Bioimpacts. 14(3):29981.
doi: 10.34172/bi.2023.29981
Original Article
Podocyte-specific proteins in urinary extracellular vesicles of patients with IgA nephropathy: Vasorin and ceruloplasmin
Negin Farzamikia Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing, 1, 2 
Seyyedeh Mina Hejazian Formal analysis, Resources, Writing – original draft, Writing – review & editing, 1
Soroush Mostafavi Writing – original draft, 3
Behzad Baradaran Conceptualization, Methodology, Validation, 4
Sepideh Zununi Vahed Formal analysis, Investigation, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing, 1, * 
Mohammadreza Ardalan Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing, 1, * 
Author information:
1Kidney Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Biotechnology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Cardiology, Hazrat-e-Rasool General Hospital, Iran University of Medical Sciences, Tehran, Iran
4Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Urinary extracellular vesicles (uEVs) can be considered biomarkers of kidney diseases. EVs derived from podocytes may reflect podocyte damage in different glomerular diseases. IgA nephropathy (IgAN) is one of the most common forms of glomerulonephritis (GN) characterized by proteinuria and hematuria. This study aimed to analyze the uEVs of IgAN patients to understand the pathophysiological processes of the disease at the protein level.
Methods:
Patients with GN [biopsy-proven IgAN (n = 16) and membranous glomerulonephritis (MGN, n = 16)], and healthy controls (n = 16) were included in this study. The uEVs were extracted, characterized, and analyzed to evaluate the protein levels of candidate markers of IgAN, including vasorin precursor, aminopeptidase N, and ceruloplasmin by western-blot analysis.
Results:
Higher levels of both podocytes and EVs-related proteins were observed in the pooled urine samples of GN patients compared to the healthy controls. In IgAN patients, uEV-protein levels of vasorin were statistically lower while levels of ceruloplasmin were significantly higher compared to MGN (P = 0.002, P = 0.06) and healthy controls, respectively (P = 0.020, P= 0.001).
Conclusion:
Different levels of the studied proteins in uEVs may indicate podocyte injury and represent a direct association with the pathology of IgAN and MGN.
Keywords: Extracellular vesicles, IgA-nephropathy, Membranous nephropathy, Podocyte, Vasorin
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
IgA nephropathy (IgAN) is one of the most common forms of chronic kidney disease (CKD) identified by the deposition of IgA in glomerular mesangial cells. Approximately 15–40% of patients with IgAN progress to end-stage renal disease (ESRD) in 5–25 years.1,2 Evidence proposes that beyond kidney mesangial cells, indirect podocyte damage participates in glomerular injury and pathogenesis of IgAN, which is clinically manifested by proteinuria and nephritic syndrome or glomerulosclerosis failure.1,3,4
At present, despite being the gold standard diagnostic method for IgAN, renal biopsy is invasive, painful, and expensive, and it cannot be performed repeatedly to check disease severity and progression.4,5 Therefore, novel, non-invasive, and reliable biomarkers related to kidney histological damage are needed for the diagnosis and monitoring of IgAN.6,7 The use of the proteomic panel as a diagnostic biomarker has received considerable attention in recent years. The urine proteomic analysis has been broadly studied to understand the mechanisms influencing the kidney,8 type 1 diabetes,9 and acute rejection of renal allografts.10
Since 2013, plasma membrane-originated vesicles, known as extracellular vesicles (EVs), have been discovered to form under various conditions like stress, cell activation, and apoptosis. The EVs are globular-like structures 100–1000 nm in size and contain different bioactive molecules, such as signaling molecules, proteins, mRNAs, microRNAs, lipids, long non-coding RNAs, and DNA. EVs are released by the majority of cell types and can be found in all biological fluids. Urinary EVs are proper materials to discover candidate biomarkers for the diagnosis of kidney disease11-13 and they can be detected by performing liquid biopsy and reflecting the pattern and severity of renal injury.14-16
According to previous studies, EVs derived from podocytes are present in the urine of patients with different glomerulonephritis (GN) such as diabetic nephropathy,17,18 lupus nephritis,19 renal injury in preeclampsia,20 and renovascular hypertensive disorder.21 The analysis of uEVs-derived proteins from patients with kidney diseases can help detect the diagnostic biomarkers. This study aimed to analyze the IgAN-related proteins including vasorin precursor, Aminopeptidase N, and ceruloplasmin5 in uEVs of IgAN patients to detect the proper candidate biomarkers for IgAN.
Materials and Methods
Patients and sampling
In this cross-sectional study, 32 patients with GN [IgAN and membranous glomerulonephritis (MGN)] were recruited from November 2019 to October 2021. Sixteen biopsy-proven IgAN patients with an age range of 18 to 50 years were included. Moreover, 16 patients with biopsy-proven primary MGN and 16 healthy volunteers were included as control groups. MGN patients with an anti-phospholipase A2 receptor (PLA2R1) activity and those not receiving rituximab were included. Patients with secondary MGN due to cancer, drugs, infectious and systemic lupus erythematosus, and other autoimmune diseases were excluded. Demographic, biochemical, and clinical data of IgAN, MGN, and healthy participants were recorded. The whole stream of early morning urine samples was centrifuged at 300 ×g for 10 minutes at 4°C to remove all live cells and other debris, and then, kept at −80°C for the next steps.
Isolation of EVs
For the isolation of the EVs, differential steps of centrifugation were performed; 3 mL quick thawing urine samples were centrifuged at 2500×g for 10 minutes to eliminate large pellets of cell fragments including apoptotic bodies. Then the remaining supernatant was redirected to high-speed centrifugation at 25,000×g for 20 minutes (4 °C) by OptimaTM MAX-E Ultracentrifuge (Beckman Coulter Inc., USA). The pellet of EVs was washed with phosphate-buffered saline (PBS) (20X) and then the high-speed centrifugation step was repeated at 25,000×g for 20 minutes at 4 °C.22 Supernatants of the isolated EVs were considered as a negative control group. The isolated EV pellets were suspended in 750 μL of PBS (20X) and stored at -80 °C for further processing.
Characterization of EVs
Electron microscope
The purity, size, and morphology of the isolated uEVs were measured by Scanning Electron Microscope (SEM, TESCAN MIRA3 FEG-SEM, Czechia). For SEM imaging of EVs, first the samples were fixed in paraformaldehyde, and then the dehydrated samples were coated with a thin layer of gold as a conductive material for imaging. Particles larger than 100 nm were considered EVs in pooled urine samples of patients with GN.
