Bioimpacts. 12(2):115-126.
doi: 10.34172/bi.2021.39
Original Research
Nanoencapsulation of Hirudo medicinalis proteins in liposomes as a nanocarrier for inhibiting angiogenesis through targeting VEGFA in the Breast cancer cell line (MCF-7)
Amir Shakouri 1, 2  , Houman Kahroba 2, Hamed Hamishekar 1, Jalal Abdolalizadeh 3, 4, *
, Houman Kahroba 2, Hamed Hamishekar 1, Jalal Abdolalizadeh 3, 4, * 
Author information:
1Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Molecular Medicine, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
3Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Paramedical Faculty, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Breast cancer is the most serious cause of women’s death throughout the world. Using nanocarrier vehicles to the exact site of cancer upgrades the therapeutic efficiency of the drugs. Capsulation of active proteins in the vesicular liposomes’ hydrophilic core is essential to develop a therapeutic protein carrier system. We aimed to encapsulate the medicinal leech saliva extract (LSE) and assess the inhibition of angiogenesis of breast cancer cells by targeting vascular endothelial growth factor A (VEGFA).
Methods:
In this research, enhanced formulation of liposomal protein was determined by zeta potential analysis, droplet size, drug release assay, and transmission electron microscopy (TEM). Furthermore, a cytotoxicity assay of liposomal LSE was performed to determine the cytotoxic activity of components. For assessing the expression of VEGFA, P53, and hypoxia-inducible factor subunit alpha (HIF1a) genes, Real-Time PCR was applied.
Results:
Nano liposome was chosen as an enhanced formulation due to its much smaller size (46.23 nm). Liposomal LSE had more practical actions on the MCF-7 cells. As noticed by DAPI staining, apoptosis was extensively greater in treated MCF-7 cells. Wound healing assay demonstrated that MCF-7 cells could not sustain growth at the presence of liposomal LSE and expression of the VEGFA gene was declined in treated cells. Downregulation of VEGFA was evaluated with western blotting technique.
Conclusion:
It can be concluded that our investigation of the tests confirmed the fact that nano liposomal LSE is a novel promising formulation for anticancer drugs and can significantly improve the penetration of protein drugs to cancer cells.
Keywords: Angiogenesis, Breast cancer, Leech saliva extract, Liposome, Nano drug, VEGFA
Copyright and License Information
© 2022 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The advanced delivery systems are highly demanded since they are appropriate for delivering different active pharmaceutical ingredients (APIs), particularly systems with high efficiency, low risk, low toxicity, and cost.
1,2
Over the last few decades, the targeted intracellular delivery of drugs attracted remarkable attention for improving biological activity. There are different biomaterials, and it has been shown that their usage is a milestone in treating diseases with high mortality.
3,4
Proteins play a crucial role in maintaining all cellular functions.
5
Therefore, malfunctioning or inadequate protein expression in the cell is the origin of most genetic and many acquired diseases. Replacing dysfunctional macromolecules via in vitro or in vivo delivery of proteins can be the most secure and unambiguous method for treating diseases, especially cancer.
6
Therapeutic materials with a protein basis play a crucial role in the medical field for treating different disorders like cancer, diabetes, and inflammatory diseases.
7
Various APIs can be utilized well via dynamic light scattering (DLS), Nano-size drug delivery systems designed to improve the APIs delivery with poor biodistribution and pharmacokinetics.
8
For example, most of the chemotherapeutic medications include the characteristics of low pharmacokinetic profiles along with non-specific distribution in the body organs and tissues, which result in extreme systemic toxicity and side effects.
9
Therefore, better treatment of the APIs is demonstrated by nano-sized structure-based pharmaceutical formulations (e.g., liposomes,
10
polymeric nanoparticles,
11
electro sprayed particles,
12
and nano suspension
13
). Furthermore, in cancer treatment, effective penetration of anti-cancer agents encapsulated within a nanocarrier is the chief challenge owing to the solid tumors complexity.
14,15
Liposomes as the most frequently assessed nanostructures are utilized in the developed drug delivery initially found by Alee Bangham, in 1963.
16
Liposomes are artificially spherical vesicles originated from a phospholipid naturally. They involve one or more lipid bilayers with discrete aqueous space. Liposomal carriers are widely used for the delivery of therapeutic proteins, antibodies, and enzymes.
17,18
The lipid bilayers of liposomes inhibit access of extracellular and endosomal proteases in order to maintain protein stability.
19
The main advantage of nano liposome as the drug carrier is capability in dissolving other hydrophilic and hydrophobic drugs, protecting drugs against degradation, increasing bioavailability, and drug solubilizing with long term stability. Adequate interfacial space is provided by small size droplets for drug absorbing and penetrating the skin. Nano liposome becomes attractive by these features in different types of drug delivery systems including ocular, topical, intranasal, oral, and intravenous delivery systems. Recently, by nanotechnology, it is possible to utilize various definite compounds from medicinal leeches in manufacturing cosmetic and pharmaceutical products.
20-22
Based on the use of medicinal leeches in modern medicine, it is indicated that these substances are advantageous in the treatment of different skin disorders.
The medical applications of leeches are dated back to the beginning of evolution. Conventionally, in several countries with medical leech, it had been utilized for numerous human body disorders. Furthermore, several reports have revealed the uses of leech in skin sicknesses, nervous system irregularities such as brain congestion, reproductive, and urinary system difficulties. Dental problems, ocular inflammation, and hemorrhoids are also treated by leech therapy.
23,24
Medical leech salivary glands include over 100 bioactive proteins and enzymes with antiedematous, analgesic, and bacteriostatic effects; they have resolving activity, cause microcirculation disorders elimination, restoring the hurt vascular permeability of organs and tissues, hypoxia elimination, blood pressure reduction, improved immune system activity, detoxified organism, relieving it from the hostile complications like strokes and infarct, and the organism’s bioenergetic status improvement.
25
Saliva secretions of the leech contain antimetastatic agents. A protein labeled antistatic is involved in the leech saliva which prevents lung cancer colonization. There are anti-proteolytic, platelet aggregation inhibitors, and anticoagulant enzymes. In addition, anti-tumor activity is involved in other elements like hyaluronidase. It is considered that by destroying the hyaluronic acid-CD44 interaction, hyaluronidase antitumor activity may occur to some extent through pro-tumorigenic immune cell inhibition into the tumor stroma.
26
Concerning metastasis and cancer therapy, numerous scientists defined the practical applications of leech saliva extract (LSE) as an anti-metastatic and Anti-Cancer agent.
27
Other explorations defined successful synthetic hirudin preparing as an effective metastasis inhibitor of various cancer cells like pulmonary carcinoma, osteosarcoma, leukemia, and breast carcinoma. Recent studies exploring the compounds and therapeutic potential of LSE have recognized many peptides and proteins with multiple therapeutic properties containing anti-thrombotic, antimicrobial, and anti-metastatic. In vitro anti-cancer effects were revealed in breast, prostate, and lung cancer cell lines. In vivo anticancer activity of the LSE was shown in multiple breasts and prostate cancer.
28
Hirudin extracted from Hirudo medicinalis demonstrates practical anti-metastatic activity in various malignancies like pulmonary carcinoma, osteosarcoma, breast carcinoma, and leukemia.
29
Activating tumor-associated macrophages (TAMs) is the impact of hyaluronic acid (HA) accumulation throughout tumorigenesis.
30
For instance, the stromal compartments shaped in HA-high tumors are especially supplemented with TAMs, expedited by HA-CD44 interaction. HA is degraded by hyaluronidase and HA-CD44 contact is disturbed, preventing leucocyte and tumor growth in multiple HA-high cancer variations.
31
That is clear that a part of the tumoral hyaluronidase activity could occur through inhibition of HA-CD44 interaction.
32,33
HA fragments are generated by highly aggressive bladder cancers within the 30–50 saccharide size range, incredibly mutagenic for endothelial cells, and thus terribly antigenic.
34
In numerous studies, different bioactive molecules of leeches were found in their secretions. Over 100 molecules and their action modes were recognized, however, numerous more awaiting explorations exist. These molecules contain anti-tumor activity. It is believed that further indications may be emerged within more investigations owing to newly clarified impact mechanisms. In this study, a nano liposome was designed with a lipid bilayer around an aqueous phase. This system is the most widely assessed nanocarrier for transporting anticancer drugs. Embedding biocompatible substances reactive to definite stimuli in the bilayer can obtain a controlled release of drugs from liposomes. Furthermore, it is possible to use the aqueous core of liposome in loading therapeutic drugs; Thus, LSE was loaded into liposomes. Novelty of this study is encapsulating medical leech saliva proteins as a carrier for MCF7 breast cancer cells, which can affect VEGFA gene expression. Moreover, LSE has anti- cancer activity with a decrease in the expression of VEGFA via HIF1a activation pathway, for transporting these proteins inside cell liposomes. The drug release from liposomes is assessed, and the impacts of the combined liposomal -LSE treatment for cancer cells are evaluated. Hence, this paper provides a vision, and takes widely into account the action modes.
Materials and Methods
Materials
The main substances in our research were purchased as follows: Ethanol 96%; chloroform, glycerin, and cholesterol from Merck (Germany); Medical Leeches from Hakim Razi Herbal Medical Clinic (HRHMC) and soybean lecithin from Lipoid. Phosphate buffered saline (PBS, 1X sterile solution) was acquired from AMERCO; LDH kit and 4′,6-diamidino-2-phenylindole (DAPI) from Sigma-Aldrich, and sterile double-distilled water utilized in all the tests.
Protein extraction of leech saliva
Over the investigation, the leeches were kept in starvation. The saliva extraction procedure was initiated by rinsing the leeches with distilled water, followed by a slow addition of 8 % (v/v) ethanol solution. Since the leeches are sensitive to ethanol, vomiting in response to this sensitivity causes saliva secretion followed by 15 minutes. For obtaining pure saliva and eliminating other impurities, multi-stages of centrifugation and filtering were accomplished.
35
Protein concentration was calculated by spectrophotometer utilizing absorption at 280 nm.
36
Preparation of liposome
Briefly, cholesterol and lecithin were dissolved at a molar ratio of 70:30 (21 mol:9 mol) in 5 mL of chloroform and thoroughly mixed. Adding cholesterol structural rigidity was provided for the phospholipid bilayers.
