Bioimpacts. 2025;15:30241.
doi: 10.34172/bi.30241
Review
A promising breakthrough in pancreatic cancer research: The potential of spheroids as 3D models
Nazanin Jamshidi Conceptualization, Data curation, Project administration, Validation, Visualization, Writing – original draft, 1 
Negar Jamshidi Validation, Writing – original draft, 1 
Amir Modarresi Chahardehi Data curation, Validation, Writing – review & editing, 1 
Elahe Shams Resources, 2 
Vahid Chaleshi Conceptualization, Investigation, Supervision, Writing – review & editing, 2, * 
Author information:
1Kimia Andisheh Teb Medical and Molecular Laboratory Research Co, Tehran, Iran
2Basic and Molecular Epidemiology of Gastrointestinal Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract
Pancreatic ductal adenocarcinoma (PDAC) stands as the fourth leading cause of cancer-related deaths, primarily attributable to its resistance to chemotherapy, resulting in a nearly universal fatality rate. Despite the promise exhibited by numerous drugs in preclinical studies, their subsequent failure in clinical trials underscores the inherent limitations of conventional two-dimensional cell culture models commonly employed in early drug screening endeavors. The inadequacies of two-dimensional (2D) models prompted the exploration of three-dimensional (3D) culture systems, which more faithfully recapitulate the native tumor microenvironment. These 3D systems have distinct advantages over 2D models in morphology, proliferation, drug response, and protein expression. Among these 3D platforms, tumor organoids and spheroids, generated through different methodologies, have emerged as next-generation models that closely mirror aspects of pancreatic tumor biology. This comprehensive review scrutinizes pancreatic cancer spheroids' techniques, tissue sources, and applications, offering a nuanced analysis of their advantages and limitations. By comparing these distinct 3D culture systems, researchers gain valuable insights to inform the selection of optimal model designs aligned with their specific experimental objectives. The utilization of these advanced models holds significant promise for enhancing the clinical relevance of both in vitro and in vivo cancer research, thereby contributing to the development of improved therapeutics against pancreatic cancer.
Keywords: 3D cell culture, Spheroid, Tumor microenvironment, Pancreatic cancer, In vitro cancer model
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
None.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) stands out as a highly malignant form of human cancer, exhibiting an escalating incidence. The current therapeutic approach for PDAC involves a combination of surgery, chemotherapy, and radiation therapy in select cases, achieving long-term survival only for a minority of patients.1 The dense desmoplastic stroma, distinguished by an overabundance of fibroblasts, extracellular matrix, and immune cells, is a defining feature of PDAC. This stromal milieu plays a pivotal role in disease progression and therapy response and is a distinctive PDAC feature. The complex interaction among tumor cells and diverse stromal components along multiple signaling pathways governs the tumor microenvironment's (TME) development.2 The significance of this interaction underscores the necessity for a suitable in vitro technique that accurately recapitulates the complexity of pancreatic TME cells, thereby advancing our understanding of pancreatic cancer development and facilitating the discovery of effective treatments. Conventional two-dimensional cell cultures, while valuable, need to catch up in capturing the intricate multicellular interactions within the TME that drive PDAC progression in vivo. Challenges persist despite the deployment of animal models in preclinical trials to address these limitations. For instance, the extended duration and engraftment issues associated with drug examination on animal models can impede timely research outcomes.3
Recently, there has been much interest in three-dimensional (3D) cell models since they can accurately mimic the characteristics of tumors in living organisms. This makes them a valuable tool that fills the gap between standard two-dimensional (2D) cell culture methods, and in vivo models.4 3D cell cultures facilitate cell-to-cell and cell-to-matrix interactions, mirroring the physiological conditions experienced by cells in vivo. This approach has gained prominence as one of the most favored methods in drug development.
Moreover, employing human cells in 3D culture models can reduce reliance on alternative models, such as mouse models, which are often associated with high costs and may not precisely depict therapeutic efficacy and drug adverse effects.5,6 Various 3D models for cell culture have emerged, including organoids, spheroids, organ-on-a-chip, 3D scaffolds, hydrogels, 3D bioprinting, tumor microenvironment models, tumor organoids, and tumor spheres (Table 1).
Table 1.
Various 3D cell culturing approaches and applications
|
Model
|
Key Features Modeled
|
Preparation method
|
References
|
| Spheroid |
simple
-
-
- Nutrient/oxygen gradient
-
|
|
7
|
| Organoids |
|
|
8
|
| Organ-on-chip |
|
|
9
|
| 3D scaffolds |
|
|
10,11
|
| Hydrogels |
|
simple
-
- Crosslinked hydrophilic polymers
|
12,13
|
| 3D Bioprinting |
|
simple
-
- Computer-controlled deposition
|
14
|
| Tumor Microenvironment Models |
simple
-
-
- Stromal cell interactions
|
simple
-
- Co-cultures with stroma, immune cells, vasculature
|
15
|
| Tumor Organoids |
simple
-
- Intra-tumor heterogeneity
-
- Patient-specific profiles
|
simple
-
- Patient-derived 3D models
|
16
|
| Tumor Spheres |
simple
-
-
- Cancer stem cell enrichment
|
simple
-
- Anchorage-independent spheroids
|
17
|
ECM: extra cellular matrix.
To develop more effective treatments for PDAC, it is essential to use research models that accurately recreate the native tumor microenvironment and heterogeneity. This review summarizes the latest strides in utilizing 3D spheroid models derived from PDAC tissues for disease modeling, drug development, and personalized medicine applications. Our synthesis encompasses critical studies showcasing the capabilities of these models in faithfully reproducing tumor heterogeneity, microenvironment interactions, and therapeutic responses. Furthermore, we address the challenges inherent in these next-generation culture systems and outline future directions to enhance their clinical translatability.
Spheroid
Sutherland and colleagues pioneered the concept of spheroids in the early 1970s. Since then, various models and creation methods have been developed.18 The formation of spheroids involves spontaneous cell aggregation followed by cell surface integrin binding to the extracellular matrix (ECM). As cells upregulate E-cadherin, accumulating on the cell surface, intercellular E-cadherin interactions lead to the compact structure characteristic of spheroids. Growth factors, oxygen, and nourishment are a few variables that impact this process.19,20
Distinct cell lines contribute to the variability in the structure and morphology of spheroids based on their cellular sources.21 Additionally, the morphology of spheroids is impacted by the technique used and the primary cell origin.21,22 For instance, Luka et al. demonstrated that metastatic cell lines of colon cancer, when cultured in a laminin-rich extracellular matrix (IrECM), formed grape-like spheroids. In contrast, colon cancer cells derived from primary tumor tissue exhibited a round morphology.23 In addition, 25 breast cancer cell lines were further categorized by Kenni and colleagues into four types of 3D spheroids: circular, mass, stellate, and grape-like.24 Each spheroid type presents specific characteristics, such as weak cell-cell interactions and an aggressive phenotype for round, grape-like, and stellate spheroids.25-27
There are several known approaches to spheroid creation, with several of these approaches having been fine-tuned for controlled mass manufacturing.28-30 There are different sources for producing spheroids, with three common models:
-
Multicellular spheroids from tumor cell lines,
-
Oncospheres representing cancer stem cell (CSCs) growth,
-
Mechanical and enzymatic tumor tissue dissociation produces organotypic multicellular spheres.31,32
The biological and pathologic properties of the tumor cell line are significantly influenced by the types of cell lines used, originating from pancreatic cancer, donor patients, and the region of derivation. These factors should be carefully considered when designing in vitro investigations. Numerous studies have explored spheroid construction with varying degrees of success and inconclusive outcomes.
Challenges and limitations
Tumor spheroids, such as multicellular tumor spheroids (MCTSs), are becoming more commonly utilized as 3D models in vitro for pharmacological investigations, notably in the field of cancer research. 3D cell cultures provide a more accurate depiction of the tumor environment in living organisms, as opposed to conventional 2D cell cultures. This makes them highly useful for many applications like as drug screening, drug design, drug targeting, drug toxicity assessment, and validation of drug delivery techniques.33 Nevertheless, despite the benefits they offer, the use of tumor spheroids in pharmaceutical research is accompanied by many obstacles and restrictions.
Reproducibility and standardization
The lack of consistency in their manufacturing is one of the key obstacles to employing tumor spheroids. Both scaffold-based and scaffold-free techniques have been developed for the production of MCTSs. Cell requirements and the relevant biological inquiry often dictate the approach taken.34 This heterogeneity might cause changes in the characteristics of the spheroids formed, which affects the repeatability of results across multiple experiments.
Size and testing performance
The effectiveness of tumor spheroids in testing can also be influenced by their size. An inadequate supply of nutrients and oxygen, for instance, might cause bigger spheroids to develop a necrotic core, which in turn can impact the efficacy of anti-cancer medications. Furthermore, spheroids of different sizes might affect drug penetration and distribution, which can provide misleading findings.35
Complexity of TME
Tumor spheroids are more closely related to the in vivo tumor environment than 2D cultures, although they do not entirely recreate the complexity of the TME. For example, most spheroids are formed up of tumor cells alone and do not comprise other critical components of the tumor microenvironment, such as fibroblasts, adipocytes, and immune cells.33 Because interactions between tumor cells and various other cell types can greatly impact the effectiveness of medication delivery and the therapeutic value of therapy, this restriction can impact the accuracy of drug testing findings.33
Data analysis
Data generated by research examining cancer cell metabolism and cell cycle abnormalities using tumor spheroids can be enormous, necessitating sophisticated methods for comprehensive analysis.34
Spheroid of pancreatic cancer cell lines
This section discusses several studies on spheroid generation derived from pancreatic cancer cell lines. While most PDAC cell lines could form spheroids, Sipos et al noted that MiaPaCa-2 "totally failed to develop as spheroids" because it disaggregated after harvesting.36 In 2013, Yeon et al. documented the creation of tumor spheroids (TS) using human pancreatic cancer cells (Aspc-1, PANC-1, Capan-2) in concave polydimethylsiloxane (PDMS) microwell plates. They evaluated their appropriateness as a model for testing the effectiveness of anticancer treatments. TS formation was observed in the three mentioned cell lines, exhibiting varying necrosis within the spheroids. PANC-1 spheroids, with a spherical shape, rough surface, and distinctive adhesion structures, were effectively formed on concave microwell plates without noticeable necrosis. Drug resistance-associated compounds, including MT1-MMP, TGF-b1, CTGF, collagen type I, laminin, and fibronectin, were detected in PANC-1 spheroids grown in concave microwells. TGF-b1, CTGF, and MT1-MMP are crucial molecules in pancreatic cancer associated with poor prognosis and therapy resistance.37 The study also demonstrated the necessity of epidermal growth factor (EGF) for the 3D culture of Capan-2 cells. In contrast, the monolayer culture of these cells did not require EGF. This dependence may be attributed to EGF's significant role in pancreatic cancer development and its overexpression in pancreatic cancer.38
In contrast to earlier findings by Sipos et al and Wen et al, another study established a 3D spheroid-based cultivation method for pancreatic cancer cell lines, specifically MiaPaCa-2 and PANC-1, to conduct pharmacological testing. Their models exhibited reproducibility and ease of manipulation, indicating that 3D cell culture has the potential to serve as an intermediary between 2D cell cultures and in vivo models in the medication research and evaluation method for pancreatic cancer.39 Ware et al successfully generated spheroids by combining two established techniques: the hanging drop method and using methyl cellulose (MC) in the media. This method enhanced the compactness of the spheroids while preventing their separation into smaller components. Five pancreatic cancer cell lines—PANC-1, MiaPaCa-2, Capan-1, BxPC-3, and Aspc1—could be effectively transformed into spheroids.40
Various cell lines exhibit distinct spheroidal features, likely attributed to differences in their inherent cellular characteristics. For instance, BxPC-3 and Capan-1 displayed similar shapes and developmental patterns, generating densely packed spheroids that were one-third to one-half the size of those formed by AsPc-1, PANC-1, and MIA-PaCa-2.40 Conversely, AsPc-1, PANC-1, MIA-PaCa-2 spheroids, and BxPc-3 and Capan-1 spheroids demonstrated a comparable phenotype.41 Despite the importance of the MIA-PaCa-2 cell line in pancreatic cancer studies, generating and maintaining homogenous and stable MIA-PaCa-2 spheroids has proven challenging. Researchers, including Cavo and colleagues, have explored various methods to overcome these challenges, such as round-bottom wells, hanging drop, and Matrigel embedding, in the presence and absence of methylcellulose in multiple mediums. Based on their results, a hydrophobic base with a methylcellulose-enriched medium may produce MIA-PaCa-2 spheroids.42 Genomic research also suggests that the shape and size of spheroids may reflect distinct genetic states in the cells that produce them.42 While studies demonstrate the feasibility of generating spheroids from different cell lines, there is no standardized procedure, and researchers are focused on refining existing methods. 3D spheroids have been the subject of much research due to their promise as a model for screening anticancer drugs. For example, Longati et al, pancreatic cancer spheroids such as AsPC-1, BxPC-3, Capan-1, and PANC-1 are superior to 2D-cultured versions of these cells when it comes to drug testing due to their chemo-resistant phenotype and matrix-rich composition.43
Additional studies have explored nanoparticle permeation in 3D multicellular spheroid models of pancreatic cancers, investigating factors such as size, surface charge, PEG decorating, and other physicochemical features.44 Researchers have also generated spheroids from pancreatic tumor stroma cells, particularly pancreatic stellate cells, to study drug efficacy, given their role in PDAC progression and drug resistance.45 Improved spheroid homogeneity and stability and more accurate assessment of drug responses are anticipated outcomes of future research that uses more suitable cell line models, such as primary cells with established mutation pathways.
