Bioimpacts. 14(4):28902.
doi: 10.34172/bi.2023.28902
Original Article
Design and implementation of a lab-on-a-chip for assisted reproductive technologies
Firooz Safaefar Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing, 1 
Javad Karamdel Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 2, * 
Hadi Veladi Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, 3, * 
Masoud Maleki Formal analysis, Methodology, Resources, Validation, Visualization, 1, 4 
Author information:
1Department of Biomedical Engineering, Faculty of Technical and Engineering, South Tehran Branch, Islamic Azad University, Tehran, Iran
2Department of Electrical Engineering, Faculty of Technical and Engineering, South Tehran Branch, Islamic Azad University, Tehran, Iran
3Microsystem Fabrication Laboratory, Faculty of Electrical and Computer Engineering, University of Tabriz, Tabriz, Iran
4Department of Biology, Islamic Azad University, Tabriz Branch, Tabriz, Iran
Abstract
Introduction:
The microfluidic device is highly optimized to remove oocytes from the cumulus-corona cell mass surrounding them. Additionally, it effectively captures and immobilizes the oocytes, aiding in assessing their quality and facilitating the injection of sperm into the oocyte. In this study, a novel microfluidic chip was designed and manufactured using conventional soft lithography methods.
Methods:
This research proposes the utilization of a microfluidic chip as a substitute for the conventional manual procedures involved in oocyte denudation, trapping, and immobilization. The microfluidic chip was modeled and simulated using COMSOL Multiphysics® 5.2 software to optimize and enhance its design and performance. The microfluidic chip was fabricated using conventional injection molding techniques on a polydimethylsiloxane substrate by employing soft lithography methods.
Results:
A hydrostatic force was applied to guide the oocyte through predetermined pathways to eliminate the cumulus cells surrounding the oocyte. The oocyte was subsequently confined within the designated trap region by utilizing hydraulic resistance along the paths and immobilized by applying vacuum force.
Conclusion:
The application of this chip necessitates a lower level of operator expertise compared to enzymatic and mechanical techniques. Moreover, it is feasible to continuously monitor the oocyte's state throughout the procedure. There is a reduced need for cultural media compared to more standard approaches.
Keywords: Microfluidic chip, Lab-on-a-chip, Assisted reproductive technology, COMSOL Multiphysics 5.2
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Assisted reproductive technology (ART) stands as a remarkable achievement in medical science, offering hope to infertile families by facilitating the conception of children.1 Significant advancements have been made in this domain over the past few decades. Presently, most ART procedures are conducted manually, with the success of these procedures heavily relying on the expertise of embryologists and laboratory specialists. Nevertheless, the existing equipment used in infertility laboratories poses challenges due to its incompatibility with the dimensions of oocytes and sperm. Moreover, the high costs associated with assisted reproductive techniques have limited access to this technology for low-income groups.
In recent times, microfluidic technology has emerged as a promising field with a growing array of applications. Researchers have explored the use of microfluidic chips to address the limitations of traditional methods in infertility treatment.2,3 Microfluidic systems offer several advantages, such as reduced media consumption and precise control and manipulation of cells due to their small size.4,5 These systems have been extensively investigated across various applications, including in vitro oocyte Maturation,6 cell rotation,7-9 cell trapping,10,11 sperm behavior analysis,12 sperm motility evaluation and selection,13-16 cumulus removal,17,18 fertilization,19 embryo culture, and embryo selection.20
In this context, microfluidic systems' ultimate objective is to create a lab-on-a-chip (LOC) platform that can be easily employed in infertility treatment laboratories. Notably, previous research has yielded promising results in this direction. For instance, Ma et al developed a microfluidic device that integrated multiple steps of in vitro fertilization (IVF) on a single chip, encompassing oocyte positioning, sperm screening, fertilization, medium replacement, and embryo culture.21 Heo et al proposed an on-chip flow actuation system that facilitated the loading and culture of embryos in micro-wells, albeit without considering the fertilization process.22 Another study by Heydari et al. It included the construction of a microfluidic microchip for sperm sorter, which uses spermatozoa intrinsic behaviors to select superior sperm.23 Intracytoplasmic sperm injection (ICSI), a technique where a single sperm is directly injected into the oocyte for fertilization, has demonstrated the potential to cause unexpected damage due to the manipulation of both sperm and oocyte.24 In the embryology laboratories of ART centers, a crucial step in preparing oocytes for micromanipulation, such as ICSI, involves denudation to remove the surrounding cumulus cell mass. Traditionally, denudation is achieved through enzymatic or mechanical methods. The enzymatic technique employs the hyaluronidase enzyme to digest the hyaluronic acid between cumulus cells, while the mechanical approach involves pipetting. Both methods heavily rely on the operator’s skills and can be time-consuming and labor-intensive, especially during mass ICSI operations.
Additionally, denudation procedures may pose a risk of damaging valued oocytes. Moreover, oocyte immobilization is essential for research purposes and microinjection procedures involving sperm into the oocyte cytoplasm and oocyte sampling. These tasks typically require micro-manipulator systems for holding needles, necessitating sophisticated facilities and skilled embryologists, ultimately increasing process costs. To simplify these processes and alleviate the dependence on manual interventions, our previous research introduced a chip designed to remove cumulus cells surrounding the oocyte, with laboratory tests demonstrating its efficiency.25 In continuation of our group's research to achieve the mentioned goals, we have proposed an integrated microfluidic chip to replace the manual operations of denudation, trapping, and immobilization of oocytes. Building upon this foundation, our current work aims to investigate replacing manual procedures in the embryology laboratory with microfluidic chips. Specifically, we present a novel chip that combines oocyte denudation and trapping functionalities, enabling the immobilization of oocytes for ICSI and research purposes.
Despite the promising potential of microfluidic chips in laboratories, their adoption faces significant challenges for embryologists. These challenges include complexities in use, uncertainties surrounding work safety, deviations from natural conditions, and uncertainties related to the potential harm of electric fields on oocytes and sperm if employing such fields. To address these concerns, a LOC specifically tailored for infertility applications has been designed and fabricated in this study. This LOC comprises four interconnected units, facilitating sample denudation, trapping, culturing, and holding for IVF and ICSI applications. The design was computationally optimized using finite element analysis through COMSOL Multiphysics software, with particular attention to oocyte dimensions and medium flow behavior in micro-channels to achieve optimal results. Subsequently, the chip was fabricated using soft lithography techniques.
In conclusion, integrating microfluidic technology in infertility treatment laboratories holds immense promise for advancing assisted reproductive techniques. Through the development of the proposed chip, it is intended to contribute to the enhancement of fertility procedures while addressing the challenges and concerns associated with microfluidic applications in this domain.
Materials and Methods
Physical concept
The oocyte, enveloped by cumulus cells (Fig. 1), requires their removal during the IVF and ICSI procedures. In embryology laboratories, this is accomplished either manually or enzymatically using a stripper. However, both of these methods subject the oocyte to high stress and lead to increased consumption of culture medium. This research has focused on two key aspects, namely, optimizing the design of micro-channels with appropriate shapes to minimize stress on the oocyte during its passage through these channels and establishing minimal requirements for culture medium to facilitate the crucial removal of cumulus cells around the oocyte as a preparatory step for IVF and ICSI processes.
