Bioimpacts. 2025;15:30187.
doi: 10.34172/bi.30187
Review
Investigating the function and targeting of MET protein as an oncogene kinase in pancreatic ductal adenocarcinoma: A microarray data integration
Nahid Askari Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing, 1 
Morteza Hadizadeh Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, 2 
Mohammad Sina Writing – original draft, 3 
Sepideh Parvizpour Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, 4, * 
Seyedeh Zahra Mousavi Conceptualization, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, 5, * 
Mohd Shahir Shamsir Investigation, Writing – review & editing, 6
Author information:
1Department of Biotechnology, Institute of Sciences and High Technology and Environmental Sciences, Graduate University of Advanced Technology, Kerman, Iran
2Physiology Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran
3A. Nocivelli Institute for Molecular Medicine, Department of Molecular and Translational Medicine, University of Brescia, 25123 Brescia, Italy
4Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Molecular Genetics, Faculty of Biological Sciences, Tarbiat Modares University, Tehran, Iran
6Bioinformatics Research Group (BIRG), Department of Biosciences, Faculty of Science, Universiti Teknologi Malaysia, Skudai, Johor, Malaysia
Abstract
Introduction:
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal disease with a poor prognosis. Kinase proteins are essential regulators of cellular processes and potential targets for drug development.
Methods:
Integration of multiple microarray datasets was screened to find differentially expressed kinases (DE-Kinases) across adjacent normal and tumor tissue samples in PDAC. The most effective kinase for drug design and docking in this study was selected by investigating biological mechanisms and survival analyses. Forty phytochemicals were extracted from the yellow sweet clover, Melilotus officinalis (Linn.) Pall, and were then subjected to in silico screening and molecular docking studies against a specific potent kinase.
Results:
MET, PAK3, and PDK4 were identified as the DE-Kinases. After examining the pathways and biological processes, up-regulated MET had the most significant survival analysis and became our primary kinase for drug design and docking in this study. Four of the extracted phytocompounds of Melilotus officinalis (Linn.) Pall that exhibited high binding affinities with MET and were selected for toxicity analysis. Finally, the stability and mobility of the two nontoxic compounds that passed the toxicity test (dicumarol PubChem CID: 54676038 and melilotigenin PubChem CID: 14059499) were studied by molecular dynamics simulation.
Conclusion:
This study's results identified two phytochemicals in yellow sweet clover that could be used to develop an anticancer drug, but experimental evaluation is necessary to confirm their efficacy.
Keywords: Gene expression, Pancreatic ductal adenocarcinoma, Melilotus officinalis (Linn.) Pall, Yellow sweet clover, Melilotigenin, Dicumarol
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This study was supported by the Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences (#73175).
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a severe malignancy ranked as the fourth leading cause of cancer-related deaths.1,2 At the time of diagnosis, most patients have incurable metastatic conditions with an overall 5-year survival rate of less than 10%.2 It is predicted that PDAC will become the second-greatest cause of cancer-related death globally during the next two decades.3 Various elements contribute to the high mortality rate caused by PDAC.4 These include the advanced stage at which it is typically diagnosed and the limited availability of efficacious systemic treatments.5 Currently, clinical and pathological features possess limited predictive utility in forecasting the prognosis of patients with metastatic, locally advanced, or resectable subgroups of PDAC.6,7 It is imperative to develop efficient systemic therapeutic approaches for PDAC. Advancements in molecular biology and genomic technologies, including transcriptomic analyses, have led to substantial progress in the diagnosis and therapeutic interventions for PDAC.5 Transcriptomic analyses in cancer genomics contribute to advancing the field of precision medicine by identifying clinically transcriptomic biomarkers.8,9 Abundant transcriptomic data is publicly accessible in The Gene Expression Omnibus (GEO) database, which consists of open-access, high-throughput gene expression data with various functional genomics datasets.10 GEO contains data from microarray technology, a potent tool for studying global gene expression in human cancers. This technique has provided essential insights into the progression, prognosis, and therapeutic response of many cancer types.11,12 The UALCAN database has been shown to have the ability to make connections between gene expression and the overall survival of individuals.13 Very few cancer genes encode targets that are suitable for drug development. Protein kinases are among the most important targets for small-molecule inhibitors against cancer.5 They belong to a group of 500 genes encoding protein kinase enzymes responsible for facilitating protein phosphorylation.14,15 Kinase enzymes regulate and maintain proliferation, cell cycle, apoptosis, motility, growth, and differentiation in a cell by transferring a phosphate group.16 Misregulated kinase activity can lead to profound alterations in these processes and is identified as a potential carcinogenic process.14 Given the essential role of kinases in cell biology and their significant contribution to various cancer types, there are numerous ongoing studies on kinase inhibitors in research and therapeutic contexts.14,17 Previous investigations have employed a multi-database analysis methodology to identify essential gene networks and anticipate prospective drug candidates. Several studies have generated various multi-modal molecular profiles for PDAC that show promise in terms of prognostic potential and cross-validation and generalization18-21; however, more systematic analyses of data are required to develop feasible clinical applications.22 A meta-analysis of previously published data on PDAC provides the ability to integrate related research.23,24
In the past few decades, among the diverse treatment approaches used for different types of cancers, chemotherapy has emerged as the predominant method for cancer treatment. Chemotherapeutic agents are typically classified into two groups based on their source: natural products (such as those derived from plants) and synthetic compounds. Despite the expensive production costs of synthetic chemotherapeutics, they have not demonstrated the anticipated efficacy in cancer treatment. Conversely, plant-derived chemotherapeutic agents have demonstrated promising outcomes in treating various diseases, including some types of cancer. The anticancer properties of over 3000 plant species have been acknowledged globally. The anticancer activities demonstrated by the bioactive compounds found in these plants result in the scavenging of free radicals, antioxidant effects, stimulation of apoptosis, halting of the cell cycle, and suppression of angiogenesis.
Melilotus officinalis (Linn.) Pall is an annual herb containing chemical compounds with antioxidative, anti-inflammatory, antitumor, and various pharmaceutical properties. This herb, commonly known as yellow sweet clover, belongs to the Melilotus genus of the Fabaceae family and is widely distributed worldwide. Studies have demonstrated the inhibitory effects of Melilotus officinalis on MCM7, MCF-7, PC3M, and other tumor cell lines. For example, research has shown that the ethanolic extract of Melilotus officinalis directly inhibits the growth of MCF-7 cancer cells through its antioxidant properties and enhancement of the expression of the p53 gene.25 Additionally, the inhibitory effects of two novel benzoic acid compounds from this herb on PC-3M prostate cancer cells have been documented. In silico studies reveal the potential of rosmarinic acid and melilotigenin to serve as anticancer agents against the MCM7 protein.26
This study aimed to identify the differentially expressed kinase genes (DEKGs) in PDAC and to screen for potential anticancer compounds from the yellow sweet clover plant, Melilotus officinalis. The study used transcriptome data from three public datasets of PDAC tumor samples and adjacent non-tumor samples and performed a meta-analysis to find consistent DEKGs across the datasets. The study also performed survival analysis to evaluate the prognostic value of DEKGs in PDAC patients. The study then used virtual screening and molecular docking techniques to test phytochemicals from the yellow sweet clover plant against the DEKGs and selected four compounds that showed strong binding affinities with the target proteins to discover novel biomarkers and drug candidates for PDAC, a highly fatal disease with a poor prognosis.
Materials and Methods
Data collection
To search the mRNA expression datasets, the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo) was searched using the following keywords: "pancreatic adenocarcinoma," "Homo sapiens," '[porgn: txid9606]' and 'expression profiling by array.' After completing an extensive search, the researchers selected and analyzed three GSE profiles (GSE28735, GSE62452, and GSE183795). The datasets contained PDAC samples and were the expression of transcripts on the GPL6244 (Affymetrix Human Gene 1.0 ST array [transcript (gene) version]). Subsequently, the KinHub database (http://www.kinhub.org/kinases.html) was used to collect all human kinase genes and report them in a table.
