Bioimpacts. 10(4):209-215.
doi: 10.34172/bi.2020.27
Original Research
Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: A randomized clinical trial
Khalil Ansarin 1, 2  , Ramin Tolouian 3, Mohammadreza Ardalan 4, *
, Ramin Tolouian 3, Mohammadreza Ardalan 4, *  , Ali Taghizadieh 2, 5, Mojtaba Varshochi 6, Soheil Teimouri 5, Tahere Vaezi 5, Hamed Valizadeh 2, 5, Parviz Saleh 4, Saeid Safiri 7, 8
, Ali Taghizadieh 2, 5, Mojtaba Varshochi 6, Soheil Teimouri 5, Tahere Vaezi 5, Hamed Valizadeh 2, 5, Parviz Saleh 4, Saeid Safiri 7, 8  , Kenneth R. Chapman 9
, Kenneth R. Chapman 9 
Author information:
1Rahat Breath and Sleep Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Tuberculosis and Lung Disease Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Division of Nephrology, University of Arizona, Tucson, AZ, USA
4Kidney Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Internal Medicine, School of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
6Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
7Department of Community Medicine, School of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
8Physical Medicine and Rehabilitation Research Center, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
9Asthma and Airway Center, University Health Network, University of Toronto, Toronto, ON, Canada
Abstract
Introduction:
Bromhexine is a potential therapeutic option in COVID-19, but no data from a randomized clinical trial has been available. The present study aimed to evaluate the efficacy of bromhexine in intensive care unit (ICU) admission, mechanical ventilation, and mortality in patients with COVID-19.
Methods:
An open-label randomized clinical trial study was performed in Tabriz, North-West of Iran. They were randomized to either the treatment with the bromhexine group or the control group, in a 1:1 ratio with 39 patients in each arm. Standard therapy was used in both groups and those patients in the treatment group received oral bromhexine 8 mg three times a day additionally. The primary outcome was a decrease in the rate of ICU admissions, intubation/mechanical ventilation, and mortality.
Results:
A total of 78 patients with similar demographic and disease characteristics were enrolled. There was a significant reduction in ICU admissions (2 out of 39 vs. 11 out of 39, P = 0.006), intubation (1 out of 39 vs. 9 out of 39, P = 0.007) and death (0 vs. 5, P = 0.027) in the bromhexine treated group compared to the standard group. No patients were withdrawn from the study because of adverse effects.
Conclusion:
The early administration of oral bromhexine reduces the ICU transfer, intubation, and the mortality rate in patients with COVID-19. This affordable medication can easily be administered everywhere with a huge positive impact(s) on public health and the world economy. Altogether, the verification of our results on a larger scale and different medical centers is strongly recommended.
Trial Registration:
IRCT202003117046797N4; https://irct.ir/trial/46969.
Keywords: Bromhexine hydrochloride, COVID-19 disease, SARS-CoV2, TMPRSS2, COVID-19 pneumonia, COVID-19 treatment
Copyright and License Information
© 2020 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS- CoV- 2) disease has emerged as a multifaceted disease with varied pathologies involving multiple organs. The COVID-19 infection can manifest in degrees from a predicted 50% asymptomatic illness, to 20% of cases having severe illness.
1
According to Johns Hopkins University Coronavirus Resource Center, the mortality rates vary between 4.5-16% in the top 10 most affected countries. As of mid-June 2020, the number of deaths surpassed 438,000 individuals throughout the world. The virus can aggravate an inflammation cascade and cause a cytokine storm. This is one of the major mechanisms that can cause the deterioration of the general condition in patients with COVID-19 infection.
2
Multiple different therapeutic options like anti-malaria, HIV medications, antivirals, and steroids have been tried with limited results. The most notable was the compassionate use of remedisivir in the setting of COVID-19 infection. The original data initially encouraged the FDA to approve this medication for use in COVID-19 infections, but further clinical trials have not been able to support significant clinical benefit. Currently, no therapeutic agents have been proven to be effective in reducing the mortality and treatment of patients with COVID-19.
3-5
SARS-CoV-2 attaches to the angiotensin-converting enzyme 2 (ACE2) receptor of the host cell via its spike protein. The virus has two pathways in which it can enter the cell, either Endocytosis or Non-endocytosis. Today, the newer viruses tend to use the non-endocytosis pathway which is activated by transmembrane serine protease 2 (TMPRSS2).
6
Blocking the TMPRSS2 and the non-endocytosis pathway would be an interesting therapeutic option in preventing virus entry into the cell and therefore, treating the disease. Nafamostat and camostat mesylate, medications that are used to treat chronic pancreatitis are potential candidates for blocking virus entry by inhibiting TMPRSS2.
7,8
There is some concern about the low distribution of these drugs in the respiratory system. As shown in Fig. 1, another TMPRSS2 inhibitor with a greater distribution capacity into the lung tissue is bromhexine hydrochloride.
9,10
It is an old over-the-counter medication that has been used for several decades as a mucolytic agent. The current study, an open-label, randomized clinical trial, examined the efficacy of early start of oral bromhexine, in the intensive care unit (ICU) admission, rate of mechanical ventilation, and mortality in patients with COVID-19 pneumonia.
