Bioimpacts. 11(2):135-146.
doi: 10.34172/bi.2021.21
Original Research
Can early treatment of twitcher mice with high dose AAVrh10-GALC eliminate the need for BMT?
Mohammad A Rafi *  , Paola Luzi , David A Wenger
, Paola Luzi , David A Wenger 
Author information:
Department of Neurology, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, PA 19107, USA
Abstract
Introduction:
Krabbe disease (KD) is an autosomal recessive disorder caused by mutations in the galactocerebrosidase (GALC) gene resulting in neuro-inflammation and defective myelination in the central and peripheral nervous systems. Most infantile patients present with clinical features before six months of age and die before two years of age. The only treatment available for pre-symptomatic or mildly affected individuals is hematopoietic stem cell transplantation (HSCT). In the animal models, combining bone marrow transplantation (BMT) with gene therapy has shown the best results in disease outcome. In this study, we examine the outcome of gene therapy alone.
Methods:
Twitcher (twi) mice used in the study, have a W339X mutation in the GALC gene. Genotype identification of the mice was performed shortly after birth or post-natal day 1 (PND1), using polymerase chain reaction on the toe clips followed by restriction enzyme digestion and electrophoresis. Eight or nine-day-old affected mice were used for gene therapy treatment alone or combined with BMT. While iv injection of 4 × 1013 gc/kg of body weight of viral vector was used originally, different viral titers were also used without BMT to evaluate their outcomes.
Results:
When the standard viral dose was increased four- and ten-fold (4X and 10X) without BMT, the lifespans were increased significantly. Without BMT the affected mice were fertile, had the same weight and appearance as wild type mice and had normal strength and gait. The brains showed no staining for CD68, a marker for activated microglia/macrophages, and less astrogliosis than untreated twi mice.
Conclusion:
Our results demonstrate that, it may be possible to treat human KD patients with high dose AAVrh10 without blood stem cell transplantation which would eliminate the side effects of HSCT.
Keywords: Krabbe disease, AAVrh10, Gene therapy, Twitcher mice, Bone marrow transplantation, Combined therapy, Myelination
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Globoid cell leukodystrophy (GLD) or Krabbe disease (KD) is a lysosomal disorder caused by the deficiency of galactocerebrosidase (GALC) activity.
1
The disease is inherited in an autosomal recessive manner and presents in the most severe infantile form by 6 months of age, followed by death usually by 2 years of age.
2
While the infantile form of the disease is most common, later onset and adult patients with KD are also diagnosed. In the absence of GALC activity not only galactosylceramide (galcer), an important component of myelin, cannot be degraded but galactosylsphingosine or psychosine also accumulates to a toxic level.
3-5
There are several animal models for this disease, including mouse, dog, and monkey models that are being used for the treatment trials.
6-8
Twitcher (twi) mouse, the murine model of KD, has been extensively used to understand the pathogenesis of this disease and in many treatment trials.
9
This has resulted in various outcomes in improving the myelination process and extension of life. However, none of these approaches has resulted in a complete correction of the clinical features and pathological aspects of the disease. For complete correction of the disease, adequate GALC activity needs to be delivered to the CNS and PNS. Gene therapy seems the most promising in addressing this need, but the blood brain barrier (BBB) remains an issue.
10-15
Hematopoietic stem cell transplantation (HSCT) is currently the “standard of care” for human patients.
16,17
Besides providing some GALC enzyme to different CNS cell types, it may play an anti-inflammatory role. GALC-expressing macrophages from transplanted HSCs play pivotal role in cleaning up the undegraded myelin debris and content of the globoid cells.
18
In animal models of the disease, BMT has shown a synergistic effect when combined with other treatment strategies.
9
The mechanism of such synergistic effect has also been the subject of several studies.
19,20
While the lack of GALC activity in the CNS and PNS is the main cause of the disease, inflammation within the nervous system is also a characteristic of KD.
21
We have previously highlighted the anti-inflammatory role of BMT in murine model of this disease.
22
In the current article, we assess the abilities of bone marrow (BM) cells and AAVrh10-mGALC to deliver GALC activity to critical tissues and their ability to prevent or decrease neuro-inflammation and extend the lives of the treated mice. An increase of GALC activity in brain following BMT was originally reported by several authors.
23-26
However, the anti-inflammatory role of BM cells in the treatment of the KD may have been overstated in past decades while their GALC-supplying capacity has been understated.
20,27,28
Following our recent publication on the effect of viral dose and timing of treatment of twi mice,
29
we were interested in exploring the effect of high viral dose alone in their treatment. The results demonstrate that early gene therapy at a sufficiently high dose can supply high GALC activity to the brain and other tissues and possibly prevent neuro-inflammation before it has started. In the current paper, besides iv injection of our standard viral dose (4 × 1013 gc/kg of body weight, called 1X dose), we have increased viral dose four times (4X = 1.6 × 1014 gc/kg of body weight) and ten times (10X = 4 × 1014 gc/kg of body weight) alone or combined with BMT. The results could indicate that iv injection of AAVrh10-mGALC alone supplied at high dose at an early time point could be successful in treating human patients without the need for HSCT.
Materials and Methods
Animal procedures
All studies in mice were completed in accordance with approved protocols from the Institutional Animal Care and Use Committee (IACUC) at Jefferson Medical College. Twi mice used in the study were originally obtained from the Jackson Laboratory. These mice are in the C57BL/6 background and have a W339X mutation in the GALC gene. Genotype identification of the mice was performed immediately after birth or on post-natal day 1 (PND1)using polymerase chain reaction (PCR) as previously described.
12,30
Toe clips were used for DNA extraction and genotyping. PCR products were digested with EcoRV restriction enzyme and analyzed by electrophoresis on 2.5% MetaPhor agarose gel (Lonza Inc. Allendale, NJ, USA). Treated mice were monitored daily for the first week after the treatment was started and any abnormal signs related to the procedure was noted. Body weight was recorded weekly throughout their lives. Treated mice were allowed to survive as long as humanely possible or sacrificed at different time points for analysis. If deemed moribund (inactive and with unexpected weight loss) the mice were euthanatized by carbon dioxide and the age was recorded.
Generation of AAVrh10-mGALC vector
Construction of the AAVrh10-mGALC vector was previously reported.
13
Briefly, pCB7plasmid, which is an enhanced version of AAV2 vector, was received from the Institute for Human Gene Therapy at the University of Pennsylvania. Murine GALC cDNA was cloned into EcoRI site of this plasmid, downstream from the human CMV-enhancer/chicken β-actin hybrid promoter. The integrity of the ITRs was confirmed by sequencing and restriction enzyme analysis using SmaI and NcoI. The functionality of the construct was verified by in vitro cell transfection and measurement of GALC enzyme activity. Viral packaging and purification of the product were accomplished by the Institute for Human Gene Therapy and the vector was called AAVrh10-mGALC.
12,13
Viral titer was determined by PCR of the simian virus 40 poly(A) sequence.
31,32
The viral titer of the vector batch of AAVrh10-mGALC in use is 6×1013 genomic equivalents/mL.
Viral delivery
Viral injections were carried out on a light box to facilitate visualizing the tail vein. The young mice were cryo-anesthetized on ice before the injections. Iv injection was done through the tail vein using a 28G insulin syringe.
28
The success of injection was verified by noting blanching of the vein.
12
After the injection, pups were warmed and returned to their cage. Mice that died within a few days of the injection (less than 10%) were not included in the study.
Bone marrow transplantation (BMT)
The methodology for BM preparation and BMT was previously described.
28
Briefly, BMT recipient mice were myelo-suppressed using busulfan (Sigma-Aldrich, St. Louis, MO, USA). A 3 mg/mL solution of busulfan was prepared by initially dissolving it in dimethyl sulfoxide (Sigma-Aldrich) and adding sterile phosphate-buffered saline to bring the final concentration to 30%. Eight or nine-day-old affected mice were weighed, and 30 mg/kg of body weight of the busulfan solution was injected intraperitoneally (ip). BM cells from the donor mice were obtained by flushing tibiae and femora using ice-cold Hepes buffered Hanks’ balanced salt solution (Mediatech, Manassas, VA, USA). The cells were counted, centrifuged, and resuspended in Dulbecco's modified Eagle's medium (Sigma-Aldrich). Twenty-four hours after busulfan injection, mice received an ip injection of 3–4 × 107 BM cells in a total volume of 0.2 mL. For at least two weeks after BMT, mice were provided with drinking water containing 500 μg/mL Neomycin (Sigma-Aldrich). Mice that received BMT and died less than PND30 were not included in these studies. Initially BM cells from non-carrier donors were used, but in some later studies BM cells from carrier mice were also used.
Preparation of BM cells for GALC activity test
To harvest BM cells from treated or untreated twi mice, wild type and heterozygote mice, cell isolation steps for BMT were followed as previously described.
28
Briefly, BM cells were harvested by flushing tibiae and femora using ice-cold Hepes buffered Hanks’ balanced salt solution (Mediatech, Manassas, VA, USA). 28-G sterile needle were used for rinsing the bones. Collected cells were filtered through a 70-µm nylon web to extract the maximum quantity of BM cells, rinsed repeatedly with phosphate buffered saline (PBS) (Sigma-Aldrich), centrifuged and resuspended in 1 mL of Dulbecco's modified Eagle's medium.
Preparation of BM-derived monocytes/macrophages
BM cells were prepared as described above. To differentiate these cells towards monocytes the protocol proposed by Wagner et al
33
was followed. Harvested cells were counted and seeded on 6-well ultra-low-attachment surface plates to prevent permanent adhesion to the bottom of the plate on a concentration of 106 cells per ml with up to 6 ml per well. Plates were supplemented with 20 ng/mL macrophage colony stimulating factor (M-CSF) (Sigma-Aldrich) to promote cell differentiation. The 6-well plates were incubated at 37°C and 5% carbon dioxide for 5 days and observed daily. After 5 days, 60%-80% of the cells were expected to be differentiated to monocytes.
Harvesting the cultured BM cells and tail vein injection
Cells were harvested in ice-cold PBS containing 0.5% bovine serum albumin (BSA) (Sigma-Aldrich) and 2-mM EDTA (Sigma-Aldrich). kept in 4°C by gentle pipetting and detaching with cold harvesting solution. Cells were counted and suspended in 0.9% NaCl at a concentration of 10 × 106/mL.
