Bioimpacts. 12(1):3-7.
doi: 10.34172/bi.2021.23931
Mini Review
Krabbe disease: A personal perspective and hypothesis
Mohammad A. Rafi * 
Author information:
Emeritus Professor, Department of Neurology, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, PA 19107, USA
Abstract
Introduction:
Krabbe disease (KD) or globoid cell leukodystrophy (GLD) is one of the lysosomal disorders affecting central and peripheral nervous systems (CNS and PNS). It is caused by mutations on the galactocerebrosidase (GALC) gene. Affected individuals accumulate undegraded substrates and suffer from neuroinflammation.
Methods:
Hematopoietic stem cell transplantation (HSCT) has been partially successful in treating patients with KD when accomplished prior to the onset of symptoms. The success is credited to the ability of the hematopoietic stem cells in providing some GALC enzyme to the CNS and eradicating potential neuroinflammation. Combination of the HSCT with some other GALC-providing strategies has shown synergistic effects in the treatment of the mouse model of this disease.
Results:
Here, the possibility of eliminating HSCT in the treatment of human patients and replacing it with a single therapy that will provide sufficient GALC enzyme to the nervous systems is suggested. Such treatment, if started during the asymptomatic stage of the disease, not only may eradicate the enzyme deficiency, but may also keep any neuroinflammation at bay.
Conclusion:
Successful treatment of the KD may be possible by restoring consistent and sufficient GALC expression in CNS and PNS.
Keywords: Krabbe disease, GLD, GALC, Gene therapy, Bone marrow transplantation, BMT
Copyright and License Information
© 2022 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Krabbe disease (KD) or globoid cell leukodystrophy (GLD) is a recessive genetic disease caused by the deficiency of a lysosomal enzyme called galactocerebrosidase (GALC). KD is a neurodegenerative disorder with progressive demyelination and oligodendrocytes apoptosis. While there are late onset and adult forms of this disease, it is primarily an infantile malady. Affected infants have no symptoms at birth but begin to develop signs of disease before about six months of age. Irritability and stiffness increase with time and the affected, untreated child rarely lives beyond two years of age.
1
There are several animal models for this disease.
2
Twitcher (twi) mouse, the murine model of the KD has been the subject of many treatment trials. Bone marrow transplantation (BMT) was the first treatment strategy applied in twi mice in 1984
3
and this resulted in a significant extension of life. Human patients have been treated by hematopoietic stem cell transplantation (HSCT)
4
and this treatment has become the standard of care since then. The other treatment trials practiced in twi mice include neural progenitor/neural stem cell transplantation,
5-7
mesenchymal stem cell transplantation,
8
pharmacological chaperone therapy,
9
substrate reduction therapy,
10
enzyme replacement therapy,
11,12
antioxidant therapy,
13
anti-inflammatory therapies,
14
gene therapy,
15-19
and various combinations of these treatments.
20-28
Among these combinatorial therapies, some demonstrated synergistic effect in improving the lifespan of the treated mice. At the same time, some of these treatments resulted in little, if any, increase in lifespan and some had significant side effects limiting their use in humans. A closer look at these combinational therapies applied reveals that the synergistic effect occurs when both treatments involved in the combination, either contributed to the enzyme delivery to the CNS
20-25
or played a role in the substrate reduction approach.
26
These findings highlight the importance of delivering sufficient GALC to the nervous system or reducing the undegraded materials. Such a crucial role of GALC has been demonstrated recently in twi mice
29,30
and the canine model of GLD.
31
Based on the results of the different combinational therapies, outcomes of the recent studies, and serious side effects of myeloablation required for cell transplantation, the aim of this article is to hypothesize that BMT/HSCT can entirely be eliminated from GLD treatment without being excessively concerned about the neuroinflammation or other secondary hits, such as psychosine toxicity. This, of course, requires supplying adequate GALC activity by another approach, such as gene therapy, in a timely manner.
Role of BMT in combination with any enzyme supplying methodology
Combination of BMT and gene therapy or any other type of enzyme delivery system to the CNS, has shown strong synergistic effect reported by us and other colleagues.