Microarray data processing and integrative data integration
The R statistical programming language was used in all data processing and integration stages. The datasets discussed were from a refined platform (Affymetrix). After combining two data sets, principal component analysis (PCA) and boxplot were used to investigate whether the batch effect was eliminated. As the final product of the data integration, a unit expression matrix (the combination of the two datasets in this study) was generated.
Identification of differentially expressed genes
We extracted differentially expressed genes (DEGs) from a unit expression matrix comparing "pancreatic adenocarcinoma with normal tissue." DEGs were identified using the R program limma.27 We used a log2 fold change ≥ |1| and an adjusted P-value of 0.05 to assess whether DEGs were statistically significant. This work focused on DEG kinase in PDAC cancer to see if they could be converted to oncogenes. Therefore, we used the Venny 2.0 tool (https://bioinfogp.cnb.csic.es/tools/venny/index2.0.2.html) to identify genes that were both kinase and DEGs in this study (DE-Kinase).
Gene Ontology (GO) and pathway enrichment analyses
The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and Gene Ontology (GO), including the biological process (BP) terms, were used to recognize biological mechanisms. In the present study, the ClusterProfiler and GOPlot packages in R were run to attitude KEGG pathways and BP enrichment analyses of DE-Kinase in this study.28
Survival analysis
UALCAN (http://ualcan.path.uab.edu/index.html) is an interactive website that enables an in-depth examination of TCGA gene expression data. Kaplan-Meier analyses play a prominent and essential role within the UALCAN platform. They offer valuable insights and analyses in cancer research.29 P values of less than 0.05 were considered statistically significant for survival analysis.
Protein and ligand retrieval
The Protein Data Bank (PDB) is a repository that contains information on the three-dimensional structures of large biological molecules. The RCSB PDB was used to obtain the 3D structure of the target protein.30 We also used two different databases to obtain the phytochemicals from the yellow sweet clover plant, scientifically known as Melilotus officinalis (Linn.) Pall. The first database was IMPPAT, a comprehensive collection of Indian medicinal plants and their associated phytochemicals. IMPPAT helps in the discovery of natural product-based drugs using cheminformatic methods. The second database was Dr. Duke's database, which provides information on the medicinal properties of phytocompounds in humans.31 AutoDock software was used to process the acquired compounds.
Molecular docking study
Molecular docking studies involve computational techniques to predict how two molecules, such as a drug and a protein, might interact. The AutoDock Vina tool from PyRx virtual screening software was used to determine the binding pose of the ligand-protein pair. This software is widely used in computer-aided drug design (CADD) to find the optimal binding mode of a specific drug to its target protein. The PyRx software provides a reliable and easy-to-use docking tool for CADD by screening libraries of compounds against the selected target protein.32 The complexes with the lowest binding energy (highest with a negative sign) in units of kcal/mol were selected for further analysis.
Pharmacokinetics study
The Swiss-ADME online server was used to evaluate the drug candidate's absorption, distribution, metabolism, and excretion (ADME) characteristics.33 This analysis is essential for predicting the drug's potential behavior in the body before it undergoes clinical testing. Optimizing the ADME profile is essential to ensuring the drug's success in both clinical and commercial settings. The ADME properties of the compounds were estimated based on their physicochemical properties, such as hydrophobicity, lipophilicity, interaction with the gastrointestinal environment, and ability to cross the blood-brain barrier. These properties affect how the drug is eliminated from the body through urine and feces.
Toxicity analysis
In order to guarantee the safety of the drug candidate, it was subjected to toxicity testing as an essential part of its development process. The toxicity test is a crucial stage in drug development for acquiring the compound's safety profile. The preclinical toxicity test aims to anticipate specific risks in the compound's profile, including mutagenicity, carcinogenicity, and immunotoxicity, both quantitatively and qualitatively.34 The admetSAR 2.0 online server was utilized to assess the toxicity of the selected phytochemicals. Furthermore, the ProTox-II server was employed to investigate the compound's toxic effects further by examining various pathways related to toxicity, such as those involving nuclear receptors and stress responses.
The protein-ligand interaction visualization
After the molecular docking study, pharmacokinetics search, and toxicity analysis, BIOVIA Discovery Studio Visualizer software was used to visualize and analyze the binding interaction between the ligands and the protein. This software is a powerful tool for visualizing and analyzing molecular structures and their properties. It is commonly used in chemistry, biochemistry, and drug discovery to examine and manipulate molecular models. We also used the software to calculate the binding energy, hydrogen bonds, and other interaction parameters of each ligand-protein pair. We used these parameters to evaluate the stability and specificity of the binding interaction and to identify the key residues involved in the binding site.
Molecular dynamics simulation
The complex structures underwent 150 nanoseconds of MD simulations using the GROMACS 5.1.4 software within a Linux environment to assess the stability of the chosen candidate compounds binding to the target protein. A simple point-charge water molecule was utilized to address the system, with an orthorhombic periodic boundary box shape set at a distance of 10 Å on both sides to maintain a specific volume. Suitable ions such as Na + and Cl− with a salt concentration of 0.15 M were randomly placed in the solvated system to neutralize it electrically. Following the construction of the solvated system containing the protein in complex with the ligand, the system was minimized and relaxed using the default protocol implemented within the GROMOS 96 43a1 force field parameters. The system was gently heated at a low temperature (t = 0.1 ps) and pressure (t = 0.5 ps) using Berendsen algorithms to achieve the equilibrium geometry at 300 K. All simulations were carried out under constant temperature and pressure conditions with a non-bonded cut-off of 1.4 Å. The molecular dynamics simulation lasted for 150 ns at 300 K, with bond length constraints enforced using LINCS and electrostatic interactions handled using the particle mesh Ewald method. The simulation followed the NPT (constant number of particles, pressure, and temperature) criteria. Throughout the simulation, the frame was saved every 1.0 ps. The stabilized structure was extracted from the system's trajectory to assess the reliability of protein geometry and structural folding. Subsequently, the protein's dynamic behavior and structural changes were analyzed by calculating the root mean square deviation (RMSD) to investigate the structural stability of complexes.35
Results
Data collection and expression analysis
Table 1 provides details of the three GEO data sets used in this study. We detected 536 human kinase genes in the KinHub database (Supplementary file 1, Table S1). The integrated analysis of the three GEO datasets resulted in 277 DEGs, which were divided into 163 upregulated genes and 114 downregulated genes between tumor samples (n = 253) and adjacent non-tumor samples (n = 208) (Supplementary file 2, Table S2). By comparing upregulated DEGs with human protein kinases, three genes, i.e., MET (MET proto-oncogene, receptor tyrosine kinase), PAK3 (p21 (RAC1) activated kinase 3), and PDK4 (pyruvate dehydrogenase kinase 4), were identified as the final list. In this study, MET expression was upregulated, while the PAK3 and PDK4 genes were down-regulated.
Table 1.
