Bioimpacts. 14(5):29946.
doi: 10.34172/bi.2024.29946
Original Article
The effects of Fe2O3 nanoparticles on catalytic function of human acetylcholinesterase: size and concentration role
Samaneh Rashtbari Conceptualization, Data curation, Investigation, Validation, Visualization, Writing – original draft, , # 
Zahra Hassanpour Aydinlou Conceptualization, Investigation, Writing – original draft, , # 
Leila Sadeghi Conceptualization, Data curation, Project administration, Supervision, Validation, Visualization, Writing – review & editing, , * 
Author information:
Department of Animal Biology, Faculty of Natural Science, University of Tabriz, Tabriz, Iran
#Samaneh Rashtbari and Zahra Hassanpour Aydinlou are equal contributors to this work and designated as co-first authors.
Abstract
Introduction:
Fe2O3 NPs can enter cells quickly, pass through the blood-brain barrier and interact with macromolecules. These materials are widely used in different fields, so their risk assessment is among the most critical issues. Acetylcholinesterase (AChE) is a cholinergic enzyme in central and peripheral nervous systems.
Methods:
In this work, the possible effects of Fe2O3 NPs on the structure and catalytic activity of AChE were investigated using circular dichroism (CD), surface plasmon resonance (SPR), and fluorescence spectroscopies.
Results:
The outcomes demonstrated that 5 nm Fe2O3 NPs inhibit AChE activity through mixed mechanism. While 50 nm Fe2O3 NPs caused an enhancement in the catalytic activity up to 60 nM. However, higher concentrations of Fe2O3 NPs (above 60 nM) hindered the enzyme activity via mixed mechanism. Fluorescence analysis showed that NPs can quench the fluorescence intensity of AChE that refer to conformational changes. Furthermore, CD results showed that Fe2O3 NPs can reduce the α-helix and β-sheet contents of the enzyme and decrease the stability of AChE. Also, the SPR data analysis showed that the affinity between AChE and Fe2O3 NPs decreased with rising temperature. After treatment with Fe2O3 NPs, the catalytic activity of AChE was assessed in HepG2 cell lines, and the results confirmed the inhibitory effects of Fe2O3 NPs on AChE activity in vivo.
Conclusion:
These findings provide helpful information about the impact of Fe2O3 NPs on the structure and function of AChE and could offer new insights into the risk assessment of the medical application of nanoparticles.
Keywords: Acetylcholinesterase, Fe2O3 nanoparticles, Fluorescence spectroscopy, Circular dichroism
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
None to be stated.
Introduction
In recent years, the use of various nanoparticles (NPs) has expanded widely in different areas, including medicine, engineering, catalysis, and environmental remediation.1 This is due to the unique properties of these materials, such as their electrical, optical, chemical, magnetic, and magneto-optical properties.2-4 Given the widespread use of NPs, risk assessment of these materials is critical, and their toxicity has become one of the main challenges among researchers. So far, different kinds of NPs have been synthesized and developed. Iron oxide NPs (which consist of maghemite (γ-Fe2O3) and/or magnetite (Fe3O4) particles) are among the most important nanomaterials.5
Fe2O3 NPs have gained considerable attention because of their unique intrinsic features, such as outstanding biocompatibility and superior magnetic properties.6,7 These NPs are widely used in a variety of fields, such as sensing technologies, memory storage devices, magnetic separation, magnetic labeling, catalytic processes, and biomedicine (heating for hyperthermia treatments, providing contrast effects for magnetic imaging, and remotely controlling the delivery of targeted drugs).8-10
NPs are small particles with large surface-to-volume ratios.11,12 It has been accepted that the small size of NPs can cause these materials to enter cells quickly, pass through the blood-brain barrier, and interact with different kinds of proteins and enzymes.13 In general, the conformation and function of enzymes and proteins are widely associated with their tertiary structure and protein dysfunction can lead to various diseases and disorders.14
Acetylcholinesterase (AChE), a critical serine hydrolase, is secreted into the synaptic space by postsynaptic cholinergic neurons.15 AChE is a crucial enzyme for the growth and operation of the central nervous system. This enzyme also has a significant impact on neurodevelopment and hematological differentiation.16,17 In the synaptic space, it promotes the breakdown of acetylcholine into choline and acetate.18 According to studies, the inhibition of AChE leads to an accumulation of acetylcholine in the synaptic cleft. It disrupts the neurotransmitter levels in the synapses, which overexcites nicotinic and muscarinic acetylcholine receptors and impairs neurotransmission.19,20
According to the previous experiment, Fe2O3 NPs could change brain proteome and affect the cholinergic function of a rat's brain.21 Therefore, the main objective of the present work was to investigate the possible effects of Fe2O3 NPs on the structure and catalytic activity of AChE via spectroscopic methods. On the other hand, the activity of AChE extracted from HepG2 cell lines was evaluated after exposure to Fe2O3 NPs with two different sizes (5 and 50 nm).
Materials and Methods
Materials
Human acetylcholinesterase enzyme (AChE), 5,5’-dithio-bis-(2-nitrobenzoic) acid (DTNB), acetylthiocholine iodide ethanaminium, and Fe2O3 NPs (Fe2O4 NPs; with the size of 5 and 50 nm), N-ethyl-N-(3-dimethyl aminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS), NaCl, and NaOH were provided from Sigma Aldrich Company (St. Louis, MO, USA).
Methods
Preparation and characterization of NPs
Fe2O3 NPs were prepared from Sigma-Aldrich Company and dispersed by sonication (10 min, 750 W, and 20 kHz) in phosphate buffer (pH 7) before use. Size distribution and NPs dispersion were evaluated by the dynamic light scattering (DLS) and TEM methods. Fig. 1 shows Fe2O3 NP in crystalline phase with 5 and 50 nm sizes. In this work, the high-purity Fe2O3 NPs (99%) were used without coating. The prepared particles had γ-Fe2O3 crystalline phase with spherical morphology. Also, the specific surface areas of Fe2O3 NP with 5 nm and 50 nm were 450–2000 m2/g and 50–245 m2/g, respectively.
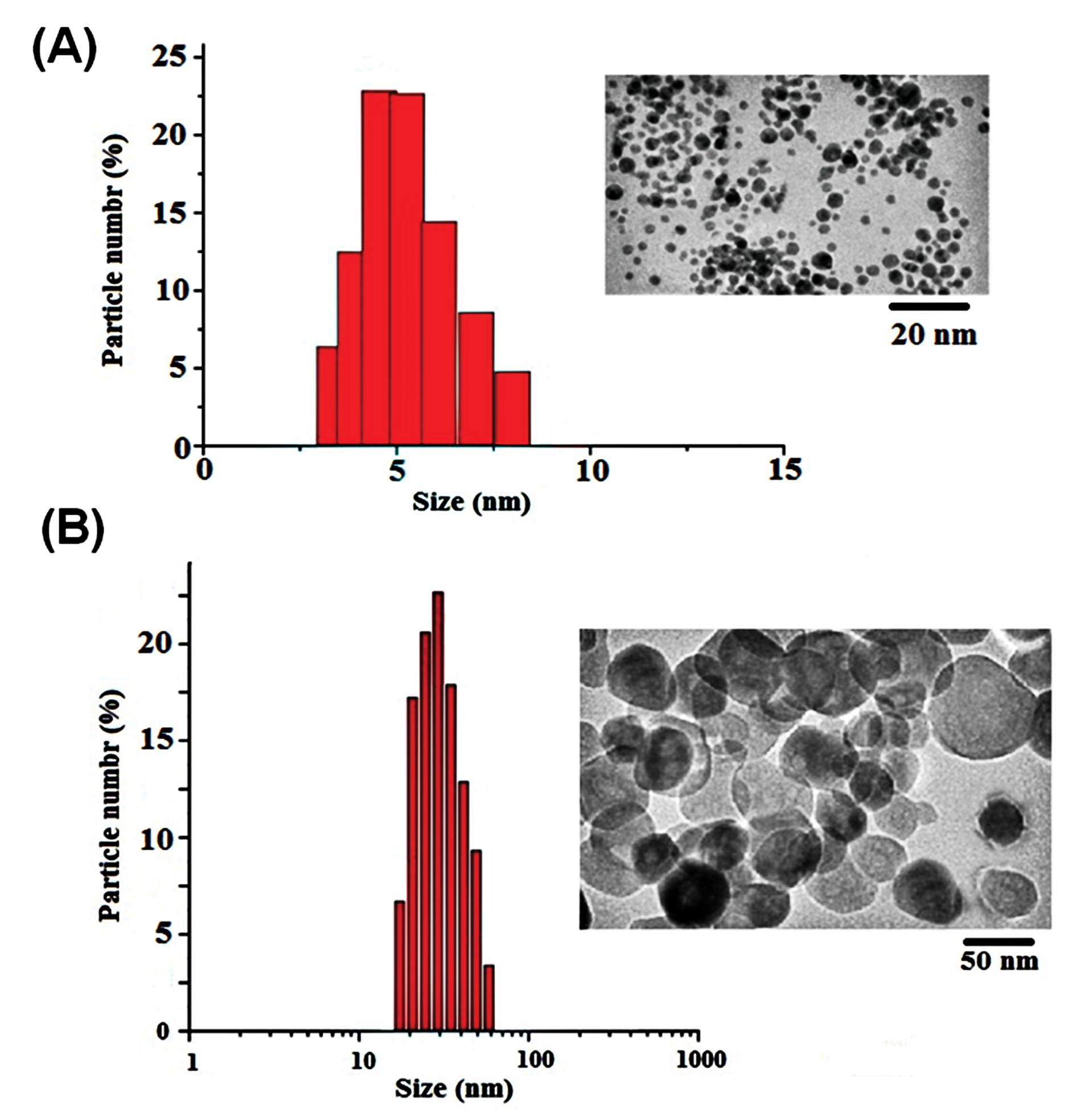
Fig. 1.
