Bioimpacts. 11(4):245-252.
doi: 10.34172/bi.2021.32
Original Research
Cell cycle arrest and anti-cancer potential of probiotic Lactobacillus rhamnosus against HT-29 cancer cells
Najme Dehghani 1  , Farzaneh Tafvizi 1, *
, Farzaneh Tafvizi 1, *  , Parvaneh Jafari 2
, Parvaneh Jafari 2
Author information:
1Department of Biology, Parand Branch, Islamic Azad University, Parand, Iran
2Microbiology Department, Faculty of Science, Arak Branch, Islamic Azad University, Arak, Iran
Abstract
Introduction:
Nowadays, probiotic bacteria have been considered as a factor in the prevention and treatment of cancer, especially by induction of apoptosis. This study aimed to evaluate the cytotoxic, anti-proliferative, and apoptotic effects of the supernatant of probiotic Lactobacillus rhamnosus on HT-29 cell line.
Methods
: Molecular identification of probiotic L. rhamnosus was carried out using specific primers of 16S rRNA gene and sequencing. HT-29 cells were treated with different concentrations of bacterial supernatants at 24, 48, and 72 hours. MTT assay, Annexin V-FITC, real-time PCR, cell cycle analysis, and DAPI staining tests were conducted to evaluate the induction of apoptosis. The level of cyclin D1 protein was measured by immunocytochemistry method.
Results:
The supernatant of L. rhamnosus inhibited the growth of HT-29 cancer cells in a dose- and time-dependent manner. The results of flow cytometry confirmed apoptotic cell death. Probiotic bacterial supernatant caused up-regulation of pro-apoptotic genes including caspase-3, caspase-9, and Bax. In addition, they resulted in down-regulation of Bcl2 and a decrease in expression levels of cyclin D1, cyclin E, and ERBB2 genes. Cancer cells were arrested in the G0/G1 phase of the cell cycle. The results of immunocytochemistry showed significant down-regulation of cyclin D1 protein during the 48 hours treatment with bacterial supernatant compared to the untreated cells.
Conclusion:
The supernatant of probiotic L. rhamnosus has a great potential to inhibit the proliferation of HT-29 cells and the induction of apoptosis. L. rhamnosus might be used as a biological anti-cancer factor in the prevention and treatment of colon cancer.
Keywords: Apoptosis, Colon cancer, Lactobacillus rhamnosus, Probiotic, Supernatant
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Cancer is the second most common cause of death worldwide after cardiovascular disease. Colorectal cancer (CRC) is one of the four most common cancers in the world. CRC is the most common type of gastrointestinal cancer, and it is the third and fourth most common cancer among Iranian males and females, respectively.
1
Resistance to programmed cell death and uncontrolled cell proliferation are the key features of cancer cells. Therefore, a factor that can induce apoptosis in cancer cells can be used as an anti-cancer medication.
2
Current cancer treatments often kill healthy cells and cause toxicity and side effects for the patients. In addition, resistance to chemotherapy has become a major problem. Therefore, it is necessary to find new methods and therapeutic compounds for cancer treatment.
3
Studies have shown that at least half of all cancers are due to the existence of some compounds in dietary patterns.
4
Therefore, the nutritional compounds including probiotics and their relationship to human health are of interest to many researchers.
5
Probiotics are non-pathogenic micro-organisms that have beneficial effects on the host’s health and appear to have anti-tumor effects as well.
6,7
The use of these beneficial bacteria in sufficient amounts regulates the microflora of the digestive system.
8
Although probiotics are used to manage and control certain digestive disorders including diarrhea, infections, and inflammation, their role in the prevention and treatment of colon cancer remains under investigation.
9
Considering the effect of intestinal microflora on reducing the progression of colon cancer, it is important to produce novel products that, in addition to cancer-preventing properties, inhibit the growth of cancer cells while having no harmful effects on the healthy cells. Therefore, probiotics are a good choice for this purpose.
Probiotic bacteria can play an important role in preventing colon cancer as chemopreventive agents and reduce the risk of cancer by stimulating the immune system, changes in intestinal microflora, increased antioxidant activity, cell cycle control, and degradation of potential carcinogens such as the mutagenic compounds and secondary bile acids produced by the intestinal bacteria.
10-12
According to the previous studies, non-living probiotic bacteria including heat-killed probiotics, peptidoglycans, exopolysaccharide extracts, cellular extracts, and other cellular components exhibit potential anti-cancer activity.
13-18
Several studies have been conducted to investigate the probiotics and their products' cytotoxic effects and inhibitory activity on cancer cells proliferation.
19-21
Anti-proliferative activity is highly strain-dependent and differs widely from one strain to another. Moreover, using different bacterial components produces different results such as inhibition of cancer cells proliferation, induction of apoptosis, and anti-cancer properties.
22
Therefore, probiotic bacteria can be used as biological treatments in the future.
22
The present study aimed to investigate the anti-proliferative, apoptotic, and cell cycle arrest effects of indigenous probiotic L. rhamnosus as an effective biological compound on HT-29 cells.
Materials and Methods
Bacterial strain
Strain isolation and characterization
The bacterial strain used in this study (L. rhamnosusstrain Y5) was isolated in our previous study from traditional and non-industrial yogurt from Arak province, Iran.
23
The safety and efficacy of the strain were assessed based on the WHO guidelines for the evaluation of probiotics in food.
24
Molecular identification
DNA extraction, 16S rRNA gene amplification, and sequencing
Bacterial genomic DNA was extracted from MRS broth cultures by DNA extraction kit (MBST, Tehran, Iran) according to the instructions. The quantity and quality of the extracted DNAs were determined by DeNovix DS-11 Spectrophotometer (DeNovix Technologies, Wilmington, Delaware, USA). The PCR amplification was carried out in a total volume of 25 μL containing 12.5 μL Ampliqon master mix buffer, 50 ng DNA, and 0.5 μL (0.4 μM) of 16S rRNA gene-specific primers (27 F (5′-GAGAGTTTGATCCTGGCTCAG-3′), 1495 R (5′-CTACGGCTACCTTGTTACGA-3′)). The PCR condition was optimized using a BioRad PCR system (Bio-Rad, USA) with the following program: pre-denaturation step at 94°C for 5 minutes, 30 cycles of denaturation at 94°C for 60 seconds, annealing at 56°C for 60 seconds; and final extension at 72°C for 10 minutes.
The PCR products were run on 1.5% agarose gel, stained with DNA safe stain and visualized using a Gel Doc device (UVITECH, UK). Finally, the PCR products were sent for sequencing (Bioneer Company, South Korea). Then, the sequences of PCR products were blasted for molecular identification in GenBank database (http://www.ncbi.nlm.nih.gov).