19,20
The mechanism of such synergistic effect has also been the subject of multiple studies
20,21
with no clear conclusion. In previous publications, we have emphasized the anti-inflammatory role of BMT/HSCT in the treatment of the twi mice or human patients.
14-19
In our latest publications,
29,30
we have also acknowledged the GALC delivery capacity and anti-inflammatory properties of BM cells. However, here, we are highlighting the GALC delivery role of BMT over its anti-inflammatory function. The GALC delivery capability of BMT combined with another GALC source appear to exhibit either additive or synergistic effects in these experiments without involvement of any anti-inflammatory action from BM cells. The logic behind such hypotheses is that there is no plausible neuroinflammation at the early stage of the disease. In other words, the anti-inflammation property of the BM cells is still there but not in use because of the timing. As we have shown previously, a) treatment of the twi mice with anti-inflammatory drugs failed to demonstrate any positive outcome in treating these mice at a young age,
14
b) decreasing viral dose in half with regular BMT, compared to injecting full viral dose along with BMT using BM cells from heterozygote mice resulted in similar reduced lifespan (Fig. 1).
29
This confirms the pivotal role of GALC activity provided by BMT and gene therapy. Another point worth mentioning here, is that AAVrh10, and potentially some other AAV serotypes, is/are capable of in vivo and in vitro transducing the BM cells.
30
This is an additional reason for eliminating BMT in combination with gene therapy, as we are hitting two targets when using high dose gene therapy alone: delivering enzyme to the nervous system directly and via the transduced BM cells indirectly.
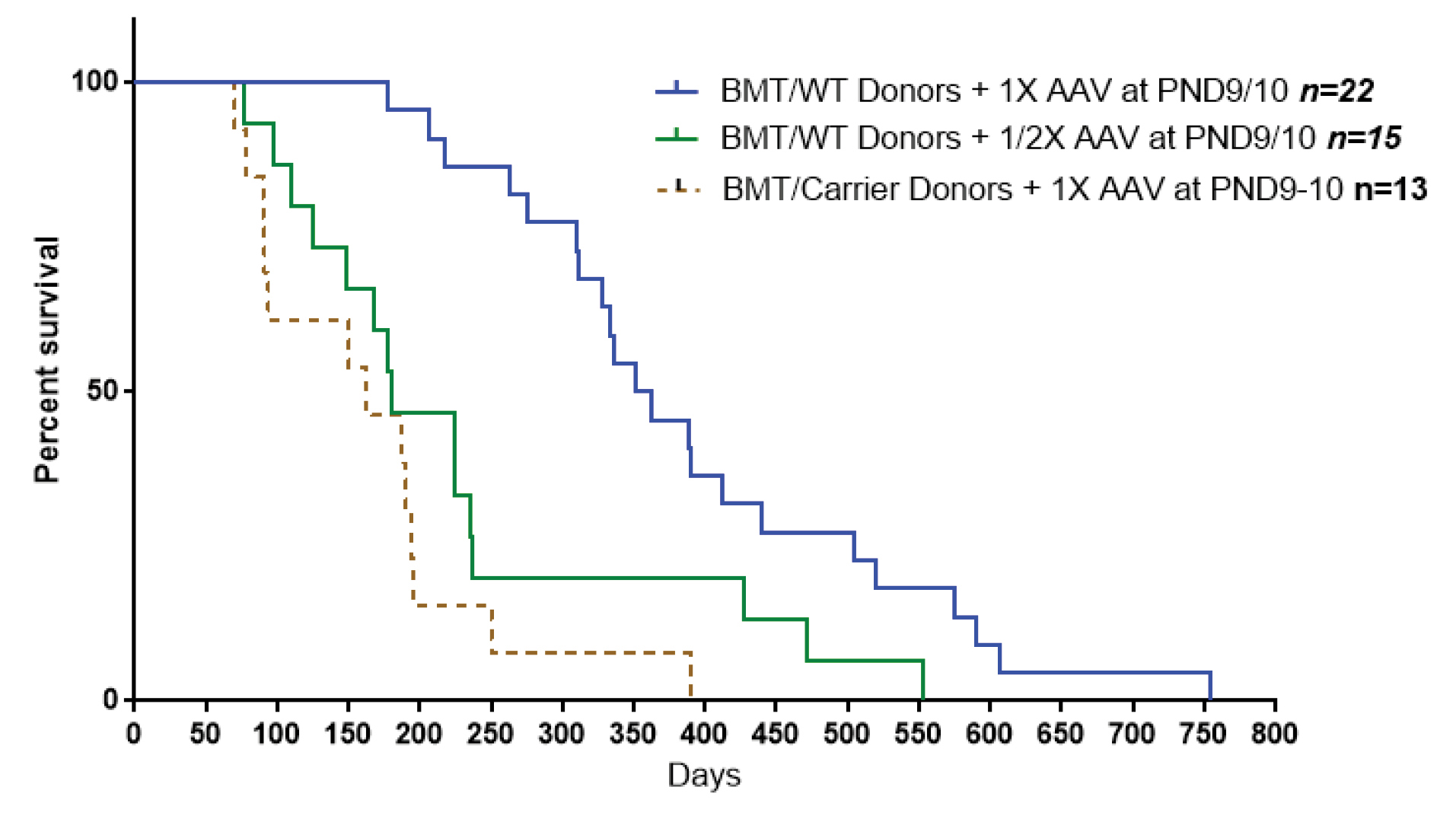
Fig. 1.
Cutting viral dose in half or using BM cells from heterozygote mouse in a combination of BMT and gene therapy treatments results in a similar reduction in lifespan of the treated mice.
30
.
Cutting viral dose in half or using BM cells from heterozygote mouse in a combination of BMT and gene therapy treatments results in a similar reduction in lifespan of the treated mice.
30
Importance of the enzyme level in the CNS and time of the treatment
Enzyme level: As the ability of the current AAV serotypes in crossing the blood brain barrier (BBB) is limited,
32
the level of GALC delivered to the CNS will directly depend on the number of the viral particles reaching the brain. With high viral dose, as shown by us
30
and Bradbury et al,
31