37
Via rotary evaporation at 35◦C, the chloroform was eliminated from the mixture to create thin lipid layers over the flask wall. The flask with the lipid films was freeze-dried for 12 hours for complete removal of the residual chloroform. Prior to addition to the flask comprising the lipid films, 6 mg of the protein being encapsulated was dissolved in 6 mL of 10 mM PBS (pH 7.4) for 5 minutes at 37°C. Then, the combination was completely hydrated in the rotary evaporator at ambient temperature for 30 minutes and sonicated 3 times for 10 seconds each for entirely detaching the lipid layers from the flask wall. The protein–liposome mixture was exposed to 10 freeze-thaw cycles in liquid nitrogen and a 45°C water bath. Ultimately, to create liposomes with a predefined diameter, extruding the mixture was performed 15 times via a mini-extruder armed with a 100 nm, 200 nm, or 400 nm polycarbonate membrane filter. In this stage, MLVs (multi-lamellar vesicles) were converted to LUVs (big unilamellar vesicles) with relatively narrow, monodisperse diameter distributions near the selected pore size.
38
Over extruding, the temperature was kept over the lecithin’s phase transition temperature cautiously to avoid pollution.
39
Each test group had continuous lipid film extrusion and formation processes. Created liposomes were kept at 4◦C (Fig. 1).
40
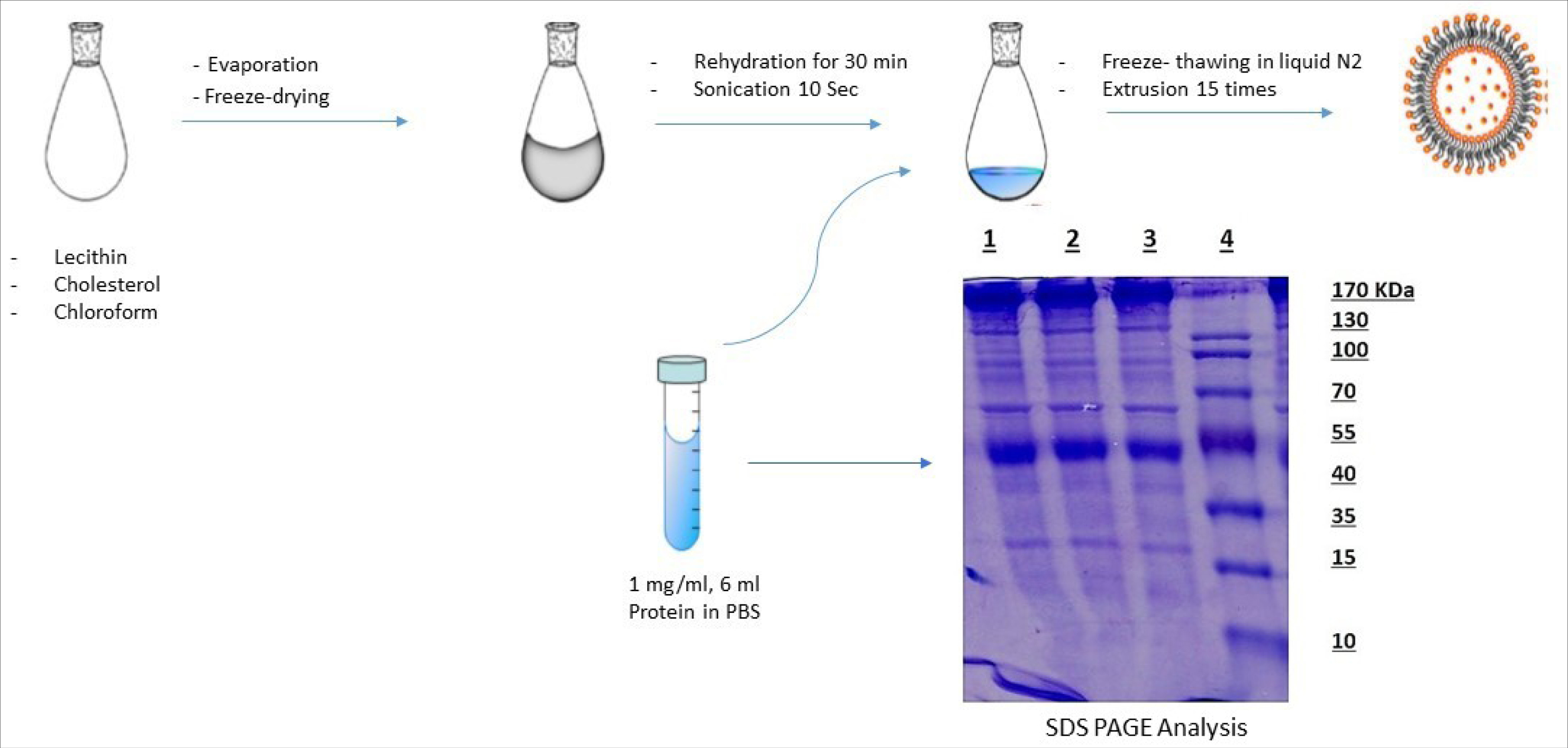
Figure 1.
Schematic representation of the liposome preparation process. SDS-PAGE analysis of leech saliva extract (Coomassie blue staining). Lanes 1-3: medical leech saliva extract. Lane 4: Molecular Weight Size marker.
.
Schematic representation of the liposome preparation process. SDS-PAGE analysis of leech saliva extract (Coomassie blue staining). Lanes 1-3: medical leech saliva extract. Lane 4: Molecular Weight Size marker.
Nano liposome droplet size analysis
The size distribution, surface charge, and mean particle size measurements of the zeta potential of polyampholyte-modified and unmodified liposomes were analyzed through DLS analysis utilizing a Zeta sizer 3000 (Malvern Instruments, Worcestershire, UK) with a 135° scattering angle. The polydispersity index (PDI) and mean particle diameter (Z-average) were determined based on the particle size distribution. The liposomes’ colloidal suspension diluted with PBS and the particle size were analyzed at a temperature of 25°C and a scattering angle of 135°C. The liposomes were dispersed in PBS. Data were attained as the mean of over three measurements on various specimens.
41,42
Liposome pH determination
The pH (25°C) values of liposomes were measured via a pH meter (HACH digital pH meter, Loveland, NY, USA).
43
Transmission electron microscopy
Transmission electron microscopy (TEM) was utilized for observing the morphology of droplets (Zeiss, Jena, Germany) at 100 kV. Before the analysis, the optimized liposomes were diluted ten times with distilled water. A drop of the diluted specimen was placed over a 200-mesh film grid for drying at room temperature. Using a 2% phosphotungstic acid solution, the specimen was stained. It was then left to dry for 2 minutes prior to observing with the electron microscope.
44
Scanning electron microscopy
Utilizing a scanning electron microscope (SEM; Hitachi S-510, Hitachi-High Technologies Europe GmbH, Krefeld, Germany), the tests were performed. Conductive carbon tabs were used to pipet the liposome containing LSE onto SEM pin stubs, and a laminar airflow hood to dry it. After fixing the samples with 2% glutaraldehyde for one hour, they were immersed in distilled water three times and stained with 2% osmium tetroxide for the following hour. Then, they were re-immersed three times in distilled water, dehydrated with increasing concentrations of ethanol, and then thoroughly dehydrated through supercritical drying utilizing a Bal-Tec CPD 030 Critical Point Dryer (Bal-Tec AG, Balzers, Liechtenstein). The specimens were ultimately sputtered thrice with gold at 10 mA and 13.3 Pa Argon by the use of an Edwards S150 sputter coater (Edwards Vacuum, Crawley, UK), and evaluated under 5.3 × 10-4 Pa vacuum by the use of a scanning electron microscope with an emission current of 30 A and a 5 kV accelerating voltage. A DISS 5 digital image gaining system (Point Electronic GmbH, Halle, Germany) was used to record the micrographs digitally.
45
In vitro drug release assay
Placing 5 mL of liposomal LSE suspensions into dialysis bags (Sigma) with a 10 KDa molecular weight cut off, the solution was dialyzed in 50 mL of PBS (pH 7.4, 37°C) in a closed container. Dialysis solution aliquots were taken at various time intervals and the same PBS volume was replaced. Ultimately, the samples drug concentration was calculated utilizing UV–visible spectroscopy. The following equation calculated the cumulative drug release percentage:
in which M total shows the overall quantity of trapped LSE in the nano liposome, Vb represents the release media’s volume (50 mL), Vr shows the sample volume eliminated at various time intervals (Vr = 1 mL), and Cn is the LSE concentration in the eliminated sample each time.
Cancer cell culture and cytotoxicity assay of liposomal LSE
The human breast adenocarcinoma cell line (MCF-7) was attained from American Type Cell Collection (ATCC). The cells were cultured at a primary inoculum cell concentration of 104 cells/cm2 in 15 mL Roswell Park Memorial Institute medium (RPMI) with 10% FBS (v/v) strep in Corning® 75 cm2 cell culture flask. Incubation was performed for the cultured cells at 37°C in a 5 % CO2 humidified atmosphere.
46,47
The result of the mixture of liposomal LSE and Etoposide treatment on cell ability of MCF-7 cells was quantified utilizing an LDH assay. One hundred microliters of diluted liposomal LSE at 50 µg/mL was inserted into the treated plate. Then 10 µL of diluted Etoposide was added to control positive plate. After 24 and 48 hours of incubation, the LDH solution was introduced into wells in the 96-well plate. Ultimately, a Quant ELISA Reader (Bio-Tek Instruments, Maharashtra, India) was used to read the optic density of the plate at 630 nm and 570 nm as the reference wavelength.
48
The test was repeated at least for three times with triplicate specimens for each test. The proliferation percentage was determined as:
Cell viability percentage = (ODsample/ODcontrol) × 100%
DNA fragmentation assay
DAPI could be a DNA -binding fluorescent coloring used to envision DNA fragmentation. First of all, using 4% paraformaldehyde cells were fixed within the microfluidic channels for 10 minutes at room temperature, after washing process with PBS, 0.3% Triton X-100 permeabilized the cells for 10 minutes, they were stained by DAPI for 15 minutes. Apoptosis was noticeable by morphology, chromatin concentration, DNA strand damage, apoptotic bodies, or nuclear DNA fragmentation.
49
Cell migration assay
A scratch test was utilized to assess cell migration capability. First, MCF-7 and HUVEC cells were planted into 1.5 ml medium in each well of a 24-well plate. Then, the cells were grown over a confluent layer (48 hours), and a scratch was then created in each well utilizing a pipette tip. Afterward, rinsing the cells was slightly performed with PBS, then liposomal LSE was inserted into the respective wells. An image was captured at time point 0. Then, the cells were incubated in 5% CO2 at 37°C, and the images were attained after 24 hours. To reduce the potential effect of proliferation over the scratch closing, the 24-hour time point was selected.