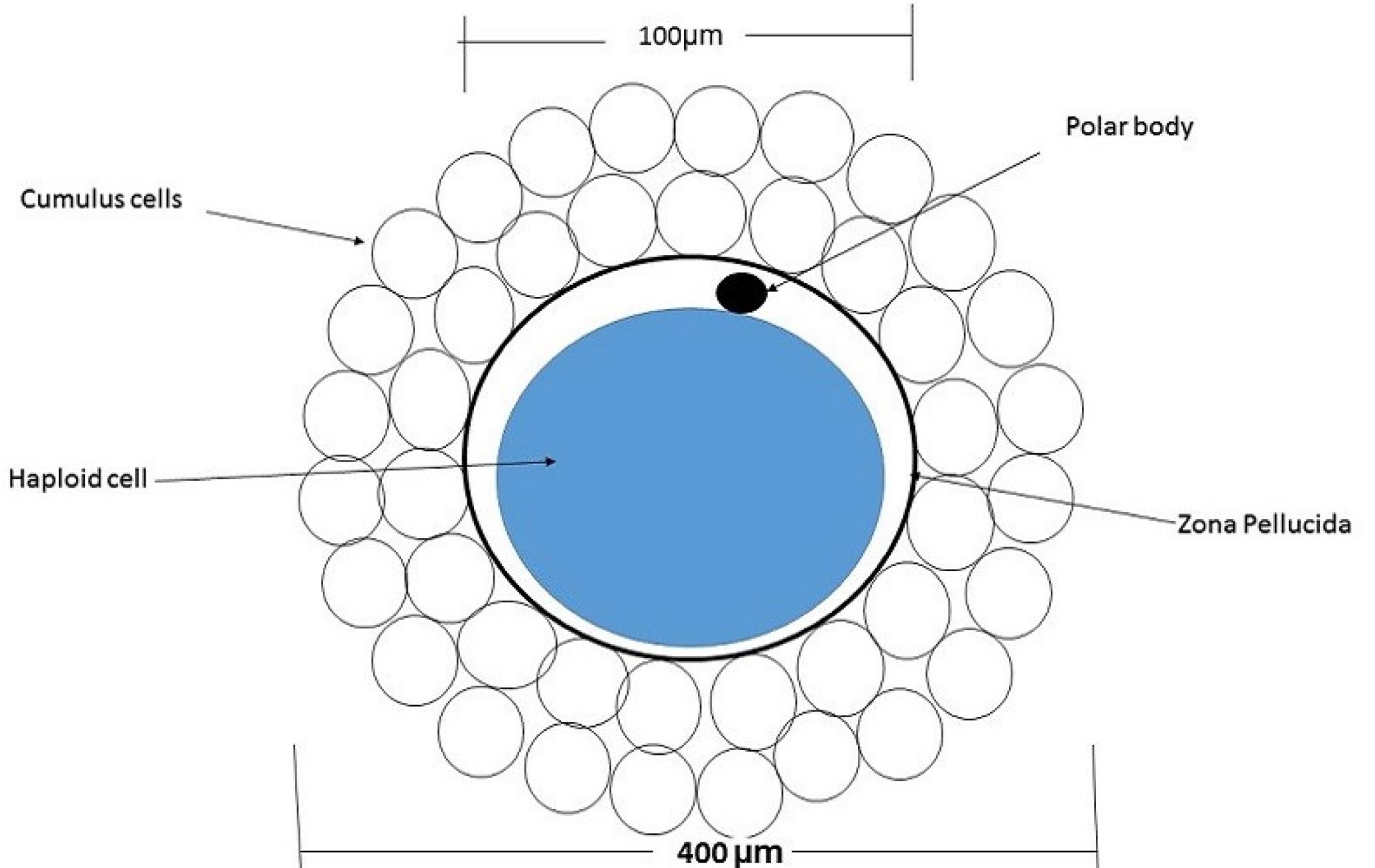
Fig. 1.
Diagram of a mammalian oocyte with cumulus cells.
.
Diagram of a mammalian oocyte with cumulus cells.
The second part of the study involves the structural design of a trap mechanism to aid in the IVF and ICSI procedures. This model is based on hydrodynamic flow manipulation, enabling the immobilization of the oocyte using a vacuum channel for ICSI and research applications. Currently, expensive cell manipulator systems (Figs. 2A, 2B), operated by highly skilled personnel, are employed for cell immobilization and processing, contributing to the high costs associated with IVF treatments.
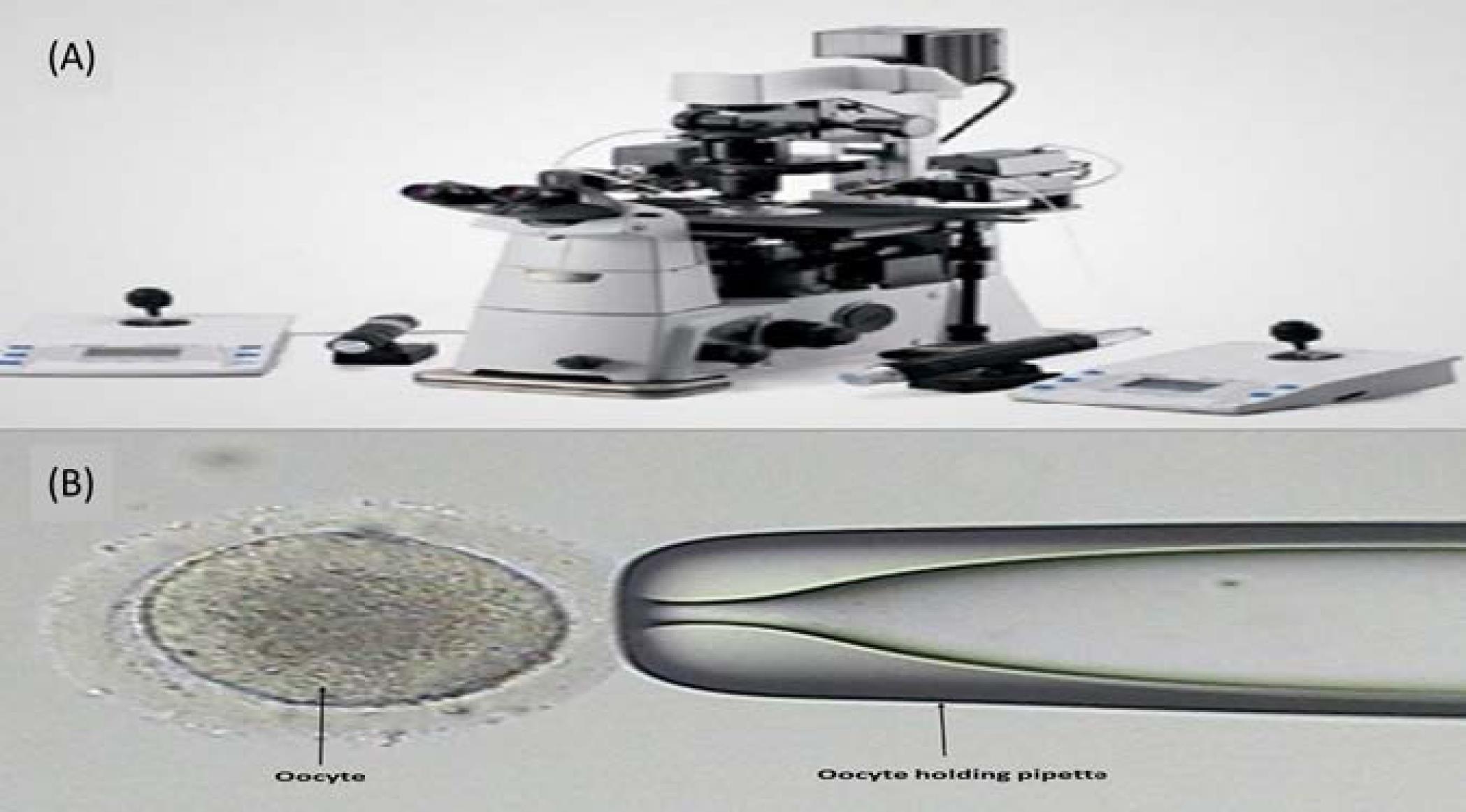
Fig. 2.
(A) Eppendorf cell manipulation system and (B) oocyte holding pipette.
.