there is higher GALC activity in the CNS, meaning that a higher number of viral particles have reached the brain, resulting in a much better outcome. While the level of the supplied enzyme to the nervous systems is pivotal for successful therapy, the goal should be supplying enough GALC activity to the nervous system not overwhelming it.
Treatment time: An encouraging point about this disease is that both human patients and animal models enjoy an asymptomatic period after birth. All human patients and animal models of GLD are normal at birth until a certain time, depending on the species. No pathological abnormalities, such as CNS inflammation, nor increased psychosine, seem to be at an alarming level during these asymptomatic periods. This timeframe provides a sufficient window of opportunity to initiate treatments in a preventive manner. To take advantage of this asymptomatic period, the at-risk children should be determined as soon as possible. Such identification is being done by newborn screening (NBS) programs in several states of the USA.
33
In the case of the twi mice, the symptoms appear at postnatal day 21 (PND21). Therefore, the safe period for any intervention will be from PND0 until about PND18. For instance, we have shown successful results when treatment was completed between PND8 to PND18. It’s reasonably assumed that for any treatment, earlier is better. This approach may prevent pathological changes in the CNS and PNS prior to becoming clinically intractable and irreversible. However, when there is a wide window of treatment opportunity, determining the optimal window of opportunity may be preferable to avoid potential complications. For instance, treatment started in utero, may be possible but could result in unforeseen complications.
Treatment options
While the goal is to find a safe and efficacious therapy for human patients, for the sake of simplicity and immediate evaluation of the potential outcomes, the treatment options are largely discussed on twi mice. Furthermore, as the most promising way of supplying GALC enzyme to the CNS seems to be under the umbrellas of gene therapy and gene editing technologies, here, the discussion will be limited to the gene therapy options. While gene therapy has great potential for the treatment of neurological diseases, the biggest hurdle has been the transfer of the viral vectors to the CNS. The BBB blocks delivery of most drugs to the brain. Currently, several newly developed AAV serotypes such as AAV9 and AAVrh10 and their variants such as AAV-PHP.B and AAV-PHP.eB can cross the BBB of mice though with low efficiency.