50
Real-time fluorescent quantitative polymerase chain reaction
TRIzol was used to extract the overall RNA of the two types of cells based on the protocol of the manufacturer and was reverse transcriptized based on the instruction of the manufacturer. The reaction system of real-time fluorescent quantitative PCR contained 1 μL sense primer, 10 μL SYBR, 1 μL cDNA, 7 μL dH2O, and 1 μL antisense primer. The specimens were examined in triplicate for the internal control and target gene. Table 1 shows the designed PCR primers. The quantitative test was performed 40 cycles under the circumstances of 95°C for 20 seconds, 60°C for 30 seconds, and 95°C for 3 minutes. By determining the average of each sample group, the relative transcript level of Vascular Endothelial Growth Factor A (VEGFA), P53, hypoxia-inducible factor Subunit Alpha (HIF1a) was examined as ΔCq = target gene Cq − reference gene Cq; ΔΔCq = target gene ΔCq − standard values ΔCq; and the relative copy of target gene = 2−△△Cq, and GAPDH gene was applied as an interior control for analysis of genes.
Table 1.
Primers used for Real time PCR of human breast cancer cells (MCF-7)
|
Gene
|
GeneBank No
|
Primer
|
|
| VEGFA |
NM-001171627 |
Forward |
AGGGCAGAATCATCACGAAGT |
| Reverse |
AGGGTCTCGATTGGATGGCA |
| P53 |
NM_001126117 |
Forward |
TCAAGACTGGCGCTAAAAGT |
| Reverse |
TTTCAGGAAGTAGTTTCCATAGGT |
| HIF1a |
NM-181054 |
Forward |
ATCCATGTGACCATGAGGAAATG |
| Reverse |
TCGGCTAGTTAGGGTACACTTC |
| GAPDH |
NM-001256799 |
Forward |
AATGAATGGGCAGCCGTTAG |
| Reverse |
TTGGAACATGTAAACCATGTAGTTG |
Western blot analysis
RIPA buffer was used for lysing the cells (1% Triton X-100, 150 mM NaCl, 10 mM Tris–HCl, pH 7.4, 1 mM EDTA, 2 mM NaF). Protein concentrations were determined using a Bradford assay. Protein samples (50 μg) were analyzed by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore Corporation, Billerica, MA, USA). Membranes were washed in Tris-buffered saline containing Tween 20 (TBST) and 5% skim milk was applied to block PVDF for 30 minutes at room temperature, and incubated with the primary antibody at 4°C overnight. The membranes were incubated in secondary antibodies for 1 hour at room temperature. After washing, proteins were detected using enhanced chemiluminescence (Millipore Corporation). Finally, gel was analyzed with Image J software to evaluate protein bands and their expression.
51
Statistical analyses
One-way analysis of variance was applied via Prism 9 software. P< 0.05 was selected as a statistically significant variance. Triplicate tests were done in all experiments and all samples were analyzed in duplicate. The data were expressed as mean ± SD.
Results
Protein extraction
The existence of proteins in leech extracted saliva was studied through SDS-PAGE analysis. Over 25 types of proteins and peptides with a molecular weight within the range of 10-170 kDa were stained with Coomassie blue and silver nitrate (Fig. 1).
Analysis of liposome size and stability
DLS determines the particles’ hydrodynamic radius in a solution. Based on the findings, the various liposomes have the same sizes of almost 100 nm, which is an outstanding size value for a nanocarrier for intravenous administration. The sizes do not essentially rely on the lipid character or the presence or absence of the proteins.
52
The outcomes of DLS indicated that Z- Average was 49.26, and PDI was 0.145 nm (Fig. 2). The homogeneity grade in a particle solution size is determined by the PDI. A specimen is regarded homogeneous by PDI lower than 0.3. The attained PDI values are ≤0.3, representing the prepared liposomes’ homogeneity based on their sizes.
53
The particles with zeta potentials of <−30 mV are generally stable, hence, they overcome the natural tendency to aggregate. By low zeta potential, the repulsion may be exceeded by the attractive forces, and liposome droplets probably break and flocculate.
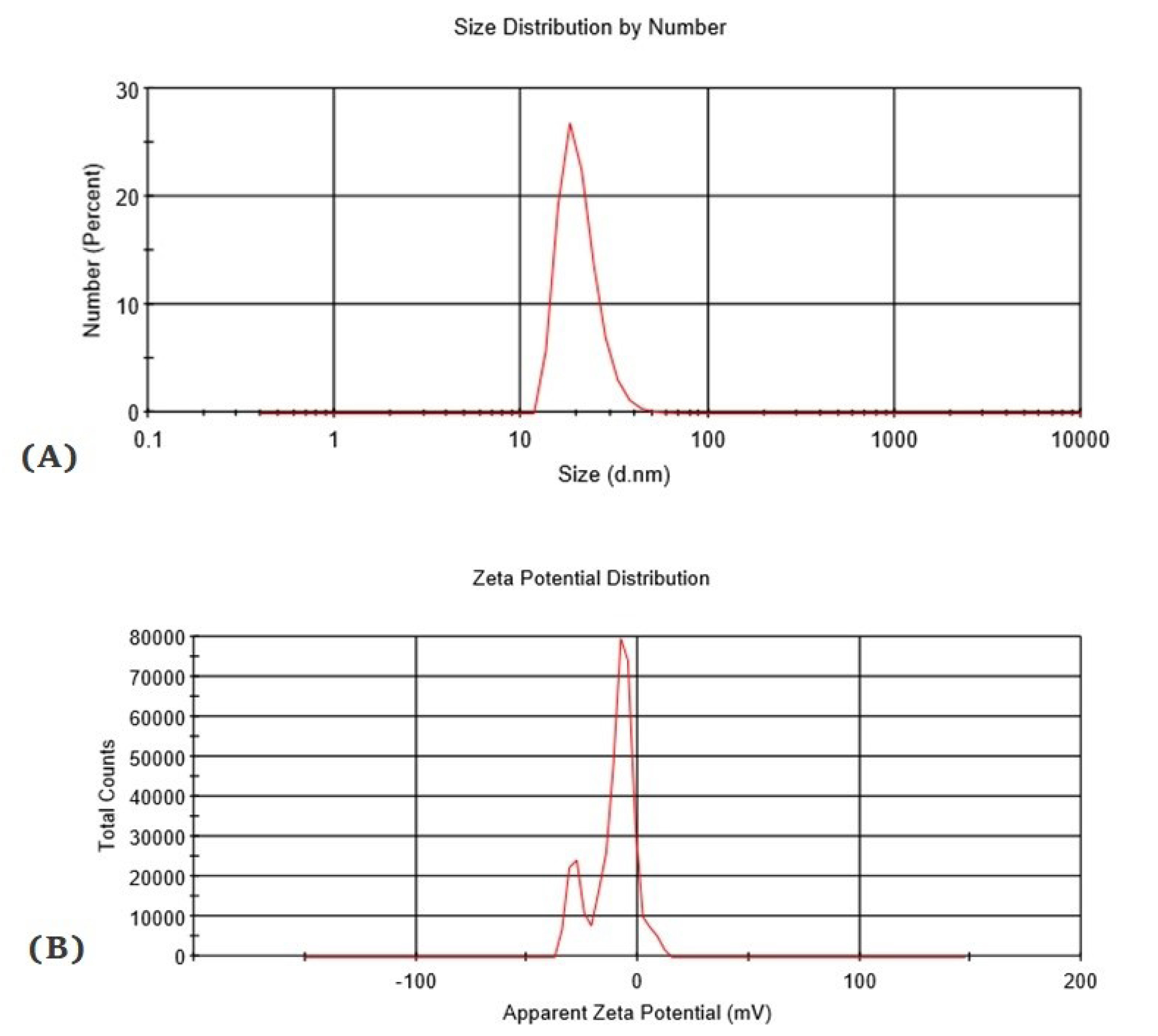
Figure 2.
DLS analysis of liposomes. Particle size distribution of liposomal protein were analyzed by DLS [size distributions by intensity] at 0 day. The graph (A) demonstrated that Z-Average (d. nm) is about 49.26, PDI is 0.145 and zeta potential represented in graph (B) result quality is good.
.
DLS analysis of liposomes. Particle size distribution of liposomal protein were analyzed by DLS [size distributions by intensity] at 0 day. The graph (A) demonstrated that Z-Average (d. nm) is about 49.26, PDI is 0.145 and zeta potential represented in graph (B) result quality is good.
Stability studies
To assess the impact of gravity on nano liposome stability, a centrifugation test was performed. Followed by centrifugation for 30 minutes at 3500 g, no sign of phase separation was found in all nano liposome specimens indicating that nano liposome had excellent stability over the gravitational separation force. The storage temperature is a key factor in the quality of protein since they normally have high sensitivity to heat and are unstable and rapidly degradable in high temperatures. Hence, it is essential to store the nano liposome at low temperatures (around 4°C). The optimized nano liposome is stored at 4°C. The rate of hydrolysis of nano liposome is considerably affected by pH value. Mainly, hydrolysis can be prevented by the pH value ranges within 6.5-8.5. Fig. 3 displays the pH variations of improved nano liposome stored at 4°C, over a 30-day storage period. The primary pH of the optmized nano liposome stored at 4°C on day 0 was 6.5. On day 30, only a slight alteration in pH was found at 4°C. At 4°C, it is possible to minimize both lipid hydrolysis and oxidation. Consequently, nano liposome droplets can be protected from oxidation and hydrolysis. Hence, the prepared nano liposome was physically stable. No considerable alteration was found in the optimized particle size of nano liposome at temperatures up to 40°C, being under 200 nm (Fig. 3B).

Figure 3.
Stability studies of liposomes. (A) pH changes of optimized nano liposome at 4°C. (B) Droplet size of nano liposome, duration: 30-day period of storage. (C) TEM photograph of stable formulation displays measurement of droplet size less than 50 nm. (D) SEM analysis of nano liposome at 120 nm. Photograph demonstrated that morphology of liposomes is spherical standard. The white spheres are to guide the eyes.
.
Stability studies of liposomes. (A) pH changes of optimized nano liposome at 4°C. (B) Droplet size of nano liposome, duration: 30-day period of storage. (C) TEM photograph of stable formulation displays measurement of droplet size less than 50 nm. (D) SEM analysis of nano liposome at 120 nm. Photograph demonstrated that morphology of liposomes is spherical standard. The white spheres are to guide the eyes.