Cultivating pancreatic cancer cell lines into spheroids, three-dimensional cell clusters suitable for diverse analyses like live cell staining and imaging, is a valuable approach. Nevertheless, creating and manipulating stable and robust spheroids from pancreatic cancer cell lines, including the challenging MIA-PaCa-2, poses a significant challenge.46 Several methods and models have been developed to address this issue (Fig. 1).
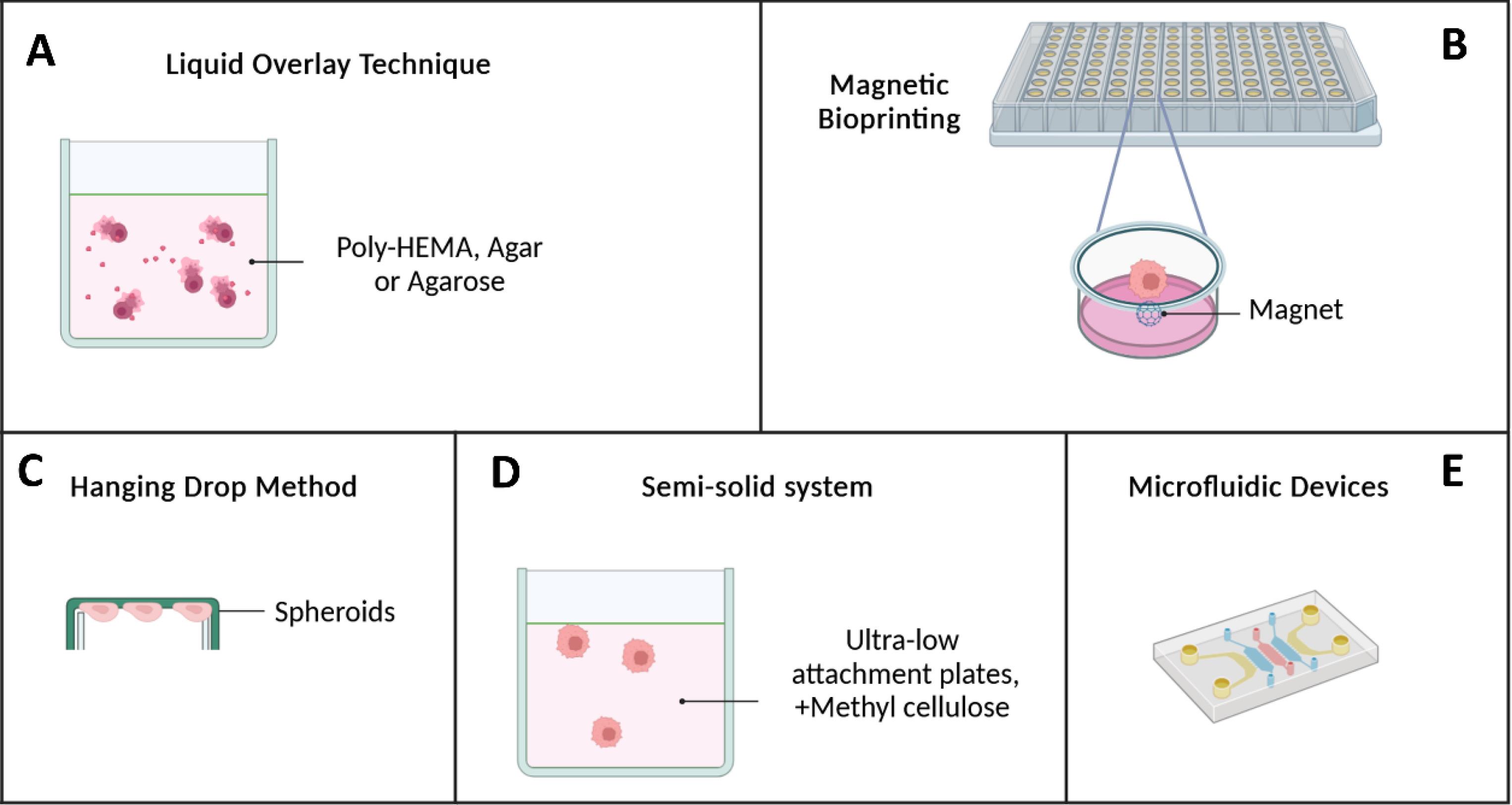
Fig. 1.
Techniques for generating pancreatic cancer spheroids. A) Liquid Overlay Technique: Cells are seeded onto plates coated to prevent adhesion and then aggregate into spheroids. Agitation prevents spheroid fusion; B) Magnetic Bioprinting: Spheroids containing magnetized cells are printed using bioinks; C) Hanging Drop Method: Cells aggregate into spheroids by suspending drops containing cells from an inverted plate. Spheroid size can be controlled by cell number per drop; D) Semi-Solid System: Cells are cultured in ultra-low attachment plates with methylcellulose and growth factors to promote spheroid formation; and E) Microfluidic Devices: Microfluidic systems enable controlled spheroid formation through automated processing steps.
.
Techniques for generating pancreatic cancer spheroids. A) Liquid Overlay Technique: Cells are seeded onto plates coated to prevent adhesion and then aggregate into spheroids. Agitation prevents spheroid fusion; B) Magnetic Bioprinting: Spheroids containing magnetized cells are printed using bioinks; C) Hanging Drop Method: Cells aggregate into spheroids by suspending drops containing cells from an inverted plate. Spheroid size can be controlled by cell number per drop; D) Semi-Solid System: Cells are cultured in ultra-low attachment plates with methylcellulose and growth factors to promote spheroid formation; and E) Microfluidic Devices: Microfluidic systems enable controlled spheroid formation through automated processing steps.
Liquid overlay technique (LOT)
This method primarily relies on non-adhesive sheets covered by poly 2-hydroxyethyl methacrylate (poly-HEMA),22,47-49 agar, or agarose.50,51 These materials prevent cells from adhering to the plate, prompting them to adhere to each other and form spheroids.52,53 Plates covered with agar or agarose or low-adhesive surfaces create cell suspensions. Cells aggregate more efficiently when the plate is continuously shaken on a shaker. Liquid overlay is a straightforward and widely used method, applicable even in 96-well plates. The real-time observation of spheroid formation is a notable advantage. However, it is necessary to have more influence over the dimension and form of the produced spheroids when using the liquid overlay approach, which is a challenge.54 Table 2 summarizes the LOT method's pros and drawbacks.
Table 2.
Advantages and disadvantages of coating material used in the LOT method
|
Material
|
Advantage
|
Disadvantage
|
Ref.
|
| Agar |
-
Straightforward, low-cost, and relatively simple to handle
-
Post-processing directly in the plates is very advantageous in high-throughput experiments.
-
Inexpensive
-
Dissolve in water and serum-free media.
-
Enables optical microscopy monitoring of spheroid development by allowing single cells to spontaneously self-assemble.
-
Preventing the loss of MCTS as a result of the MCTS being accidentally removed from the system.
|
-
Used to test a specific number of cancer medicines.
-
Dissolved in a high temperature
-
Agar can affect cell growth and other qualities when coated plates are stored for only a few days.
-
In a drug assay, there are no repeatable results.
-
Heterogeneous spheroids are labor-intensive and time-consuming to mass-produce.
|
53,55-57
|
| Agarose |
-
Simple to perform and can be sterilized using an autoclave or UV light.
-
Inexpensive
-
Allows for accurate MCTS size adjustment and large-scale MCTS generation
-
Allows for optical microscopy monitoring of spheroid formation.
-
Allow for spontaneous self-assembly of single cells.
-
Scalability
-
The process results in the formation of irregular 3D cellular aggregates of varying sizes and forms.
-
Preventing the loss of MCTS as a result of the medium being accidentally removed with it.
|
-
Dissolve in a hot environment.
-
Coated plates are only good for a few days and cannot be reused. They are used for short-term cultures and have a wide size distribution.
-
Unreliable drug assay results; labor-intensive and time-consuming; difficult to mass-produce
-
Heterogeneous spheroids Some cell lines have a hard time producing spheroids.
|
58,59
|
Poly
(2-hydroxyethyl methacrylate) (pHEMA) |
-
Performed simple and relatively easy handling very useful in high-throughput experiments direct post processing in plates
-
Storing the coat plate and solution at 4 oC for several months to prevent the loss of MCTS due to unintended removal of the MCTS and medium.
|
-
High expense; long-term cultivation problematic; 95 percent ethanol preparation
-
Coated plates must be stored for several months and are labor-intensive and time-consuming to mass-produce.
-
Heterogeneous spheroids Some cell lines have a hard time producing spheroids.
|
60-62
|
By tailoring culture substrates to individual researchers' needs, scientists have more leeway regarding experiment design and budget. Commercial low-adherence culture ware, on the other hand, provides an efficient and easy-to-use alternative. Many different kinds of cells, including cancer cells, may be effectively transformed into spheroids using these commercially accessible techniques,63 dental papilla cells,64 mesenchymal stem cells (MSCs),65 and a mixture of heterogeneous cell types.66 Notable products in the market include UpCellTM, NunclonTM, NuncTM, SpheraTM, Corning Ultra-Low Attachment surfaces, Lipidure® -COAT, CELLSTAR®, and Nanocluster.