32
Most of the injected viral vectors get trapped in peripheral organs such as the heart, skeletal muscles, and liver. Only a fraction of these viral particles succeeds in crossing the BBB and reaching the brain tissues. Therefore, the ideal solution for the current hurdle will be developing viral vectors with a higher capacity of crossing the BBB. However, in the absence of such viral vectors, alternative potentially useful approaches can be considered:
simple
-
1) Increasing the viral load: We have recently demonstrated the enhancing effect of such an increase.
29,30
Our regular dose of intravenous injection of AAVrh10-mGALC in twi mice was 4×1013gc/kg of body weight. With this injection dose without BMT, the lifespan of the twi mice was increased from about 40 days to 72 days. A four-fold and ten-fold increase of this injection dose (1.6×1014gc/kg and 4×1014gc/kg), raised the median lifespan of the twi mice to 180 days and 280 days respectively without any apparent toxicity. The 4×1014gc/kg dose may not be the upper limit that could be administered. However, at some point, toxicity may become a factor to be considered.
-
2) Double injection: If increasing viral dose over 4×1014gc/kg is not tolerated, because of immune system constraints or risk of neoplastic changed in the liver, the same dose can be injected in two different intervals (e.g., PND10 and 15 in twi mice). Of course, the second injection needs to be done in a short interval before any potential immune reactions develop after the first injection.
-
3) Combination of two viral serotypes: As discussed above, currently, AAVrh10 and AAV9 are the two available serotypes that can cross the BBB more efficiently than the other serotypes. However, the mechanisms utilized by these viruses to enter the CNS are unknown.
32
If injected within a safe time frame (for instance, PND10 and 15 in twi mice), it may result in a better outcome. The advantage of this method, relative to the multiple injections of one serotype, is that different serotypes may utilize alternating or distinct pathways in crossing the BBB and, therefore, using different serotypes will increase the overall delivery rate to the CNS while decreasing any potential of immune response.
-
4) Mobilizing the innate BM cells before viral injection: AAVrh10 is capable of in vivo transducing the BM cells.
30
It has also been shown that mobilizing the BM cells into the bloodstream significantly increases the transduction rate after adenovirus injection.
34
This could happen for the AAV viral vectors too. The transduced cells return to the bone marrow compartment for engraftment.
34
-
5) Temporarily opening the BBB prior to viral injection: It has been known that the BBB can be temporarily opened for drug delivery. Focused ultrasound strategy is one such method that can transiently increase BBB permeability for certain impenetrable drugs.
35
Mannitol mediated osmotic destruction of the BBB is another strategy to assist viral vectors entering the CNS. Such approach has been shown effective in treatment of type IIIB mucopolysaccharide disease.
36
-
6) Generating an engineered AAV-GALC construct: Recently, several studies have demonstrated that molecular engineering of a transgene significantly enhances the ability of the viral construct to cross the BBB. Addition of the signal peptide from the highly secreted iduronate-2-sulfatase and the BBB-binding domain from the Apolipoprotein B (ApoB-BD) to the viral construct have been reported in mouse model of mucopolysaccharidoses type IIIA to increase the expression of the transgene and facilitate crossing the BBB.
37
Similar improvement in crossing BBB has also been shown in twi mice.
38,39
Such methodologies can be combined with higher viral dose injection to improve the outcome.
-
7) Developing a carrier-mediated method of crossing the BBB: Several carrier-mediated strategies have been developed to assist the viral particles in crossing the BBB. Some of these practical strategies have been summarized by Bellettato and Scarpa.
40
In receptor-mediated transcytosis approaches, viral vectors interact directly with extracellular receptors of the capillary endothelial cells to facilitate crossing the BBB.
32
Discussion
The initial idea of BMT in treatment of the mouse model of KD, was based on the ability of the BM cells to secrete the missing enzyme that can be taken up by the enzyme-deficient cells via cross-correction.
