Dr. Ali Pourali is a research scientist at Food and Drug Safety Research Center, Tabriz University of Medical Sciences (TUOMS). Having B.Sc. and M.Sc. degrees in Chemistry, he obtained his Ph.D. in the
field of Pharmaceutical Nanotechnology from TUOMS Faculty of Pharmacy and at the Research Center for
Pharmaceutical Nanotechnology under Prof. Omidi’s supervision in 2019, working on nanoscale biosensor
for early detection of cardiovascular diseases.
Professor Yadollah Omidi has a Ph.D. degree in Pharmaceutical Sciences (2003, Cardiff University, UK) and completed a postdoctoral program (2004) at Cardiff University. He is currently working as a full professor at Nova Southeastern University College of Pharmacy, Florida. Prof. Omidi’s research in advanced targeted diagnosis and therapy of diseases have resulted in over 300 published papers in international journals, 27 book chapters, and a few patents. His H-index is 58 and he has consecutively been listed among top 1%
highly cited scientists worldwide by WoS-ESI.
Abstract
Summary
The molecular marker, cardiac troponin (cTn) is a complex protein that is attached to tropomyosin on the actin filament. It is an essential biomolecule in terms of the calcium-mediated regulation of the contractile apparatus in myofibrils, the release of which is an indication of the dysfunction of cardiomyocytes and hence the initiation of ischemic phenomena in the heart tissue. Fast and accurate analysis of cTn may help the diagnosis and management of acute myocardial infarction (AMI), for which electrochemical biosensors and microfluidics devices can be of great benefit. This editorial aims to highlight the importance of cTn as vital biomarkers in AMI diagnosis.
Keywords: Acute myocardial infarction, Biosensor, Cardiac troponin, Cardiomyocytes, Electrochemical analysis, Microfluidics
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
According to the World Health Organization (WHO) reports, cardiovascular diseases (CVDs) are the most important cause of mortality among both men and women worldwide. It is estimated that the number of people dying from CVDs will raise by about 23 million annually.1 The myocardial infarction (MI), as the most common type of CVDs, can occur when the coronary artery is blocked and the blood supplementary to the heart is defected – a phenomenon so-called ischemia. As a result, the myocardial muscle can get injured.2 Unfortunately, the muscular damage in the heart tissue is an irreversible detrimental phenomenon that can be initiated by various factors and result in sudden progression for up to 85% within the first two hours of the emergence. As a result, accurate diagnosis and immediate treatment are of vital importance to enhance the survival rate.2