Preparation of active crude supernatant
The bacterial strain was cultured on MRS broth and incubated at 37°C for 24, 48 and 72 hours under a microaerophilic atmosphere (5% CO2). The growth of bacteria was identified by measuring the optical density at 600 nm. Viable cells count was enumerated by pour plating of serially diluted of culture on MRS agar with 2 replicates. After incubation time, the number of bacteria was identified by the following equation:
which N is the total viable cell count per mL; ∑C is the sum of colonies counted on all the dishes retained; n1 is the number of dishes retained in the first dilution; n2 is the number of dishes retained in the second dilution; and d is the dilution factor corresponding to the first dilution
Crude cell-free supernatant (CFS) was prepared by centrifugation of the incubated culture (24, 48, and 72 hours) at 13 000 rpm for 10 minutes. CFS was filtered through 0.22 µm nitrocellulose membrane. Supernatant small aliquots were stored frozen at -20°C until analysis.
Cell line, culture medium, and MTT assay
The human colorectal adenocarcinoma HT-29 cell line was purchased from the Iranian Biological Resource Center (IBRC C10097). Briefly, 1×104 cell/well were seeded into 96-well plates followed by incubation overnight in a CO2 incubator at 37°C. Then, the cells were treated with concentrations of probiotic bacterial supernatant (0.0038, 0.0192, 0.096, 0.48, 2.4, 12.00, 60.00, and 1000 mg/mL) for 24, 48 and 72 hours. In order to determine the cytotoxicity of probiotic bacterial supernatant against HT-29 cells, the MTT [3-[4,5-dimethylthiazol-2-yl]- 2,5-diphenyltetrazolium bromide] colorimetric method was used. Then, the MTT was added into the wells and the reaction mixture was kept at 37°C in a 5% CO2 for 4 hours. The MTT dye was then removed and the formazan crystals were dissolved in dimethyl sulfoxide (DMSO). Finally, the absorbance was recorded at 570 nm using an ELISA Reader (Organon Teknika, Netherlands) and cell viability was determined. According to these results, IC50 values and cell viability percentage were reported.
Detection of apoptosis by Annexin V-FITC
The detection of apoptosis/necrosis ratio in HT-29 cells was carried out using Annexin V-FITC kit (Roch, Penzberg, Germany) according to the manufacturer’s instructions. The HT-29 cells (1×105 cell/well) were treated with IC50 values of bacterial supernatant for 48 and 72 hours. The untreated HT-29 cells were used as a control. Finally, the apoptotic/necrotic cells ratios were measured. Flow cytometry device (Biocompare, San Francisco, USA) was used for cellular analysis.
Cell cycle analysis
The HT-29 cells were seeded into 6-well plates (1×106 cell/well) and incubated for 24 hours. Then, the semi-confluent cells were treated with IC50 values of bacterial supernatant for 48 and 72 hours. Following the treatment, the cells were washed twice with PBS. The cells were then harvested by centrifuging at 1500 rpm for 5 minutes. Subsequently, cell pellets were suspended in 250 µL of propidium iodide (PI) staining solution and kept at room temperature for 30 minutes. The profile of the cell cycle was determined using a BD FACScan Cell Flow Cytometer (Becton Dickinson, Franklin Lake, USA). The data obtained were subjected to analysis by Flow Jo software and reported as the percentage of the cell cycle.
Gene expression
The HT-29 cells were incubated with IC50 of bacterial supernatant for 48 and 72 hours. Then, RNA extraction and cDNA synthesis were carried out according to CinnaGen and Revert AidTM First Strand cDNA Synthesis Kit protocol (Fermentas, Burlington, Canada), respectively. The expression level of 𝛽-actin (housekeeping gene), caspase-3, caspase-9, Bax, Bcl2, cyclin D1, cyclin E, and ERBB2 genes was determined according to SYBER Green method. A detail of Real-time PCR reaction and the thermal program have been described in the previous study.
14
The sequences of primers are presented in Table 1.
Table 1.
Primers sequences of target genes
|
Genes
|
Primer sequence
|
|
β-actin
|
Forward:5’- TCCTCCTGAGCGCAAGTAC-3’
Revers: 5’- CCTGCTTGCTGATCCACATCT-3’
|
|
Caspase 3
|
Forward:5'- CATACTCCACAGCACCTGGTTA-3′
Revers: 5'- ACTCAAATTCTGTTGCCACCTT-3'
|
|
Caspase 9
|
Forward: 5’-CATATGATCGAGGACATCCAG-3
Revers: 5’-TTAGTTCGCAGAAACGAAGC-3’
|
|
Cyclin D1
|
Forward:5'- CAGATCATCCGCAAACACGC-3′
Revers: 5'- AAGTTGTTGGGGCTCCTCAG-3'
|
|
ERBB2
|
Forward:5'- TTCCCTAAGGCTTTCAGTACC -3′
Revers: 5'- GAGTCTTTGTGGATTCTGAGG -3'
|
|
Cyclin E
|
Forward:5'- CTCCAGGAAGAGGAAGGCAA-3′
Revers: 5'- TTGGGTAAACCCGGTCATCA-3'
|
|
Bax
|
Forward:5'- GAGCTGCAGAGGATGATTGC-3′
Revers: 5'- AAGTTGCCGTCAGAAAACATG-3'
|
|
Bcl2
|
Forward:5'- ATTGGGAAGTTTCAAATCAGC-3′
Revers: 5'- CAGTCTACTTCCTCTGTGATGTTG-3'
|
DAPI staining and cyclin D1 protein detection
The HT-29 cells were seeded, treated and incubated for 48 hours. Then paraformaldehyde (4%) was used for the cell fixation. Afterward, the cells were permeabilized with Triton X-100 (0.1%) for 30 minutes and washed with PBS and then were incubated by goat serum for 45 minutes. After that, the cells were incubated by primary antibody (anti-cyclin D1, ab8451, UK) overnight at 4oC and washed with PBS 3 times. Then the cells were incubated by secondary antibody (Goat anti-rabbit, SC-2012, UK) for 1.5 hours at 37oC. After 3 times washing with PBS they were stained with 4,6-diamidino-2-phenylindole (DAPI) for 10 minutes. Finally, the apoptotic cells were observed by an inverted fluorescence microscope (Olympus, Hamburg, Germany).
Five different microscopic fields were considered for analysis. Percentage levels of Cyclin D1 protein were estimated according to the threshold by ImageJ version 1.52h software.
Statistical analysis
The data were analyzed using one-way analysis of variance (ANOVA) employing SPSS 19.0 software (SPSS Inc., Chicago, USA). The evaluation of expression levels of the genes in the treated and untreated cells was determined by Tukey's HSD post hoc test. The results were expressed as mean ± standard deviation (SD) of three replicates and P value<0.05 was considered as significant.
Results
Strain characterization and molecular identification
The isolated strain was gram-positive, oxidase-negative, catalase-negative and non-motile bacillus with no hemolytic activity. Our results showed that isolated strain had up to 87% resistance after 3 hours treatment in pH=3. Based on the growth rate in bile salt complemented MRS broth, this strain was non-sensitive to bile salts with Cinh=0.48.
Similarity analysis of partial 16S rDNA sequence of the isolate was carried out using the BLAST search tool, which revealed that the isolate belonged to the lactic acid bacteria (LAB) family. A similarity rate of about 99% was observed with L. rhamnosus.