(A) Eppendorf cell manipulation system and (B) oocyte holding pipette.
Our proposed design is a functional LOC that aims to simplify the essential steps of embryology laboratories while minimizing human intervention. This device efficiently performs oocyte denudation and immobilization, thus reducing the overall cost of infertility treatment. Furthermore, it offers an automated approach that reduces dependence on the skills of individual embryologists (Fig. 3).
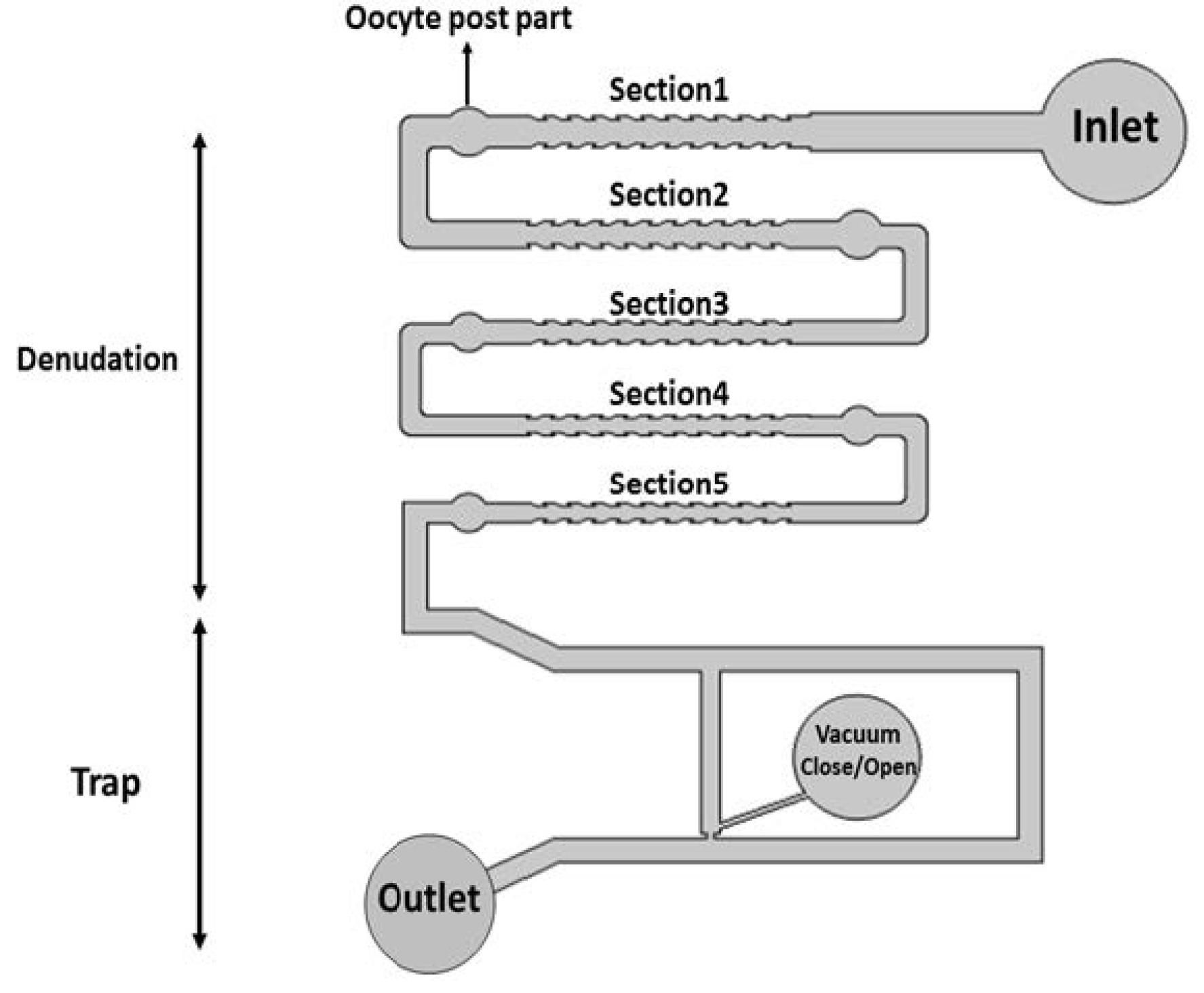
Fig. 3.
Schematic diagrams of oocyte denudation and holding chip.
.
Schematic diagrams of oocyte denudation and holding chip.
Theoretical concept
According to the dimensions of the micro-channels (length ~4-6.5 mm, width ~120-400 µm, and height =250 µm), the fluid specifications (Dynamic viscosity; µ=0.799e-3 Ns/m2 and fluid density; ρ=997 kg/m3), and the fluid flow rate (0.16 mL/min), the Reynolds number can be calculated as follows:
Where Dh and V are the hydraulic dimensions and fluid velocity, respectively, in this design, always Re <10, and movement of the liquid inside the micro-channels follows a laminar flow. The proposed devices are governed by a general fluidic model based on the steady-state Navier-Stokes equation, representing an incompressible Newtonian fluid26:
(2)
Computational fluid dynamics is employed to analyze the wall shear stress as a function of the channel width and flow rate. Given that the channel length significantly exceeds the channel height (L ~4-6.5 mm, W ~120-400 µm, and H = 250 µm), a two-dimensional (2D) simulation proves effective for modeling the system. Utilizing these conditions, the maximum shear stress applied to the cell (τs) can be determined using equation (3):27
where H, μ, and Q represent the channel height, the medium dynamic viscosity, and the volumetric flow rate, respectively.
Computational model
The current study used COMSOL Multiphysics 5.2 software to model and simulate the microfluidic chip, optimize its design, enhance performance, and estimate flow profiles and shear stresses within the denudation device. Finite element models were constructed, and 2D simulations were conducted to investigate the flow behavior in the microfluidic chip. The optimal design layout is illustrated in Fig. 3.
Five corrugated channel profiles (Section 1-5) were devised in the denudation sections of the chip. These channels were designed with a gradual decrease in width from section 1 to section 5, aiming to facilitate the removal of cumulus cells around the oocyte and simplify the denudation process for IVF or ICSI procedures. This design ensures a smooth removal of cumulus cells with minimal damage to the oocyte. In the post-parts, the velocity line graph (Fig. 4) depicts reduced speed, allowing for the suspension of the oocyte by controlling the liquid inlet pressure.