With the introduction of more sensitive cardiac biomarkers, the European Society of Cardiology (ESC) and the American College of Cardiology (ACC) collaborated to redefine MI using a biochemical and clinical approach and reported that myocardial injury detected by abnormal biomarkers in the setting of acute myocardial ischemia should be labeled as MI.3 Consequently, the determination of biomarker concentration has a pivotal role and as an indispensable factor has been the cornerstone in the diagnosis of acute MI (AMI). Thus, so far, various markers have been vigorously investigated, including cardiac troponins (cTn), myoglobin (MYO), creatine kinase-MB (CK-MB).4-9 Of these, the cTn biomarker has also been exploited to diagnose AMI in chronic kidney disease.10 These molecular markers are considered as the well-established gold standard for the diagnosis of AMI, while some of them are still under investigation.11,12
Of various molecular markers, cTn biomarkers have widely been studied. The 3-part complex of cTn (I, T, and C) are attached to the tropomyosin on the actin filament, which is necessary for the calcium-mediated regulation of the contractile apparatus in myofibrils (Fig. 1). Given that the troponin C (cTnC) biomarker is very similar in the skeletal and cardiac muscle tissues, it is not specific to the heart tissue and is not utilized in assays for the AMI diagnosis.13 However, the cTnT and cTnI biomarkers are distinct between skeletal and cardiac muscles because they are coded by different genes.14 As a result, the cTnI and cTnT biomarkers have equal cardiac specificity, and currently, they are considered as “gold standard” biomarkers for the diagnosis of AMI in clinical practice. In addition, these biomarkers are released in the early initiation of heart failure, showing high viability in the bloodstream and good sensitivity for cardiac cell necrosis.15
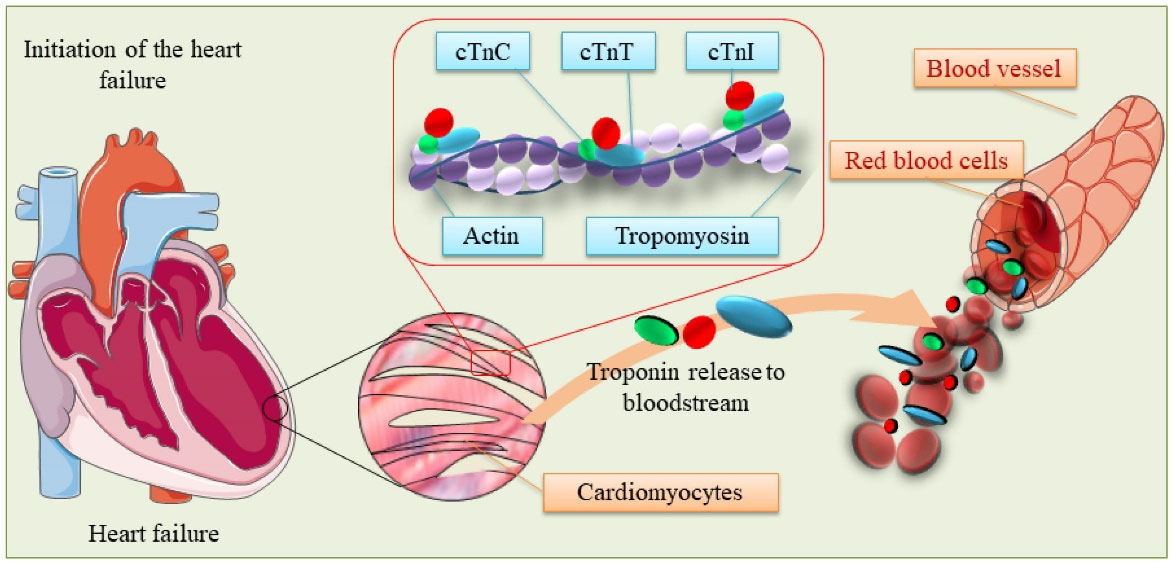
Fig. 1.
The schematic representation of the cardiac troponin (cTns) and myofibrils functions. The cTn biomarker, consisting of three parts (I, T, and C), is attached to tropomyosin on the actin filament of cardiomyocytes. In myofibrils, cTn plays an essential role in the calcium-mediated regulation of the contractile apparatus. Upon the emergence of damages in the heart tissue, the cTn biomarkers are released into the bloodstream, the release of which can be measured by various techniques such as electrochemical biosensors, and microfluidic devices. Note: not drawn to scale.
.
The schematic representation of the cardiac troponin (cTns) and myofibrils functions. The cTn biomarker, consisting of three parts (I, T, and C), is attached to tropomyosin on the actin filament of cardiomyocytes. In myofibrils, cTn plays an essential role in the calcium-mediated regulation of the contractile apparatus. Upon the emergence of damages in the heart tissue, the cTn biomarkers are released into the bloodstream, the release of which can be measured by various techniques such as electrochemical biosensors, and microfluidic devices. Note: not drawn to scale.