Western blotting
According to MISEV (Minimal information for studies of extracellular vesicles) guidelines, western blotting is the most common method of protein assay in EVs.23 This method was performed by lysing of EVs in 250 μL of protein lysis buffer (1X, St. Louis, USA). The protein concentration was measured using the Bradford method (Protein assay dye reagent, Biorad, CA, USA). An equal volume of solubilized protein (12 mL) was loaded for each sample on 10% sodium dodecyl-sulfate-polyacrylamide gel (SDS-PAGE). Separated proteins were transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, Massachusetts, United States) and blocked with 2% nonfat-dried milk for 75 minutes. For characterizing the EVs, extracellular–specific markers including TSG-101, flotillin-1, ALiX, and CD-9 along with histone H1 (HIST1H, as a negative marker of EVs) were studied. Moreover, two podocyte-specific protein markers (nephrin and podocalyxin) were studied in urine samples of glomerulonephritis patients and normal controls. These proteins were detected by Santa Cruz Biotechnology mouse monoclonal anti-Nephrin (sc-377246), anti-Podocalyxin (sc-23904), anti-TSG101 (sc-136111), anti-Fotillin (sc-74566), anti-CD9 (sc-13118), anti-ALiX (sc-53540), and anti-HIST1H (sc-514856), 1: 1000.
Evaluating IgAN -specific proteins in urinary EVs
Podocyte-derived specific protein markers of IgAN were assessed by Santa Cruz Biotechnology mouse monoclonal anti-ceruloplasmin (SC-365206), anti-vasorin (SC-517034), anti-aminopeptidase N (sc-13536), and Elabscience biotechnology Rabbit Polyclonal anti-ANPEP (E-AB-61048). After incubation with primary antibodies, membranes were washed (3×) and then incubated with the secondary antibodies (1:10000), mouse anti-rabbit IgG-HRP (sc-2357) and anti-Mouse m-IgGκBP-HRP (sc-516102). The antibody-antigen reactions were visualized using chemiluminescence (GE Healthcare, NJ, USA). The proteins’ bands were detected and measured by enhanced chemiluminescence (ECL). The normalization of western blots was done by β-actin to remove any fragments due to aggregated proteins.24
Statistical analysis
The normality of variables was determined using the Shapiro-Wilk test and the results were reported as mean± standard deviation or median (minimum-maximum). A one-way ANOVA test followed by post hoc analysis was applied to compare the differences between the studied groups. Qualitative variables were reported as number (percentage) and then compared by the chi-square test. SPSS software version 21.0 was applied to data analysis. A P value below 0.05 was considered a meaningful result.
Results
Demographic information
During this study, urine samples of 32 GN patients (16 patients with IgAN and 16 patients with MGN), and 16 healthy controls were collected. IgAN and MGN groups had mean ages of 36.38 ± 8.18 and 42.88 ± 13.84, respectively. The mean age of healthy controls was 41.21 ± 5.82. There was no significant difference between the mean age of the studied patient groups (P= 0.89). Moreover, gender distribution (Male/Female ratio) in IgAN, MGN, and healthy controls were 10/6, 11/5, and 7/9, respectively and there were no statistically significant differences between them (P= 0.156). Moreover, it was revealed that 12.5% and 37.5% of IgAN patients had diabetes mellitus (n= 2), and cardiovascular disorders (n= 6), respectively. One patient with IgAN had an inflammatory disease. Some other demographic and clinical information such as body mass index (BMI), estimated glomerular filtration rate (eGFR), and levels of serum creatinine, urea, and hemoglobin are represented in Table 1. It was revealed that eGFR, BMI, and proteinuria levels of IgAN patients were significantly lower than MGN patients (P= 0.013, 0.011, and <0.001, respectively) and hemoglobin and serum creatinine levels were higher than MGN patients (P= 0.044 and 0.026, respectively).
Table 1.
Demographic information of the studied participants
|
Characteristics
|
IgAN (n= 16)
|
MGN (n= 16)
|
Healthy controls (n= 16)
|
P valuea
|
| Gender |
|
|
|
0.156 |
| Male |
10 (62.5%) |
11 (68.75%) |
7 (43.75%) |
|
| Female |
6 (37.5%) |
5 (31.25%) |
9 (56.25%) |
|
| Age (y) |
36.38±8.18 |
42.88±13.84 |
41.21±5.82 |
0.089 |
| BMI |
25.25 (22-31.8) |
27.90 (19.16-36.33) |
24.31 (21-28.5) |
0.011 |
| eGFR (60 mL/min/1.73 m2) |
56.11 (6.58-133.92) |
88.45 (32.22-129.38) |
75.50 (65.42-110) |
0.013 |
| Urea (mg/dL) |
39.5 (21-212) |
23 (12-92) |
31 (20-38) |
0.095 |
| Hemoglobin (g/dL) |
13.83±1.57 |
13.78±2.83 |
12.46±1.72 |
0.044 |
| Serum creatinine (mg/dL) |
1.34 (0.75-7.29) |
1.00 (0.5-2.49) |
1.12 (0.84-1.21) |
0.026 |
| Proteinuria (mg/24h) |
646.45 (230-1420) |
3916.5 (1280-7224) |
140 (0-200) |
<0.001 |
| Medications |
| Losartan |
6 (37.5%) |
5 (31.25%) |
0 (0%) |
0.674 |
| Losartan+ Atorvastatin |
1 (6.25%) |
2 (12.5%) |
0 (0%) |
| Underlying disorders |
| Diabetes mellitus |
2 (12.5%) |
0 (0%) |
0 (0%) |
<0.001 |
| Cardiovascular disorders |
6 (37.5%) |
0 (0%) |
0 (0%) |
<0.001 |
| Inflammatory diseases |
1 (6.25%) |
0 (0%) |
0 (0%) |
<0.001 |
aThe data of IgAN and MGN groups were compared and a P-value less than 0.05 was considered statistically significant. Quantitative variables are reported as mean ± standard deviation or median (minimum-maximum) and qualitative variables are reported as number (percentage).
BMI: body mass index, eGFR: estimated glomerular filtration rate, IgAN: IgA nephropathy, MGN: membranous glomerulonephritis.