This study used information from three GEO datasets
|
No. of GEO profile
|
Platform
|
Samples
|
Stage
|
|
Tumor tissue
|
Adjacent non-tumor
|
Normal Pancreas
|
Stage I
|
Stage II
|
Stage III
|
Stage IV
|
Not reported
|
| GSE28735 |
GPL6244 |
45 |
45 |
- |
- |
- |
- |
- |
90 |
| GSE62452 |
GPL6244 |
69 |
61 |
- |
7 |
84 |
26 |
13 |
- |
| GSE183795 |
GPL6244 |
139 |
102 |
3 |
11 |
143 |
32 |
13 |
45 |
The Affymetrix company utilized all mRNA platforms for its research
Gene Ontology (GO) and pathway enrichment analysis
The clusterProfiler and GOplot packages were utilized to find the enriched pathways and GO with P values < 0.05, shown by dot and chord plots, respectively. No significant biological pathway was found for the PDK4 gene. However, pathway analysis indicated that the renal cell carcinoma, axon guidance, focal adhesion, and Ras signaling pathways were most prevalent in MET and PAK3 (Fig. 1A). Besides, regulation of protein polymerization, regulation of actin filament organization, protein polymerization, regulation of actin cytoskeleton organization, and regulation of supramolecular fiber organization were significant in the GO category (Fig. 1B).
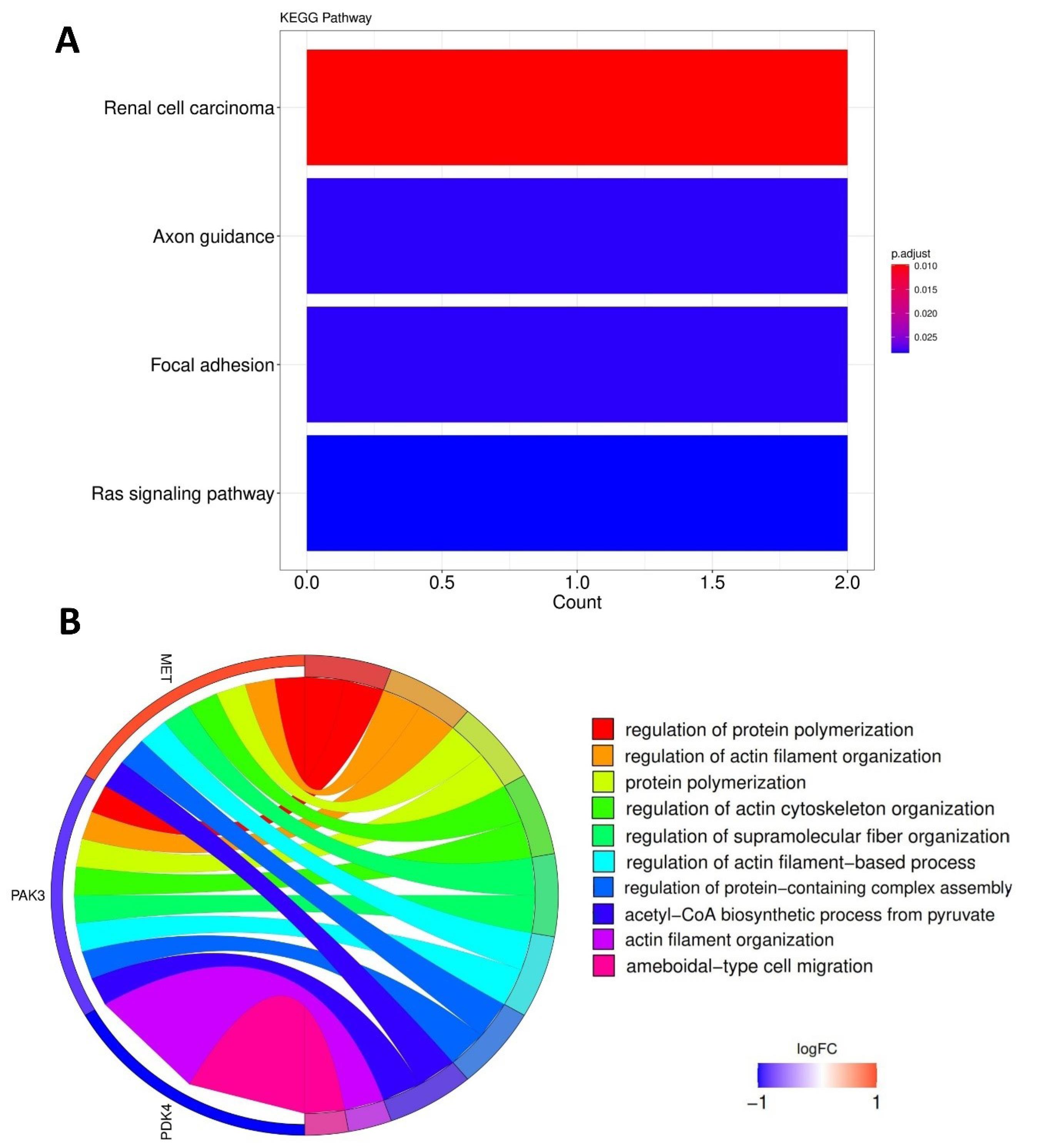
Fig. 1.
Functional enrichment analysis of DE-kinase. Top KEGG pathways (A); Top GO terms (B).
.
Functional enrichment analysis of DE-kinase. Top KEGG pathways (A); Top GO terms (B).
Screening DE-Kinases by survival analysis
The overall survival of DE-Kinases was analyzed using UALCAN. The results showed that the PDK4 survival analysis was insignificant (P =0.11). MET (P =0.0001) and PAK3 (P =0.022) were two of the kinases that we were able to identify among the DE-Kinases (Fig. 2). Patients who had higher expression of MET and lower expression of PAK3 had worse overall survival among PDAC patients. Considering the high expression of MET and the most significant value of survival analysis and its significant impact on survival in this study, MET was associated with shorter overall survival and predicted a poor prognosis. Due to its high expression and significant effect on the system's survival, this kinase can be a very suitable therapeutic target.
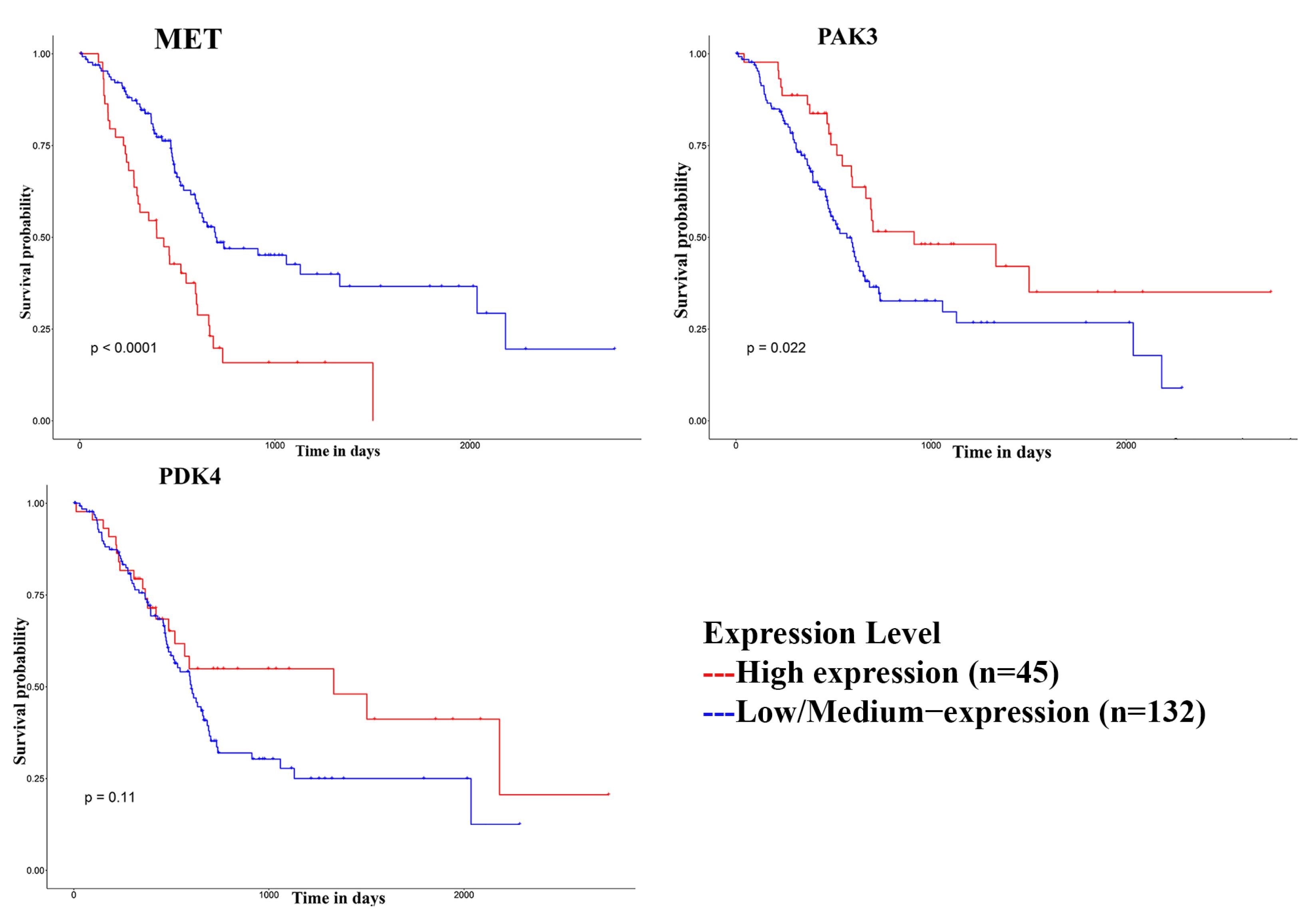
Fig. 2.
