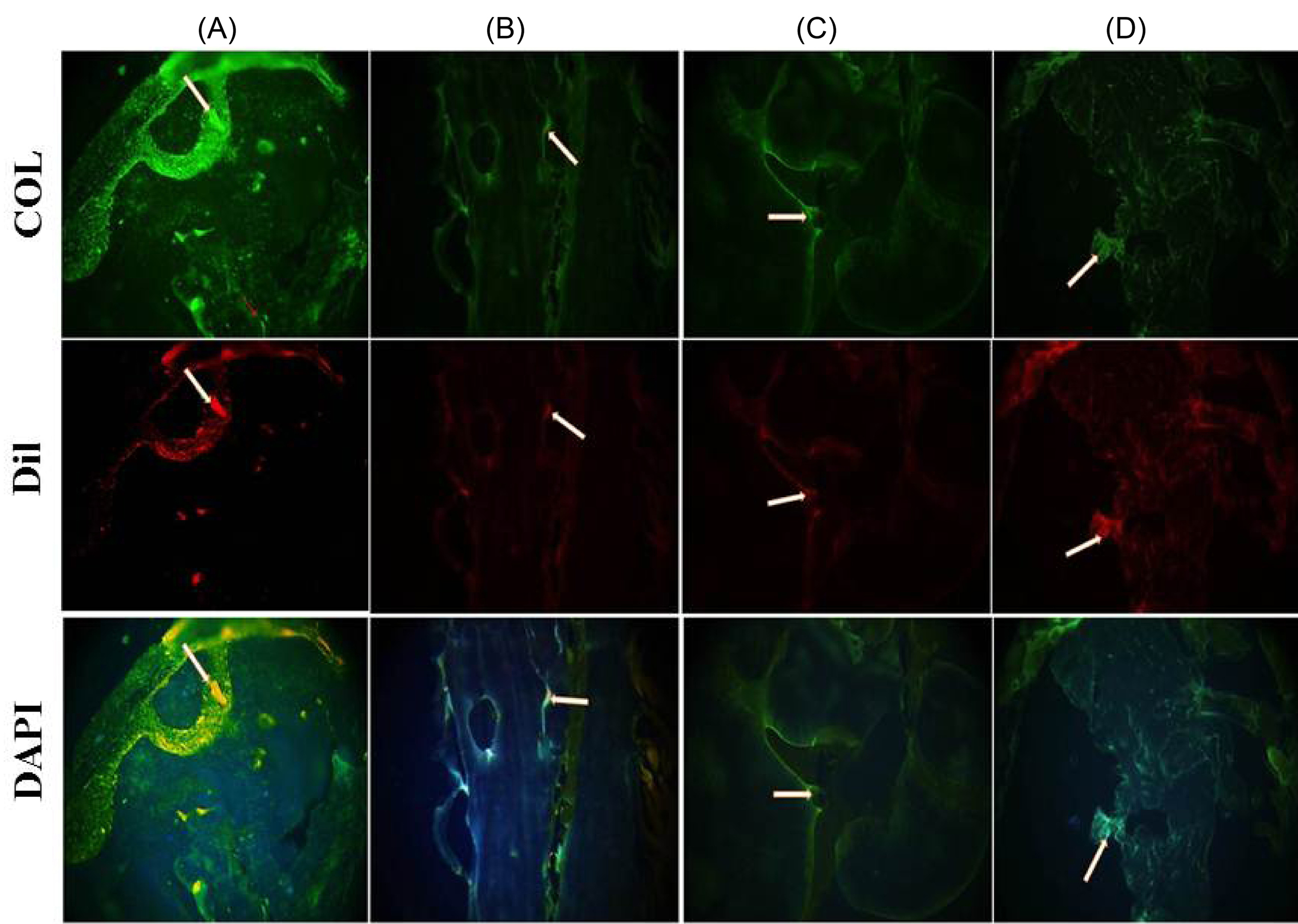
Fig. 8.