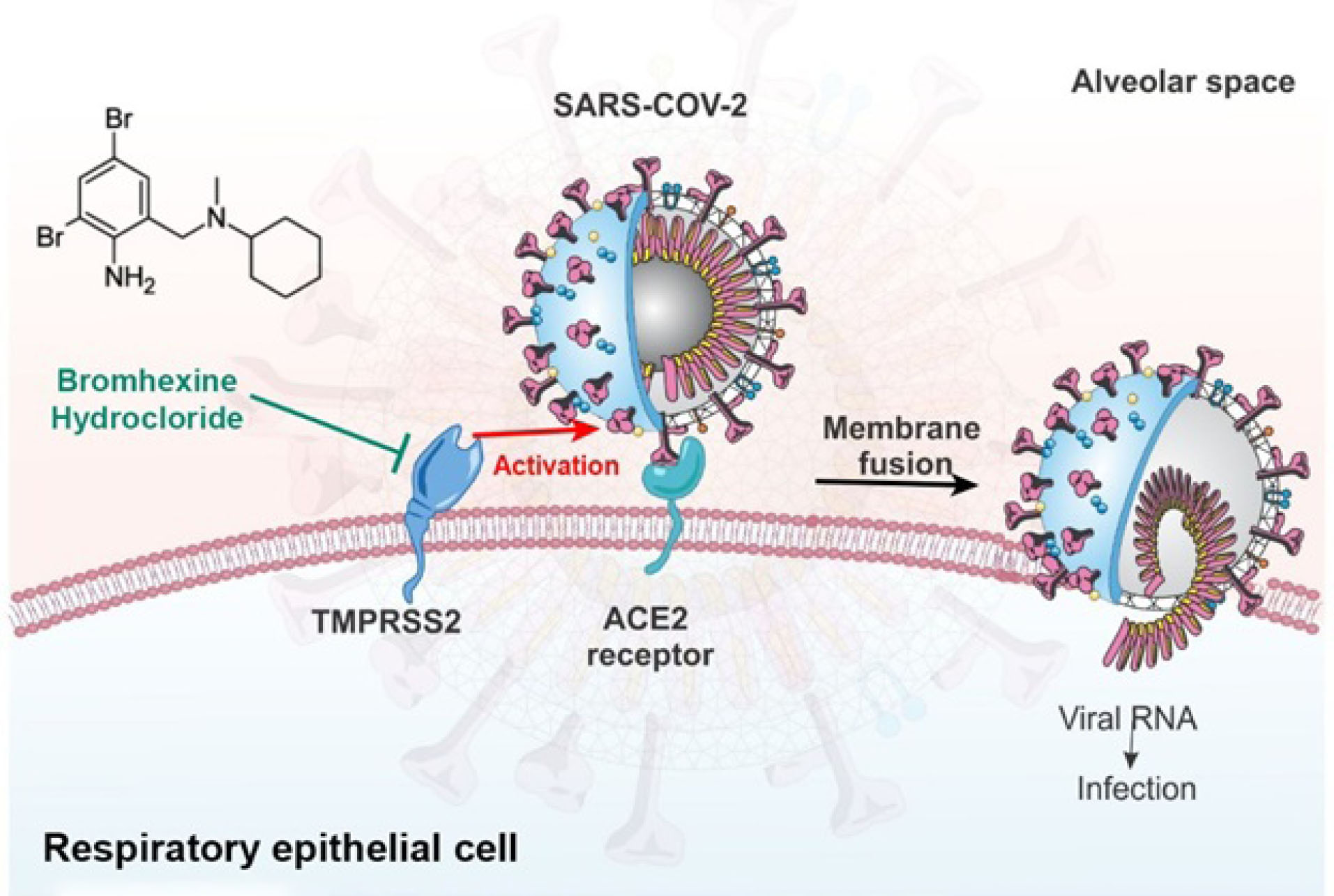
Fig. 1.
TMPRSS2 protease system of host cell wall priming viral spike protein and facilitating viral cell entry via ACE2 receptor blocked by bromhexine.
.
TMPRSS2 protease system of host cell wall priming viral spike protein and facilitating viral cell entry via ACE2 receptor blocked by bromhexine.
Materials and Methods
Patients were recruited in University Hospital from April 18, 2020, to May 19, 2020. The study was completed on May 28, 2020. Written informed consent was obtained from all study participants. This clinical trial was an open-label, randomized clinical study. Eighty-nine patients with a diagnosis of COVID-19 pneumonia were screened. Eleven of the patients were excluded. [9 not meeting inclusion criteria, 2 declined]. A total of 78 patients were enrolled.
The diagnosis of COVID-19 pneumonia was made by a board-certified pulmonologist based on clinical symptoms and signs, as well as chest CT findings compatible with the COVID-19 pneumonia pattern. Although PCR testing became available within a few weeks following the start of the outbreak, its use was not relied upon for entry into the trial given the lag period for the availability of results and a high number of false-negative results.
The subjects, based on inclusion and exclusion criteria, were randomized (1:1) via balanced block randomization into treatment and non-treatment (standard) groups [39 persons per each group] by epidemiologists of the study. Clinicians and patients were not blind to treatment assignment.
Inclusion criteria
Inclusion criteria were the following: hospital admission, 18 years old or greater, chest imaging and clinical symptoms consistent with COVID-19 pneumonia, willingness to participate in the study, signed informed consent, and no participation in other clinical trials.
Exclusion criteria
Exclusion criteria were the following: Pregnancy or lactation, patients with chronic respiratory disease that pre-existence symptoms or consolidations may interfere with an accurate diagnosis of COVID-19, and a history of allergy to bromhexine and patients with cancer.