Characterization of EVs
The evaluation of the morphological properties of EVs showed that multiple particles >100 nm in size (101.56, 108.88, 112.95, 117.03, 127.90, 149.45, and 156.23 nm) exist in different views of urine samples of GN patients, considered as EVs (Fig. 1A-D).
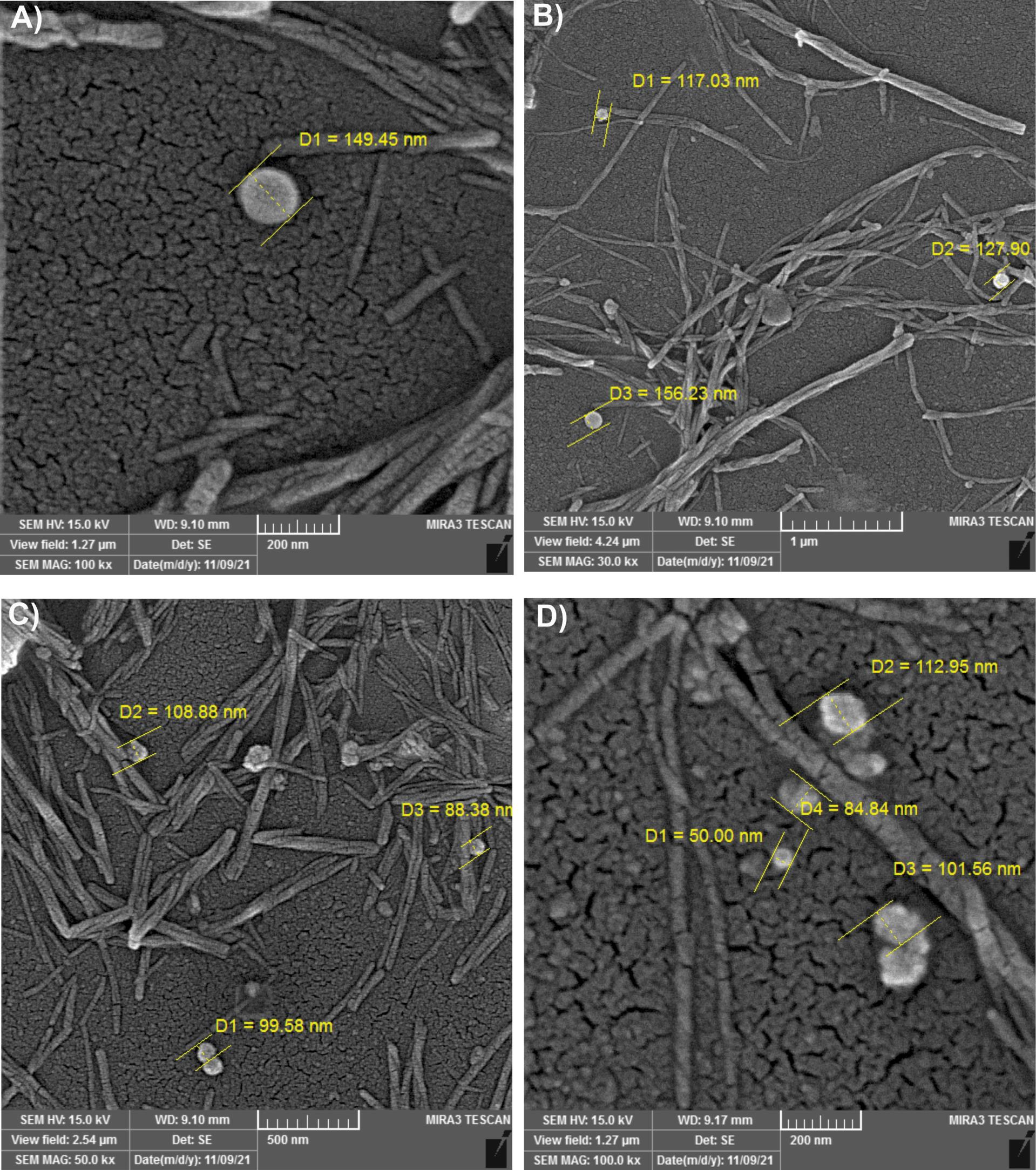
Fig. 1.
Characterization of extracellular vesicles in urine samples of glomerulonephritis patients by SEM. (A-D) Scanning electron microscope (SEM) of isolated particles. Particles larger than 100 nm were considered extracellular vesicles (EVs) in pooled urine samples of GN (glomerulonephritis) patients.
.
Characterization of extracellular vesicles in urine samples of glomerulonephritis patients by SEM. (A-D) Scanning electron microscope (SEM) of isolated particles. Particles larger than 100 nm were considered extracellular vesicles (EVs) in pooled urine samples of GN (glomerulonephritis) patients.
EVs specific markers including TSG-101, flotillin-1, ALiX, and CD-9 along with HIST1H and podocyte-specific proteins (nephrin and podocalyxin) were determined by western blotting and reported as their target density compared to the controls. The results exhibited that there were higher levels of both podocyte- and EVs-related proteins in pooled urine samples of GN patients compared to the healthy control and supernatant (negative control) samples (Fig. 2A-D).