Preparation of indomethacin solution
Indomethacin (Sigma-Aldrich) was dissolved in polyethylene glycol/Tween 20 (Sigma-Aldrich) (95:5 v/v) at a concentration of 0.4 mg/mL, then, diluted to 1:200 or 1:100 in drinking water to have 2 ug/mL and 4 ug/mL. The drinking water was changed twice a week.
Tissue preparation for GALC assay
Tissues from treated and untreated mice were removed immediately after carbon dioxide euthanasia. The tissues were immediately homogenized in distilled water using a Polytron apparatus (Brinkmann Instruments, Westbury, NY, USA) or frozen quickly and stored at −80°C. Protein concentration was determined following the method of Lowry et al.
34
GALC activity was measured using [3H]galactosylceramide substrate, according to our published method.
35
GALC activity was expressed as nmol substrate hydrolyzed/h/mg protein.
Behavioral studies
Treated mice were weighed and examined for any signs of tremor, weakness, or gait disturbance daily during weekdays. Some of these mice were subjected to evaluation of their walking pattern and capability to hang upside down from a wire screen.
Walking pattern
A blind tunnel made from a polyvinyl chloride pipe with 34 cm length, 4 cm wide, and 3 cm high was used to record mice footprints. The front paws of the mice were dipped in nontoxic red food color, and back paws in blue food color. The mouse to be evaluate was placed at one of the tunnel’s end on a blank paper so that it could walk down the tunnel towards the light source. The piece of paper with the colored paw prints was retained for comparative analysis. An 80-day-old normal mouse and 42-day-old untreated affected mouse were used as the controls.
Wire hanging test
Hanging test was used to assess strength and coordination. The mouse was placed on top of a wired mesh that was slowly turned over and maintained horizontally 35 cm above a thick layer of soft bedding. The length of time until the mice fell from the wire was recorded. Each testing consisted of three trials with one-minute intervals from which the timing scores were averaged. The ability to hang upside down and move around for 90 seconds was considered normal. Wild-type mice of different ages were able to hang and move around for at least 90 seconds. Untreated twi mice were unable to hang for any length of time after 25 days of age.
Immunohistochemistry
Frozen brain sections were thawed at room temperature and fixed for 15 minutes in freshly made 4% paraformaldehyde (PFA)/PBS. These tissues were permeabilized with 0.5% Triton X-100 for 10 minutes then treated with blocking reagent from Vector Laboratories (Burlingame, CA) for one hour. Tissues were then incubated with the rat anti-CD68 (BioRad, Hercules, CA) or mouse anti-GFAP (Millipore, MA, USA) for 90 minutes at room temperature to label macrophages/microglia and astrocytes. The targeted antigens were visualized by incubating the sections with the secondary anti-rabbit or anti-rat antibodies (Alexa 488 from Molecular Probes, Eugene, OR, USA) for 2 hours at 25°C. The immunostained slides were mounted with Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA, USA) as a nuclear marker. Although coronal sections from the entire brain were monitored, most sections studied were from the cortex area.
Statistical analysis
Survival analysis was performed using GraphPad Prism 7.0 software (GraphPad Software Inc., San Diego, CA, USA). The survival curves were analyzed using the log-rank (Mantel-Cox) test. (* P< 0.05, ** P< 0.01, *** P< 0.001, **** P< 0.0001).
Results
Evaluating the effects of treating twi mice with BM cells from wild-type and heterozygous mice plus AAVrh10-mGALC
The current belief about the role of BM cells in the treatment of twi mice and human KD patients is that these cells supply some GALC activity to the central and peripheral nervous system (CNS and PNS) in addition to their role in preventing neuro-inflammation. We found this to be in contrast with our recent findings when BM cells from heterozygous donors were used in combination with gene therapy.
29
Treating mice with BM cells from wild-type donors and a 1X viral dose (4 ×1013 gc/kg) results in a longer lifespan compared to mice receiving the same viral dose and BM cells from heterozygous mice. In addition, the survival rate of mice treated with BM cells from heterozygous mice along with a 1X viral dose, is comparable to the survival rate of the mice treated with BM cells from wild-type mice but receiving half of the viral dose (2 × 1013 gc/kg) (Fig. 1). Such findings show the importance of GALC activity delivered by BM cells in the twi mouse treated with combined therapy. Studies have shown no significant histological or behavioral differences between wild-type and heterozygous mice although heterozygous mice may have decreased ability to repair myelin following treatment with cuprizone.
36,37
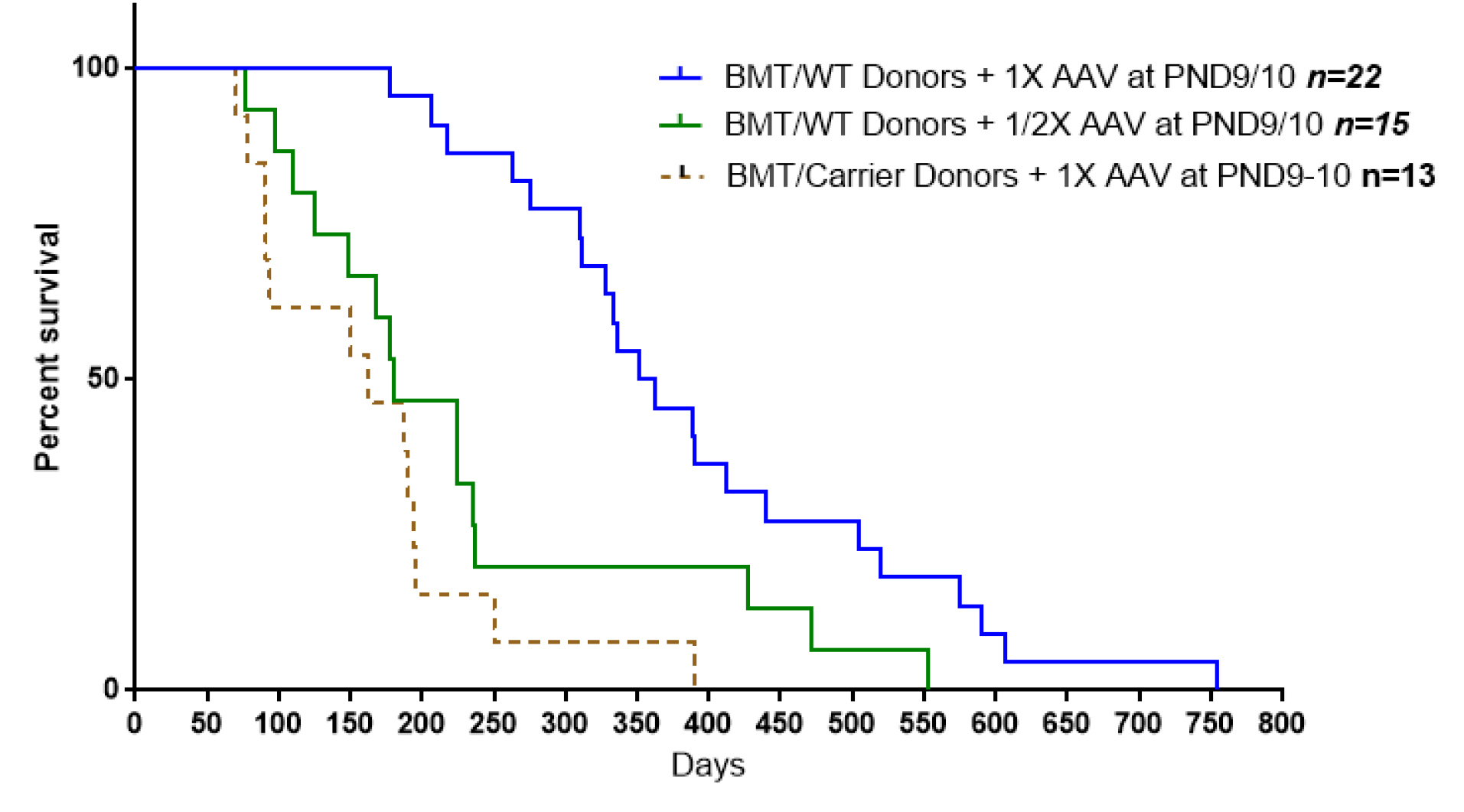
Fig. 1.
Comparison of the survival outcomes from twi mice receiving 1X viral dose (4 x 1013 gc/kg) plus BM cells from wild-type mice and twi mice receiving the same viral dose but BM cells from heterozygote mice. Another group of twi mice received BMT from wild-type mice but half of the viral dose (2 x 1013 gc/kg). While the survival curves of the treatment groups using ½ viral dose or BM cells from carrier mice are not significantly different from each other (p=0.9999), the survival curves of these two groups are significantly different from the survival curve of mice receiving 1X viral dose and BM cells from wild-type mice (P < 0.001and P < 0.0001 respectively).
.
Comparison of the survival outcomes from twi mice receiving 1X viral dose (4 x 1013 gc/kg) plus BM cells from wild-type mice and twi mice receiving the same viral dose but BM cells from heterozygote mice. Another group of twi mice received BMT from wild-type mice but half of the viral dose (2 x 1013 gc/kg). While the survival curves of the treatment groups using ½ viral dose or BM cells from carrier mice are not significantly different from each other (p=0.9999), the survival curves of these two groups are significantly different from the survival curve of mice receiving 1X viral dose and BM cells from wild-type mice (P < 0.001and P < 0.0001 respectively).
We examined the GALC activities of the enriched BM cells from wild-type and carrier mice. The overall GALC activity of these cells was much higher than expected. As Table 1 shows the mean GALC activity measured in BM cells from wild-type mice, prepared as they are routinely prepared for BMT, was 4.4 nmol/h/mg protein, ranging between 3.0 and 6.2 nmol/h/mg protein. It was also the highest GALC activity of all other organs examined in these mice, except for the kidneys where the mean GALC activity was 7.8 nmol/h/mg protein. The average GALC activity measured in BM cells of the heterozygous mice was 2.1 nmol/h/mg protein about half of the values from wild-type mice, as expected (Table 1).
Table 1.