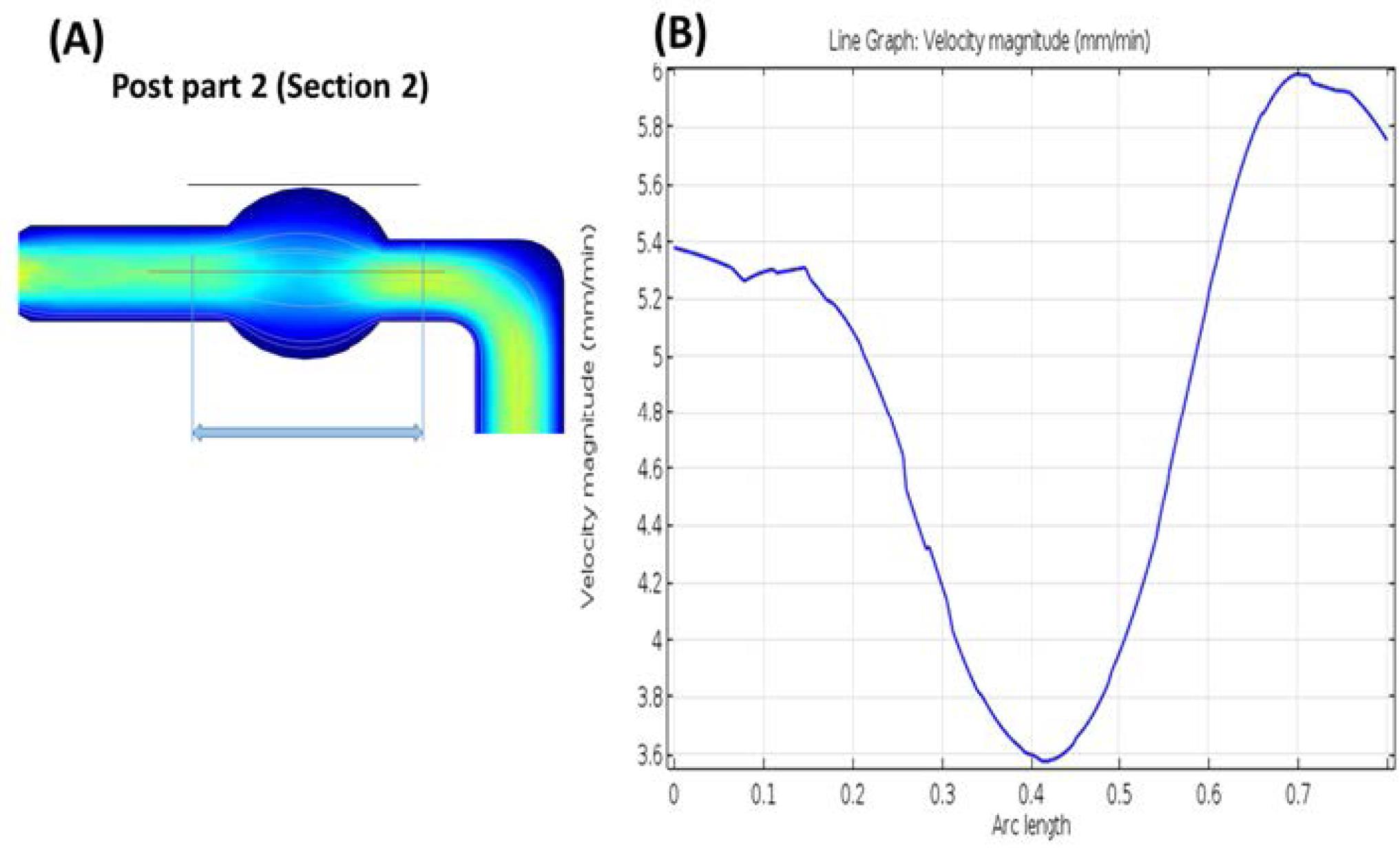
Fig. 4.
Fluid velocity (mm/min) in the post-part.
.
Fluid velocity (mm/min) in the post-part.
Within the trap section, a structure was designed based on the dimensions of the oocyte, effectively preserving the oocyte using the hydraulic resistance of the micro-channel path (Figs. 5A, 5B, and 5C). The dimensions of the microchannels are provided in Table 1.
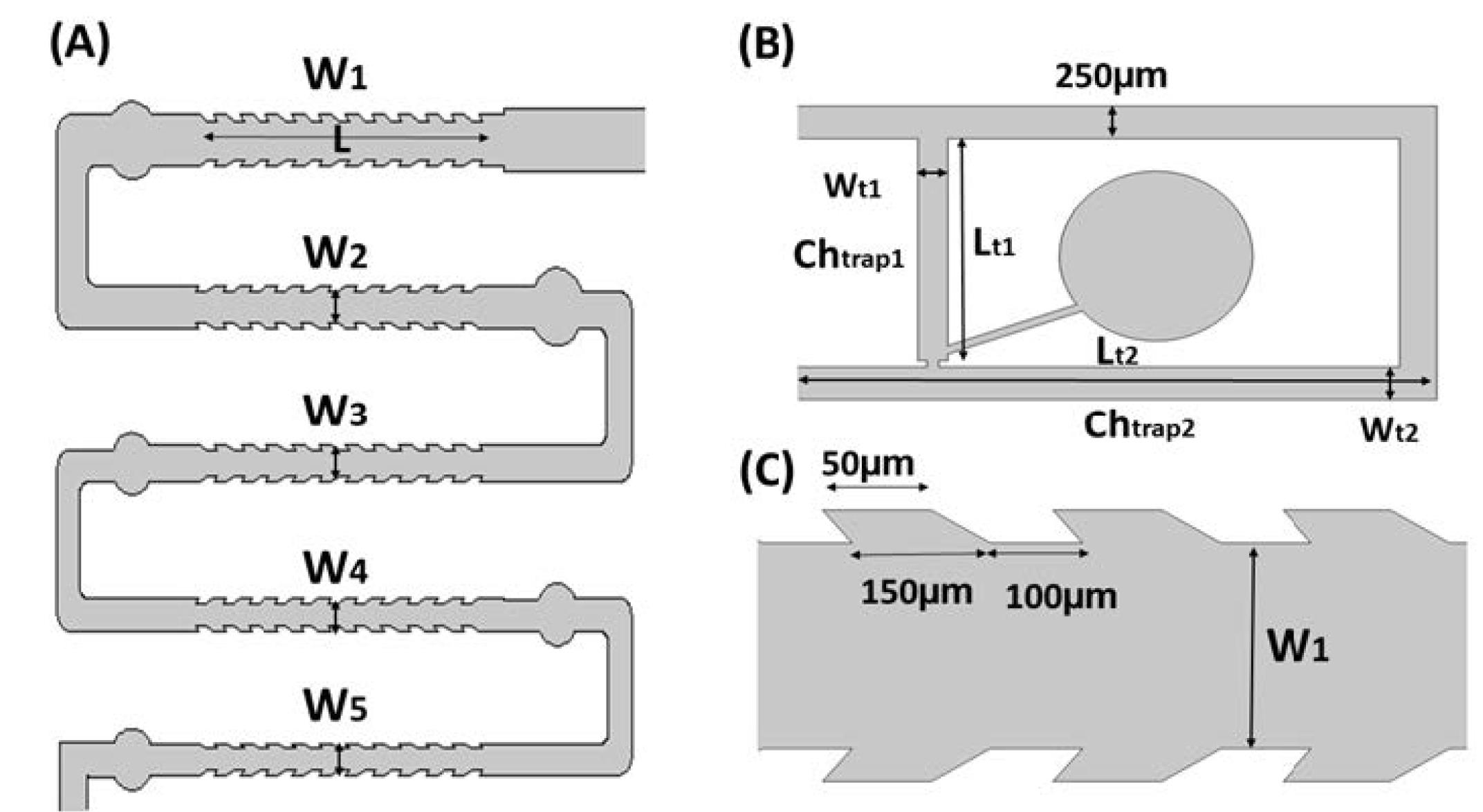
Fig. 5.
(A) Denudation part of the chip, (B) Jagged-surface micro-channels, and (C) Trap part of the chip
.
(A) Denudation part of the chip, (B) Jagged-surface micro-channels, and (C) Trap part of the chip
Table 1.
Dimensions of the micro-channels
|
Denudation part width, length, height
|
Trap part
|
|
Section 1
|
Section 2
|
Section 3
|
Section 4
|
Section 5
|
Chtrap 1
|
Chtrap 2
|
W1
250 µm |
W2
200 µm |
W3
170 µm |
W4
150 µm |
W5
140 µm |
Wt1
200 µm |
Wt2
250 µm |
| L=3 mm |
Lt1
1.75 mm |
Lt2
6.5 mm |
Height of all micro-channels: H = 250 µm
The fluidic behavior of the medium inside the micro-channels was analyzed and simulated. The applied flow rate at the inlet was 0.16 mL/min, well-suited to the range of biological medium, with no slip and no penetration as the boundary conditions on the walls. The simulation of the trap section in the absence of an oocyte revealed that, due to the low hydraulic resistance of the trap section compared to the main path, the medium mainly flows to the output from this part. However, in the presence of an oocyte, the simulation shows an increase in path resistance, causing the medium to flow from the main path, effectively trapping the oocyte in the proposed channel (Fig. 6).