A pancreatic cancer spheroid model was created using human pancreatic cancer cell lines MIAPaCa-2 and PANC-1 to assess the effects of chemotherapeutic treatments using the LOT approach. Cells at passage >20 were detached from the bottom of the dish using 0.05% trypsin. Using agarose-coated 96-well culture plates (50 mL 1.5% agarose per well), spheroids were started in a liquid overlay by seeding 1.2×103 MIAPaCa-2 cells and 1.0×103 PANC-1 cells per well in 200 mL of media. Except for a 72-hour drug treatment setup detailed in the drug treatment section, after an initiating period of 4 days, 50% of the supernatant was changed with the new medium. This process was repeated every 48 hours after that. Spheroid cell viability was evaluated using a modified acid phosphatase (APH) test. The study examined the effectiveness of gemcitabine and 5-FU in MIAPaCa-2 and PANC-1 spheroid and monolayer cultures, respectively. The results indicated that the efficacy of chemotherapy drugs in spheroid culture is lower than in monolayer culture for these two cell lines. This suggests that the size and volume of the spheroid, a good simulator of the actual tumor sample in the body, may hinder the proper signaling of drugs.67 To investigate cellular interactions at the molecular and cellular level, spheroids can be generated using soft agar. Spheroids made from cell lines MCF-7, BxPC-3, Capan-2, Panc-1, MIA PaCa-2, and Capan-1 showed that the microenvironment of 3D cultured cells is more acidic than that of 2D cells, attributed to increased phosphorylation of Tyr421 and elevated expression of cortactin. This acidity can lead to better stimulation of pro-metastasis migration of tumor cells.68
Hanging drop method
This method is a straightforward technique for forming spheroids without requiring specialized facilities. This method leverages the surface tension of cellular suspensions to optimize spheroid formation by intensifying cell-cell interactions by including various biological factors in minimal amounts.69,70 The hanging drop technique involves seeding tissue culture plates with a predetermined number of cells in the shape of tiny drops. Spheroids quickly develop after a 180° rotation in a humid atmosphere when cells gather at the drop's tip—the interface between the liquid and air—caused by gravity.69,71 While praised for its simplicity and compatibility with high-throughput screening, tracking spheroid formation and directly assessing drug perturbations can be challenging.72
One notable advantage of the hanging drop method is the concurrent culture of two or more cell lines, enabling cell-matrix interactions and cell-cell investigation. Wound healing, tumor cell interactions with stroma in aggressiveness and cancer, fetal development, and tissue engineering all rely on these interactions.73 However, limitations such as a restricted suspension volume and inadequate nutrients pose challenges for long-term culture. Consequently, spheroids formed using this method often need to be transferred to other plates, affecting their integrity and time-consuming.74,75
As mentioned previously, in 2016, Ware et al41 used five human PDAC cell lines—BxPC-3, PANC-1, Capan-1, AsPc-1, and MIA-PaCa-2—to create a spheroid model enriched with human pancreatic stellate cells. Adding pancreatic stellate cells enhances the simulation of the dense microenvironment characteristic of pancreatic tumors. This team used a hybrid approach, combining the hanging drop method with methyl cellulose as a medium component. The findings revealed notable differences between spheroids grown with pancreatic stellate cells and those formed with only PDAC cell lines. Spheroids cultivated with pancreatic stellate cells exhibited increased density, compactness, and more collagen than those produced using PDAC cell lines alone. Beyond similarities in collagen content, spheroids that incorporated pancreatic stellate cells closely resembled orthotopic tumors regarding the expression of KI67 and HIF-1α.41
Magnetic bioprinting
Another innovative approach for maintaining cell cohesion until spheroid formation involves using magnetic force. Magnetic 3D bioprinting enables the magnetization of cells with biocompatible nanoparticles, which are subsequently printed onto multi-well forms. Once nearby, these magnetized cells aggregate to form a spheroid, which can be harvested for subsequent biophysical and biochemical studies. In a study by Noel et al, NanoShuttle, comprising iron oxide, poly-L-lysine, and gold nanoparticles, was employed. The Patu8902 cell line was labeled with NanoShuttle, which electrostatically binds to the plasma membrane and is spontaneously released after approximately one week. The specific membrane receptor involved in this binding has yet to be discovered. Notably, using meager magnetic forces (30 pN) ensures sufficient force for cell accumulation without compromising cell survival, metabolism, or proliferation.76
Semi-solid system
The production of non-specific cell aggregations results from the mobility of individual cells, which is one of the constraints of spheroid formation.77 The semi-solid culture method addresses this issue by incorporating methylcellulose into the spheroid culture medium, thereby limiting the excessive mobility of cells. In this approach, cells are seeded in ultra-low attachment plates, and the culture medium is supplemented with methylcellulose and growth factors. After 11 days, spheroids are formed. Yang et al successfully generated spheroids from the PANC-1 cell line using this method. These spheroids exhibited enhanced proliferation, differentiation, migration, and invasion properties compared to conventional 2D cultures.78
Patterned surfaces and microfluidic devices
In 1970, microfluidics emerged as a versatile technology with applications across various industries, including cell isolation,79 biological and diagnostic sensors,80 pharmacological experiments,81 DNA extraction,82 and the formation of spheroids. Microfluidic chips consist of inlets and outlets connected by microchannels or chambers within a bulk material. The microchannel network directs, mixes, or splits liquid fluid to achieve specific applications used in biomedical and chemical settings, including microreactors, fluid mixers, cell culture, and sorting of cells and particles.83
The gas permeability, cheap cost, and ease of use of PDMS make it a popular material for microfluidic system fabrication.84-86 PDMS's transparency enables direct fluorescent imaging of proteins and cells.87-92 However, challenges such as evaporation of cell culture medium over time due to gas permeability, time-consuming system development, and repeatability issues have been associated with PDMS microfluidic systems.93,94 Microfluidic systems have been fabricated using plastic and glass materials to address these challenges.91
Recent advancements in 3D printing technology have provided solutions for spheroid formation using microfluidics. In this technique, cells grow in layers through automated systems. Polyjet, stereolithography, and extrusion-based printing are three 3D printing techniques applicable to microfluidics.92,95,96 These techniques can produce systems with channels that facilitate cell-cell interaction, mimicking in vivo conditions.92,97 Additionally, chips,69,98-101 and biosensors98 have been designed to produce spheroids. In these techniques, cells are trapped on a bed of ECM, including fibronectin and collagen.102 The accumulation of spheroids can be quantified by being placed between two electrodes, proving valuable in assessing the efficacy of medications.103 The materials used for these structures typically include silicon, glass, or plastic, with transparent materials enabling fluorescent microscopy investigations.102
Moreover, recent approaches involve using columns coated with Matrigel, poly-L-lysine, and barium chloride for spheroid culturing. This method includes suspending cells in 1% alginate, collagen, and Matrigel. The column is then introduced into the culture medium using the hanging drop method, ultimately resulting in spheroid formation at the tip of the column. This technique proves effective in assessing medication efficacy, as these columns can be placed in a medium or a 96-well plate containing the drug.104,105
Integrating pancreatic stellate cells and pancreatic tumor spheroids in a 3D collagen matrix is the goal of the microchannel plate-based co-culture paradigm put out by Lee and colleagues. By recreating chemoresistance and the epithelial-mesenchymal transition (EMT), this model attempts to mimic the in vivo TME. Findings indicated that PANC-1 cells when co-cultured with pancreatic stellate cells, doubled the number of spheroids and generated 3D tumor spheroids after five days. By cultivating pancreatic stellate cells in close quarters with cancer cells in a 3D collagen matrix, they were able to show that the two cell types interact to enhance EMT and medication resistance in the microchannel plate. One promising approach to studying EMT and treatment resistance therapeutically relevantly is the microfluidic co-culture of pancreatic stellate cells and pancreatic tumor spheroids.106 Additionally, there have been reports of spheroid models that exhibit enhanced biological complexity. These models involve the co-culturing of 3D cancer cells with one or more kinds of cells from the PDAC TME. This is a significant improvement compared to monocultures, as interactions between cells in the TME might impact the course of the illness and the effectiveness of treatment.107 See Table 3 for a comparison of different methods for pancreatic-derived spheroid formation.
Table 3.
Different methods of pancreatic-derived spheroid formation
|
|
Cell line
|
Advantage
|
Disadvantage
|
Ref.
|
| Pellet culture |
Capan-2
PDAC1, PDAC2, PDAC3, PDAC5 |
|
|
108-111
|
| LOT |
MIAPaCa2
PANC-1
BxPC-3
Capan-1 Capan-2 Panc-1 MCF-7 |
|
|
67,68,112-116
|
| Hanging drop |
AsPc-1, PANC-1, BxPC-3 MIA-PaCa2, Capan-1, |
|
|
75-78,117,118
|
| Magnetic bio printing |
Patu8902 |
|
|
79
|
| Semi-solid system |
PANC-1 |
|
|
119
|
| Patterned Surfaces and Microfluidic Devices |
PANC-1 |
-
Easy handling
-
User friendly
-
Low-cost
|
|
82,84,89,91,92,97,100,103,107,120
|
Patient-derived spheroid
Tumor-derived spheroids are created by isolating single-cell suspensions from tumor tissues using mechanical or enzymatic methods, followed by serum or serum-free medium cultivation. Various cancers, including brain,108 breast,109 lung,110 colon,111 prostate,112 pancreatic,113 and ovarian cancers, have been successfully used to generate tumor-derived spheroids.114 Enriching cancer stem cells is achieved by cultivating tumor cells with stem cell traits in a serum-free medium rich in various growth factors like progesterone, hydrocortisone, and insulin. This process promotes tumor cell proliferation while excluding non-malignant and differentiated cells. As a result, a critical feature of tumor-derived spheroids is the concentration of cancer stem cells.
Tumors are either partially physically or enzymatically dissociated into 0.3 mm pieces for use in ex vivo explant cultures, followed by cultivation in agar-covered plates with a serum-containing medium comparable to organotypic multicellular tumor spheroids.115 Although tumor spheroids are among the most fundamental 3D cell culture models, their attractiveness lies in their ability to closely mimic solid tumors' characteristics in various ways. Importantly, they interact with other cells and the ECM. Moreover, when grown beyond 500 µm, spheroids resemble non-vascularized or minimally vascularized tumors, displaying metabolic gradients. There are three distinct layers to the structure: one with cells that are actively dividing, one with cells that are resting, and finally, one with cells that are hypoxic and necroses.85 Similar to human cancers, these unique characteristics of tumor spheroids make them resistant to radiation and anti-cancer therapy. Consequently, tumor spheroids are extensively utilized in drug screening investigations.116
3D tumor sphere models can be divided into four distinct groups based on their culture methods and sphere biology: organotypic multicellular spheres (OMS), multicellular tumor spheroids (MCTS), tissue-derived tumor spheres (TDTS), and tumorspheres (Fig. 2).117
-
MCTS: These spheroids comprise tumor cells co-cultured with stromal cells such as immune cells, endothelial cells, and fibroblasts, which can be mono- or heterotypic cell populations. Cell culture in non-adherent media can produce MCTS.118
-
Tumorspheres: Also known as cancer stem cell spheres, this method isolates and propagates CSCs from tumor tissues or cancer cell lines.119
-
TDTS: This model is created by partially dissolving tumor tissue using enzymes or mechanical means. This process separates cancer cells from non-tumor cells while keeping cancer cells in touch with each other. The OMS model differ from this approach, which involves cutting primary tumor tissues.120
-
OMS: Primary tumor tissues are sliced to obtain OMS models. When compared to MCTS and tumorspheres, TDTS and OMS models do a better job of simulating tumor development and gene expression patterns. But by including stromal cells, the OMS model adds another layer of intricacy.120 OMS has great promise for personalized medicine since it is the best 3D model for evaluating a tumor's therapeutic response to treatment.150
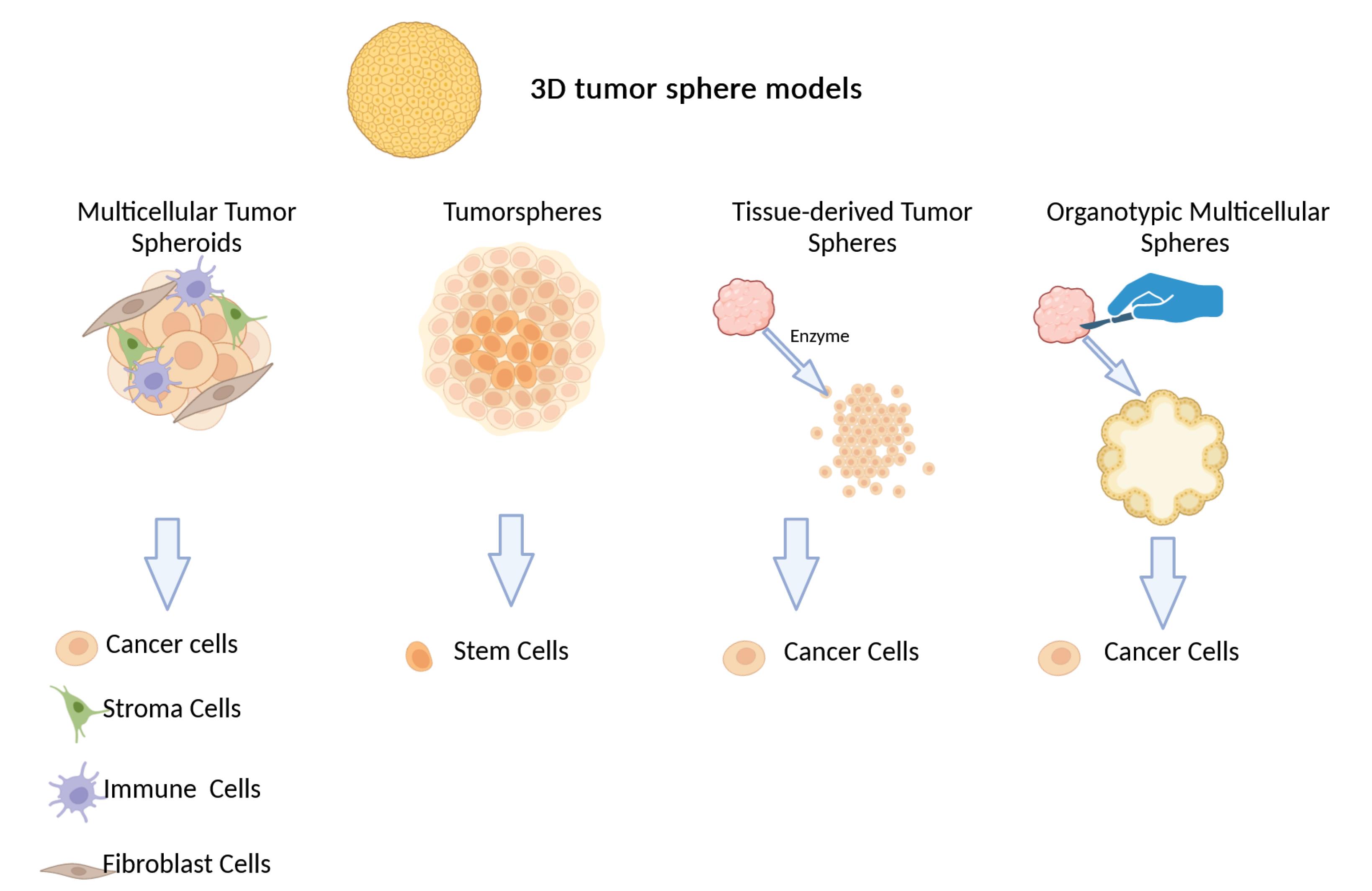
Fig. 2.