DLS analysis of Fe2O3 NPs with 5 nm (A) and 50 nm (B) in size.
.
DLS analysis of Fe2O3 NPs with 5 nm (A) and 50 nm (B) in size.
AChE activity assay
The catalytic activity of AChE in the presence of Fe2O3 NP was investigated using Ellman’s colorimetric method.22 For this purpose, additional concentrations of Fe2O3 NP (0-2500 nM) were added to the reaction solution containing phosphate buffer (0.1 M, pH 7.0) and AChE (0.7 μg/mL). After incubating for 2 hours, DTNB and acetylcholine iodide (as a substrate) were added to the mixture. DTNB and substrate had final concentrations of 0.33 mM and 1.56 mM, respectively. Following incubation of the prepared reaction mixture for 5.0 minutes at 25 °C, the rate of acetylcholine iodide hydrolysis and the formation of 5-thio-2-nitrobenzoate were measured spectrophotometrically using a UV-visible spectrometer (T-60, PG Instruments LTD., Leicestershire, UK) at 412 nm. AChE decomposes acetylcholine iodide and produces thiocholine. The interaction between thiocholine and DTNB results in 5-thio-2-nitrobenzoate production.23,24
Circular dichroism spectroscopy
To obtain insight into the potential effects of Fe2O3 NPs on the secondary structure of AChE, the circular dichroism spectroscopy(CD) spectra of AChE in the absence or presence of Fe2O3 NPs were measured in the far UV spectral region (200-250 nm) using a Jasco model spectropolarimeter at 25 °C. In this regard, different concentrations of Fe2O3 NPs (0-1000 nM) were added to 1.0 mL of 20 mM phosphate buffer solution (pH 7.4; 310 K) containing 2.0 mg/mL AChE. The prepared reaction mixtures were incubated for 5 minutes, and the far UV spectra of the samples were recorded from 200 nm to 250 nm.25 Finally, CDNN software was used to calculate the percentage of changes in the secondary structural elements of the enzyme.
Surface plasmon resonance (SPR) measurements
The kinetic parameters of the AChE-Fe2O3 NPs interaction were investigated using SPR analysis at four different temperatures (298, 303, 310, and 313 K) to obtain the rate constants and affinity between AChE and Fe2O3 NPs. All the SPR analysis was carried out on a double-circuit channel MP-SPR NaviTM 210A device with gold chips (Bio Navis Ltd. Tampere, Finland) after immobilizing AChE on the carboxymethyl dextran (CMD) Au chip. In this regard, the CMD sensor chip was washed using a 10 mM acetate buffer solution (pH=4.5) and then appended to the SPR device. Subsequently, to establish a stable baseline, the chip surface was cleaned by injecting NaCl (2 M) and NaOH (0.1 M) into the apparatus for 30 min at a circuit rate of 30 μL/min. To activate the chip surface, a solution containing 0.05 M NHS and 0.2 M EDC was injected into the device for 7 min. Then, the prepared AChE solution was introduced into channel 1. Channel 2 was used as a reference channel. Finally, the immobilization process was completed by using 0.1 M ethanolamine-HCl (pH=8.5), which was injected into the Au chip surface to block the non-specific binding sites. In addition, to investigate the binding of Fe2O3 NPs to the immobilized AChE and the analysis of kinetic and thermodynamic parameters, additional concentrations of 5 nm Fe2O3 NPs (5, 100, 200, 400, and 800 nM) and 50 nm Fe2O3 NPs (0.5, 1, 2, 4, and 8 µM) were injected into channel 1. SPR NaviTM data viewer software and Trace DrawerTM were utilized for data analysis and the calculation of the interaction parameters, respectively.
Fluorescence spectroscopy
In this work, fluorescence spectroscopy was used to evaluate the conformational changes of AChE upon interaction with Fe2O3 NPs. For this purpose, the intrinsic fluorescence intensity of AChE was measured without and with various concentrations of Fe2O3 NPs (50-1000 nM) using a spectrofluorometer (Jasco, FP-750, Kyoto, Japan) with a 1.0 cm quartz cuvette. A fixed concentration of AChE (0.5 mg/mL) was incubated with different dosages of Fe2O3 NPs (50-1000 nM) for 5 minutes at 310 K in phosphate buffer solution (pH 7.4). Then, the enzyme was excited at 280 nm, and the emission spectra of the samples were recorded in the range of 300-500 nm. The slit width for the excitation and emission was 5 nm.18
Cell culture
To examine the potential effects of Fe2O3 NPs (5 nm and 50 nm) on the catalytic activity of AChE, HepG2 cell lines were cultivated in Williams-fetal bovine serum (Williams-FBS) media containing 10% FBS, 100 U/mL ampicillin, and 100 g/mL streptomycin. The cells were seeded per well of a 12-well culture plate and treated with a 2 µM concentration of Fe2O3 NPs. The treated and untreated control cells were incubated at 5% CO2 in a humidified atmosphere of 95% air and 37 °C for 24 hours. Then, the inhibitory effect of Fe2O3 nanoparticles on AChE activity was evaluated by preparing cell lysate.26 The experiments were repeated three times.
Statistical evaluation
All of the experimental data were analyzed by version 11 of SPSS software, and the expression of the data was done as mean ± standard deviation (SD). One-way variance (ANOVA) was used for the statistical analysis, followed by a multiple-range test using Dennett's approach. Differences at P < 0.05 were considered significant results.
Results and Discussion
Because it may regulate the cholinergic neurotransmitter in the synaptic cleft, AChE is a crucial enzyme for the nervous system's proper operation. Therefore, it is one of the main target enzymes in neural toxicity and progressive neurological disorders such as Alzheimer's. Fe2O3 NPs are applied in various scientific fields and different industries.27 Applications of these NPs in different sizes have been rising in recent years. Despite the wide range of applications of NPs, particularly in medicine, their neurotoxicity poses challenges. Therefore, investigating the toxicity of NPs on various macromolecules, such as enzymes, proteins, and nucleic acids, is of great importance.