It is extremely important to diagnose AMI at the beginning stages of its initiation before its progression, which makes it possible to succeed in the treatment and recovery of patients. To this end, therefore, it is necessary to develop simple and sensitive biosensors to quantitatively and quickly detect cTn biomarkers at very low levels in biological fluids, including blood, plasma, and even saliva samples. Currently, biosensing platforms are developing with the use of new technologies, such as printed and fine electrodes, microfluidic devices, and nanostructures to fulfill these demands. The sensitive assays for the detection of cTn biomarkers have excellent diagnostic performance, which might improve the early diagnosis of AMI, particularly in patients with a recent onset of chest pain (see clinical trial NCT00470587 at ClinicalTrials. gov).16 The main question is the know-how the precise measurement of these biomarkers as early as possible. Of various approaches, the electrochemical transducers, as the most frequently utilized platforms in the development of biosensors, have greatly benefited from the capability of miniaturization, excellent sensitivity, and specificity. They have successfully been applied in the blood glucose biosensors developments as the leading commercial biosensor. The name electrochemical biosensing is used for a molecular sensing event, in which a close couple occurs between the biological recognition element at the surface of the electrode transducer and the target analyte. We have successfully implemented electrochemical sensing technologies for the detection and monitoring of various molecular markers.17-25 We envision that both the labeled and label-free electrochemical sensing technologies are simple and cost-effective approaches that can be simply developed and used for the detection of various entities at molecular and cellular levels, including cTn biomarkers. Once combined with microfluidic techniques, the electrochemical sensing approaches can provide reproducible results and valid clinical outcomes. The electrochemical biosensors have been classified into three main groups of (i) amperometric, (ii) potentiometric, and (iii) impedimetric approaches. Each method involves a variety of techniques with unique properties. For instance, amperometric biosensors may be more attractive because of their high sensitivity and wide linear range. Valuable mounting researches using microchips and new sensing approaches coupled with different technological innovations have paved the way towards widespread clinical applications of electrochemical biosensing devices.26 Despite all the advancements in the field of electrochemical sensors/biosensors, we believe that the diagnosis of biological molecular markers such as cTn can be further advanced using different technologies and materials, including dispensable paper-based sensors, screen-printed systems, molecularly imprinted electrodes, DNA-/enzyme-based sensors, quartz crystal microbalance, micro-/nano-cantilever sensors, surface plasmon resonance sensors, microfluidics such as microfluidic paper-based analytical devices (microPADs), and multianalyte sensing systems.27-30 Furthermore, much needs to be learned from mother nature sensing systems at the cellular level in the future. At this stage, the assimilation of advanced nanomaterials and biomaterials into the electrochemical biosensors might enable us to detect any biological target with great sensitivity and selectivity. While the main advancements are based on the modification of electrodes with different entities, we should devise much more humanized sensing systems by integrating different dominions of science and technologies, in particular microfluidic-based systems. Collectively, the future biosensors and immunosensors can be revolved not only through immobilization and interface potentials of nanobiomaterials but also by engineering much more stimuli-responsive systems which are similar to mother nature sensing mechanisms.
Funding sources
None to be stated.
Ethical statement
None to be stated.
Competing interests
Yadollah Omidi acts as the Editor-in-Chief of Bioimpacts. The peer-review process of this editorial has been done based on COPE and ICMJE guidelines.
References
- World Health Organization. Cardiovascular Diseases Fact Sheet. WHO; 2017.