The overall survival curve of DE-Kinases.
.
The overall survival curve of DE-Kinases.
Protein and ligand retrieval and preparation
The structure of the Met domain protein kinase (7mo7.pdb) was obtained from the PDB. Various structures of the Met protein kinase can be found in the PDB, and from these, 7mo7 was selected due to its longest sequence length and absence of mutations. A set of 40 phytochemical compounds derived from the yellow sweet clover plant (Melilotus officinalis) was obtained from IMPPAT and Dr. Duke's databases. Molecular docking analysis was done to identify the most effective interaction between the Met domain protein kinase (7mo7) and the phytochemical compounds. The AutoDock Vina wizard from the PyRx software was used to examine the 40 compounds. The results showed binding affinities ranging from -4.5 to -11.9 kcal/mol (Supplementary file 3, Table S3). The compounds with lower binding affinity compared to the control compound were selected. However, capmatinib had an inhibitory effect on Met domain protein kinase and a binding affinity of -10.5 kcal/mol as determined by PyRx. The top four compounds, PubChem CID: 54676038 (-10.8 kcal/mol), PubChem CID: 14059499 (-11.9 kcal/mol), PubChem CID: 18646 (-9.9 kcal/mol), and PubChem CID: 5280343 (-9.5 kcal/mol), exhibited better binding affinities than the control ligand capmatinib (-9.2 kcal/mol). More information on these four compounds can be found in Table 2.
Table 2.
The docking information of the four best ligands and lapatinib (control)
|
Compound ID
|
Chemical name
|
Chemical Formula
|
Docking score
(kcal/mol)
|
PubChem CID:
54676038 |
Dicumarol |
C19H12O6 |
-10.8 |
PubChem CID:
14059499 |
Melilotigenin |
C6H46O5 |
-11.9 |
PubChem CID:
18646 |
2-Methoxyanthraquinone |
C15H10O3 |
-9.7 |
PubChem CID:
5280343 |
Quercetin |
C15H10O7 |
-9.5 |
PubChem
CID: 25145656 |
Capmatinib |
C23H17FN6O |
-9.2 |
Pharmacokinetics study
The SwissADME server assessed the ADME properties of the selected compounds based on their interaction with the target macromolecules. These properties reveal the advantages and disadvantages of a drug candidate, such as how it is taken up, distributed, broken down, and eliminated by the body. The ADME properties of the four compounds are shown in Table 3. These properties are essential for determining how a drug acts in the human body and are essential factors to consider during the drug design process to ensure successful clinical trials. A drug molecule's molecular weight and topological polar surface area (TPSA) affect its permeability across biological barriers. Permeability decreases with higher molecular weight, while permeability increases with lower TPSA. The lipophilicity of a drug molecule, measured by the logarithm of the inorganic and aqueous phase partition coefficient (LogP), influences its absorption in the human body. Lower absorption is associated with higher LogP values. Water solubility, measured by the LogS parameter, also affects drug absorption, with lower values indicating higher solubility. The ability of a drug molecule to cross the membrane bilayer is influenced by the number of hydrogen bond donors and acceptors being within an appropriate range. Additionally, the number of rotatable bonds influences the oral bioavailability of compounds, with an optimal range near 10. Based on their pharmacokinetic properties, three compounds (PubChem CIDs 5280343, 14059499, and 5467038) demonstrated effectiveness and potency.
Table 3.
Pharmacokinetics analysis results of the four selected compounds
|
|
Properties
|
PubChem CID:
54676038
|
PubChem CID:
14059499
|
PubChem CID:
5280343
|
PubChem CID:
18646
|
| Physicochemical properties |
Molecular Weight (g/mol) |
336.29 |
270.28 |
302.24 |
238.24 |
| Heavy atoms |
25 |
20 |
22 |
18 |
| Arom. heavy atoms |
20 |
12 |
16 |
12 |
| Rotatable bonds |
2 |
1 |
1 |
1 |
| H-bond acceptors |
6 |
4 |
7 |
3 |
| H-bond donors |
2 |
1 |
5 |
0 |
| Lipophilicity |
Log Po/w |
2.6 |
2.52 |
1.63 |
2.75 |
| Water solubility |
Log S (ESOL) |
Soluble |
Soluble |
Soluble |
Moderately |
| Pharmacokinetics |
GI absorption |
High |
High |
High |
High |
| Drug-likeness |
Lipinski |
Yes |
Yes |
Yes |
Yes |
| Medi. Chemistry |
Synth. Accessibility |
3.37 |
3.54 |
3.23 |
2.28 |
Toxicity test
The admetSAR online server was used to assess the toxicity of the selected phytochemical compounds. Table 4 shows the results of the toxicity tests, which revealed that none of the compounds had the potential to cause cancer. Most compounds did not inhibit hERG toxicity, which is significant because hERG inhibition can result in dangerous heart rhythm abnormalities. However, one compound, identified as PubChem CID: 18646, did show inhibitory effects on hERG.
Table 4.
Toxicity test analysis results of the selected four compounds
|
Compound ID
|
PubChem CID: 54676038
|
PubChem CID: 14059499
|
PubChem CID: 5280343
|
PubChem CID: 18646
|
| hERG toxicity |
No |
No |
No |
Yes
|
| AMES toxicity |
No |
No |
Yes
|
No |
| Carcinogenicity |
No |
No |
No |
No |
| PGI |
No |
No |
No |
Yes
|
| Rat (LD50) |
2.32 |
2.01 |
3.02 |
3.02 |
| Hepatotoxicity |
Inactive |
Inactive |
Inactive |
Inactive |
| Immunotoxicity |
Inactive |
Inactive |
Inactive |
Inactive |
| Mutagenicity |
Inactive |
Inactive |
Inactive |
Inactive |
| Cytotoxicity |
Inactive |
Inactive |
Inactive |
Inactive |
| Aryl hydrocarbon Receptor |
Inactive |
Inactive |
Inactive |
Inactive |
| Androgen Receptor |
Inactive |
Inactive |
Inactive |
Inactive |
On the other hand, this compound exhibited inhibitory effects on P-glycoprotein (PGP), which limits the entry of drugs into cells and is highly expressed in various cells. Regarding genotoxicity, all of the compounds passed the Ames tests except for PubChem CID: 5280343, which showed potential genotoxicity by causing reverse mutations. Additionally, the LD50 values, which measure immediate or acute toxicity, were within an acceptable range for all compounds. Further assessment using the ProTox-II server indicated that the compounds were not toxic regarding hepatotoxicity, immunotoxicity, mutagenicity, cytotoxicity, aryl hydrocarbon receptor, and androgen receptor.