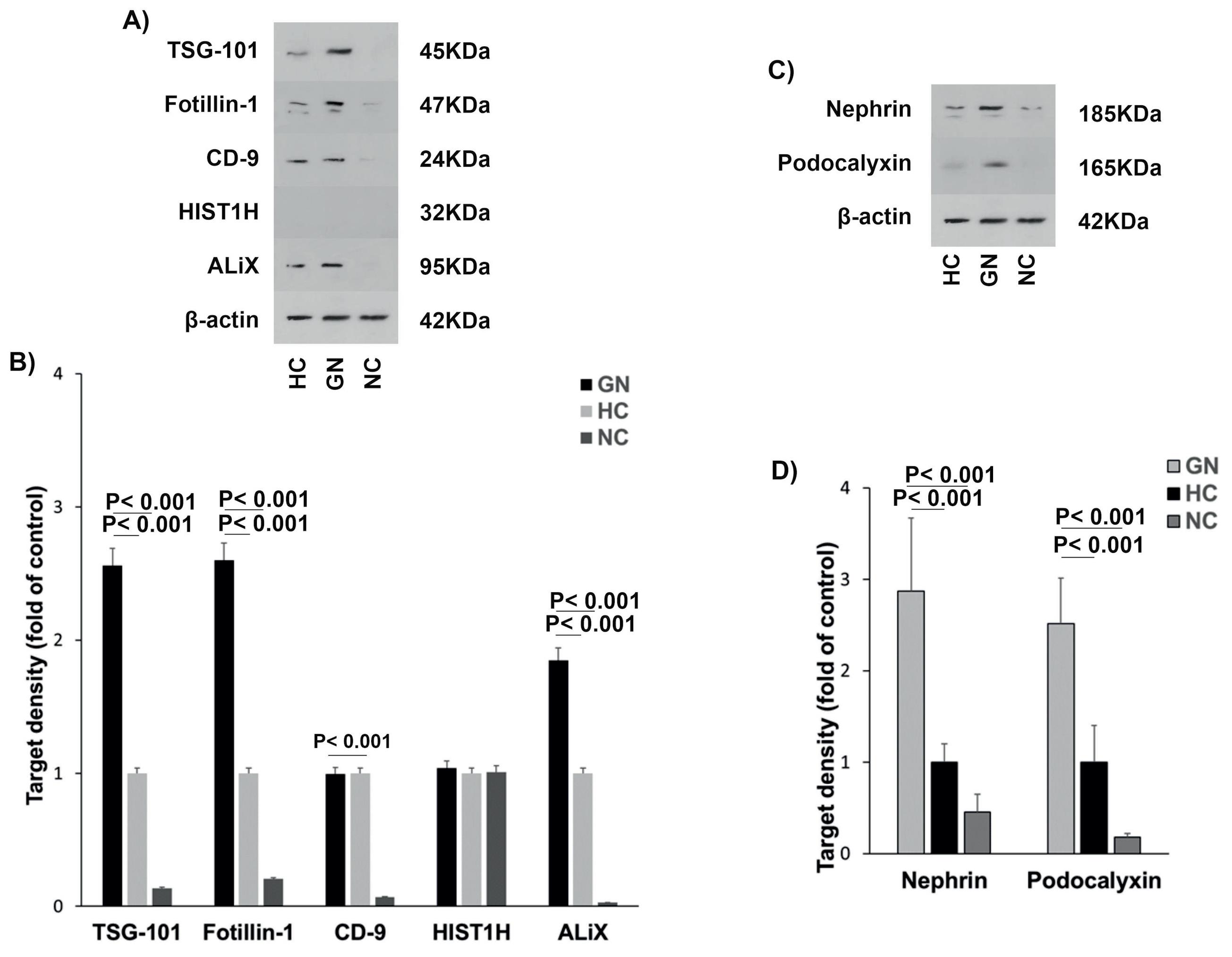
Fig. 2.
Characterization of urinary EVs derived from podocyte. (A-B) Extracellular vesicle markers (TSG-101, fotillin-1, CD9, HIST1H, ALiX) and (C-D) podocyte-specific proteins (Nephrin, and podocalyxin). β-actin was an internal control. HC: healthy control, HIST1H: Histone H1, GN: glomerulonephritis, NC: negative control, TSG101: Tumor susceptibility gene 101.
.
Characterization of urinary EVs derived from podocyte. (A-B) Extracellular vesicle markers (TSG-101, fotillin-1, CD9, HIST1H, ALiX) and (C-D) podocyte-specific proteins (Nephrin, and podocalyxin). β-actin was an internal control. HC: healthy control, HIST1H: Histone H1, GN: glomerulonephritis, NC: negative control, TSG101: Tumor susceptibility gene 101.
Protein analysis of EVs
Protein levels of vasorin, aminopeptidase N, and ceruloplasmin related to the IgAN pathogenesis were determined by western blotting in urine samples of IgAN patients and compared to their levels in MGN patients, healthy, and negative controls (Fig. 3A-B). It was observed that protein levels of vasorin (P= 0.001) and ceruloplasmin (P< 0.001) were significantly different in IgAN patients compared to the MGN, healthy, and negative groups. These results displayed that vasorin levels were statistically lower in IgAN patients compared to the MGN (P= 0.002) and healthy controls (P= 0.020). In addition, ceruloplasmin levels were higher in IgAN patients compared to the MGN (P= 0.06), healthy (P= 0.001), and negative (P= 0.008) controls. However, levels of aminopeptidase N were not statistically different between the studied groups (P= 0.054, Fig. 3C-D).
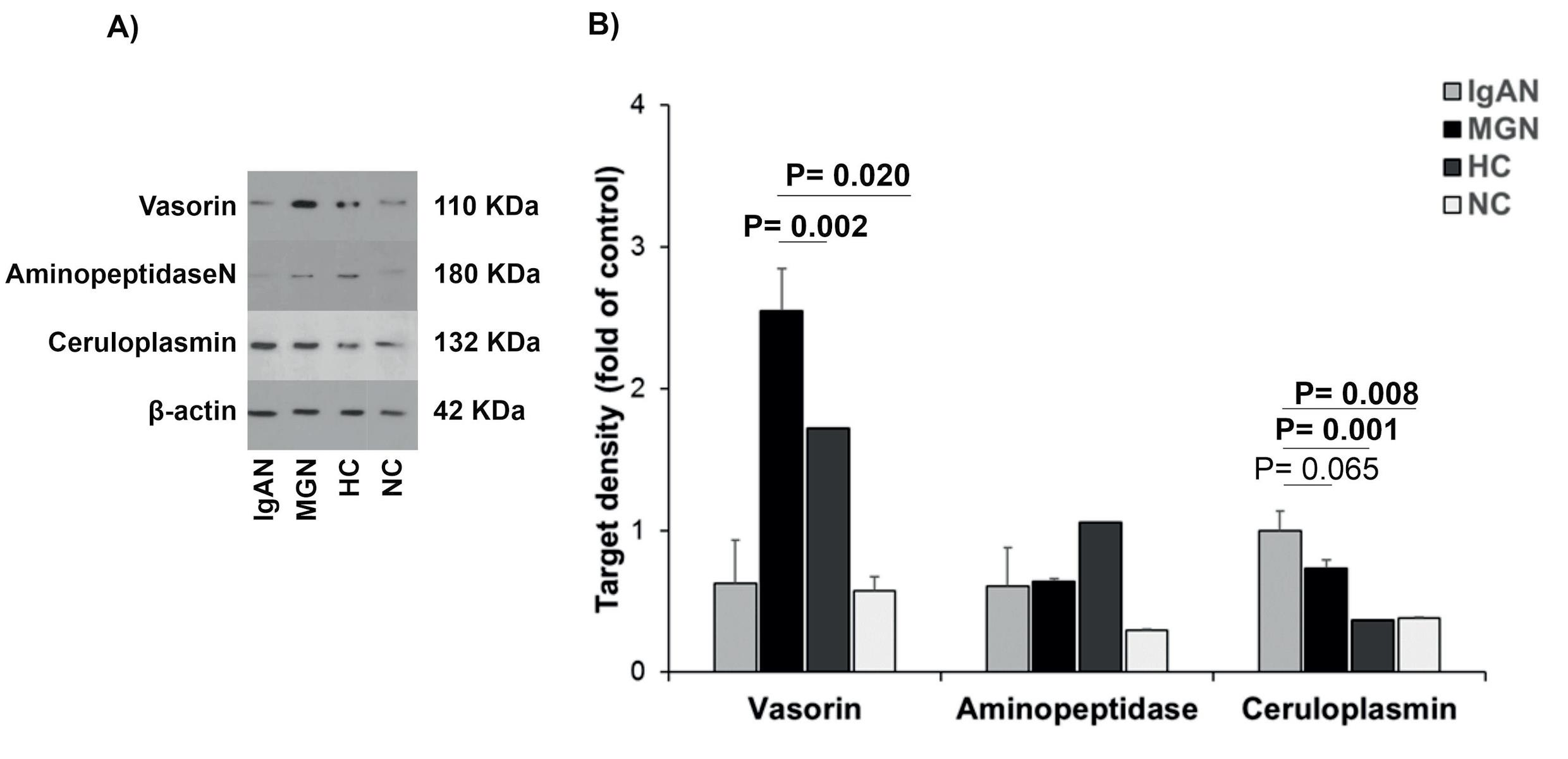
Fig. 3.
The analysis of podocyte-derived EV proteins in urine samples of patients with glomerulopathy. (A) Proteins related to the IgAN (Vasorin, aminopeptidase N, and ceruloplasmin) were determined by western blotting and (B) their density were compared in urine samples of IgAN patients compared to MGN patients, healthy controls, and negative controls. β-actin was considered an internal control. Results obtained from One-way ANOVA and post-HOC analysis and P-value< 0.05 was considered significant. EVs: extracellular vesicles, NC: negative control, HC: healthy control, IgAN: IgA nephropathy, MGN: membranous glomerulonephritis.
.
The analysis of podocyte-derived EV proteins in urine samples of patients with glomerulopathy. (A) Proteins related to the IgAN (Vasorin, aminopeptidase N, and ceruloplasmin) were determined by western blotting and (B) their density were compared in urine samples of IgAN patients compared to MGN patients, healthy controls, and negative controls. β-actin was considered an internal control. Results obtained from One-way ANOVA and post-HOC analysis and P-value< 0.05 was considered significant. EVs: extracellular vesicles, NC: negative control, HC: healthy control, IgAN: IgA nephropathy, MGN: membranous glomerulonephritis.
Discussion
Urinary EV protein levels of vasorin and ceruloplasmin had significant differences in the IgAN, MGN, and healthy control groups. The density of vasorin decreased while the density of ceruloplasmin increased significantly in the IgAN group compared to the MGN and normal groups. The target density pattern of the identified proteins in uEVs was in concordance with podocyte and glomerular injury, indicating a direct association with the pathology of IgAN and MGN.