Standard arm
Patients received treatment based on the Iranian national COVID-19 treatment protocol and best practice guidelines at that time and also the “Hydroxychloroquine 200 mg/d for two weeks” in addition to supportive and symptomatic therapy.
Treatment arm
The treatment arm received oral bromhexine hydrochloride 8 mg three times a day for two weeks after randomization in addition to standard therapy.
Outcome measures
The primary outcomes were the improvement in the rate of ICU admissions, intubation/mechanical ventilation, and 28-days mortality. The secondary clinical outcomes were as follows, clinical improvement of symptoms including fever, dyspnea, and weakness, assessment of C-reactive protein (CRP), lactate dehydrogenase (LDH), neutrophil/lymphocyte ratio (NLR), and length of stay in the hospital. The clinical improvement was defined as the improvement of the patient’s symptoms to the point that we were able to discharge the subject home and complete treatment as an outpatient.
Clinical symptoms of the subjects included fever, cough, dyspnea, lassitude, myalgia, and GI symptoms, (nausea, vomiting, and diarrhea) which were ascertained by a physician blinded to the treatment allocation. A structured questionnaire for admission baseline and again at the end of the treatment period was used. In all patients, the NLR, CRP, and LDH levels were measured and recorded before the treatment onset and again at the conclusion of treatment. Oxygen saturation was monitored daily and chest X-rays were done as needed. Clinical stability and oxygen saturation ≥90% in room air for 48 hours were criteria for discharge home with isolation and all patients were followed for 28 days total.
The criteria for the ICU admission were the worsening of respiratory distress assessed by the physician, hemodynamic instability requiring vasopressors, Oxygen desaturation <85% that was not responsive to low flow oxygen therapy. The Apache score of all subjects was calculated.
Statistical analysis
Assuming 80% power, two-sided testing at α = 0.05, the anticipated ICU admission rate of 35% in the control arm and 5% in the treatment arm, a minimum sample size of 27 subjects per group was calculated. The quantitative and qualitative variables were reported as mean ± standard deviation and number (%) respectively. The normally and non-normally distributed quantitative variables were compared between groups using The Independent Sample t- test and Mann-Whitney, respectively. Besides, the qualitative variables were compared between two groups using the Chi-Square test, and Fisher Exact test was applied where the data sparsity was observed. All the statistical analysis was conducted using SPSS software (version 26, SPSS Inc., Chicago, IL, USA) and P <0.05 was considered significant. Missing data for the secondary outcomes were not imputed. Only observed values were used for data analysis and presentation.
Results
Eighty-nine patients with proven COVID-19 pneumonia were screened. Eleven of the patients were excluded (i.e., 9 not meeting the inclusion/exclusion criteria, 2 declined). A total of 78 patients were enrolled in this randomized clinical trial. They were assigned to either the treatment with the bromhexine group or the standard treatment group in a 1:1 ratio with 39 patients in each arm. No attrition in either arm (Fig. 2)
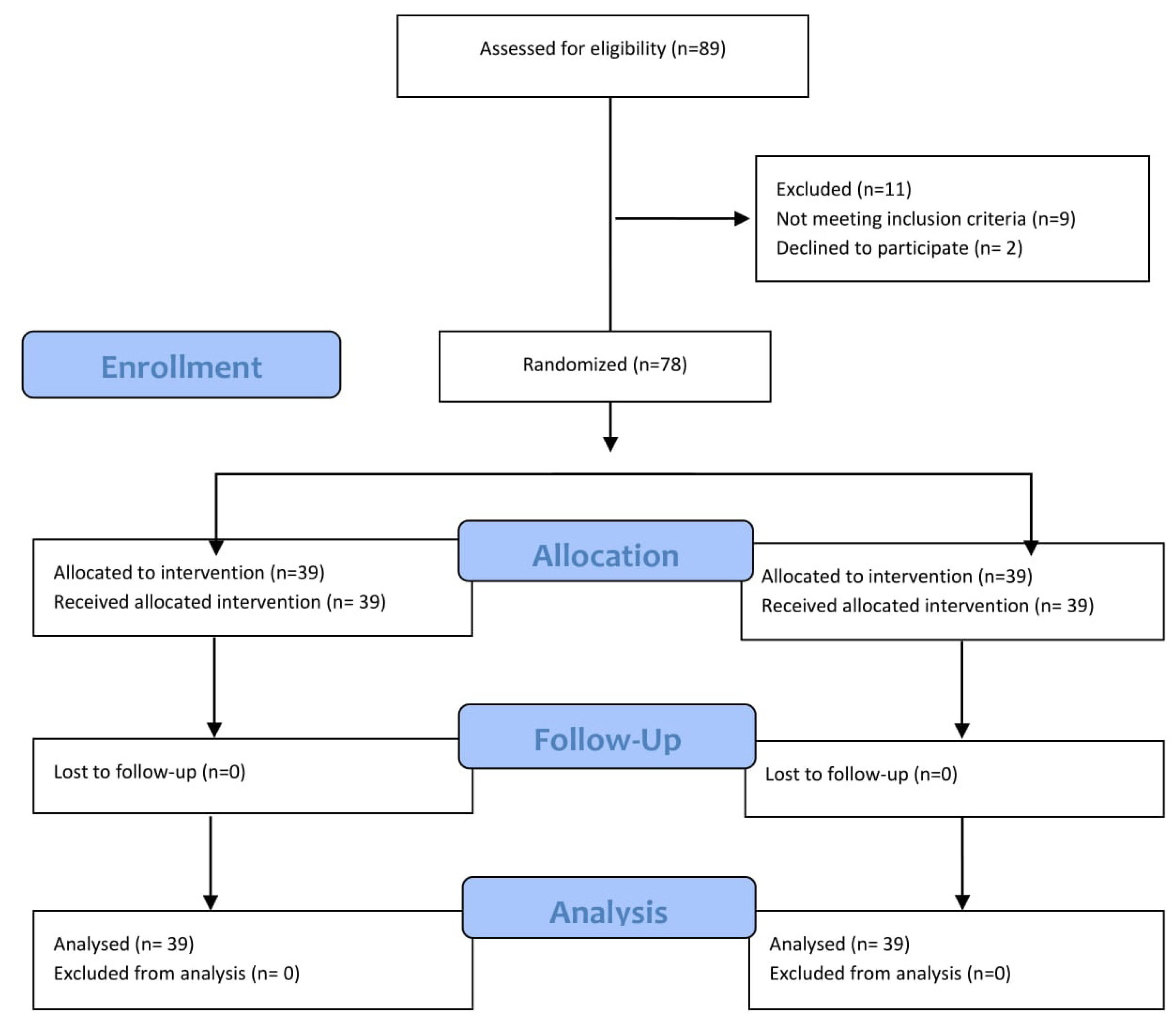
Fig. 2.