Protein-ligand interaction visualization
After the pharmacokinetics study and toxicity tests, the two compounds PubChem CID: 14059499, PubChem CID: 54676038 were selected. Then, the BIOVIA Discovery Studio Visualizer software was employed to visualize and analyze the binding interaction between these two ligands and proteins. Fig. 3 shows the interactions between PubChem CID: 14059499, PubChem CID: 54676038, and PubChem CID: 25145656 (control).
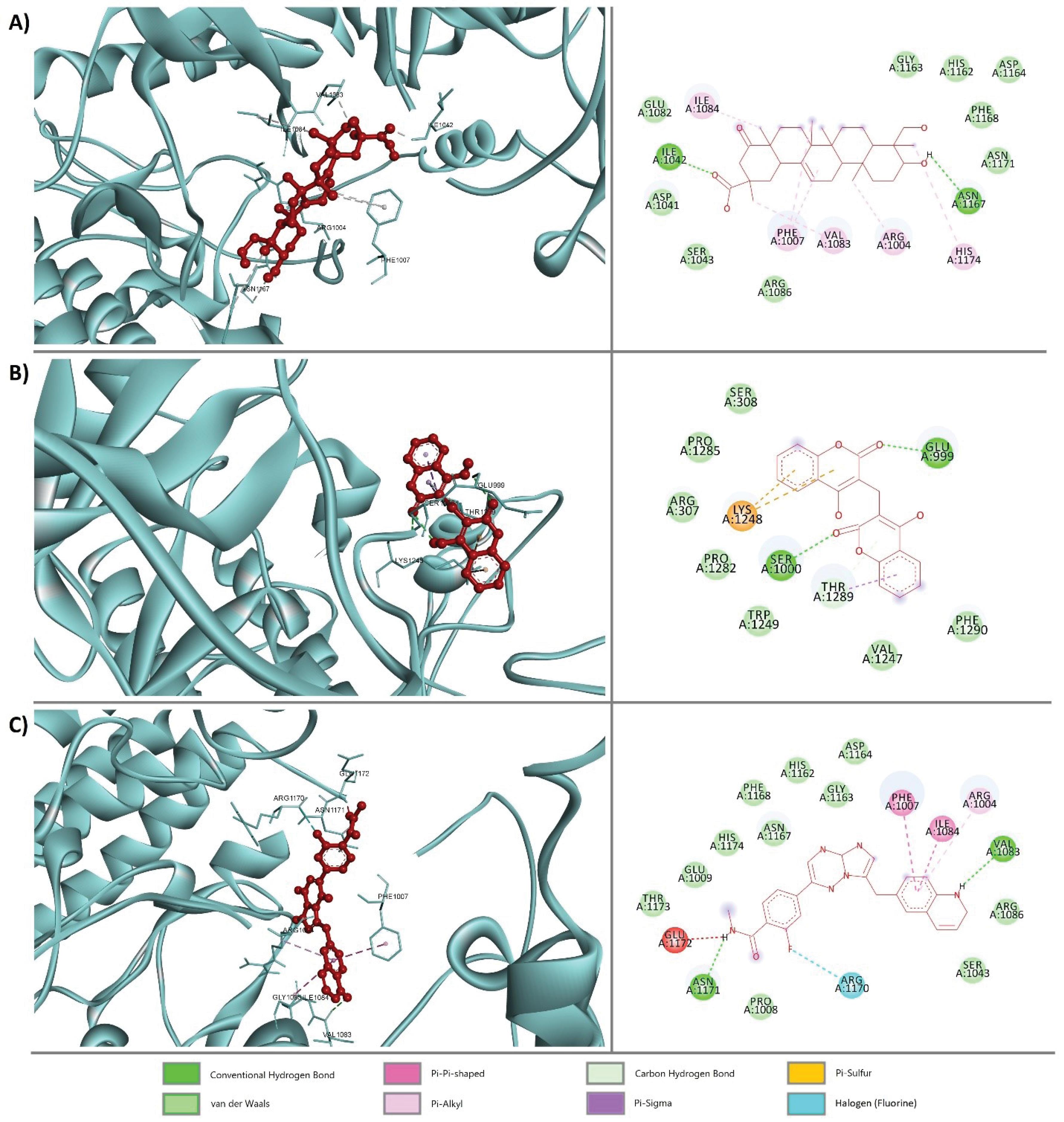
Fig. 3.
3D (left side) and 2D (right side) visualization of protein-ligand interaction between the MET protein and (A) PubChem CID: 14059499, (B) PubChem CID: 54676038, and (C) PubChem CID: 25145656 (control).
.
3D (left side) and 2D (right side) visualization of protein-ligand interaction between the MET protein and (A) PubChem CID: 14059499, (B) PubChem CID: 54676038, and (C) PubChem CID: 25145656 (control).
MD simulation analysis
To study the stability and mobility of the structure of PubChem CID:54676038 and PubChem CID:14059499 as two nontoxic complexes that passed the toxicity test, as well as capmatinib PubChem CID: 25145656 as the control compound, a 150 ns MD simulation at 300 K was performed. The global behavior of the models was analyzed based on the RMSD, which is a crucial parameter for evaluating the molecule's stability during the simulation. Fig. 4 represents the RMSD plot for the complex structures during simulation. The figures show that both complexes are more stable during the simulation than the control compound.
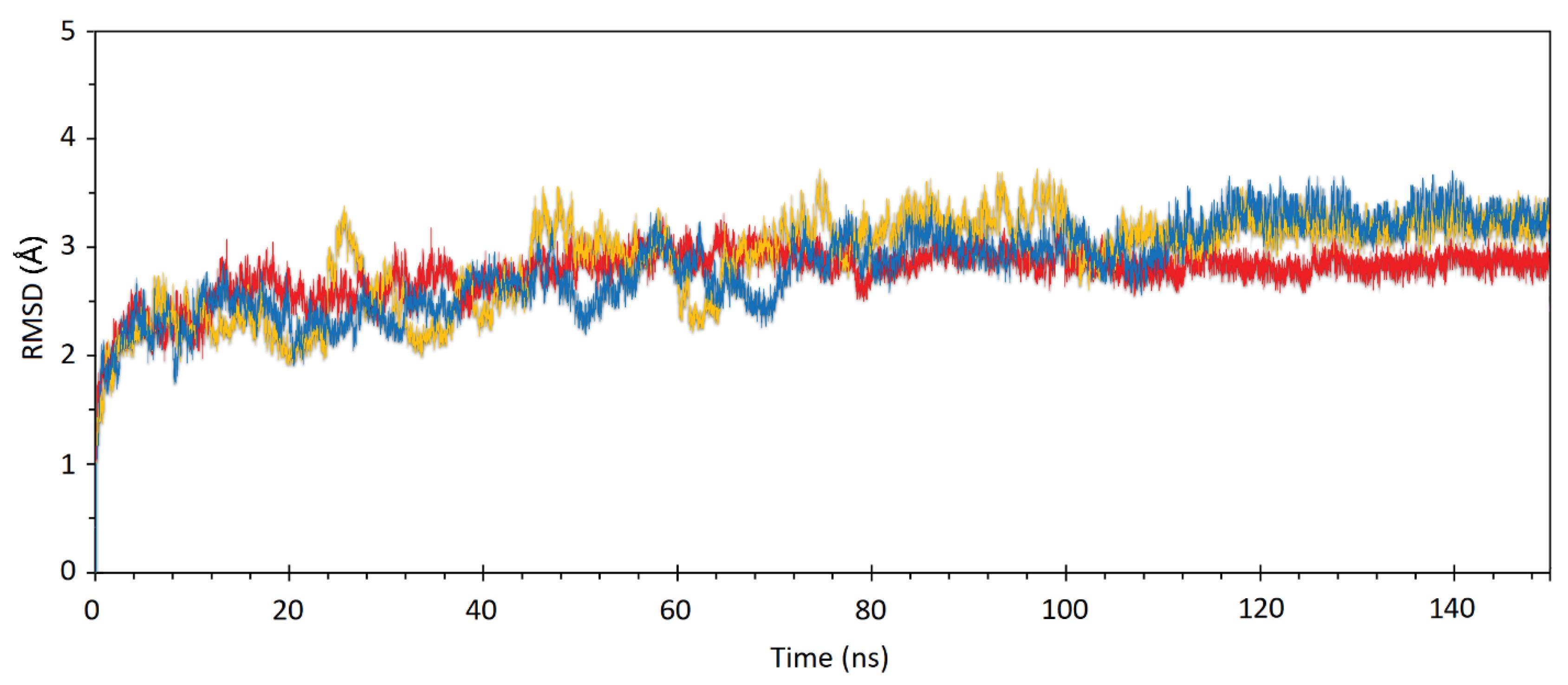
Fig. 4.