Immunohistochemical analysis for collagen type I detection. Fluorescent microscope images showing the signals from anti-collagen I (green), Dil (red) and DAPI (blue) in constructs of the A) AF-MSCs + pPCL + PRP, B) AF-MSCs + PCL + PRP, C) AF-MSCs + pPCL, D) AF-MSCs + PCL. Scale bar = 200µm.
.
Immunohistochemical analysis for collagen type I detection. Fluorescent microscope images showing the signals from anti-collagen I (green), Dil (red) and DAPI (blue) in constructs of the A) AF-MSCs + pPCL + PRP, B) AF-MSCs + PCL + PRP, C) AF-MSCs + pPCL, D) AF-MSCs + PCL. Scale bar = 200µm.
Discussion
Medical progress and improved living condition have led to an increase in life expectancy. However, the increasing life span will be accompanied by new challenges such as age-related diseases and poor quality of life. Cardiovascular disease, dementia, cancer, stroke and osteoarthritis are among the serious diseases whose prevalence is increasing with age.
36,37
In addition to the mentioned disorders, bone fracture and associated complications are one of the debilitating problems which can affect the general health of the elderly. Autologous bone graft is a gold standard of bone repairing treatment, but in the aged patients' prognosis of this treatment, modality is poor due to reduced or nearly disappeared differentiation and proliferation capacity of stem cells.
12
In order to meet bone formation demands, tissue engineering has introduced de novo methods.
38
Tissue engineering deals with the usage of a mixture of cells, as well as biomaterial, biochemical or physicochemical factors to recover or replace functional tissues and organs. Amniotic fluid stem cells have been of great interest for many reasons, including the ability of differentiation into various cell types, lack of ethical issues and tumorigenicity after transplantation and expression of immunosuppressive factors (such as CD59) which might make AF-MSCs resistant to rejection, thereby inhibiting the proliferation of T Lymphocytes.
39
As a result, AF-MSCs can survive after allogenic transplantation compared to other cells without using suppressive therapies. It seems that these cells are an excellent alternative for allogeneic transplantation because of their low immunogenicity.
39
Also, tissue engineering considers the use of a scaffold as a sub-field of biomaterials, for the cell support and formation of new practical tissue. Biocompatible fibrous scaffolds are increasingly employed for tissue engineering due to advantages such as higher surface-to-volume ratio than traditional scaffolds that enhance the cells-scaffold interactions.
40
Biocompatible and fibrous scaffold, PCL that can be a good choice for medical applications. With a combination of inherent features and unique fibrous structure, PCL can be a useful scaffold for tissue engineering.
41
In the present study, the AF-MSCs were cultivated on a PCL scaffold and transplanted in the defected site of a bone in the rat model. We demonstrated the presence of collagen type I with a great expression in cell-scaffold groups. Im et al reported developed bone formation in hydroxyapatite (HA) scaffold seeded with MSCs compared to the empty and scaffold-only groups.
42
In a previous study, the stem cell-collagen constructs were maintained in the osteogenic medium for 1 week before in vivo implantation. Four weeks after the cranial implantation they reported new bone formation in all groups but the most relevant differences in defect correction were shown by stem cell-collagen samples.
4
In our work, after 8 weeks of transplantation, the most successful formation of new bone tissue was seen in the cell-scaffold construct (AF-MSCs + PCL scaffold) compared to the PCL scaffold alone.
One approach to tissue engineering is the use of growth factors. Researchers have employed the growth factor to boost repair and bone regeneration in tissue engineering.
29,43
In this regard, platelet-rich plasma (PRP), as a rich source of growth factors, has attracted many scientists. Due to the different methods for PRP isolation and the inherent differences between blood transducers in the terms of platelet size and concentration, there are controversies between scientists about the therapeutic capability of PRP.
44-46
Numerous researches have shown that PRP does not enhance new bone formation in a critical size bone defect.