3
While the in vivo “cross-correction” of GALC has been recently questioned by Weinstock et al,
41
independent of the mechanism of BMT, as shown by us
16-19
and others,
42
delivering sufficient GALC to the CNS and PNS via any methodology is the key for the effective treatment of KD. Such delivery should be accomplished at the pre-symptomatic stage to prevent accumulation of the toxic materials. Currently, HSCT is the “standard of care” treatment for human patients. However, newer technologies of delivering GALC to the nervous system have emerged and their application alone or in combination with BMT in animal models of KD have been the subject of many studies. These technologies, including enzyme replacement therapy, neural-stem cell transplantation, as well as gene therapy, have improved our understanding of the disease and helped to better recognize the unmet needs for treating this disease. Combination of these new methods with BMT in animal models, revealed an interesting synergistic effect on the lifespan of the treated mice. These very encouraging results in animal models paved the way for the current clinical trial in humans (Forge Biologics, see NCT04693598). However, with such promising combinational therapy on human patients, the study on the animal models did not stop. While acknowledging the anti-inflammatory property of the HSCT, its synergistic effect with other treatments highlights the importance of supplying extra GALC enzyme to the nervous system. Considering the side effects of the BMT/HSCT, such as infertility and overall abnormal growth, replacing it with other enzyme-providing treatment options may be preferable. Two independent studies in two different animal models of KD
30,31
have already demonstrated that increasing GALC expression in the CNS and PNS via gene therapy, can substitute the GALC delivery share of the BMT and, therefore, have validated that BMT can completely be eliminated from the combinational therapies.
Conclusion
According to the current state of the knowledge and what was discussed above, it appears that exogenous BMT in animal models of KD or HSCT in human patients may soon be obsolete. Restoring consistent and sufficient GALC expression in the CNS and PNS via any methodology may be a safe and efficacious treatment for KD. The subject remains for further validation.
Acknowledgments
The author would like to thank Dr. David A. Wenger, Professor of Neurology at Thomas Jefferson University, and Dr. M. Reza Sadaie, Consultant, Senior Biomedical Scientist at NovoMed Consulting, for their insight and editing of the manuscript.
Funding sources
The study was partially funded by Legacy of Angels Foundation.
Ethical statement
There is no ethical issue to be considered.
Competing interests
The author declares no conflicts of interest.
Author’s Note
My interest in Krabbe disease began in 1987 when I joined Professor David A. Wenger’s laboratory at Thomas Jefferson University, Philadelphia. This was soon after Dr. Wenger had established the Diagnostic and Research Laboratories for lysosomal storage diseases. While we initiated working on several lysosomal disorders, our research was mostly focused on Krabbe disease. The breakthrough was when Dr. Yue Qun Chen joined our research team. With his pivotal efforts, we purified and characterized GALC, the missing enzyme in Krabbe disease. This opened the way for the cloning of cDNA of this protein for human and several animal models of this disease. Since then, beside the continuous efforts toward disease-related studies such as characterization of the gene and mutational analysis, the aim was focused on exploring the treatment options for this devastating disease. In this brief article, shortly after my retirement, I wanted to share my experiences and views and provide hypothesis toward potential enhancements of the current clinical trial in humans.
Review Highlights
What is the current knowledge?
simple
-
√ BMT/HSCT has the capacity of providing some GALC activity to the CNS.
-
√ The success of BMT/HSCT was mostly credited to the ability of the BM cells in eradicating neuroinflammation in the CNS.
What is new here?
simple
-
√ The positive role of BMT/HSCT relies on its ability to provide substantial amount of GALC enzyme to the CNS and PNS.
-
√ AAVrh10 is capable of in vivo transduction of bone marrow cells.
-
√ BMT/HSCT may be eliminated completely by supplying sufficient GALC activity to the CNS and PNS via any other methodologies.
References
- Wenger, DA, Escolar, ML, Luzi, P, Rafi, MA. Krabbe disease (globoid cell leukodystrophy). In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, eds. The Online Metabolic and Molecular Bases of Inherited Diseases. McGraw Hill; 2013.
