Bioimpacts. 2025;15:30224.
doi: 10.34172/bi.2024.30224
Review
Oil-in-water nanoemulsions for glaucoma treatment: An insight into the latest trends
Ankita Kishore Writing – original draft, 1 
Alok Kumar Mahor Conceptualization, Supervision, Writing – review & editing, 1, * 
Niraj Kumar Singh Methodology, Validation, 2
Prem Prakash Singh Methodology, Resources, 1
Priyanka Rathore Methodology, 1 
Kuldeep Kumar Bansal Conceptualization, Writing – review & editing, 3, * 
Author information:
1Institute of Pharmacy, Bundelkhand University, Jhansi, India
2Division of Pharmacology, Institute of Pharmaceutical Research, GLA University, Mathura 281406, India
3Pharmaceutical Sciences Laboratory, Faculty of Science and Engineering, Abo Akademi University, 20520 Turku, Finland
Abstract
Glaucoma is a serious eye disease characterized by elevated intraocular pressure, which can ultimately lead to blindness, making it the second leading cause of blindness worldwide, following cataracts. The condition is associated with various risk factors and primarily affects the optic nerve. To treat glaucoma, a range of approaches, both traditional and innovative, have been employed. Recently, there has been a significant focus on nanoemulsions as a promising avenue for treatment. This review underscores the advantages of using oil-in-water nanoemulsions for ocular drug delivery, showcasing their superiority in terms of enhanced bioavailability and stability compared with other dispersion systems. This review also delves into the limitations inherent in traditional drug formulations, elucidates the mechanisms governing drug release, explores the pivotal role of surfactants, and examines the landscape of granted patents in this domain. By addressing these critical aspects, the review offers invaluable insights into the treatment of glaucoma, shedding light on innovative approaches that hold great promise in the fight against this debilitating eye condition. During our search, it was noticed that despite the existence of commendable research in the field of ocular nanoemulsions, particularly in the context of glaucoma along with granted patents, the commercialized nanoemulsion formulations for glaucoma is not yet exist.
Keywords: Glaucoma, Nanoemulsion, Ophthalmic, Cationic surfactants, Ostwald ripening
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This study received no funding.
Introduction
Glaucoma is a group of eye conditions that damage the optic nerve, vital for transmitting visual information to the brain. Typically, the damage is due to elevated intraocular pressure (IOP), which is often caused by an imbalance between the production and drainage of the eye fluid (aqueous humor). The build-up of fluid can result from issues with the outflow of intraocular fluid from the iris (the coloured part of the eye). This condition can result in gradual vision loss and, if left untreated, can lead to blindness.1,2 Various factors, such as irritation or blockage of the drainage pathway, can lead to inadequate fluid outflow.3 After cataracts, glaucoma is the second-largest cause of visual loss.4 Overcoming barriers to effectively deliver drugs to the eyes involves tackling various challenges. These include tight junctions in the corneal epithelium, the natural blinking reflex, increased tear production, difficulties in absorption through the cornea and conjunctiva, and limited capacity of the conjunctival sac.
Glaucoma is a neurodegenerative disease, that affects the retinal nerve fibers and optic nerve head, and is a leading cause of global visual impairment. Projections suggest that by 2040, approximately 111.8 million individuals worldwide will be affected.5,6 Glaucoma vision loss is accompanied by stiletto field losses that advance from one retinotopic region to the next. The responsiveness to IOP, which measures the generation and outflow of aqueous fluid in the anterior eye, is associated with these impairments.7
Two types of glaucoma can be considered as major types, (a) open-angle glaucoma, and (b) closed-angle glaucoma. The first type which is primary open-angle glaucoma (POAG) is the most common (3). A critical pathological risk factor for POAG involves intraocular pressure levels, which are produced within the anterior chamber of the eye due to resistance in draining aqueous humor from the trabecular meshwork and the inner wall of Schlemm’s canal.8 Generally, the breadth of the angle between the cornea and iris permits normal aqueous fluid outflow from the anterior chamber to the drainage channels of the trabecular meshwork. Finally, the issue with fluid outflow was caused by the malfunction of the trabecular meshwork, which becomes blocked by cell debris, including inflammatory or blood cells. The most prevalent type of glaucoma is primary open-angle glaucoma (or POAG), which is linked to increased resistance in the aqueous outflow channels.9 Often, there are no immediate or acute signs or symptoms and no discomfort. The only symptoms that worsen over time are narrowing of the visual field and alterations in the optics nerve.10 Secondary, closed-angle glaucoma, characterized by the narrowing or occlusion of the outflow route, hinders the drainage of aqueous fluid, potentially leading to sudden spikes in IOP and significant ocular discomfort.11,12 Another type of glaucoma is congenital glaucoma, which manifests in babies with symptoms such as excessive tearing, light sensitivity, a fear of light, and eyelid retraction. These signs are often accompanied by a noticeable enlargement and cloudiness of the cornea.13,14 The study of glaucoma and its consequences has been the focus of numerous investigations.15 Fig. 1 illustrates a comparison between eyes with normal and increased IOP, whereas the second comparison, shown in Fig. 2, distinguishes between open-angle and closed-angle glaucoma.
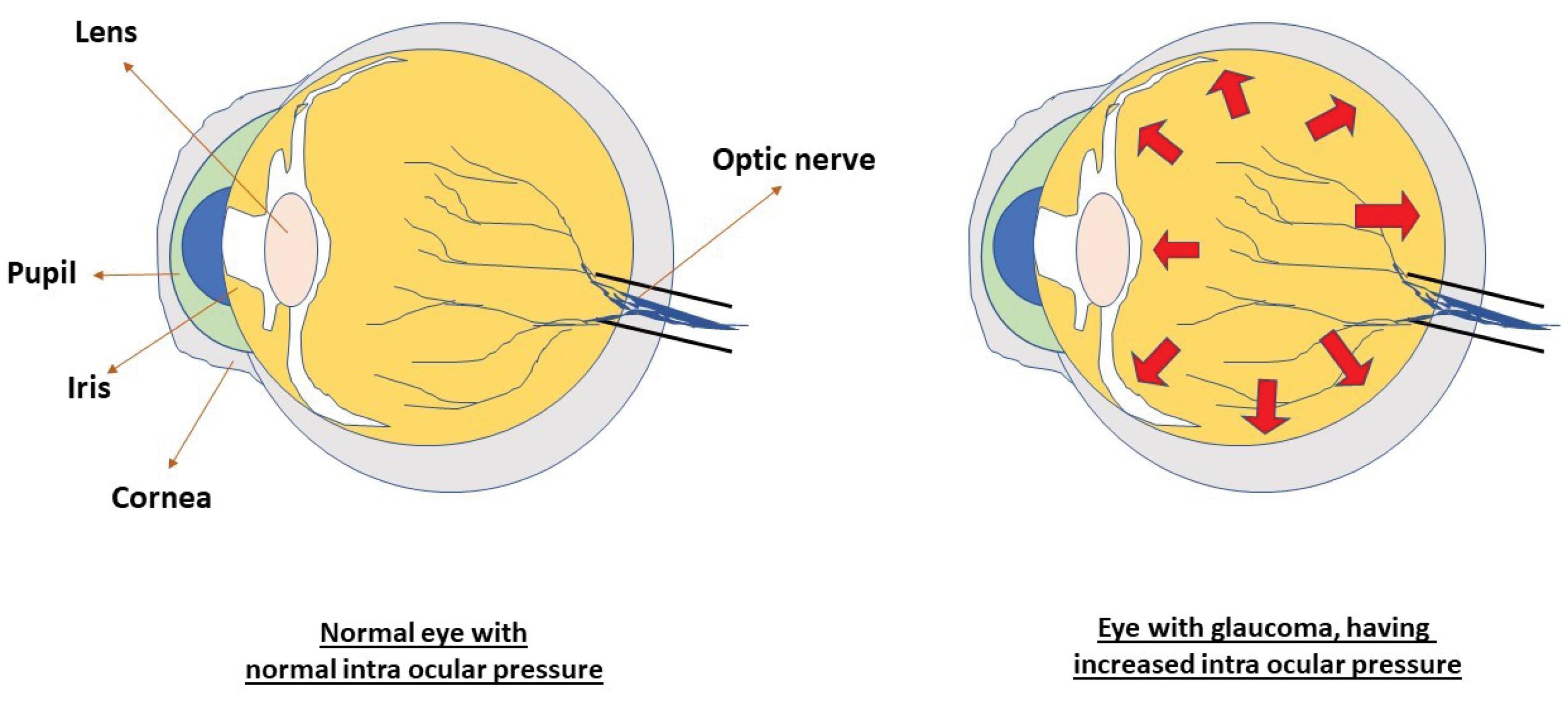
Fig. 1.
Diagram showing intraocular pressure in normal eyes and eyes with glaucoma.
.
Diagram showing intraocular pressure in normal eyes and eyes with glaucoma.
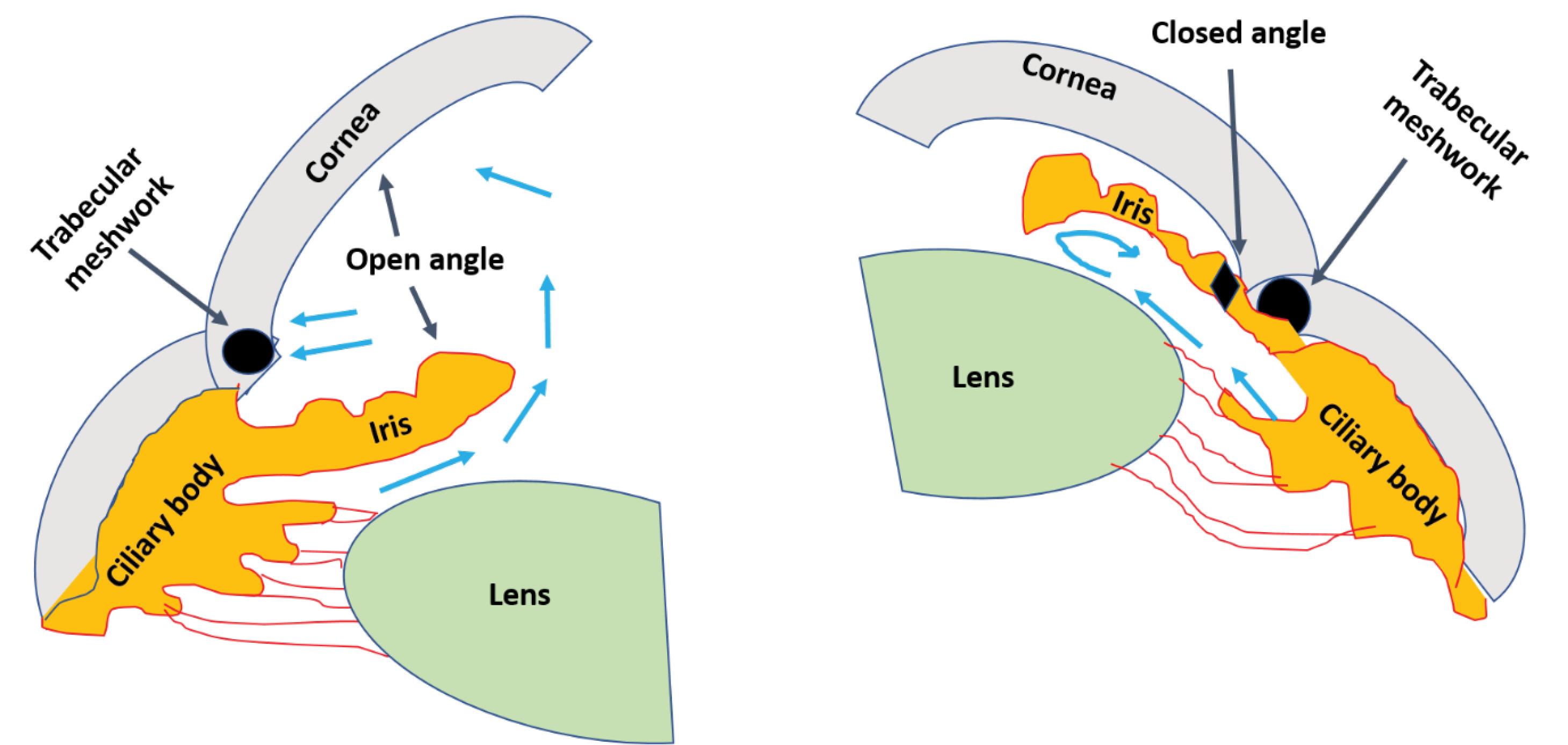
Fig. 2.