47,48
Unlike the above results, several studies reported that the PRP has positive effects on bone regeneration.
49-51
In our study, histological results indicated the formation of blood vessels in groups with PRP. It seems that the growth factors present in PRP
30-33
increase the viability and bone differentiation of transplanted cells, contribute to the formation of blood vessels, and promote nutrient exchange at the site of the lesion. In the previous study, the PCL scaffolds were coated with PRP and showed that this construct improves MSCs adhesion and proliferation.
52
However our results confirmed that the PRP treatment has positive effects on bone repair and on blood vessel formation in vivo.
One of the most important approaches to bone tissue engineering is the simultaneous use of cells, scaffolds and growth factors. In the present study, the effect of AF-MSCs, PCL scaffold and PRP were studied. Results showed that the higher level of new blood vessels and collagen were expressed in AF-MSCs, PCL fibrous scaffold and PRP group. Kasten et al reported that the bone regeneration was enhanced with the combination of bone marrow-derived MSCs, scaffold and PRP.
53
Agacayak and colleagues demonstrated that the combination of BM-MSCs, PRP and biphasic calcium phosphate was found to be more effective in inducing osteogenesis in critical size bone defects.
54
However, it seems that the formation of blood vessels in the site of lesion is caused by the growth factors released by PRP, which provides a ground for food metabolism, increased viability, and bone differentiation of grafted cells.
Conclusion
In this study, the simultaneous application of PCL scaffold, AF-MSCs and PRP has a positive effect on the regeneration of bone tissue. Furthermore, the PRP can affect blood vessel formation and collagen type I expression in the transplanted scaffold.
Acknowledgments
The authors would like to thank the Deputy of the Research and Technology, ZUMS.
Funding sources
The financial support for this work by Deputy of the Research and Technology, ZUMS (Grant No: A‐12–892‐4).
Ethical statement
The ethical approval of the study was obtained from the Ethics Committee of the Zanjan University of Medical Sciences (Ethical Number: ZUMS.REC.1394.81).
Competing interests
None declared.
Authors’ contribution
ZG: Draft preparation, writing and reviewing, data analysis and presentation. OS: Advisor, experimental design, data analysis, writing and reviewing. YM: Advisor, study validation, writing and reviewing. AE: Advisor and provision of study materials and equipment. SN: Conceptualization, experimental design, data analysis, facility of study materials and equipment, study justification, supervision, writing and reviewing draft preparation.
Research Highlights
What is the current knowledge?
simple
-
√ Bone regeneration and new blood vessels form in the bone defect site by the simultaneous application of PCL fibrous scaffold + AF-MSCs + PRP
What is new here?
simple
-
√ The simultaneous application of nanofibrous scaffold + AF-MSCs + PRP for bone regeneration medicine.
-
√ Positive effects of scaffold, stem cells and growth factor for repairing of bone defect.