GALC activity of different tissues from wild-type and heterozygous mice
|
Mice age
|
BM
|
Brain
|
Cereb
|
SC
|
SN
|
Liver
|
Heart
|
Muscle
|
Kidney
|
|
GALC activity*of different tissues from wild-type mice
|
| 70-d |
3.0 |
1.0 |
0.7 |
0.3 |
0.4 |
1.1 |
0.4 |
0.2 |
9.0 |
| 80-d |
3.7 |
0.8 |
1.0 |
0.8 |
0.7 |
1.6 |
3.4 |
0.8 |
6.5 |
| 120-d |
4.3 |
1.7 |
0.8 |
1.0 |
0.8 |
1.5 |
0.6 |
0.4 |
6.2 |
| 80-d |
4.2 |
1.5 |
NA |
NA |
NA |
NA |
NA |
NA |
8.9 |
| 150-d |
5.0 |
0.6 |
NA |
NA |
NA |
NA |
NA |
NA |
8.4 |
| 110-d |
6.2 |
1.8 |
NA |
NA |
NA |
NA |
NA |
NA |
8.0 |
| Mean values |
4.40 |
1.23 |
0.83 |
0.70 |
0.63 |
1.36 |
1.46 |
0.46 |
7.83 |
|
GALC activity*of different tissues from heterozygote mice
|
| 30-d |
1.7 |
1.2 |
0.8 |
0.8 |
0.8 |
2.1 |
0.4 |
0.4 |
- |
| 45-d |
2.1 |
0.9 |
1.0 |
0.7 |
1.0 |
1.3 |
1.3 |
0.8 |
3.4 |
| 150-d |
3.1 |
1.0 |
0.9 |
0.6 |
0.3 |
0.9 |
0.2 |
0.4 |
3.2 |
| 150-d |
2.0 |
0.7 |
0.5 |
0.5 |
0.1 |
0.9 |
0.2 |
0.7 |
2.7 |
| 218-d |
1.8 |
0.5 |
0.6 |
0.1 |
0.03 |
1.7 |
0.1 |
0.6 |
2.5 |
| Mean values |
2.14 |
0.86 |
0.76 |
0.54 |
0.44 |
1.38 |
0.44 |
0.58 |
2.95 |
BM cells used for GALC assays were prepared as described in Materials and Methods. We also assayed GALC activity of differentiated monocytes/macrophages from wild type mice that were prepared according to the method described by Wagner et al.
33
GALC activity of these cells after being in differentiation media for 5 and 8 days was 8.4 and 10.1 nmol/h/mg protein.
Effect of increased viral dose in gene therapy treatment alone
Previously, we reported the critical role of viral dose in combinational therapy of twi mice.
29
Here, we report the results of increased viral dose without BMT.
Survival, weight gain, and strength
Fig. 2A shows the extension of the survival of twi mice as the viral dose increases. Using our initially published 1X viral dose alone the median survival age was 72 days. The median survival is 351 days when that viral dose is combined with BMT, clearly showing the synergistic effect of this combination. However, increasing the viral dose 4X without BMT raised the median survival age to about 180 days. A 10X viral dose without BMT increased the median survival age to 280 days without any apparent toxicity. Longest living mouse treated with a 10X viral dose at PND10 was sacrificed at age 430 days.
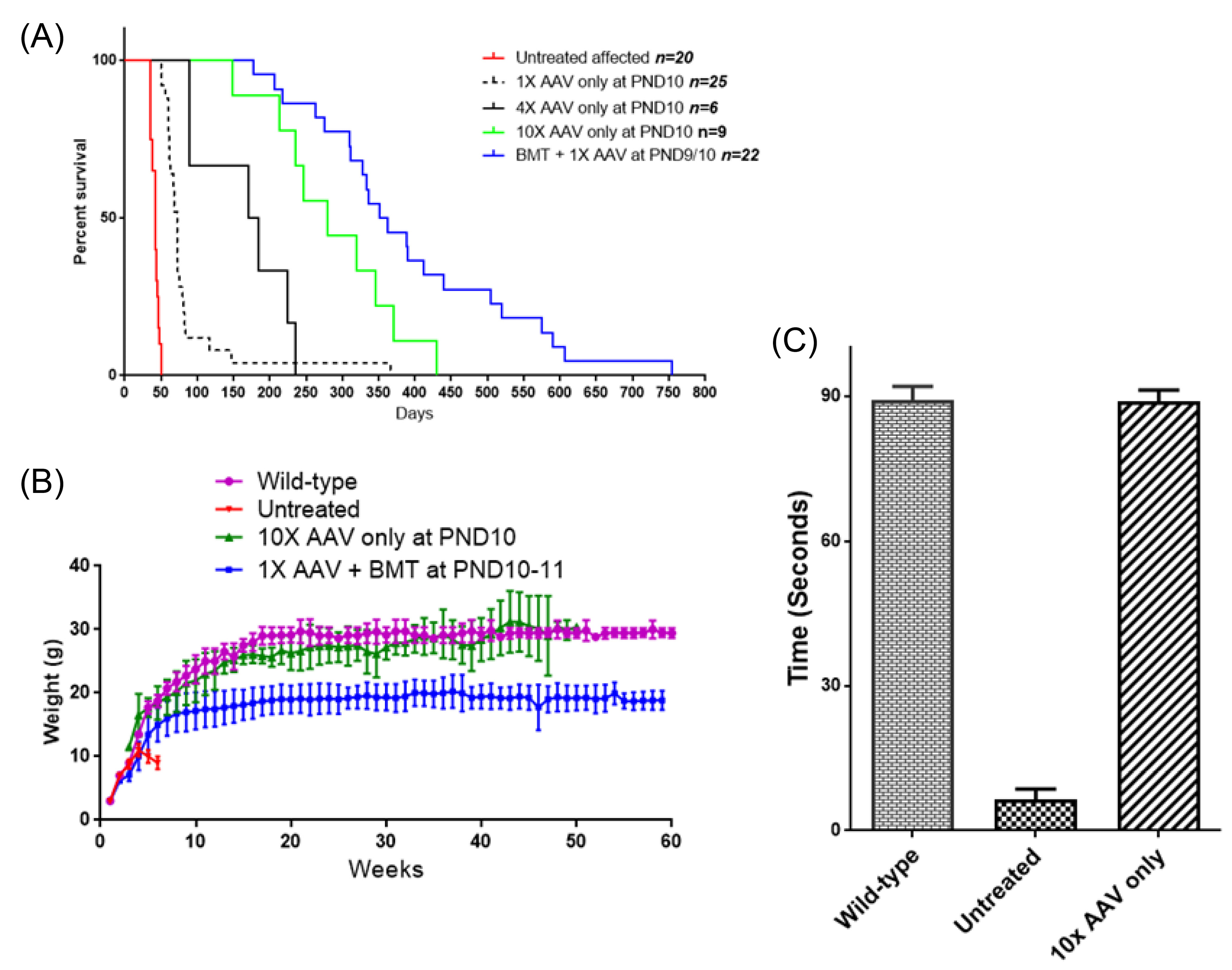
Fig. 2.
Comparing various treatments of twi mice for survival, weight gain and strength. (A) Survival of twi mice treated with AAVrh10 alone at different doses compared to mice treated with combination therapy and untreated mice. Between 1X and 10X viral dose the survival was highly significant (P < 0.0001). Comparing 10X alone and 1X + BMT survival was much less significant (P < 0.05). (B) Weighs of the mice treated with 10X AAVrh10 alone (n=9), 1X + BMT (n=16), untreated affected (n=9) and wild type mice (n=4). (C) Hang time of mice treated with 10X AAVrh10 alone (n=2) tested between 250 and 300 days compared to untreated affected and wild type mice.
.
Comparing various treatments of twi mice for survival, weight gain and strength. (A) Survival of twi mice treated with AAVrh10 alone at different doses compared to mice treated with combination therapy and untreated mice. Between 1X and 10X viral dose the survival was highly significant (P < 0.0001). Comparing 10X alone and 1X + BMT survival was much less significant (P < 0.05). (B) Weighs of the mice treated with 10X AAVrh10 alone (n=9), 1X + BMT (n=16), untreated affected (n=9) and wild type mice (n=4). (C) Hang time of mice treated with 10X AAVrh10 alone (n=2) tested between 250 and 300 days compared to untreated affected and wild type mice.
Body weights of the treated mice have been monitored as an indicator of general health. Fig. 2B shows the growth and weight gain of the differently treated mice compared to the untreated affected mice and wild-type mice. While there is a significant difference in weight gains of the mice treated with a combination of 1X AAV plus BMT compared to wild-type mice (P<0.0001), there is no significant difference between the twi mice receiving 10X AAV alone compared to wild-type mice. The lower body weights in the mice receiving combined treatment likely reflect the effects of the busulfan used for myelosuppression.
A hanging and grip test, an indicator of strength and coordination, was measured in wild-type mice, untreated twi mice and affected mice treated with 10X AAVrh10-mGALC as described in Materials and Methods. Untreated twi mice have impaired motor function and are unable to complete any hanging test after 3-4 weeks of age. However, mice treated with a high viral dose alone, tested between 250 and 300 days of age, did as well as the wild-type mice (Fig. 2C). These mice were able to hang on the inverted screen for at least 90 seconds, the maximum time allowed for wild-type mice.
Physical appearance and behavior
All mice treated with a high viral dose alone had normal appearance compared to wild-type mice, with no tremor or twitching. Some presented mild wobbling at terminal age or about a week before death. Mice receiving BMT alone or BMT plus viral therapy show side effects probably from the busulfan, including ruffled fur and smaller size. The mice treated with 10X viral dose alone presented with shiny, black coats similar to wild-type mice in addition to normal body weight. Fig. 3A shows one such treated mouse with two mice that were treated with BMT plus viral therapy. Mice treated with the higher viral dose alone, demonstrated normal behavior for almost their entire life. This included continuous exploratory movements, climbing the walls of the cage, and having a normal walking pattern. Fig. 3B compares the gait pattern of a 260-day-old mouse treated with 10X viral dose alone to an 80-day-old wild-type mouse and an untreated 42-day-old twi mouse. Another major drawback of BMT in the treatment of twi mice is the gonadotoxicity of busulfan and other myeloablative agents used in the BMT procedure causing infertility in male and female mice.
38
Similar findings have been reported in human patients receiving HSCT.