- Wenger DA. Murine, canine and non-human primate models of Krabbe disease. Mol Med Today 2000; 6:449-51. [ Google Scholar]
- Yeager AM, Brennan S, Tiffany C, Moser HW, Santos GW. Prolonged survival and remyelination after hematopoietic cell transplantation in the twitcher mouse. Science 1984; 225:1052-4. doi: 10.1126/science.6382609 [Crossref] [ Google Scholar]
- Krivit W, Shapiro EG, Peters C, Wagner JE, Cornu G, Kurtzberg J. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. N Engl J Med 1998; 338:1119-1126. [ Google Scholar]
- Taylor RM, Snyder EY. Widespread engraftment of neural progenitor and stem-like cells throughout the mouse brain. Transplant Proc 1997; 29:845-847. [ Google Scholar]
- Strazza M, Luddi A, Carbone M, Rafi MA, Costantino-Ceccarini E, Wenger DA. Significant correction of pathology in brains of twitcher mice following injection of genetically modified mouse neural progenitor cells. Mol Genet Metab 2009; 97:27-34. [ Google Scholar]
- Neri M, Ricca A, di Girolamo I, Alcala’-Franco B, Cavazzin C, Orlacchio A. Neural stem cell gene therapy ameliorates pathology and function in a mouse model of globoid cell leukodystrophy. Stem Cells 2011; 29:1559-1571. [ Google Scholar]
- Ripoll Ripoll, CB CB, Flaat Flaat, M M, Klopf-Eiermann Klopf-Eiermann, J J, Fisher-Perkins Fisher-Perkins, JM JM, Trygg Trygg, CB CB, Scruggs Scruggs. Mesenchymal lineage stem cells have pronounced anti-inflammatory effects in the twitcher mouse model of Krabbe’s disease. Stem Cells 2011; 29:67-77. [ Google Scholar]
- Berardi Berardi, AS AS, Pannuzzo Pannuzzo, G G, Graziano Graziano, A A, Costantino-Ceccarini Costantino-Ceccarini, E E, Piomboni Piomboni, P and Luddi, A A. Pharmacological chaperones increase residual β-galactocerebrosidase activity in fibroblasts from Krabbe patients. Mol Genet Metab 2014; 112:294-301. [ Google Scholar]
- Biswas S, LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr Res 2002; 51:40-47. [ Google Scholar]
- Lee WC, Courtenay A, Troendle FJ, Stallings-Mann ML, Dickey CA, DeLucia MW. Enzyme replacement therapy results in substantial improvements in early clinical phenotype in a mouse model of globoid cell leukodystrophy. FASEB J 2005; 19:1549-1551. [ Google Scholar]
- Lee WC, Tsoi YK, Troendle FJ, DeLucia MW, Ahmed Z, Dicky CA. Single-dose intracerebroventricular administration of galactocerebrosidase improves survival in a mouse model of globoid cell leukodystrophy. FASEB J 2007; 21:2520-2527. [ Google Scholar]
- Paintlia MK, Singh I, Singh AK. Effect of vitamin D3 intake on the onset of disease in murine model of human Krabbe disease. J Neurosici Res 2015; 93:28-42. [ Google Scholar]
- Luzi P, Abraham RM, Rafi MA, Curtis M, Hooper DC, Wenger DA. Effects of treatments on inflammatory and apoptotic markers in the CNS of mice with globoid cell leukodystrophy. Brain Res 2009; 1300:146-58. doi: 10.1016/j.brainres.2009.09.017 [Crossref] [ Google Scholar]
- Rafi MA, Zhi Rao H, Passini MA, Curtis M, Vanier MT, Zaka M. AAV-mediated expression of galactocerebrosidase in brain results in attenuated symptoms and extended life span in murine models of globoid cell leukodystrophy. Mol Ther 2005; 11:734-44. doi: 10.1016/j.ymthe.2004.12.020 [Crossref] [ Google Scholar]