In vitro 3D tumor sphere models for cancer research. From left to right: MCTS, spheroids of only tumor cells like cancer cell lines, useful for studying inherent behaviors like invasion, proliferation, and drug response. Tumorspheres, generated by isolating and amplifying cancer stem cells from tumor tissues or cancer cell lines. TDTS, created by partially dissociating tumor tissue enzymatically or mechanically to separate cancer cells from non-tumor cells while maintaining cancer cell connections. OMS, organotypic multicellular spheroids derived from patient tumor samples, representing a personalized model for assessing therapeutic response.
.
In vitro 3D tumor sphere models for cancer research. From left to right: MCTS, spheroids of only tumor cells like cancer cell lines, useful for studying inherent behaviors like invasion, proliferation, and drug response. Tumorspheres, generated by isolating and amplifying cancer stem cells from tumor tissues or cancer cell lines. TDTS, created by partially dissociating tumor tissue enzymatically or mechanically to separate cancer cells from non-tumor cells while maintaining cancer cell connections. OMS, organotypic multicellular spheroids derived from patient tumor samples, representing a personalized model for assessing therapeutic response.
Applications of different 3D models
As previously mentioned, many approaches are used to create three-dimensional spheroids. In this section, various 3D models have been applied to studying pancreatic cancer. According to the literature, each of these models has distinct uses. Spheroids find applications in preclinical domains, gene expression evaluation, and protein investigation. 3D tumor models generated by spherical microplates mimic thein vivo conditions of the microenvironment, allowing spheroids to grow as monocellular entities or in conjunction with other cells in the microenvironment. This model offers a better opportunity for predicting the effectiveness of cancer medications. The MCTS model in pancreatic cancer is employed for investigating hypoxia markers,40 different miRNA expression,37 protein levels,37-122 and drug responses.37,123-125
In co-culture methods, various behaviors of pancreatic cancer cells, including aggression126,127, immigration,128 proliferation,129 signaling pathways,130 and drug resistance,129,131 can be investigated. Device-based 3D models, such as biosensors and chips, are utilized in pancreatic cancer to evaluate hypoxia,132 pharmacological responses,106,134 and expression markers and factors.106 Recreating the in vivo environment has led to tumor spheroids or tumoroids gaining prominence.135,136 Recently, novel platforms have been developed as combinations that do not require microfluidics for fabricating spheroids.137-141 For example, microcells made by micro-molding or photolithography techniques can produce spheroids of specific sizes and compositions.69,137,142 Furthermore, platforms with weak attachment surfaces were designed utilizing non-adhesive materials, such as PDMS143-145 or agarose.146,147 These platforms allowed the formation of spheroids in a controlled and simple environment. A small sample size, incompatible culture media, and the inability to retrieve any samples are all potential limitations of this method. However, these models are highly effective in medical screening and are simple.71,141,142,148,149
An essential part of the TME in pancreatic cancer is the presence of pancreatic stellate cells, which are involved developing treatment resistance and the advancing the disease. Lee et al designed a culture model based on microchannels for producing spheroids with pancreatic stellate cells in a 3D collagen matrix, allowing the examination of epithelial-mesenchymal transfer and drug resistance. They designed microchannels using collagen so spheroids could grow with pancreatic stellate cells in a 3D pattern. An advantageous paradigm for therapeutically testing EMT and treatment resistance was suggested, which involves collaborating pancreatic tumor stellate cells with pancreatic tumor spheroid microfluidics.106
On the other hand, the initiation of pancreatitis and pancreatic cancer follows different patterns. Acinar cells of the exocrine pancreas are positioned beneath the ductal cells and reconstruct the 3D structure of pancreatic tissue. However, the molecular mechanism of 3D structure formation still needs to be better understood. Therefore, Hakobyan et al designed a spheroid 3D model of pancreatic cancer using laser-assisted bioprinting. This model could be applied to determine the phenotypic evolution of cancer cells over time through visual analysis of phenotypic features.150
Moreover, Monteiro et al devised a platform for evaluating drug resistance in 3D culture, employing classified spheroid models that mimic stromal cells. These models exhibit repeatable morphology and feature molecular biomarkers such as TGF, FGF-2, IL-1, and MMP9—essential elements secreted in the stratified microenvironment spheroid (STAMS) 3D models of human pancreatic cancer. By incorporating STAMS into an ECM-mimetic hydrogel matrix, this model reflects increased therapeutic resistance and mimics the architectural characteristics of PDAC stroma in vitro.151
Bio-fabrication of 3D spheroid models
Understanding the biological behavior of tissues, organs, and tumors holds significant promise for disease treatment in the medical field.152,153 A major obstacle for tissue engineering is producing organs that mimic healthy and diseased tissues with a dense population of live cells.154,155 Most engineered tissues to date have been relatively thin (< 2 mm) to facilitate oxygen and nutrient transfer, and remove cell debris.154
3D printing has emerged as a groundbreaking technology in tissue generation within medicine. Stents and splints are only two medical gadgets significantly benefiting from this technique.156 In 3D bioprinting, the precise arrangement of layers consisting of biological, chemical-biological, and living cells is utilized to construct 3D structures by controlling the positioning of functional components. The resulting material from this technique, which incorporates cells, is called bioink. Despite their differences, all bioprinters can print cell masses, cells encased in hydrogel or other viscous fluids, and cells housed in microcarriers.157,158 Bioprinters encompass laser-assisted devices, extrusion devices, and printers resembling inkjet technology (Fig. 3).
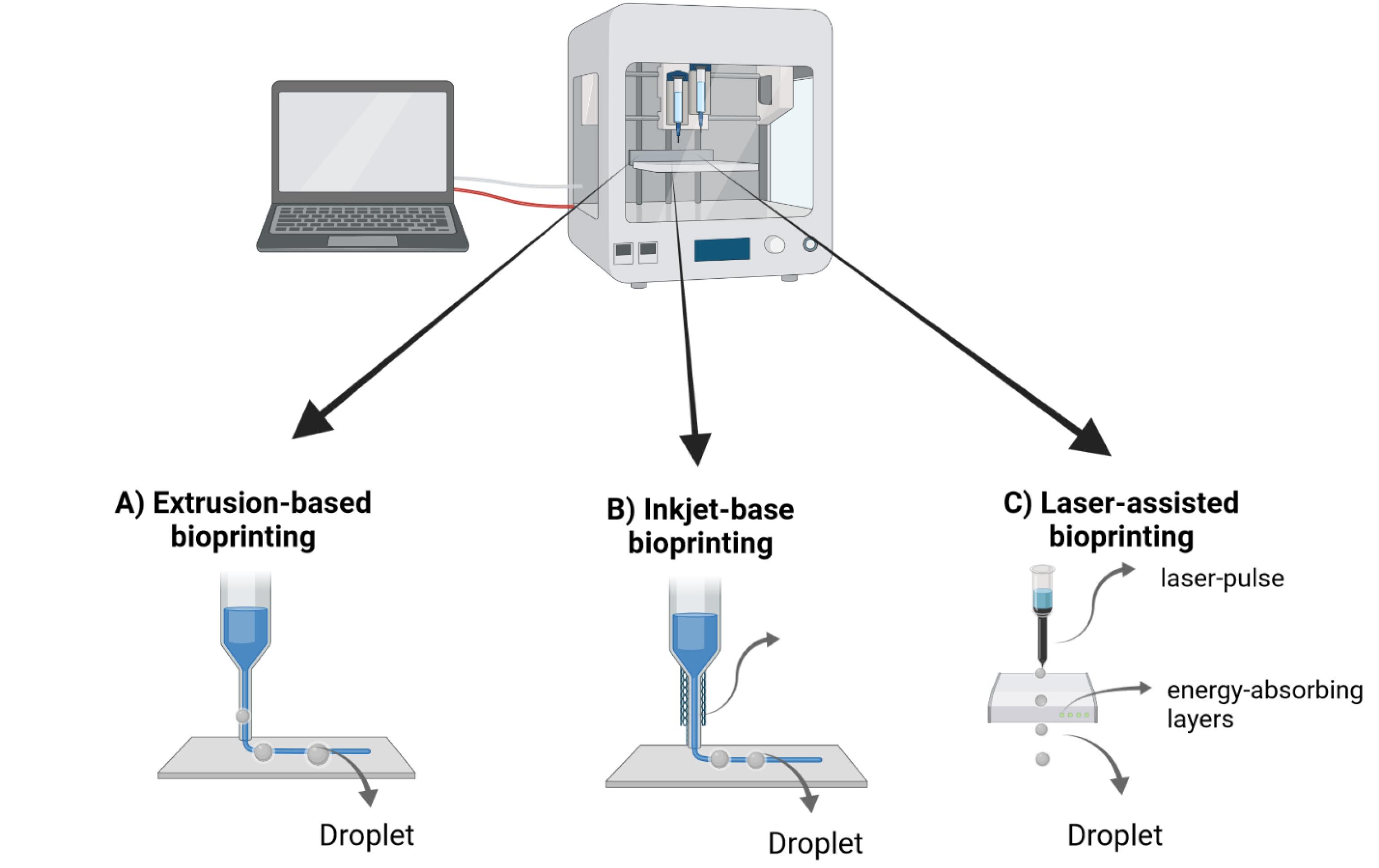
Fig. 3.
The three primary bio-printing techniques: A) Extrusion-based bioprinting involves depositing bio-inks in layers through a controlled extrusion system. B) Inkjet-based bioprinting uses controlled droplet ejection to pattern biomaterials with high resolution. C) Laser-assisted bioprinting uses focused laser energy to position biomaterials with precision, enabling construction of intricate biological structures.
.
The three primary bio-printing techniques: A) Extrusion-based bioprinting involves depositing bio-inks in layers through a controlled extrusion system. B) Inkjet-based bioprinting uses controlled droplet ejection to pattern biomaterials with high resolution. C) Laser-assisted bioprinting uses focused laser energy to position biomaterials with precision, enabling construction of intricate biological structures.
In extrusion-based deposition, syringe-like instruments with polymeric or hydrogel characteristics are employed to fabricate 3D structures, typically guided by pneumatic pressure or computer-controlled mechanical pistons.159 As a drop-by-drop method, inkjet printing generates the 3D structure by combining hydrogel and cell-friendly biomaterials in drops.160,161 The laser-induced forward transfer (LIFT) technique involves cells forming a 3D structure as drops using a laser.162-164 According to the recent classification by Moroni et al., bio-fabrication is categorized as 3D bioprinting.165 3D bioprinting in bio-fabrication enables the creation of 3D cancer models by printing live cells and "bio-ink" ECM together. This process allows for accurate manipulation of the position and development of primary cells. This may lead to a suitable tissue structure.166-168 In this method, bio-ink consists of cells surrounded by a matrix printed in a specific pattern. Consequently, cells self-organize, and 3D cell growth occurs. Different types of hydrogels, both natural and manufactured, including Matrigel, fibrin, collagen, PEG, alginate, and gelatin methacrylate, can be utilized in 3D bioprinting for to investigate cell function and activity.169-173
Recently, HeLa cells have been 3D bio-printed in a hydrogel, creating a cervical cancer model.173 The cells within the 3D tumor bio-print demonstrate elevated growth rates, a heightened inclination to create spheroids, enhanced matrix metalloproteinase expression, and superior resistance to chemical challenges compared to cells cultured in a 2D culture. These results mirror in vivo responses, making this model suitable for assessing the efficacy of medications. The 3D human cancer model is a valuable tool for investigating the behavior of healthy and diseased cells, and for medication screening.174,175 However, this technique has some limitations, such as the time-consuming nature of cellular assembly, taking weeks or days. Additionally, these models lack the capacity for the simultaneous culture of two or three cell types.166,172,176
Concluding remarks
Pancreatic cancer continues to be one of the most lethal forms of cancer, often associated with a grim prognosis. Conventional 2D cell cultures have been widely employed in fundamental research and drug development; however, their ability to accurately predict the efficacy of novel treatments is constrained. More clinically relevant cell models are urgently needed to improve the success of drug development and provide deeper biological insights.