Transmission electron microscopy
Based on the TEM analysis, it was observed that formulation droplets were homogeneous and spherical shape. It appeared that droplets in the optimized liposome were dark, and in the bright background (Fig. 3C). Based on these findings, it was confirmed that numerous droplets were in the nano size range (less than 200 nm). In conclusion, droplets of nano liposomes were well-dispersed without droplet aggregation. According to the morphological investigation utilizing TEM, it was found that liposome was spherical with some deviations. It characteristically implied the use of the routine technique for preparing the samples in TEM. Based on the measurements of droplet size, the droplets measured over 200 nm. Nevertheless, there was no uniform size distribution of liposomes. Theoretically, by adding a drop of nano liposome to a TEM copper grid and letting it dry, particles may be rearranged over drying.
Scanning electron microscopy
SEM micrographs were very helpful with the structural establishment of these new liposomes. The micrographs showed that these liposomes are mostly spherical, and they are 50–100 nm in size, as confirmed by the DLS measurements. Given the affinity of lecithin phospholipids, identifying the stained structures under an electron microscope is easier even at a larger magnification.
54
The liposomes scaled in the micrographs resembled in size those in the DLS measurements (Fig. 3D).
In vitro drug release
Fig. 4 represents the profile of the drug release of the LSE. Around 50% of the total LSE loaded on liposome was released in 150 hours in a sustained manner implying the relative stability of the liposomes at 37ºC. Moreover, it was found that the LSE is more slow-releasing, which could be caused by the interactions between LSE proteins and the hydrophobic region of phosphatidylcholine in liposomes. All data were compared with LSE and PBS as a control.
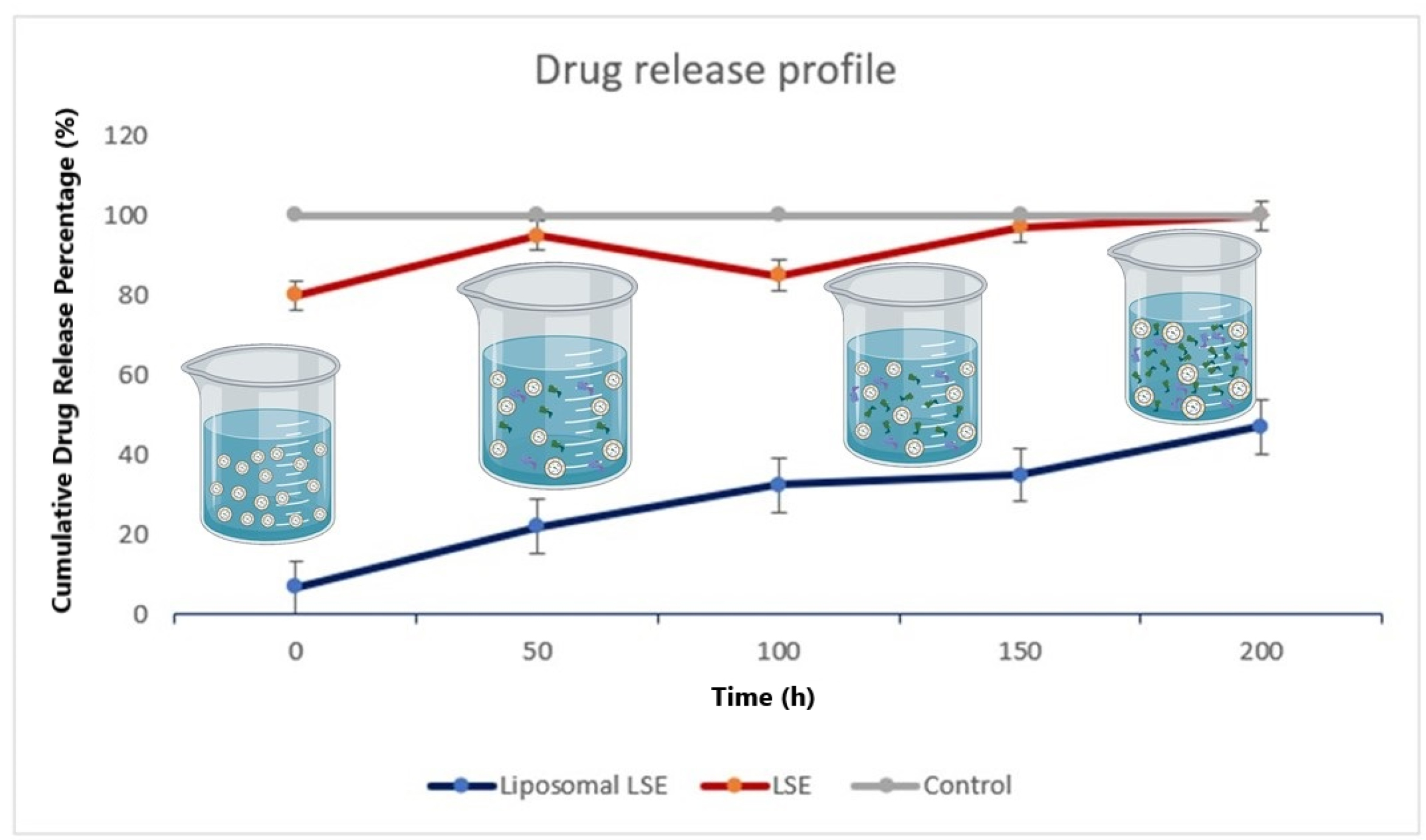
Figure 4.
Drug release assay. In vitro drug release assay for liposomal protein in different periods of time. Line blue indicated that after 50 hours, 40% of drug was released.
.
Drug release assay. In vitro drug release assay for liposomal protein in different periods of time. Line blue indicated that after 50 hours, 40% of drug was released.
Cytotoxicity assay
Results demonstrated that LSE had a significant anti-proliferation activity against MCF-7 cell line. The concentration of the total protein of LSE inhibiting the growth of 50% of the treated cells after 24 and 48 hours of incubation was 80.52 μg/mL. On the other hand, the anti-proliferation effect of nano liposomal LSE was compared to LSE effect, and all of them was compared with Triton X 100 that kills cells. Nano liposome LSE kills 97% of cancer cells, but leech saliva was about 50%. Besides that, Leech saliva and liposomal LSE had about 10% anti-proliferation activity against the HUVEC cell line as a normal cell (Fig. 5).
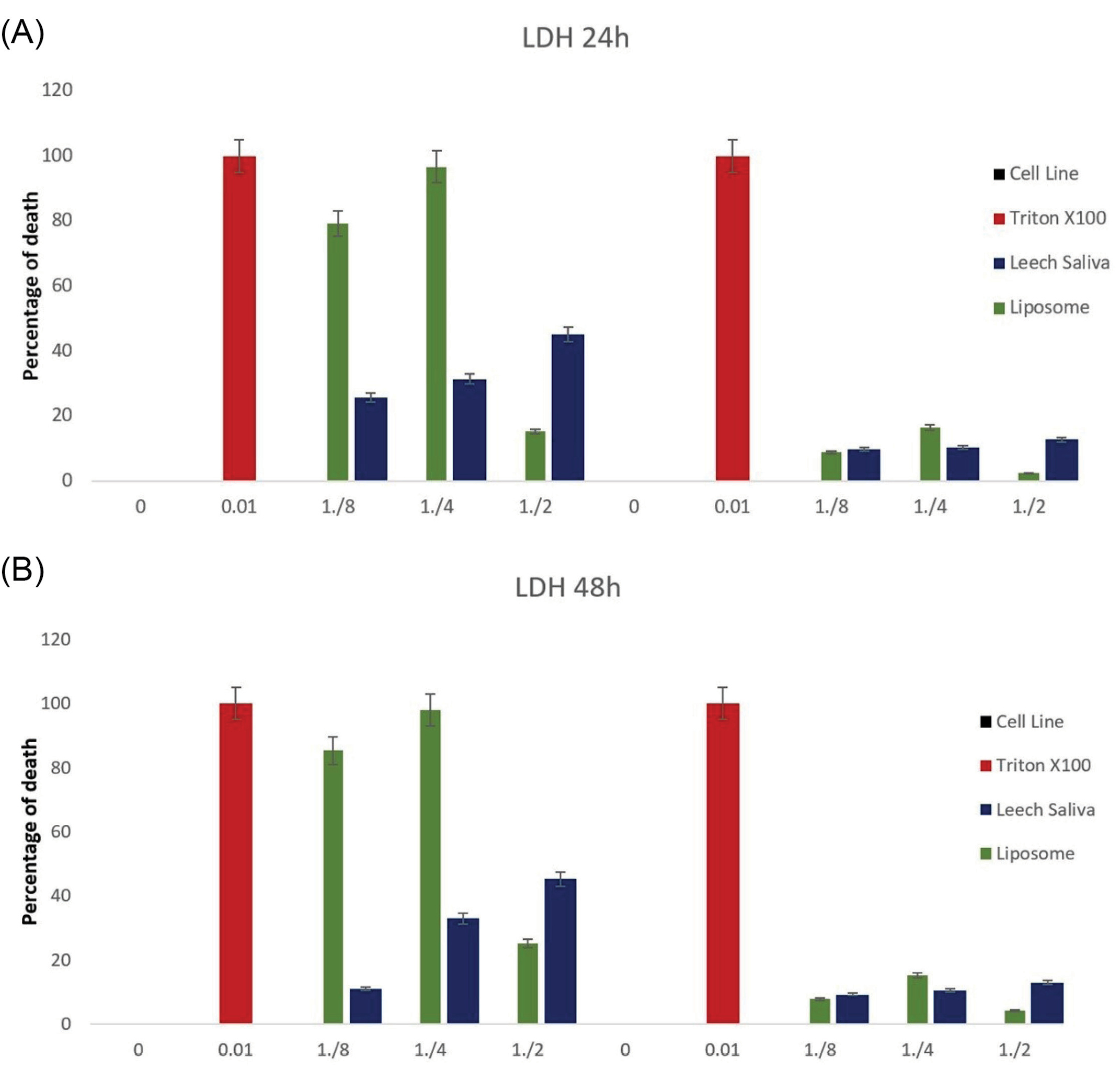
Figure 5.
LDH Test for MCF-7 as breast cancer cells and HUVEC cell line as control group. (A) Treatment after 24 hours. (B) Treatment after 48 hours. In tow times confirmed that 97% of cancer cells were destroyed by nano liposomal LSE and 50% of cancer cells were destroyed only by LSE with no effect on HUVEC cell line.
.
LDH Test for MCF-7 as breast cancer cells and HUVEC cell line as control group. (A) Treatment after 24 hours. (B) Treatment after 48 hours. In tow times confirmed that 97% of cancer cells were destroyed by nano liposomal LSE and 50% of cancer cells were destroyed only by LSE with no effect on HUVEC cell line.