References
- Ben-David D, Kizhner TA, Kohler T, Muller R, Livne E, Srouji S. Cell-scaffold transplant of hydrogel seeded with rat bone marrow progenitors for bone regeneration. J Craniomaxillofac Surg 2011; 39:364-71. doi: 10.1016/j.jcms.2010.09.001 [Crossref] [ Google Scholar]
- Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 2012; 40:363-408. [ Google Scholar]
- Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med 2014; 25:2445-61. doi: 10.1007/s10856-014-5240-2 [Crossref] [ Google Scholar]
- Maraldi T, Riccio M, Pisciotta A, Zavatti M, Carnevale G, Beretti F. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res Ther 2013; 4:53. doi: 10.1186/scrt203 [Crossref] [ Google Scholar]
- Ben-David D, Kizhner TA, Kohler T, Müller R, Livne E, Srouji S. Cell-scaffold transplant of hydrogel seeded with rat bone marrow progenitors for bone regeneration. Journal of Cranio-Maxillofacial Surgery 2011; 39:364-71. doi: 10.1016/j.jcms.2010.09.001 [Crossref] [ Google Scholar]
- Perez JR, Kouroupis D, Li DJ, Best TM, Kaplan L, Correa D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front Bioeng Biotechnol 2018; 6:105. doi: 10.3389/fbioe.2018.00105 [Crossref] [ Google Scholar]
- Terella A, Mariner P, Brown N, Anseth K, Streubel SO. Repair of a calvarial defect with biofactor and stem cell-embedded polyethylene glycol scaffold. Arch Facial Plast Surg 2010; 12:166-71. doi: 10.1001/archfacial.2010.37 [Crossref] [ Google Scholar]
- Sakkas A, Wilde F, Heufelder M, Winter K, Schramm A. Autogenous bone grafts in oral implantology-is it still a "gold standard"? A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent 2017; 3:23. doi: 10.1186/s40729-017-0084-4 [Crossref] [ Google Scholar]
- Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioactive Materials 2017; 2:224-47. doi: 10.1016/j.bioactmat.2017.05.007 [Crossref] [ Google Scholar]
- Fernandez de Grado G, Keller L, Idoux-Gillet Y, Wagner Q, Musset AM, Benkirane-Jessel N. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J Tissue Eng 2018; 9:2041731418776819. doi: 10.1177/2041731418776819 [Crossref] [ Google Scholar]
- Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res 2014; 9:18. doi: 10.1186/1749-799x-9-18 [Crossref] [ Google Scholar]
- Zong C, Xue D, Yuan W, Wang W, Shen D, Tong X. Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur Cell Mater 2010; 20:109-20. [ Google Scholar]
- Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev 2008; 60:184-98. doi: 10.1016/j.addr.2007.08.041 [Crossref] [ Google Scholar]
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 2008; 121:255-64. doi: 10.1242/jcs.006064 [Crossref] [ Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011; 3. doi: 10.1101/cshperspect.a005058 [Crossref]
- Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res 2014; 163:268-85. doi: 10.1016/j.trsl.2013.11.003 [Crossref] [ Google Scholar]
- O'Brien FJ. Biomaterials & scaffolds for tissue engineering. Materials Today 2011; 14:88-95. doi: 10.1016/S1369-7021(11)70058-X [Crossref] [ Google Scholar]
- Murugan R, Ramakrishna S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng 2007; 13:1845-66. doi: 10.1089/ten.2006.0078 [Crossref] [ Google Scholar]
- Ghassemi T, Shahroodi A, Ebrahimzadeh MH, Mousavian A, Movaffagh J, Moradi A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch Bone Jt Surg 2018; 6:90-9. [ Google Scholar]
- Malikmammadov E, Tanir TE, Kiziltay A, Hasirci V, Hasirci N. PCL and PCL-based materials in biomedical applications. J Biomater Sci Polym Ed 2018; 29:863-93. doi: 10.1080/09205063.2017.1394711 [Crossref] [ Google Scholar]