Effects of Fe2 O3 NPs on catalytic activity of AChE
Due to their unique physiochemical properties, Fe2O3 NPs are widely used in many in vivo and in vitro research projects.28 These materials are one of the most significant nanomaterials because of their extensive use in magnetic resonance imaging (MRI), ultrasound, optical imaging, X-ray imaging, drug delivery, gene delivery, etc.29-31 Previous studies confirmed that this type of nanomaterials causes harsh oxidative damage in living systems.32 By considering previous results and physiological signs of Fe2O3 NPs poisoning, the main objective of this study was to study the possible effects of Fe2O3 NPs in two different sizes, 5 nm and 50 nm, on the structure and catalytic activity of AChE. As shown in Fig. 2A, the Fe2O3 NPs with a 5 nm size inhibited the enzyme activity in a dose-dependent manner. AChE's catalytic activity was shown to be affected in two ways by 50 nm Fe2O3 NPs (Fig. 2B), and a continuous decrease of enzyme activity was observed with increasing concentrations of 5 nm Fe2O3 NPs. The results indicated that the enzyme's activity increased significantly with increasing the Fe2O3 NPs concentration (up to 60 nM). However, at higher concentrations than 60 nM, a reduction in enzyme activity was observed. It can be concluded that the particles of large size interfere with or compete at the catalytic site of the enzymes and inhibit their action. This suggests that competition with the substrate due to the hydrophobicity of the particle and NPs forming micelles with the microsomal membrane leads to a change in membrane integrity and, thus, enzyme inactivation.33 In addition, with increasing the size of nanoparticles, their entrance into the active site of the enzymes reduces. Therefore, the conformation of the active site cannot be influenced by particles of large size.34
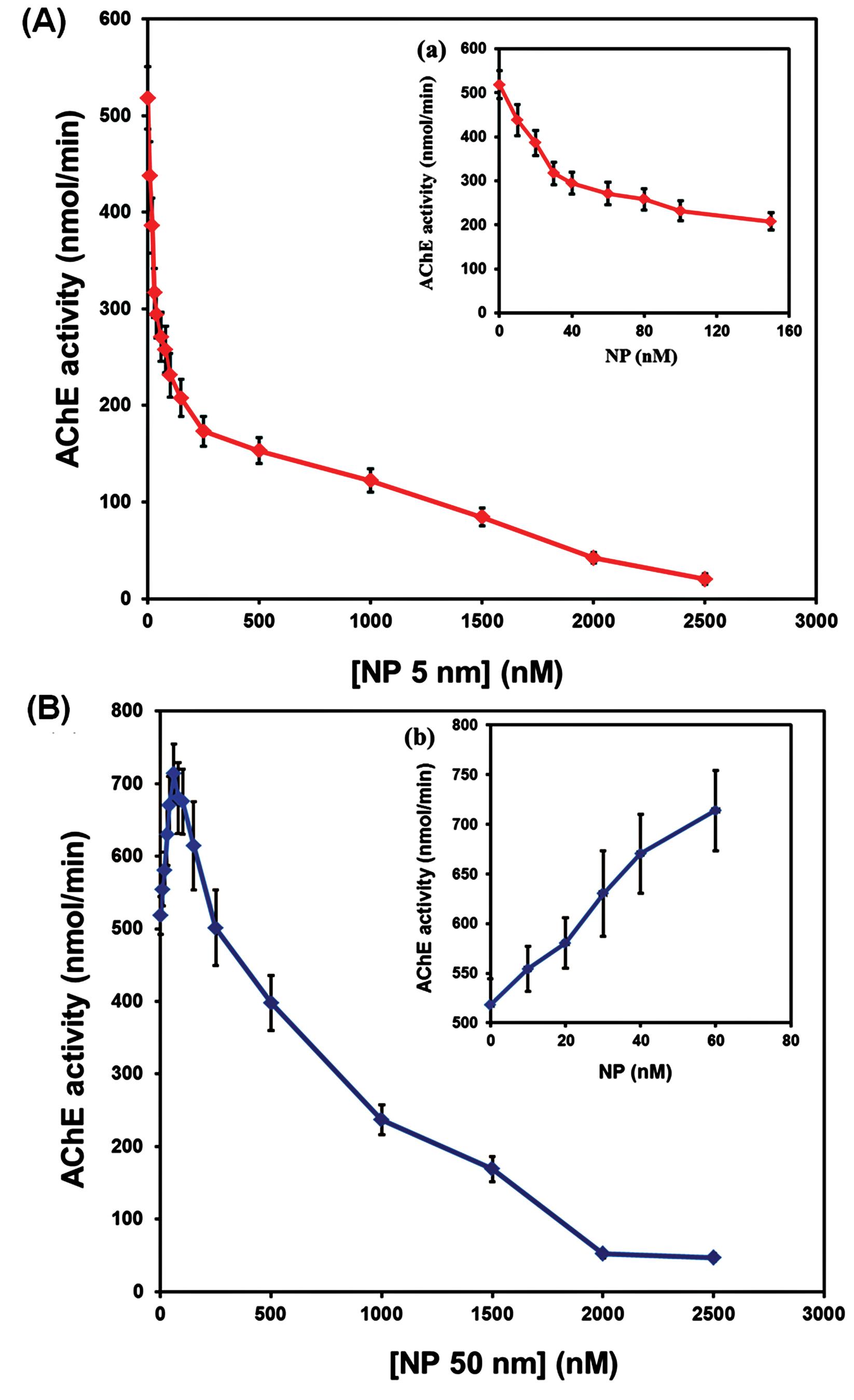
Fig. 2.
AChE catalytic activity in the presence of Fe2O3 NPs of size 5 nm (A) and 50 nm (B) at 25 °C. Data was shown as mean±SD.
.
AChE catalytic activity in the presence of Fe2O3 NPs of size 5 nm (A) and 50 nm (B) at 25 °C. Data was shown as mean±SD.
In addition, the inhibition type of the Fe2O3 NPs (5 and 50 nm) in the AChE action was evaluated. In this regard, AChE was incubated with different concentrations of 5 nm Fe2O3 NPs (0, 60, and 150 nM) and 50 nm Fe2O3 NPs (0, 700, and 1000 nM) and then the activity of the AChE was recorded spectrophotometrically. The results indicated that the catalytic activity of AChE was inhibited in the presence of increasing concentrations of Fe2O3 NPs through a mixed-type mechanism (Fig. 3). The calculated Km and Vmax values are listed in Table 1. As shown in Fig. 3A and B, increasing the 5 nm Fe2O3 NPs concentration mainly increased the Kmvalue from 1.81±0.09 mM for the free enzyme to 2.43±0.11 mM for the AChE-Fe2O3 NPs complex. Also, the Vmax values decreased from 793.15±56.87 nmol/min for the free AChE to 364.28±23.65 nmol/min for the AChE upon interaction with Fe2O3 NPs. On the other hand, 50 nm Fe2O3 NPs caused a reduction in the Km (from 1.81±0.09 mM to 1.31±0.05 mM) and Vmax (from 793.15±56.87 nmol/min to 207.17±13.21 nmol/min) values of AChE (Fig. 3C and D). According to these results and based on the data reported in Table 1, it was concluded that Fe2O3 NPs (5 nm and 50 nm) are able to inhibit the catalytic activity of AChE through mixed mechanism of inhibition.35 According to the results 5 nm NPs could bind to the free enzyme more than enzyme-substrate complex that refer to a competitive-noncompetitive inhibition while the 50 nm NPs prefer to bind to the enzyme-substrate complex (noncompetitive-uncompetitive type of inhibition).
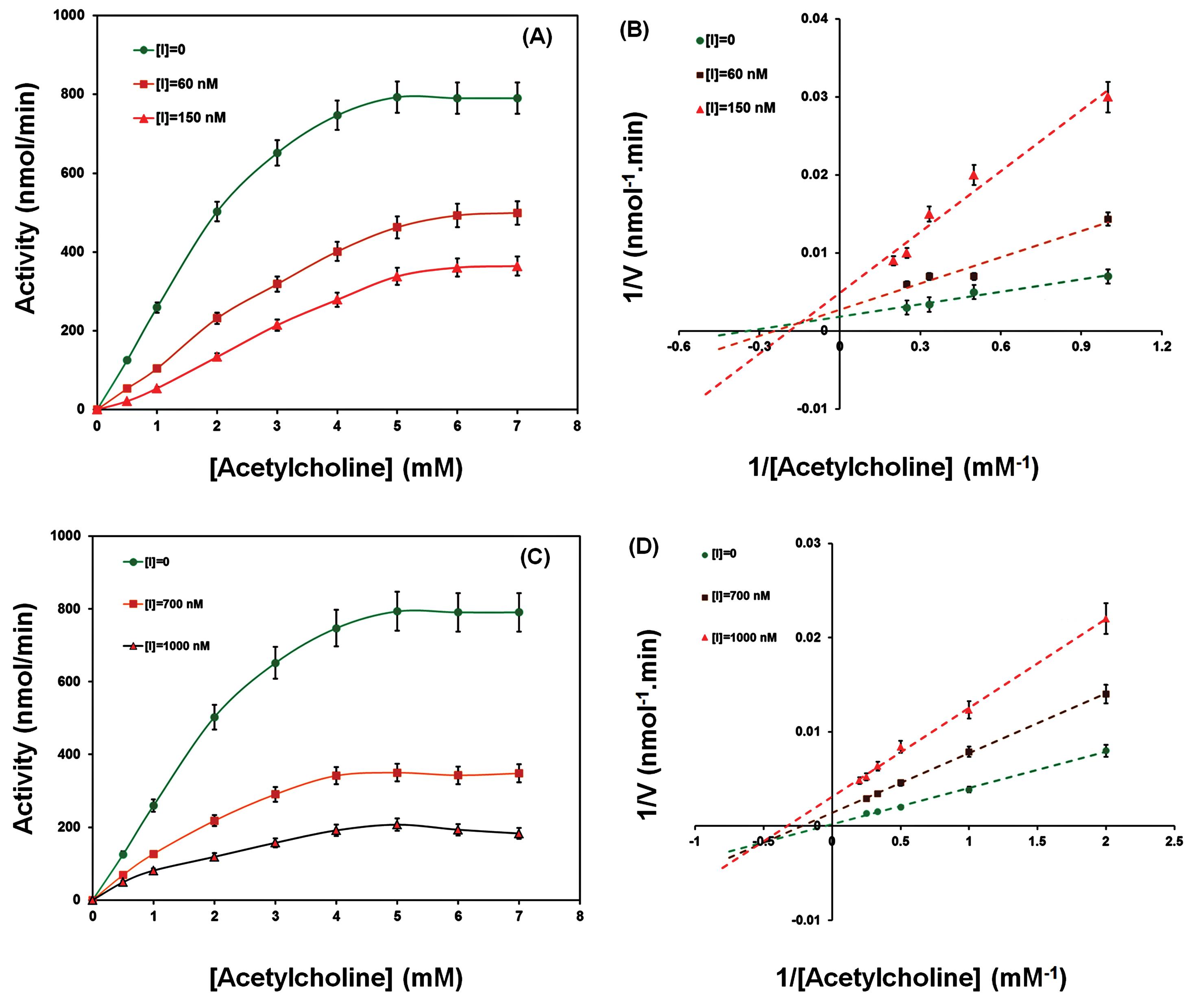
Fig. 3.
Michaelis-Menten and Lineweaver-Burk plots of AChE with and without various concentrations of Fe2O3 NPs with 5 nm (A and B) and 50 nm (C and D) in size.
.
Michaelis-Menten and Lineweaver-Burk plots of AChE with and without various concentrations of Fe2O3 NPs with 5 nm (A and B) and 50 nm (C and D) in size.