Comparison between open- and closed-angle glaucoma. The main difference is in the location of fluid collection.
.
Comparison between open- and closed-angle glaucoma. The main difference is in the location of fluid collection.
Glaucoma can also be considered as a set of ocular neuropathic conditions that together account for the world’s leading cause of irreversible blindness, where blindness is caused by the degeneration of neural tissues in the optic projection from the retina to the brain.16,17 Glaucoma is becoming more common as the population ages; by 2040, nearly 112 million people will be affected worldwide.9
Traditional ophthalmic solutions, employed over an extended period, have limitations in terms of therapeutic effectiveness. Challenges include drug elimination before corneal contact, drainage influenced by gravity, nasolacrimal system drainage, absorption by the conjunctiva, and a deficiency in controlled release and bioadhesive properties.18 The non-adherence of patients to medications is also one of the main problems in the treatment of glaucoma. Glaucoma-induced blindness can be avoided if the disease can be diagnosed at a very early stage and by taking medications timely.19 Effective treatment of glaucoma requires patients to maintain good adherence and persistence in controlling their intraocular pressure. Patients must be well-informed about their condition.20 Improving patient adherence necessitates establishing effective communication between healthcare providers and patients and implementing long-term strategies to enhance adherence.21 Poor adherence often stems from factors such as forgetfulness, challenges with applying eye drops, and difficulties adhering to medication schedules.22
Researchers are currently exploring novel drug delivery systems to overcome the limitations of conventional ophthalmic dosage forms and improve patient compliance. One promising system under investigation is nanoemulsions, which have the potential to overcome these limitations. Nanoemulsions are liquid dispersion systems with nanoscale phases containing oil, water, and a surfactant. Specifically, cationic oil-in-water nanoemulsions are preferred in ophthalmic applications because of their superior adherence to the ocular surface and transparent appearance. These nanoemulsions can carry hydrophobic drugs, and the inclusion of cationic surfactants enhances their ability to penetrate ocular surfaces. This resulted in improved bioavailability and extended the shelf life of the formulation. Ocular nanoemulsions are gaining attention as a promising option in the realm of novel ophthalmic drug delivery systems, particularly in glaucoma. Studies suggest that nanoemulsions loaded with anti-glaucoma drugs can effectively overcome the challenges associated with targeting drug delivery to the anterior chamber of the eye.23-27
Thus, in this review, we embrace the applicability of oil-in-water nanoemulsions in enhancing the bioavailability of hydrophobic drugs. Furthermore, this study focuses on nanoemulsion stability, drug release, and stabilization mechanisms. Cationic nanoemulsions have additional advantages in overcoming ocular barriers. An explanation of some of these agents and their comparison has been discussed. The article also encompasses recent research conducted and granted patents in the field of nanoemulsion. Safety and toxicity considerations in formulation have been summarized based on published studies. Unlike other existing review articles, our goal was to consolidate critical information about ocular nanoemulsions in single article. We investigated the correlation between marketed ocular nanoemulsion preparations, patents granted for glaucoma, and ongoing research in ophthalmic nanoemulsions. The collected information revealed a gap in current knowledge and researchers can leverage these data to assess the current status of nanoemulsion in the field of glaucoma.
Conventional ocular drug delivery systems used for treating glaucoma
Ocular drug delivery systems are intended to be used in the eyes. Several drug delivery systems are available to treat various ocular problems in different parts of the eye. The following are some drug delivery systems specific to glaucoma treatment.
Numerous drugs with antiglaucoma properties are accessible, but a significant challenge lies in devising effective delivery systems that target the eye. This article is dedicated to the treatment of glaucoma and other ocular disorders using nanoemulsion-based drug-loaded delivery systems. The traditional drug delivery systems mentioned below have been utilized for the treatment of glaucoma over an extended period.
Conventional drug delivery systems
Numerous traditional dosage forms are commercially available and have been employed for an extended period to treat glaucoma, each operating through distinct mechanisms (Fig. 3).
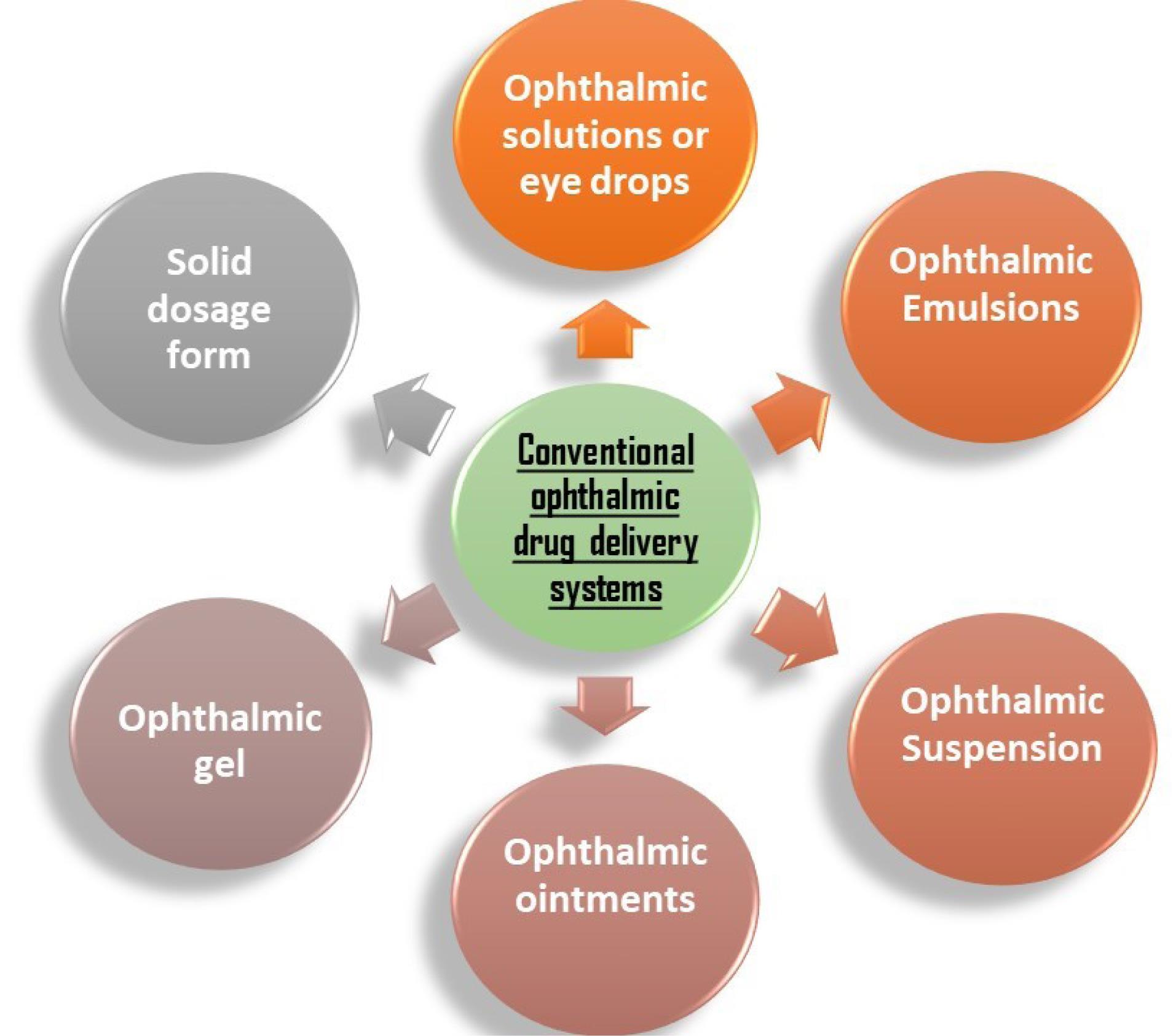
Fig. 3.
Conventional drug delivery systems for treating glaucoma.
.
Conventional drug delivery systems for treating glaucoma.