- Schantz JT, Hutmacher DW, Chim H, Ng KW, Lim TC, Teoh SH. Induction of ectopic bone formation by using human periosteal cells in combination with a novel scaffold technology. Cell Transplant 2002; 11:125-38. [ Google Scholar]
- Liao HT, Chen JP, Lee MY. Bone Tissue Engineering with Adipose-Derived Stem Cells in Bioactive Composites of Laser-Sintered Porous Polycaprolactone Scaffolds and Platelet-Rich Plasma. Materials (Basel) 2013; 6:4911-29. doi: 10.3390/ma6114911 [Crossref] [ Google Scholar]
- Labet M, Thielemans W. Synthesis of polycaprolactone: a review. Chem Soc Rev 2009; 38:3484-504. doi: 10.1039/b820162p [Crossref] [ Google Scholar]
- Cananzi M, Atala A, De Coppi P. Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reprod Biomed Online 2009; 18 Suppl 1:17-27. [ Google Scholar]
- Kaviani A, Perry TE, Dzakovic A, Jennings RW, Ziegler MM, Fauza DO. The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg 2001; 36:1662-5. doi: 10.1053/jpsu.2001.27945 [Crossref] [ Google Scholar]
- Baghaban Eslaminejad M, Jahangir S. Amniotic fluid stem cells and their application in cell-based tissue regeneration. Int J Fertil Steril 2012; 6:147-56. [ Google Scholar]
- Ghionzoli M, Cananzi M, Zani A, Rossi CA, Leon FF, Pierro A. Amniotic fluid stem cell migration after intraperitoneal injection in pup rats: implication for therapy. Pediatr Surg Int 2010; 26:79-84. doi: 10.1007/s00383-009-2504-x [Crossref] [ Google Scholar]
- Klemmt PA, Vafaizadeh V, Groner B. The potential of amniotic fluid stem cells for cellular therapy and tissue engineering. Expert Opin Biol Ther 2011; 11:1297-314. doi: 10.1517/14712598.2011.587800 [Crossref] [ Google Scholar]
- De Witte TM, Fratila-Apachitei LE, Zadpoor AA, Peppas NA. Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen Biomater 2018; 5:197-211. doi: 10.1093/rb/rby013 [Crossref] [ Google Scholar]
- Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med 2008; 1:165-74. doi: 10.1007/s12178-008-9032-5 [Crossref] [ Google Scholar]
- Lynch SE, Nixon JC, Colvin RB, Antoniades HN. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A 1987; 84:7696-700. doi: 10.1073/pnas.84.21.7696 [Crossref] [ Google Scholar]
- Sabarish R, Lavu V, Rao SR. A Comparison of Platelet Count and Enrichment Percentages in the Platelet Rich Plasma (PRP) Obtained Following Preparation by Three Different Methods. J Clin Diagn Res 2015; 9:Zc10-2. doi: 10.7860/jcdr/2015/11011.5536 [Crossref] [ Google Scholar]
- Gobbi A, Karnatzikos G, Mahajan V, Malchira S. Platelet-rich plasma treatment in symptomatic patients with knee osteoarthritis: preliminary results in a group of active patients. Sports Health 2012; 4:162-72. doi: 10.1177/1941738111431801 [Crossref] [ Google Scholar]
- Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang 2009; 97:110-8. doi: 10.1111/j.1423-0410.2009.01188.x [Crossref] [ Google Scholar]
- Nadri S, Soleimani M. Comparative analysis of mesenchymal stromal cells from murine bone marrow and amniotic fluid. Cytotherapy 2007; 9:729-37. doi: 10.1080/14653240701656061 [Crossref] [ Google Scholar]
- Jaul E, Barron J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front Public Health 2017; 5:335. doi: 10.3389/fpubh.2017.00335 [Crossref] [ Google Scholar]
- Brown GC. Living too long: the current focus of medical research on increasing the quantity, rather than the quality, of life is damaging our health and harming the economy. EMBO Rep 2015; 16:137-41. doi: 10.15252/embr.201439518 [Crossref] [ Google Scholar]
- Black CRM, Goriainov V, Gibbs D, Kanczler J, Tare RS, Oreffo ROC. Bone Tissue Engineering. Cur Mol Biol Rep 2015; 1:132-40. doi: 10.1007/s40610-015-0022-2 [Crossref] [ Google Scholar]