Patient enrollment and treatment assignment.
.
Patient enrollment and treatment assignment.
The demographic and disease characteristics of the treatment and standard groups were similar (Table 1). The mean age was 58.4±13.7 years among the treated arm and 61.1 ±16.1 in the standard arm. In terms of gender, 43 (54.4%) were male, and the percentage of males in the treatment and standard groups was 48% and 61%, respectively. No significant difference was noted in the comorbid conditions of diabetes mellitus and hypertension between the treatment and control groups. There was no significant difference in the time-lag of symptom initiation to hospital admission in the treatment (7.7±4.1 days) arm or the standard (8.3±5.8 days) arm (P =0.632). The body mass index (BMI) and APACHE score in both groups were almost identical.
Table 1.
Demographic and clinical characteristics in patients treated with bromhexine-hydrochloride and standard group
|
|
Standard group (n=39)
|
Treatment group (n=39)
|
P
value
|
|
Demographic characteristics
|
| Age(year) |
61.1 ±16.1 |
58.4±13.7 |
0.43a
|
| Gender (male/female) |
24/15 |
19/20 |
0.255b
|
|
BMI (kg/m2)
|
27.8±4.3 |
27.6±4.4 |
0.812a
|
| Diabetes mellitus, No. (%) |
10 (25.6%) |
16 (41.0%) |
0.150b
|
| Hypertension, No. (%) |
20 (51.3%) |
19 (48.7%) |
0.821 |
|
Baseline clinical characteristics
|
| APACHE Score |
10.2±4.6 |
9.3 ±2.9 |
0.295a
|
| Time-lag of symptom initiation to hospital admission (days) |
8.3±5.8 |
7.7±4.1 |
0.632 |
| SpO2 (%) |
88.5±4.4 |
87.8±4.6 |
0.550a
|
| Fever, No. (%) |
18 (46.2%) |
19 (48.7%) |
0.821b
|
| Cough, No. (%) |
26 (66.7%) |
30 (76.9%) |
0.314b
|
| Dyspnea, No. (%) |
28 (71.8%) |
26 (66.7%) |
0.624b
|
| Lassitude, No. (%) |
24 (61.5%) |
18 (46.2%) |
0.173b
|
| Myalgia, No. (%) |
27 (69.2%) |
17 (43.6%) |
0.022b
|
| Headache, No. (%) |
11 (28.2%) |
7 (17.9%) |
0.282b
|
| GI symptoms, No. (%) |
13 (33.3%) |
16 (41.0%) |
0.482b
|
| NLR (IQR) |
5.5 (8.1) |
4.7 (4.2) |
0.526c
|
| Positive CRP, No. (%) |
33 (91.7%) |
29 (87.9%) |
0.450d
|
| LDH U/L |
470.0 (262.5) |
507.0 (177.0) |
0.742c
|
Abbreviations: APACHE, acute physiology and chronic health evaluation; Spo2, saturation of peripheral oxygen; GI, Gastrointestinal; NLR, neutrophil lymphocyte ratio; CRP, C-reactive protein; LDH, lactate dehydrogenase; ICU, intensive care unit.
aIndependent samples t test; bChi-squared test; cCalculated with Mann-Whitney; dFisher's exact test
The values shown were based on total number of patients who contributed values.
Primary clinical outcome
There was a significant difference in the 28-day mortality, no patient died (0%) in the treatment group, while five patients died (12.8%) in the standard group; P = 0.027 (Fig. 3). Among patients in the treatment arm with bromhexine, two patients were admitted to ICU (5.1%) while in the standard arm, eleven patients (28.2%) were transferred to ICU. There was a significant difference in the ICU admission between the treatment and the standard group (P = 0.006). Moreover, among patients in bromhexine arm, one patient received mechanical ventilation (2.6%), while in the standard arm nine patients (23.1%) were intubated and underwent mechanical ventilation. There was also a highly significant difference in the intubation and mechanical ventilation between the two groups (P = 0.007) as shown in Table 2.