- Lin D, Fantz CR, Levy B, Rafi MA, Vogler C, Wenger DA. AAV2/5 vector expressing galactocerebrosidase ameliorates CNS disease in the murine model of globoid-cell leukodystrophy more efficiently than AAV2. Mol Ther 2005; 12:422-30. doi: 10.1016/j.ymthe.2005.04.019 [Crossref] [ Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Curtis MT, Wenger DA. Extended normal life after AAVrh10-mediated gene therapy in the mouse model of Krabbe disease. Mol Ther 2012; 20:2031-42. doi: 10.1038/mt.2012.153 [Crossref] [ Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Luddi A, Curtis MT, Wenger DA. Intravenous injection of AAVrh10-GALC after the neonatal period in twitcher mice results in significant expression in the central and peripheral nervous systems and improvement of clinical features. Mol Genet Metab 2015; 114:459-66. doi: 10.1016/j.ymgme.2014.12.300 [Crossref] [ Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Wenger DA. Long-term Improvements in Lifespan and Pathology in CNS and PNS After BMT Plus One Intravenous Injection of AAVrh10-GALC in Twitcher Mice. Mol Ther 2015; 23:1681-90. doi: 10.1038/mt.2015.145 [Crossref] [ Google Scholar]
- Lin Lin, D D, Donsante Donsante, A A, Macauley Macauley, S S, Levy Levy, B B, Vogler Vogler, C and Sands, MS (2007). Central nervous system-directed AAV2/5-mediated gene therapy synergizes with bone marrow transplantation in the murine model of globoid-cell leukodystrophy. Mol Ther 2007; 15:44-52. [ Google Scholar]
- Reddy AS, Kim JH, Hawkins-Salsbury JA, Macauley SL, Tracy ET, Vogler CA. Bone marrow transplantation augments the effect of brain- and spinal cord-directed adeno-associated virus 2/5 gene therapy by altering inflammation in the murine model of globoid-cell leukodystrophy. J Neurosci 2011; 31:9945-57. doi: 10.1523/JNEUROSCI.1802-11.2011 [Crossref] [ Google Scholar]
- Galbiati Galbiati, F F, Givogri Givogri, MI MI, Cantuti Cantuti, L L, Rosas Rosas, AL AL, Cao Cao, H H, van Breemen. Combined hematopoietic and lentiviral gene-transfer therapies in newborn Twitcher mice reveal contemporaneous neurodegeneration and demyelination in Krabbe disease. J Neurosci Res 2009; 87:1748-1759. [ Google Scholar]
- Qin EY, Hawkins-Salsbury JA, Jiang X, Reddy AS, Farber NB, Ory DS. Bone marrow transplantation increases efficacy of central nervous system-directed enzyme replacement therapy in the murine model of globoid cell leukodystrophy. Mol Genet Metab 2012; 107:186-196. [ Google Scholar]
- Hawkins-Salsbury JA, Shea L, Jiang X, Hunter DA, Guzman AM, Reddy AS. Mechanism-based combination treatment dramatically increases therapeutic efficacy in murine globoid cell leukodystrophy. J Neurosci 2015; 35:6495-6505. [ Google Scholar]
- Ricca A, Rufo N, Ungari S, Morena F, Martino S, Kulik W. Combined gene/cell therapies provide long-term and pervasive rescue of multiple pathological symptoms in a murine model of globoid cell leukodystrophy. Hum Mol Genet 2015; 15; 24:3372-89. doi: 10.1093/hmg/ddv086 [Crossref] [ Google Scholar]
- Biswas S, LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr Res 2002; 51:40-47. [ Google Scholar]
- Hawkins-Salsbury JA, Qin EY, Reddy AS, Vogler CA, Sands MS. Oxidative stress as a therapeutic target in globoid cell leukodystrophy. Exp Neurol 2012; 237:444-52. doi: 10.1016/j.expneurol.2012.07.013 [Crossref] [ Google Scholar]