Topical solutions or eye drops
The most practical, secure, immediately effective, patient-compliant, non-invasive, and comfortable method for administering ocular medications is topical drops.28 Tonicity, pH, stability, viscosity, sterility, and the choice of a preservative are the most crucial aspects to consider when making an ophthalmic solution.29 One study has shown that ophthalmic solutions, after opening for 30 days, can vary in pH, which is generally not statistically meaningful for the solutions.30
Topical emulsions
The bioavailability and solubility of pharmaceuticals can be enhanced using an emulsion-based formulation strategy.31 Emulsions, especially microemulsions, appear to be rapidly moving toward commercial applications and may become the preferred delivery system for lipid-soluble ophthalmic medications.32 Ophthalmic emulsions are intricate systems used to transport medications that are poorly penetrable to the eye, which is a complicated organ with several potential target tissues, depending on the condition.33
Topical suspension
Eye drops for drugs that are difficult to dissolve are commonly made as suspensions.34 The basic requirements and indications for liquid and semisolid vehicles containing dispersed drugs are the same as those for regular eye drops, ointments, and hydrogels that include dissolved medications.32
Topical ointments
A blend of semisolid and solid hydrocarbons with a melting temperature at physiological ophthalmic temperature makes up ophthalmic ointments.35 Ointments aid in maintaining drug release and increasing ophthalmic bioavailability.36
Topical gel
It is of two types: hydrophilic polymer-based aqueous gels (hydrogels) and stimuli-responsive polymer-based aqueous gels.37 However, this formulation has not been well received due to hazy vision.38 This problem can be solved by using an in situ gel-forming ocular drug delivery system made of polymers that demonstrate a sol-to-gel phase transition in the eyes in response to a change in a particular physiochemical parameter.39 The abovementioned conventional topical formulations were limited in their actions because of various ophthalmic barriers.40 These barriers protect the eyes from the external environment and materials, causing the externally applied drug concentration to decrease at the place of application within a few seconds. Thus the time needed for absorption of the drug was also reduced. Therefore, scientists have huge opportunities to overcome these barriers and increase the contact time of formulations on the eye surface.41
Solid dosage form (tablets)
The doctor may recommend an oral tablet, typically a carbonic anhydrase inhibitor, if the above-discussed conventional topical dosage forms alone fail to reduce eye pressure to the required level.42,43 Many conventional drug delivery systems are commercially available for glaucoma, but these conventional forms have basic formulation-related limitations. Owing to these limitations, some advancements are required to enhance formulation efficacy. Therefore, we are moving toward novel drug delivery-based systems. Table 1 summarizes a few commercial formulations that are used for treating glaucoma along with their limitations.
Table 1.
Commercial products and limitations of conventional ophthalmic drug delivery systems
|
Conventional ophthalmic drug delivery system
|
Commercial products
|
Limitations of the conventional drug delivery system
|
Ref.
|
| Ophthalmic Solutions or Eye Drops |
Alphagan solutions (brimonidine tartarate), Combigan solutions (brimonidine & timolol), Lumigan solutions (bimatoprost), etc. |
Rapid removal of the drug due to lacrimal secretions, low bioavailability, frequent instillation, quick drainage, and sustainable action. |
44,45
|
| Ophthalmic Emulsions |
Xelpros (latanoprost) |
Toxicity (greater concentration) and stability are influenced by the choice of co-surfactant, aqueous, and organic phase. |
44
|
| Ophthalmic Suspension |
Azopt suspension (Brinzolamide), symbrinza (brimonidine & brinzolamide), |
Irritation due to the presence of particles and loss of drug solution. |
44
|
| Ophthalmic Ointment |
Lacort (Chloramphenicol & Hydrocortisone) |
Blurring vision, poor patient compliance, eyelid stickiness, and the partition coefficient restrict drug selection. |
45
|
| Ophthalmic Gel |
Oilopine-HS (pilocarpine HCl) |
Only triggered by temperature, pH, and ionic strength after the use of matted eyelids, with no control on the rate of diffusion. |
37,46
|
| Solid dosage forms |
Diamox (acetazolamide) and neptazane (methazolamide) |
Blood–ocular barriers, limit drug penetration, and drug loss due to liver metabolism and kidney clearance. |
47,48
|
Conventional dosage forms for treating glaucoma face some typical problems such as short time for drug retention, corneal penetration, formulation removal from eyes, and low bioavailability. that inhibit them from imparting sufficient therapeutic effect for treatment. The challenges faced in conventional dosage forms for glaucoma include the following:
-
Limited drug retention: Conventional eye drops face challenges in retaining the administered drug within the eye. Factors such as tear drainage, blinking, and nasolacrimal drainage contribute to reduced drug retention on the ocular surface.
-
Ineffective corneal penetration: The corneal epithelium with its tight junctions poses a barrier to effective drug penetration. Achieving sufficient drug levels in the cornea is challenging with conventional formulations.
-
Short residence time: Conventional dosage forms are prone to rapid elimination from the ocular surface, leading to a short residence time. This limits the duration of the therapeutic action.
-
Systemic absorption: There is a risk of systemic absorption of the drug, especially when administered through eye drops. This can lead to systemic side effects and may not be ideal for patients with certain medical conditions.
-
Limited bioavailability: Precorneal factors, such as tear dilution and drainage, contribute to the limited bioavailability of drugs delivered through conventional dosage forms. This may result in suboptimal therapeutic outcomes.
-
Lack of Controlled Release: Conventional eye drops lack controlled release mechanisms, leading to fluctuations in drug concentrations. This can affect the sustained therapeutic effect required for glaucoma management.
-
Patient compliance: The need for frequent administration of eye drops poses challenges related to patient compliance. Patients may forget or find it inconvenient to adhere to the prescribed dosing regimen.
-
Bioadhesion issues: Conventional formulations may lack sufficient bioadhesive properties, making it challenging for the drug to adhere to the ocular surface and prolong contact time.
Topical hypotensive medications are ineffective; 20% of the prescribed quantity is squandered, and each eye may require up to 3.7 drops of medication per dose. Drug waste is also a result of the ocular surface’s poor absorption capacity. Topical dosages have 1%–10% bioavailability because of the cornea’s rapid and poor absorption of the medication. IOP may be ineffectively controlled as a result, and the illness may worsen. Sustained release of novel drug delivery systems is being investigated as a potential solution to these problems.49 Addressing these challenges has led to the exploration of novel drug delivery systems, such as nanoemulsions and other nanotechnology-based approaches, to enhance the efficacy and patient compliance in glaucoma therapy.
Nanoemulsions: Characteristics and composition
When two or more immiscible phases are mechanically sheared with the appropriate surfactants, at least one of the phases is dispersed in the other phases, creating a thermodynamically or kinetically stable system known as a nanoemulsion.
Nanoemulsions are unique in their composition, featuring a biocompatible, nonpolar oil phase with structural similarities to substances commonly found in the human body and food. In pharmaceutical applications, oil-in-water nanoemulsions are favored because of their ability to effectively retain lipophilic drugs within the internal phase.50-52 These nanoemulsions leverage primary and secondary surfactants,53 in conjunction with a high zeta potential, to generate repulsion forces among their components, thereby ensuring superior thermodynamic stability among the dispersed globules.54 The benefits of nanoemulsions extend beyond conventional emulsions. Notably, they offer heightened thermodynamic stability, reduced viscosity, and improved drug bioavailability because of the larger surface area of their globules. It is important to note that nanoemulsions excel in terms of biocompatibility, which sets them apart from certain nanoparticle-based systems that may pose potential toxicity risks due to the materials employed in their formulations. This exceptional compatibility is attributed to the presence of lipids and water in the nanoemulsions, making them an ideal choice for various pharmaceutical applications.55,56
Significance of droplet size and zeta potential in nanoemulsions
The stability of nanoemulsion hinges on factors such as droplet size, surface charge, and emulsifier composition.57,58 These elements collectively dictate the overall stability. A well-calibrated emulsifier blend establishes a flexible interface between immiscible liquids, effectively suspending small globules of the dispersed phase.55 These droplets, endowed with elasticity, can withstand high deformation levels, thereby enhancing stability. Smaller, uniformly sized droplets further fortify stability by minimizing the impact of gravitational forces. Moreover, a robust intermolecular repulsive force driven by a higher surface charge keeps smaller droplets separated. During elastic collisions, approaching droplets experience a force that propels them apart, thereby thwarting coalescence. This charge-driven repulsion mechanism sustains all charged nanodroplets in a continuous Brownian motion.59 A similar charge-based repulsion mechanism stabilizes mono-surfactant-based nanoemulsions, as shown in Fig. 4, to clarify the stabilization process.
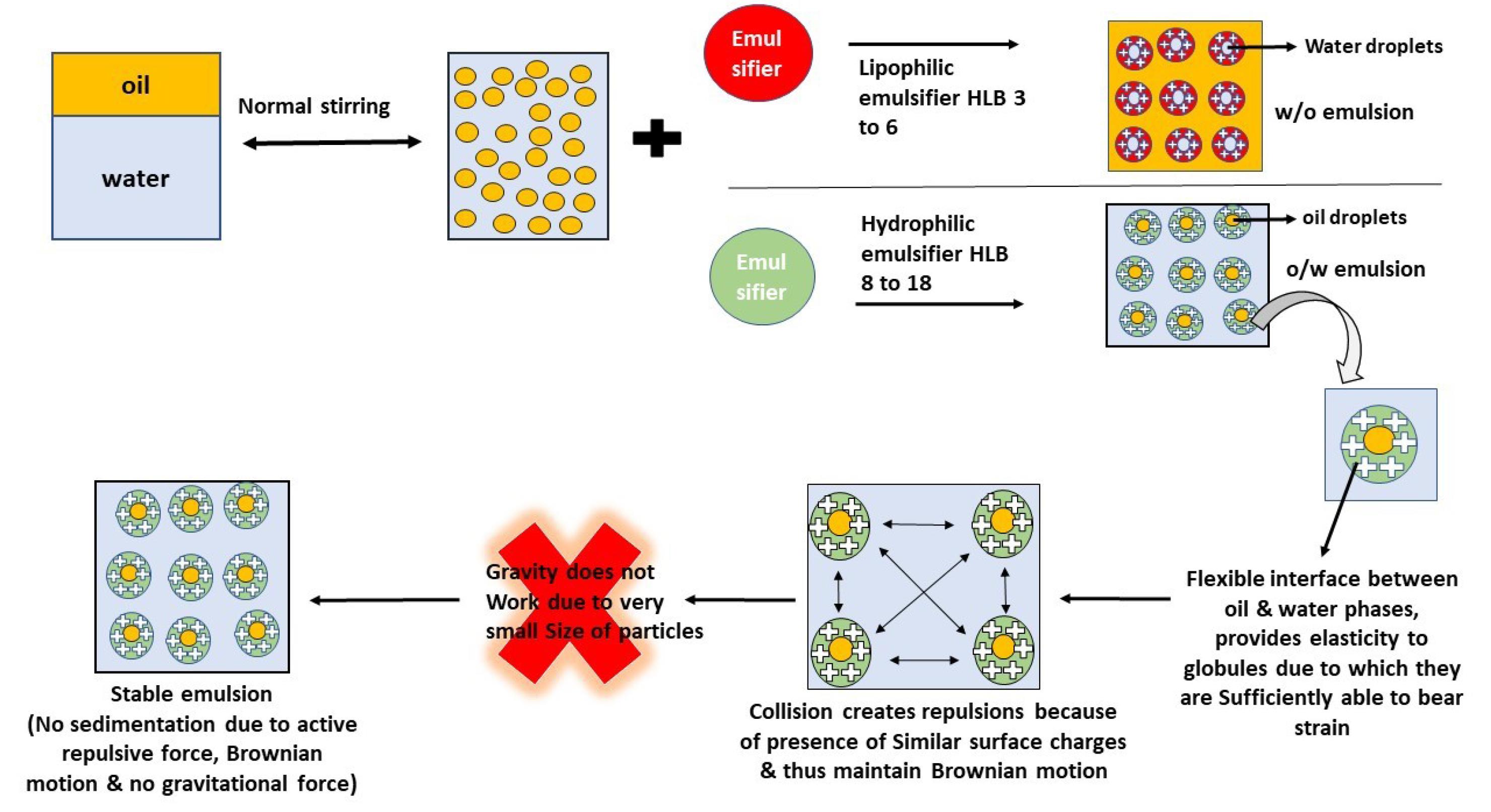
Fig. 4.