According to 16S rDNA sequencing, our isolate was identified as L. rhamnosus and registered in GenBank under accession number Lactobacillus sp. strain YJ1 (MK453288.1).
Cell viability and cytotoxicity
The 72-hour bacterial supernatant was used for all biological tests and to evaluate the apoptosis. The cytotoxic effects and anti-proliferative activity of the bacterial supernatant on the HT-29 cells for 24, 48, and 72 hours were presented in Fig. 1. The results revealed that an increase in the concentration of supernatant and the period of cell treatment increased cytotoxic effects and reduction of proliferative activity of HT-29 cells. Moreover, 99% inhibition was detected in HT-29 cancer cells after 72 hours at a concentration of 30 mg/mL. The IC50 values of bacterial supernatant were 1.95, 0.25, and 0.053 mg/mL after 24, 48, and 72 hours, respectively. As the treatment time increased, the IC50 decreased.
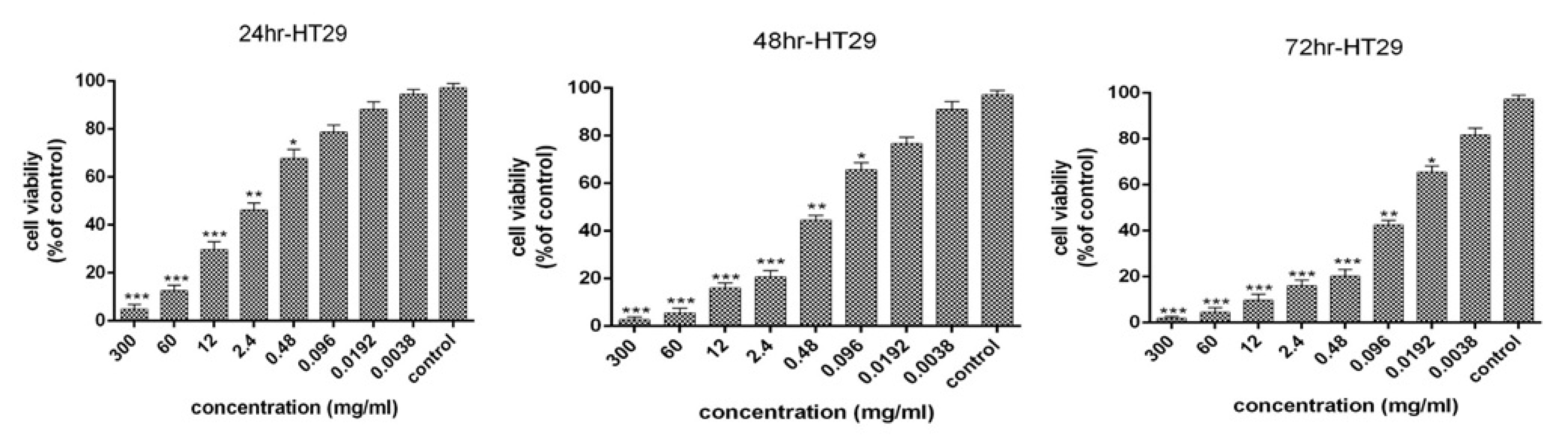
Fig. 1.
Cell viability of HT-29 cancer cells following treatment with L. rhamnosus after 24,48 and 72 h (results are reported as viability in comparison with control group [***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05: ]).
.
Cell viability of HT-29 cancer cells following treatment with L. rhamnosus after 24,48 and 72 h (results are reported as viability in comparison with control group [***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05: ]).
Apoptosis induction in HT-29 cells
Annexin V-FITC kit and flow cytometry were used for a more detailed examination of cell death (apoptosis or necrosis). As shown in Fig. 2, after simultaneous staining of the cells with Annexin V-FITC and PI, early apoptotic cells absorbed Annexin V-FITC, necrotic cells absorbed PI, and late apoptotic cells absorbed both Annexin V- FITC and PI.
Induction of apoptosis in HT-29 cells after treatment with bacterial supernatant was observed at both 48 and 72 hours compared to the untreated cells, so that after 48 and 72 hours, 58.2% and 35.35% apoptosis occurred in the cells, respectively. The cancer cells showed a significant increase in apoptosis in 48 hours than in 72 hours (P<0.001).
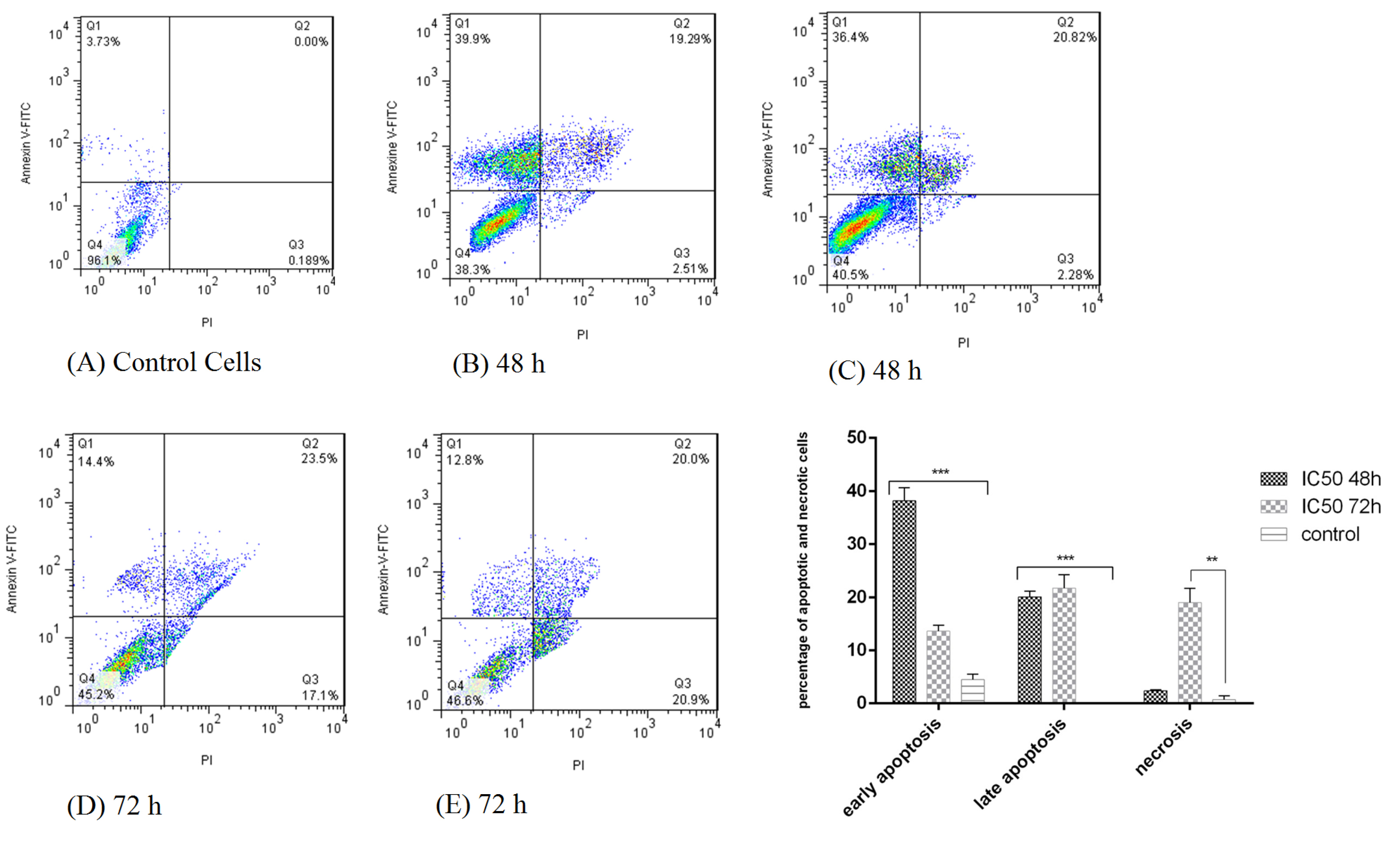
Fig. 2.