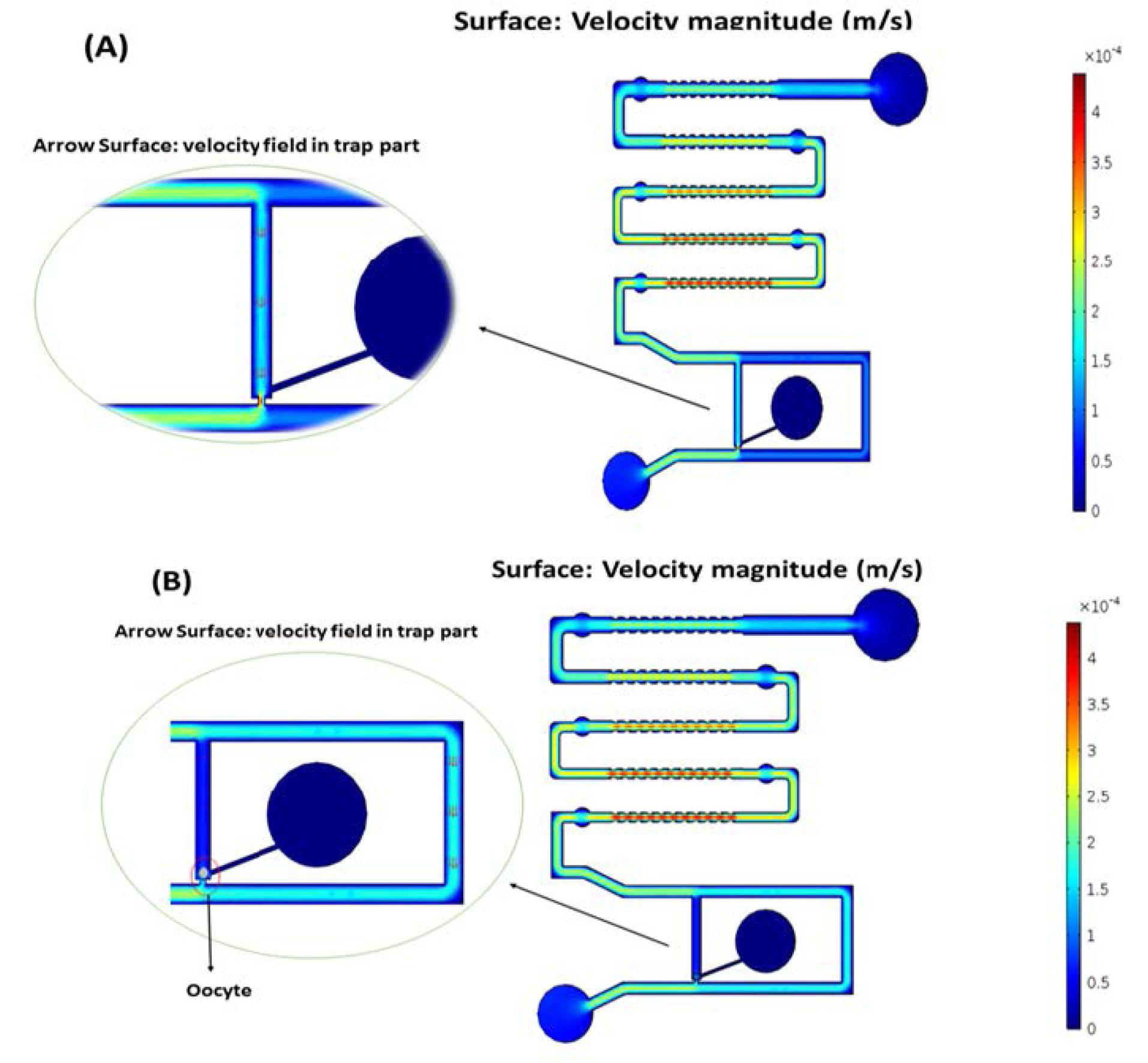
Fig. 6.
Medium velocity magnitude (m/s): (A) Without the oocyte in the trap channel and (B) Presence of the oocyte in the trap channel.
.
Medium velocity magnitude (m/s): (A) Without the oocyte in the trap channel and (B) Presence of the oocyte in the trap channel.
To prevent oocyte damage during denudation and trapping, it is essential to ensure that the shear stress does not exceed the maximum allowed value (1.2 dyne/cm2 or 0.12 Pa).28 Table 2 presents the maximum and minimum shear stress points calculated using COMSOL Multiphysics 5.2 software in the micro-channels. The oocyte’s movement along the channel and its shear stress were estimated using the particle tracing tool in COMSOL. Due to changes in the oocyte’s diameter during denudation, the precise study of oocyte shear stress in the channels is challenging. Hence, the investigation here focuses solely on shear stress within the channels.
Table 2.
Maximum and minimum shear stress on the walls of channels
| X (µm) |
Y (µm) |
Shear stress (Pa) |
| 4325.0 |
631.84 |
1.5098E-11 (Min) |
| 2425.2 |
1598.9 |
0.0016289 (Max) |
Fabrication and sample preparation
The fabrication process began by thoroughly cleaning a glass substrate using deionized water and 70% ethanol. A 250-μm-thick scotch tape served as the spacer layer, while UV resin (Loxeal 3024) was applied to the glass and evenly spread using Doctor Blading. The structure was then exposed to UV sources (400 nm) for 35 seconds by utilizing a predesigned photomask (Fig. 7A), followed immediately by development in isopropyl alcohol (IPA). Subsequently, the mold underwent a debris-cleaning step with an IPA jet wash and post-exposure to the same UV source for an additional 7 minutes. To improve the molding process, a thin layer of 10% polyvinyl alcohol (PVA) was coated using a small brush and dried in a convection oven at 80 ºC for 20 minutes.
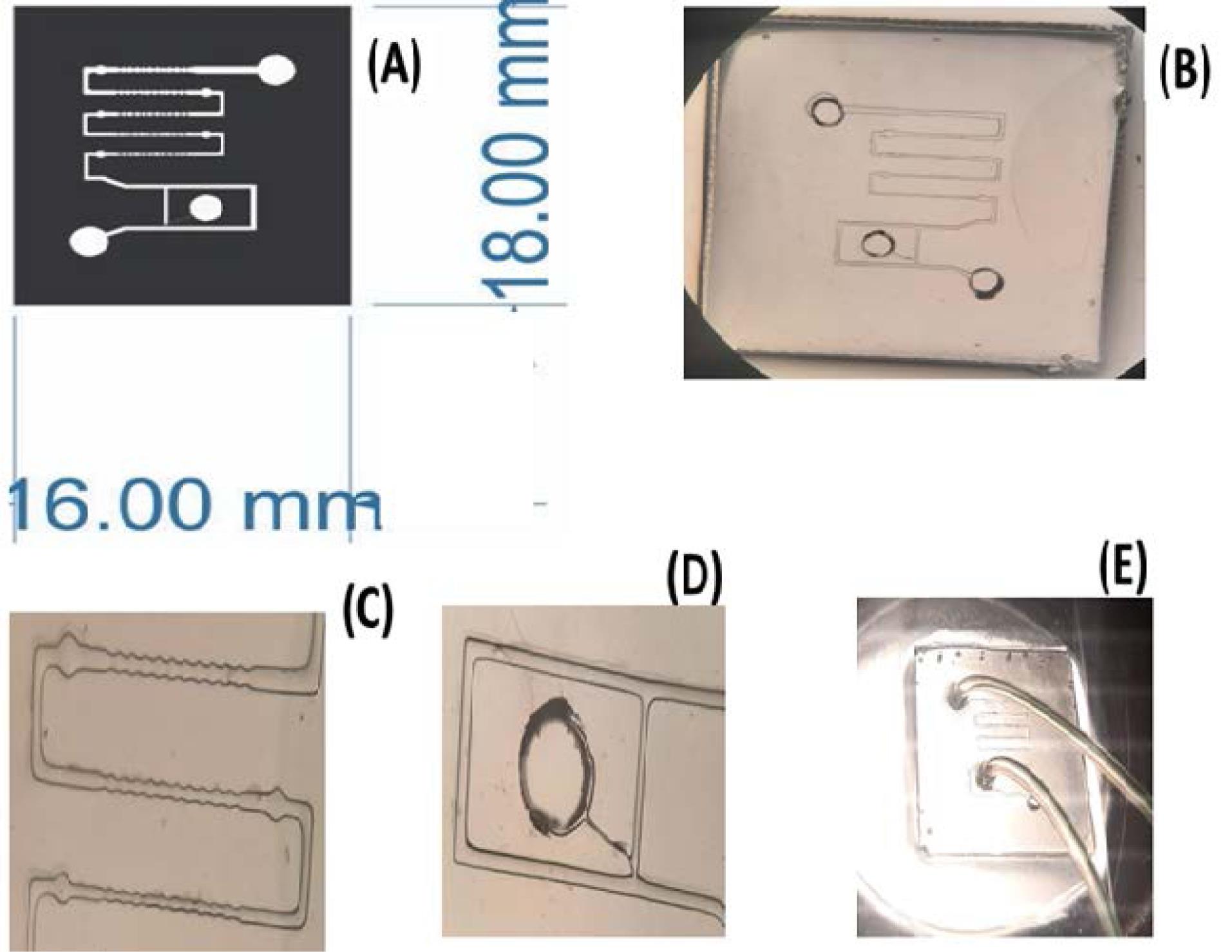
Fig. 7.