DAPI staining
Over morphological examination, a high cell death incidence was found, particularly with 50 µg/mL and 12.5 µg/mL treated cells. DAPI staining was performed for evaluating the impact of liposomal LSE on the cell nucleus of the treated and control cells. Dosages of 10, 25, and 50% of liposomal LSE for 24 hours post-treatment were used to perform DAPI staining. Based on treating with a 10% dose, it was shown slight nuclear disintegration in comparison with control cells; however, treating with 25% revealed a considerable nuclear death quantity. The dose of 50% indicated a remarkable significant quantity of cell death and nuclear disintegration (Fig. 6).
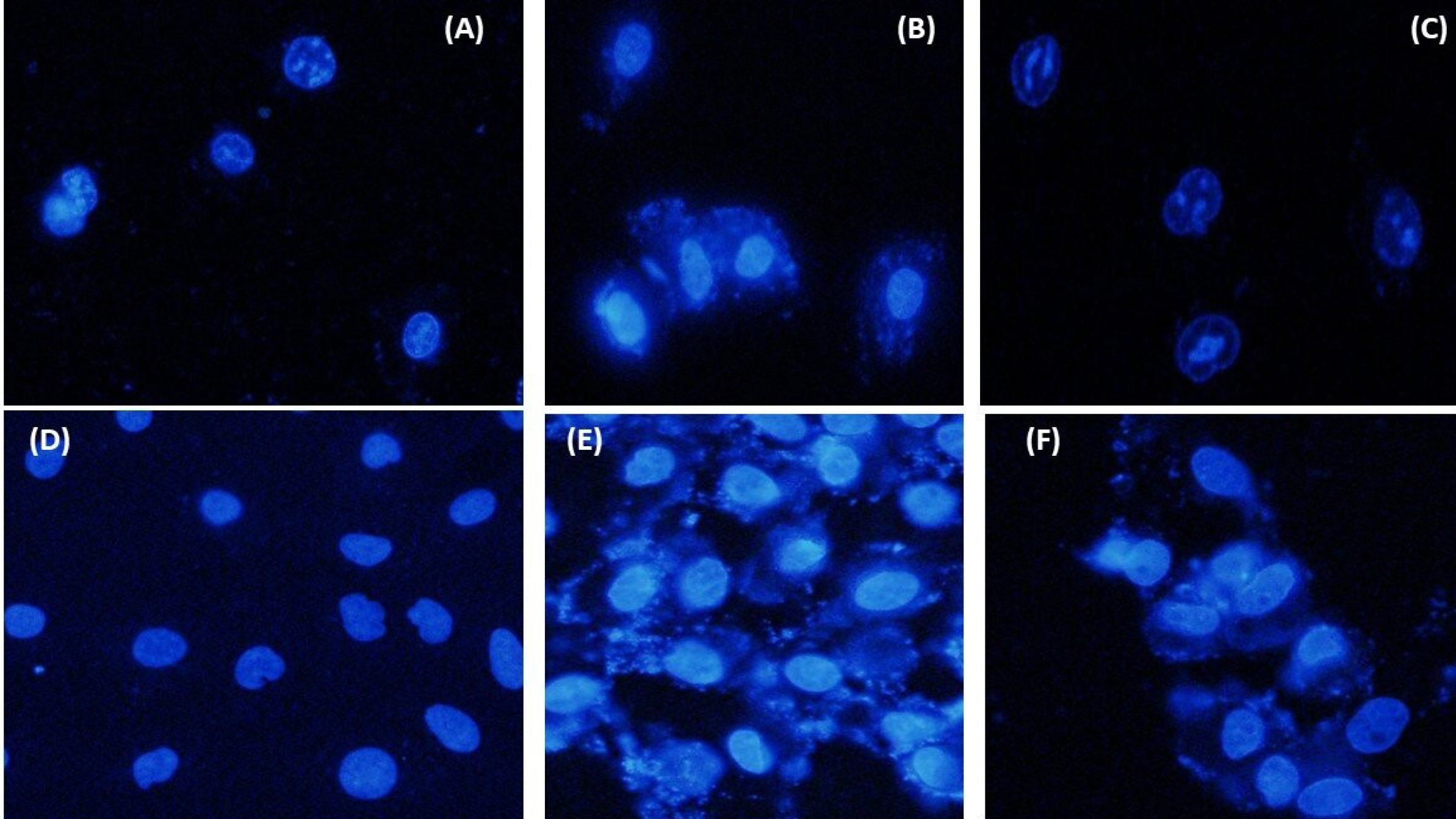
Figure 6.
Fluorescentmicroscopic images of MCF-7 cells stained with 4’,6-diamidino-2-phenylindole (DAPI) (blue color); (1) MCF-7 cells were treated with liposomal LSE, (2) MCF-7 cells without treatment, and (3) positive control treated with Etoposide. (4) HUVEC treated with liposomal LSE, (5) without treatment as a negative control, and (6) positive control treated with Etoposide.
.
Fluorescentmicroscopic images of MCF-7 cells stained with 4’,6-diamidino-2-phenylindole (DAPI) (blue color); (1) MCF-7 cells were treated with liposomal LSE, (2) MCF-7 cells without treatment, and (3) positive control treated with Etoposide. (4) HUVEC treated with liposomal LSE, (5) without treatment as a negative control, and (6) positive control treated with Etoposide.
Cell migration assay
The ability of MCF-7 and HUVEC cells to migrate was examined at three time points (4, 24, and 48 hours); through wound healing assays indicated that the scratches' width was progressively decreased (Fig. 7). Based on multiple comparisons of the 48-hour scratch repair rates, it was found that the rates in the experimental groups were considerably lower compared to the control group (all P values were <0.05; 1-way ANOVA). The scratch band healed almost entirely without treatment. Conversely, the migration of MCF-7 treated with liposomal LSE was predominantly inhibited. These results showed that liposomal LSE could suppress the migration of MCF-7 cells (Fig. 8A).
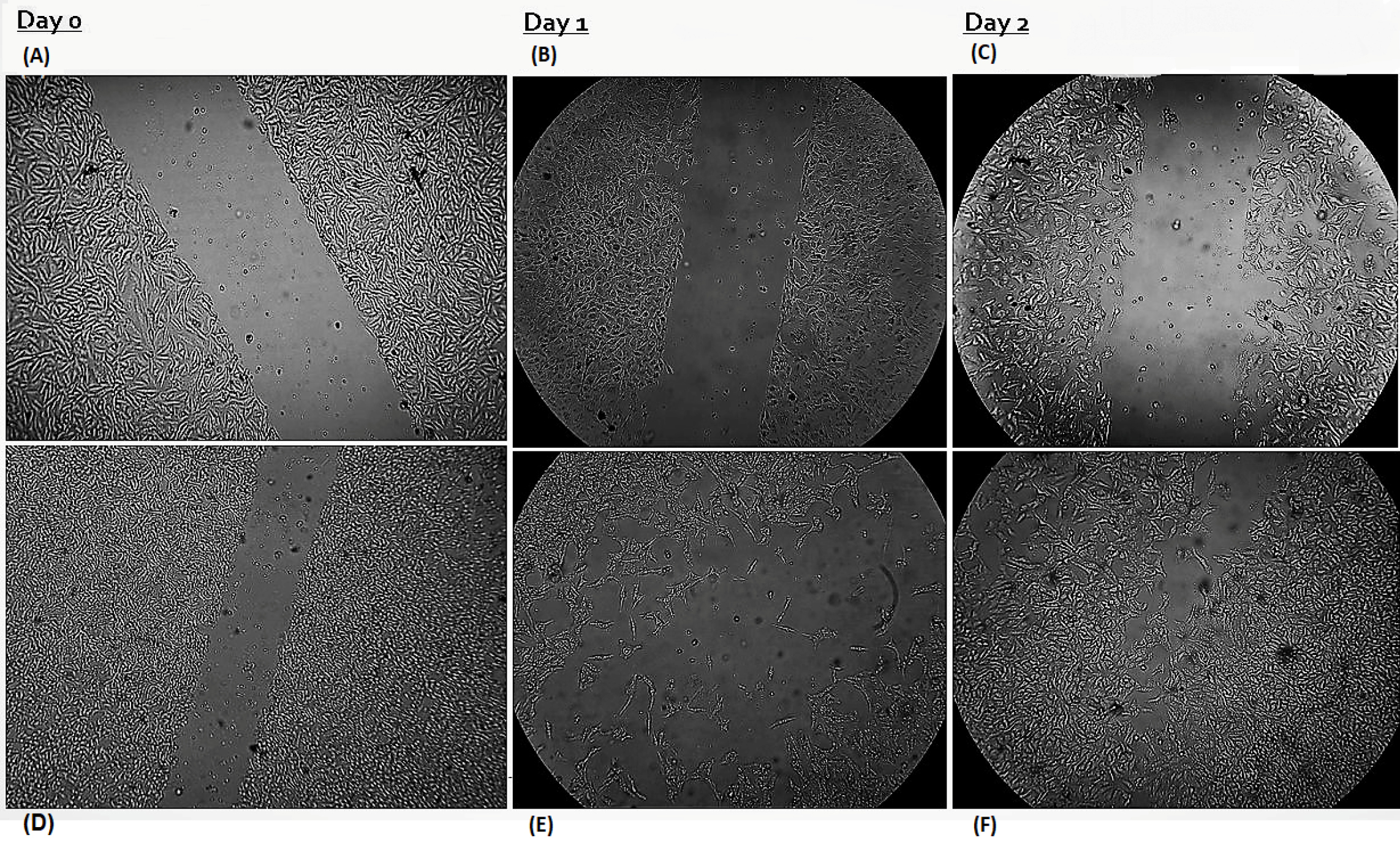
Figure 7.
The inhibitory effect of liposomal LSE on the migration of MCF-7 and HUVEC cells. MCF-7 cells were treated with or without liposomal LSE for 24 or 48 hours. Wounds were created in the cultured cells and images were taken with a microscope. (A) MCF-7 cells were treated with liposomal LSE. (B) and (C) shows no migration activity in 48 hours. (D) HUVEC cells as a negative control. (E) and (F) The untreated control cells grew and migrated more rapidly in 24 and 48 hours
.