39
Male and female affected twi mice treated with a high viral dose alone maintain their full fertility, delivering several litters of all affected pups (Fig. 3C). The maturity age and the size of the litters are comparable to wild-type mice
40
(Fig. 3D).
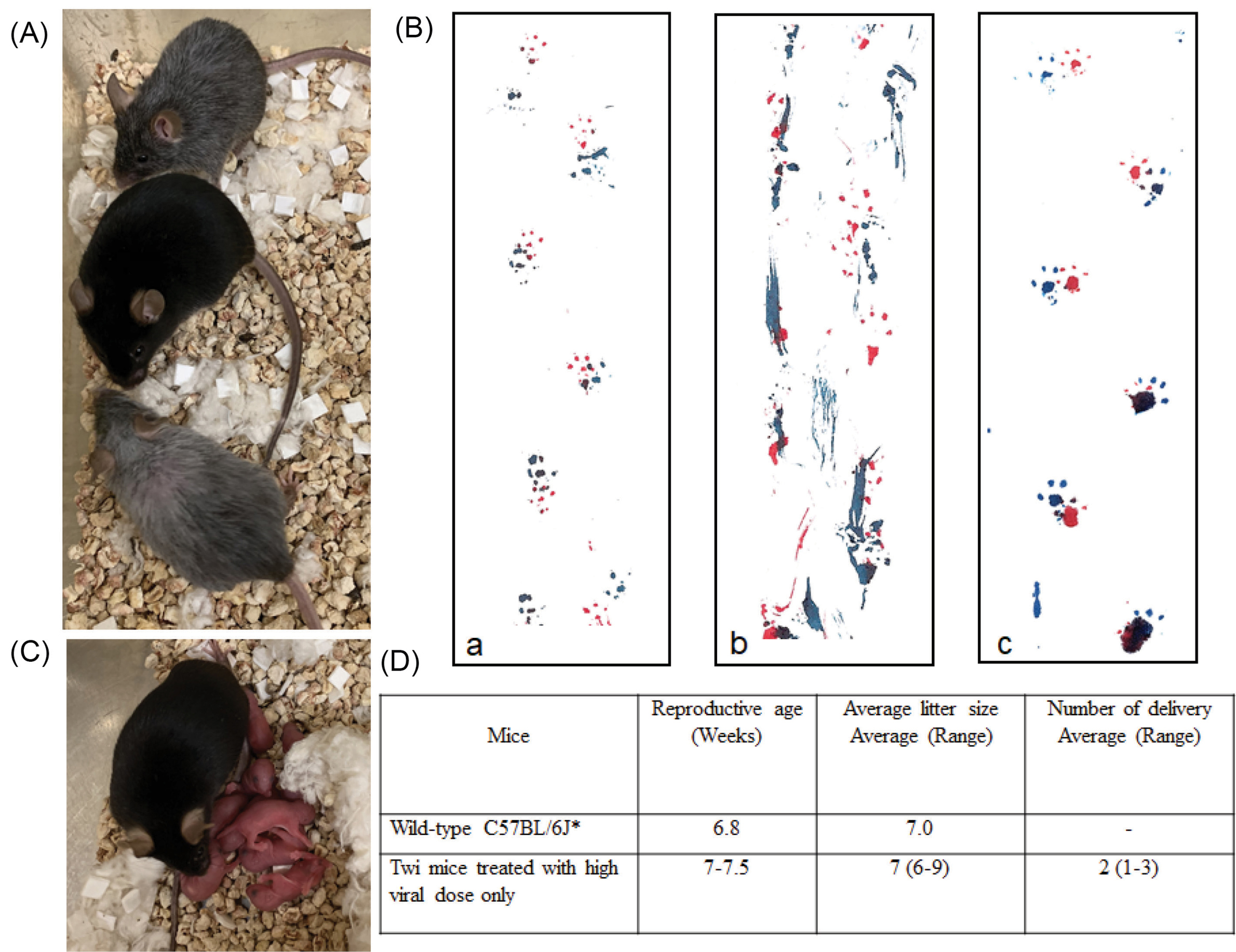
Fig. 3.
Effects of various treatments on physical appearance, gait and fertility. (A) The physical appearance of a mouse treated with the 10X viral dose (middle) is compared to age-matched mice (250-days old) treated with 1X viral dose + BMT. While the mice receiving combined therapy are smaller in size and have grey fur, the mice treated with the higher viral dose alone have a normal appearance and a normal coat. (B) Footprints of an 80-day-old wild-type mouse (a) and a 42-day-old untreated affected mouse (b) and a 260-day-old mouse treated with 10X viral dose (c). (C) The second litter resulting from the mating of affected mice treated with 10X viral dose only. All pups are affected since both parents are genetically homozygote for the twi mutation. (D) Reproductive age and litter size of female mice treated with 10X viral dose compared to wild-type mice.
.
Effects of various treatments on physical appearance, gait and fertility. (A) The physical appearance of a mouse treated with the 10X viral dose (middle) is compared to age-matched mice (250-days old) treated with 1X viral dose + BMT. While the mice receiving combined therapy are smaller in size and have grey fur, the mice treated with the higher viral dose alone have a normal appearance and a normal coat. (B) Footprints of an 80-day-old wild-type mouse (a) and a 42-day-old untreated affected mouse (b) and a 260-day-old mouse treated with 10X viral dose (c). (C) The second litter resulting from the mating of affected mice treated with 10X viral dose only. All pups are affected since both parents are genetically homozygote for the twi mutation. (D) Reproductive age and litter size of female mice treated with 10X viral dose compared to wild-type mice.
Pathological assessment
Antibodies against CD68, a marker of inflammation, and glial fibrillary acidic protein (GFAP) are used to compare the brain sections of mice treated with 10X viral dose to 80-day-old wild-type mouse and 42-day-old untreated twi mouse (Figs. 4 and 5). In contrast to the untreated twi mouse, the brain sections of the 90-day-old and 76-day-old AAV-treated mice are free of activated microglial/macrophages and are comparable to the wild-type mice (Fig. 4). In Fig. 5, the sections from the same mice stained with the anti-GFAP antibody are shown. As shown in Fig. 5B, the untreated twi mouse demonstrates an increased number of hypertrophic reactive appearing astrocytes. The astrocytic densities of the 90-day-old and 76-day-old mice treated with 10X viral dose are comparable to the wild-type mouse (Fig. 5A).
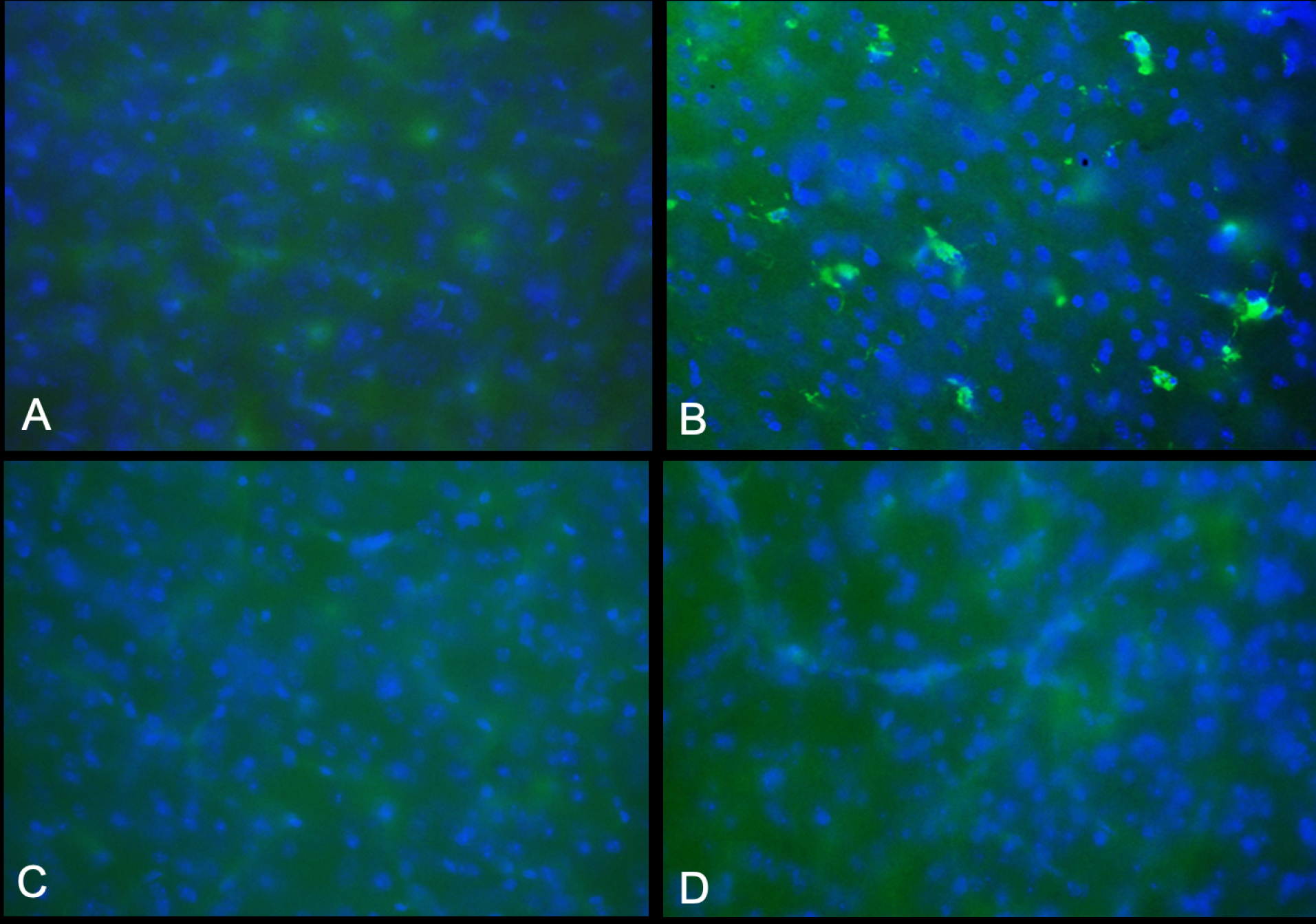
Fig. 4.