Table 1.
The calculated apparent Km and Vmax values for AChE in the presence of Fe2O3 NPs
|
Ligand
|
[NPs] (nM)
|
Km (nM)
|
Vmax (nmol/min)
|
| Fe2O3 NPs(5 nm) |
0 |
1.81±0.09 |
793.15±56.87 |
| 60 |
2.11±0.11 |
499.27±32.72 |
| 150 |
2.43±0.11 |
364.28±23.65 |
| Fe2O3 NPs(50 nm) |
0 |
1.81±0.09 |
793.15±56.87 |
| 700 |
1.60±0.07 |
348.09±21.47 |
| 1000 |
1.31±0.05 |
207.17±13.21 |
Data was represented as mean ± SD.
Fluorescence spectroscopy studies
Fluorescence spectroscopy is one of the most simple, sensitive, and inexpensive methods for evaluating the interactions between macromolecules and ligands, such as protein-ligand interactions. Three different aromatic amino acid residues, including tyrosine (Tyr), tryptophan (Trp), and phenylalanine (Phe), exist in the AChE structure.36 The intrinsic fluorescence intensity of AChE comes from these residues, which are very sensitive to rearrangements in the polarity of their environment.18 In this work, the effect of additional concentrations of Fe2O3 NPs on the emission intensity of AChE was investigated. The maximal emission spectrum of the AChE was observed at 360 nm, a characteristic of Trp residues in a slightly hydrophilic environment. The results showed that Fe2O3 NPs can reduce an enzyme's intrinsic emission by changing its conformation and the polar micro-region of aromatic amino acids (Fig. 4). The fluorescence quenching effects of AChE in the presence of Fe2O3 NPs depict a less compact structure due to increased distances between the fluorophore molecules as well as their more significant interactions with the hydrophilic environment that result in fluorescence quenching.36 The study also showed no shift in the position of the Trp residues, which means that although the inhibitor interacted very closely with AChE to quench fluorescence, the secondary structures of the enzyme may not have been altered.37 It is suggested that a new non-fluorescent complex was formed between AChE and Fe2O3 NPs. The tertiary structure of AChE, in particular the substrate entrance gate of the active site, may be impacted by these particles, which inhibits the enzyme's catalytic activity.
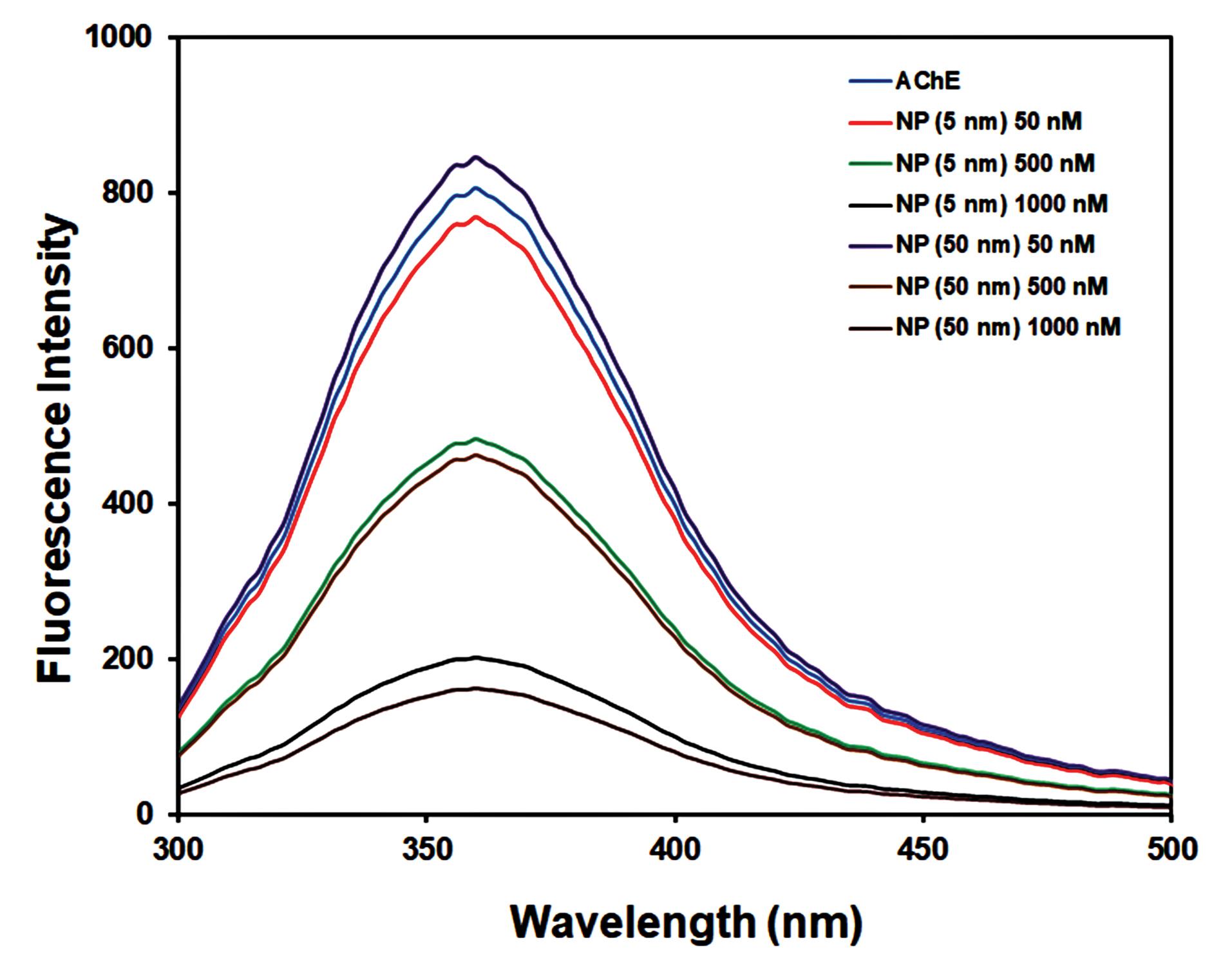
Fig. 4.
Fluorescence spectra of AChE without and with different concentrations (50, 500, and 1000 nM) of Fe2O3 NPs (5 nm, and 50 nm).
.
Fluorescence spectra of AChE without and with different concentrations (50, 500, and 1000 nM) of Fe2O3 NPs (5 nm, and 50 nm).
CD spectroscopy studies
CD spectroscopy is one of the acceptable methods for monitoring the conformational and structural alternations in the secondary structure of different macromolecules, such as proteins induced via binding to specific molecular substances.38 To look into potential impacts on the secondary structure of AChE, a specific concentration (2 mg/mL) of AChE was incubated with various concentrations of Fe2O3 NPs (50–1000 nM) for 3 min, and then, the CD spectra of the samples were recorded in the range of 200-250 nm. In general, the CD spectra of AChE show two main negative bonds, which are located at 208 nm and 222 nm.39 These negative bands are related to π ⟶ π* and n ⟶ π* transitions of amide groups in α-helical structure, respectively. Also, a negative band around 217 nm corresponds to the β-sheets.18 Fig. 5 shows the CD spectra of AChE upon interaction with Fe2O3 NPs. As shown in this figure, after incubating with Fe2O3 NPs, the α-helix and β-sheets contents decreased in the AChE structure. According to Table 2, the native AChE showed a preponderance of 30.1% α-helix, 21.9% β-sheets, 15.2 % β-turn, and 32.8% unordered structures. However, the percentages of these elements changed upon interaction with Fe2O3 NPs of different sizes. The results indicated that the contents of α-helix were decreased to 18.3% and 10.3% in the presence of Fe2O3 NPs with 5 nm and 50 nm (1000 nM), respectively. In addition, Fe2O3 NPs with 5 nm and 50 nm reduced the percentages of β-sheets to 13.8% and 8.5%, respectively. So, these results showed that Fe2O3 NPs can reduce the stability of AChE by decreasing the α-helical structures that refer to the denaturing of proteins in the presence of NPs. The outcomes indicated the alterations in conformation and secondary structure of AChE in the presence of Fe2O3 NPs with a 50 nm size were higher than those of 5 nm Fe2O3 NPs. Due to the existence of the active site of AChE between α-helix and β-sheet domains, it can be concluded that the catalytic activity of the enzyme can be influenced by any changes in their conformations and contents.18 The structure of the active site may also be impacted by AChE's secondary conformational changes, which impact the substrate entrance gate and enzyme activity.40 Therefore, it is suggested that the active site rearrangement caused by Fe2O3 NPs may eventually result in substrate traffic at the entrance gate.