The inhibitory effect of liposomal LSE on the migration of MCF-7 and HUVEC cells. MCF-7 cells were treated with or without liposomal LSE for 24 or 48 hours. Wounds were created in the cultured cells and images were taken with a microscope. (A) MCF-7 cells were treated with liposomal LSE. (B) and (C) shows no migration activity in 48 hours. (D) HUVEC cells as a negative control. (E) and (F) The untreated control cells grew and migrated more rapidly in 24 and 48 hours
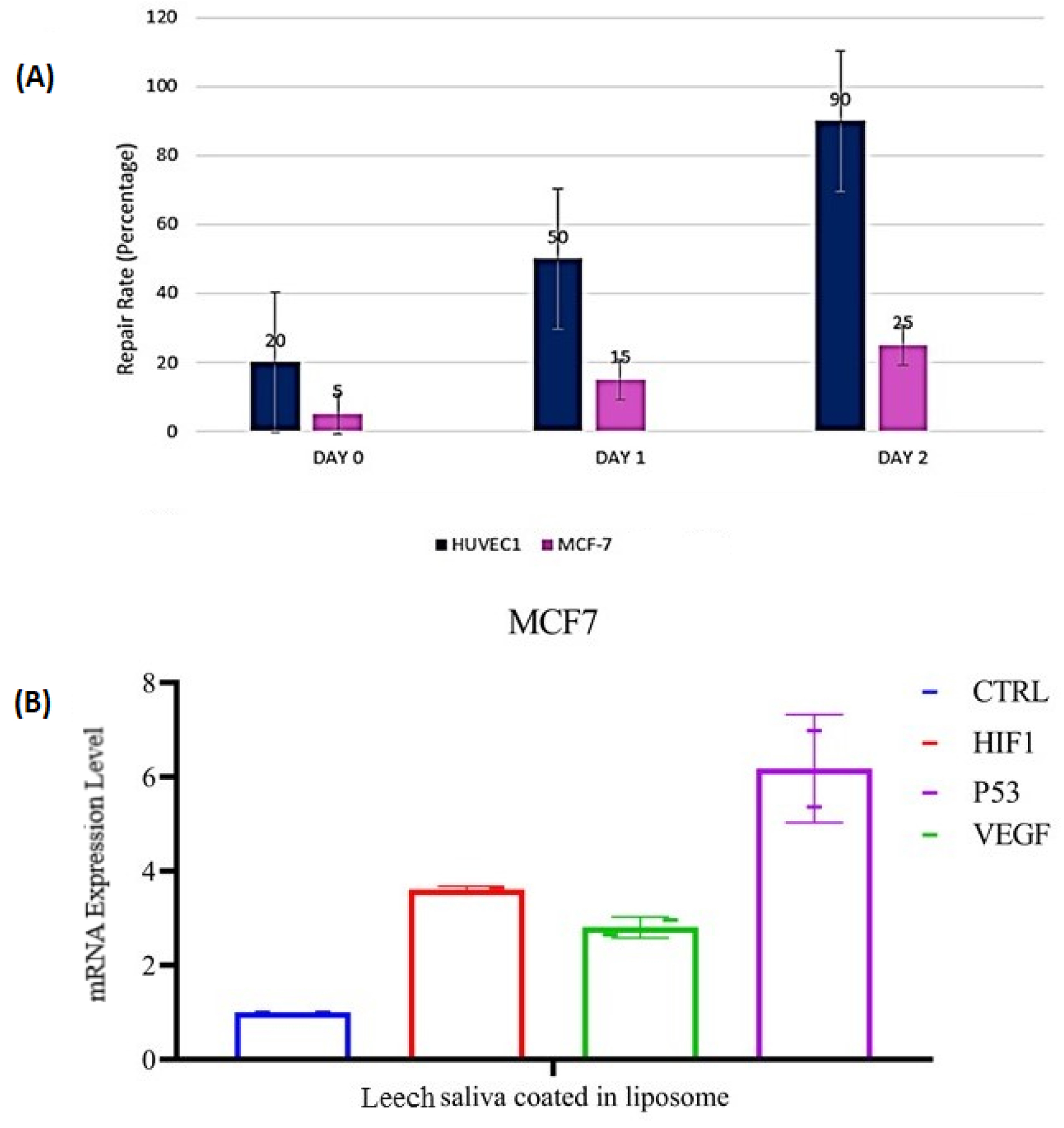
Figure 8.
(A) Wound healing assays. Images of cells taken at 0, 24, and 48 hours after scratching in wound healing assays; quantitative analysis indicated that a liposomal LSE inhibited MCF-7 cell migration. (B) Gene expression patterns of VEGF, HIF1a, and P53 genes in MCF-7 and Huvec cells. GAPDH was used as a housekeeping gene. Effects of liposomal LSE on pro/anti-apoptotic genes: Levels of mRNA of P53 and HIF1a were increased significantly in MCF-7 (P < 0.05) compared with HUVEC cell lin. Level of VEGFA reduced significantly in MCF-7 cell line.
.
(A) Wound healing assays. Images of cells taken at 0, 24, and 48 hours after scratching in wound healing assays; quantitative analysis indicated that a liposomal LSE inhibited MCF-7 cell migration. (B) Gene expression patterns of VEGF, HIF1a, and P53 genes in MCF-7 and Huvec cells. GAPDH was used as a housekeeping gene. Effects of liposomal LSE on pro/anti-apoptotic genes: Levels of mRNA of P53 and HIF1a were increased significantly in MCF-7 (P < 0.05) compared with HUVEC cell lin. Level of VEGFA reduced significantly in MCF-7 cell line.
Detection of VEGFA, Hif1a, P53 expression in the cells via real-time fluorescent quantitative PCR
Utilizing the 2−△△C analysis technique, the data were analyzed. Based on the results, it was indicated a remarkable reduction in the VEGFA mRNA expression level in treated MCF7 cells in comparison to the negative control (*P < 0.05). No statistically significant difference was found in expressing VEGFA between black control MCF-7 cells and negative control cells. Based on the expression of VEGFA in MCF-7 cells, mRNA expression of VEGFA inhibition rate was 53%. Our data indicated that VEGFA expression was decreased in breast cancer cells (MCF7). Fig. 8B shows a comparison between different cells.
Expression of VEGFA in cells
To evaluate the expression of the VEGFA protein, we examined the levels of VEGFA in different cells by Western blot analysis. Our data indicated that VEGFA protein was present at high levels in the MCF-7 (negative control), at moderate levels in the HUVEC cells (negative control), and at low levels in the treated MCF-7 with liposomal LSE. GAPDH was also used as a control protein and graph demonstrated that in treated cells with liposomal LSE, expression of VEGFA is a responsible factor to upregulate cancer cells (Fig. 9).
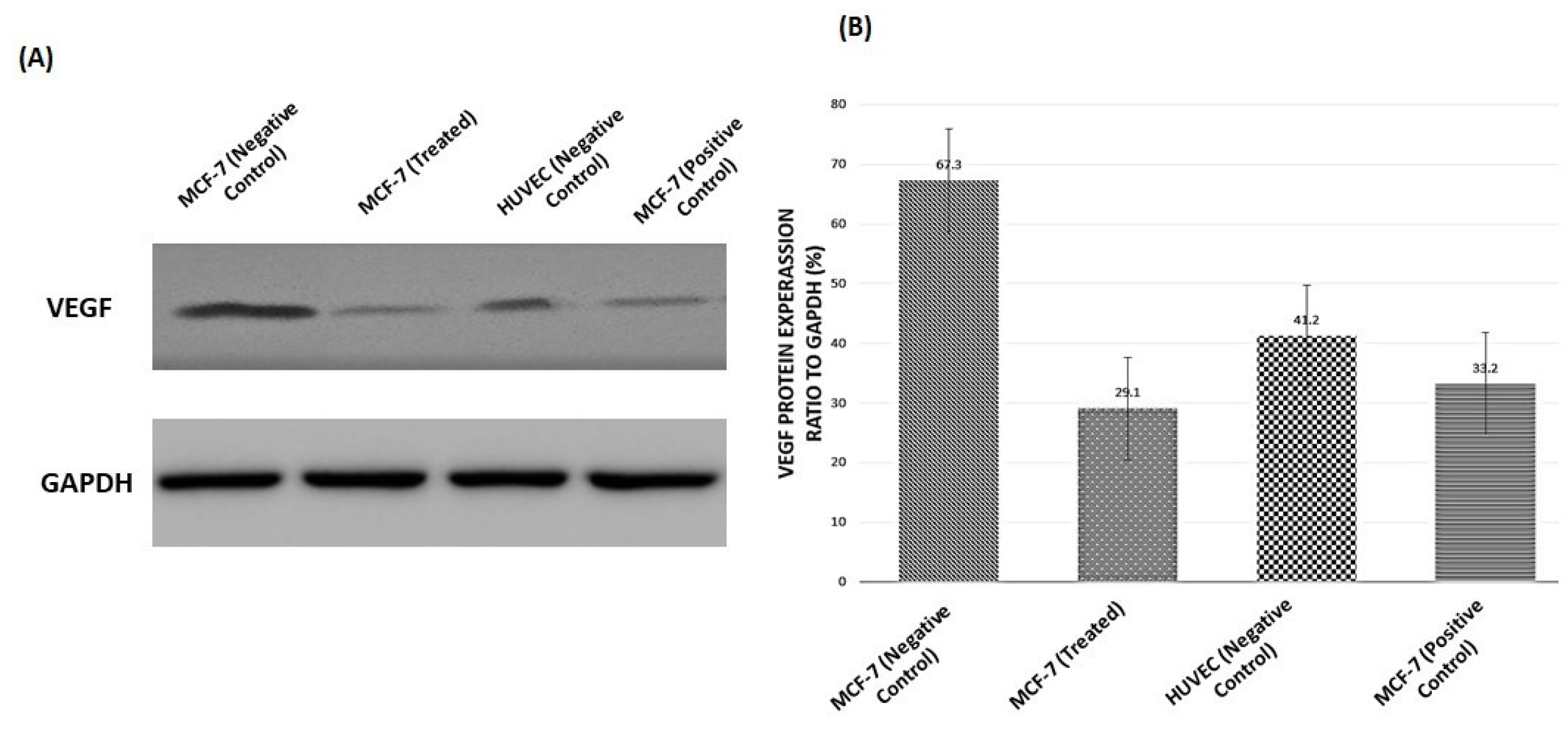
Figure 9.
VEGFA expression in MCF-7 and HUVEC cells. (A) VEGFA protein levels were analyzed using western blotting. GAPDH levels were calculated as a loading control. (B) Graph demonstrated VEGFA protein expression relative to that of GAPDH. All data corresponded to the mean ± SD of three independent experiments.
.
VEGFA expression in MCF-7 and HUVEC cells. (A) VEGFA protein levels were analyzed using western blotting. GAPDH levels were calculated as a loading control. (B) Graph demonstrated VEGFA protein expression relative to that of GAPDH. All data corresponded to the mean ± SD of three independent experiments.
Discussion
Protein delivery into cells is a powerful strategy for the development of new therapeutics since it can replace poorly expressed or dysfunctional proteins, minimizing off-target effects. However, efficient delivery of protein is essential to achieve this goal. First and foremost, we need to focus on what happens once proteins are inside the cell.