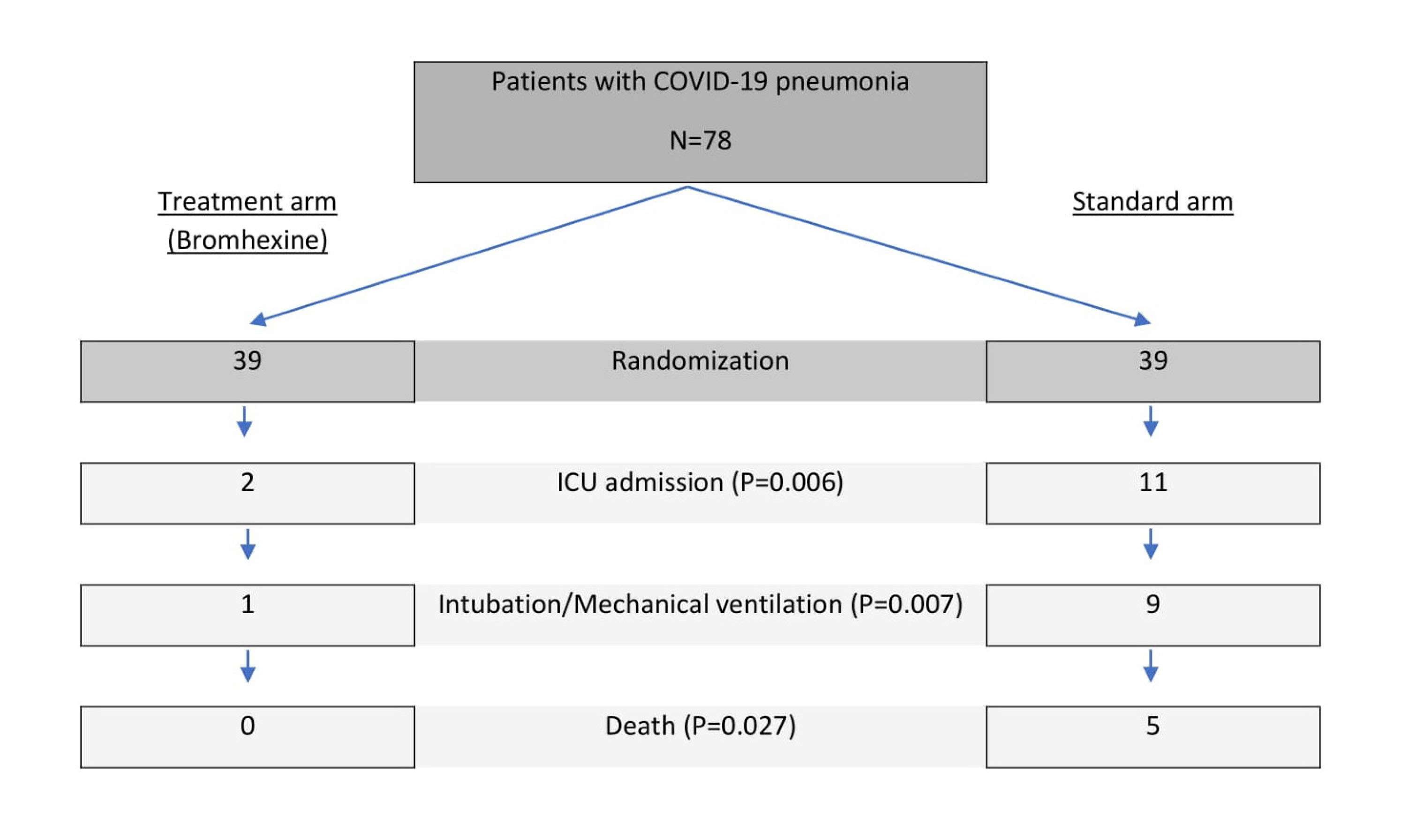
Fig. 3.
Patient enrollment and primary outcomes.
.
Patient enrollment and primary outcomes.
Table 2.
Post-treatment clinical characteristics in patients treated with bromhexine and standard group
|
Demographic characteristics
|
Standard group (n=39)
|
Treatment group (n=39)
|
P
value
|
|
Primary outcomes
|
| Mortality, No. (%) |
5 (12.8) |
0 (0) |
0.027d
|
| ICU admission, No. (%) |
11 (28.2) |
2 (5.1) |
0.006b
|
| Mechanical ventilation, No. (%) |
9 (23.1) |
1 (2.6) |
0.007b
|
|
Secondary outcomes
|
| Fever, No. (%) |
3 (10.0) |
0 (0) |
0.125d
|
| Cough, No. (%) |
12 (40.0) |
2 (6.9) |
0.003b
|
| Dyspnea, No. (%) |
14 (48.3) |
1 (3.4) |
<0.001b
|
| Lassitude, No. (%) |
10 (34.5) |
2 (6.9) |
0.010b
|
| Myalgia, No. (%) |
4 (13.8) |
3 (10.3) |
0.500d
|
| Headache, No. (%) |
3 (10.0) |
0 (0) |
0.125d
|
| GI symptoms, No. (%) |
1 (3.3) |
4 (13.8) |
0.166d
|
| NLR (IQR) |
3.0 (6.3) |
1.7 (1.0) |
0.052c
|
| Positive CRP, No. (%) |
9 (81.8) |
0 (0) |
<0.001d
|
| LDH U/L mean ±SD |
445.3±115.2 |
363.2±83.6 |
0.056a
|
| Hospital stay (days), mean ±SD |
8.1 ±5.5 |
7.6 ±3.5 |
0.587a
|
Abbreviations: APACHE, acute physiology and chronic health evaluation; Spo2, saturation of peripheral oxygen; GI, Gastrointestinal; NLR, neutrophil-lymphocyte ratio; IQR, interquartile range; CRP, C-reactive protein; LDH, lactate dehydrogenase; ICU, intensive care unit.
aIndependent Samples t-test; b Chi-squared test; dFisher's exact test, dCalculated with Mann-Whitney;
The values shown were based on the total number of patients who contributed values.