The RMSD of the MET protein in interaction with PubChem CID: 25145656 (yellow) (control structure), PubChem 14059499, and (B) PubChem CID: 54676038 during 100 ns MD simulation.
.
The RMSD of the MET protein in interaction with PubChem CID: 25145656 (yellow) (control structure), PubChem 14059499, and (B) PubChem CID: 54676038 during 100 ns MD simulation.
Discussion
PDAC is a highly lethal and incurable metastatic malignancy of the pancreas, and the 5-year survival rate is approximately 11%.31 The majority of patients with pancreatic tumors arrive with advanced illness as a result of the silent nature of PDAC development. Even the patients who undergo surgery are faced with metastatic spread within two years. As there are currently no efficient, targeted treatments for PDAC, it is critical to better understand the biology of pancreatic tumors and find novel and effective approaches for therapy.32 Clinical management of cancer must target the genes with the metastasis transformational capacity. One of these category genes is kinases. Many kinases participate in cell transformation, tumor cell initiation, and proliferation.33 This study aimed to identify potential natural MET kinase inhibitors, a crucial regulator of pancreatic cancer development and progression. We performed an integrated analysis of three GEO datasets to find differentially expressed kinases (DE-Kinases) between tumor and non-tumor samples. We found three DE-Kinases, MET, PAK3, and PDK4, significantly associated with pancreatic cancer. Using molecular docking analysis, we then screened 40 phytochemical compounds derived from the yellow sweet clover plant (Melilotus officinalis) for their binding affinity with the MET kinase. We selected four compounds that showed better binding affinity than the control compound capmatinib, a known MET kinase inhibitor. We further evaluated these compounds' pharmacokinetics and toxicity properties using in silico prediction tools. We found that three compounds had favorable drug-like characteristics and low toxicity. However, one compound had inhibitory effects on hERG and PGP, which may affect cardiac safety and cellular uptake.
MET is a tyrosine kinase receptor that regulates vital processes such as cell signaling, growth, and differentiation. MET also promotes cancer development and metastasis by stimulating cell proliferation, invasion, migration, and angiogenesis.34,35 This hypothesis is supported by the observation that wild-type MET overexpression is associated with highly aggressive and metastatic tumors.36 MET signaling is naturally activated by the interaction of the MET receptor and its ligand hepatocyte growth factor (HGF). This interaction leads to the receptor dimerization and phosphorylation of two tyrosine residues, Tyr1234 and Tyr1235, in the MET kinase catalytic domain. The phosphorylated MET activates a network of intracellular proteins, including PI3K, PLCc1, GrB2, GaB1, and STAT3. As a result, MET triggers the activation of some important physiological processes in cancer development, including the PI3K/Akt, STAT3, SRC/FAK, and MAPK/ERK signaling pathways.37 MET has been reported to be highly upregulated in pancreatic cancer tissues and positively correlated with PD-L1 expression levels. The expression levels of MET and PD-L1 are related to metastatic progression, tumor TNM stage, and overall survival in pancreatic cancer. MET inhibition facilitates lymphocyte infiltration into pancreatic tumors, and combining MET inhibitors with PD-1/PD-L1 blockage has demonstrated notably improved health in mouse models of pancreatic cancer.38 Consistent with the above report content, the survival analysis result from the UALCAN database showed that the patients with higher MET expression levels had worse overall survival rates than healthy individuals.
PAK3 is a serine-threonine kinase that has a role in glioma formation and could be a therapeutic target. PAK3 is highly expressed in proneural glioblastoma tumors and cell lines. PAK3 expression is linked to neuronal differentiation, lower proliferation, and longer survival in glioblastoma. Our survival analysis from UALCAN showed that lower PAK3 expression has been associated with worse survival in healthy individuals. PAK3 inhibition increases glioma growth in nude mice.39 It has been shown that PAK3 regulates the Akt-GSK3-β-catenin pathway in pancreatic cancer cells. PAK3 depletion blocks tumor sphere formation and β-catenin stability. PAK3 overexpression increases Akt signaling and β-catenin expression. PAK3 inhibition reduces tumor growth in vivo.40
Pyruvate dehydrogenase kinase 4 (PDK4) is another gene differentially expressed in our study. PDK4 is an enzyme that inactivates pyruvate dehydrogenase (PDH) by adding a phosphate group. PDH is an enzyme that converts pyruvate into acetyl-CoA, a key molecule for cell growth. By inhibiting PDH, PDK4 increases the amount of pyruvate in the cell, which is then converted into lactate by another enzyme called lactate dehydrogenase. This process also recycles NAD + , which is needed for making ATP, the cell's energy currency, through a pathway called aerobic glycolysis. This pathway helps cancer cells to grow and proliferate faster.41 The heart, skeletal muscles, kidneys, and pancreatic islets all have significant levels of PDK4 gene expression.
PDK4 is implicated in cancer development according to several lines of evidence. In several human malignancies, including breast, ovarian, colon, and lung tumors, PDK4 mRNA expression is markedly reduced.42 PDK4 has different roles in different types of cancers. Some studies have shown that PDK4 can stop the growth of prostate, lung, and liver cancers. Other studies have shown that PDK4 can help the growth of colorectal and lung cancers. These studies suggest that PDK4's function depends on the type of tissue or cell it is in.43-47 A study showed that individuals with pancreatic cancer with high PDK4 gene expression had a better prognosis than those with low PDK4 gene expression due to the inhibition of cancer stem cell characteristics in PDAC.48 Another study on the KIS compounds used as inhibitors of PDK4 demonstrated that, given its effect on shifting glucose metabolism, PDK4 is a desirable target for cancer therapy. In both subcutaneous xenograft and orthotopic pancreatic tumor models in nude mice, KIS37 inhibited the development of pancreatic cancer cells by inhibiting PDK4.49
Additionally, a small drug called cryptotanshinone (CPT), which blocks PDK4 activity, prevents tumorigenesis of KRAS-activated human pancreatic and colorectal cancer cells.50 PDK4 acts as a critical gene responsible for ferroptosis resistance in PDAC cells. By limiting pyruvate oxidation, which prevents pancreatic cancer cells from synthesizing fatty acids or oxidizing lipids, PDK4 helps avoid ferroptosis. By preventing pyruvate dehydrogenase-dependent pyruvate oxidation, PDK4 prevents ferroptosis. System XC drugs' anticancer efficacy is increased by PDK4 inhibition in vitro in the appropriate preclinical animal models.51 However, the survival analysis through UALCAN did not show a significant difference between pancreatic and healthy patients. Together, MET, PAK3, and PDK4 kinases play essential roles in promoting cancer and can be potential targets for cancer therapy. Our findings suggest that the yellow sweet clover plant may be a promising source of natural inhibitors of MET kinase for pancreatic cancer treatment. Our study also demonstrates the usefulness of computational methods for screening and evaluating potential drug candidates from natural sources. However, our study has some limitations and challenges that must be addressed in future research.