55
Leech therapy is a key method that is clinically utilized for patients with different kinds of cancers; however, tumor cell resistance to this agent is still the key obstacle in effective cancer treatment. Assessing the molecular mechanisms included in signaling the VEGFA can contribute to designing novel strategies in cancer treatment.
56
Our approach is further corroborated by our promising results, hence, the anti-cancer activity of medical LSE can be obtained through incorporating into liposomes. Here, a protein extracted from medicinal leech saliva is studied for formulating a kind of vital and original nano liposome. Further detailed investigations and optimization are needed for clarifying the mechanism of incorporation of liposomes to enhance absorption proteins, to allow targeting and further enhancements to increment the formulation stability. Hence, it will be possible to develop an effective nature-derived anti-cancer formulation to offer a substitute for the presently available therapy.
57
The present study was the first work studying the use of medical leech saliva proteins inside liposomes as an anticancer drug. In each traditional and trendy therapeutic approaches, leeches are promising within the treatment of varied diseases. Except for the recognized anti-metastatic activity of leech saliva, here, we initially reported that the liposomal LSE achieved from the H. medicinalis had an anti-proliferative activity against the human breast adenocarcinoma cell line (MCF-7). DAPI staining of cells proved that liposomal LSE had an anti-tumor activity, and the Scratching test demonstrated that drugs had an anti-proliferative activity.
Furthermore, expression of VEGFA, HIF1a, and P53 illustrated that liposomal LSE has an anti-cancer effect. Hence, additional works still are required in this regard to separate and determine the active principle, to check the mechanism of action, and to judge its effect on different cell line varieties. We first applied liposomal LSE for breast cancer cells (MCF-7 cell line). Followed by determining the cytotoxicity in treated cells and control cells, the impacts of high concentrations of liposomal LSE were assessed on treated-MCF-7 cells for investigating mRNA expression levels of VEGFA, HIF1a, and P53. Our results revealed that low expression of VEGFA in MCF-7 cells reduced cell migration, which is evident from wound healing assay, and also western blotting showed that expression of VEGFA gene was declined and HIF1a and P53 increased in treated cells.
Conclusion
Our study proposes that mechanisms of liposomal leech saliva proteins for downregulation of VEGFA are special. Comprehensive data and optimization to enable active targeting and further developments to raise the stability of the drug are essential to enable the development of an operative nature anti- cancer formulation, which can provide an alternative to the accessible antitumor agents. Furthermore, our data add new illustration of biological activity of leech saliva in the expression of VEGFA to tumor angiogenesis. This also highlights the position of studying leech therapy with other features. Present data together with the previous results demonstrate an impressive impact of medical leech saliva proteins inhibition on tumor growth and angiogenesis. This is one of the first studies demonstrating the in vitro antitumor activity of leech saliva proteins in MCF-7 cells. The anti-cancer effect of leech saliva can be further improved by the use of combined therapy, thus making it a hopeful remedy for administration of breast cancer patients.
Future perspective
Protein delivery into cells is a potentially powerful strategy for developing new therapeutics since it can replace poorly expressed or dysfunctional proteins, minimizing off-target effects. However, efficient delivery of protein is essential to achieve this goal. First and foremost, we need to focus more on what happens once proteins are inside the cell. Improved methods for endosomal escape as well as mechanisms that avoid endocytosis altogether provide the promise of considerable improvement beyond current vehicles. Thinking further ahead, efficient access to the cytosol will enable intracellular targeting, providing another strategy that will further increase the efficacy of protein-based therapy. The various secretions of LSE are individually been extracted and synthesized by recombinant techniques. These compounds have been studied for various aspects like molecular structure, cellular and molecular activity, physiological functions, and their specific therapeutic actions on various disease conditions. Hopefully, this may assist in the development of various specific therapeutic agents for a variety of diseases.
Acknowledgments
The authors would like to express their gratitude to all the staff of the Drug Applied Research Center of Tabriz University of Medical Sciences and University of Tabriz, Tabriz, Iran for their contribution and sincere support.
Funding sources
This research was funded by personal sources.
Ethical statement
The Ethics Committee of Drug Applied Research Center of Tabriz University of Medical Sciences has approved this study (Reference Number: 421799/AS).
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interests.
Authors’ contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AS, HK. The first draft of the manuscript was written by AS and all authors commented on previous versions of the manuscript and HH and JA were supervisors of this project. All authors read and approved the final manuscript.
Research Highlights
What is the current knowledge?
√ Nowadays medical leech therapy is well known all around the world and majority of people apply medical leech for a wide range of diseases without any information about its saliva proteins.
What is new here?
√ In this paper we aimed to evaluate medical leech proteins as cancer inhibitor by using nano liposome as the carrier to cells for the first time. Nano drug was evaluated on MCF7 cells and expression of VEGFA, HIF1a, and P53 were recognized by real-time PCR and western blotting.
References
- Olusanya TOB, Haj Ahmad RR, Ibegbu DM, Smith JR, Elkordy AA. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018; 23:907. doi: 10.3390/molecules23040907 [Crossref] [ Google Scholar]
- Wieland K, Ramer G, Weiss VU, Allmaier G, Lendl B, Centrone A. Nanoscale chemical imaging of individual chemotherapeutic cytarabine-loaded liposomal nanocarriers. Nano Research 2019; 12:197-203. [ Google Scholar]
- Crommelin DJA, van Hoogevest P, Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release 2020; 318:256-63. doi: 10.1016/j.jconrel.2019.12.023 [Crossref] [ Google Scholar]
- Kiaie SH, Mojarad-Jabali S, Khaleseh F, Allahyari S, Taheri E, Zakeri-Milani P. Axial pharmaceutical properties of liposome in cancer therapy: Recent advances and perspectives. Int J Pharm 2020; 581:119269. doi: 10.1016/j.ijpharm.2020.119269 [Crossref] [ Google Scholar]
- Tajbakhsh A, Hasanzadeh M, Rezaee M, Khedri M, Khazaei M, ShahidSales S. Therapeutic potential of novel formulated forms of curcumin in the treatment of breast cancer by the targeting of cellular and physiological dysregulated pathways. J Cell Physiol 2018; 233:2183-92. doi: 10.1002/jcp.25961 [Crossref] [ Google Scholar]
- Ray M, Lee YW, Scaletti F, Yu R, Rotello VM. Intracellular delivery of proteins by nanocarriers. Nanomedicine (Lond) 2017; 12:941-52. doi: 10.2217/nnm-2016-0393 [Crossref] [ Google Scholar]
- Belfiore L, Saunders DN, Ranson M, Thurecht KJ, Storm G, Vine KL. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. J Control Release 2018; 277:1-13. doi: 10.1016/j.jconrel.2018.02.040 [Crossref] [ Google Scholar]
- Lujan H, Griffin WC, Taube JH, Sayes CM. Synthesis and characterization of nanometer-sized liposomes for encapsulation and microRNA transfer to breast cancer cells. Int J Nanomedicine 2019; 14:5159-73. doi: 10.2147/IJN.S203330 [Crossref] [ Google Scholar]
- Tang M, Svirskis D, Leung E, Kanamala M, Wang H, Wu Z. Can intracellular drug delivery using hyaluronic acid functionalised pH-sensitive liposomes overcome gemcitabine resistance in pancreatic cancer?. J Control Release 2019; 305:89-100. doi: 10.1016/j.jconrel.2019.05.018 [Crossref] [ Google Scholar]
- Kieler-Ferguson HM, Chan D, Sockolosky J, Finney L, Maxey E, Vogt S. Encapsulation, controlled release, and antitumor efficacy of cisplatin delivered in liposomes composed of sterol-modified phospholipids. Eur J Pharm Sci 2017; 103:85-93. doi: 10.1016/j.ejps.2017.03.003 [Crossref] [ Google Scholar]
- Balzus B, Sahle FF, Honzke S, Gerecke C, Schumacher F, Hedtrich S. Formulation and ex vivo evaluation of polymeric nanoparticles for controlled delivery of corticosteroids to the skin and the corneal epithelium. Eur J Pharm Biopharm 2017; 115:122-30. doi: 10.1016/j.ejpb.2017.02.001 [Crossref] [ Google Scholar]
- Mehta P, Al-Kinani AA, Haj-Ahmad R, Arshad MS, Chang MW, Alany RG. Electrically atomised formulations of timolol maleate for direct and on-demand ocular lens coatings. Eur J Pharm Biopharm 2017; 119:170-84. doi: 10.1016/j.ejpb.2017.06.016 [Crossref] [ Google Scholar]
- Chen MJ, Hui H-W, Lee T, Kurtulik P, Surapaneni S. Nanosuspension of a poorly soluble drug via microfluidization process. Google Patents; 2017.
- Takechi-Haraya Y, Goda Y, Sakai-Kato K. Control of Liposomal Penetration into Three-Dimensional Multicellular Tumor Spheroids by Modulating Liposomal Membrane Rigidity. Mol Pharm 2017; 14:2158-65. doi: 10.1021/acs.molpharmaceut.7b00051 [Crossref] [ Google Scholar]
- Askarizadeh A, Butler AE, Badiee A, Sahebkar A. Liposomal nanocarriers for statins: A pharmacokinetic and pharmacodynamics appraisal. J Cell Physiol 2019; 234:1219-29. doi: 10.1002/jcp.27121 [Crossref] [ Google Scholar]
- Bangham AD. Physical Structure and Behavior of Lipids and Lipid Enzymes. In: Paoletti R, D Kritchevsky, editors. Advances in Lipid Research. Elsevier; 1963. p. 65-104.