Staining of activated microglia/macrophages in CNS. Brain sections from the 90-day-old (image C) and 76-days-old (image D) twitcher mice treated with high viral dose (4x 1014 gc/kg) only are compared to the 42-day-old untreated twitcher (B) and 80-day-old wild-type mice (A). All images are from PFA-fixed frozen sections stained with CD68 antibody. No CD68-positive cells were detected in virally treated twi mice (original magnification ×400).
.
Staining of activated microglia/macrophages in CNS. Brain sections from the 90-day-old (image C) and 76-days-old (image D) twitcher mice treated with high viral dose (4x 1014 gc/kg) only are compared to the 42-day-old untreated twitcher (B) and 80-day-old wild-type mice (A). All images are from PFA-fixed frozen sections stained with CD68 antibody. No CD68-positive cells were detected in virally treated twi mice (original magnification ×400).
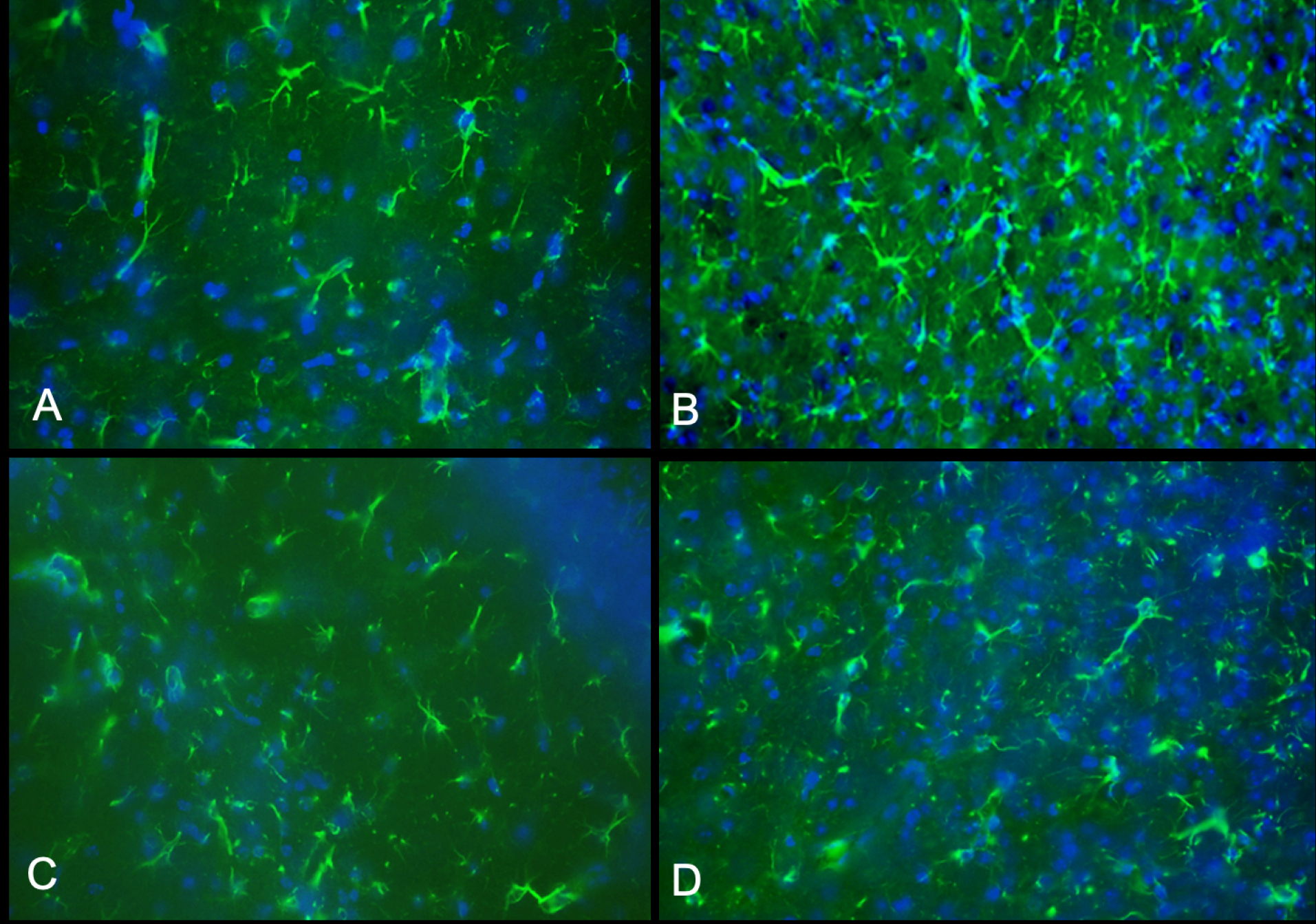
Fig. 5.
Astrogliosis. All images are from PFA-fixed frozen sections stained with anti-glial fibrillary acidic protein (GFAP) antibody that detects astrocytes. Image (A) is from a 80-day-old wild-type mouse, image (B) from 42-day-old untreated twi, image (C) and (D) from the 90-day-old and 76-day-old mice respectively treated with 10X viral dose (all original magnifications are ×400). As shown in images (C) and (D), the astrocyte distribution is comparable to the wild-type mouse.
.
Astrogliosis. All images are from PFA-fixed frozen sections stained with anti-glial fibrillary acidic protein (GFAP) antibody that detects astrocytes. Image (A) is from a 80-day-old wild-type mouse, image (B) from 42-day-old untreated twi, image (C) and (D) from the 90-day-old and 76-day-old mice respectively treated with 10X viral dose (all original magnifications are ×400). As shown in images (C) and (D), the astrocyte distribution is comparable to the wild-type mouse.
Ability of AAVrh10 to transduce certain tissues including BM cells following iv injection
We measured the GALC activity in certain tissues, including BM cells, from twi mice treated with iv gene therapy alone or gene therapy combined with BMT (Table 2). BM cells from twi mice treated with 10X AAVrh10-mGALC alone exhibit GALC activity as high or higher than mice treated with BMT plus 1X and 4X AAVrh10-mGALC. GALC expression in these cells could be detected 48 hours after viral injection and remains persistent during the lifespan of the virally treated mice. To our knowledge, no published study has described in vivo transduction of BM cells by any AAV vector. However, several ex vivo studies using different AAV serotypes including AAV1 and AAV6 have shown a weak transduction of BM cells in culture.
41-44
When BM cells from heterozygous mice are used for transplantation with the 1X viral dose, the measured GALC activity in nervous tissues was about half of the activity measured when BM cells from wild type mice were used with 1X viral dose (Table 3). The activity measured is similar to the activity when BM cells from wild type mice were used with ½X viral dose.
Table 2.
GALC activity* in certain tissues from mice treated by different methods
|
Mice treatment**
|
Brain
|
Sciatic nerve
|
Heart
|
BM cells
|
| BMT + 1X AAV |
0.9, 1.4 |
1.3, 23.0 |
470.2, 553.7 |
13.9, 19.8 |
| BMT + 4X AAV |
3.0, 3.5 |
1.8, 4.6 |
441.0, 515.4 |
12.1, 13.7 |
| 10X AAV only |
2.3-5.9 |
8.6-18.8 |
488.6-626.9 |
15.8-44.2 |
| Wild-type |
0.6-1.8 |
0.4-0.8 |
0.4-3.4 |
3.0-6.2 |
| Untreated twi |
0-0.1 |
0.1-0.2 |
0-0.2 |
0-0.1 |
* nmol/h/mg protein.
** Two mice analyzed in “BMT + 1X AAV” group were at PND218 and 362, two mice in group “BMT + 4X AAV” were at PND105 and 341 and five mice in group “10X AAV alone” were at PND37, 54, 90, 111 and 150.
Table 3.
GALC activity in nervous tissues based on BM donor type and viral dose
|
Treatment
|
Average of GALC activity* n=6-13
|
|
BM donor
|
Viral dose
|
Brain
|
Cerebellum
|
Sciatic Nerve
|
| Wild-type mice |
1X viral dose |
1.7 |
1.6 |
5.9 |
| Wild-type mice |
1/2X viral dose |
0.8 |
1.0 |
2.0 |
| Wild-type mice |
No viral injection |
0.9 |
1.0 |
1.4 |
| Heterozygote mice |
1X viral dose |
0.8 |
1.1 |
2.7 |
Ability of AAVrh10-mGALC to transduce BM cells in vitro
Following the detection of the in vivo transduction of BM cells after iv injection of AAVrh10-mGALC, the question was whether such ability could be demonstrated in BM cells in tissue culture. To this end, the harvested BM cells from twi mice were cultured in monocyte isolation media (see Materials and Methods). Under such conditions, BM cells differentiate towards the monocyte/macrophage state.
33
The transductions were done by adding 3 x 1010 gc/mL of AAVrh10-mGALC to the medium and keeping them at 37o C in a cell-culture incubator for several days. The success of the transduction was determined by evaluating the GALC activity of the cells at different time points. Increased GALC activity of BM cells could be detected 48 hours after transduction and continued to increase with time. The GALC activity of the transduced BM cells from twi mice could reach as high as 23.0 nmol/h/mg protein after being in culture for 5 days and 46.5 nmol/h/mg protein after being in culture for eight days. This may be the result of the continuous GALC expression or possibly due to expressed GALC being taken up from the cultured media according to the cross-correction phenomena reported earlier.
45
Effects of anti-inflammatory drugs on the progression of the disease
Given that neuro-inflammation is a component of KD, HSCT has been recognized as an effective option to lower the inflammation by delivering multiple anti-inflammatory cytokines. However, as HSCT/BMT comes with undesired side effects, we were interested to see if a safe and similarly effective option could be found to replace the current methodology. To this end indomethacin, a well-known non-steroidal anti-inflammatory drug was chosen to evaluate its potential in eliminating neuro-inflammation and slowing disease progression in combination with gene therapy. In a comparative study of several non-steroidal anti-inflammatory drugs, indomethacin was found to decrease the levels of several factors involved in inflammation.
22
Therefore, following iv injection of a 1X viral dose on PND10, indomethacin was also supplied in the drinking water with a concentration of 2 ug to 4 ug/mL starting on PND14 and continued for 4 weeks. No tangible positive result was obtained from the total of six mice treated this way. Treated mice lived between 54 and 67 days, which was similar to the treatment with a 1X viral dose alone.