Secondary clinical outcomes
There was also a significant difference in the secondary outcome of this study (Fig. 4). Improvement of cardinal respiratory symptoms such as cough and dyspnea within two weeks among the two groups were assessed: Dyspnea remained in 3.4% in the treatment group vs 48.3% in the standard group P ≤ 0.001 and cough remained in the treatment group 6.9% vs 40.0% in the standard group, P = 0.003). Similar results for lassitude between the two groups were observed (6.9% vs 34.5%, P = 0.010). At baseline, there was no significant difference in the frequency of fever, headache, and gastrointestinal symptoms between the two groups.
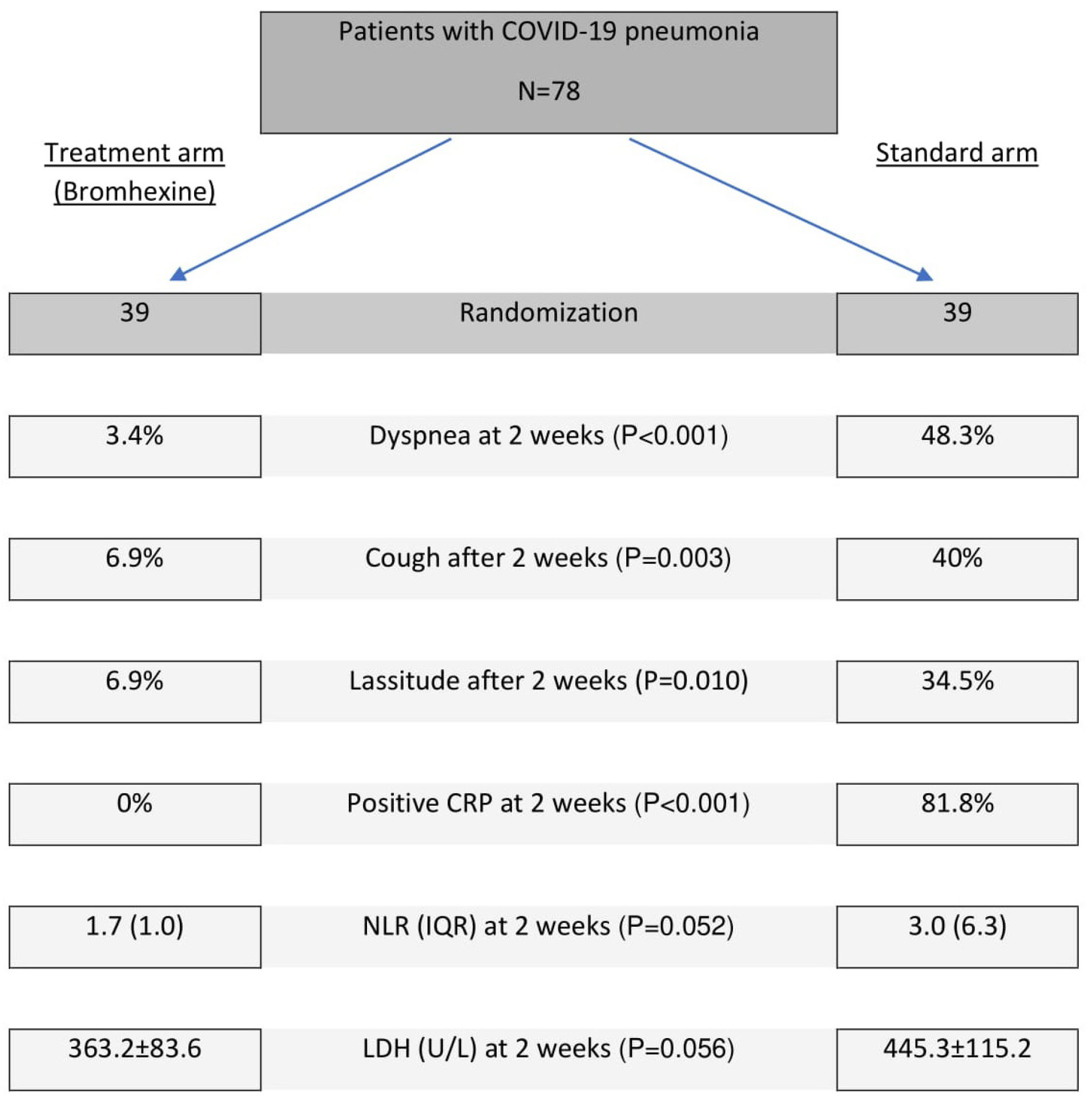
Fig. 4.
Patient enrollment and secondary outcomes.
.