- Kim K, Park C, Kwon D, Kim D, Meyyappan M, Jeon S. Silicon nanowire biosensors for detection of cardiac troponin I (cTnI) with high sensitivity. BiosensBioelectron 2016; 77:695-701. doi: 10.1016/j.bios.2015.10.008 [Crossref] [ Google Scholar]
- Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000; 36:959-69. doi: 10.1016/s0735-1097(00)00804-4 [Crossref] [ Google Scholar]
- Perrone MA, Storti S, Salvadori S, Pecori A, Bernardini S, Romeo F. Cardiac troponins: are there any differences between T and I?. J Cardiovasc Med (Hagerstown) 2021; 22:797-805. doi: 10.2459/JCM.0000000000001155 [Crossref] [ Google Scholar]
- Lippi G, Cervellin G, Sanchis-Gomar F. Predicting mortality with cardiac troponins: recent insights from meta-analyses. Diagnosis (Berl) 2021; 8:37-49. doi: 10.1515/dx-2019-0061 [Crossref] [ Google Scholar]
- Clerico A, Padoan A, Zaninotto M, Passino C, Plebani M. Clinical relevance of biological variation of cardiac troponins. Clin Chem Lab Med 2021; 59:641-52. doi: 10.1515/cclm-2020-1433 [Crossref] [ Google Scholar]
- Rotondo C, Corrado A, Colia R, Maruotti N, Sciacca S, Lops L. Possible role of higher serum level of myoglobin as predictor of worse prognosis in Sars-Cov 2 hospitalized patients. A monocentric retrospective study. Postgrad Med 2021; 133:688-93. doi: 10.1080/00325481.2021.1949211 [Crossref] [ Google Scholar]
- Al Fatease A, Haque M, Umar A, Ansari SG, Alhamhoom Y, Muhsinah AB. Label-Free Electrochemical Sensor Based on Manganese Doped Titanium Dioxide Nanoparticles for Myoglobin Detection: Biomarker for Acute Myocardial Infarction. Molecules 2021; 26:4252. doi: 10.3390/molecules26144252 [Crossref] [ Google Scholar]
- Wei W, Zhang L, Zhang Y, Tang R, Zhao M, Huang Z. Predictive value of creatine kinase MB for contrast-induced acute kidney injury among myocardial infarction patients. BMC Cardiovasc Disord 2021; 21:337. doi: 10.1186/s12872-021-02155-7 [Crossref] [ Google Scholar]
- Kraus D, von Jeinsen B, Tzikas S, Palapies L, Zeller T, Bickel C. Cardiac Troponins for the Diagnosis of Acute Myocardial Infarction in Chronic Kidney Disease. J Am Heart Assoc 2018; 7:e008032. doi: 10.1161/JAHA.117.008032 [Crossref] [ Google Scholar]
- Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ 2005; 173:1191-202. doi: 10.1503/cmaj/051291 [Crossref] [ Google Scholar]
- Garg P, Morris P, Fazlanie AL, Vijayan S, Dancso B, Dastidar AG. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med 2017; 12:147-55. doi: 10.1007/s11739-017-1612-1 [Crossref] [ Google Scholar]
- Lewandrowski K, Chen A, Januzzi J. Cardiac markers for myocardial infarction: a brief review. Pathology Patterns Reviews 2002; 118:S93-S9. [ Google Scholar]
- Apple FS. Tissue specificity of cardiac troponin I, cardiac troponin T and creatine kinase-MB. Clin Chim Acta 1999; 284:151-9. doi: 10.1016/s0009-8981(99)00077-7 [Crossref] [ Google Scholar]
- Daubert MA, Jeremias A. The utility of troponin measurement to detect myocardial infarction: review of the current findings. Vasc Health Risk Manag 2010; 6:691. doi: 10.2147/vhrm.s5306 [Crossref] [ Google Scholar]
- Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361:858-67. doi: 10.1056/NEJMoa0900428 [Crossref] [ Google Scholar]
- Akbari Nakhjavani S, Afsharan H, Khalilzadeh B, Ghahremani MH, Carrara S, Omidi Y. Gold and silver bio/nano-hybrids-based electrochemical immunosensor for ultrasensitive detection of carcinoembryonic antigen. BiosensBioelectron 2019; 141:111439. doi: 10.1016/j.bios.2019.111439 [Crossref] [ Google Scholar]