- Pantalone A, Antonucci I, Guelfi M, Pantalone P, Usuelli FG, Stuppia L. Amniotic fluid stem cells: an ideal resource for therapeutic application in bone tissue engineering. Eur Rev Med Pharmacol Sci 2016; 20:2884-90. [ Google Scholar]
- Smith IO, Liu XH, Smith LA, Ma PX. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2009; 1:226-36. doi: 10.1002/wnan.26 [Crossref] [ Google Scholar]
- Mochane M, Simon Motsoeneng T, Rotimi Sadiku E, Teboho M, Sefadi J. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl Sci 2019; 9:2205. doi: 10.3390/app9112205 [Crossref] [ Google Scholar]
- Im JY, Min WK, You C, Kim HO, Jin HK, Bae JS. Bone regeneration of mouse critical-sized calvarial defects with human mesenchymal stem cells in scaffold. Lab Anim Res 2013; 29:196-203. doi: 10.5625/lar.2013.29.4.196 [Crossref] [ Google Scholar]
- Azevedo HS, Pashkuleva I. Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Adv Drug Deliv Rev 2015; 94:63-76. doi: 10.1016/j.addr.2015.08.003 [Crossref] [ Google Scholar]
- Nagata MJ, Messora M, Pola N, Campos N, Vieira R, Esper LA. Influence of the ratio of particulate autogenous bone graft/platelet-rich plasma on bone healing in critical-size defects: a histologic and histometric study in rat calvaria. J Orthop Res 2010; 28:468-73. doi: 10.1002/jor.21027 [Crossref] [ Google Scholar]
- Weibrich G, Kleis WK, Hitzler WE, Hafner G. Comparison of the platelet concentrate collection system with the plasma-rich-in-growth-factors kit to produce platelet-rich plasma: a technical report. Int J Oral Maxillofac Implants 2005; 20:118-23. [ Google Scholar]
- Sanchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants 2003; 18:93-103. [ Google Scholar]
- Sarkar MR, Augat P, Shefelbine SJ, Schorlemmer S, Huber-Lang M, Claes L. Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials 2006; 27:1817-23. doi: 10.1016/j.biomaterials.2005.10.039 [Crossref] [ Google Scholar]
- Plachokova AS, van den Dolder J, Stoelinga PJ, Jansen JA. Early effect of platelet-rich plasma on bone healing in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res 2007; 18:244-51. doi: 10.1111/j.1600-0501.2006.01327.x [Crossref] [ Google Scholar]
- Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbuhl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials 2008; 29:3983-92. doi: 10.1016/j.biomaterials.2008.06.014 [Crossref] [ Google Scholar]
- Rai B, Oest ME, Dupont KM, Ho KH, Teoh SH, Guldberg RE. Combination of platelet-rich plasma with polycaprolactone-tricalcium phosphate scaffolds for segmental bone defect repair. J Biomed Mater Res A 2007; 81:888-99. doi: 10.1002/jbm.a.31142 [Crossref] [ Google Scholar]
- He F, Chen Y, Li J, Lin B, Ouyang Y, Yu B. Improving bone repair of femoral and radial defects in rabbit by incorporating PRP into PLGA/CPC composite scaffold with unidirectional pore structure. J Biomed Mater Res A 2015; 103:1312-24. doi: 10.1002/jbm.a.35248 [Crossref] [ Google Scholar]
- Diaz-Gomez L, Alvarez-Lorenzo C, Concheiro A, Silva M, Dominguez F, Sheikh FA. Biodegradable electrospun nanofibers coated with platelet-rich plasma for cell adhesion and proliferation. Mater Sci Eng C Mater Biol Appl 2014; 40:180-8. doi: 10.1016/j.msec.2014.03.065 [Crossref] [ Google Scholar]
- Kasten P, Beverungen M, Lorenz H, Wieland J, Fehr M, Geiger F. Comparison of platelet-rich plasma and VEGF-transfected mesenchymal stem cells on vascularization and bone formation in a critical-size bone defect. Cells Tissues Organs 2012; 196:523-33. doi: 10.1159/000337490 [Crossref] [ Google Scholar]
- Agacayak S, Gulsun B, Ucan MC, Karaoz E, Nergiz Y. Effects of mesenchymal stem cells in critical size bone defect. Eur Rev Med Pharmacol Sci 2012; 16:679-86. [ Google Scholar]