BMT using BM cells with supra-normal GALC activity
Multiple attempts at transplanting in vivo-transduced BM cells expressing supra-normal GALC activity failed engraftment. The isolated BM cells from twi mice that had been treated with a 10X dose of AAV vector had GALC activity between 16 and 44 nmol/h/mg protein (Table 2). This is well above GALC activity in BM cells of wild type mice (Table 1). Transplantation failure was previously reported by Gentner et al
46
using high GALC-expressing BM cells following in vitro transduction by a lentiviral vector. As the transplantation failed, they concluded that higher than normal GALC activity must be toxic for hematopoietic stem cells and early progenitor cells. However, following the iv injection of 10X AAVrh10-mGALC, the GALC activity in BM cells is much higher than BM cells from wild type mice (Table 2) yet mice treated in this manner live much longer than twi mice treated by BMT or with 1X AAVrh10-mGALC alone. This indicates that their BM is functioning normally although it has very high GALC activity.
Discussion
KD is caused by the deficiency of GALC activity resulting from mutations in the GALC gene. Several animal models, including mice, dogs, and rhesus monkeys, have also been identified.
6-8
The mouse model of this disease has a mutation causing a premature stop codon in the GALC gene.
30
Mice homozygous for this mutation usually die between 35 and 45 days.
BMT treatment of twi mice has resulted in increased lifespan as reported by several studies.
23-26,28
Some authors believe that the effectiveness of BMT is mostly due to the ability of the BM cells to deliver GALC activity to the critical tissues,
25-27
while others consider their anti-inflammation properties as an additional beneficial factor.
20,27,28
Adding gene therapy to BMT has resulted in a much better outcome as reported by us and others.
10,19,20,27-29,47
However, the mechanism of such synergistic effect has not been clearly understood. As discussed below, the data presented in this paper suggest that HSCT/BMT may not be necessary for successful treatment of twi mice or Krabbe patients if a high dose of viral vector alone is timely delivered to the critical tissues. This will result in sufficient GALC expression in CNS before the accumulation of toxic metabolites that will trigger neuro-inflammation.
Timing of the pathological damages causing inflammation in the CNS of twi mice
Twi mice are indistinguishable from wild-type littermates until PND20-21 and have similar immune response capability as wild-type mice.
48
Neurologic features including tremors and ataxic gait appear around day 20.
49
Evidence of hypomyelination in the PNS also has been reported around PND15-20.
50
The earliest changes in microglial morphology was not seen until two weeks of age.
51
Therefore, twi mice treated with BMT may benefit from the anti-inflammatory role of the infiltrated macrophages in the CNS only when the disease has progressed. The same may be true in affected human patients. Supplying enough GALC activity at the earlier time, when the mouse or human patient is pre-symptomatic, could prevent disease progression and avert CNS inflammation (Figs. 4 and 5). This can be done by GALC-expressing macrophages produced by HSCT/BMT or by transducing endogenous macrophages by other strategies such as gene therapy. The advantage of in vivo virally-transduced macrophages is that they can rapidly deliver high GALC activity to degrade myelin debris and prevent globoid cell formation.
As noted in the Results section, the addition of indomethacin in the drinking water did not have any synergistic effect when these mice were also treated with gene therapy. These experiments may indicate the absence of neuro-inflammation at the time of the treatment if sufficient GALC is delivered to the CNS. Therefore, in addition to the anti-inflammatory role of HSCT/BMT discussed in our earlier study,
22
BMT also supplies some GALC activity when combined with viral gene therapy. Hence, with earlier delivery of sufficient GALC activity there may be less myelin degradation, neuro-inflammation and globoid cell formation.
We have recently shown that a single iv injection of AAVrh10-mGALC combined with BMT can result in comparable outcomes when implemented between PND10 and PND18 in twi mice.
29
The most consistent outcomes in our experiments are seen when mice are treated between PND10 and PND15. A similar pre-symptomatic period to start treatment may be available for human patients.
In vivo transduction of BM cells
Table 2 shows the increase in GALC activity of BM cells following iv injection of AAVrh10-mGALC with or without BMT. The GALC activity in BM cells of twi mice treated with 10X AAV alone is as high or higher than BM cells from twi mice treated with BMT plus 1X or 4X AAV. This could indicate that BMT is not needed to deliver GALC activity to nervous tissues. While it takes about seven days for the macrophages to be differentiated and infiltrated in the CNS following BMT,
52
it takes less time for endogenous BM cells to be transduced following iv viral injection and to reach the CNS after differentiation.
Avoiding the adverse side effects of the myeloablative regimens
The undesirable side effects of the myeloablative regimens required for HSCT/BMT are significant. In our experiments, all of the mice treated with busulfan before BMT have lower body weight, infertility, and depigmentation of their fur (Fig. 3). Similar side effects of busulfan treatment have also been reported by Yeager et al.
53
Busulfan and cyclophosphamide are currently the most widely used in the myeloablative regimen of allogeneic stem cell transplantation in human patients. In addition to side effects of these drugs, human transplant recipients can also suffer from varying degrees of graft-versus-host disease.
54
As can be seen in Figs. 2 and 3 mice treated with high dose gene therapy alone have the same weight, normal appearance and fertility as wild type mice. Therefore, the use of gene therapy alone at a sufficient dose and before neuro-inflammation has occurred may replace the need for blood stem cell transplantation.
Delivery of GALC activity to twi mice via gene therapy versus BMT
In the present study, we measured the GALC activity in certain tissues, including BM cells, in wild type mice, untreated twi mice as well as twi mice treated with combined therapy and 10X viral gene therapy alone (Table 2). Clearly, the BM cells of twi mice treated with gene therapy have higher GALC activity than wild type mice.
Our recent publication
29
showed that mice treated with BM cells from heterozygous mice plus 1X viral dose had decreased survival compared to when BM cells from wild-type mice were used. This suggested that BM cells from wild type mice were delivering more GALC activity to critical tissues. This is borne out by the data shown in Table 3. While the anti-inflammatory cytokines delivered by BM cells from wild-type and heterozygote mice should be similar, the GALC activity of BM cells from heterozygote mice is about half of the value from wild-type mice (Table 1). As shown in Fig. 1 the survival curve of these mice is comparable to the survival of the mice receiving BMT from wild-type mice but injected with1/2X viral dose. The GALC activity measured in the CNS of mice treated with a 1X viral dose plus BM cells from wild-type mice is higher than in mice treated with BM cells from wild type mice alone and mice treated with 1X viral dose and BM cells from heterozygous mice (Table 3). Therefore, GALC activity in nervous tissues of twi mice treated with combined therapy comes from both the viral vector transducing endogenous brain cells and macrophages recruited from the blood after BMT.
There appeared to be a failure of engraftment into twi mice when isolated BM cells expressing high GALC activity following iv AAVrh10-mGALC injection were used for transplantation. As mentioned in the Results section, similar transplantation failure was also reported by Gentner et al
46
when the authors used lentvirally-transduced BM cells with supra-normal GALC activity. The authors’ interpretation was that higher than normal GALC activity may be toxic for hematopoietic stem cells and early progenitor cells. Suppressing GALC expression in these cells via incorporation of the miR-126 sequence in the viral vector resulted in a successful transplantation. However, average survival with this treatment was only 88 days. In our experiments, all of the treatment approaches involving iv injection of AAVrh10-mGALC have resulted in higher than normal GALC expression in BM cells of the treated mice (Table 2) with no apparent toxicity. The reason for unsuccessful transplantation using BM cells over-expressing GALC activity may be caused by the bone structure and BM niche composition. As reported by Katayama and Frenette,
55
galcer, the main substrate for GALC enzyme, plays an essential role in BM niche structure and function. These authors have shown that galcer is crucial in establishing lymphoid-supportive niches and differentiation of lymphoid precursor cells in the BM. The synthesis of galcer is initiated by the addition of UDP-galactose to ceramide in a reaction catalyzed by UDP-galactose: ceramide galactosyltransferase (CGT). As shown by Katayama and colleagues, CGT-deficient mice lacking galcer exhibit aberrant nerve conduction and disturbed BM niche function.
56
In the case of supra-normal GALC expression, destruction of the BM niches via hydrolyzing BM galcer, could affect the BM’s engraftment. Interestingly, blocking GALC expression by “stem cell-specific miRNAs” enabled transplantation, not by preventing the cellular apoptosis caused by GALC toxicity, but by safeguarding the BM niches’ functionality and allowing engraftment.
46
Given that the mice treated with a 10X viral dose alone benefitted from an extension of healthy and active life, one can reasonably conclude that any treatment strategy must deliver an adequate amount of missing enzyme to the CNS and the PNS before pathology has occurred. Unlike the mice treated with a combination of gene therapy and BMT, these mice are fertile and capable of delivering several litters with up to nine pups in each litter. All of these pups are genetically affected when both parents are affected and treated with gene therapy alone.
It is noteworthy that current gene therapy research suggests that AAVrh10 and AAV9 are the most efficient viral vectors in crossing the BBB.
57
However, following systemic injection an overwhelming number of viral particles are trapped in peripheral organs and only a small fraction may reach the CNS and PNS. In the absence of an ideal viral vector with higher capability of crossing the BBB and transducing the critical cells, one practical way of eliminating BMT is by raising the viral dose. As demonstrated in this study, the iv injection of 4 ×1014 gc/kg body weight of AAVrh10-mGALC, has been tolerated very well in the mouse model of GLD with very encouraging outcomes and no apparent evidence of toxicity. Perhaps the viral dose may be increased even further without any toxicity. In support of the presented data, other recent publications have shown significant correction of neurologic disease using gene therapy alone without BM cell transplantation. When high dose AAV9 is injected intrathecally at an early time point, there is a significant extension of life in the dog model of KD without the need for BMT.
58
Previously, a single iv injection of rAAV vector containing aspartoacylase cDNA alone at an early age, showed significant effectiveness in treating the mouse model of Canavan disease.
59
Also, a single iv injection of a self-complementary AAV9 vector expressing the Hexb cDNA in Sandhoff disease mice has also been shown to be safe, prevent disease development and result in a normal lifspan.
60
However, some recent studies have documented the deaths of patients who received high-dose AAV gene therapy.