- Ebrahimi M, Johari-Ahar M, Hamzeiy H, Barar J, Mashinchian O, Omidi Y. Electrochemical impedance spectroscopic sensing of methamphetamine by a specific aptamer. Bioimpacts 2012; 2:91-5. doi: 10.5681/bi.2012.013 [Crossref] [ Google Scholar]
- Johari-Ahar M, Rashidi MR, Barar J, Aghaie M, Mohammadnejad D, Ramazani A. An ultra-sensitive impedimetric immunosensor for detection of the serum oncomarker CA-125 in ovarian cancer patients. Nanoscale 2015; 7:3768-79. doi: 10.1039/c4nr06687a [Crossref] [ Google Scholar]
- Saberian-Borujeni M, Johari-Ahar M, Hamzeiy H, Barar J, Omidi Y. Nanoscaled aptasensors for multi-analyte sensing. Bioimpacts 2014; 4:205-15. doi: 10.15171/bi.2014.015 [Crossref] [ Google Scholar]
- Majidi MR, Omidi Y, Karami P, Johari-Ahar M. Reusable potentiometric screen-printed sensor and label-free aptasensor with pseudo-reference electrode for determination of tryptophan in the presence of tyrosine. Talanta 2016; 150:425-33. doi: 10.1016/j.talanta.2015.12.064 [Crossref] [ Google Scholar]
- Karami P, Majidi MR, Johari-Ahar M, Barar J, Omidi Y. Development of screen-printed tryptophan-kynurenine immunosensor for in vitro assay of kynurenine-mediated immunosuppression effect of cancer cells on activated T-cells. BiosensBioelectron 2017; 92:287-93. doi: 10.1016/j.bios.2016.11.010 [Crossref] [ Google Scholar]
- Akbari Nakhjavani S, Khalilzadeh B, Samadi Pakchin P, Saber R, Ghahremani MH, Omidi Y. A highly sensitive and reliable detection of CA15-3 in patient plasma with electrochemical biosensor labeled with magnetic beads. BiosensBioelectron 2018; 122:8-15. doi: 10.1016/j.bios.2018.08.047 [Crossref] [ Google Scholar]
- Samadi Pakchin P, Ghanbari H, Saber R, Omidi Y. Electrochemical immunosensor based on chitosan-gold nanoparticle/carbon nanotube as a platform and lactate oxidase as a label for detection of CA125 oncomarker. BiosensBioelectron 2018; 122:68-74. doi: 10.1016/j.bios.2018.09.016 [Crossref] [ Google Scholar]
- Hashemzadeh S, Omidi Y, Rafii-Tabar H. Amperometric lactate nanobiosensor based on reduced graphene oxide, carbon nanotube and gold nanoparticle nanocomposite. Mikrochim Acta 2019; 186:680. doi: 10.1007/s00604-019-3791-0 [Crossref] [ Google Scholar]
- Wang J. Electrochemical biosensors: towards point-of-care cancer diagnostics. BiosensBioelectron 2006; 21:1887-92. doi: 10.1016/j.bios.2005.10.027 [Crossref] [ Google Scholar]
- Kimmel DW, LeBlanc G, Meschievitz ME, Cliffel DE. Electrochemical sensors and biosensors. Anal Chem 2012; 84:685-707. doi: 10.1021/ac202878q [Crossref] [ Google Scholar]
- Abdollahi Aghdam A, Majidi MR, Veladi H, Omidi Y. Microfluidic-based separation and detection of synthetic antioxidants by integrated gold electrodes followed by HPLC-DAD. Microchem J 2019; 149:104059. doi: 10.1016/j.microc.2019.104059 [Crossref] [ Google Scholar]
- Abdollahi-Aghdam A, Majidi MR, Omidi Y. Microfluidic paper-based analytical devices (microPADs) for fast and ultrasensitive sensing of biomarkers and monitoring of diseases. Bioimpacts 2018; 8:237-40. doi: 10.15171/bi.2018.26 [Crossref] [ Google Scholar]
- Fathi F, Rashidi MR, Omidi Y. Ultra-sensitive detection by metal nanoparticles-mediated enhanced SPR biosensors. Talanta 2019; 192:118-27. doi: 10.1016/j.talanta.2018.09.023 [Crossref] [ Google Scholar]