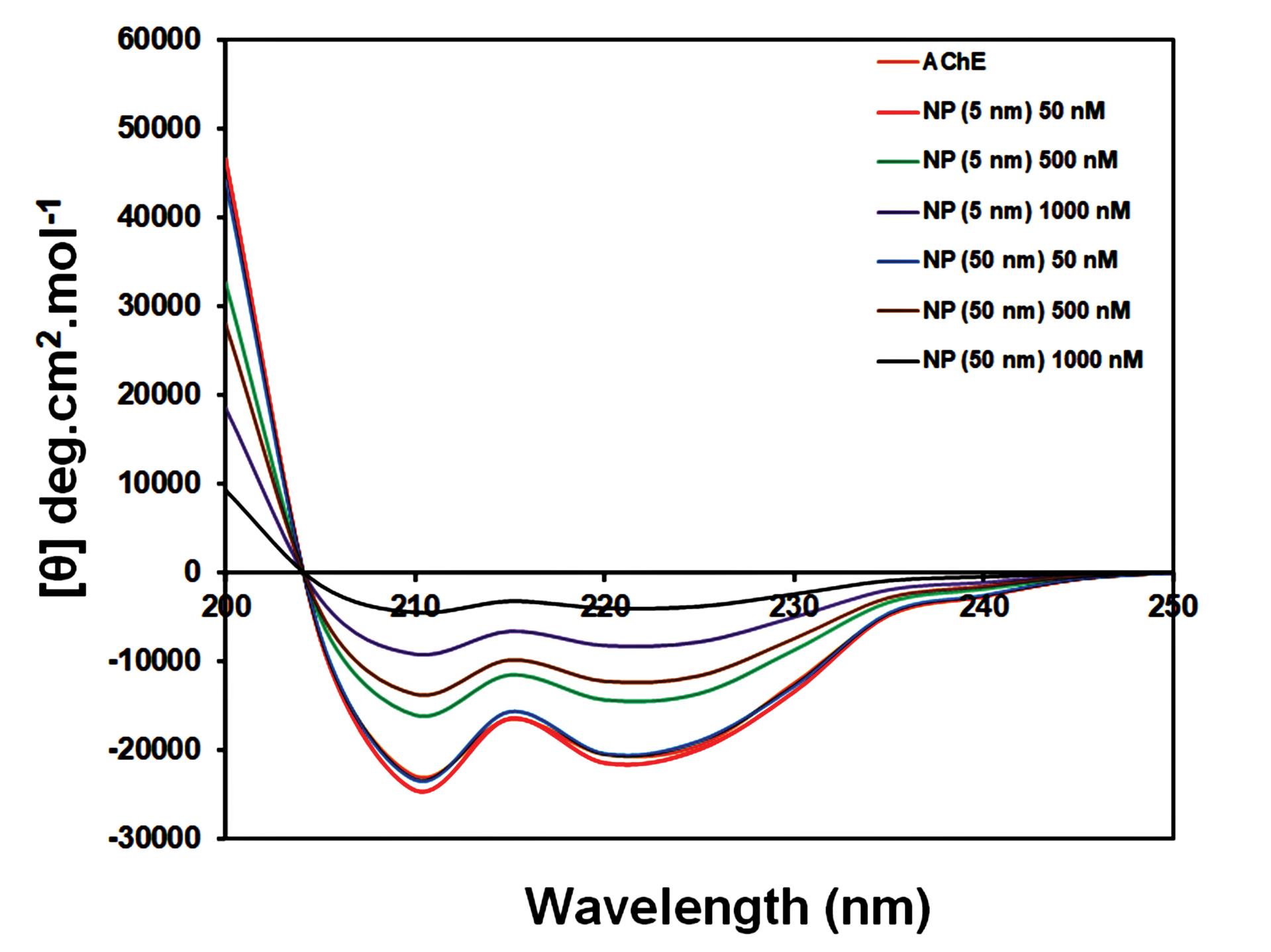
Fig. 5.
CD spectra of AChE in the absence and presence of additional concentrations (50, 500, and 1000 nM) of Fe2O3 NPs.
.
CD spectra of AChE in the absence and presence of additional concentrations (50, 500, and 1000 nM) of Fe2O3 NPs.
Table 2.
Content of secondary structure elements of AChE upon interaction with Fe2O3 NPs at room temperature
|
|
[NPs] (nM)
|
Secondary structure content in AChE (%)
|
|
α-helix
|
β-sheet
|
β-turns
|
Random coil
|
| Fe2O3 NPs(5 nm) |
0 |
30.1 |
21.9 |
15.2 |
32.8 |
| 50 |
31.8 |
22.3 |
15.9 |
30.0 |
| 500 |
24.6 |
20.1 |
18.8 |
36.5 |
| 1000 |
18.3 |
13.8 |
22.1 |
45.8 |
| Fe2O3 NPs(50 nm) |
50 |
30.2 |
21.7 |
14.9 |
33.2 |
| 500 |
22.6 |
18.9 |
21.5 |
37 |
| 1000 |
10.3 |
8.5 |
12.9 |
68.3 |
SPR results
The kinetic parameters of the interaction of Fe2O3 NPs with immobilized AChE were assessed using the SPR technique. These parameters reveal the affinity between a ligand and a macromolecule. To this end, different concentrations of Fe2O3 NPs were injected into the chip surface, and then the SPR signals were recorded. Fig. 6 shows the SPR sensorgram of the binding of AChE with Fe2O3 NPs at 298K (the SPR sensorgrams at 303, 310, and 313 K are not shown). It is clear that with increasing the concentration of Fe2O3 NPs the binding signal gradually increased, too. The equilibrium constant (KD) values were calculated, and the findings are summarized in Table 3. According to this table, the low values of KDindicate a high affinity between ligands and AChE.41 Additionally, the findings showed that the KD values increased as the temperature rose, supporting that the affinity between AChE and Fe2O3 NPs and the reaction rate decreased with rising temperature.42
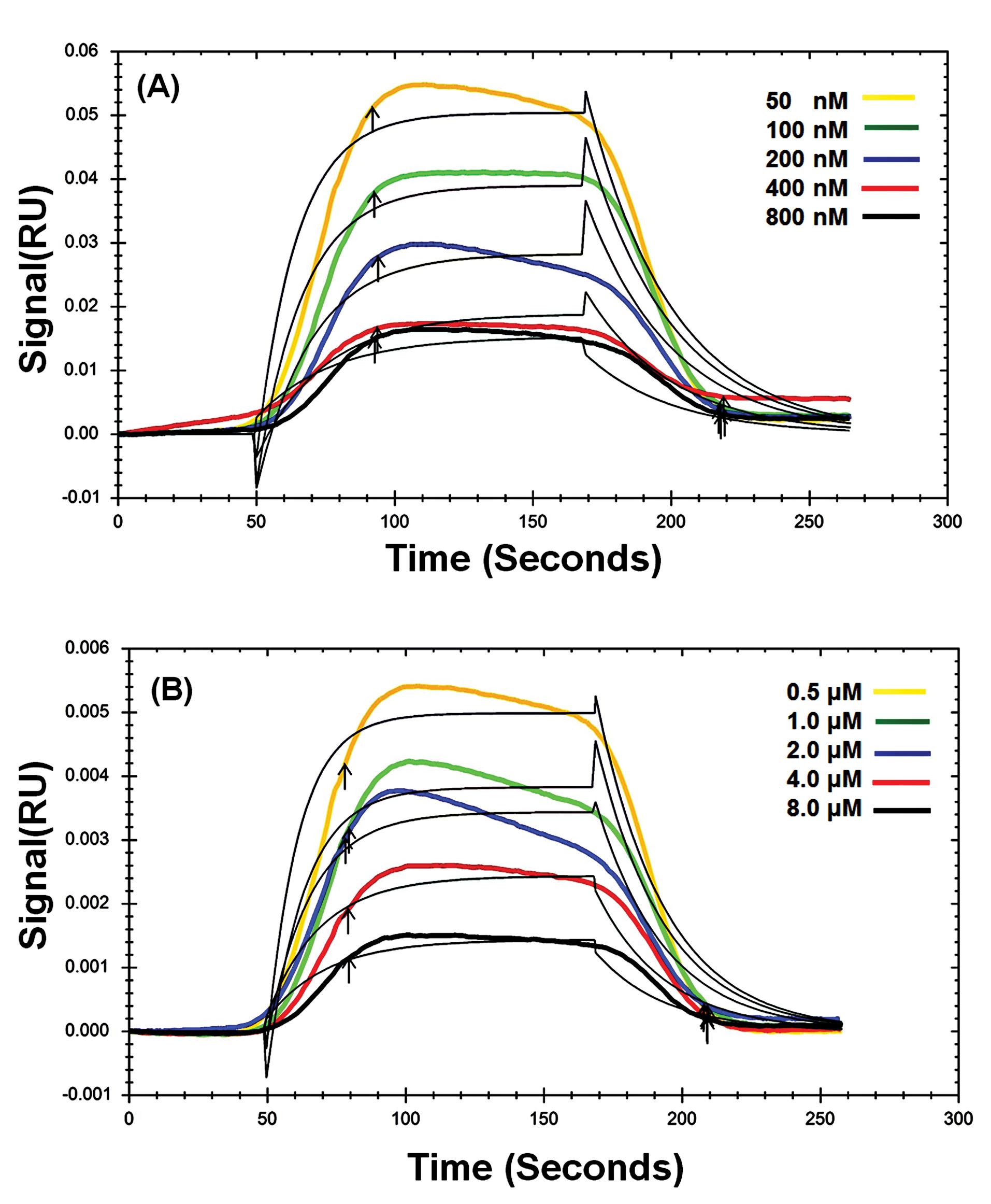
Fig. 6.