Early diagnosis of IgAN by the identification of non-invasive biomarkers helps the patients have treatment at a proper time. Recent studies have emphasized the role of urinary extracellular proteomic analysis in the detection of relevant biomarkers in different GNs.25,26 Since uEVs represent their original cells, they are more reliable biomarkers27 to follow the pathogenesis of renal injury.28 In this study, the characterization of uEVs membranous markers (TSG-101, flotillin-1, ALiX, CD-9) and two podocyte-specific markers were evaluated in urine samples of the participants.
The high density of TSG-101, flotillin-1, and ALiX was observed in the glomerulonephritis group compared to the healthy controls, reflecting an increased number of uEVs. TSG101 participates in endosomal sorting component required for transport (ESCRT) pathway as a stable internal marker of uEVs, and the high density of this protein demonstrates the presence of high levels of EVs in urine. Similar to the results of Wu et al, in this work the density of TSG101 was higher in patients with GN than that in the other two groups.29 The most known markers of the membrane exosomes or microvesicles, CD9 and ALiX are secreted by different cell types.30 Analyzing the density of urinary EVs protein markers showed that the density of CD9 was much more than flotillin.31 However, in our study, there were high levels of flotillin-1 in GN patients than in the healthy group, and the CD9 density was almost the same between the two groups.
Podocyte-specific podocalyxin and nephrin markers of uEVs have been reported in a couple of recent studies and their density was higher in patients with early podocyte injury compared to the healthy controls.32 Flow cytometry analysis also indicated that these biomarkers are present in EVs of patients with diabetic nephropathy, other GNs,33 and renal cell carcinoma.34 Likewise, our western blot analysis in urine samples of patients with glomerulonephritis showed a high expression of nephrin and podocalyxin, confirming an increased number of EVs derived from podocytes due to podocyte damage.35
Among 1877 urinary exosomal proteins, four proteins including vasorin precursor, aminopeptidase N, ceruloplasmin, and α-1-antitrypsin were introduced as specific biomarkers for discriminating IgAN from TBMN (thin basement membrane nephropathy).5 The extracellular domain of vasorin is an inhibitor of transforming growth factor-beta (TGF-β) and has a protecting role against apoptosis, inflammation, fibrosis, and modulating immunity.36 Ikeda et al indicated an increase in protein levels of vasorin negatively modulates the TGF-β signaling pathway in TBMN that induces cell responses against vessel injury.37 A decreased level of vasorin is reported in the urinary proteomics and exosomes of IgAN patients compared to healthy controls.5,38 Similar to these results, we observed decreased levels of vasorin in uEVs of IgAN patients compared to the MGN and normal groups.