Patient enrollment and secondary outcomes.
The patients in the bromhexine group showed improvement in the level of LDH (363.2±83.6 vs. 445.3±115.2; P = 0.056), NLR [1.7 (1.0) vs. 3.0 (6.3); P =0.052] and CRP [0% vs. 81.8%; P < 0.001)] within two weeks compared to the standard group (Table 2).
There was no significant difference in the length of stay in the hospital [from randomization to discharge] between the two groups (treatment 7.6±3.5 days and standard 8.1±5.5 days; P = 0.587).
Adverse Events
No major adverse events were found.
Discussion
The data presented in this clinical trial confirmed that the early-onset treatment with oral bromhexine 8 mg three times a day not only effectively mitigated the respiratory symptoms, but also significantly decreased the rate of ICU admissions, intubation, mechanical ventilation, and mortality in COVID-19 disease. This is a very significant finding that has a direct correlation with the outcome of the disease. The difference in the number of deaths in the bromhexine arm (0 death) vs. the standard arm (5 deaths, 12.8%) is reflective of the efficacy of this medication in reducing mortality when started early in the course of the disease.
COVID-19 infection, like the original SARS and MERS diseases, has a strong affinity to the lung parenchyma. The clinical manifestations vary from a simple cough to full-blown acute respiratory disease (ARDS). Hypoxemia and oxygen desaturation, which have often been unresponsive to the usual therapeutic measures have led the patients to ICUs which often includes initiation of ventilation support and death.
11
Hypercapnia due to neuromuscular involvement, thrombotic events despite anticoagulation, and cardiac involvement might be contributing factors in the deteriorating respiratory condition leading to ICU admission and death.
12-14
It has been observed that there is usually rapid deterioration of the general condition within the first 24 hours of intubation and mechanical ventilation in patients with COVID-19 infection, having a direct correlation with poor outcomes.
15,16
This observation might encourage intensivists to adopt a higher threshold for intubating patients with COVID-19 infection. It is prudent to consider ICU admission and intubation/mechanical ventilation in this patient population as going into the danger zone rather than a bridge to recovery. Therefore, any intervention that prevents ICU admission, may have a high impact on mortality rates.
The length of hospital stay in the treatment arm was 7.6±3.5 days and in the standard group was 8.1±5.5 days. The big margin between the minimum and maximum days of hospitalization in both groups could not provide a significant P-value.
The data analysis of this clinical trial also showed that cardinal respiratory symptoms (cough, lassitude, and dyspnea) in patients with COVID-19 disease who received bromhexine treatment was remarkably less than the standard group. These findings may reflect the lower intensity of pulmonary involvement in the group of patients who received bromhexine.
SARS-CoV-2 activates several inflammatory and cytokine cascades in the human body. The CRP is a marker of inflammation which is suggested to be used in the screening of the patients suspected of COVID-19 disease. CRP is an acute-phase reactant that is synthesized by the liver under the trigger of IL-6. The plasma half-life of CRP is 19 hours.
17
Higher CRP has been linked to unfavorable aspects of COVID-19 disease such as ARDS development.
18,19
In our clinical trial CRP was positive in 87.9% and 91.7% of patients in treatment and standard arms respectively, before the initiation of any treatment. Re-checking CRP two weeks after treatment showed complete improvement in CRP in 100% of patients in the bromhexine group while CRP remained positive in 83.3% of patients in the standard treatment arm. This is an interesting outcome in this clinical trial which indicates the effective impact of bromhexine in controlling inflammation.
Another lab finding that predicts the severity of illness is the NLR. It has been shown that the NLR of >3.3 is predictive of admission to ICU.
20
In our study, the NLR was higher than 3.5 in both groups before the treatment. In the bromhexine treated arm, by the end of the treatment, NLR was lower compared to the standard group but surprisingly, it was not statistically significant (P =0.052). Larger scale trials might be able to reveal the significant reduction of NLR with bromhexine.
LDH is an intracellular enzyme and presents in all cells, including the lung. Patients with COVID-19 disease release high amounts of LDH into circulation due to cytokine release and lung damage. The LDH level increases in patients with COVID-19 infection and returns to normal with the improvement of the disease. The LDH is a predictor of severity and mortality in hospitalized patients.
21
In our study, the LDH levels in both groups were high on admission, but the LDH level was found to be lower in the patients in the bromhexine arm as compared to the standard group at the end of the therapy, however it was not statistically significant (P =0.056). The low levels of LDH in the bromhexine group are indicative of the less cell damage and therefore, better outcomes during COVID-19 pneumonia.
Conclusion
This study revealed the efficacy of early oral administration of bromhexine hydrochloride in the reduction of mortality of patients with COVID-19 disease. Patients treated with bromhexine may experience a milder course of the disease, and thus, the need for ICU transfer, intubation, and mechanical ventilation can be obviated. Along the same line, decreasing CRP after initiation of bromhexine is in favor of the efficacy of this medication in controlling inflammation and mitigating the disease. Bromhexine is a very inexpensive, affordable, safe, and over-the-counter medication in most countries that can easily be administered in remote and underserved areas. Despite the relatively small sample size and a single-center experience, our results are very encouraging, highlighting a potential breakthrough point in the management of patients with COVID-19 disease if confirmed after verification in large-scale clinical trials. Considering the current state of the pandemic, it may be a game-changer and can have a huge positive impact on resolving public health issues as well as on the world economy.