Flow cytometry plot for evaluating apoptosis induced by L. rhamnosusafter 48 and 72 h in HT-29 cells. (A) control cells (untreated HT-29 cells); (B,C) treatment of HT-29 cells by bacterial supernatant after 48 h; (D,E) treatment of HT-29 cells by bacterial supernatant after 72 h.
.
Flow cytometry plot for evaluating apoptosis induced by L. rhamnosusafter 48 and 72 h in HT-29 cells. (A) control cells (untreated HT-29 cells); (B,C) treatment of HT-29 cells by bacterial supernatant after 48 h; (D,E) treatment of HT-29 cells by bacterial supernatant after 72 h.
Cell cycle analysis
Treatment of HT-29 cells with bacterial supernatants prevented the cells from crossing the G0/G1 phase to subsequent stages of the cell cycle and arrested them. Also, the percentage of cell population in the sub G1 phase increased significantly compared to the untreated cells (Fig. 3). The cell population in the sub-G1 phase was 30.67% and 55.75% after 48 and 72 hours, respectively compared to the untreated cells.
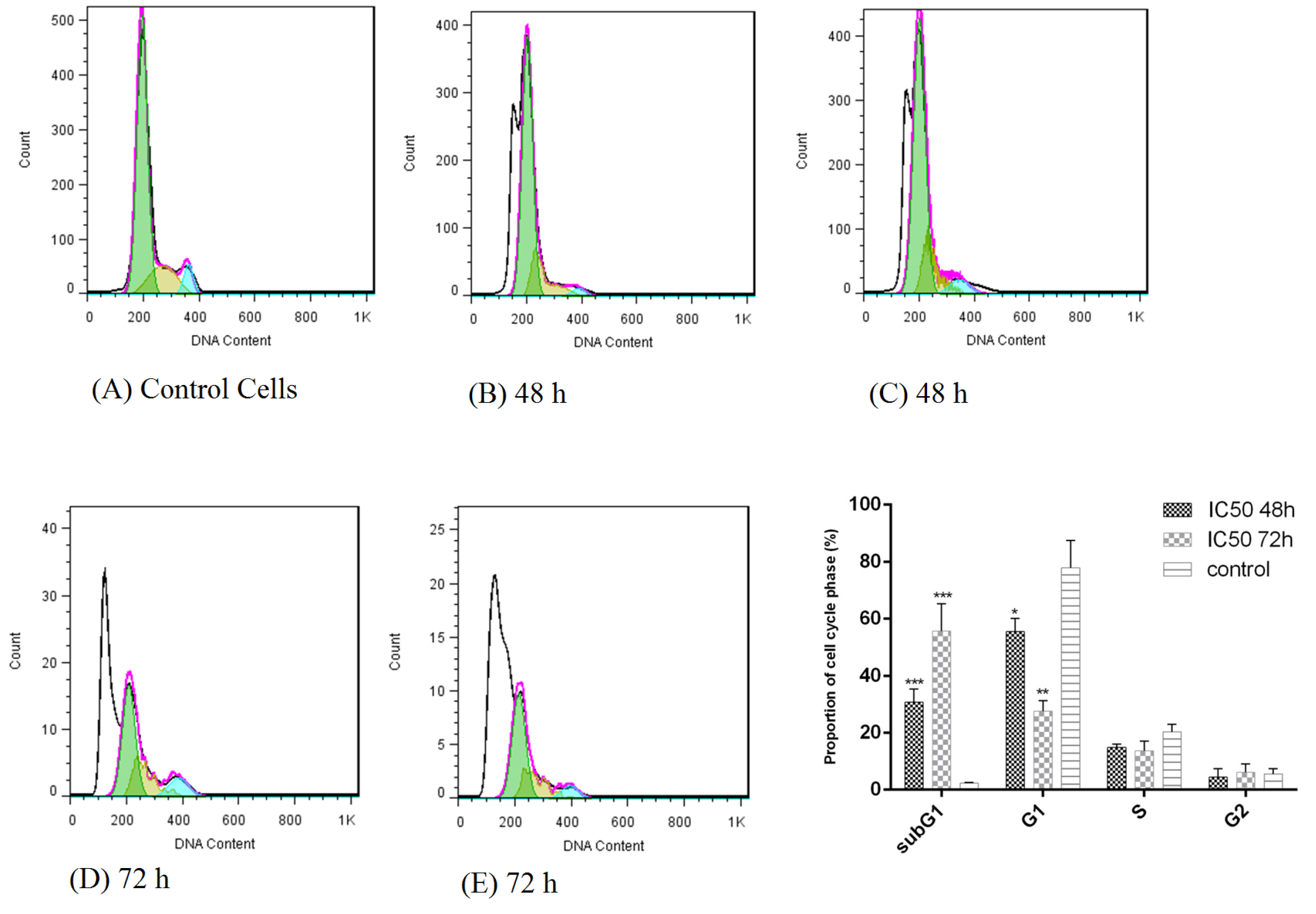
Fig. 3.
Cell cycle analysis of HT-29 cancer cells following treatment with L. rhamnosus for 48 and 72 h. (A) control cells (untreated HT-29 cells); (B,C) treatment of HT-29 cells by bacterial supernatant after 48 h; (D,E) treatment of HT-29 cells by bacterial supernatant after 72 h.
.
Cell cycle analysis of HT-29 cancer cells following treatment with L. rhamnosus for 48 and 72 h. (A) control cells (untreated HT-29 cells); (B,C) treatment of HT-29 cells by bacterial supernatant after 48 h; (D,E) treatment of HT-29 cells by bacterial supernatant after 72 h.