Diversein vivo and in vitro models have been established for pancreatic cancer. Unlike 2D monolayer cultures, 3D culture systems better mimic tumors' architecture and physiological activity through enhanced cell-cell and cell-matrix interactions. Current 3D approaches include multicellular spheroids, organoids, co-cultures, and microfluidic systems. Spheroid models demonstrate greater chemoresistance and expression of tumor microenvironment components compared to 2D cultures. While technical challenges of scale and reproducibility have limited spheroid generation, new technologies are emerging to enable large-scale, standardized spheroid production critical for downstream applications.
Both spheroids and organoids offer advantages over 2D models by recreating physiologically relevant tissue and tumor conditions. These 3D culture systems more faithfully reconstruct the in vivo microenvironment, providing higher predictive value for testing therapeutic strategies. While organoids emphasize recapitulating native tissue organization and heterogeneity, spheroids focus on tumor cell proliferation and drug responses. The optimal 3D model depends on specific research questions and goals. In the future, interconnected spheroid and organoid models could provide insight into human tissue interactions in vitro. 3D culture will continue to have tremendous potential to advance pancreatic cancer research and therapy development.
Review Highlights
What is the current knowledge?
√ Pancreatic ductal adenocarcinoma (PDAC) is highly malignant with limited treatment options. More predictive preclinical models are needed to improve drug development.
√ Conventional 2D cell cultures lack the complexity of the tumor microenvironment. 3D culture systems better recapitulate in vivo architecture and interactions.
√ Spheroids focus on proliferation and drug response, while organoids emphasize tissue organization and heterogeneity.
√ Emerging techniques enable large-scale, standardized spheroid production critical for drug screening.
What is new here?
√ Multicellular tumor spheroids demonstrate enhanced chemoresistance and expression of microenvironment components versus 2D cultures.
√ Both spheroids and organoids offer advantages over 2D models, but optimal choice depends on specific research questions.
√ Continued 3D culture development has tremendous potential to advance pancreatic cancer research and therapy.
√ 3D bioprinting shows promise for creating customizable models, but challenges remain regarding speed and co-culture.
√ Co-culture of tumor spheroids with stromal cells adds complexity approaching in vivo conditions.
√ Microfluidic models allow dynamic investigation of cell interactions within physiologically relevant microenvironments.
Acknowledgment
All figures were created with “BioRender.com” (free version).
Competing Interests
The authors declare no conflict of interest.
Ethical Statement
Not applicable.
References
- Hu ZI, O’Reilly EM. Therapeutic developments in pancreatic cancer. Nat Rev Gastroenterol Hepatol 2023; 5:1-8. doi: 10.1038/s41575-023-00840-w [Crossref] [ Google Scholar]
- Sherman MH, Beatty GL. Tumor microenvironment in pancreatic cancer pathogenesis and therapeutic resistance. Annu Rev Pathol 2023; 18:123-48. doi: 10.1146/annurev-pathmechdis-031621-024600 [Crossref] [ Google Scholar]
- Sailer V, von Amsberg G, Duensing S, Kirfel J, Lieb V, Metzger E. Experimental in vitro, ex vivo and in vivo models in prostate cancer research. Nat Rev Urol 2023; 20:158-78. doi: 10.1038/s41585-022-00677-z [Crossref] [ Google Scholar]
- Kyriakopoulou K, Koutsakis C, Piperigkou Z, Karamanos NK. Recreating the extracellular matrix: novel 3D cell culture platforms in cancer research. FEBES J 2023; 290:5238-5247. doi: 10.1111/febs.16778 [Crossref] [ Google Scholar]
- Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V. 2D and 3D cell cultures–a comparison of different types of cancer cell cultures. Arch Med Sci 2018; 14:910-9. doi: 10.5114/aoms.2016.63743 [Crossref] [ Google Scholar]
- Zanoni M, Piccinini F, Arienti C, Zamagni A, Santi S, Polico R. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Adv Exp Med Biol 2016; 6:19103. doi: 10.1007/978-3-030-58174-9_11 [Crossref] [ Google Scholar]
- Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, De Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol 2013; 31:108-15. doi: 10.1016/j.tibtech.2012.12.003 [Crossref] [ Google Scholar]
- Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet 2018; 19:671-87. doi: 10.1038/s41576-018-0051-9 [Crossref] [ Google Scholar]
- Lin A, Sved Skottvoll F, Rayner S, Pedersen‐Bjergaard S, Sullivan G, Krauss S. 3D cell culture models and organ‐on‐a‐chip: Meet separation science and mass spectrometry. Electrophoresis 2020; 41:56-64. doi: 10.1002/elps.201900170 [Crossref] [ Google Scholar]
- Haycock JW. 3D cell culture: a review of current approaches and techniques: Springer. Methods Mol Biol 2011; 695:1-15. doi: 10.1007/978-1-60761-984-0_1 [Crossref] [ Google Scholar]
- Greiner AM, Richter B, Bastmeyer M. Micro‐engineered 3D scaffolds for cell culture studies. Macromol Biosci 2012. 12: 1301-14. 10.1002/mabi.201200132.
- Cushing MC, Anseth KS. Hydrogel cell cultures. Science 2007; 316:1133-4. doi: 10.1126/science.1140171 [Crossref] [ Google Scholar]
- Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 2009; 103:655-63. doi: 10.1002/bit.22361 [Crossref] [ Google Scholar]
- Jian H, Wang M, Wang S, Wang A, Bai S. 3D bioprinting for cell culture and tissue fabrication. Bio-Des Manuf 2018; 1:45-61. doi: 10.1007/s42242-018-0006-1 [Crossref] [ Google Scholar]
- Wu M, Swartz MA. Modeling tumor microenvironments in vitro. J Biomech Eng 2014; 136:021011. doi: 10.1115/1.4026447 [Crossref] [ Google Scholar]
- Yuki K, Cheng N, Nakano M, Kuo C. Organoid models of tumor immunology. Trends Immunol 2020; 41:652-64. doi: 10.1016/j.it.2020.06.010 [Crossref] [ Google Scholar]
- Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K. Tumor‐derived spheroids: relevance to cancer stem cells and clinical applications. Cancer Sci 2017; 108:283-9. doi: 10.1111/cas.13155 [Crossref] [ Google Scholar]
- Sutherland RM, McCredie JA, Inch WR. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst 1971; 46:113-20. [ Google Scholar]
- Steinberg MS. Differential adhesion in morphogenesis: a modern view. Current Opinion In Genetics & Development 2007; 17:281-6. doi: 10.1016/j.gde.2007.05.002 [Crossref] [ Google Scholar]
- Lin R-Z, Chou L-F, Chien C-CM, Chang H-Y. Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and β1-integrin. Cell Tissue Res 2006; 324:411-22. doi: 10.1007/s00441-005-0148-2 [Crossref] [ Google Scholar]
- Froehlich K, Haeger J-D, Heger J, Pastuschek J, Photini SM, Yan Y. Generation of multicellular breast cancer tumor spheroids: comparison of different protocols. J Mammary Gland Biol Neoplasia 2016; 21:89-98. doi: 10.1007/s10911-016-9359-2 [Crossref] [ Google Scholar]
- Wei X, Senanayake TH, Warren G, Vinogradov SV. Hyaluronic acid-based nanogel–drug conjugates with enhanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors. Bioconjug Chem 2013; 24:658-68. doi: 10.1021/bc300632w [Crossref] [ Google Scholar]
- Luca AC, Mersch S, Deenen R, Schmidt S, Messner I, Schäfer K-L. Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS One 2013; 8:e59689. doi: 10.1371/journal.pone.0059689 [Crossref] [ Google Scholar]
- Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol 2007; 1:84-96. doi: 10.1016/j.molonc.2007.02.004 [Crossref] [ Google Scholar]
- Sodunke TR, Turner KK, Caldwell SA, McBride KW, Reginato MJ. Micropatterns of Matrigel for three-dimensional epithelial cultures. Biomaterials 2007; 28:4006-16. doi: 10.1016/j.biomaterials.2007.05.021 [Crossref] [ Google Scholar]
- Härmä V, Virtanen J, Mäkelä R, Happonen A, Mpindi J-P, Knuuttila M. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PloS one 2010; 5:e10431. doi: 10.1371/journal.pone.0010431 [Crossref] [ Google Scholar]
- Chitcholtan K, Asselin E, Parent S, Sykes PH, Evans J. Differences in growth properties of endometrial cancer in three dimensional (3D) culture and 2D cell monolayer. Exp Cell Res 2013; 319:75-87. doi: 10.1016/j.yexcr.2012.09.012 [Crossref] [ Google Scholar]
- Panchalingam KM, Jung S, Rosenberg L, Behie LAJScr, therapy therapy. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: a review. 2015; 6:1-10. doi: 10.1186/s13287-015-0228-5 [Crossref]
- Fonoudi H, Ansari H, Abbasalizadeh S, Larijani MR, Kiani S, Hashemizadeh S. A universal and robust integrated platform for the scalable production of human cardiomyocytes from pluripotent stem cells. Stem Cells Transl Med 2015; 4:1482-94. doi: 10.5966/sctm.2014-0275 [Crossref] [ Google Scholar]
- Petry F, Salzig D. Large-scale production of size-adjusted β-cell spheroids in a fully controlled stirred-tank reactor. Processes 2022; 10:861. doi: 10.3390/pr10050861 [Crossref] [ Google Scholar]
- Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia 2015; 17:1-15. doi: 10.1016/j.neo.2014.12.004 [Crossref] [ Google Scholar]
- Gilazieva Z, Ponomarev A, Rutland C, Rizvanov A, Solovyeva V. Promising applications of tumor spheroids and organoids for personalized medicine. Cancers (Basel) 2020; 12:2727. doi: 10.3390/cancers12102727 [Crossref] [ Google Scholar]
- Roy M, Alix C, Bouakaz A, Serriere S, Escoffre JM. Tumor Spheroids as Model to Design Acoustically Mediated Drug Therapies: A Review. Pharmaceutics 2023; 15:806. doi: 10.3390/pharmaceutics15030806 [Crossref] [ Google Scholar]
- Mitrakas AG, Tsolou A, Didaskalou S, Karkaletsou L, Efstathiou C, Eftalitsidis E. Applications and Advances of Multicellular Tumor Spheroids: Challenges in Their Development and Analysis. Int J Mol Sci 2023; 24:6949. doi: 10.3390/ijms24086949 [Crossref] [ Google Scholar]
- El Harane S, Zidi B, El Harane N, Krause KH, Matthes T, Preynat-Seauve O. Cancer Spheroids and Organoids as Novel Tools for Research and Therapy: State of the Art and Challenges to Guide Precision Medicine. Cells 2023; 12:1001. doi: 10.3390/cells12071001 [Crossref] [ Google Scholar]
- Sipos B, Möser S, Kalthoff H, Török V, Löhr M, Klöppel G. A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform. Virchows Archiv 2003; 442:444-52. doi: 10.1007/s00428-003-0784-4 [Crossref] [ Google Scholar]
- Yeon S-E, No DY, Lee S-H, Nam SW, Oh I-H, Lee J. Application of concave microwells to pancreatic tumor spheroids enabling anticancer drug evaluation in a clinically relevant drug resistance model. PloS one 2013; 8:e73345. doi: 10.1371/journal.pone.0073345 [Crossref] [ Google Scholar]
- Dufau I, Frongia C, Sicard F, Dedieu L, Cordelier P, Ausseil F. Multicellular tumor spheroid model to evaluate spatio-temporal dynamics effect of chemotherapeutics: application to the gemcitabine/CHK1 inhibitor combination in pancreatic cancer. BMC Cancer 2012; 12:15. doi: 10.1186/1471-2407-12-15 [Crossref] [ Google Scholar]
- Wen Z, Liao Q, Hu Y, You L, Zhou L, Zhao Y. A spheroid-based 3-D culture model for pancreatic cancer drug testing, using the acid phosphatase assay. Braz J Med Biol Res 2013; 46:634-42. doi: 10.1590/1414-431X20132647 [Crossref] [ Google Scholar]
- Ware MJ, Colbert K, Keshishian V, Ho J, Corr SJ, Curley SA. Generation of homogenous three-dimensional pancreatic cancer cell spheroids using an improved hanging drop technique. Tissue Eng Part C: Methods 2016; 22:312-21. doi: 10.1089/ten.TEC.2015.0280 [Crossref] [ Google Scholar]
- Ware MJ, Keshishian V, Law JJ, Ho JC, Favela CA, Rees P. Generation of an in vitro 3D PDAC stroma rich spheroid model. Biomaterials 2016; 108:129-42. doi: 10.1016/j.biomaterials.2016.08.041 [Crossref] [ Google Scholar]
- Cavo M, Delle Cave D, D’Amone E, Gigli G, Lonardo E, Del Mercato LL. A synergic approach to enhance long-term culture and manipulation of MiaPaCa-2 pancreatic cancer spheroids. Sci Rep 2020; 10:1-11. doi: 10.1038/s41598-020-66908-8 [Crossref] [ Google Scholar]
- Longati P, Jia X, Eimer J, Wagman A, Witt M-R, Rehnmark S. 3D pancreatic carcinoma spheroids induce a matrix-rich, chemoresistant phenotype offering a better model for drug testing. BMC Cancer 2013; 13:1-13. doi: 10.1186/1471-2407-13-95 [Crossref] [ Google Scholar]
- Durymanov M, Kroll C, Permyakova A, Reineke J. Role of endocytosis in nanoparticle penetration of 3d pancreatic cancer spheroids. Mol Pharmaceutics 2019; 16:1074-82. doi: 10.1021/acs.molpharmaceut.8b01078 [Crossref] [ Google Scholar]
- Liu X, Gundel B, Li X, Liu J, Wright A, Lohr M. 3D heterospecies spheroids of pancreatic stroma and cancer cells demonstrate key phenotypes of pancreatic ductal adenocarcinoma. Transl Oncol 2021; 14:101107. doi: 10.1016/j.tranon.2021.101107 [Crossref] [ Google Scholar]
- Polat A, Gokturk D. An alternative approach to tracing the volumic proliferation development of an entire tumor spheroid in 3D through a mini-Opto tomography platform. Micron 2022; 152:103173. doi: 10.1016/j.micron.2021.103173 [Crossref] [ Google Scholar]
- Yang Q, Yang Y, Li L, Sun W, Zhu X, Huang Y. Polymeric nanomedicine for tumor-targeted combination therapy to elicit synergistic genotoxicity against prostate cancer. ACS Appl Mater Interfaces 2015; 7:6661-73. doi: 10.1021/am509204u [Crossref] [ Google Scholar]
- Lei H, Hofferberth SC, Liu R, Colby A, Tevis KM, Catalano P, et al. Paclitaxel-loaded expansile nanoparticles enhance chemotherapeutic drug delivery in mesothelioma 3-dimensional multicellular spheroids. J Thorac Cardiovasc Surg 2015; 149: 1417-25. e1. 10.1016/j.jtcvs.2015.02.020.