Dose-response sensorgrams of AChE in the presence of Fe2O3 NPs with 5 nm (A) and 50 nm (B) in size at 298 K.
.
Dose-response sensorgrams of AChE in the presence of Fe2O3 NPs with 5 nm (A) and 50 nm (B) in size at 298 K.
Thermodynamic analysis
It has been reported that various acting forces, such as hydrogen bonds, electrostatic forces, Vander Waals forces, and hydrophobic interactions, play a critical role in the complex formation between a ligand and a macromolecule.38,43 In the present work, the thermodynamic parameters were calculated using equations 1 and 2.
Here, R (8.314 J/mol/K) is the universal gas constant and T denotes the absolute temperature, respectively.
The plot of lnKD against 1/T (Van’t Hoff plot) was constructed and then the slope and the intercept of the plot was used for the calculation of ΔH and ΔS, respectively (Fig. 7). The calculated values are reported in Table 3. Based on these data, it can be concluded that Fe2O3 NPs bind AChE non-spontaneously (ΔG>0). Also, the positive values of ΔH means that the system has gotten energy from the surroundings in the form of heat. In addition, the reaction was an endothermic since the products have a greater energy level than the reactants and the net heat was absorbed. A positive ΔH and negative ΔS cause a positive ΔG so the reaction is not spontaneous.
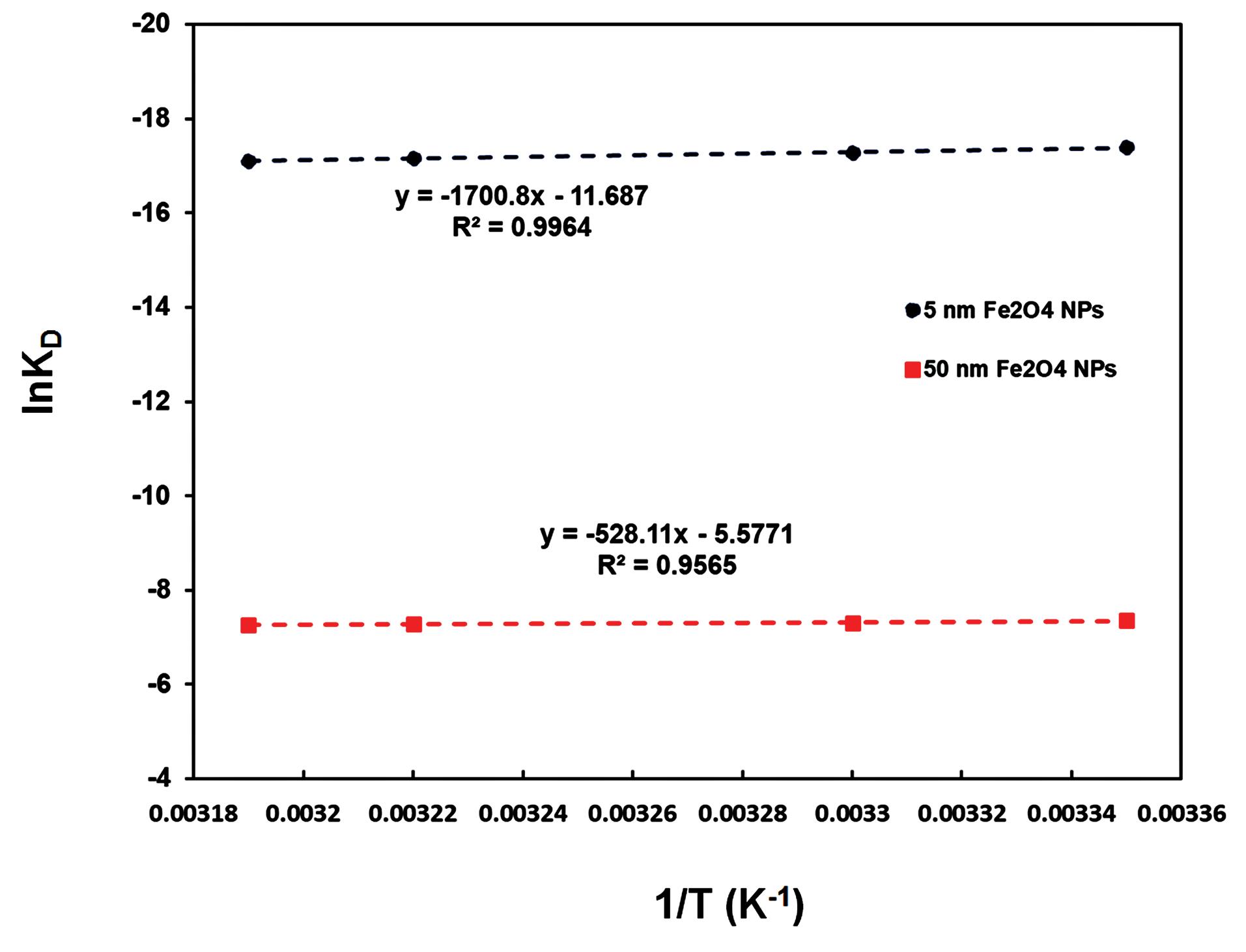
Fig. 7.
Van't Hoff curves for the binding of Fe2O3 NPs with 5 nm (A) and 50 nm (B) in size to AChE.
.
Van't Hoff curves for the binding of Fe2O3 NPs with 5 nm (A) and 50 nm (B) in size to AChE.
Table 3.
Equilibrium constants (KD) for binding of Fe2O3 NPs (5 nm and 50 nm) to AChE at different temperatures
|
Sample
|
T (K)
|
KD (M)
|
ΔH (kJ/mol)
|
ΔS (kJ/mol)
|
ΔG (kJ/mol/ k)
|
| Fe2O3 NPs(5 nm) |
298 |
2.8 × 10-8 |
14.14 |
-0.097 |
43.09 |
| 303 |
3.1 × 10-8 |
43.57 |
| 310 |
3.5 × 10-8 |
44.26 |
| 313 |
3.7 × 10-8 |
44.55 |
| Fe2O3 NPs(50 nm) |
298 |
6.4 × 10-4 |
4.39 |
-0.046 |
18.20 |
| 303 |
6.7 × 10-4 |
18.43 |
| 310 |
6.9 × 10-4 |
18.76 |
| 313 |
7.0 × 10-4 |
18.90 |
AChE activity of HepG2 cells
In this work, HepG2 cells were utilized as a model to investigate the impact of Fe2O3 NPs on AChEcatalyticactivity inside the cells. For this purpose, the prepared cell lysates were used to assess the catalytic activity of AChE. The findings are shown in Fig. 8. It can be seen from this figure that Fe2O3 NPs caused a decrease in AChE activity. However, the inhibitory effect of Fe2O3 NPs with a 5 nm size (65 %) was significantly higher than that of a 50 nm size (20%). Therefore, it can be concluded that Fe2O3 NPs inhibit the catalyticactivity of AChEin a size-dependent manner. Based on these results, nanoparticles with a smaller size can easily cross the blood-brain barrier, interact with various neurological targets such as AChE, and cause neurotoxic effects.44 According to this study, due to their interaction with AChE, Fe2O3 NPs (of various sizes) may not be safe or even be neurotoxic. However, the small particles have a more significant neurotoxic effect on the native structure and catalyticactivity of AChE.44,45
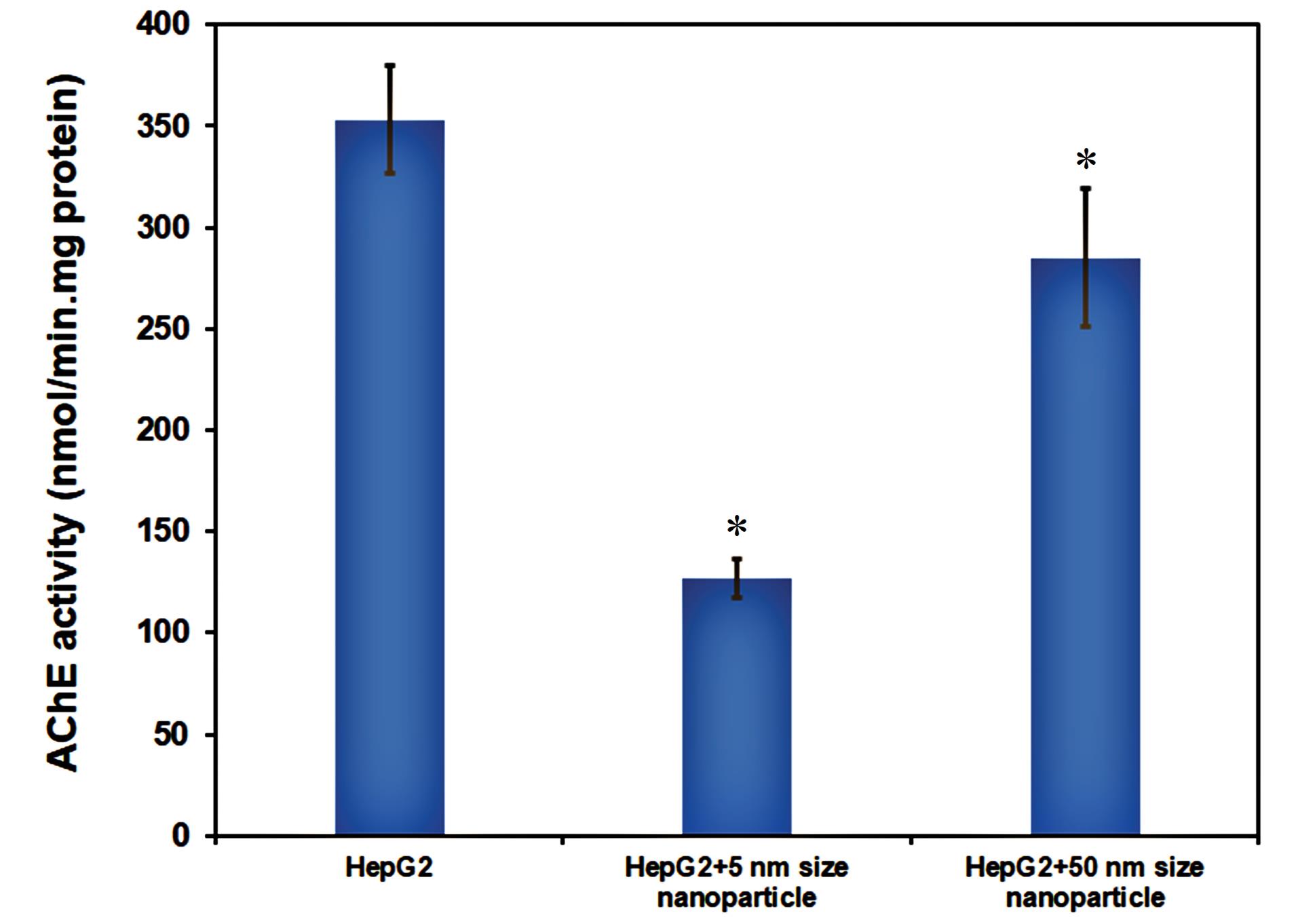
Fig. 8.