- Kandzija N, Khutoryanskiy VV. Delivery of Riboflavin-5'-Monophosphate Into the Cornea: Can Liposomes Provide Any Enhancement Effects?. J Pharm Sci 2017; 106:3041-9. doi: 10.1016/j.xphs.2017.05.022 [Crossref] [ Google Scholar]
- Vallejo LF, Rinas U. Strategies for the recovery of active proteins through refolding of bacterial inclusion body proteins. Microb Cell Fact 2004; 3:11. doi: 10.1186/1475-2859-3-11 [Crossref] [ Google Scholar]
- Jahanban-Esfahlan R, de la Guardia M, Ahmadi D, Yousefi B. Modulating tumor hypoxia by nanomedicine for effective cancer therapy. J Cell Physiol 2018; 233:2019-31. doi: 10.1002/jcp.25859 [Crossref] [ Google Scholar]
- Mohamadi Saani S, Abdolalizadeh J, Zeinali Heris S. Ultrasonic/sonochemical synthesis and evaluation of nanostructured oil in water emulsions for topical delivery of protein drugs. Ultrason Sonochem 2019; 55:86-95. doi: 10.1016/j.ultsonch.2019.03.018 [Crossref] [ Google Scholar]
- Jameera Begam A, Jubie S, Nanjan MJ. Estrogen receptor agonists/antagonists in breast cancer therapy: A critical review. Bioorg Chem 2017; 71:257-74. doi: 10.1016/j.bioorg.2017.02.011 [Crossref] [ Google Scholar]
- Barar J, Omidi Y. Surface modified multifunctional nanomedicines for simultaneous imaging and therapy of cancer. Bioimpacts 2014; 4:3-14. doi: 10.5681/bi.2014.011 [Crossref] [ Google Scholar]
- Porshinsky BS, Saha S, Grossman MD, Beery Ii PR, Stawicki SP. Clinical uses of the medicinal leech: a practical review. J Postgrad Med 2011; 57:65-71. doi: 10.4103/0022-3859.74297 [Crossref] [ Google Scholar]
- Cherniack EP, Cherniack E. Bugs as drugs, part two: worms, leeches, scorpions, snails, ticks, centipedes, and spiders. Altern Med Rev 2011; 16:50-8. [ Google Scholar]
-
Shakouri A, Wollina U. Time to Change Theory; Medical Leech from a Molecular Medicine Perspective Leech Salivary Proteins Playing a Potential Role in Medicine. Adv Pharm Bull 2020. 10.34172/apb.2021.038
- Singh SK, Rajoria K. Medical leech therapy in Ayurveda and biomedicine - A review. J Ayurveda Integr Med 2019. doi: 10.1016/j.jaim.2018.09.003 [Crossref]
- Merzouk A, Ghawi A, Abdualkader A, Abdullahi A, Alaama M. Anticancer effects of medical Malaysian leech saliva extract (LSE). Pharm Anal Acta S 2012; 15:2-6. [ Google Scholar]
- Ammar A, Guns E, Kucuk O, Abdualkader A, Alaama M, Uddin AH, et al. Mechanism of anticancer activity of BPS-001 (lyophilized leech saliva extract). AACR; 2017.
- Dong P, Rakesh KP, Manukumar HM, Mohammed YHE, Karthik CS, Sumathi S. Innovative nano-carriers in anticancer drug delivery-a comprehensive review. Bioorg Chem 2019; 85:325-36. doi: 10.1016/j.bioorg.2019.01.019 [Crossref] [ Google Scholar]
- Huang Z, Zhao C, Chen Y, Cowell JA, Wei G, Kultti A. Recombinant human hyaluronidase PH20 does not stimulate an acute inflammatory response and inhibits lipopolysaccharide-induced neutrophil recruitment in the air pouch model of inflammation. J Immunol 2014; 192:5285-95. [ Google Scholar]
- Kobayashi N, Miyoshi S, Mikami T, Koyama H, Kitazawa M, Takeoka M. Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization. Cancer Res 2010; 70:7073-83. doi: 10.1158/0008-5472.CAN-09-4687 [Crossref] [ Google Scholar]
- Huang Z, Zhao C, Radi A. Characterization of hyaluronan, hyaluronidase PH20, and HA synthase HAS2 in inflammation and cancer. Inflammation and Cell Signaling 2014; 1:e306. [ Google Scholar]
- Kultti A, Li X, Jiang P, Thompson CB, Frost GI, Shepard HM. Therapeutic targeting of hyaluronan in the tumor stroma. Cancers 2012; 4:873-903. [ Google Scholar]
- Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev 2006; 106:818-39. doi: 10.1021/cr050247k [Crossref] [ Google Scholar]
- Shakouri A, Adljouy N, Balkani S, Mohamadi M, Hamishehkar H, Abdolalizadeh J. Effectiveness of topical gel of medical leech (Hirudo medicinalis) saliva extract on patients with knee osteoarthritis: A randomized clinical trial. Complement Ther Clin Pract 2018; 31:352-9. doi: 10.1016/j.ctcp.2017.12.001 [Crossref] [ Google Scholar]
- Alaama M, AlNajjar M, Abdualkader A, Mohammad A, Merzouk A. Isolation and analytical characterization of local Malaysian leech saliva. IIUM Engineering Journal 2011; 12:51-9. [ Google Scholar]
- Jeon JY, Hwang SY, Cho SH, Choo J, Lee EK. Effect of cholesterol content on affinity and stability of factor VIII and annexin V binding to a liposomal bilayer membrane. Chem Phys Lipids 2010; 163:335-40. doi: 10.1016/j.chemphyslip.2010.01.005 [Crossref] [ Google Scholar]
- Di Domenico M, Pozzi D, Palchetti S, Digiacomo L, Iorio R, Astarita C. Nanoparticle-biomolecular corona: A new approach for the early detection of non-small-cell lung cancer. J Cell Physiol 2019; 234:9378-86. doi: 10.1002/jcp.27622 [Crossref] [ Google Scholar]
- Sen K, Banerjee S, Mandal M. Dual drug loaded liposome bearing apigenin and 5-Fluorouracil for synergistic therapeutic efficacy in colorectal cancer. Colloids Surf B Biointerfaces 2019; 180:9-22. doi: 10.1016/j.colsurfb.2019.04.035 [Crossref] [ Google Scholar]
- Ghafari M, Haghiralsadat F, Khanamani Falahati-Pour S, Zavar Reza J. Development of a novel liposomal nanoparticle formulation of cisplatin to breast cancer therapy. J Cell Biochem 2020; 121:3584-92. doi: 10.1002/jcb.29651 [Crossref] [ Google Scholar]
- Vakili-Ghartavol R, Mombeiny R, Salmaninejad A, Sorkhabadi SMR, Faridi-Majidi R, Jaafari MR. Tumor-associated macrophages and epithelial-mesenchymal transition in cancer: Nanotechnology comes into view. J Cell Physiol 2018; 233:9223-36. doi: 10.1002/jcp.27027 [Crossref] [ Google Scholar]
- Ahmed S, Fujita S, Matsumura K. Enhanced protein internalization and efficient endosomal escape using polyampholyte-modified liposomes and freeze concentration. Nanoscale 2016; 8:15888-901. doi: 10.1039/c6nr03940e [Crossref] [ Google Scholar]
- Khataee A, Arefi-Oskoui S, Samaei L. ZnFe-Cl nanolayered double hydroxide as a novel catalyst for sonocatalytic degradation of an organic dye. Ultrason Sonochem 2018; 40:703-13. doi: 10.1016/j.ultsonch.2017.08.014 [Crossref] [ Google Scholar]
- Khataee A, Eghbali P, Irani-Nezhad MH, Hassani A. Sonochemical synthesis of WS2 nanosheets and its application in sonocatalytic removal of organic dyes from water solution. Ultrason Sonochem 2018; 48:329-39. doi: 10.1016/j.ultsonch.2018.06.003 [Crossref] [ Google Scholar]
- de Matos MBC, Miranda BS, Rizky Nuari Y, Storm G, Leneweit G, Schiffelers RM. Liposomes with asymmetric bilayers produced from inverse emulsions for nucleic acid delivery. J Drug Target 2019; 27:681-9. doi: 10.1080/1061186X.2019.1579819 [Crossref] [ Google Scholar]
- Nagaraju S, Truong D, Mouneimne G, Nikkhah M. Microfluidic Tumor-Vascular Model to Study Breast Cancer Cell Invasion and Intravasation. Adv Healthc Mater 2018; 7:e1701257. doi: 10.1002/adhm.201701257 [Crossref] [ Google Scholar]
- Minnelli C, Cianfruglia L, Laudadio E, Galeazzi R, Pisani M, Crucianelli E. Selective induction of apoptosis in MCF7 cancer-cell by targeted liposomes functionalised with mannose-6-phosphate. J Drug Target 2018; 26:242-51. doi: 10.1080/1061186X.2017.1365873 [Crossref] [ Google Scholar]
- Ziko L, Saqr AA, Ouf A, Gimpel M, Aziz RK, Neubauer P. Antibacterial and anticancer activities of orphan biosynthetic gene clusters from Atlantis II Red Sea brine pool. Microb Cell Fact 2019; 18:56. doi: 10.1186/s12934-019-1103-3 [Crossref] [ Google Scholar]
- Khodabakhsh F, Muyldermans S, Behdani M, Kazemi-Lomedasht F. Liposomal delivery of vascular endothelial growth factor/receptors and their inhibitors. J Drug Target 2020; 28:379-85. doi: 10.1080/1061186X.2019.1693578 [Crossref] [ Google Scholar]
- Fu M, Tang W, Liu JJ, Gong XQ, Kong L, Yao XM. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J Drug Target 2020; 28:245-58. doi: 10.1080/1061186X.2019.1656725 [Crossref] [ Google Scholar]
- Menina S, Eisenbeis J, Kamal MAM, Koch M, Bischoff M, Gordon S. Bioinspired Liposomes for Oral Delivery of Colistin to Combat Intracellular Infections by Salmonella enterica. Adv Healthc Mater 2019; 8:e1900564. doi: 10.1002/adhm.201900564 [Crossref] [ Google Scholar]
- Moyá ML, López-López M, Lebrón JA, Ostos FJ, Pérez D, Camacho V. Preparation and characterization of new liposomes Bactericidal activity of cefepime encapsulated into cationic liposomes. Pharmaceutics 2019; 11:69. [ Google Scholar]
- Clayton KN, Salameh JW, Wereley ST, Kinzer-Ursem TL. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics 2016; 10:054107. doi: 10.1063/1.4962992 [Crossref] [ Google Scholar]
- Pinnapireddy SR, Duse L, Strehlow B, Schafer J, Bakowsky U. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids Surf B Biointerfaces 2017; 158:93-101. doi: 10.1016/j.colsurfb.2017.06.022 [Crossref] [ Google Scholar]
- Zheng Y, Li Z, Chen H, Gao Y. Nanoparticle-based drug delivery systems for controllable photodynamic cancer therapy. Eur J Pharm Sci 2020; 144:105213. doi: 10.1016/j.ejps.2020.105213 [Crossref] [ Google Scholar]
- Rawal S, Patel MM. Threatening cancer with nanoparticle aided combination oncotherapy. J Control Release 2019; 301:76-109. doi: 10.1016/j.jconrel.2019.03.015 [Crossref] [ Google Scholar]
- Rolle F, Bincoletto V, Gazzano E, Rolando B, Lollo G, Stella B. Coencapsulation of disulfiram and doxorubicin in liposomes strongly reverses multidrug resistance in breast cancer cells. Int J Pharm 2020; 580:119191. doi: 10.1016/j.ijpharm.2020.119191 [Crossref] [ Google Scholar]