Conclusion
In conclusion, phytochemical compounds melilotigenin PubChem 14059499 and dicumarol PubChem CID: 54676038 may have therapeutic potential as a source of natural inhibitors of MET kinase in pancreatic cancer. The results also provided insights into these compounds' molecular mechanisms and pharmacological properties. We hope our study will inspire further research on developing natural inhibitors of MET kinase for pancreatic cancer treatment. However, as future work, we suggest experimental work to evaluate the results of the in silico study.
Review Highlights
What is the current knowledge?
√ Kinase proteins are important regulators of cancer cells and potential targets for drug discovery.
What is new here?
√ Phytochemicals of Yellow sweet clover has the possibility for developing an anticancer drug.
Competing Interests
The authors declare no conflict of interest.
Ethical Statement
There is none to be disclosed.
Supplementary files
Supplementary file 1 contains Table S1.
(pdf)
Supplementary file 2 contains Table S2.
(pdf)
Supplementary file 3 contains Table S3.
(pdf)
References
- Grossberg AJ, Chu LC, Deig CR, Fishman EK, Hwang WL, Maitra A. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin 2020; 70:375-403. doi: 10.3322/caac.21626 [Crossref] [ Google Scholar]
- Anwar M. The Association Between Smoking and the Risk of Pancreatic Cancer-The Norwegian Women and Cancer (NOWAC) Study [thesis]. UiT Norges Arktiske Universitet; 2021.
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74:2913-21. doi: 10.1158/0008-5472.can-14-0155 [Crossref] [ Google Scholar]
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet 2016; 388:73-85. doi: 10.1016/s0140-6736(16)00141-0 [Crossref] [ Google Scholar]
- Xiao Y, Zhang B, Cloyd JM, Xu G, Du S, Mao Y. Gene signature and connectivity mapping to assist with drug prediction for pancreatic ductal adenocarcinoma. Surg Oncol 2022; 44:101849. doi: 10.1016/j.suronc.2022.101849 [Crossref] [ Google Scholar]
- Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014; 270:248-60. doi: 10.1148/radiol.13131184 [Crossref] [ Google Scholar]
- Tempero MA, Arnoletti JP, Behrman SW, Ben-Josef E, Benson AB 3rd, Casper ES, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2012. 10: 703-13. 10.6004/jnccn.2012.0073.
- Supplitt S, Karpinski P, Sasiadek M, Laczmanska I. Current achievements and applications of transcriptomics in personalized cancer medicine. Int J Mol Sci 2021; 22:1422. doi: 10.3390/ijms22031422 [Crossref] [ Google Scholar]
- Horak P, Heining C, Kreutzfeldt S, Hutter B, Mock A, Hüllein J. Comprehensive genomic and transcriptomic analysis for guiding therapeutic decisions in patients with rare cancers. Cancer Discov 2021; 11:2780-95. doi: 10.1158/2159-8290.cd-21-0126 [Crossref] [ Google Scholar]
- Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res 2013; 41:D991-5. doi: 10.1093/nar/gks1193 [Crossref] [ Google Scholar]
- Bumgarner R. Overview of DNA microarrays: types, applications, and their future. Curr Protoc Mol Biol 2013; Chapter 22: Unit 22.1. 10.1002/0471142727.mb2201s101.
- Hryciuk B, Szymanowski B, Bieńkowski M, Perdyan A, Korwat A, Winnik K. Consistency in biomarkers expression between matched tissue microarray cores from primary gallblader and ovarian cancers. Oncol Clin Pract 2019; 15:85-8. doi: 10.5603/ocp.2019.0011 [Crossref] [ Google Scholar]
- Chandrashekar DS, Bashel B, Balasubramanya SA, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017; 19:649-58. doi: 10.1016/j.neo.2017.05.002 [Crossref] [ Google Scholar]
- Cicenas J, Zalyte E, Bairoch A, Gaudet P. Kinases and cancer. Cancers (Basel) 2018; 10:63. doi: 10.3390/cancers10030063 [Crossref] [ Google Scholar]
- Bhullar KS, Lagarón NO, McGowan EM, Parmar I, Jha A, Hubbard BP. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer 2018; 17:48. doi: 10.1186/s12943-018-0804-2 [Crossref] [ Google Scholar]
- Hunter T, Cooper JA. Protein-tyrosine kinases. Annu Rev Biochem 1985; 54:897-930. doi: 10.1146/annurev.bi.54.070185.004341 [Crossref] [ Google Scholar]
- Yang BB, Lum P, Chen A, Arends R, Roskos L, Smith B. Pharmacokinetic and pharmacodynamic perspectives on the clinical drug development of panitumumab. Clin Pharmacokinet 2010; 49:729-40. doi: 10.2165/11535970-000000000-00000 [Crossref] [ Google Scholar]
- Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 2011; 17:500-3. doi: 10.1038/nm.2344 [Crossref] [ Google Scholar]
- Donahue TR, Tran LM, Hill R, Li Y, Kovochich A, Calvopina JH. Integrative survival-based molecular profiling of human pancreatic cancer. Clin Cancer Res 2012; 18:1352-63. doi: 10.1158/1078-0432.ccr-11-1539 [Crossref] [ Google Scholar]
- Stratford JK, Bentrem DJ, Anderson JM, Fan C, Volmar KA, Marron JS. A six-gene signature predicts survival of patients with localized pancreatic ductal adenocarcinoma. PLoS Med 2010; 7:e1000307. doi: 10.1371/journal.pmed.1000307 [Crossref] [ Google Scholar]
- Zhang G, Schetter A, He P, Funamizu N, Gaedcke J, Ghadimi BM. DPEP1 inhibits tumor cell invasiveness, enhances chemosensitivity and predicts clinical outcome in pancreatic ductal adenocarcinoma. PLoS One 2012; 7:e31507. doi: 10.1371/journal.pone.0031507 [Crossref] [ Google Scholar]
- Haider S, Wang J, Nagano A, Desai A, Arumugam P, Dumartin L. A multi-gene signature predicts outcome in patients with pancreatic ductal adenocarcinoma. Genome Med 2014; 6:105. doi: 10.1186/s13073-014-0105-3 [Crossref] [ Google Scholar]
- Atay S. Integrated transcriptome meta-analysis of pancreatic ductal adenocarcinoma and matched adjacent pancreatic tissues. PeerJ 2020; 8:e10141. doi: 10.7717/peerj.10141 [Crossref] [ Google Scholar]
- Zhao L, Li Y, Zhang Z, Zou J, Li J, Wei R. Meta-analysis based gene expression profiling reveals functional genes in ovarian cancer. Biosci Rep 2020; 40:BSR20202911. doi: 10.1042/bsr20202911 [Crossref] [ Google Scholar]
- Liu YT, Gong PH, Xiao FQ, Shao S, Zhao DQ, Yan MM. Chemical constituents and antioxidant, anti-inflammatory and anti-tumor activities of Melilotus officinalis (Linn) Pall. Molecules 2018; 23:271. doi: 10.3390/molecules23020271 [Crossref] [ Google Scholar]