- Ivascu A, Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. Journal of Biomolecular Screening 2006; 11:922-32. [ Google Scholar]
- Lee JY, Chung SJ, Cho HJ, Kim DD. Phenylboronic acid‐decorated chondroitin sulfate A‐based theranostic nanoparticles for enhanced tumor targeting and penetration. Adv Funct Mater 2015; 25:3705-17. doi: 10.1002/adfm.201500680 [Crossref] [ Google Scholar]
- Costa EC, Gaspar VM, Coutinho P, Correia IJ. Optimization of liquid overlay technique to formulate heterogenic 3D co‐cultures models. Biotechn Bioeng 2014; 111:1672-85. doi: 10.1002/bit.25210 [Crossref] [ Google Scholar]
- Kyffin JA, Cox CR, Leedale J, Colley HE, Murdoch C, Mistry P. Preparation of primary rat hepatocyte spheroids utilizing the liquid‐overlay technique. Curr Protoc Toxicol 2019; 81:e87. doi: 10.1002/cptx.87 [Crossref] [ Google Scholar]
- Metzger W, Sossong D, Bächle A, Pütz N, Wennemuth G, Pohlemann T. The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells. Cytotherapy 2011; 13:1000-12. doi: 10.3109/14653249.2011.583233 [Crossref] [ Google Scholar]
- Costa EC, de Melo‐Diogo D, Moreira AF, Carvalho MP, Correia IJ. Spheroids formation on non‐adhesive surfaces by liquid overlay technique: Considerations and practical approaches. Biotechnol J 2018; 13:1700417. doi: 10.1002/biot.201700417 [Crossref] [ Google Scholar]
- Sgouros G, Yang W-H, Enmon R. Spheroids of prostate tumor cell lines. In: Prostate Cancer Methods and Protocols. Springer; 2003. p. 79-88. 10.1385/1-59259-372-0:79.
- Sarisozen C, Abouzeid AH, Torchilin VPJEJoP. The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur J Pharm Biopharm 2014; 88:539-50. doi: 10.1016/j.ejpb.2014.07.001 [Crossref] [ Google Scholar]
- Jou CH, Chen WC, Yang MC, Hwang MC, Chou WL, Lin SM. In vitro biocompatibility of three‐dimensional chitosan scaffolds immobilized with chondroitin‐6‐sulfate. Polym Adv Technol 2008; 19:377-84. doi: 10.1002/pat.1020 [Crossref] [ Google Scholar]
- Huang B-W, Gao J-QJ. Application of 3D cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. J Control Release 2018; 270:246-59. doi: 10.1016/j.jconrel.2017.12.005 [Crossref] [ Google Scholar]
- Metzger W, Sossong D, Putz N, Wennemuth G, Pohlemann T, Oberringer M. The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells. Cytotherapy 2011; 13:1000-2. doi: 10.3109/14653249.2011.583233 [Crossref] [ Google Scholar]
- Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc 2013; 8:1391. doi: 10.1038/nprot.2013.076 [Crossref] [ Google Scholar]
- Phung YT, Barbone D, Broaddus VC, Ho M. Rapid generation of in vitro multicellular spheroids for the study of monoclonal antibody therapy. J Cancer 2011; 2:507. doi: 10.7150/jca.2.507 [Crossref] [ Google Scholar]
- Lawrenson K, Grun B, Gayther SA. Heterotypic three-dimensional in vitro modeling of stromal-epithelial interactions during ovarian cancer initiation and progression. J Vis Exp 2012; 28(66):e4206. doi: 10.3791/4206 [Crossref] [ Google Scholar]
- Yoshii Y, Furukawa T, Waki A, Okuyama H, Inoue M, Itoh M. High-throughput screening with nanoimprinting 3D culture for efficient drug development by mimicking the tumor environment. Biomaterials 2015; 51:278-89. doi: 10.1016/j.biomaterials.2015.02.008 [Crossref] [ Google Scholar]
- Yamamoto M, Kawashima N, Takashino N, Koizumi Y, Takimoto K, Suzuki N. Three-dimensional spheroid culture promotes odonto/osteoblastic differentiation of dental pulp cells. Arch Oral Biol 2014; 59:310-7. doi: 10.1016/j.archoralbio.2013.12.006 [Crossref] [ Google Scholar]
- Yamaguchi Y, Ohno J, Sato A, Kido H, Fukushima T. Mesenchymal stem cell spheroids exhibit enhanced in vitro and in vivo osteoregenerative potential. BMC Biotechnol 2014; 14:1-10. doi: 10.1186/s12896-014-0105-9 [Crossref] [ Google Scholar]
- Kim S-A, Lee EK, Kuh H-J. Co-culture of 3D tumor spheroids with fibroblasts as a model for epithelial–mesenchymal transition in vitro. Exp Cell Res 2015; 335:187-96. doi: 10.1016/j.yexcr.2015.05.016 [Crossref] [ Google Scholar]
- Wen Z, Liao Q, Hu Y, You L, Zhou L, Zhao Y. A spheroid-based 3-D culture model for pancreatic cancer drug testing, using the acid phosphatase assay. Braz J Med Biol Res 2013; 46:634-42. doi: 10.1590/1414-431X20132647 [Crossref] [ Google Scholar]
- Stock K, Borrink R, Mikesch JH, Hansmeier A, Rehkamper J, Trautmann M. Overexpression and Tyr421-phosphorylation of cortactin is induced by three-dimensional spheroid culturing and contributes to migration and invasion of pancreatic ductal adenocarcinoma (PDAC) cells. Cancer Cell Int 2019; 19:77. doi: 10.1186/s12935-019-0798-x [Crossref] [ Google Scholar]
- Lin RZ, Chang HYJT. Recent advances in three‐dimensional multicellular spheroid culture for biomedical research. Biotechnol J 2008; 3:1172-84. doi: 10.1002/biot.200700228 [Crossref] [ Google Scholar]
- Patel NR, Aryasomayajula B, Abouzeid AH, Torchilin VP. Cancer cell spheroids for screening of chemotherapeutics and drug-delivery systems. Ther Deliv 2015; 6:509-20. doi: 10.4155/tde.15.1 [Crossref] [ Google Scholar]
- Benien P, Swami A. 3D tumor models: history, advances and future perspectives. Future Oncol. 2014; 10: 1311-27. 10.2217/fon.13.274
- Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging-drop method. Tissue Engin. Springer; 2007. p. 141-51. 10.1007/978-1-59745-443-8_8.
- Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp 2011: 51: 2720. 10.3791/2720.
- Leung BM, Lesher-Perez SC, Matsuoka T, Moraes C, Takayama S. Media additives to promote spheroid circularity and compactness in hanging drop platform. Biomater Sci 2015; 3:336-44. doi: 10.1039/c4bm00319e [Crossref] [ Google Scholar]
- Panek M, Grabacka M, Pierzchalska M. The formation of intestinal organoids in a hanging drop culture. Cytotechnology 2018; 70:1085-95. doi: 10.1007/s10616-018-0194-8 [Crossref] [ Google Scholar]
- Noel P, Munoz R, Rogers GW, Neilson A, Von Hoff DD, Han H. Preparation and metabolic assay of 3-dimensional spheroid co-cultures of pancreatic cancer cells and fibroblasts. J Vis Exp 2017; 126:e56081. doi: 10.3791/56081 [Crossref] [ Google Scholar]
- Kim DE, Lee YB, Shim HE, Song JJ, Han JS, Moon KS. Application of hexanoyl glycol chitosan as a non-cell adhesive polymer in three-dimensional cell culture. ACS Omega 2022; 7:18471-80. doi: 10.1021/acsomega.2c00890 [Crossref] [ Google Scholar]
- Yang Z, Zhang Y, Tang T, Zhu Q, Shi W, Yin X. Transcriptome profiling of PANC-1 spheroid cells with pancreatic cancer stem cells properties cultured by a Novel 3D Semi-Solid System. Cell Physiol Biochem 2018; 47:2109-25. doi: 10.1159/000491479 [Crossref] [ Google Scholar]
- Benavente-Babace A. Design, fabrication and optimization of a multifunctional microfluidic platform for single-cell analyses. Mújica, Maite y Pérez, Eva [Tesis doctoral]. Universidad de Navarra; 2014.