AChE activity assessment in HepG2 cell lines in the absence and presence of Fe2O3 NPs. Data was shown as mean±SD and star symbols show significant difference (P<0.05) in comparison with the control (without NPs).
.
AChE activity assessment in HepG2 cell lines in the absence and presence of Fe2O3 NPs. Data was shown as mean±SD and star symbols show significant difference (P<0.05) in comparison with the control (without NPs).
Conclusion
In this work, the inhibitory effects of Fe2O3 NPs (with two different sizes, 5 nm, and 50 nm) on the catalytic activity of AChE were investigated, and the obtained results indicated that Fe2O3 NPs can inhibit AChE activity in a dose-dependent manner. The results confirmed the inhibitory effect of 5 nm Fe2O3 NPs on AChE activity via mixed mechanism. However, it was observed that the AChE activity was increased in the presence of 50 nm Fe2O3 NPs (up to 60 nM) and then decreased. Also, conformational studies were performed using fluorescence and CD spectroscopy analysis. The results suggested that Fe2O3 NPs could change the secondary and tertiary structures of the enzyme by reducing the amount of α-helix and β-sheet, leading to the unfolding of the enzyme structure. According to the results, the conformational changes of the enzyme in the presence of 50 nm Fe2O3 NPs were higher than those of 5 nm Fe2O3 NPs. On the other hand, the interaction between AChE and Fe2O3 NPs was studied using the SPR method, and the results indicated that the KD values increased with rising temperature, suggesting a reduction in the affinity of AChE towards Fe2O3 NPs. Thermodynamic studies revealed that the AChE/Fe2O3 NPs complex was formed through a nonspontaneous process. In addition, the effect of Fe2O3 NPs on AChEcatalyticactivity was evaluated in HepG2 cell lines, and the obtained results showed that the inhibitory effect of Fe2O3 NPs with a 5 nm size was significantly higher than that of a 50 nm size, possibly due to increased surface area to volume ratio. This study provided new insights into the impact of Fe2O3 NPs on the function and structure of AChE, and a novel mechanism of Fe2O3 NPs poisoning in the dysfunction of the cholinergic system. Considering the increasing application of NPs in different fields and specific features of NPs such as high reactivity, penetration through the blood-brain barrier, and oxidative damage, our results provide strong reasons why the application of NPs should be limited in all aspects of life. It is valuable to notice that the results of this study could help extend the knowledge of utilizing Fe2O3 NPs for poisoning in the neural system.
Research Highlights
What is the current knowledge?
√ During the last decade, nanotechnology has had extensive applications as nanomedicine in the medical field.
√ Hence, it is essential to establish the toxicity, safety, and risks involved in the use of nanoparticles.
What is new here?
√ The effect of Fe2O3 NPs on AChE activity depends on the size of the nanoparticles.
√ The Fe2O3 NPs nanoparticles in small sizes inhibit enzyme activity, but in larger sizes, they show a dual effect on enzyme activity.
√ The Fe2O3 NPs inhibit the enzyme activity in vivo.
Competing Interests
We wish to confirm that there are no known conflicts of interest associated with this publication.
Ethical Statement
None to be stated.
References
- Srivastava V, Gusain D, Sharma YC. Critical review on the toxicity of some widely used engineered nanoparticles. Ind Eng Chem Res 2015; 54:6209-33. doi: 10.1021/acs.iecr.5b01610 [Crossref] [ Google Scholar]
- Mirzajani F, Motevalli SM, Jabbari S, Siadat SOR, Sefidbakht Y. Recombinant acetylcholinesterase purification and its interaction with silver nanoparticle. Protein Expr Purif 2017. 136: 58-65. 10.1016/j.pep.2017.05.007.
- Rashtbari S, Dehghan G, Khataee S, Amini M, Khataee A. Dual enzymes-mimic activity of nanolayered manganese-calcium oxide for fluorometric determination of metformin. Chemosphere 2022; 291:133063. doi: 10.1016/j.chemosphere.2021.133063 [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G, Amini M, Khorram S, Khataee A. A sensitive colori/fluorimetric nanoprobe for detection of polyphenols using peroxidase-mimic plasma-modified MoO3 nanoparticles. Chemosphere 2022; 295:133747. doi: 10.1016/j.chemosphere.2022.133747 [Crossref] [ Google Scholar]
- Dietrich J, Enke A, Wilharm N, Konieczny R, Lotnyk A, Anders A. Energetic Electron-Assisted Synthesis of Tailored Magnetite (Fe3O4) and Maghemite (γ− Fe2O3) Nanoparticles: Structure and Magnetic Properties. Nanomaterials 2023; 13:786. [ Google Scholar]
- Mahmoudi M, Shokrgozar MA, Sardari S, Moghadam MK, Vali H, Laurent S. Irreversible changes in protein conformation due to interaction with superparamagnetic iron oxide nanoparticles. Nanoscale 2011; 3:1127-38. doi: 10.1039/c0nr00733a [Crossref] [ Google Scholar]
- Dulińska-Litewka J, Łazarczyk A, Hałubiec P, Szafrański O, Karnas K, Karewicz A. Superparamagnetic iron oxide nanoparticles—Current and prospective medical applications. Materials 2019; 12:617. doi: 10.3390/ma12040617 [Crossref] [ Google Scholar]
- Nguyen MD, Tran H-V, Xu S, Lee TR. Fe3O4 nanoparticles: structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl Sci 2021; 11:11301. [ Google Scholar]
- Srinoi P, Chen Y-T, Vittur V, Marquez MD, Lee TR. Bimetallic nanoparticles: enhanced magnetic and optical properties for emerging biological applications. Appl Sci 2018; 8:1106. [ Google Scholar]
- Wu K, Saha R, Su D, Krishna VD, Liu J, Cheeran MC-J. Magnetic-nanosensor-based virus and pathogen detection strategies before and during COVID-19. ACS Appl Nano Mater 2020; 3:9560-80. doi: 10.1021/acsanm.0c02048 [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G, Amini M. An ultrasensitive label-free colorimetric biosensor for the detection of glucose based on glucose oxidase-like activity of nanolayered manganese-calcium oxide. Anal Chim Acta 2020; 1110:98-108. doi: 10.1016/j.aca.2020.03.021 [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G, Khorram S, Amini M, Khataee A, Yoon Y. Plasma modified Co3O4 nanoparticles for catalytic degradation process through enhanced peroxidase-like activity. J Ind Eng Chem 2023; 121:114-123. doi: 10.1016/j.jiec.2023.01.015 [Crossref] [ Google Scholar]
- Wu Z, Zhang B, Yan B. Regulation of enzyme activity through interactions with nanoparticles. Int J Mol Sci 2009; 10:4198-209. doi: 10.3390/ijms10104198 [Crossref] [ Google Scholar]
- Radman M. Dysfunction and toxicity of damaged proteins in the etiology of aging and age-related degenerative and malignant diseases. Croat Med J 2020; 61:159-66. doi: 10.3325/cmj.2020.61.159 [Crossref] [ Google Scholar]
- Aletaha N, Dehghan G, Sadeghi L, Rashtbari S, Khataee A. Binding mechanism of perphenazine/thioridazine with acetylcholinesterase: Spectroscopic surface plasmon resonance and molecular docking based analysis. J Mol Liq 2023; 377:121547. doi: 10.1016/j.molliq.2023.121547 [Crossref] [ Google Scholar]
- Campanha HM, Carvalho F, Schlosser PM. Active and peripheral anionic sites of acetylcholinesterase have differential modulation effects on cell proliferation, adhesion and neuritogenesis in the NG108-15 cell line. Toxicol Lett 2014; 230:122-31. doi: 10.1016/j.toxlet.2014.03.012 [Crossref] [ Google Scholar]