Ceruloplasmin acts as an antioxidant in oxidizing the toxicity of iron.39 It is assumed that the ceruloplasmin is expressed in a group of the parietal epithelial cells of glomeruli and protected podocytes from the toxic effects of molecules like ferrous iron.40 This acute-phase protein is upregulated under inflammatory situations such as a systemic form of IgAN (Henoch-Schönlein purpura) by peroxidation of lipids.41 Moreover, a higher level of ceruloplasmin was found in the urinary exosomes of patients with IgAN42 and diabetic nephropathy.43 The comparison of the urinary expression of ceruloplasmin between IgAN, other CKD groups, and TBMN indicates its diagnostic value for early detection of podocyte injury.44 Our results indicated a higher expression of ceruloplasmin in IgAN and MGN patients compared to the normal control group; however, the differences in its density were not statistically significant between IgAN and MGN patients.
Aminopeptidase N, a membrane-bound metalloproteinase, is widely expressed in various human cells and tissues.45 This enzyme can regulate the function of the immunoglobulin G receptor (FcγR) as a signal regulator. Proteomic analysis indicated that aminopeptidase N was significantly higher in urine samples of patients with glomerular injury compared to the healthy controls.46,47 This protein was established as a biomarker of proximal tubular injury.48 Through the interaction of uEVs with proximal tubular cells, the pro-fibrotic response is induced in glomerulonephritis.49 Moon et al reported a decreased expression of aminopeptidase N in urinary exosomes of patients with IgAN compared to TBMN and healthy controls.5 Likewise, in the current study, the levels of this biomarker were lower in uEVs of the IgAN and MGN groups compared to healthy controls; however, they were not statistically significant.
Although uEVs have significant potential for application as biomarkers in GNs, one of the most certain limitations was their isolation method. High-speed centrifugation utilizes as a common method for EV isolation but there may be some non-EV impurities that cause a low yield of isolated specific proteins during this method.50 It is recommended to develop other techniques to remove these impurities and increase the value of the obtained results. This urinary proteomic analysis of EVs derived from podocytes could be applied at the early diagnosis of IgAN by liquid biopsy.
There are some limitations in this study: (1) a small sample size of controls and patients was the main limitation of this study, (2) other primary glomerular diseases such as minimal change disease, and focal segmental glomerulosclerosis were not included in this study as control groups, (3) this study did not evaluate the diagnostic value of the studied podocyte-derived IgAN proteins due to a small sample size, (4) proteomic analysis of uEVs was not performed, (5) the percentage of podocyte-derived EVs from whole uEVs was not determined, and (6) there was an age difference between the study groups that could be a confounding factor. However, this study has some strengths; at the time of sample collection, all IgAN and MGN patients had not been on therapy; so, the changes in protein levels reflect the pathophysiology of kidney glomeruli. In addition, there were strict criteria for patient selection and it seems that most of the proportion of uEVs was from the injured podocyte. It is suggested to evaluate a big panel of IgAN‐specific proteins in different uEVs (exosomes, migrasomes,51 and microparticles) in larger cohorts and compare the results between different glomerular diseases.
Conclusion
In conclusion, the appearance of EVs derived from podocytes in the urine is an indicator of podocyte damage during the progression of IgAN and MGN. Moreover, differential protein expression of uEVs was observed in IgAN patients in comparison to MGN patients and healthy controls. Further large-scale prospective studies are warranted to confirm the clinical application of podocyte-derived EVs.
Research Highlights
What is the current knowledge?
√ Urinary extracellular vesicles (uEVs) are altered in different kidney diseases.
√ The appearance of uEVs derived from podocytes is an indicator of podocyte damage.
What is new here?
√ Higher excretion of uEVs was seen in patients with IgAN and MGN due to podocyte injury.
√ Different levels of uEV-derived vasorin and ceruloplasmin were seen in IgAN patients compared to MGN.
Competing interests
The authors declared that there was no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Statement
This cross-sectional study was performed according to the Declaration of Helsinki and approved by National Institute for Medical Research Development (NIMAD), Iran, Tehran (Ethical code: IR.NIMAD.REC.1398.368). Written informed consent was obtained from all participants.
Funding
This study was funded by National Institute for Medical Research Development (NIMAD) (#Grant NO. 988064).
References
- Trimarchi H, Coppo R. Podocytopathy in the mesangial proliferative immunoglobulin A nephropathy: new insights into the mechanisms of damage and progression. Nephrol Dial Transplant 2019; 34:1280-5. doi: 10.1093/ndt/gfy413 [Crossref] [ Google Scholar]
- Menon MC, Chuang PY, He JC. Role of podocyte injury in IgA nephropathy. Contrib Nephrol 2013; 181:41-51. doi: 10.1159/000348461 [Crossref] [ Google Scholar]
- Matovinović MS. Matovinović MS1Pathophysiology and classification of kidney diseases. Journal of the International Federation of Clinical Chemistry 2009; 20:2. [ Google Scholar]
- Duan Z-Y, Cai G-y, Bu R, Lu Y, Hou K, Chen X-M. Selection of urinary sediment miRNAs as specific biomarkers of IgA nephropathy. Sci Rep 2016; 6:1-10. doi: 10.1038/srep23498 [Crossref] [ Google Scholar]
- Moon PG, Lee JE, You S, Kim TK, Cho JH, Kim IS. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. J Proteomics 2011; 11:2459-75. doi: 10.1002/pmic.201000443 [Crossref] [ Google Scholar]
- Marshall T, Williams KM. Clinical analysis of human urinary proteins using high resolution electrophoretic methods. J Electrophoresis 1998; 19:1752-70. doi: 10.1002/elps.1150191037 [Crossref] [ Google Scholar]
- Wu HH, Goldys EM, Pollock CA, Saad S. Exfoliated Kidney Cells from Urine for Early Diagnosis and Prognostication of CKD: The Way of the Future?. Int J Mol Sci 2022; 23:7610. doi: 10.3390/ijms23147610 [Crossref] [ Google Scholar]
- Meier M, Kaiser T, Herrmann A, Knueppel S, Hillmann M, Koester P. Identification of urinary protein pattern in type 1 diabetic adolescents with early diabetic nephropathy by a novel combined proteome analysis. J diabetes 2005; 19:223-32. doi: 10.1016/j.jdiacomp.2004.10.002 [Crossref] [ Google Scholar]
- Wittke S, Haubitz M, Walden M, Rohde F, Schwarz A, Mengel M. Detection of acute tubulointerstitial rejection by proteomic analysis of urinary samples in renal transplant recipients. Am J Transplant 2005; 5:2479-88. doi: 10.1111/j.1600-6143.2005.01053.x [Crossref] [ Google Scholar]
- Celis JE, Wolf H, Østergaard M. Bladder squamous cell carcinoma biomarkers derived from proteomics. J Electrophoresis 2000; 21:2115-21. doi: 10.1002/1522-2683(20000601)21:11<2115::AID-ELPS2115>3.0.CO;2-K [Crossref] [ Google Scholar]
- Delrue C, De Bruyne S, Speeckaert R, Speeckaert MM. Urinary Extracellular Vesicles in Chronic Kidney Disease: From Bench to Bedside? Diagnostics (Basel) 2023; 13. 10.3390/diagnostics13030443.