61
It should be noted that the patients who died following high-dose gene therapy had different diseases and pre-existing liver disease and were treated with different AAV vectors. While some pathological changes were documented in the livers of twi mice treated with combined BMT plus iv AAVrh10-mGALC, there was no evidence of neoplastic changes.
29
In conclusion, it appears that AAVrh10-mGALC injected iv at a high enough dose before significant pathology has taken place has the ability to deliver GALC activity to the CNS and PNS without the need for BMT/HSCT and to extend life without the side effects of HSCT. With further study, it may be possible to safely treat human patients with KD using only viral gene therapy.
Conclusion
Previous studies have shown that BMT together with a single intra-venous injection of AAVrh10-GALC results in a much better outcome than either treatment alone in twi mice. Those studies also showed that timing and dosing is critical to a successful outcome. It has been postulated that BM cells provide some GALC activity to many tissues as well providing an anti-inflammatory component to the treatment. The addition of the viral vector results in higher GALC activity being expressed in many tissues, including the central and peripheral nervous systems, leading to much improved myelination and greatly extended life in treated mice. As the drugs used for myelo-suppression before BMT have significant side effects, it was considered that the early injection of high dose viral vector alone could prevent the inflammatory component of this disease and therefore eliminate the need for BMT. The results show that when a viral dose of 4 ×1014 gc/kg is injected at PND10 there is a significant extension of life, about 7 times that of untreated mice, normal appearance and strength and normal fertility. In addition there is much less activated microglia/macrophages indicating less inflammation and less astrogliosis. These studies indicate that it may be possible to treat patients with KD with high dose viral vector alone without the need for blood stem cell transplantation and the drugs used in that therapy.
Acknowledgments
We thank Han Zhi Rao for her technical assistance.
Funding sources
This research was sponsored in part by a grant from The Legacy of Angels Foundation.
Ethical statement
The authors declare no ethical issue to be considered.
Competing interests
The authors declare no conflicts of interests.
Authors’ contribution
MAR, PL and DAW planned the studies, assisted in performing the necessary assays and analysis of the data. All contributed to the writing and editing of the manuscript.
Research Highlights
What is the current knowledge?
simple
-
√ KD is an autosomal recessive lysosomal disorder.
-
√ KD is caused by mutations in the GALC gene.
-
√ Low GALC activity causes defective myelination in the peripheral and central nervous systems.
-
√ HSCT in pre-symptomatic human patients is the only effective treatment at this time.
-
√ The twi mouse is a useful model to study pathogenesis and treatment of KD.
-
√ BMT has shown to have a synergistic effect when combined with other treatment strategies such as gene therapy in the animal models.
What is new here?
simple
-
√ Intra-venous (iv) injection of AAVrh10-mGALC gene alone at a sufficient viral dose and before neuro-inflammation has occurred resulted in extended normal life in twi mice.
-
√ Iv AAvrh10 gene therapy alone raised GALC activity in heart, brain, sciatic nerve and BM cells.
-
√ BM cells with high GALC activity from in vivo transduction were not successful in treating twi mice when used for BMT.
-
√ With further study, this approach may eliminate the need for HSCT and its side effects in human patients.
References
- Wenger DA, Rafi MA, Luzi P. Krabbe disease: One Hundred years from the bedside to the bench to the bedside. J Neurosci Res 2016; 94:982-9. doi: 10.1002/jnr.23743 [Crossref] [ Google Scholar]
- Wenger DA, Rafi MA, Luzi P. Molecular genetics of Krabbe disease (globoid cell leukodystrophy): diagnostic and clinical implications. Hum Mutat 1997; 10:268-79. doi:
10.1002/(SICI)1098-1004(1997)10:4<268::AID-HUMU2>3.0.CO;2-D [Crossref] [ Google Scholar]
- Miyatake T, Suzuki K. Globoid cell leukodystrophy: additional deficiency of psychosine galactosidase. Biochem Biophys Res Commun 1972; 48:539-43. doi: 10.1016/0006-291x(72)90381-6 [Crossref] [ Google Scholar]
- Svennerholm L, Vanier MT, Mansson JE. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J Lipid Res 1980; 21:53-64. [ Google Scholar]
- O'Sullivan C, Dev KK. Galactosylsphingosine (psychosine)-induced demyelination is attenuated by sphingosine 1-phosphate signalling. J Cell Sci 2015; 128:3878-87. doi: 10.1242/jcs.169342 [Crossref] [ Google Scholar]
- Kobayashi T, Yamanaka T, Jacobs JM, Teixeira F, Suzuki K. The Twitcher mouse: an enzymatically authentic model of human globoid cell leukodystrophy (Krabbe disease). Brain Res 1980; 202:479-83. doi: 10.1016/0006-8993(80)90159-6 [Crossref] [ Google Scholar]
- Baskin GB, Ratterree M, Davison BB, Falkenstein KP, Clarke MR, England JD. Genetic galactocerebrosidase deficiency (globoid cell leukodystrophy, Krabbe disease) in rhesus monkeys (Macaca mulatta). Lab Anim Sci 1998; 48:476-82. [ Google Scholar]
- Fletcher TF, Kurtz HJ, Low DG. Globoid cell leukodystrophy (Krabbe type) in the dog. J Am Vet Med Assoc 1966; 149:165-72. [ Google Scholar]
- Li Y, Sands MS. Experimental therapies in the murine model of globoid cell leukodystrophy. Pediatr Neurol 2014; 51:600-6. doi: 10.1016/j.pediatrneurol.2014.08.003 [Crossref] [ Google Scholar]
- Karumuthil-Melethil S, Marshall MS, Heindel C, Jakubauskas B, Bongarzone ER, Gray SJ. Intrathecal administration of AAV/GALC vectors in 10-11-day-old twitcher mice improves survival and is enhanced by bone marrow transplant. J Neurosci Res 2016; 94:1138-51. doi: 10.1002/jnr.23882 [Crossref] [ Google Scholar]
- Lin D, Fantz CR, Levy B, Rafi MA, Vogler C, Wenger DA. AAV2/5 vector expressing galactocerebrosidase ameliorates CNS disease in the murine model of globoid-cell leukodystrophy more efficiently than AAV2. Mol Ther 2005; 12:422-30. doi: 10.1016/j.ymthe.2005.04.019 [Crossref] [ Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Curtis MT, Wenger DA. Extended normal life after AAVrh10-mediated gene therapy in the mouse model of Krabbe disease. Mol Ther 2012; 20:2031-42. doi: 10.1038/mt.2012.153 [Crossref] [ Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Luddi A, Curtis MT, Wenger DA. Intravenous injection of AAVrh10-GALC after the neonatal period in twitcher mice results in significant expression in the central and peripheral nervous systems and improvement of clinical features. Mol Genet Metab 2015; 114:459-66. doi: 10.1016/j.ymgme.2014.12.300 [Crossref] [ Google Scholar]
- Rafi MA, Zhi Rao H, Passini MA, Curtis M, Vanier MT, Zaka M. AAV-mediated expression of galactocerebrosidase in brain results in attenuated symptoms and extended life span in murine models of globoid cell leukodystrophy. Mol Ther 2005; 11:734-44. doi: 10.1016/j.ymthe.2004.12.020 [Crossref] [ Google Scholar]
- Shen JS, Meng XL, Yokoo T, Sakurai K, Watabe K, Ohashi T. Widespread and highly persistent gene transfer to the CNS by retrovirus vector in utero: implication for gene therapy to Krabbe disease. J Gene Med 2005; 7:540-51. doi: 10.1002/jgm.719 [Crossref] [ Google Scholar]
- Krivit W, Shapiro EG, Peters C, Wagner JE, Cornu G, Kurtzberg J. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. N Engl J Med 1998; 338:1119-26. doi: 10.1016/j.bbmt.2019.09.003 [Crossref] [ Google Scholar]
- Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med 2005; 352:2069-81. doi: 10.1056/NEJMoa042604 [Crossref] [ Google Scholar]
-
Weinstock NI, Shin D, Dhimal N, Hong X, Irons EE, Silvestri NJ, et al. Macrophages Expressing GALC Improve Peripheral Krabbe Disease by a Mechanism Independent of Cross-Correction. Neuron 2020. 10.1016/j.neuron.2020.03.031
- Lin D, Donsante A, Macauley S, Levy B, Vogler C, Sands MS. Central nervous system-directed AAV2/5-mediated gene therapy synergizes with bone marrow transplantation in the murine model of globoid-cell leukodystrophy. Mol Ther 2007; 15:44-52. doi: 10.1038/sj.mt.6300026 [Crossref] [ Google Scholar]
- Reddy AS, Kim JH, Hawkins-Salsbury JA, Macauley SL, Tracy ET, Vogler CA. Bone marrow transplantation augments the effect of brain- and spinal cord-directed adeno-associated virus 2/5 gene therapy by altering inflammation in the murine model of globoid-cell leukodystrophy. J Neurosci 2011; 31:9945-57. doi: 10.1523/JNEUROSCI.1802-11.2011 [Crossref] [ Google Scholar]
- Suzuki K. Globoid cell leukodystrophy (Krabbe's disease): update. J Child Neurol 2003; 18:595-603. doi: 10.1177/08830738030180090201 [Crossref] [ Google Scholar]
- Luzi P, Abraham RM, Rafi MA, Curtis M, Hooper DC, Wenger DA. Effects of treatments on inflammatory and apoptotic markers in the CNS of mice with globoid cell leukodystrophy. Brain Res 2009; 1300:146-58. doi: 10.1016/j.brainres.2009.09.017 [Crossref] [ Google Scholar]
- Hoogerbrugge PM, Poorthuis BJ, Romme AE, van de Kamp JJ, Wagemaker G, van Bekkum DW. Effect of bone marrow transplantation on enzyme levels and clinical course in the neurologically affected twitcher mouse. J Clin Invest 1988; 81:1790-4. doi: 10.1172/JCI113521 [Crossref] [ Google Scholar]
- Hoogerbrugge PM, Suzuki K, Suzuki K, Poorthuis BJ, Kobayashi T, Wagemaker G. Donor-derived cells in the central nervous system of twitcher mice after bone marrow transplantation. Science 1988; 239:1035-8. doi: 10.1126/science.3278379 [Crossref] [ Google Scholar]
- Ichioka T, Kishimoto Y, Brennan S, Santos GW, Yeager AM. Hematopoietic cell transplantation in murine globoid cell leukodystrophy (the twitcher mouse): effects on levels of galactosylceramidase, psychosine, and galactocerebrosides. Proc Natl Acad Sci U S A 1987; 84:4259-63. [ Google Scholar]
- Yeager AM, Brennan S, Tiffany C, Moser HW, Santos GW. Prolonged survival and remyelination after hematopoietic cell transplantation in the twitcher mouse. Science 1984; 225:1052-4. doi: 10.1126/science.6382609 [Crossref] [ Google Scholar]
- Hawkins-Salsbury JA, Shea L, Jiang X, Hunter DA, Guzman AM, Reddy AS. Mechanism-based combination treatment dramatically increases therapeutic efficacy in murine globoid cell leukodystrophy. J Neurosci 2015; 35:6495-505. doi: 10.1523/JNEUROSCI.4199-14.2015 [Crossref] [ Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Wenger DA. Long-term Improvements in Lifespan and Pathology in CNS and PNS After BMT Plus One Intravenous Injection of AAVrh10-GALC in Twitcher Mice. Mol Ther 2015; 23:1681-90. doi: 10.1038/mt.2015.145 [Crossref] [ Google Scholar]
- Rafi MA, Luzi P, Wenger DA. Conditions for combining gene therapy with bone marrow transplantation in murine Krabbe disease. Bioimpacts 2020; 10:105-15. doi: 10.34172/bi.2020.13 [Crossref] [ Google Scholar]
- Sakai N, Inui K, Tatsumi N, Fukushima H, Nishigaki T, Taniike M. Molecular cloning and expression of cDNA for murine galactocerebrosidase and mutation analysis of the twitcher mouse, a model of Krabbe's disease. J Neurochem 1996; 66:1118-24. doi: 10.1046/j.1471-4159.1996.66031118.x [Crossref] [ Google Scholar]
- Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N, Wilson JM. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol 2001; 75:6199-203. [ Google Scholar]
- Passini MA, Wolfe JH. Widespread gene delivery and structure-specific patterns of expression in the brain after intraventricular injections of neonatal mice with an adeno-associated virus vector. J Virol 2001; 75:12382-92. doi: 10.1128/JVI.75.24.12382-12392.2001 [Crossref] [ Google Scholar]
- Wagner M, Koester H, Deffge C, Weinert S, Lauf J, Francke A. Isolation and intravenous injection of murine bone marrow derived monocytes. J Vis Exp 2014. doi: 10.3791/52347 [Crossref]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265-75. [ Google Scholar]
-
Wenger DA, Williams C. Screening for lysosomal disorders. In: Hommes FA, ed. Techniques in Diagnostic Human Biochemical Genetics. New York, NY: Wiley-Liss; 1991. p. 587-617.