- Young PP, Fantz CR, Sands MS. VEGF disrupts the neonatal blood-brain barrier and increases life span after non-ablative BMT in a murine model of congenital neurodegeneration caused by a lysosomal enzyme deficiency. Exp Neurol 2004; 188:104-14. doi: 10.1016/j.expneurol.2004.03.007 [Crossref] [ Google Scholar]
- Rafi MA, Luzi P, Wenger DA. Conditions for combining gene therapy with bone marrow transplantation in murine Krabbe disease. Bioimpacts 2020; 10:105-15. doi: 10.34172/bi.2020.13 [Crossref] [ Google Scholar]
- Rafi MA, Luzi P, Wenger DA. Can early treatment of twitcher mice with high dose AAVrh10-GALC eliminate the need for BMT?. Bioimpacts 2021; 11:135-46. doi: 10.34172/bi.2021.21 [Crossref] [ Google Scholar]
- Bradbury AM, Bagel JH, Nguyen D, Lykken EA, Pesayco Salvador J, Jiang X. Krabbe disease successfully treated via monotherapy of intrathecal gene therapy. J Clin Invest 2020; 130:4906-20. doi: 10.1172/JCI133953 [Crossref] [ Google Scholar]
- Liu D, Zhu M, Zhang Y, Diao Y. Crossing the blood-brain barrier with AAV vectors. Metab Brain Dis 2021; 36:45-52. doi: 10.1007/s11011-020-00630 [Crossref] [ Google Scholar]
- Gelb MH. Newborn Screening for Lysosomal Storage Diseases: Methodologies, Screen Positive Rates, Normalization of Datasets, Second-Tier Tests, and Post-Analysis Tools. Int J Neonatal Screen 2018; 4:23. doi: 10.3390/ijns4030023 [Crossref] [ Google Scholar]
- Richter M, Saydaminova K, Yumul R, Krishnan R, Liu J, Nagy EE. In vivo transduction of primitive mobilized hematopoietic stem cells after intravenous injection of integrating adenovirus vectors. Blood 2016; 128:2206-17. [ Google Scholar]
- Burgess A, Shah K, Hough O, Hynynen K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev Neurother 2015; 15:477-91. doi: 10.1586/14737175.2015.1028369 [Crossref] [ Google Scholar]
- McCarty DM, DiRosario J, Gulaid K, Muenzer J, Fu H. Mannitol-facilitated CNS entry of rAAV2 vector significantly delayed the neurological disease progression in MPS IIIB mice. Gene Ther 2009; 16:1340-52. doi: 10.1038/gt.2009.85 [Crossref] [ Google Scholar]
- Sorrentino NC, D'Orsi L, Sambri I, Nusco E, Monaco C, Spampanato C. A highly secreted sulphamidase engineered to cross the blood-brain barrier corrects brain lesions of mice with mucopolysaccharidoses type IIIA. EMBO Mol Med 2013; 5:675-90. [ Google Scholar]
- Pan X, Sands SA, Yue Y, Zhang K, LeVine SM, Duan D. An Engineered Galactosylceramidase Construct Improves AAV Gene Therapy for Krabbe Disease in Twitcher Mice. Hum Gene Ther 2019; 9:1039-1051. [ Google Scholar]
- Ricca A, Cascino F, Morena F, Martino S, Gritti A. In vitro Validation of Chimeric β-Galactosylceramidase Enzymes with Improved Enzymatic Activity and Increased Secretion. Front Mol Biosci 2020; 7:167. doi: 10.3389/fmolb.2020.00167 [Crossref] [ Google Scholar]
- Bellettato CM, Scarpa M. Possible strategies to cross the blood-brain barrier. Ital J Pediatr 2018; 44(Suppl 2):131. doi: 10.1186/s13052-018-0563-0 [Crossref] [ Google Scholar]
- Weinstock NI, Shin D, Dhimal N, Hong X, Irons EE, Silvestri NJ. Macrophages Expressing GALC Improve Peripheral Krabbe Disease by a Mechanism Independent of Cross-Correction. Neuron 2020; 107:65-81. doi: 10.1016/j.neuron.2020.03.031 [Crossref] [ Google Scholar]
- Mikulka CR, Sands MS. Treatment for Krabbe's disease: Finding the combination. J Neurosci Res 2016; 94:1126-37. doi: 10.1002/jnr.23822 [Crossref] [ Google Scholar]