- Lee JM, Zhang M, Yeong WY. Characterization and evaluation of 3D printed microfluidic chip for cell processing. Microfluid Nanofluidics 2016; 20:5. doi: 10.1007/s10404-015-1688-8 [Crossref] [ Google Scholar]
- Carrell CS, McCord CP, Wydallis RM, Henry CS. Sealing 3D-printed parts to poly (dimethylsiloxane) for simple fabrication of microfluidic devices. Anal Chim Acta 2020; 1124:78-84. doi: 10.1016/j.aca.2020.05.014 [Crossref] [ Google Scholar]
- Rival A, Jary D, Delattre C, Fouillet Y, Castellan G, Bellemin-Comte A. An EWOD-based microfluidic chip for single-cell isolation, mRNA purification and subsequent multiplex qPCR. Lab on a Chip 2014; 14:3739-49. doi: 10.1039/C4LC00592A [Crossref] [ Google Scholar]
- Xu Y, Qi F, Mao H, Li S, Zhu Y, Gong J. In-situ transfer vat photopolymerization for transparent microfluidic device fabrication. Nat Commun 2022; 13:1-11. doi: 10.1038/s41467-022-28579-z [Crossref] [ Google Scholar]
- Torino S, Corrado B, Iodice M, Coppola G. PDMS-based microfluidic devices for cell culture. Inventions 2018; 3:65. doi: 10.3390/inventions3030065 [Crossref] [ Google Scholar]
- Nunes AS, Barros AS, Costa EC, Moreira AF, Correia IJ. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol Bioeng 2019; 116:206-26. doi: 10.1002/bit.26845 [Crossref] [ Google Scholar]
- Wang L, Yu L, Grist S, Cheung KC, Chen DD. Different in vitro cellular responses to tamoxifen treatment in polydimethylsiloxane-based devices compared to normal cell culture. J Chromatogr B Analyt Technol Biomed Life Sci 2017; 1068:105-11. doi: 10.1016/j.jchromb.2017.09.041 [Crossref] [ Google Scholar]
- Liu J, Zheng H, Dai X, Poh PS, Machens H-G, Schilling AF. Transparent PDMS Bioreactors for the Fabrication and Analysis of Multi-Layer Pre-vascularized Hydrogels Under Continuous Perfusion. Front Bioeng Biotechnol 2020; 8:568934. doi: 10.3389/fbioe.2020.568934 [Crossref] [ Google Scholar]
- Nielsen JB, Hanson RL, Almughamsi HM, Pang C, Fish TR, Woolley AT. Microfluidics: Innovations in materials and their fabrication and functionalization. Anal Chem 2019; 92:150-68. doi: 10.1021/acs.analchem.9b04986 [Crossref] [ Google Scholar]
- Knowlton S, Yu CH, Ersoy F, Emadi S, Khademhosseini A, Tasoglu S. 3D-printed microfluidic chips with patterned, cell-laden hydrogel constructs. Biofabrication 2016; 8:025019. doi: 10.1088/1758-5090/8/2/025019 [Crossref] [ Google Scholar]
- Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RM. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron 2015; 63:218-31. doi: 10.1016/j.bios.2014.07.029 [Crossref] [ Google Scholar]
- Gale BK, Jafek AR, Lambert CJ, Goenner BL, Moghimifam H, Nze UC. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018; 3:60. doi: 10.3390/inventions3030060 [Crossref] [ Google Scholar]
- Waheed S, Cabot JM, Macdonald NP, Lewis T, Guijt RM, Paull B. 3D printed microfluidic devices: enablers and barriers. Lab on a Chip 2016; 16:1993-2013. doi: 10.1039/C6LC00284F [Crossref] [ Google Scholar]
- Khot MI, Levenstein MA, de Boer GN, Armstrong G, Maisey T, Svavarsdottir HS. Characterising a PDMS based 3D cell culturing microfluidic platform for screening chemotherapeutic drug cytotoxic activity. Sci Rep 2020; 10:15915. doi: 10.1038/s41598-020-72952-1 [Crossref] [ Google Scholar]
- Heo YS, Cabrera LM, Song JW, Futai N, Tung Y-C, Smith GD. Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly (dimethylsiloxane) devices. Anal Chem 2007; 79:1126-34. doi: 10.1021/ac061990v [Crossref] [ Google Scholar]
- Chen C, Mehl BT, Munshi AS, Townsend AD, Spence DM, Martin RS. 3D-printed microfluidic devices: fabrication, advantages and limitations—a mini review. Anal Methods 2016; 8:6005-12. doi: 10.1039/C6AY01671E [Crossref] [ Google Scholar]
- Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem 2014; 86:3240-53. doi: 10.1021/ac403397r [Crossref] [ Google Scholar]
- Castiaux AD, Pinger CW, Hayter EA, Bunn ME, Martin RS, Spence DM. PolyJet 3D-printed enclosed microfluidic channels without photocurable supports. Anal Chem 2019; 91:6910-7. doi: 10.1021/acs.analchem.9b01302 [Crossref] [ Google Scholar]
- Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 2014; 12:207-18. doi: 10.1089/adt.2014.573 [Crossref] [ Google Scholar]
- Fang Y, Eglen RM. Three-dimensional cell cultures in drug discovery and development. SLAS Discov 2017; 22:456-72. doi: 10.1177/1087057117696795 [Crossref] [ Google Scholar]
- Bianco M, Zizzari A, Priore P, Moroni L, Metrangolo P, Frigione M. Lab-on-a-brane for spheroid formation. Biofabrication 2019; 11:021002. doi: 10.1088/1758-5090/ab0813 [Crossref] [ Google Scholar]
- Lopa S, Piraino F, Kemp RJ, Di Caro C, Lovati AB, Di Giancamillo A. Fabrication of multi‐well chips for spheroid cultures and implantable constructs through rapid prototyping techniques. Biotechnol Bioeng 2015; 112:1457-71. doi: 10.1002/bit.25557 [Crossref] [ Google Scholar]
- Ben-Yoav H, Melamed S, Freeman A, Shacham-Diamand Y, Belkin S. Whole-cell biochips for bio-sensing: integration of live cells and inanimate surfaces. Crit Rev Biotechnol 2011; 31:337-53. doi: 10.3109/07388551.2010.532767 [Crossref] [ Google Scholar]
- Kloß D, Fischer M, Rothermel A, Simon JC, Robitzki AA. Drug testing on 3D in vitro tissues trapped on a microcavity chip. Lab Chip 2008; 8:879-84. doi: 10.1039/B800394G [Crossref] [ Google Scholar]
- Lee DW, Yi SH, Jeong SH, Ku B, Kim J, Lee M-Y. Plastic pillar inserts for three-dimensional (3D) cell cultures in 96-well plates. Sensors 2013; 177:78-85. doi: 10.1016/j.snb.2012.10.129 [Crossref] [ Google Scholar]
- Kang J, Lee DW, Hwang HJ, Yeon S-E, Lee M-Y, Kuh H-J. Mini-pillar array for hydrogel-supported 3D culture and high-content histologic analysis of human tumor spheroids. Lab Chip 2016; 16:2265-76. doi: 10.1039/c6lc00526h [Crossref] [ Google Scholar]
- Lee J-H, Kim S-K, Khawar IA, Jeong S-Y, Chung S, Kuh H-JJJoE. Microfluidic co-culture of pancreatic tumor spheroids with stellate cells as a novel 3D model for investigation of stroma-mediated cell motility and drug resistance. J Exp Clin Cancer Res 2018; 37:1-12. doi: 10.1186/s13046-017-0654-6 [Crossref] [ Google Scholar]
- Velliou E, Gupta P, Ricci C, Danti S. Chapter 11 - Biomaterial-based in vitro models for pancreatic cancer. In: Kundu SC, RL Reis, editors. Biomaterials for 3D Tumor Modeling. Elsevier; 2020. p. 235-49. 10.1016/B978-0-12-818128-7.00011-3.
- Quereda V, Hou S, Madoux F, Scampavia L, Spicer TP, Duckett D. A cytotoxic three-dimensional-spheroid, high-throughput assay using patient-derived glioma stem cells. SLAS Discov 2018; 23:842-9. doi: 10.1177/2472555218775055 [Crossref] [ Google Scholar]
- Halfter K, Hoffmann O, Ditsch N, Ahne M, Arnold F, Paepke S. Testing chemotherapy efficacy in HER2 negative breast cancer using patient-derived spheroids. J Transl Med 2016; 14:1-14. doi: 10.1186/s12967-016-0855-3 [Crossref] [ Google Scholar]
- Della Corte CM, Barra G, Ciaramella V, Di Liello R, Vicidomini G, Zappavigna S. Antitumor activity of dual blockade of PD-L1 and MEK in NSCLC patients derived three-dimensional spheroid cultures. J Exp Clin Cancer Res 2019; 38:1-12. doi: 10.1186/s13046-019-1257-1 [Crossref] [ Google Scholar]
- Jeppesen M, Hagel G, Glenthoj A, Vainer B, Ibsen P, Harling H. Short-term spheroid culture of primary colorectal cancer cells as an in vitro model for personalizing cancer medicine. PloS One 2017; 12:e0183074. doi: 10.1371/journal.pone.0183074 [Crossref] [ Google Scholar]
- Linxweiler J, Hammer M, Muhs S, Kohn M, Pryalukhin A, Veith C. Patient-derived, three-dimensional spheroid cultures provide a versatile translational model for the study of organ-confined prostate cancer. J Cancer Res Clin Oncol 2019; 145:551-9. doi: 10.1007/s00432-018-2803-5 [Crossref] [ Google Scholar]
- Tomás-Bort E, Kieler M, Sharma S, Candido JB, Loessner D. 3D approaches to model the tumor microenvironment of pancreatic cancer. Theranostics 2020; 10:5074. doi: 10.7150/thno.42441 [Crossref] [ Google Scholar]
- Raghavan S, Mehta P, Ward MR, Bregenzer ME, Fleck EM, Tan L. Personalized medicine–based approach to model patterns of chemoresistance and tumor recurrence using ovarian cancer stem cell spheroids. Clin Cancer Res 2017; 23:6934-45. doi: 10.1158/1078-0432.CCR-17-0133 [Crossref] [ Google Scholar]
- Ryu N-E, Lee S-H, Park H. Spheroid culture system methods and applications for mesenchymal stem cells. Cells 2019; 8:1620. doi: 10.3390/cells8121620 [Crossref] [ Google Scholar]
- Nath S, Devi GR. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacology & therapeutics 2016; 163:94-108. [ Google Scholar]
- Anonymous Anonymous. Organoid model advances pancreatic cancer research. Cancer Discov 2015; 5:218-9. doi: 10.1158/2159-8290.Cd-nb2015-008 [Crossref] [ Google Scholar]
- Riffle S, Hegde RSJJoE, Research CC. Modeling tumor cell adaptations to hypoxia in multicellular tumor spheroids. J Exp Clin Cancer Res 2017; 36:1-10. doi: 10.1186/s13046-017-0570-9 [Crossref] [ Google Scholar]
- Johnson S, Chen H, Lo P-KJB-p. In vitro tumorsphere formation assays. 2013; 3:e325-e.
- Weiswald L-B, Bellet D, Dangles-Marie VJN. Spherical cancer models in tumor biology. 2015; 17:1-15. doi: 10.21769/bioprotoc.325 [Crossref]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 2001; 294:1708-12. doi: 10.1126/science.1064829 [Crossref] [ Google Scholar]
- Gagliano N, Celesti G, Tacchini L, Pluchino S, Sforza C, Rasile M. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma: Characterization in a 3D-cell culture model. World J Gastroenterol 2016; 22:4466. doi: 10.3748/wjg.v22.i18.4466 [Crossref] [ Google Scholar]
- Feng H, Ou B-c, Zhao J-k, Yin S, Lu A-g, Oechsle E. Homogeneous pancreatic cancer spheroids mimic growth pattern of circulating tumor cell clusters and macrometastases: displaying heterogeneity and crater-like structure on inner layer. J Cancer Res Clin Oncol 2017; 143:1771-86. doi: 10.1007/s00432-017-2434-2 [Crossref] [ Google Scholar]
- Asthana A, Kisaalita WS. Microtissue size and hypoxia in HTS with 3D cultures. Drug Discov Today 2012; 17:810-7. doi: 10.1016/j.drudis.2012.03.004 [Crossref] [ Google Scholar]
- Firuzi O, Che PP, El Hassouni B, Buijs M, Coppola S, Löhr M. Role of c-MET inhibitors in overcoming drug resistance in spheroid models of primary human pancreatic cancer and stellate cells. Cancers 2019; 11:638. doi: 10.3390/cancers11050638 [Crossref] [ Google Scholar]
- Froeling FE, Mirza TA, Feakins RM, Seedhar A, Elia G, Hart IR. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, β-catenin, and Ezrin expression in tumor cells. Am J Pathol 2009; 175:636-48. doi: 10.2353/ajpath.2009.090131 [Crossref] [ Google Scholar]
- Seino T, Kawasaki S, Shimokawa M, Tamagawa H, Toshimitsu K, Fujii M, et al. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell 2018; 22: 454-67. e6. 10.1016/j.stem.2017.12.009.
