Bioimpacts. 2025;15:30264.
doi: 10.34172/bi.30264
Short Communication
Annexin A1, calreticulin and high mobility group box 1 are elevated in secondary progressive multiple sclerosis: Does immunogenic cell death occur in multiple sclerosis?
Mohammad Saeid Hejazi Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, 2, , * # 
Sevda Jafari Data curation, Investigation, Resources, Validation, Writing – original draft, Writing – review & editing, 3, #
Soheila Montazersaheb Data curation, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing, 1
Ommoleila Molavi Data curation, Resources, Validation, Writing – original draft, Writing – review & editing, 1, 2
Vahid Hosseini Investigation, 1
Mahnaz Talebi Data curation, Investigation, 4
Masoud Nikanfar Data curation, Investigation, 5, *
Author information:
1Molecular Medicine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
3Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Neuroscience Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
5Razi Hospital, Tabriz University of Medical Sciences, Tabriz, Iran
#These authors contributed equally and should be considered co-first authors.
Abstract
Introduction:
Multiple sclerosis (MS) is a chronic neuroinflammatory diseases characterized by demyelination of the nerve fibers. Immunogenic cell death (ICD) is a process, during which damaged and stressed cells release danger-associated molecular patterns (DAMPs) activating immune responses. This study aimed to elucidate the induction of ICD in MS diseases.
Methods:
To achieve this goal, the level of DAMPs including Annexin A1 (ANXA1), calreticulin and HMGB1 was measured in the cerebrospinal fluid (CSF) of a secondary progressive multiple sclerosis (SPMS) patient in comparison to control group.
Results:
Results showed significant upregulation (more than two-fold) of ANXA1, calreticulin (CRT) and HMGB1 in the CSF of the patient.
Conclusion:
Although further studies are suggested in this regard, this data could imply induction of ICD in MS. The proposed ICD might trigger immune response against neural cells resulting in neuroinflammation and demyelination in CNS in MS. Our observation could suggest inclusion of ICD interfering treatments in routine MS therapy.
Keywords: Multiple sclerosis, Immune response, DAMPs, Inflammatory response, ICD
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This work was financially supported by the Molecular Medicine Research center, Tabriz University of Medical Sciences, Tabriz, Iran (Pazhoohan ID: 73602).
Introduction
Multiple sclerosis (MS) is characterized by demyelination of the central nervous system (CNS).1 Although, various environmental and genetic factors are involved in MS onset, the role of Epstein-Barr Virus (EBV) infection is approved as an environmental causal agent triggering the disease.2,3 Approximately 2.5 million people worldwide are affected by this disease, which causes substantial neurological disabilities in young adults.1 It has been shown that infiltration of immune cells from the periphery into the CNS results in localized inflammation, demyelination, and axonal damage. Clinical manifestations of MS include cognitive deficits, sensory abnormalities, paralysis, and ocular symptoms that correlate with relapse and remission of the disease. However, the symptoms vary depending on the affected area within the CNS. The disorder manifests in four distinct phenotypes based on its progression and course: relapsing-remitting MS (RRMS), secondary progressive MS (SPMS), primary progressive MS (PPMS), and progressive-remitting MS (PRMS).4,5
Researchers are currently focused on two paradigms, the "outside-in" and "inside-out" to explore the basic etiology and pathophysiological theory of MS. Each paradigm is studied by distinct experimental animal models. The "outside-in" model suggests that MS originates from peripherally-induced inflammatory and autoimmune attacks targeting damaged myelin. Conversely, the "inside-out" theory proposes that primary cell degeneration, particularly in oligodendrocytes (OLs) within the CNS, elicits subsequent reactive inflammatory/autoimmune reactions against myelin fragments.6-9 In this regard, a concept involving a cell death model and a positive feedback loop driven by damage-associated molecular patterns (DAMPs) has been proposed for chronic inflammatory demyelination. This paradigm aims to align with the "inside-out" theory of MS.
Immunogenic cell death (ICD) is a process by which dying cells release danger signals that can activate both innate and adaptive immune responses. ICD occurs in response to infection following the release of pathogen-associated molecular patterns (PAMPs) and also happens in a sterile environment next to the release of danger-associated molecular patterns (DAMPs). DAMPs are endogenous molecules considered as danger signals capable of eliciting the innate and adaptive immune responses, thereby enhancing overt autoimmune disorders. Therefore, ICD is considered not only as a defense against infection, but also a mechanism for clearing damaged and stressed cells.10-13 ICD is a subset of regulated cell death (RCD) triggering immune responses.11,13 Various types of cell death such as apoptosis, pyroptosis, autophagy-dependent cell death, necroptosis and ferroptosis have been identified as significant contributors to the development of neuroinflammatory and neurodegenerative disorders in the CNS.14 Environmental factors such as EBV infections, smoking and UVR involved in 70% of all autoimmune diseases have been shown to promote forms of cell death leading to the induction of DAMPs.13,15 One instance involves microglia activation triggered by DAMPs like extracellular ATP, which have been observed to intensify neuroinflammation in conditions like Alzheimer's disease.16,17 Additionally, it is shown that heat shock protein 70 (HSP70) is upregulated in MS.18 It is shown that high mobility group box 1 (HMGB1) released from astrocytes promotes experimental autoimmune encephalomyelitis (EAE) onset, and increases the disease score and demyelination.19 Compelling evidence suggests that oxidative stress results in the release of specific DAMPs, such as calreticulin (CRT), and cell death leading to neurodegeneration in MS.20,21 To date, there have been no direct reports regarding ICD induction in the CNS of individuals diagnosed with MS.