Gene expression analysis
The pro-apoptotic genes; caspase-3, caspase-9 and Baxwere up-regulated after 48 and 72 hours. Cyclin D1, which is involved in cell cycle progression, was down-regulated after 48 and 72 hours. The decrease in Bcl2 expression as an anti-apoptotic gene after 48 hours (P<0.01) and ErBb2 an anti-proliferation gene and cyclin E gene were significant after 72 hours (P<0.05). Gene expression pattern showed that apoptosis at 48 hours was stronger than 72 hours (Fig. 4). Interestingly, the results of gene expression confirmed the results of apoptosis induction using the flow cytometry method.
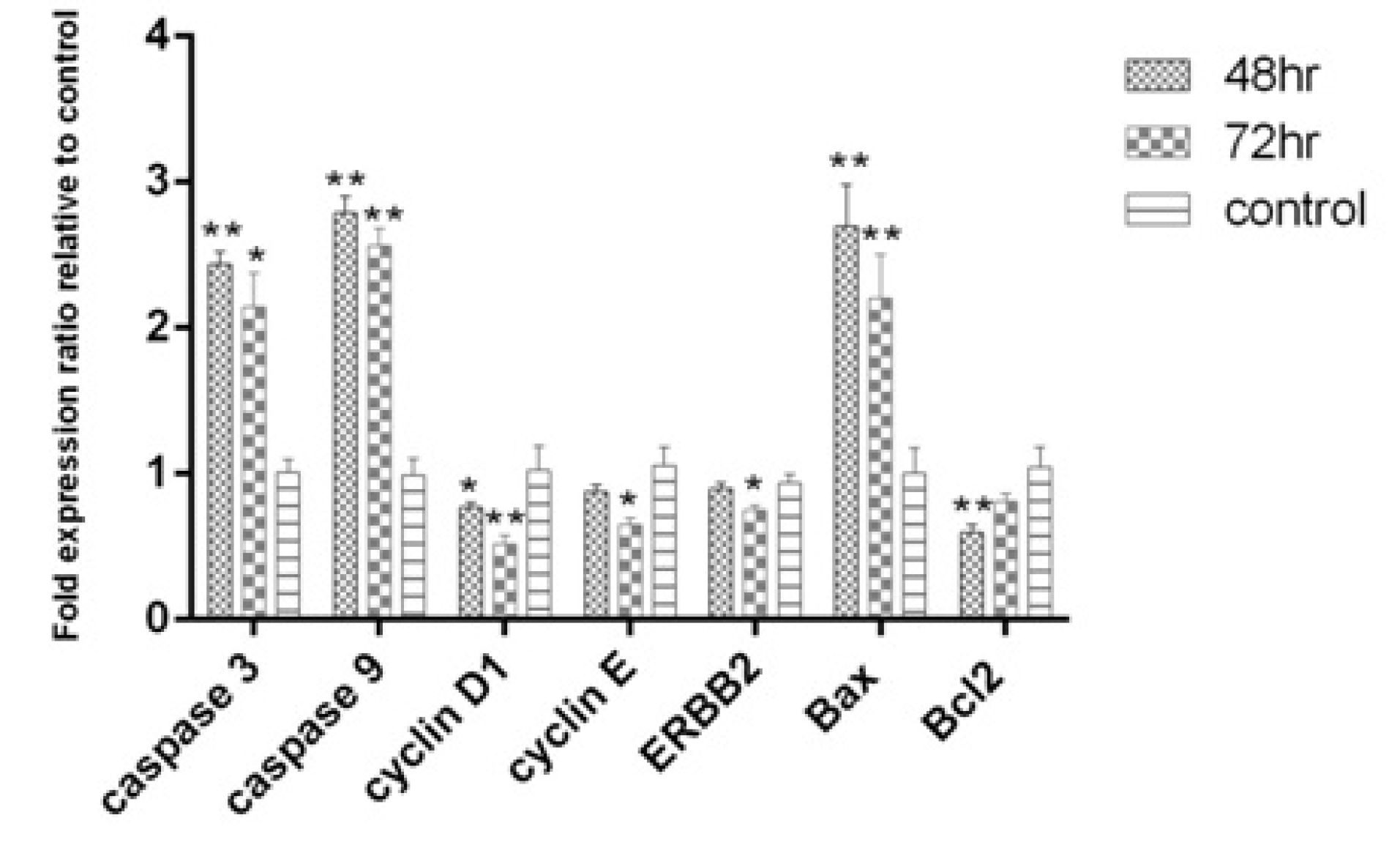
Fig. 4.
Expression levels of Caspase 3, Caspase 9, Cyclin D1, Cyclin E and ERBB2 after 48 and 72 hr of treatment with L. rhamnosus in the HT-29 cell line.
.
Expression levels of Caspase 3, Caspase 9, Cyclin D1, Cyclin E and ERBB2 after 48 and 72 hr of treatment with L. rhamnosus in the HT-29 cell line.
Cyclin D1 protein expression
The level of cyclin D1 protein expression was assessed by immunocytochemistry. The expression of cyclin D1 marker was reported as a percentage in 48 hours treatment of the cells with bacterial supernatant compared to the control group. Cyclin D1 protein expression decreased significantly in the cancer cells treated with bacterial supernatant compared to the untreated cells (Fig. 5). The expression of cyclin D1 in treated cells was estimated to be about 43% compared to the control group (78%).
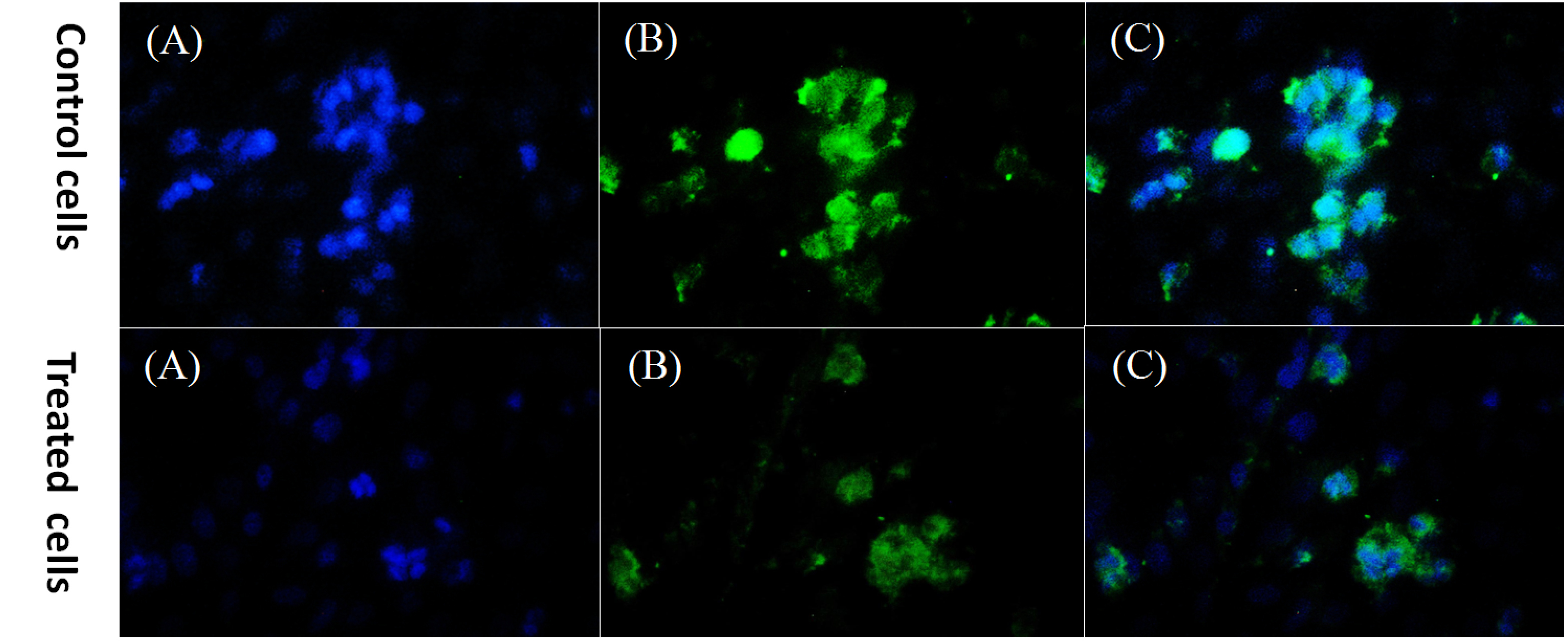
Fig. 5.