- Ashcroft J, Leighton P, Elliott TR, Hosgood SA, Nicholson ML, Kosmoliaptsis V. Extracellular vesicles in kidney transplantation: a state-of-the-art review. Kidney Int 2022; 101:485-97. doi: 10.1016/j.kint.2021.10.038 [Crossref] [ Google Scholar]
- Farzamikia N, Baradaran B, Mostafavi S, Ahmadian E, Hosseiniyan Khatibi SM, Zununi Vahed S. Podocyte-derived microparticles in IgA nephropathy. Biomed Pharmacother 2021; 141:111891. doi: 10.1016/j.biopha.2021.111891 [Crossref] [ Google Scholar]
- Burger D, Thibodeau J-F, Holterman C, Burns KD, Kennedy CR. Abstract 28: Formation of Podocyte-Derived Microparticles in Diabetic Glomerular Injury. American-Heart-Association High Blood Pressure; 2013. p. A28-A.
- Burger D, Oleynik P. Isolation and characterization of circulating microparticles by flow cytometry. In: Hypertension (Dallas, Tex: 1979): Springer; 2017. p. 271-81.
- Grange C, Bussolati B. Extracellular vesicles in kidney disease. Nat Rev Nephrol 2022; 18:499-513. doi: 10.1038/s41581-022-00586-9 [Crossref] [ Google Scholar]
- Lytvyn Y, Xiao F, Kennedy CR, Perkins BA, Reich HN, Scholey JW. Assessment of urinary microparticles in normotensive patients with type 1 diabetes. J Diabetologia 2017; 60:581-4. doi: 10.1007/s00125-016-4190-2 [Crossref] [ Google Scholar]
- Huang SJ, Zhang Y, Wang GH, Lu J, Chen PP, Zhang JX. Deposition of platelet-derived microparticles in podocytes contributes to diabetic nephropathy. Int Urol Nephrol 2023; 55:355-66. doi: 10.1007/s11255-022-03332-z [Crossref] [ Google Scholar]
- Lu J, Hu ZB, Chen PP, Lu CC, Zhang JX, Li XQ. Urinary podocyte microparticles are associated with disease activity and renal injury in systemic lupus erythematosus. BMC nephrology 2019; 20:1-9. doi: 10.1186/s12882-019-1482-z [Crossref] [ Google Scholar]
- Gilani SI, Anderson UD, Jayachandran M, Weissgerber TL, Zand L, White WM. Urinary extracellular vesicles of podocyte origin and renal injury in preeclampsia. J Am Soc Nephrol 2017; 28:3363-72. doi: 10.1681/ASN.2016111202 [Crossref] [ Google Scholar]
- Kwon SH, Woollard JR, Saad A, Garovic VD, Zand L, Jordan KL. Elevated urinary podocyte-derived extracellular microvesicles in renovascular hypertensive patients. Nephrol Dial Transplant 2017; 32:800-7. doi: 10.1093/ndt/gfw077 [Crossref] [ Google Scholar]
- Sullivan KM, Scholey J, Moineddin R, Sochett E, Wicklow B, Elia Y. Urinary podocyte-derived microparticles in youth with type 1 and type 2 diabetes. Diabetologia 2021; 64:469-75. doi: 10.1007/s00125-020-05297-z [Crossref] [ Google Scholar]
- Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018; 7:1535750. doi: 10.1080/20013078.2018.1535750 [Crossref] [ Google Scholar]
- Santucci L, Bruschi M, Del Zotto G, Antonini F, Ghiggeri GM, Panfoli I. Biological surface properties in extracellular vesicles and their effect on cargo proteins. Sci Rep 2019; 9:13048. doi: 10.1038/s41598-019-47598-3 [Crossref] [ Google Scholar]
- Pisitkun T, Shen R-F, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 2004; 101:13368-73. doi: 10.1073/pnas.0403453101 [Crossref] [ Google Scholar]
- Li S, Hao H, Li R, Guo S. Urinary Exosomal MicroRNAs as New Noninvasive Biomarkers of IgA Nephropathy. Tohoku J Exp Med 2022; 256:215-23. doi: 10.1620/tjem.256.215 [Crossref] [ Google Scholar]
- Blijdorp CJ, Burger D, Llorente A, Martens-Uzunova ES, Erdbrügger U. Extracellular Vesicles as Novel Players in Kidney Disease. J Am Soc Nephrol 2022; 33:467-71. doi: 10.1681/ASN.2021091232 [Crossref] [ Google Scholar]
- Erdbrügger U, Blijdorp CJ, Bijnsdorp IV, Borràs FE, Burger D, Bussolati B. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J Extracell Vesicles 2021; 10:e12093. doi: 10.1002/jev2.12093 [Crossref] [ Google Scholar]
- Wu F, Chen Y-Y, Huang J-X, Wang K-Y, Xu H-S, Lin D. Significance of Mannan Binding Lectin-Associated Serine Protease 2 in Urinary Extracellular Vesicles in IgA Nephropathy. Clin Investig Med 2022; 45:E47-54. doi: 10.25011/cim.v45i3.38876 [Crossref] [ Google Scholar]
- Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009; 9:4997-5000. doi: 10.1002/pmic.200900351 [Crossref] [ Google Scholar]
- Dhondt B, Geeurickx E, Tulkens J, Van Deun J, Vergauwen G, Lippens L. Unravelling the proteomic landscape of extracellular vesicles in prostate cancer by density-based fractionation of urine. J Extracell Vesicles 2020; 9:1736935. doi: 10.1080/20013078.2020.1736935 [Crossref] [ Google Scholar]
- Zhang L-H, Zhu X-Y, Eirin A, Nargesi AA, Woollard JR, Santelli A. Early podocyte injury and elevated levels of urinary podocyte-derived extracellular vesicles in swine with metabolic syndrome: role of podocyte mitochondria. Am J Physiol Renal Physiol 2019; 317:F12-F22. doi: 10.1152/ajprenal.00399.2018 [Crossref] [ Google Scholar]