Stabilization mechanism of the nanoemulsion.
.
Stabilization mechanism of the nanoemulsion.
The gravitational pull of the globules is determined by their mass and size, whereas the repelling force is influenced by the strength of the zeta potential. Zeta potential values can serve as predictors of the stability of nanoemulsions. The pharmacokinetics and biodistribution of nanoemulsions are similarly affected by droplet size. Reduced droplet size provides a longer blood residence time, indicating a size-dependent effect on the duration of circulation. Thus, the zeta potential and droplet size are important parameters to be considered in the case of a good nanoemulsion.60
Considerations concerning the development of ocular Nanoemulsions
When developing ophthalmic drug delivery systems, it is crucial to consider various barriers, challenges, and influencing factors, all of which are detailed in Table 2. These principles also apply to the development of ophthalmic nanoemulsions, making them a versatile and valuable option for enhancing drug delivery.
Table 2.
Barriers, challenges, and consideration factors during the development of topical ocular drug delivery systems
61-67
|
Ophthalmic barriers to ocular drug delivery
|
Challenges in the development of topical ocular drug delivery
|
Parameters to be considered when developing ocular drug delivery systems
|
-
Tight junction of the corneal epithelium
-
Very small volume of the conjunctival sac (30 µl)
-
Reflex blinking of eyes (5-7 times in a minute)
-
Biotransformation of drugs in ocular tissues
-
Increased tear production
-
High rate of tear fluid exchange
-
High evaporation route of the tear fluid
-
Corneal thickness
-
Absorption through the cornea
-
Absorption through the conjunctiva
-
Drug-Protein binding
-
Efflux pumps
-
Nasolacrimal efflux
-
Lymphatic clearance
-
Conjunctival blood flow
|
-
Absorption (>5% bioavailability)
-
Development of dosage forms of drugs with limited aqueous solubility.
-
Ease of use (low discomfort)
-
Limited excipient availability concerning the safety of the eyes
-
Delivery of the drug to the posterior segment of the eye. (Often invasive therapy is preferred)
|
-
Anatomical structure and functions of the eye
-
Tear compositions that can affect drug absorption.
-
Presence of any other ocular diseases
-
Compatibility with patient expectations
-
Comfortable to the eyes
|
Advantages of nanoemulsions in improving bioavailability and penetration of the ocular surface
Nanoemulsions play a vital role in the formulations of hydrophobic drugs, especially those of the oil-in-water type. In this configuration, oil droplets serve as carriers for hydrophobic drugs, thereby improving their bioavailability through increased surface area and penetration. However, integrating hydrophobic ocular drugs into nanoemulsions poses several challenges because of the eye’s intricate anatomy and physiology. The eye is highly sensitive to foreign substances and rapidly removes them from its surface. Additionally, the lacrimal gland consistently secretes tear fluid, accounting for approximately 16 % of the total tear volume per minute under normal conditions.68
The challenges in delivering drugs to the eyes create two significant issues. First, conventional aqueous eye drops are not suitable for the integration of hydrophobic drugs. Second, the eye’s drainage systems swiftly eliminates eye drops, with approximately 80% removed within minutes, demanding frequent dosing for sustained therapeutic effects. In response, nanoemulsions have emerged as promising remedies for ocular drug delivery. The Novasorb product series has effectively employed nanoemulsions.69 Earlier methods aimed at boosting solubility inadvertently reduced pharmacokinetics and bioavailability while elevating toxicity levels in the system.70
To make the formulation more sustainable, other secondary formulations can also be developed by using this basic nanoemulsion as its primary formulation. For example, Tau et aldeveloped an ocular nanoemulsion for latanoprost, another hydrophobic drug. This nanoemulsion contained a mild preservative, potassium sorbate, and avoided the use of the surfactant benzalkonium chloride, which is known to cause ocular toxicity with long-term use. A comparison between this gentle nanoemulsion and an ophthalmic solution containing benzalkonium chloride revealed that the former was milder and better tolerated.27
Prolonged drug release
The process of drug release from a nanoemulsion typically consists of three distinct steps, as depicted in Fig. 5. This process is influenced by various factors, including the components of the formulation, the characteristics of the active pharmaceutical ingredients, and the surrounding environmental conditions. The steps of drug release can be described as follows:
simple
-
a) Initially, the drug transitions from the oil phase to the surfactant layer and subsequently into the aqueous phase (the mucous layer of the tear film is hydrophilic) during drug release from the nanoemulsion.
-
b) As the solubilized portion of the drug moves out of the oil phase and encounters the surrounding water, nanoprecipitation occurs.
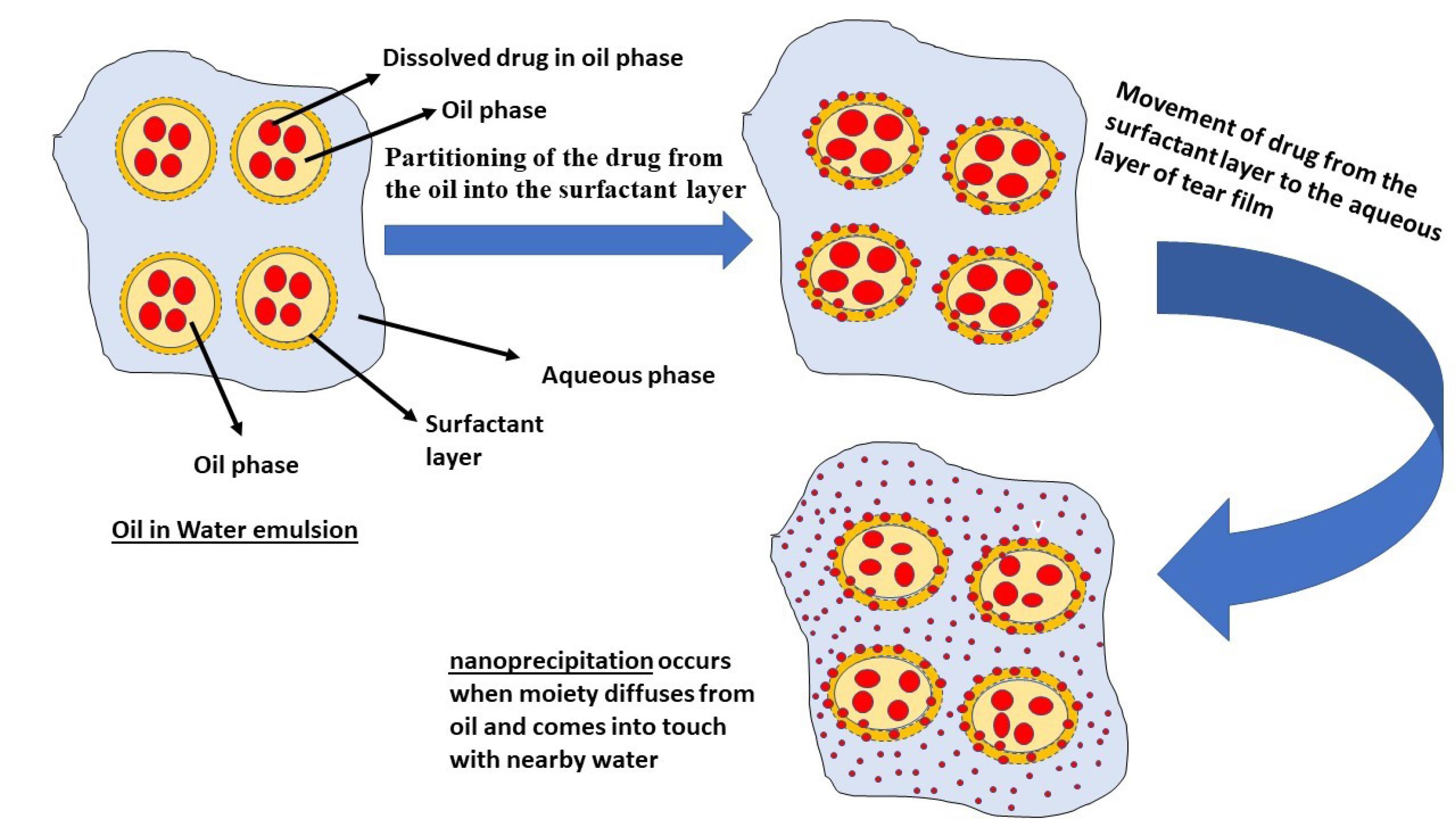
Fig. 5.
Release steps of drug from nanoemulsion to aqueous layer of tear film.
.
Release steps of drug from nanoemulsion to aqueous layer of tear film.
This nanoprecipitation event results in a significant increase in the drug’s surface area, consequently expediting its disintegration, as described by the Noye–Whitney equation.
Additionally, nanoemulsions frequently require less surfactant than conventional colloidal dispersions while preserving many benefits and are thus anticipated to overwhelm the commercial dug delivery barrage.71 The nanoemulsion becomes unstable because of the shift in droplet size caused by Ostwald ripening. Stabilizing the oil-water interface during emulsification is a major challenge in functionalizing the surface of nanoemulsions.54
Commercialized nanoemulsion products for the treatment of ocular diseases
Novagali Pharma is a French ophthalmology-focused biopharmaceutical firm that was acquired by Santen Pharmaceutical in 2011.72 The formulation and development of ophthalmic products is the core area of expertise of this company. Its products are among some of the most popular ophthalmic products, and they are still improving their formulations using advanced and modified technologies.64
Some important formulations of Novagali Pharma are Novasorb® and Catioprost®and Cationorm®, which are being used for treating glaucoma and have successfully entered clinical trial phases. Novasorb®is a cationic nanoemulsion containing latanoprost as an active pharmaceutical ingredient for treating glaucoma.64 It contains benzalkonium chloride as a cationic surfactant, which increases formulation adherence time with a negatively charged ocular surface and increases the formulation’s effectiveness.73
Formulation development encountered several challenges. One primary hurdle involved the careful selection of suitable cationic agents. Limited options existed because only a few cationic agents had official approval for use in ophthalmic formulations, given the potential risk of ocular toxicity and cell damage. The zeta potential emerged as a crucial parameter for stabilizing the formulation, imparted by these chosen cationic agents. Throughout the development process, it became evident that maintaining the zeta potential above +10 mV was vital for the stability of the nanoemulsion. To enhance stability, the zeta potential was optimized to fall within the range of +20 mV to +40 mV. The primary objective was to select the most appropriate cationic surfactant capable of providing a sufficiently high zeta potential at the lowest concentration while also aligning with regulatory standards.