- Assis CRD, Linhares AG, Oliveira VM, França RCP, Santos JF, Carvalho E. Effect of ions on the activity of brain acetylcholinesterase from tropical fish. Journal of Coastal Life Medicine 2015; 3:505-14. doi: 10.12980/JCLM.3.2015J5-11 [Crossref] [ Google Scholar]
- Yekta R, Sadeghi L, Dehghan G. The inefficacy of donepezil on glycated-AChE inhibition: Binding affinity, complex stability and mechanism. Int J Biol Macromol 2020; 160:35-46. doi: 10.1016/j.ijbiomac.2020.05.177 [Crossref] [ Google Scholar]
- Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 2013; 11:315-35. doi: 10.2174/1570159X11311030006 [Crossref] [ Google Scholar]
- Hayden KM, Norton MC, Darcey D, Østbye T, Zandi PP, Breitner J. Occupational exposure to pesticides increases the risk of incident AD: the Cache County study. Neurology 2010; 74:1524-30. doi: 10.1212/WNL.0b013e3181dd4423 [Crossref] [ Google Scholar]
- Askri D, Cunin V, Ouni S, Béal D, Rachidi W, Sakly M. Effects of iron oxide nanoparticles (γ-Fe2O3) on liver, lung and brain proteomes following sub-acute intranasal exposure: A new toxicological assessment in rat model using iTRAQ-based quantitative proteomics. Int J Mol Sci 2019; 20:5186. doi: 10.3390/2Fijms20205186 [Crossref] [ Google Scholar]
- Ellman GL, Courtney KD, Andres Jr V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7:88-95. doi: 10.1016/0006-2952(61)90145-9 [Crossref] [ Google Scholar]
- Worek F, Eyer P, Thiermann H. Determination of acetylcholinesterase activity by the Ellman assay: a versatile tool for in vitro research on medical countermeasures against organophosphate poisoning. Drug Test Anal 2012; 4:282-91. doi: 10.1002/dta.337 [Crossref] [ Google Scholar]
- Yousefi Babadi V, Sadeghi L, Shirani K, Malekirad AA, Rezaei M. The toxic effect of manganese on the acetylcholinesterase activity in rat brains. J Toxicol 2014; 2014:946372. doi: 10.1155/2014/946372 [Crossref] [ Google Scholar]
- Patil DN, Patil SA, Sistla S, Jadhav JP. Comparative biophysical characterization: A screening tool for acetylcholinesterase inhibitors. PLoS One 2019; 14:e0215291. doi: 10.1371/journal.pone.0215291 [Crossref] [ Google Scholar]
- Pérez-Aguilar B, Vidal CJ, Palomec G, García-Dolores F, Gutiérrez-Ruiz MC, Bucio L. Acetylcholinesterase is associated with a decrease in cell proliferation of hepatocellular carcinoma cells. Biochim Biophys Acta Mol Basis Dis 2015; 1852:1380-7. doi: 10.1016/j.bbadis.2015.04.003 [Crossref] [ Google Scholar]
- Van Nhan L, Ma C, Rui Y, Cao W, Deng Y, Liu L. The effects of Fe2O3 nanoparticles on physiology and insecticide activity in non-transgenic and Bt-transgenic cotton. Front Plant Sci 2016; 6:1263. doi: 10.3389/fpls.2015.01263 [Crossref] [ Google Scholar]
- Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev 2008; 60:1252-65. doi: 10.1016/j.addr.2008.03.018 [Crossref] [ Google Scholar]
- Hemalatha T, Prabu P, Gunadharini DN, Gowthaman MK. Fabrication and characterization of dual acting oleyl chitosan functionalised iron oxide/gold hybrid nanoparticles for MRI and CT imaging. Int J Biol Macromol 2018; 112:250-7. doi: 10.1016/j.ijbiomac.2018.01.159 [Crossref] [ Google Scholar]
- Unsoy G, Yalcin S, Khodadust R, Gunduz G, Gunduz U. Synthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applications. J Nanopart Res 2012; 14:1-13. doi: 10.1007/s11051-012-0964-8 [Crossref] [ Google Scholar]
- Al-Hakkani MF, Gouda GA, Hassan SH. A review of green methods for phyto-fabrication of hematite (α-Fe2O3) nanoparticles and their characterization, properties, and applications. Heliyon 2021; 7:e05806. doi: 10.1016/j.heliyon.2020.e05806 [Crossref] [ Google Scholar]
- Sadeghi L, Tanwir F, Babadi VY. In vitro toxicity of iron oxide nanoparticle: Oxidative damages on Hep G2 cells. Exp Toxicol Pathol 2015; 67:197-203. doi: 10.1016/j.etp.2014.11.010 [Crossref] [ Google Scholar]
- Fröhlich E, Kueznik T, Samberger C, Roblegg E, Wrighton C, Pieber TR. Size-dependent effects of nanoparticles on the activity of cytochrome P450 isoenzymes. Toxicol Appl Pharmacol 2010; 242:326-32. doi: 10.1016/j.taap.2009.11.002 [Crossref] [ Google Scholar]
- Chen W-Q, Wu W-J, Yu Y-Q, Liu Y, Jiang F-L. New Insights on the Size-Dependent Inhibition of Enzymes by Gold Nanoparticles. Langmuir 2023; 39:9595-603. doi: 10.1021/acs.langmuir.3c01367 [Crossref] [ Google Scholar]
- Agarwal PK. Enzymes: An integrated view of structure, dynamics and function. Microbial cell factories 2006; 5:1-12. doi: 10.1186/1475-2859-5-2 [Crossref] [ Google Scholar]
- Asen ND, Okagu OD, Udenigwe CC, Aluko RE. In vitro inhibition of acetylcholinesterase activity by yellow field pea (Pisum sativum) protein-derived peptides as revealed by kinetics and molecular docking. Front Nutr 2022; 9:1021893. doi: 10.3389/fnut.2022.1021893 [Crossref] [ Google Scholar]
- Malta SM, Batista LL, Silva HCG, Franco RR, Silva MH, Rodrigues TS. Identification of bioactive peptides from a Brazilian kefir sample, and their anti-Alzheimer potential in Drosophila melanogaster. Sci Rep 2022; 12:11065. doi: 10.1038/s41598-022-15297-1 [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G, Yekta R, Jouyban A. Investigation of the binding mechanism and inhibition of bovine liver catalase by quercetin: Multi-spectroscopic and computational study. Bioimpacts 2017; 7:147. doi: 10.15171/bi.2017.18 [Crossref] [ Google Scholar]
- Xiang J, Yu C, Yang F, Yang L, Ding H. Conformation-activity studies on the interaction of berberine with acetylcholinesterase: Physical chemistry approach. Prog Nat Sci 2009; 19:1721-5. doi: 10.1016/j.pnsc.2009.07.010 [Crossref] [ Google Scholar]
- Colletier JP, Fournier D, Greenblatt HM, Stojan J, Sussman JL, Zaccai G. Structural insights into substrate traffic and inhibition in acetylcholinesterase. EMBO J 2006; 25:2746-56. doi: 10.1038/sj.emboj.7601175 [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G, Sadeghi L, Sareminia L, Iranshahy M, Iranshahi M. Interaction of bovine serum albumin with ellagic acid and urolithins A and B: Insights from surface plasmon resonance, fluorescence, and molecular docking techniques. Food Chem Toxicol 2022; 162:112913. doi: 10.1016/j.fct.2022.112913 [Crossref] [ Google Scholar]
- Khataee S, Dehghan G, Yekta R, Rashtbari S, Maleki S, Khataee A. The protective effect of natural phenolic compound on the functional and structural responses of inhibited catalase by a common azo food dye. Food Chem Toxicol 2022; 160:112801. doi: 10.1016/j.fct.2021.112801 [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G, Yekta R, Jouyban A, Iranshahi M. Effects of resveratrol on the structure and catalytic function of bovine liver catalase (BLC): spectroscopic and theoretical studies. Adv Pharm Bull 2017; 7:349. doi: 10.15171/apb.2017.042 [Crossref] [ Google Scholar]
- Khatoon A, Khan F, Ahmad N, Shaikh S, Rizvi SMD, Shakil S. Silver nanoparticles from leaf extract of Mentha piperita: eco-friendly synthesis and effect on acetylcholinesterase activity. Life Sci 2018; 209:430-4. doi: 10.1016/j.lfs.2018.08.046 [Crossref] [ Google Scholar]
- Cabaleiro-Lago C, Lundqvist M. The effect of nanoparticles on the structure and enzymatic activity of human carbonic anhydrase I and II. Molecules 2020; 25:4405. doi: 10.3390/molecules25194405 [Crossref] [ Google Scholar]