Acknowledgments
The authors would like to thank Dr. Sepideh Zununi Vahed for submitting the trial to IRCT. Dr. Majid Sadigh and Dr. Ramin Ahmadi for reviewing, Dr. Audrey Tolouian for her contribution in editing the final version of the manuscript.
Funding sources
This study was supported by Tabriz University of Medical Sciences, Tabriz, Iran.
Ethical statement
The study was approved by the Ethics Committee of the Tabriz University of Medical Sciences, Tabriz, Iran (IR.TBZMED.REC.1399.013).
Competing interests
The authors declare no conflict of interest in publishing this paper. This study not supported by any grant money from a pharmaceutical company or for-profit organization.
Authors’ contribution
KA, MRA and SS designed the study. KA, RT, MRA, AT, ST and SS contributed to the protocol development. KA, MRA, AT, MV, ST, TV, HV and PS collected the data. Data analysis and interpretation were conducted by KA, MRA and SS. The initial manuscript was drafted by KA, MRA, RT, SS and KRC. All authors critically revised the manuscript for important intellectual content and approved it for submission.
Research Highlights
What is the current knowledge?
simple
-
√ Starting from the emergence of the COVID-19 disease in
January 2020 an effective treatment was sought diligently and
avidly all over the world to treat and decrease morbidity and
mortality of this disease with no clear advancement and no
remarkably effective drug detected at hand so far.
What is new here?
simple
-
√ This study intended to verify for the first time the efficacy
of the bromhexine hydrochloride as a TMPRSS2 inhibitor in
various aspects of COVID-19 disease. The trial revealed that
the drug, if started relatively early in the course of the disease,
is effective in providing clinical improvement of the patients
with obviating ICU admissions, intubation and mechanical
ventilations, and also in reduction of the mortalities of the
disease.
References
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497-506. doi: 10.1016/S0140-6736(20)30183-5 [Crossref] [ Google Scholar]
- Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest 2020; 130:2202-5. doi: 10.1172/jci137647 [Crossref] [ Google Scholar]
- Grein J, Myers RP, Brainard D. Compassionate Use of Remdesivir in Covid-19 Reply. N Engl J Med 2020:382. doi: 10.1056/NEJMc2015312 [Crossref]
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC. Remdesivir for the Treatment of Covid-19 - Preliminary Report. N Engl J Med 2020. doi: 10.1056/NEJMoa2007764 [Crossref]
- Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395:1569-78. doi: 10.1016/S0140-6736(20)31022-9 [Crossref] [ Google Scholar]
- Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R, Nunneley JW. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res 2015; 116:76-84. doi: 10.1016/j.antiviral.2015.01.011 [Crossref] [ Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181:271-80 e8. doi: 10.1016/j.cell.2020.02.052 [Crossref] [ Google Scholar]
- Shen LW, Mao HJ, Wu YL, Tanaka Y, Zhang W. TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections. Biochimie 2017; 142:1-10. doi: 10.1016/j.biochi.2017.07.016 [Crossref] [ Google Scholar]
- Depfenhart M, de Villiers D, Lemperle G, Meyer M, Di Somma S. Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy?. Intern Emerg Med 2020:1-12. doi: 10.1007/s11739-020-02383-3 [Crossref]
- Lucas JM, Heinlein C, Kim T, Hernandez SA, Malik MS, True LD. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov 2014; 4:1310-25. doi: 10.1158/2159-8290.cd-13-1010 [Crossref] [ Google Scholar]
- Kashani KB. Hypoxia in COVID-19: Sign of Severity or Cause for Poor Outcomes. Mayo Clin Proc 2020; 95:1094-6. doi: 10.1016/j.mayocp.2020.04.021 [Crossref] [ Google Scholar]
- Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA 2020. doi: 10.1001/jama.2020.6825 [Crossref]
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020. doi: 10.1056/NEJMoa2015432 [Crossref]
- Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med 2020; 382:2012-22. doi: 10.1056/NEJMoa2004500 [Crossref] [ Google Scholar]
- Ñamendys-Silva SA. Respiratory support for patients with COVID-19 infection. Lancet Respir Med 2020; 8:e18. doi: 10.1016/S2213-2600(20)30110-7 [Crossref] [ Google Scholar]
- Meng L, Qiu H, Wan L, Ai Y, Xue Z, Guo Q. Intubation and Ventilation amid the COVID-19 Outbreak: Wuhan's Experience. Anesthesiology 2020; 132:1317-32. doi: 10.1097/ALN.0000000000003296 [Crossref] [ Google Scholar]
- Berger D, Bölke E, Seidelmann M, Beger HG. Time-scale of interleukin-6, myeloid related proteins (MRP), C reactive protein (CRP), and endotoxin plasma levels during the postoperative acute phase reaction. Shock 1997; 7:422-6. doi: 10.1097/00024382-199706000-00006 [Crossref] [ Google Scholar]
- Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol 2020; 127:104370. doi: 10.1016/j.jcv.2020.104370 [Crossref] [ Google Scholar]
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020. doi: 10.1001/jamainternmed.2020.0994 [Crossref]
- Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol 2020; 84:106504. doi: 10.1016/j.intimp.2020.106504 [Crossref] [ Google Scholar]
- BM H, G A, J W, Benoit S, Vikse J, Plebani M. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am J Emerg Med 2020. doi: 10.1016/j.ajem.2020.05.073 [Crossref]