Quaternary ammonium compounds have emerged as promising candidates, serving dual roles as preservatives and surfactants. Acting as preservatives, their positive charge allowed them to bind to the negatively charged surfaces of microorganisms, damaging their cell walls. However, it was noted that this positive charge also posed a potential risk of damaging ocular cells. The challenge was to strike a balance by selecting a cationic surfactant that effectively met both criteria.
Sznitowska discovered that the preservative action of quaternary ammonium compounds could be minimized by incorporating them into emulsions. In this context, most of their components become bound within the emulsion, reducing the presence of the free part in the aqueous phase and limiting its preservative action. Novagali Pharma adopted benzalkonium chloride and cetalkonium chloride (a lipophilic derivative of benzalkonium chloride) as innovative cationic nanovectors.
The high lipophilic nature of Cetalkonium chloride’s facilitated its interaction with the oily phase, contributing to the high zeta potential of its droplets. This design minimized the amount of cetalkonium chloride available to induce ophthalmic toxicity. Studies indicated that buffering the formulation was unnecessary, as low-pH formulations were well tolerated by the eyes when quickly returned to normal ocular pH. Droplet size influences absorption, which occurs through diffusion. Various physicochemical characterization tests, including pH, zeta potential, surface tension, and interfacial tests, were conducted to assess formulation stability.
For drug loading, it is preferable to choose drug candidates that are nonionizable and lipophilic (with a log P value of 2 to 3) when preparing emulsions. Nanocarriers typically have a mechanism for active ingredient uptake, which can be explained in two ways:
-
When nanodroplets are intact and adhere to the mucin layer in close contact with corneal epithelial cells, they can enter the ocular cell membrane via two mechanisms: Active or passive diffusion, where nanodroplets release the active ingredients outside the cells.
-
When nanodroplets are in their intact form, they can be taken up by pinocytosis, phagocytosis, or endocytosis.
The use of benzalkonium chloride (BAK) in glaucoma eye solutions retains the solution over an extended period. However, it has also been demonstrated to cause and exacerbate the signs and symptoms of dry eye disease in patients. Cationic nanoemulsions, even without active ingredients, offer benefits for the eyes because of their basic composition, including oil, water, surfactants, and glycerol. This composition helps prevent tear evaporation, keeping the eyes lubricated and moisturized. As a result, a preservative-free and active ingredient-free emulsion has been globally commercialized to address dry eye symptoms.
Novasorb®, which contains benzalkonium chloride as a preservative, can induce signs and symptoms of dry eye disease. In contrast, Cationorm®, an artificial tear based on Novasorb®, can offer relief, particularly in cases of glaucoma-associated dry eye disease.73 Cationorm stands out among other artificial tears because it is preservative-free and contributes to the enhancement of the tear film’s condition through its unique composition. The positive charge on the oil droplets in Cationorm promotes adherence to the ocular surface, ensuring a prolonged duration of action. Its oil composition targets the lipid layer of the tear film, whereas the aqueous phase facilitates even distribution across the ocular surface, providing extended moisturization compared with other artificial tears. This mechanism effectively safeguards and nurtures the tear film.74
Kinnunen et al conducted a study assessing the tolerability of Cationorm using in vitro testing on human corneal epithelial cells (HCE cells). The HCE cell culture line was used to evaluate the impact of Cationorm on cytotoxicity, cellular morphology, and inflammatory responses. The findings demonstrated its excellent tolerability compared with prior eye drops containing benzalkonium chloride.75
Catioprost®, a cationic emulsion containing latanoprost as the active pharmaceutical ingredient, stands out because it is entirely free of the preservative benzalkonium chloride. It has been successfully compared with latanoprost-containing solutions containing benzalkonium chloride. The results indicated that both formulations were effective, with the benzalkonium chloride-free latanoprost cationic emulsion reducing conjunctival hyperemia by 42%. In addition, Catioprost® was found to be equally potent as Xalatan but with an enhanced safety profile. In comparison with benzalkonium chloride-free Travatan Z®, which contains travoprost in a 0.004% solution, Catioprost® demonstrated superior efficacy in reducing intraocular pressure and improving conditions related to ocular surface disease.76
Emerging Nanoemulsion Technologies for ocular delivery and patents granted in glaucoma field
Several nanoemulsions are available in the market not only for glaucoma but also for other ocular problems, as listed in Table 3.
Table 3.
FDA-approved and under clinical trial nanoemulsions used for the delivery of medications at the ocular surface
|
Marketed product
|
API
|
Targeted ocular disease
|
FDA approval/clinical trial phase
|
Manufacturer
|
Ref.
|
| Ikervis® |
Cyclosporin A |
Keratitis associated with dry eye disease |
FDA approved |
Santen |
77
|
| Restasis |
Cyclosporin A |
Chronic dry eye |
FDA approved |
Allergen |
33
|
| Durezol |
difluprednate |
Inflammation of eye |
FDA approved |
Alcon |
78
|
| Systane |
Propylene glycol-based eye drop nanoemulsion |
Dry eye treatment |
Phase IV |
Alcon |
79
|
| SVT-15473 |
Clobetasol propionate |
Inflammation and pain in paediatric patients after cataract surgery |
Phase III |
Salvate laboratories |
52
|
| Cyclokat |
Cyclosporin A |
Dry eye |
- |
- |
80
|
| Verkazia |
Cyclosporine |
Vernal keratoconjunctivitis |
FDA approved |
Santen |
80
|
| SBI-100 |
Proprietary prodrug of tetrahydrocannabinol |
Primary open-angle Glaucoma |
Phase II |
Skye Biosciene |
81
|
| - |
Brimonidine Tartrate |
Dry eye disease |
Phase III |
- |
82
|
Patents granted for ocular nanoemulsions
Table 4 lists the patents that have been granted to the ocular nanoemulsions that have been involved in treating glaucoma directly or indirectly.
Table 4.
List of patents in the last decade related to ocular nanoemulsions
|
Patent No.
|
Patent Title
|
Publication date
|
Ref.
|
| CN116725954A |
Nanometer medicinal preparation with antioxidant function and its use for treating glaucoma |
12/09/2023 |
83
|
| TW202247845A |
Eye nanoemulsion composition containing prostaglandin derivative has better stability to provide convenience in the preservation |
16/12/2022 |
84
|
| CN115463088A |
Ophthalmic nanoemulsion composition containing prostaglandin derivative |
12/12/2022 |
85
|
| US 20220125634 A1 |
Implantable ocular drug delivery devices and methods |
28/04/2022 |
86
|
| BR102014024497B1 |
Nanoemulsion compositions containing melaleuca leucadendron extract and/or fractions and use |
23/11/2021 |
87
|
| WO2021240376 A2 |
Ophthalmic nanoemulsion compositions |
02/12/2021 |
88
|
| WO2020240451A1 |
In situ gelling nanoemulsion of brinzolamide |
03/12/2020 |
89
|
| US20200197522A1 |
A pharmaceutical composition comprising brinzolamide |
25/06/2020 |
90
|
| US 20200315965A1 |
Nanoemulsion concentrate formulations and methods |
08/10/2020 |
91
|
| KR20200053205A |
A surfactant-free type ophthalmic nano-emulsion composition, and the manufacturing method thereof |
18/05/2020 |
92
|
| BR102014024497A2 |
Nanoemulsified compositions of fructose leucadendron and/or pilocarpine extract and/or fractions and uses |
21/11/2018 |
93
|
| EP2978409B1 |
Ophthalmic composition, method for preparing the same, and use of the same |
01/10/2018 |
94
|
| US9801891B2 |
Compositions and methods for lowering intraocular pressure |
31/10/2017 |
95
|
| AU2010220321B2 |
Anionic oil-in-water emulsions containing prostaglandins and uses thereof |
20/08/2015 |
96
|
| JP5722803B2 |
Cationic oil-in-water emulsions containing prostaglandins and their use |
27/05/2015 |
97
|
| US8414904B2 |
Ophthalmic oil-in-water emulsions containing prostaglandins |
9/04/2013 |
98
|
Challenges and considerations concerning the formulation for developing ophthalmic nanoemulsions
Safety concerns and toxicity evaluation
Safety concerns
Because of their potential for better therapeutic efficacy and drug delivery, ophthalmic nanoemulsions intended for use in the eyes have drawn attention. However, when creating and applying ophthalmic nanoemulsions, particular safety issues must be considered. Some major safety issues with this formulation and related considerations are tabulated in Table S1 (Supplementary file 1).
Toxicity evaluation
The toxicological evaluation of ocular nanoemulsions entails a thorough examination of the potential side effects and safety concerns associated with the use of these formulations in ophthalmic applications. The vital phases and concerns for toxicity assessment of ophthalmic nanoemulsions are summarized in Table S2 (Supplementary file 1).
Potential side effects and mitigation strategies
Owing to their unique formulation and administration method, ophthalmic nanoemulsions for eye application may have possible side effects. Ocular irritation, blurred vision, increased tear production, conjunctival hyperemia, photophobia, foreign body sensation, dry eye sensation, systemic absorption, corneal oedema, ocular discharge, corneal deposits, changes in IOP, visual disturbances, and allergic reactions are examples of these symptoms.99,100 Ocular irritation can be modified by the choice of surfactants and excipients, and preclinical and clinical investigations should analyze the risk of irritation.57
To reduce these side effects, formulation design, preclinical testing, and close monitoring during clinical trials are essential.70 Throughout the development process, patient safety and ocular tolerability should be prioritized, and regulatory bodies should be involved to ensure the approval of safe and effective ophthalmic nanoemulsions. Regular intraocular pressure monitoring and adherence to regulatory criteria are critical for maintaining the safety and effectiveness of ophthalmic nanoemulsions. In addition, careful formulation design, extensive preclinical and clinical testing, and ongoing monitoring are required. Collaboration with regulatory bodies, healthcare providers, and patients is critical for ensuring the safety and efficacy of medications.101 Choosing non-irritating surfactants and excipients, conducting comprehensive preclinical studies minimizing vision effects, selecting hypoallergenic materials, conducting skin patch testing, monitoring intraocular pressure, excluding individuals with known glaucoma, ensuring contact lens compatibility, maintaining tear film stability minimizing photophobia, providing clear instructions, reporting unusual side effects and adhering to safety guidelines are all important factors.102
Current trends in nanoemulsions use in glaucoma
Nanoemulsions, known for their potential to enhance drug delivery, have garnered considerable attention in diverse pharmaceutical applications. Researchers are actively exploring the uncharted territories of nanoemulsions, particularly in the context of glaucoma treatment. Table S3 (Supplementary file 1) provides an overview of the research conducted in this area from 2017 to 2023.