(A) Photolithography mask, (B) Microfluidic chip, (C) Denudation channels, (D) Trap part, and (E) Final microfluidic chip with injection tubes.
.
(A) Photolithography mask, (B) Microfluidic chip, (C) Denudation channels, (D) Trap part, and (E) Final microfluidic chip with injection tubes.
Concurrently, a polydimethylsiloxane (PDMS) mixture in a 1:10 ratio, weighing 2 g, was prepared and degassed in a desiccator for 20 minutes to remove any air bubbles. This PDMS mixture was then manually poured into the mold and left in a convection oven at 70 ºC overnight for curing. The next day, the cured PDMS was gently peeled off in a 70 ºC water bath, effectively removing the PVA and safely releasing the PDMS. Subsequently, surface activation was performed by exposing the PDMS to O2 plasma for approximately 15 minutes to enhance its bonding to the glass. Paper clamps were employed to ensure firm layer adhesion. Before injection, the chip is subjected to a sterilization process, which includes cleaning the chip with 70% alcohol, then for 30 minutes in a 37°C incubator to remove the alcohol from the chip altogether, and finally, UV sterilization for 1 hour. Immature oocyte samples were tested in the embryology lab of the ART center. Initially, hyaluronidase 80 was added to the oocyte and placed in Ham’s f10 medium to loosen the surrounding cells, and the microfluidic chip was prepared for testing. Fig. 7B depicts the final chip at various scales.
Results and Discussion
The present LOC was introduced to replace automatic methods instead of conventional manual methods in embryology laboratories, which was the goal of our research group. To test the chip in the embryology laboratory, Ham's f10 medium was first injected into the chip using a syringe pump. Then, the immature human oocyte was directed to the microchip channels with a constant flow rate of 0.16 mL/min and using hydrostatic force from the predicted paths were crossed. As a result, the jagged walls of the micro-channels removed the cumulus cells around the oocyte. At each stage, circular posts allow for checking the condition of the oocyte under an inverted microscope by stopping the injection of the syringe pump at those places. After the oocyte passed through the denudation area, it finally reached the trap chip, and according to the simulations, the oocyte, by following the direction of liquid flow, was trapped in the trap part of the chip and placed in the lower part of the trap. It was then immobilized using vacuum force through a mouth-controlled pipette. Finally, by applying positive pressure at the inlet intended for suction, the oocyte was directed from inside the chip to the outlet and exited the chip. Laboratory experiments (Fig. 8) confirmed the efficiency of the introduced oocyte cumulus cell denudation and trapping steps.
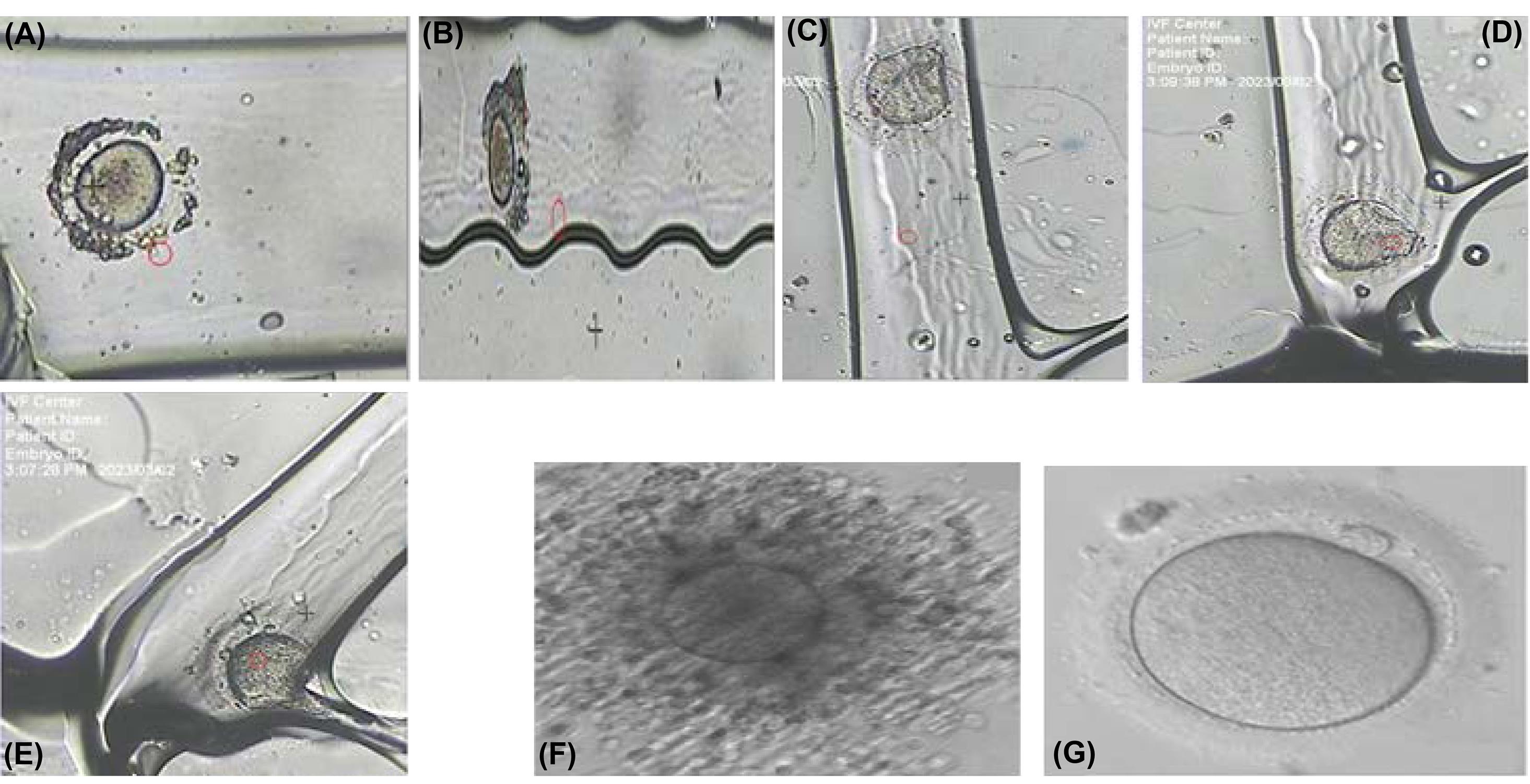
Fig. 8.