- Musante L, Saraswat M, Duriez E, Byrne B, Ravidà A, Domon B. Biochemical and physical characterisation of urinary nanovesicles following CHAPS treatment. PloS one 2012; 7:e37279. doi: 10.1371/journal.pone.0037279 [Crossref] [ Google Scholar]
- Raimondo F, Morosi L, Corbetta S, Chinello C, Brambilla P, Della Mina P. Differential protein profiling of renal cell carcinoma urinary exosomes. Mol Biosyst 2013; 9:1220-33. doi: 10.1039/c3mb25582d [Crossref] [ Google Scholar]
- Lu J, Hu ZB, Chen PP, Lu CC, Zhang JX, Li XQ. Urinary levels of podocyte-derived microparticles are associated with the progression of chronic kidney disease. Ann Transl Med 2019; 7:445. doi: 10.21037/atm.2019.08.78 [Crossref] [ Google Scholar]
- Lin S-Y, Chang C-H, Wu H-C, Lin C-C, Chang K-P, Yang C-R. Proteome profiling of urinary exosomes identifies alpha 1-antitrypsin and H2B1K as diagnostic and prognostic biomarkers for urothelial carcinoma. Sci Rep 2016; 6:1-12. doi: 10.1038/srep34446 [Crossref] [ Google Scholar]
- Ikeda Y, Imai Y, Kumagai H, Nosaka T, Morikawa Y, Hisaoka T. Vasorin, a transforming growth factor β-binding protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo. Proc Natl Acad Sci U S A 2004; 101:10732-7. doi: 10.1073/pnas.0404117101 [Crossref] [ Google Scholar]
- Samavat S, Kalantari S, Nafar M, Rutishauser D, Rezaei-Tavirani M, Parvin M. Diagnostic urinary proteome profile for immunoglobulin a nephropathy. Iran J Kidney Dis 2015; 9:239-48. [ Google Scholar]
- Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien J-P, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci 2002; 22:6578-86. doi: 10.1523/JNEUROSCI.22-15-06578.2002 [Crossref] [ Google Scholar]
- Wiggins J. Podocytes and glomerular function with aging. Semin Nephrol 2009; 29:587-93. doi: 10.1016/j.semnephrol.2009.07.012 [Crossref] [ Google Scholar]
- Ece A, Kelekçi S, Hekimoğlu A, Kocamaz H, Balık H, Yolbaş İ. Neutrophil activation, protein oxidation and ceruloplasmin levels in children with Henoch-Schönlein purpura. Pediatr Nephrol 2007; 22:1151-7. doi: 10.1007/s00467-007-0475-5 [Crossref] [ Google Scholar]
- Miyata T, Nangaku M, Suzuki D, Inagi R, Uragami K-i, Sakai H. A mesangium-predominant gene, megsin, is a new serpin upregulated in IgA nephropathy. J Clin Invest 1998; 102:828-36. doi: 10.1172/JCI2450 [Crossref] [ Google Scholar]
- Gudehithlu KP, Hart P, Joshi A, Garcia-Gomez I, Cimbaluk DJ, Dunea G. Urine exosomal ceruloplasmin: a potential early biomarker of underlying kidney disease. Clin Exp Nephrol 2019; 23:1013-21. doi: 10.1007/s10157-019-01734-5 [Crossref] [ Google Scholar]
- Hwang VJ, Ulu A, Van Hoorebeke J, Weiss RH. Biomarkers in IgA nephropathy. Biomark Med 2014; 8:1263-77. doi: 10.2217/bmm.14.92 [Crossref] [ Google Scholar]
- Stefanovič V, Vlahovič P, Ardaillou N, Ronco P, Ardaillou R. Cell surface aminopeptidase A and N activities in human glomerular epithelial cells. Kidney Int 1992; 41:1571-80. doi: 10.1038/ki.1992.227 [Crossref] [ Google Scholar]
- Holdt-Lehmann B, Lehmann A, Korten G, Nagel H-R, Nizze H, Schuff-Werner P. Diagnostic value of urinary alanine aminopeptidase and N-acetyl-β-D-glucosaminidase in comparison to α1-microglobulin as a marker in evaluating tubular dysfunction in glomerulonephritis patients. J Clinicachimica acta 2000; 297:93-102. doi: 10.1016/s0009-8981(00)00237-0 [Crossref] [ Google Scholar]
- Mitic B, Lazarevic G, Vlahovic P, Rajic M, Stefanovic V. Diagnostic value of the aminopeptidase N, N-acetyl-beta-D-glucosaminidase and dipeptidylpeptidase IV in evaluating tubular dysfunction in patients with glomerulopathies. Ren Fail 2008; 30:896-903. doi: 10.1080/08860220802359048 [Crossref] [ Google Scholar]
- Mitic B, Lazarevic G, Vlahovic P, Rajic M, Stefanovic V. Diagnostic value of the aminopeptidase N, N-Acetyl-β-d-glucosaminidase and dipeptidylpeptidase IV in evaluating tubular dysfunction in patients with glomerulopathies. J Ren Fail 2008; 30:896-903. doi: 10.1080/08860220802359048 [Crossref] [ Google Scholar]
- Munkonda MN, Akbari S, Landry C, Sun S, Xiao F, Turner M. Podocyte-derived microparticles promote proximal tubule fibrotic signaling via p38 MAPK and CD36. J Extracell Vesicles 2018; 7:1432206. doi: 10.1080/20013078.2018.1432206 [Crossref] [ Google Scholar]
- Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int 2018; 2018. 10.1155/2018/8545347.
- Ardalan M, Hosseiniyan Khatibi SM, Rahbar Saadat Y, Bastami M, Nariman-Saleh-Fam Z, Abediazar S. Migrasomes and exosomes; different types of messaging vesicles in podocytes. Cell Biol Int 2022; 46:52-62. doi: 10.1002/cbin.11711 [Crossref] [ Google Scholar]