Future perspectives and research directions
Nanoemulsions, a type of nanoscale drug delivery system, offer distinct advantages, particularly for enhancing drug solubility, especially for BCS class II and IV drugs. Various formulation techniques are available, allowing customization based on the formulation needs. Cationic nanoemulsions are particularly beneficial because they enhance adherence to ocular cells, and prolong drug effects. Nanoemulsions transform drugs into nanoprecipitated forms, increasing their surface area and boosting their solubility and bioavailability. The use of nanoemulsion-based delivery methods can improve the water dispersibility, stability, and bioavailability of hydrophobic drugs. However, they must be carefully constructed to achieve the desired functional characteristics.
In current ocular drug delivery trends, punctal plugs, implants, contact lenses, and fornix rings loaded with nanoemulsions offer convenience and versatility. Research predominantly favors the use of Tween 80 as the surfactant for nanoemulsion development, with Transcutol-P and Pluronic also gaining popularity. The removal of preservatives from nanoemulsion formulations is a growing trend to minimize ocular toxicity during prolonged use. Researchers have focused on improving adherence to the ocular surface to achieve longer-lasting effects and increased bioavailability. In situ gelling systems employing nanoemulsions as primary formulations are also gaining prominence. Stabilizing emulsions is essential, especially concerning surfactant choice, to counteract destabilization mechanisms. For this purpose, the use of the hydrophobic, viscous polymer "poly(δ-decalactone)" (PDL)103,104 is recommended. A combination of ionic and nonionic surfactants shows promise in facilitating the delivery of hydrophobic drugs. In addition, the selection of cationic agents is crucial for enhancing the adherence of nanoemulsions to the corneal epithelium.
Nanoemulsions have shown potential in various fields, including drug delivery, biomedical imaging, vaccine delivery, nutraceutical, cosmetics, agriculture, food and beverage industry, environmental remediation, antimicrobial applications, and regulatory guidelines. Interdisciplinary collaboration and advancements in nanotechnology will shape the future of nanoemulsion-based technologies, which require long-term safety and biocompatibility studies.
Interdisciplinary collaboration is vital for advancing nanoemulsion-based technologies, that span fields such as chemistry, biology, medicine, materials science, and engineering. These collaborations can lead to innovative solutions, breakthrough discoveries, and commercially viable products. Effective communication between experts from different disciplines can significantly enhance the progress of nanoemulsion-based technologies.
Concluding remarks
According to recent data from the World Health Organization (August 10, 2023), glaucoma affects approximately 7.7 million individuals out of 1 billion worldwide and ranks as the second leading cause of irreversible blindness.105 Glaucoma primarily stems from optic nerve damage due to elevated intraocular pressure and is often caused by hindrances in aqueous fluid outflow. Some patients with glaucoma exhibit normal or low blood pressure, possibly due to reduced ocular perfusion pressure from physiological changes or antihypertensive medication, contributing to optic nerve damage. Research continues to uncover unidentified factors related to glaucoma.
Artificial intelligence has played a crucial role in the diagnosis and management of glaucoma. For treatment, traditional and innovative drug delivery systems are available. Novel systems improve drug bioavailability by regulating intraocular pressure (10-21 mm Hg) and enhancing drug adherence to the ocular surface.
Many marketed nanoemulsion formulations are available for ophthalmic diseases such as dry eye disease and inflammation. Many research articles are available on nanoemulsion-based glaucoma treatment, however, very few nanoemulsions are available in the market for glaucoma. A notable gap in this field is the limited commercialization of patented research. The market availability of nanoemulsions designed for glaucoma treatment, particularly those employing nanoscale drug delivery systems, remains limited.
Review Highlights
√ The existing literature on ophthalmic nanoemulsions has predominantly addressed barriers, challenges, preparation methods, and diverse applications of novel drug delivery systems for glaucoma.
√ This research investigates the effectiveness of oil-in-water nanoemulsions in enhancing the bioavailability of hydrophobic drugs compared with conventional eye drops.
√ This study compiles information on conducted research, granted patents, and the impact of nanoemulsions and nanoemulsion-based in-situ gels on glaucoma, encompassing safety concerns and toxicity evaluations. This article aims to consolidate this wealth of information into one comprehensive resource, with a specific emphasis on targeting nanoemulsions to the ocular surface for glaucoma treatment.
Acknowledgements
The authors from the Institute of Pharmacy, Bundelkhand University, Jhansi, India, express their gratitude to the university officials for providing an outstanding working environment and support.
Competing Interests
All authors declare that there are no conflicts of interest.
Data Availability Statement
No data were used to support the findings of this study.
Ethical Statement
Not applicable.
Supplementary files
Supplementary file 1 contains Table S1 and S3.
(doc)
References
- Ranadive F, Surti AZ, Patel H. Predicting Glaucoma Diagnosis Using AI. Intelligent Systems Reference Library 2022; 206:51-76. doi: 10.1007/978-3-030-76732-7_3 [Crossref] [ Google Scholar]
- Siggers JH, Ethier CR. Fluid Mechanics of the Eye. Annu Rev Fluid Mech 2011; 44:347-72. doi: 10.1146/ANNUREV-FLUID-120710-101058 [Crossref] [ Google Scholar]
- Braunger BM, Fuchshofer R, Tamm ER. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur J Pharm Biopharm 2015; 95:173-81. doi: 10.1016/J.EJPB.2015.04.029 [Crossref] [ Google Scholar]
- Mayo Clinic Guide to Better V. Glaucoma - Symptoms and causes - Mayo Clinic. 2020; Available from: https://www.mayoclinic.org/diseases-conditions/glaucoma/symptoms-causes/syc-20372839.
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014; 121:2081-90. doi: 10.1016/J.OPHTHA.2014.05.013 [Crossref] [ Google Scholar]
- Lin Y, Jiang B, Cai Y, Luo W, Zhu X, Lin Q. The Global Burden of Glaucoma: Findings from the Global Burden of Disease 2019 Study and Predictions by Bayesian Age–Period–Cohort Analysis. J Clin Med 2023; 12:1828. doi: 10.3390/JCM12051828 [Crossref] [ Google Scholar]
- Qing G, Zhang S, Wang B, Wang N. Functional MRI Signal Changes in Primary Visual Cortex Corresponding to the Central Normal Visual Field of Patients with Primary Open-Angle Glaucoma. Invest Ophthalmol Vis Sci 2010; 51:4627-34. doi: 10.1167/IOVS.09-4834 [Crossref] [ Google Scholar]
- Dillinger AE, Guter M, Froemel F, Weber GR, Perkumas K, Stamer WD. Intracameral Delivery of Layer-by-Layer Coated siRNA Nanoparticles for Glaucoma Therapy. Small 2018; 14:1803239. doi: 10.1002/SMLL.201803239 [Crossref] [ Google Scholar]
- Calkins DJ. Adaptive responses to neurodegenerative stress in glaucoma. Prog Retin Eye Res 2021; 84:100953. doi: 10.1016/J.PRETEYERES.2021.100953 [Crossref] [ Google Scholar]
- Tezel G. Molecular regulation of neuroinflammation in glaucoma: Current knowledge and the ongoing search for new treatment targets. Prog Retin Eye Res 2021; 100998. 10.1016/J.PRETEYERES.2021.100998.
- Khazaeni B, Khazaeni L. Acute Closed Angle Glaucoma. StatPearls [serial on the Internet]. 2017: Available from: http://europepmc.org/books/NBK430857.
- Prum Jr BE, Herndon Jr LW, Moroi SE, Mansberger Mph SL, Stein Ms JD, Lim MC. Primary Angle Closure Preferred Practice Pattern® Guidelines. Ophthalmology 2016; 123:P1-P40. doi: 10.1016/j.ophtha.2015.10.049 [Crossref] [ Google Scholar]
- Mandal A, Chakrabarti D. Update on congenital glaucoma. Indian J Ophthalmol 2011; 59:S148-S. doi: 10.4103/0301-4738.73683 [Crossref] [ Google Scholar]
- Badawi AH, Al-Muhaylib AA, Al Owaifeer AM, Al-Essa RS, Al-Shahwan SA. Primary congenital glaucoma: An updated review. Saudi Journal of Ophthalmology 2019; 33:382-8. doi: 10.1016/J.SJOPT.2019.10.002 [Crossref] [ Google Scholar]
- Dastjerdi S, Akgöz B, Civalek Ö. On the shell model for human eye in Glaucoma disease. Int J Eng Sci 2021; 158:103414. doi: 10.1016/J.IJENGSCI.2020.103414 [Crossref] [ Google Scholar]
- Iyer J, Vianna JR, Chauhan BC, Quigley HA. Toward a new definition of glaucomatous optic neuropathy for clinical research. CurrOpinOphthalmol 2020; 31:85-90. doi: 10.1097/ICU.0000000000000644 [Crossref] [ Google Scholar]
- Wareham LK, Risner ML, Calkins DJ. Protect, Repair, and Regenerate: Towards Restoring Vision in Glaucoma. Current Ophthalmology Reports 2020; 8:301-10. doi: 10.1007/S40135-020-00259-5/FIGURES/1 [Crossref] [ Google Scholar]
- Almeida H, Amaral MH, Lobão P, Lobo JMS. In situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discovery Today 2014; 19:400-12. doi: 10.1016/J.DRUDIS.2013.10.001 [Crossref] [ Google Scholar]