(A) Oocyte at the entrance of section 1 in the chip, (B) Oocyte in the denudation channel in section 2, (C) Oocyte at the entrance of the trap channel, (D) Oocyte at the end of the trap channel, (E) Immobilization of the oocyte using a vacuum in the trap channel, (F) Oocyte before denudation, (G) Oocyte after denudation in the chip
.
(A) Oocyte at the entrance of section 1 in the chip, (B) Oocyte in the denudation channel in section 2, (C) Oocyte at the entrance of the trap channel, (D) Oocyte at the end of the trap channel, (E) Immobilization of the oocyte using a vacuum in the trap channel, (F) Oocyte before denudation, (G) Oocyte after denudation in the chip
Compared to conventional and manual methods used in embryology laboratories, enzymatic and mechanical methods are utilized for the denudation part, which can harm the oocyte if the oocyte remains in the enzyme longer.29,30 In mechanical denudation, a stripper is normally employed, which can cause problems for the oocyte if the stress is high during the mechanical operation.31,32 Generally, conventional methods are inefficient and time-consuming. Unlike conventional methods, our introduced LOC faces the oocyte in an environment of almost the same scale, thus minor stress is applied to the oocyte. The state of the oocyte can always be observed during denudation. The amount of culture medium is much less than conventional methods due to the structure’s small size, and it is more efficient. To immobilize the oocyte in conventional methods, expensive micro-manipulator devices are necessary. In addition to this expensive equipment, in conventional methods, user skill is highly important, and the results depend entirely on the user's skill. However, the introduced LOC immobilizes the oocyte at the lowest cost and does not require high user skills, such as the micromanipulation method. To the best of our knowledge, no single chip performing denudation and oocyte immobilization has been reported so far. However, works that only perform the denudation and immobilization of oocytes have been published separately. Recently, Mokhtare et al published an ultrasound oocyte denudation chip.33 Although the introduced chip had a high speed in denudation, the safety of the oocyte in the face of the direct radiation of ultrasound waves from a highly close distance is still unknown, and this concern is a serious obstacle to its use in laboratories. Other works attempted oocyte cumulus cell denudation by employing suction force to remove cumulus cells around the oocyte.18 Nonetheless, it required vacuum pumps to control the channels and lacked complete coverage of the oocyte environment. It is exposed to vacuum pressure for a long time, and damage is possible to the oocyte. One of the functions of our proposed chip is to trap and immobilize the oocyte without complicated equipment in a way that does not damage the oocyte. Other reported oocyte trapping works were designed for cell dimensions below 10 microns, and complete cell immobilization was not envisioned, often involving intricate structures compared to our proposed design.10,11 The primary oocyte before and after denudation using this microfluidic chip are shown in Figs. 8F and 8G. Eight out of ten oocyte cells were denuded, and the result denudation of two oocytes was done incompletely. Of course, it should be noted that immature oocytes were selected for the test, which is usually tricky to denude. All ten oocytes utilized for the test were immobilized in the trap section. The final appearance quality of the oocytes used in the tests demonstrates that this chip does not harm the oocytes. Since in this chip, much less stress is applied to the oocytes than manual methods, and the chip is fabricated of biocompatible materials, this chip is entirely safe for the oocyte. The comparison of the proposed LOC and conventional manual methods for denudation and holding oocyte are provided in Table 3.
Table 3.
Comparison of LOC and manual conventional methods
|
Method
|
Manual methods LOC
|
| Culture medium (volume) |
150-200 λ |
16 λ |
| Test time Denudation & holding oocyte |
~5 min |
~20 s |
| Stress on oocyte |
High |
Low |
| Reproducibility |
Low |
High |
| Cost |
High |
Low |
| Dependence of the results on the skill of the operator |
High |
Low |
Conclusion
In this study, we successfully introduced a microfluidic chip designed to efficiently remove cumulus cells around the oocyte while also allowing for the trapping and immobilization of the oocyte for research and manipulation purposes. Compared to enzymatic and mechanical methods, this chip requires minimal operator skills, making it a user-friendly alternative. Additionally, the chip enables continuous monitoring of the oocyte’s condition throughout the process. Remarkably, culture medium consumption is significantly reduced to only one-fourth of what is used in conventional methods.
The fabrication process of the microfluidic chip involves standard injection molding over a PDMS substrate, resulting in a substantial cost reduction compared to traditional techniques. This microfluidic chip presents a viable and reliable substitute for current procedures in embryology laboratories. It holds promise for enhancing the efficiency of oocyte denudation, trapping, and immobilization procedures, thereby advancing the field of assisted reproductive techniques. By streamlining these crucial steps, the chip contributes to the optimization and cost-effectiveness of ART procedures in infertility treatments. With further optimization and refinement, this microfluidic chip has the potential to revolutionize current practices in the field of embryology and bring forth significant benefits to patients seeking fertility treatments. Investigation of the acceptable amount of suction to immobilize the oocyte and the fertilization quality of the oocytes used in this LOC, besides the expansion of the chip function, is one of the future works of our group.
Research Highlights
What is the current knowledge?
√ During the last decade, the research and design of microfluidic chips have offered promising applications in the clinical analysis of biological fluids.
√ The application of microfluidic devices in assisted reproductive technology is still limited.
What is new here?
√ The proposed microfluidic chip is a simple, efficient, and cost-effective device for the oocyte’s cumulus cell denudation and immobilization.
√ This work hopes to replace the manual processes of embryology laboratories with automatic and low-cost microfluidic chips.
Acknowledgment
We are grateful to the microfabrication laboratory, Faculty of Electrical and Computer Engineering University of Tabriz, for microfabrication assistance and the embryology laboratory of the ART Center, Jahad Daneshgahi Tabriz, for preparing the chip testing conditions.
Competing Interests
The authors declare that they have no competing interests.
Ethical Statement
There are none to be declared.
Funding
None.
References
- Bhandari HM, Choudhary MK, Stewart JA. An overview of assisted reproductive technology procedures. Obstet Gynaecol 2018. 20: 167-76. 10.1111/tog.12509.
- Kashaninejad N, Shiddiky MJA, Nguyen NT. Assisted Reproductive Technology: Advances in Microfluidics‐Based Assisted Reproductive Technology: From Sperm Sorter to Reproductive System‐on‐a‐ChipAdv. Biosys 2018; 2:1870021. doi: 10.1002/adbi.201700197 [Crossref] [ Google Scholar]
- Nikshad Nikshad, A A, Aghlmandi Aghlmandi, A A, Safaralizadeh Safaralizadeh, R R, Aghebati-Maleki Aghebati-Maleki, L L, Warkiani Warkiani, ME ME, Khiavi Khiavi, FM FM. Advances of microfluidic technology in reproductive biology. Life Sci 2021; 265:118767. doi: 10.1016/j.lfs.2020.118767 [Crossref] [ Google Scholar]
- Luo T, Fan L, Zhu R, Sun D. Microfluidic single-cell manipulation and analysis: Methods and applications. Micromachines 2019; 10:104. doi: 10.3390/mi10020104 [Crossref] [ Google Scholar]
- Weng, Lindong. IVF-on-a-Chip: Recent Advances in Microfluidics Technology for In Vitro Fertilization. SLAS Technol 2019; 24: 373-385 10.1177/2472630319851765.