DAPI staining and Cyclin D1 protein detection in HT-29 treated cells and control cells (untreated HT-29 cells). A: nuclei stained by DAPI, B: staining for Cyclin D1, C: a merge of DAPI and Cyclin D1 staining, magnification: ×400.
.
DAPI staining and Cyclin D1 protein detection in HT-29 treated cells and control cells (untreated HT-29 cells). A: nuclei stained by DAPI, B: staining for Cyclin D1, C: a merge of DAPI and Cyclin D1 staining, magnification: ×400.
Discussion
The usage of human microbiota is one of the new ways of treating and preventing CRC.
25
Research studies have found a complex relationship between the intestinal microflora and the development of cancer and its effects on cancer treatment. The studies on mice and humans have also suggested that the microbiome is associated with colon cancer.
26-28
Studies have shown that probiotics are one of the most important factors in regulating intestinal microbiota. The specific types of probiotics have been identified that are used to improve and treat a number of diseases such as diabetes, obesity, gastrointestinal infections, and colon cancers.
29
Probiotics are considered dietary supplements and contain non-pathogenic microorganisms that promote health in the host.
30
In the present study, the isolation and identification of the indigenous probiotic bacterium L. rhamnosus and its cytotoxic and anti-cancer effects on HT-29 cells were investigated. The results showed that L. rhamnosus was capable of inhibiting HT-29 cell proliferation and inducing apoptosis. Anti-proliferative activity and apoptosis induction are dependent on the time and dose of probiotic bacterial supernatant. Induction of apoptosis was observed after 48 and 72 hours compared to the control group, but the induction of apoptosis at 48 hours was stronger than 72 hours. Besides, the analysis of the cell cycle showed that probiotic bacterial supernatant increased the peak of sub-G1 and cell cycle arrest in G0/G1 phase, and finally prevented cell proliferation. The expression level of pro-apoptotic genes like Bax was also increased. It seems that increased expression levels of Bax led to the activation of the intrinsic mitochondrial pathway, increased caspase-3and caspase-9, and release of cytochrome cand apoptosis induction. Similarly, the expression of the anti-apoptotic Bcl2 gene and the genes involved in cell cycle progression (cyclin D1 and cyclin E) decreased. There has also been a decrease in ERBB2 gene expression that is involved in cell proliferation. It is also reported to have over-expression in many cancers.
31
In addition, decreased expression of cyclin D1 protein in HT-29 cells treated with probiotic bacteria was confirmed. The results of the present study were in line with the results of other similar studies mentioned as follows.
A study reported that Bb12 L. rhamnosus GG and B. lactis supernatants could induce apoptosis by reducing Bcl2, regulating Bak, reducing HT-29 cell growth, and preventing cell proliferation by arresting cells in the G0/G1 phase.
32
In a study similar to ours, the supernatant of Bacillus polyfermenticus suppressed colony formation of HT-29, decreased mRNA levels and expression of ERBB2, ERBB3 proteins. It also decreased the cyclin D1 expression levels in HT-29 cells.
33
Increased chromatin condensation and apoptotic bodies formation, increased caspase activity, and release of cytochrome c was observed following the effect of fermented milk supernatant of Propionibacterium freudenreichii on HGT-1 cancer cells.
34
De Marco et al examined epithelial cells of HT-29 after a period of treatment with supernatants of L. acidophilus, L. casei, L. lactis, L. reuteri,and Saccharomyces boulardii.The results showed anti-inflammatory effects of probiotics. They also found that the metabolites of these bacteria could down-regulate the expression of IL-8 and PGE-2 in HT-29 cells.
35
Thirabunyanon et al studied the effects of Enterococcus faeciumRM11 and L. fermentum RM2 probiotics supernatant on Caco-2 cells and observed decreased cancer cell viability.
36
The supernatants of L. delbrueckiiCU/22 have been shown to increase superoxide radicals and bacterial hydrogen peroxide, and subsequently increase in HT-29 cells apoptosis preventing cancer progression.
37
In vitro studies have also shown that cell-free supernatants of L. casei probiotic and L. rhamnosus GG reduce the invasion of colon cancer cells and prevent metastasis of CRC cells. These probiotics inhibit matrix metalloproteinase 9 activity and increase the level of ZO-1 (adhesion protein) in metastatic colorectal carcinoma cells in humans.
38
The supernatants derived from L. fermentum were shown to be useful in preventing CRC by increasing the levels of short-chain fatty acids that are involved in colon health and the production of anti-carcinogenic compounds.
39
The study by An and Ha also showed that combination therapy with L. plantarum supernatant and 5-Fluouracil (the current medication used in CRC chemotherapy) could increase the chemical sensitivity of cancer cells and reduce the size of the colonospheres by the inactivation of Wnt/𝛽-catenin signaling. They found that the supernatant of this bacterium could selectively inhibit 5-fluouracil -resistant cancer cells in the HCT-116 and HT-29 cancer cell lines.
40
Another study has also suggested the effect of probiotic L. delbrueckii supernatants on preventing G1 cell proliferation, induction of apoptosis, and reducing matrix metalloproteinase 9, which is associated with cancer cell invasion. It has also been suggested that probiotic bacteria can be used as a potent anti-tumor agent in the treatment of cancers.
41
The supernatant of bacteria degenerates the stability of the chromatin within the nucleus by increasing the reactive oxygen species and lipid peroxidation, as well as decreasing the mitochondrial membrane potential, increasing caspase-3 activity and reducing Bcl-2 anti-apoptotic activity.
42-45
Considering the results reported by all these studies, it might be concluded that probiotic bacteria can be introduced as effective supplements in the treatment and prevention of cancers, especially CRC because they are both inexpensive and easily available.