- Rahić O, Tucak A, Omerović N, Sirbubalo M, Hindija L, Hadžiabdić J. Novel Drug Delivery Systems Fighting Glaucoma: Formulation Obstacles and Solutions. Pharmaceutics 2020; 13:1-58. doi: 10.3390/pharmaceutics13010028 [Crossref] [ Google Scholar]
- Schwartz GF, Quigley HA. Adherence and Persistence with Glaucoma Therapy. SurvOphthalmol 2008; 53:19038625. doi: 10.1016/j.survophthal.2008.08.002 [Crossref] [ Google Scholar]
- Robin AL, Muir KW. Medication adherence in patients with ocular hypertension or glaucoma. Expert Rev Ophthalmol 2019; 14:199-210. doi: 10.1080/17469899.2019.1635456 [Crossref] [ Google Scholar]
- Newman-Casey PA, Robin AL, Blachley T, Farris K, Heisler M, Resnicow K. The Most Common Barriers to Glaucoma Medication Adherence: A Cross-Sectional Survey. Ophthalmology 2015; 122:1308-16. doi: 10.1016/j.ophtha.2015.03.026 [Crossref] [ Google Scholar]
- Sharma Y, Chahar K, Mishra L, Kumari L, Singla A, Patel P. Recent overviews on the drug delivery aspects and applications of brinzolamide for the management of glaucoma. Health Sciences Review 2023; 6:100083. doi: 10.1016/J.HSR.2023.100083 [Crossref] [ Google Scholar]
- Ismail A, Nasr M, Sammour O. Nanoemulsion as a feasible and biocompatible carrier for ocular delivery of travoprost: Improved pharmacokinetic/pharmacodynamic properties. Int J Pharm 2020; 583:119402. doi: 10.1016/J.IJPHARM.2020.119402 [Crossref] [ Google Scholar]
- Mahboobian MM, Mohammadi G, Mohammadi M. Thermosensitive brinzolamide in situ gel nanoemulsions, in vitro and ex vivo evaluation. Biointerface Res Appl Chem 2021; 11:7754-64. doi: 10.33263/BRIAC111.77547764 [Crossref] [ Google Scholar]
- Dhahir RK, Al-Nima AM, Al-Bazzaz FY. Nanoemulsions as Ophthalmic Drug Delivery Systems. Turk J Pharm Sci 2021; 18:652-64. doi: 10.4274/tjps.galenos.2020.59319 [Crossref] [ Google Scholar]
- Tau J, Passerini MS, del Papa M, Aguilar A, Berra A. A novel ophthalmic latanoprost 0005% nanoemulsion: a cytotoxicity study. Graefe's Archive for Clinical and Experimental Ophthalmology 2022; 260:1941-6. doi: 10.1007/s00417-021-05536-y [Crossref] [ Google Scholar]
- Mroczkowska S, Benavente-Perez A, Negi A, Sung V, Patel SR, Gherghel D. Primary Open-Angle Glaucoma vs Normal-Tension Glaucoma: The Vascular Perspective. JAMA Ophthalmol 2013; 131:36-43. doi: 10.1001/2013.JAMAOPHTHALMOL.1 [Crossref] [ Google Scholar]
- Riegelman S, Vaughan DG. ophthalmic solutions By. J Am Pharm Assoc 1955; 19:474-7. doi: 10.1016/S0095-9561(15)30205-X [Crossref] [ Google Scholar]
- Garcia-Valldecabres M, López-Alemany A, Refojo MF. pH Stability of ophthalmic solutions. Optometry - Journal of the American Optometric Association 2004; 75:161-8. doi: 10.1016/S1529-1839(04)70035-4 [Crossref] [ Google Scholar]
- Patel A. Ocular drug delivery systems: An overview. World J Pharmacol 2013; 2:47. doi: 10.5497/wjp.v2.i2.47 [Crossref] [ Google Scholar]
- Saettone MF, Giannaccini B, Monti D. Ophthalmic emulsions and suspensions. J Toxicol Cutaneous OculToxicol 2001; 20:183-201. doi: 10.1081/CUS-120001857 [Crossref] [ Google Scholar]
- Gore A, Pujara C, Attar M, Neervannan S. Ocular emulsions and dry eye:a case study of a non-biological complex drug product delivered to a complex organ to treat a complex disease. GaBI Journal 2017; 6:13-23. doi: 10.5639/gabij.2017.0601.004 [Crossref] [ Google Scholar]
- Toropainen E, Fraser-Miller SJ, Novakovic D, Del Amo EM, Vellonen KS, Ruponen M. Biopharmaceutics of Topical Ophthalmic Suspensions: Importance of Viscosity and Particle Size in Ocular Absorption of Indomethacin. Pharmaceutics 2021; 13:1-13. doi: 10.3390/pharmaceutics13040452 [Crossref] [ Google Scholar]
- Xu X, Al-Ghabeish M, Rahman Z, Krishnaiah YSR, Yerlikaya F, Yang Y. Formulation and process factors influencing product quality and in vitro performance of ophthalmic ointments. Int J Pharm 2015; 493:412-25. doi: 10.1016/J.IJPHARM.2015.07.066 [Crossref] [ Google Scholar]
- Rahić O, Tucak A, Sirbubalo M, Hindija L, Hadžiabdić J, Vranić E. Opportunities to Topically Reduce Intraocular Pressure in Glaucoma. An International Journal 2021; 14:An International Journal 2021; 14. doi: 10.9734/or/2021/v14i230189 [Crossref] [ Google Scholar]
- Al-Kinani AA, Zidan G, Elsaid N, Seyfoddin A, Alani AWG, Alany RG. Ophthalmic gels: Past, present and future. Adv Drug Deliv Rev 2018; 126:113-26. doi: 10.1016/J.ADDR.2017.12.017 [Crossref] [ Google Scholar]
- Yadav KS, Rajpurohit R, Sharma S. Glaucoma: Current treatment and impact of advanced drug delivery systems. Life Sci 2019; 221:362-76. doi: 10.1016/J.LFS.2019.02.029 [Crossref] [ Google Scholar]
- Jothi M, Harikumar S, Aggarwal G. In-situ ophthalmic gels for the treatment of eye diseases. Int J Pharm Sci Res 2012; 3:1891. [ Google Scholar]
- Nettey H, Darko Y, Bamiro OA, Addo RT. Ocular Barriers. In: Addo RT, editor. Ocular Drug Delivery: Advances, Challenges and Applications. Cham: Springer International Publishing; 2016. p. 27-36.
- Baranowski P, Karolewicz B, Gajda M, Pluta J. Ophthalmic drug dosage forms: characterisation and research methods. ScientificWorldJournal 2014; 2014:861904. doi: 10.1155/2014/861904 [Crossref] [ Google Scholar]
- Aslam S, Gupta V. Carbonic Anhydrase Inhibitors. Second Edition 2022; 1:Second Edition 2022; 1. doi: 10.1016/B978-0-7020-5193-7.00054-6 [Crossref] [ Google Scholar]
- Anonymous. Glaucoma - Diagnosis and treatment - Mayo Clinic. [cited 2023 15 april]; Available from: https://www.mayoclinic.org/diseases-conditions/glaucoma/diagnosis-treatment/drc-20372846.
- Sampath Kumar KP, Bhowmik D, Paswan S, Srivastava S. Recent Challenges and Advances in Ophthalmic Drug Delivery System. Pharma Innovation 2012; 1:1-15. [ Google Scholar]
- Jumelle C, Gholizadeh S, Annabi N, Dana R. Advances and limitations of drug delivery systems formulated as eye drops. Journal of Controlled Release 2020; 321:1-22. doi: 10.1016/J.JCONREL.2020.01.057 [Crossref] [ Google Scholar]
- Peng R, Gang Q, Xiabin Xiabin, Mm L, Lv H, Qian Z. The PEG-PCL-PEG Hydrogel as an Implanted Ophthalmic Delivery System after Glaucoma Filtration Surgery; a Pilot Study. Medical Hypothesis, Discovery and Innovation in Ophthalmology 2014; 3:3. [ Google Scholar]
- Chen Y, Jiang K, Wei G, Dai Y. Medical Treatment Strategy for Glaucoma. In: Sun X, Y Dai, editors. Medical Treatment of Glaucoma. Singapore: Springer Singapore; 2019. p. 87-113.
- Yavuz B, Kompella UB. Ocular drug delivery. Handbook of Experimental Pharmacology. New York LLC: Springer; 2017. p. 57-93.
- Belamkar A, Harris A, Zukerman R, Siesky B, Oddone F, Verticchio Vercellin A. Sustained release glaucoma therapies: Novel modalities for overcoming key treatment barriers associated with topical medications. Ann Med 2022; 54:343-358. doi: 10.1080/07853890.2021.1955146 [Crossref] [ Google Scholar]
- Pandey P, Gulati N, Makhija M, Purohit D, Dureja H. Nanoemulsion: A Novel Drug Delivery Approach for Enhancement of Bioavailability. Recent Pat Nanotechnol 2020; 14:276-93. doi: 10.2174/1872210514666200604145755 [Crossref] [ Google Scholar]
- Dukovski BJ, Bračko A, Šare M, Pepić I, Lovrić J. In vitro evaluation of stearylamine cationic nanoemulsions for improved ocular drug delivery. Acta Pharm 2019; 69:621-34. doi: 10.2478/acph-2019-0054 [Crossref] [ Google Scholar]
- Singh M, Bharadwaj S, Lee KE, Kang SG. Therapeutic nanoemulsions in ophthalmic drug administration: Concept in formulations and characterization techniques for ocular drug delivery. J Controlled Release 2020; 328:895-916. doi: 10.1016/J.JCONREL.2020.10.025 [Crossref] [ Google Scholar]
- Bhalerao H, Koteshwara K, Chandran S. Design, optimisation and evaluation of in situ gelling nanoemulsion formulations of brinzolamide. Drug DelivTransl Res 2020; 10:529-47. doi: 10.1007/s13346-019-00697-0 [Crossref] [ Google Scholar]
- Changediya VV, Jani R, Kakde P. A Review on Nanoemulsions: A Recent Drug Delivery Tool. Journal of Drug Delivery and Therapeutics 2019; 9:185-91. doi: 10.22270/jddt.v9i5.3577 [Crossref] [ Google Scholar]
- Gurpreet K, Singh SK. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J Pharm Sci 2018; 80:781-9. doi: 10.4172/PHARMACEUTICAL-SCIENCES.1000422 [Crossref] [ Google Scholar]
- Qadir A, Faiyazuddin MD, Talib Hussain MD, Alshammari TM, Shakeel F. Critical steps and energetics involved in a successful development of a stable nanoemulsion. J Mol Liq 2016; 214:7-18. doi: 10.1016/j.molliq.2015.11.050 [Crossref] [ Google Scholar]
- Ibrahim SS. The Role of Surface Active Agents in Ophthalmic Drug Delivery: A Comprehensive Review. J Pharm Sci 2019; 108:1923-33. doi: 10.1016/J.XPHS.2019.01.016 [Crossref] [ Google Scholar]
- Montes de Oca-Ávalos JM, Candal RJ, Herrera ML. Nanoemulsions: stability and physical properties. CurrOpin Food Sci 2017; 16:1-6. doi: 10.1016/j.cofs.2017.06.003 [Crossref] [ Google Scholar]
- Kumar M, Bishnoi RS, Shukla AK, Jain CP. Techniques for formulation of nanoemulsion drug delivery system: A review. PrevNutr Food Sci 2019; 24:225-34. doi: 10.3746/pnf.2019.24.3.225 [Crossref] [ Google Scholar]
- Rai VK, Mishra N, Yadav KS, Yadav NP. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. Elsevier B.V; 2018. 10.1016/j.jconrel.2017.11.049.