- Oskouei BS, Zargari S, Shahabi P, Novin MG, Pashaiasl M. Design and microfabrication of an on-chip oocyte maturation system for reduction of apoptosis. Cell J 2021; 23:32. doi: 10.22074/cellj.2021.7056 [Crossref] [ Google Scholar]
- Benhal P, Chase JG, Gaynor P, Oback B, Wang W. AC electric field induced dipole-based on-chip 3D cell rotation. Lab Chip 2014; 14:2717-27. doi: 10.1039/c4lc00312h [Crossref] [ Google Scholar]
- Habaza M, Kirschbaum M, Guernth‐Marschner C, Dardikman G, Barnea I, Korenstein R. Rapid 3D refractive‐index imaging of live cells in suspension without labeling using dielectrophoretic cell rotation. Adv Sci (Weinh) 2017; 4:1600205. doi: 10.1002/advs.201600205 [Crossref] [ Google Scholar]
- Puttaswamy SV, Bhalla N, Kelsey C, Lubarsky G, Lee C, McLaughlin J. Independent and grouped 3D cell rotation in a microfluidic device for bioimaging applications. Biosens Bioelectron 2020; 170:112661. doi: 10.1016/j.bios.2020.112661 [Crossref] [ Google Scholar]
- James J, Sushmitha M, Premkumar R, Narayanamurthy V, Kalpana R, editors. Microfluidic micro-well (size and shape) by numerical optimization for single cell applications: Vertical trapping approach. 2017 International Conference on Microelectronic Devices, Circuits and Systems (ICMDCS). 2017: IEEE. 10.1109/ICMDCS.2017.8211597.
- Jin D, Deng B, Li J, Cai W, Tu L, Chen J. A microfluidic device enabling high-efficiency single cell trapping. Biomicrofluidics 2015; 9:014101. doi: 10.1063/1.4905428 [Crossref] [ Google Scholar]
- Rappa K, Samargia J, Sher M, Pino JS, Rodriguez HF, Asghar W. Quantitative analysis of sperm rheotaxis using a microfluidic device. Microfluid Nanofluidics 2018; 22:100. doi: 10.1007/s10404-018-2117-6 [Crossref] [ Google Scholar]
- Chinnasamy T, Kingsley JL, Inci F, Turek PJ, Rosen MP, Behr B. Guidance and Self‐Sorting of Active Swimmers: 3D Periodic Arrays Increase Persistence Length of Human Sperm Selecting for the Fittest. Adv Sci (Weinh) 2018; 5:1700531. doi: 10.1002/advs.201700531 [Crossref] [ Google Scholar]
- Xie L, Ma R, Han C, Su K, Zhang Q, Qiu T. Integration of sperm motility and chemotaxis screening with a microchannel-based device. Clin Chem 2010; 56:1270-8. doi: 10.1373/clinchem.2010.146902 [Crossref] [ Google Scholar]
- Zhang X, Lu Z, Sun Y, editors. Disposable micro devices for clinical ICSI. 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference. 2011: IEEE. 10.1109/TRANSDUCERS.2011.5969369.
- Huang CH, Chen CH, Huang TK, Lu F, Jen Huang JY, Li BR. Design of a gradient-rheotaxis microfluidic chip for sorting of high-quality Sperm with progressive motility. iScience 2023; 26:107356. doi: 10.1016/j.isci.2023.107356 [Crossref] [ Google Scholar]
- Weng L, Lee GY, Liu J, Kapur R, Toth TL, Toner M. On-chip oocyte denudation from cumulus–oocyte complexes for assisted reproductive therapy. Lab Chip 2018; 18:3892-902. doi: 10.1039/c8lc01075g [Crossref] [ Google Scholar]
- Zeringue H, Rutledge J, Beebe D. Early mammalian embryo development depends on cumulus removal technique. Lab Chip 2005; 5:86-90. doi: 10.1039/B316494M [Crossref] [ Google Scholar]
- Ravaux B, Garroum N, Perez E, Willaime H, Gourier C. A specific flagellum beating mode for inducing fusion in mammalian fertilization and kinetics of sperm internalization. Sci Rep 2016; 6:31886. doi: 10.1038/srep31886 [Crossref] [ Google Scholar]
- Le Gac S, Nordhoff V. Microfluidics for mammalian embryo culture and selection: where do we stand now?. Mol Hum Reprod 2017; 23:213-26. doi: 10.1093/molehr/gaw061 [Crossref] [ Google Scholar]
- Ma R, Xie L, Han C, Su K, Qiu T, Wang L. In vitro fertilization on a single-oocyte positioning system integrated with motile sperm selection and early embryo development. Anal Chem 2011; 83:2964-70. doi: 10.1021/ac103063g [Crossref] [ Google Scholar]
- Heo YS, Cabrera LM, Song JW, Futai N, Tung Y-C, Smith GD. Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly (dimethylsiloxane) devices. Anal Chem 2007; 79:1126-34. doi: 10.1021/ac061990v [Crossref] [ Google Scholar]
- Heydari A, Zabetian Targhi M, Halvaei I, Nosrati R. A novel microfluidic device with parallel channels for sperm separation using spermatozoa intrinsic behaviors. Sci Rep 2023; 13:1185. doi: 10.1038/s41598-023-28315-7 [Crossref] [ Google Scholar]
- Eichenlaub-Ritter U, Shen Y, Tinneberg HR. Manipulation of the oocyte: possible damage to the spindle apparatus. Reprod Biomed Online 2002; 5:117-124. doi: 10.1016/s1472-6483(10)61613-6 [Crossref] [ Google Scholar]
- Safaefar F, Karamdel J, Veladi H, Fallah Tafti M. Design and fabrication of a microfluidic chip for oocyte denudation. Tabriz Journal of Electerical Engineering 2022; 52:189-194. doi: 10.22034/TJEE.2022.15541 [Crossref] [ Google Scholar]
- Pelesko JA, Bernstein DH. Modeling mems and nems. CRC Press; 2002. 10.1201/9781420035292.
- Gaver DP, Kute SM. A theoretical model study of the influence of fluid stresses on a cell adhering to a microchannel wall. Biophys J 1998; 75:721-33. doi: 10.1016/S0006-3495(98)77562-9 [Crossref] [ Google Scholar]
- Xie Y, Wang F, Zhong W, Puscheck E, Shen H, Rappolee D. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol Reprod 2006; 75:45-55. doi: 10.1095/biolreprod.105.049791 [Crossref] [ Google Scholar]
- Ishizuka Ishizuka, Y Y, Takeo Takeo, T T, Nakao Nakao, S S, Yoshimoto Yoshimoto, H H, Hirose Hirose, Y Y, Sakai Sakai, Y Y. Prolonged exposure to hyaluronidase decreases the fertilization and development rates of fresh and cryopreserved mouse oocytes. J Reprod Dev 2014; 60:454-459. doi: 10.1262/jrd.2014-045 [Crossref] [ Google Scholar]
- de Moura BR, Gurgel MC, Machado SP, Marques PA, Rolim JR, de Lima MC, et al. Low concentration of hyaluronidase for oocyte denudation can improve fertilization rates and embryo quality. JBRA Assist Reprod. 2017;21:27-30 10.5935/1518-0557.
- Rienzi L, Ubaldi F, Martinez F, Iacobelli M, Minasi M. G, Ferrero S, et al. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod. 2003 18: 1289-93. 10.1093/humrep/deg274.
- Cooke S, Tyler JP, Driscoll GL. Meiotic spindle location and identification and its effect on embryonic cleavage plane and early development. Hum Reprod 2003; 18:2397-405. doi: 10.1093/humrep/deg447 [Crossref] [ Google Scholar]
- Mokhtare A, Davaji B, Xie P, Yaghoobi M, Rosenwaks Z, Lal A, Palermo G. Non-contact ultrasound oocyte denudation. Lab Chip 2022; 3:219-24. doi: 10.1039/D1LC00715G [Crossref] [ Google Scholar]