- Parvizpour S, Masoudi-Sobhanzadeh Y, Pourseif MM, Barzegari A, Razmara J, Omidi Y. Pharmacoinformatics-based phytochemical screening for anticancer impacts of yellow sweet clover, Melilotus officinalis (Linn) Pall. Comput Biol Med 2021; 138:104921. doi: 10.1016/j.compbiomed.2021.104921 [Crossref] [ Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43:e47. doi: 10.1093/nar/gkv007 [Crossref] [ Google Scholar]
- Silva JM, Perez DS, Pritchett JR, Halling ML, Tang H, Smith DI. Identification of long stress-induced non-coding transcripts that have altered expression in cancer. Genomics 2010; 95:355-62. doi: 10.1016/j.ygeno.2010.02.009 [Crossref] [ Google Scholar]
- Walter W, Sánchez-Cabo F, Ricote M. GOplot: an R package for visually combining expression data with functional analysis. Bioinformatics 2015; 31:2912-4. doi: 10.1093/bioinformatics/btv300 [Crossref] [ Google Scholar]
- Morshedian A, Razmara J, Lotfi S. A novel approach for protein structure prediction based on an estimation of distribution algorithm. Soft Comput 2019; 23:4777-88. doi: 10.1007/s00500-018-3130-0 [Crossref] [ Google Scholar]
- Chakrabarti S, Kamgar M, Mahipal A. Systemic therapy of metastatic pancreatic adenocarcinoma: Current status, challenges, and opportunities. Cancers 2002; 14:2588. [ Google Scholar]
- Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol 2015; 1263:243-50. doi: 10.1007/978-1-4939-2269-7_19 [Crossref] [ Google Scholar]
- Yamashita F, Hashida M. In silico approaches for predicting ADME properties of drugs. Drug Metab Pharmacokinet 2004; 19:327-38. doi: 10.2133/dmpk.19.327 [Crossref] [ Google Scholar]
- Raies AB, Bajic VB. In silico toxicology: computational methods for the prediction of chemical toxicity. Wiley Interdiscip Rev Comput Mol Sci 2016; 6:147-72. doi: 10.1002/wcms.1240 [Crossref] [ Google Scholar]
- Parvizpour S, Razmara J, Jomah AF, Shamsir MS, Illias RM. Structural prediction of a novel laminarinase from the psychrophilic Glaciozyma antarctica PI12 and its temperature adaptation analysis. J Mol Model 2015; 21:63. doi: 10.1007/s00894-015-2617-1 [Crossref] [ Google Scholar]
- Modica C, Tortarolo D, Comoglio PM, Basilico C, Vigna E. MET/HGF co-targeting in pancreatic cancer: a tool to provide insight into the tumor/stroma crosstalk. Int J Mol Sci 2018; 19:3920. doi: 10.3390/ijms19123920 [Crossref] [ Google Scholar]
- Moosavi F, Giovannetti E, Saso L, Firuzi O. HGF/MET pathway aberrations as diagnostic, prognostic, and predictive biomarkers in human cancers. Crit Rev Clin Lab Sci 2019; 56:533-66. doi: 10.1080/10408363.2019.1653821 [Crossref] [ Google Scholar]
- Li E, Huang X, Zhang G, Liang T. Combinational blockade of MET and PD-L1 improves pancreatic cancer immunotherapeutic efficacy. J Exp Clin Cancer Res 2021; 40:279. doi: 10.1186/s13046-021-02055-w [Crossref] [ Google Scholar]
- Magne N, Rousseau V, Duarte K, Poëa-Guyon S, Gleize V, Mutel A. PAK3 is a key signature gene of the glioma proneural subtype and affects its proliferation, differentiation and growth. Cell Oncol (Dordr) 2021; 44:1257-71. doi: 10.1007/s13402-021-00635-8 [Crossref] [ Google Scholar]
- Wu HY, Yang MC, Ding LY, Chen CS, Chu PC. p21-Activated kinase 3 promotes cancer stem cell phenotypes through activating the Akt-GSK3β-β-catenin signaling pathway in pancreatic cancer cells. Cancer Lett 2019; 456:13-22. doi: 10.1016/j.canlet.2019.04.026 [Crossref] [ Google Scholar]
- Woolbright BL, Rajendran G, Abbott E, Martin A, Didde R, Dennis K. Pyruvate dehydrogenase kinase 4 deficiency increases tumorigenesis in a murine model of bladder cancer. Cancers (Basel) 2023; 15:1654. doi: 10.3390/cancers15061654 [Crossref] [ Google Scholar]
- Qin YJ, Lin TY, Lin XL, Liu Y, Zhao WT, Li XY. Loss of PDK4 expression promotes proliferation, tumorigenicity, motility and invasion of hepatocellular carcinoma cells. J Cancer 2020; 11:4397-405. doi: 10.7150/jca.43459 [Crossref] [ Google Scholar]
- Li G, Li M, Hu J, Lei R, Xiong H, Ji H. The microRNA-182-PDK4 axis regulates lung tumorigenesis by modulating pyruvate dehydrogenase and lipogenesis. Oncogene 2017; 36:989-98. doi: 10.1038/onc.2016.265 [Crossref] [ Google Scholar]
- Liu Z, Chen X, Wang Y, Peng H, Wang Y, Jing Y. PDK4 protein promotes tumorigenesis through activation of cAMP-response element-binding protein (CREB)-Ras homolog enriched in brain (RHEB)-mTORC1 signaling cascade. J Biol Chem 2014; 289:29739-49. doi: 10.1074/jbc.M114.584821 [Crossref] [ Google Scholar]
- Choiniere J, Wu J, Wang L. Pyruvate dehydrogenase kinase 4 deficiency results in expedited cellular proliferation through E2F1-mediated increase of cyclins. Mol Pharmacol 2017; 91:189-96. doi: 10.1124/mol.116.106757 [Crossref] [ Google Scholar]
- Mengual L, Ars E, Lozano JJ, Burset M, Izquierdo L, Ingelmo-Torres M. Gene expression profiles in prostate cancer: identification of candidate non-invasive diagnostic markers. Actas Urol Esp 2014; 38:143-9. doi: 10.1016/j.acuro.2013.07.012 [Crossref] [ Google Scholar]
- Yang C, Wang S, Ruan H, Li B, Cheng Z, He J. Downregulation of PDK4 increases lipogenesis and associates with poor prognosis in hepatocellular carcinoma. J Cancer 2019; 10:918-26. doi: 10.7150/jca.27226 [Crossref] [ Google Scholar]
- Yamashita M, Kumazoe M, Onda H, Hiroi S, Shimada Y, Fujimura Y. PPAR/PDK4 pathway is involved in the anticancer effects of cGMP in pancreatic cancer. Biochem Biophys Res Commun 2023; 672:154-60. doi: 10.1016/j.bbrc.2023.06.043 [Crossref] [ Google Scholar]
- Tambe Y, Terado T, Kim CJ, Mukaisho KI, Yoshida S, Sugihara H. Antitumor activity of potent pyruvate dehydrogenase kinase 4 inhibitors from plants in pancreatic cancer. Mol Carcinog 2019; 58:1726-37. doi: 10.1002/mc.23045 [Crossref] [ Google Scholar]
- Terado T, Kim CJ, Ushio A, Minami K, Tambe Y, Kageyama S. Cryptotanshinone suppresses tumorigenesis by inhibiting lipogenesis and promoting reactive oxygen species production in KRAS-activated pancreatic cancer cells. Int J Oncol 2022; 61:108. doi: 10.3892/ijo.2022.5398 [Crossref] [ Google Scholar]
- Song X, Liu J, Kuang F, Chen X, Zeh HJ, 3rd 3rd, Kang R. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep 2021; 34:108767. doi: 10.1016/j.celrep.2021.108767 [Crossref] [ Google Scholar]