46
Conclusion
The results showed that L. rhamnosus as a probiotic strain has great potential to inhibit cancer cell growth and induce apoptotic cell death in HT-29 colon cancer cells.
It seems that L. rhamnosus supernatant could be contributing to the release of cytochrome c by activating the mitochondrial pathway and promoting apoptosis. Given that the anti-cancer effects of probiotic bacteria are genus- and strain-dependent, it is proposed that after sufficient complementary testing, these indigenous bacteria can be used as supplementary drugs for inhibiting the growth of cancer cells.
Acknowledgments
The authors would like to acknowledge the Laboratory of Islamic Azad University.
Funding sources
There is no funding.
Ethical statement
Not applicable.
Competing interests
The authors declared no conflict of interest regarding the publication of this paper.
Authors’ contribution
ND performed most of the experiments, methodology and cell culture study. FT contributed by designing the experimental plans, performing data analysis, supervision, writing, review & editing, and project administration. PJ contributed to design the experimental plan and data analysis in preparation of supernatant of probiotic bacteria and writing the original draft.
Research Highlights
What is the current knowledge?
simple
-
√ Apoptosis induction in cancerous cells is one of the most suitable methods for cancer treatment.
-
√ Finding anticancer compounds, especially apoptosis inducers, has attracted great attention in recent years.
-
√ There are limited articles and reports regarding the effect of supernatant of indigenous probiotic bacteria on cancer cells.
What is new here?
simple
-
√ The supernatant of indigenous probiotic bacteria isolated from Iranian traditional dairy products induced apoptosis in HT–29 cell line.
-
√ The supernatant of probiotic bacteria inhibited the growths of HT-29 tumor cells in a dose-, and time-dependent manner.
-
√ It is proposed that these bacteria can be used as anticancer biological products for the treatment and prevention of cancer.
References
- Moghimi-Dehkordi B, Safaee A, Zali MR. Prognostic factors in 1,138 Iranian colorectal cancer patients. Int J Colorectal Dis 2008; 23:683-8. doi: 10.1007/s00384-008-0463-7 [Crossref] [ Google Scholar]
- Tariq K, Ghias K. Colorectal cancer carcinogenesis: a review of mechanisms. Cancer Biol Med 2016; 13:120-35. doi: 10.28092/j.issn.2095-3941.2015.0103 [Crossref] [ Google Scholar]
- Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev 2003; 16:658-72. doi: 10.1128/cmr.16.4.658-672.2003 [Crossref] [ Google Scholar]
- Ross SA. Evidence for the relationship between diet and cancer. Exp Oncol 2010; 32:137-42. [ Google Scholar]
-
e Silva JPS, Freitas AC. Probiotic bacteria: fundamentals, therapy, and technological aspects. Pan Stanford; 2014.
- Kuo CH, Lin YW, Chen RS. Lipopeptides extract from Bacillus amyloliquefaciens induce human oral squamous cancer cell death. Asian Pac J Cancer Prev 2015; 16:91-6. doi: 10.7314/apjcp.2015.16.1.91 [Crossref] [ Google Scholar]
- Jeong SY, Park SY, Kim YH, Kim M, Lee SJ. Cytotoxicity and apoptosis induction of Bacillus vallismortis BIT-33 metabolites on colon cancer carcinoma cells. J Appl Microbiol 2008; 104:796-807. doi: 10.1111/j.1365-2672.2007.03615.x [Crossref] [ Google Scholar]
- Prakash S, Rodes L, Coussa-Charley M, Tomaro-Duchesneau C. Gut microbiota: next frontier in understanding human health and development of biotherapeutics. Biologics 2011; 5:71-86. doi: 10.2147/BTT.S19099 [Crossref] [ Google Scholar]
- Ehlers S, Kaufmann SH. Participants of the 99 Dahlem C. Infection, inflammation, and chronic diseases: consequences of a modern lifestyle. Trends Immunol 2010; 31:184-90. doi: 10.1016/j.it.2010.02.003 [Crossref] [ Google Scholar]
- Saikali J, Picard C, Freitas M, Holt P. Fermented milks, probiotic cultures, and colon cancer. Nutr Cancer 2004; 49:14-24. doi: 10.1207/s15327914nc4901_3 [Crossref] [ Google Scholar]
- Rafter J. Probiotics and colon cancer. Best Pract Res Clin Gastroenterol 2003; 17:849-59. doi: 10.1016/S1521-6918(03)00056-8 [Crossref] [ Google Scholar]
- de Moreno de Leblanc A, Perdigon G. The application of probiotic fermented milks in cancer and intestinal inflammation. Proc Nutr Soc 2010; 69:421-8. doi: 10.1017/S002966511000159X [Crossref] [ Google Scholar]
- Kim JY, Woo HJ, Kim YS, Kim KH, Lee HJ. Cell cycle dysregulation induced by cytoplasm of Lactococcus lactis ssp lactis in SNUC2A, a colon cancer cell line. Nutr Cancer 2003; 46:197-201. doi: 10.1207/S15327914NC4602_13 [Crossref] [ Google Scholar]
- Karimi Ardestani S, Tafvizi F, Tajabadi Ebrahimi M. Heat-killed probiotic bacteria induce apoptosis of HT-29 human colon adenocarcinoma cell line via the regulation of Bax/Bcl2 and caspases pathway. Hum Exp Toxicol 2019; 38:1069-81. doi: 10.1177/0960327119851255 [Crossref] [ Google Scholar]
- Mojibi P, Tafvizi F, Bikhof Torbati M. Cell-bound exopolysaccharide extract from indigenous probiotic bacteria induce apoptosis in HT-29 cell-line. Iran J Pathol 2019; 14:41-51. doi: 10.30699/IJP.14.1.41 [Crossref] [ Google Scholar]
- Choi SS, Kim Y, Han KS, You S, Oh S, Kim SH. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett Appl Microbiol 2006; 42:452-8. doi: 10.1111/j.1472-765X.2006.01913.x [Crossref] [ Google Scholar]
- Orlando A, Refolo MG, Messa C, Amati L, Lavermicocca P, Guerra V. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC21 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr Cancer 2012; 64:1103-11. doi: 10.1080/01635581.2012.717676 [Crossref] [ Google Scholar]
- Sadeghi-Aliabadi H, Mohammadi F, Fazeli H, Mirlohi M. Effects of Lactobacillus plantarum A7 with probiotic potential on colon cancer and normal cells proliferation in comparison with a commercial strain. Iran J Basic Med Sci 2014; 17:815-9. [ Google Scholar]
- Nouri Z, Karami F, Neyazi N, Modarressi MH, Karimi R, Khorramizadeh MR. dual anti-metastatic and anti-proliferative activity assessment of two probiotics on HeLa and HT-29 cell lines. Cell J 2016; 18:127-34. [ Google Scholar]