- Bachu RD, Chowdhury P, Al-Saedi ZHF, Karla PK, Boddu SHS. Ocular drug delivery barriers—role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018; 10:1-31. doi: 10.3390/pharmaceutics10010028 [Crossref] [ Google Scholar]
- Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. American Association of Pharmaceutical Scientists 2010; 12:348-60. doi: 10.1208/s12248-010-9183-3 [Crossref] [ Google Scholar]
- Gorantla S, Rapalli VK, Waghule T, Singh PP, Dubey SK, Saha RN. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv 2020; 10:27835-55. doi: 10.1039/D0RA04971A [Crossref] [ Google Scholar]
- Lallemand F, Daull P, Benita S, Buggage R, Garrigue J-S. Successfully Improving Ocular Drug Delivery Using the Cationic Nanoemulsion, Novasorb. J Drug Deliv 2012; 2012:1-16. doi: 10.1155/2012/604204 [Crossref] [ Google Scholar]
- Gawin-Mikołajewicz A, Nartowski KP, Dyba AJ, Gołkowska AM, Malec K, Karolewicz B. Ophthalmic Nanoemulsions: From Composition to Technological Processes and Quality Control. Mol Pharm 2021; 18:3719-40. doi: 10.1021/acs.molpharmaceut.1c00650 [Crossref] [ Google Scholar]
- Raj VK, Mazumder R, Madhra M. Ocular drug delivery system: Challenges and approaches. International Journal of Applied Pharmaceutics 2020; 12:49-57. doi: 10.22159/ijap.2020v12i5.38762 [Crossref] [ Google Scholar]
- Bachu RD, Chowdhury P, Al-Saedi ZHF, Karla PK, Boddu SHS. Ocular Drug Delivery Barriers-Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018; 10. 10.3390/pharmaceutics10010028.
- Jansook P, Hnin HM, Loftsson T, Stefánsson E. Cyclodextrin-based formulation of carbonic anhydrase inhibitors for ocular delivery – A review. Int J Pharm 2021; 606. 10.1016/j.ijpharm.2021.120955.
- Gupta A. Chapter 21 - Nanoemulsions. In: Chung EJ, L Leon, C Rinaldi, editors. Nanoparticles for Biomedical Applications. Elsevier; 2020. p. 371-84.
- Naseema A, Kovooru L, Behera AK, Kumar KPP, Srivastava P. A critical review of synthesis procedures, applications and future potential of nanoemulsions. Adv Colloid Interface Sci 2021; 287:102318. doi: 10.1016/j.cis.2020.102318 [Crossref] [ Google Scholar]
- Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK. Nanoemulsion: Concepts, development and applications in drug delivery. Journal of Controlled Release 2017; 252:28-49. doi: 10.1016/j.jconrel.2017.03.008 [Crossref] [ Google Scholar]
- News F. Novagali Pharma Presents Results of its Innovative Latanoprost Formulation for Glaucoma. 2009. Available from: https://www.biospace.com/article/releases/novagali-pharma-presents-results-of-its-innovative-latanoprost-formulation-for-glaucoma-/.
- Daull P, Amrane M, Garrigue J-S. Novasorb® Cationic Nanoemulsion and Latanoprost: the Ideal Combination for Glaucoma Management?. Glaucoma: Open Access 2017; 2:1-4. doi: 10.35248/2684-1622.17.2.107 [Crossref] [ Google Scholar]
- Jurišić Dukovski B, Ljubica J, Kocbek P, Safundžić Kučuk M, Krtalić I, Hafner A, et al. Towards the development of a biorelevant in vitro method for the prediction of nanoemulsion stability on the ocular surface. Int J Pharm 2023; 633. 10.1016/j.ijpharm.2023.122622.
- Kinnunen K, Kauppinen A, Piippo N, Koistinen A, Toropainen E, Kaarniranta K. Cationorm shows good tolerability on human HCE-2 corneal epithelial cell cultures. Exp Eye Res 2014; 120:82-89. doi: 10.1016/j.exer.2014.01.006 [Crossref] [ Google Scholar]
- Garrigue Js, Lambert G, Rabinovich L, Daull P, Serle JB. A Comparative Study of Latanoprost Cationic Emulsion and Latanoprost Aqueous Solution in Preclinical Efficacy and Safety Models. Invest Ophthalmol Vis Sci 2011; 52:238. [ Google Scholar]
- López-Cano JJ, González-Cela-Casamayor MA, Andrés-Guerrero V, Vicario -de-la-Torre M, Benítez-del-Castillo JM, Herrero-Vanrell R. New trends towards glaucoma treatment: Topical osmoprotective microemulsions loaded with latanoprost. Ocular Surface 2023; 29:314-330. doi: 10.1016/j.jtos.2023.05.013 [Crossref] [ Google Scholar]
- Shelley H, Annaji M, Smith FT, Babu RJ. Difluprednate-Hydroxypropyl-β-Cyclodextrin-Based Ophthalmic Solution for Improved Delivery in a Porcine Eye Model. Journal of Ocular Pharmacology and Therapeutics 2022; 38:92-101. doi: 10.1089/jop.2021.0073 [Crossref] [ Google Scholar]
- Yeu E, Silverstein S, Guillon M, Schulze M-M, Galarreta D, Srinivasan S. Efficacy and Safety of Phospholipid Nanoemulsion-Based Ocular Lubricant for the Management of Various Subtypes of Dry Eye Disease: A Phase IV, Multicenter Trial. Clin Ophthalmol 2020; 14:2561-2570. doi: 10.2147/OPTH.S261318 [Crossref] [ Google Scholar]
- Rawat G, Kolhe S, Rana D, Salave S, Benival D. Exploring the Therapeutic Potential of Cyclosporine for Ophthalmic Indications by Novel Carrier Systems. Crit Rev Ther Drug Carrier Syst 2023; 40:1-45. doi: 10.1615/CritRevTherDrugCarrierSyst.2022043085 [Crossref] [ Google Scholar]
- Bioscience S. FDA approves Skye Bioscience’s IND for glaucoma therapy trial. 2022; 11-3.
- Nct. Study of Brimonidine Tartrate Nanoemulsion Eye Drop Solution in the Treatment of Dry Eye Disease (DED). 2022 [cited 2023]; Available from: https://classic.clinicaltrials.gov/ct2/show/NCT03785340.
- Anonymous, inventor CN116725954A- Nanometer medicinal preparation with antioxidant function and its use for treating glaucoma. Google Patents; 2023.
- Anonymous, inventor TW202247845A- Eye nanoemulsion composition containing prostaglandin derivative has better stability to provide convenience in preservation. 2022.
- Anonymous, inventor CN115463088A- Ophthalmic nanoemulsion composition containing prostaglandin derivative. 2022.
- Voce EACC, inventor US20220125634A1- Implantable ocular drug delivery devices and methods. 2022.
- Carvalho Odhdshbdsaldmavv, Inventor Br102014024497b1 - Nanoemulsioned Compositions Containing Melaleuca Leucadendron Extract And/Or Fractions and Use. Google Patents. 2021.
- Chandran HHB, inventor WO2021240376A2 - Ophthalmic nanoemulsion compositions. Google Patents; 2021.
- Chandran HHB, inventor WO2020240451A1 - In-situ gelling nanoemulsion of brinzolamide. Google Patents; 2020.
- Bahri MVS, inventor US20200197522A1 - Pharmaceutical composition comprising brinzolamide. Google Patents; 2023.
- Detzel JM, inventor US20200315965A1 - Nanoemulsion concentrate formulations and methods. Google Patents; 2020.
- Chandran HHB, inventor KR20200053205A - A surfactant-free type ophthalmic nano-emulsion composition, and the manufacturing method thereof. Google Patents; 2020.
- Carvalho Odhdshbdsaldmavv, inventor BR102014024497A2 - nanoemulsified compositions of fructose leucadendron and / or pilocarpine extract and / or fractions and uses. Google Patents; 2018.
- LAM GWTKLZLY-NLCD, inventor EP2978409B1 - Ophthalmic composition, method for preparing the same, and use of the same. Google Patents; 2018.
- Pujara CP, inventor US9801891B2 - Compositions and methods for lowering intraocular pressure. Google Patents; 2017.
- Phillips J-SGL, inventor AU2010220321B2- Anionic oil-in-water emulsions containing prostaglandins and uses thereof. Google Patents; 2015.
- Anonymous, inventor JP5722803B2- Cationic oil-in-water emulsions containing prostaglandins and their use. Google Patents; 2015.
- Chiellini FCBS, inventor US8414904B2- Ophthalmic oil-in-water emulsions containing prostaglandins. Google Patents; 2013.
- Bacharach J, Tatham A, Ferguson G, Belalcázar S, Thieme H, Goodkin ML. Phase 3, Randomized, 20-Month Study of the Efficacy and Safety of Bimatoprost Implant in Patients with Open-Angle Glaucoma and Ocular Hypertension (ARTEMIS 2) on behalf of the ARTEMIS 2 Study Group. Drugs 2021; 81:2017-33. doi: 10.1007/s40265-021-01624-9 [Crossref] [ Google Scholar]
- Choradiya BR, Patil SB. A comprehensive review on nanoemulsion as an ophthalmic drug delivery system. J Mol Liq 2021; 339:116751. doi: 10.1016/J.MOLLIQ.2021.116751 [Crossref] [ Google Scholar]
- Chockalingam A, Xu L, Stewart S, LeMerdy M, Tsakalozou E, Fan J, et al. Protocol for evaluation of topical ophthalmic drug products in different compartments of fresh eye tissues in a rabbit model. J PharmacolToxicol Methods 2019; 96. 10.1016/j.vascn.2018.12.002.
- Ng JSC, Tan YX, Alwi NAA, Yee KM, Rashid AHA, Tan KL. In Vitro Toxicity Evaluation of New Generic Latanost® and Latacom® as an Ophthalmic Formulation. J Curr Glaucoma Pract 2021; 15:139-143. doi: 10.5005/jp-journals-10078-1319 [Crossref] [ Google Scholar]
- Pyrhönen J, Bansal KK, Bhadane R, Wilén C-E, Salo-Ahen OM, Rosenholm JM. Molecular dynamics prediction verified by experimental evaluation of the solubility of different drugs in poly (decalactone) for the fabrication of polymeric nanoemulsions. Adv Nanobiomed Res 2022; 2:1-11. doi: 10.1002/anbr.202100072 [Crossref] [ Google Scholar]
- Maru S, Verma J, Wilen C-E, Rosenholm JM, Bansal KK. Attenuation of celecoxib cardiac toxicity using Poly(δ-decalactone) based nanoemulsion via oral route. Eur J Pharm Sci 2023; 190:1-9. doi: 10.1016/j.ejps.2023.106585 [Crossref] [ Google Scholar]
- Taskar PS, Patil A, Lakhani P, Ashour E, Gul W, Elsohly MA. Δ9-Tetrahydrocannabinol Derivative-Loaded Nanoformulation Lowers Intraocular Pressure in Normotensive Rabbits. Translational Vision Science and Technology 2019; 8:1-19. doi: 10.1167/tvst.8.5.15 [Crossref] [ Google Scholar]