- Kikuta K, Masamune A, Watanabe T, Ariga H, Itoh H, Hamada S. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun 2010; 403:380-4. doi: 10.1016/j.bbrc.2010.11.040 [Crossref] [ Google Scholar]
- Majety M, Pradel LP, Gies M, Ries CHJPo. Fibroblasts influence survival and therapeutic response in a 3D co-culture model. PLoS One 2015; 10:e0127948. doi: 10.1371/journal.pone.0127948 [Crossref] [ Google Scholar]
- Froeling FE, Marshall JF, Kocher HMJJob. Pancreatic cancer organotypic cultures. J Biotech 2010; 148:16-23. doi: 10.1016/j.jbiotec.2010.01.008 [Crossref] [ Google Scholar]
- Yip D, Cho CHJB. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem Biophys Res Commun 2013; 433:327-32. doi: 10.1016/j.bbrc.2013.03.008 [Crossref] [ Google Scholar]
- Rodenhizer D, Gaude E, Cojocari D, Mahadevan R, Frezza C, Wouters BG. A three-dimensional engineered tumour for spatial snapshot analysis of cell metabolism and phenotype in hypoxic gradients. Nat Mater 2016; 15:227-34. doi: 10.1038/nmat4482 [Crossref] [ Google Scholar]
- Beer M, Kuppalu N, Stefanini M, Becker H, Schulz I, Manoli S. A novel microfluidic 3D platform for culturing pancreatic ductal adenocarcinoma cells: comparison with in vitro cultures and in vivo xenografts. Sci Rep 2017; 7:1-12. doi: 10.1038/s41598-017-01256-8 [Crossref] [ Google Scholar]
- Ramappa NK, Arcangeli A, Innocenti G, Chisci L. A system biology approach to study pancreatic ductal adenocarcinoma (PDAC) cells in in vitro culture. Universita degli Studi di Firenze: Florence, Italy; 2017.
- Young EW. Cells, tissues, and organs on chips: challenges and opportunities for the cancer tumor microenvironment. Integr Biol (Camb) 2013; 5:1096-109. doi: 10.1039/c3ib40076j [Crossref] [ Google Scholar]
- Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 2014; 32:760-72. doi: 10.1038/nbt.2989 [Crossref] [ Google Scholar]
- LaBarbera DV, Reid BG, Yoo BH. The multicellular tumor spheroid model for high-throughput cancer drug discovery. Expert Opin Drug Discov 2012; 7:819-30. doi: 10.1517/17460441.2012.708334 [Crossref] [ Google Scholar]
- Verbridge SS, Chakrabarti A, DelNero P, Kwee B, Varner JD, Stroock AD. Physicochemical regulation of endothelial sprouting in a 3D microfluidic angiogenesis model. J Biomed Mater Res A 2013; 101:2948-56. doi: 10.1002/jbm.a.34587 [Crossref] [ Google Scholar]
- Okuyama T, Yamazoe H, Mochizuki N, Khademhosseini A, Suzuki H, Fukuda J. Preparation of arrays of cell spheroids and spheroid-monolayer cocultures within a microfluidic device. J Biosci Bioeng 2010; 110:572-6. doi: 10.1016/j.jbiosc.2010.05.013 [Crossref] [ Google Scholar]
- Hardelauf H, Frimat J-P, Stewart JD, Schormann W, Chiang Y-Y, Lampen P. Microarrays for the scalable production of metabolically relevant tumour spheroids: a tool for modulating chemosensitivity traits. Lab Chip 2011; 11:419-28. doi: 10.1039/C0LC00089B [Crossref] [ Google Scholar]
- Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 2013; 18:240-9. doi: 10.1016/j.drudis.2012.10.003 [Crossref] [ Google Scholar]
- Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release 2012; 164:192-204. doi: 10.1016/j.jconrel.2012.04.045 [Crossref] [ Google Scholar]
- Kang A, Seo HI, Chung BG, Lee S-H. Concave microwell array-mediated three-dimensional tumor model for screening anticancer drug-loaded nanoparticles. Nanomedicine: NBM 2015; 11:1153-61. doi: 10.1016/j.nano.2015.02.009 [Crossref] [ Google Scholar]
- Liu T, Kempson I, de Jonge M, Howard DL, Thierry B. Quantitative synchrotron X-ray fluorescence study of the penetration of transferrin-conjugated gold nanoparticles inside model tumour tissues. Nanoscale 2014; 6:9774-82. doi: 10.1039/C4NR02100B [Crossref] [ Google Scholar]
- Ohta S, Hiramoto S, Amano Y, Sato M, Suzuki Y, Shinohara M. Production of Cisplatin-incorporating hyaluronan nanogels via chelating ligand–metal coordination. Bioconjugate chemistry 2016; 27:504-8. doi: 10.1021/acs.bioconjchem.5b00674 [Crossref] [ Google Scholar]
- Gaspar VM, Costa EC, Queiroz JA, Pichon C, Sousa F, Correia IJ. Folate-targeted multifunctional amino acid-chitosan nanoparticles for improved cancer therapy. Pharm Res 2015; 32:562-77. doi: 10.1007/s11095-014-1486-0 [Crossref] [ Google Scholar]
- Anajafi T, Scott MD, You S, Yang X, Choi Y, Qian SY. Acridine orange conjugated polymersomes for simultaneous nuclear delivery of gemcitabine and doxorubicin to pancreatic cancer cells. Bioconjug Chem 2016; 27:762-71. doi: 10.1021/acs.bioconjchem.5b00694 [Crossref] [ Google Scholar]
- Morimoto Y, Hsiao AY, Takeuchi S. Point-, line-, and plane-shaped cellular constructs for 3D tissue assembly. Adv Drug Deliv Rev 2015; 95:29-39. doi: 10.1016/j.addr.2015.09.003 [Crossref] [ Google Scholar]
- Montanez-Sauri SI, Beebe DJ, Sung KE. Microscale screening systems for 3D cellular microenvironments: platforms, advances, and challenges. Cell Mol Life Sci 2015; 72:237-49. doi: 10.1007/s00018-014-1738-5 [Crossref] [ Google Scholar]
- Hakobyan D, Médina C, Dusserre N, Stachowicz M-L, Handschin C, Fricain J-C. Laser-assisted 3D bioprinting of exocrine pancreas spheroid models for cancer initiation study. Biofabrication 2020; 12:035001. doi: 10.1088/1758-5090/ab7cb8 [Crossref] [ Google Scholar]
- Monteiro MV, Gaspar VM, Mendes L, Duarte IF, Mano JF. Stratified 3D microtumors as organotypic testing platforms for screening pancreatic cancer therapies. Small Methods 2021; 5:e2001207. doi: 10.1002/smtd.202001207 [Crossref] [ Google Scholar]
- Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn 2018; 4:27-40. doi: 10.1136/bmjstel-2017-000234 [Crossref] [ Google Scholar]
- Jang J, Yi H-G, Cho D-W. 3D printed tissue models: present and future. ACS Biomater Sci Eng 2016; 2:1722-31. doi: 10.1021/acsbiomaterials.6b00129 [Crossref] [ Google Scholar]
- Griffith CK, Miller C, Sainson RC, Calvert JW, Jeon NL, Hughes CC. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng 2005; 11:257-66. doi: 10.1089/ten.2005.11.257 [Crossref] [ Google Scholar]
- Achilli T-M, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther 2012; 12:1347-60. doi: 10.1517/14712598.2012.707181 [Crossref] [ Google Scholar]
- Bracci R, Maccaroni E, Cascinu S. Bioresorbable airway splint created with a three-dimensional printer. New Eng J Med 2013; 368:2043-5. doi: 10.1056/NEJMc1206319 [Crossref] [ Google Scholar]
- Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol 2003; 21:157-61. doi: 10.1016/S0167-7799(03)00033-7 [Crossref] [ Google Scholar]
- Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJ. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng 2007; 13:1905-25. doi: 10.1089/ten.2006.0175 [Crossref] [ Google Scholar]
- Kyle S, Jessop ZM, Al‐Sabah A, Whitaker IS. ‘Printability' of candidate biomaterials for extrusion based 3D printing: state‐of‐the‐art. Adv Healthc Mater 2017; 6:1700264. doi: 10.1002/adhm.201700264 [Crossref] [ Google Scholar]
- Catros S, Fricain J-C, Guillotin B, Pippenger B, Bareille R, Remy M. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication 2011; 3:025001. doi: 10.1088/1758-5082/3/2/025001 [Crossref] [ Google Scholar]
- Guillotin B, Guillemot F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol 2011; 29:183-90. doi: 10.1016/j.tibtech.2010.12.008 [Crossref] [ Google Scholar]
- Chrisey DB. The power of direct writing. Science 2000; 289:879-81. doi: 10.1126/science.289.5481.879 [Crossref] [ Google Scholar]
- Colina M, Serra P, Fernández-Pradas JM, Sevilla L, Morenza JL. DNA deposition through laser induced forward transfer. Biosens Bioelectron 2005; 20:1638-42. doi: 10.1016/j.bios.2004.08.047 [Crossref] [ Google Scholar]
- Dinca V, Kasotakis E, Catherine J, Mourka A, Ranella A, Ovsianikov A. Directed three-dimensional patterning of self-assembled peptide fibrils. Nano Lett 2008; 8:538-43. doi: 10.1021/nl072798r [Crossref] [ Google Scholar]
- Moroni L, Boland T, Burdick JA, De Maria C, Derby B, Forgacs G. Biofabrication: a guide to technology and terminology. Trends Biotechnol 2018; 36:384-402. doi: 10.1016/j.tibtech.2017.10.015 [Crossref] [ Google Scholar]
- Zhang YS, Duchamp M, Oklu R, Ellisen LW, Langer R, Khademhosseini A. Bioprinting the cancer microenvironment. ACS Biomater Sci Eng 2016; 2:1710-21. doi: 10.1021/acsbiomaterials.6b00246 [Crossref] [ Google Scholar]
- Belgodere JA, King CT, Bursavich JB, Burow ME, Martin EC, Jung JPJFib. Engineering breast cancer microenvironments and 3D bioprinting. Front Bioeng Biotechnol 2018; 6:66. doi: 10.3389/fbioe.2018.00066 [Crossref] [ Google Scholar]
- Swaminathan S, Hamid Q, Sun W, Clyne AM. Bioprinting of 3D breast epithelial spheroids for human cancer models. Biofabrication 2019; 11:025003. doi: 10.1088/1758-5090/aafc49 [Crossref] [ Google Scholar]
- Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen J. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat Mater 2012; 11:734-41. doi: 10.1038/s41563-021-01032-0 [Crossref] [ Google Scholar]
- Ngo MT, Karvelis E, Harley BA. Multidimensional hydrogel models reveal endothelial network angiocrine signals increase glioblastoma cell number, invasion, and temozolomide resistance. Integr Biol 2020; 12:139-49. doi: 10.1093/intbio/zyaa010 [Crossref] [ Google Scholar]
- Raeber G, Lutolf M, Hubbell J. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J 2005; 89:1374-88. doi: 10.1529/biophysj.104.050682 [Crossref] [ Google Scholar]
- Kaemmerer E, Melchels FP, Holzapfel BM, Meckel T, Hutmacher DW, Loessner D. Gelatine methacrylamide-based hydrogels: an alternative three-dimensional cancer cell culture system. 2014; 10:2551-62. doi: 10.1016/j.actbio.2014.02.035 [Crossref]
- Zhao Y, Yao R, Ouyang L, Ding H, Zhang T, Zhang K. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014; 6:035001. doi: 10.1088/1758-5082/6/3/035001 [Crossref] [ Google Scholar]
- Charbe N, McCarron PA, Tambuwala MM. Three-dimensional bio-printing: a new frontier in oncology research. World J Clin Oncol 2017; 8:21. doi: 10.5306/wjco.v8.i1.21 [Crossref] [ Google Scholar]
- Jackson SJ, Thomas GJ, mechanisms mechanisms. Human tissue models in cancer research: looking beyond the mouse. Dis Model Mech 2017; 10:939-42. doi: 10.1242/dmm.031260 [Crossref] [ Google Scholar]
- Ouyang L, Yao R, Mao S, Chen X, Na J, Sun W. Three-dimensional bioprinting of embryonic stem cells directs highly uniform embryoid body formation. Biofabrication 2015; 7:044101. doi: 10.1088/1758-5090/7/4/044101 [Crossref] [ Google Scholar]