- Scott-Hewitt NJ, Folts CJ, Hogestyn JM, Piester G, Mayer-Proschel M, Noble MD. Heterozygote galactocerebrosidase (GALC) mutants have reduced remyelination and impaired myelin debris clearance following demyelinating injury. Hum Mol Genet 2017; 26:2825-37. doi: 10.1093/hmg/ddx153 [Crossref] [ Google Scholar]
- Scott-Hewitt NJ, Folts CJ, Noble MD. Heterozygous carriers of galactocerebrosidase mutations that cause Krabbe disease have impaired microglial function and defective repair of myelin damage. Neural Regen Res 2018; 13:393-401. doi: 10.4103/1673-5374.228712 [Crossref] [ Google Scholar]
- Anjamrooz SH, Movahedin M, Mowla SJ, Bairanvand SP. Assessment of morphological and functional changes in the mouse testis and epididymal sperms following busulfan treatment. Iran Biomed J 2007; 11:15-22. [ Google Scholar]
- Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood 1996; 87:3045-52. [ Google Scholar]
- Hoffmann HM. Determination of reproductive competence by confirming pubertal onset and performing a fertility assay in mice and rats. J Vis Exp 2018(140):58352. doi: 10.3791/58352 [Crossref]
- Song L, Li X, Jayandharan GR, Wang Y, Aslanidi GV, Ling C. High-efficiency transduction of primary human hematopoietic stem cells and erythroid lineage-restricted expression by optimized AAV6 serotype vectors in vitro and in a murine xenograft model in vivo. PLoS One 2013; 8:e58757. doi: 10.1371/journal.pone.0058757 [Crossref] [ Google Scholar]
- Richter M, Stone D, Miao C, Humbert O, Kiem HP, Papayannopoulou T. In Vivo Hematopoietic Stem Cell Transduction. Hematol Oncol Clin North Am 2017; 31:771-85. doi: 10.1016/j.hoc.2017.06.001 [Crossref] [ Google Scholar]
- Zhong L, Li W, Li Y, Zhao W, Wu J, Li B. Evaluation of primitive murine hematopoietic stem and progenitor cell transduction in vitro and in vivo by recombinant adeno-associated virus vector serotypes 1 through 5. Hum Gene Ther 2006; 17:321-33. doi: 10.1089/hum.2006.17.321 [Crossref] [ Google Scholar]
- Song L, Kauss MA, Kopin E, Chandra M, Ul-Hasan T, Miller E. Optimizing the transduction efficiency of capsid-modified AAV6 serotype vectors in primary human hematopoietic stem cells in vitro and in a xenograft mouse model in vivo. Cytotherapy 2013; 15:986-98. doi: 10.1016/j.jcyt.2013.04.003 [Crossref] [ Google Scholar]
- Rafi MA, Fugaro J, Amini S, Luzi P, de Gala G, Victoria T. Retroviral vector-mediated transfer of the galactocerebrosidase (GALC) cDNA leads to overexpression and transfer of GALC activity to neighboring cells. Biochem Mol Med 1996; 58:142-50. doi: 10.1006/bmme.1996.0042 [Crossref] [ Google Scholar]
- Gentner B, Visigalli I, Hiramatsu H, Lechman E, Ungari S, Giustacchini A. Identification of hematopoietic stem cell-specific miRNAs enables gene therapy of globoid cell leukodystrophy. Sci Transl Med 2010; 2:58ra84. doi: 10.1126/scitranslmed.3001522 [Crossref] [ Google Scholar]
- Ricca A, Rufo N, Ungari S, Morena F, Martino S, Kulik W. Combined gene/cell therapies provide long-term and pervasive rescue of multiple pathological symptoms in a murine model of globoid cell leukodystrophy. Hum Mol Genet 2015; 24:3372-89. doi: 10.1093/hmg/ddv086 [Crossref] [ Google Scholar]
- Galbiati F, Basso V, Cantuti L, Givogri MI, Lopez-Rosas A, Perez N. Autonomic denervation of lymphoid organs leads to epigenetic immune atrophy in a mouse model of Krabbe disease. J Neurosci 2007; 27:13730-8. doi: 10.1523/JNEUROSCI.3379-07.2007 [Crossref] [ Google Scholar]
- Duchen LW, Eicher EM, Jacobs JM, Scaravilli F, Teixeira F. Hereditary leucodystrophy in the mouse: the new mutant twitcher. Brain 1980; 103:695-710. doi: 10.1093/brain/103.3.695 [Crossref] [ Google Scholar]
- Tanaka K, Nagara H, Kobayashi T, Goto I. The twitcher mouse: accumulation of galactosylsphingosine and pathology of the sciatic nerve. Brain Res 1988; 454:340-6. doi: 10.1016/0006-8993(88)90835-9 [Crossref] [ Google Scholar]
- Snook ER, Fisher-Perkins JM, Sansing HA, Lee KM, Alvarez X, MacLean AG. Innate immune activation in the pathogenesis of a murine model of globoid cell leukodystrophy. Am J Pathol 2014; 184:382-96. doi: 10.1016/j.ajpath.2013.10.011 [Crossref] [ Google Scholar]
- Duran-Struuck R, Dysko RC. Principles of bone marrow transplantation (BMT): providing optimal veterinary and husbandry care to irradiated mice in BMT studies. J Am Assoc Lab Anim Sci 2009; 48:11-22. [ Google Scholar]
- Yeager AM, Shinn C, Farmer ER, Wingard JR, Yeager MJ. Growth retardation and depigmentation of hair after high-dose busulfan and congenic hematopoietic cell transplantation in mice. Bone Marrow Transplant 1992; 9:199-204. [ Google Scholar]
- Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J 2011; 1:e16. doi: 10.1038/bcj.2011.14 [Crossref] [ Google Scholar]
- Katayama Y, Frenette PS. Galactocerebrosides are required postnatally for stromal-dependent bone marrow lymphopoiesis. Immunity 2003; 18:789-800. doi: 10.1016/s1074-7613(03)00150-x [Crossref] [ Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006; 124:407-21. [ Google Scholar]
- Tanguy Y, Biferi MG, Besse A, Astord S, Cohen-Tannoudji M, Marais T. Systemic AAVrh10 provides higher transgene expression than AAV9 in the brain and the spinal cord of neonatal mice. Front Mol Neurosci 2015; 8:36. doi: 10.3389/fnmol.2015.00036 [Crossref] [ Google Scholar]
- Bradbury AM, Bagel JH, Nguyen D, Lykken EA, Pesayco Salvador J, Jiang X. Krabbe disease successfully treated via monotherapy of intrathecal gene therapy. J Clin Invest 2020; 130:4906-20. doi: 10.1172/JCI133953 [Crossref] [ Google Scholar]
- Ahmed SS, Li H, Cao C, Sikoglu EM, Denninger AR, Su Q. A single intravenous rAAV injection as late as P20 achieves efficacious and sustained CNS Gene therapy in Canavan mice. Mol Ther 2013; 21:2136-47. doi: 10.1038/mt.2013.138 [Crossref] [ Google Scholar]
- Niemir N, Rouviere L, Besse A, Vanier MT, Dmytrus J, Marais T. Intravenous administration of scAAV9-Hexb normalizes lifespan and prevents pathology in Sandhoff disease mice. Hum Mol Genet 2018; 27:954-68. doi: 10.1093/hmg/ddy012 [Crossref] [ Google Scholar]
- High-dose AAV gene therapy deaths. Nat Biotechnol 2020; 38:910. doi: 10.1038/s41587-020-0642-9 [Crossref] [ Google Scholar]