- Taherian-Esfahani Z, Abedin-Do A, Nouri Z, Mirfakhraie R, Ghafouri-Fard S, Motevaseli E. Lactobacilli differentially modulate mTOR and Wnt/ beta-catenin pathways in different cancer cell lines. Iran J Cancer Prev 2016; 9:e5369. doi: 10.17795/ijcp-5369 [Crossref] [ Google Scholar]
- Tiptiri-Kourpeti A, Spyridopoulou K, Santarmaki V, Aindelis G, Tompoulidou E, Lamprianidou EE. Lactobacillus casei Exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS One 2016; 11:e0147960. doi: 10.1371/journal.pone.0147960 [Crossref] [ Google Scholar]
- Daniluk U. Probiotics, the new approach for cancer prevention and/or potentialization of anti-cancer treatment. J Clin Exp Oncol 2012; 1:2. doi: 10.4172/2324-9110.1000e105 [Crossref] [ Google Scholar]
- Amraii HN, Abtahi H, Jafari P, Mohajerani HR, Fakhroleslam MR, Akbari N. In vitro study of potentially probiotic lactic acid bacteria strains isolated from traditional dairy products. Jundishapur J Microbiol 2014; 7:e10168. doi: 10.5812/jjm.10168 [Crossref] [ Google Scholar]
- Morelli L, Capurso L. FAO/WHO guidelines on probiotics: 10 years later. J Clin Gastroenterol 2012; 46 Suppl:S1-2. doi: 10.1097/MCG.0b013e318269fdd5 [Crossref] [ Google Scholar]
- Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev 2012; 70 Suppl 1:S38-44. doi: 10.1111/j.1753-4887.2012.00493.x [Crossref] [ Google Scholar]
- Ambalam P, Raman M, Purama RK, Doble M. Probiotics, prebiotics and colorectal cancer prevention. Best Pract Res Clin Gastroenterol 2016; 30:119-31. doi: 10.1016/j.bpg.2016.02.009 [Crossref] [ Google Scholar]
- Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, Chen GY. The gut microbiome modulates colon tumorigenesis. MBio 2013; 4:e00692-13. doi: 10.1128/mBio.00692-13 [Crossref] [ Google Scholar]
- Raskov H, Burcharth J, Pommergaard HC. Linking gut microbiota to colorectal cancer. J Cancer 2017; 8:3378-95. doi: 10.7150/jca.20497 [Crossref] [ Google Scholar]
- Wiley NC, Dinan TG, Ross RP, Stanton C, Clarke G, Cryan JF. The microbiota-gut-brain axis as a key regulator of neural function and the stress response: Implications for human and animal health. J Anim Sci 2017; 95:3225-46. doi: 10.2527/jas.2016.1256 [Crossref] [ Google Scholar]
- Aureli P, Capurso L, Castellazzi AM, Clerici M, Giovannini M, Morelli L. Probiotics and health: an evidence-based review. Pharmacol Res 2011; 63:366-76. doi: 10.1016/j.phrs.2011.02.006 [Crossref] [ Google Scholar]
- Yu D, Hung MC. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 2000; 19:6115-21. doi: 10.1038/sj.onc.1203972 [Crossref] [ Google Scholar]
- Borowicki A, Michelmann A, Stein K, Scharlau D, Scheu K, Obst U. Fermented wheat aleurone enriched with probiotic strains LGG and Bb12 modulates markers of tumor progression in human colon cells. Nutr Cancer 2011; 63:151-60. doi: 10.1080/01635581.2010.516874 [Crossref] [ Google Scholar]
- Ma EL, Choi YJ, Choi J, Pothoulakis C, Rhee SH, Im E. The anticancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int J Cancer 2010; 127:780-90. doi: 10.1002/ijc.25011 [Crossref] [ Google Scholar]
- Cousin FJ, Jouan-Lanhouet S, Dimanche-Boitrel MT, Corcos L, Jan G. Milk fermented by Propionibacterium freudenreichii induces apoptosis of HGT-1 human gastric cancer cells. PLoS One 2012; 7:e31892. doi: 10.1371/journal.pone.0031892 [Crossref] [ Google Scholar]
- De Marco S, Sichetti M, Muradyan D, Piccioni M, Traina G, Pagiotti R. Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid Based Complement Alternat Med 2018; 2018:1756308. doi: 10.1155/2018/1756308 [Crossref] [ Google Scholar]
- Thirabunyanon M, Boonprasom P, Niamsup P. Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol Lett 2009; 31:571-6. doi: 10.1007/s10529-008-9902-3 [Crossref] [ Google Scholar]
- Strus M, Janczyk A, Gonet-Surowka A, Brzychczy-Wloch M, Stochel G, Kochan P. Effect of hydrogen peroxide of bacterial origin on apoptosis and necrosis of gut mucosa epithelial cells as a possible pathomechanism of inflammatory bowel disease and cancer. J Physiol Pharmacol 2009; 60 Suppl 6:55-60. [ Google Scholar]
- Escamilla J, Lane MA, Maitin V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr Cancer 2012; 64:871-8. doi: 10.1080/01635581.2012.700758 [Crossref] [ Google Scholar]
- Kahouli I, Malhotra M, Alaoui-Jamali M, Prakash S. In-vitro characterization of the anti-cancer activity of the probiotic bacterium Lactobacillus fermentum NCIMB 5221 and potential against colorectal cancer. J Cancer Sci Ther 2015; 7:224-35. [ Google Scholar]
- An J, Ha EM. Combination therapy of Lactobacillus plantarum supernatant and 5-fluouracil increases chemosensitivity in colorectal cancer cells. J Microbiol Biotechnol 2016; 26:1490-503. doi: 10.4014/jmb.1605.05024 [Crossref] [ Google Scholar]
- Wan Y, Xin Y, Zhang C, Wu D, Ding D, Tang L. Fermentation supernatants of Lactobacillus delbrueckii inhibit growth of human colon cancer cells and induce apoptosis through a caspase 3-dependent pathway. Oncol Lett 2014; 7:1738-42. doi: 10.3892/ol.2014.1959 [Crossref] [ Google Scholar]
- Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med 1998; 187:1261-71. doi: 10.1084/jem.187.8.1261 [Crossref] [ Google Scholar]
- Decaudin D, Marzo I, Brenner C, Kroemer G. Mitochondria in chemotherapy-induced apoptosis: a prospective novel target of cancer therapy (review). Int J Oncol 1998; 12:141-52. [ Google Scholar]
- Hofmanova J, Strakova N, Vaculova AH, Tylichova Z, Safarikova B, Skender B. Interaction of dietary fatty acids with tumour necrosis factor family cytokines during colon inflammation and cancer. Mediators Inflamm 2014; 2014:848632. doi: 10.1155/2014/848632 [Crossref] [ Google Scholar]
- Nowak A, Paliwoda A, Blasiak J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit Rev Food Sci Nutr 2019; 59:3456-67. doi: 10.1080/10408398.2018.1494539 [Crossref] [ Google Scholar]
- Hendler R, Zhang Y. Probiotics in the treatment of colorectal cancer. Medicines (Basel) 2018; 5. doi: 10.3390/medicines5030101 [Crossref]