We hypothesized there are various reasons for the incidence of ICD in MS, including EBV infection. EBV infection is approved as a main causal agent of MS.3 Taking into account the elevated levels of EBV miRNAs found in the CSF exosomes of RRMS patients, implying variation in the virus activity in RRMS22,23 and considering that infection could trigger ICD,10 in the present case study we aimed to answer this question: Does ICD happen in MS patients? In order to answer the question, we measured the level of DAMPs, including CRT, ANXA1 and HMGB1 in the cerebrospinal fluid (CSF) of the SPMS patient compared to the control group.
Materials and Methods
Participants and human ethics
All participants underwent examination at Razi Hospital in 2023. The secondary progressive multiple sclerosis (SPMS) patient met the McDonald’s criteria24 and the diagnosis was fulfilled in agreement with the recent diagnostic criteria. While the clinical differentiation between RRMS and progressive forms remains challenging, the revised “Lublin Criteria were used to distinguish these phenotypes.25 The case study consisted a total of four individuals: one with SPMS, and three with idiopathic intracranial hypertension (IIH), who served as the control group. The patient was 52-year-old female which was diagnosed as SPMS. Her Expanded Disability Status Scale (EDSS) was 8. This patient had been administered 2 vials Zytax for 6 months and Captopril 25 bid. The relevant clinical details and demographic information of the participants are presented in Table 1. The current study was approved by the ethics committee of Tabriz University of Medical Sciences with IR.TBZMED.REC.1402.348 number. Written informed consent was obtained from the SPMS patient. The IIH samples were collected from the hospital, where the IIH patients were lumber punctured as a routine treatment.
Table 1.
List of the participants enrolled in this study (n = 4)
|
Num
|
Gender
|
Age
|
Inclusion criteria
|
Disease
|
EDS*
|
Treatment and dosing
|
Start of
treatment
|
| I |
F |
26 |
Headache, Nausea, Vomiting, Diplopia |
IIH |
- |
Acetazolamide 250 mg tds, Topiramate 50 bid, Baclophen 10 bid |
2023 |
| II |
F |
46 |
Headache, Nausea, Vomiting, Diplopia |
IIH |
- |
Acetazolamide 250 bid, Propranolol 10 bid |
2023 |
| III |
F |
42 |
Headache, Nausea, Vomiting, Diplopia |
IIH |
- |
Acetazolamide 250 tid,
Topiramate 50 bid,
Acetaminophen 500 tid, prn (if required) |
2019 |
| IV |
F |
52 |
Quadriplegia Lower limbs 1/5
Upper limbs 4/5 |
SPMS |
8 |
Zytax 6 months 2 vials, Captopril 25 bid
|
2015 |
EDSS: Expanded Disability Status Scale; F: female; IIH: idiopathic intracranial hypertension; SPMS: secondary progressive MS; tds: three times per day; bid: two times per day, prn: as needed.
CSF preparation
CSF samples were collected from IIH individuals and SPMS patient without the need for additional LP. In other words, the remaining CSF samples were collected from the hospital and used in this study. The patient and the healthy control groups were matched in terms of sex and ethnicity.
Determination of the expression level DAMPs
An enzyme-linked immunosorbent assay (ELISA) was used to determine the expression levels of DAMP molecules, namely CRT, ANXA1 and HMGB1.
Human ANXA1 commercial ELISA Kit (Elabscience; E-EL-H5512) was used to determine the level of ANXA1. HMGB1 levels were determined using commercial Novus ELISA kit (cat. No. NBP2-62766). CRT levels were analyzed using Cusabio ELISA kit (Cat. No. CSB-E09787h).
CSF samples were obtained from all participants by non-traumatic lumbar puncture and stored at -80 °C until being used. Before the experiments, CSF samples were centrifuged for 10 minutes at 1500 rpm to separate the cells, and then the kits were used according to Manufacturer protocol.
To analyze HMGB1, ANXA1 and CRT levels in the samples, the micro-ELISA plate pre-coated with an antibody specific to HMGB-1, ANXA1 and CRT were employed. Following addition of the samples in the wells the wells were washed, then, biotin-conjugated antibody against the markers were pipetted into the corresponding wells. After washing the plates, streptavidin-HRP conjugate solution was added to the wells, and proceeded by washing, addition of the substrate to the wells. Finally, intensity of the developed color was measured. The measured optical density (OD) was proportional to the markers amount in the initial step. The concentration of the markers in CFS samples was calculated by comparing the OD of the samples with the standard curve. The DAMPs levels were standardized based on the protein concentration of each sample. All samples were analyzed in triplicate.
Statistics
All values are expressed as the mean ± SD. GraphPad Prism Version 9 (GraphPad Software, San Diego, Calif. USA) was used to analyze data. The data from the two groups was compared by t test. The significance level was set at P ≤ 0.05.
Results and Discussion
In order to elucidate the occurrence of ICD in SPMS, we measured three DAMP markers including CRT, ANXA1 and HMGB1 in CSF of the patient compared to the control group. Differentially elevation of these molecules not only could represent the incidence of ICD, but also could concur as upstream activators of ICD originated adaptive immunity.26 Our results revealed a significant elevation in the level of ANXA1 in the CSF of SPMS patient compared to individuals without MS, as depicted in Fig. 1. The observed increase in ANXA1 levels in the CSF of the patient suggests a potential association between ANXA1 and the pathophysiology of MS. ANXA1 is known for its involvement in ICD processes. It is involved in the functional maturation of antigen-presenting cells (APCs), particularly dendritic cells (DCs) during ICD. Upon release from apoptotic cells, ANXA1 binds to the formyl peptide receptor 1 receptor (FPR1) on APCs, facilitating a stable interaction between the APC and the dying cancer cell. This function of ANXA1 enables the uptake of antigens and their cross-presentation on major histocompatibility complex class I (MHC I), ultimately leading to the activation of immune responses.27-29
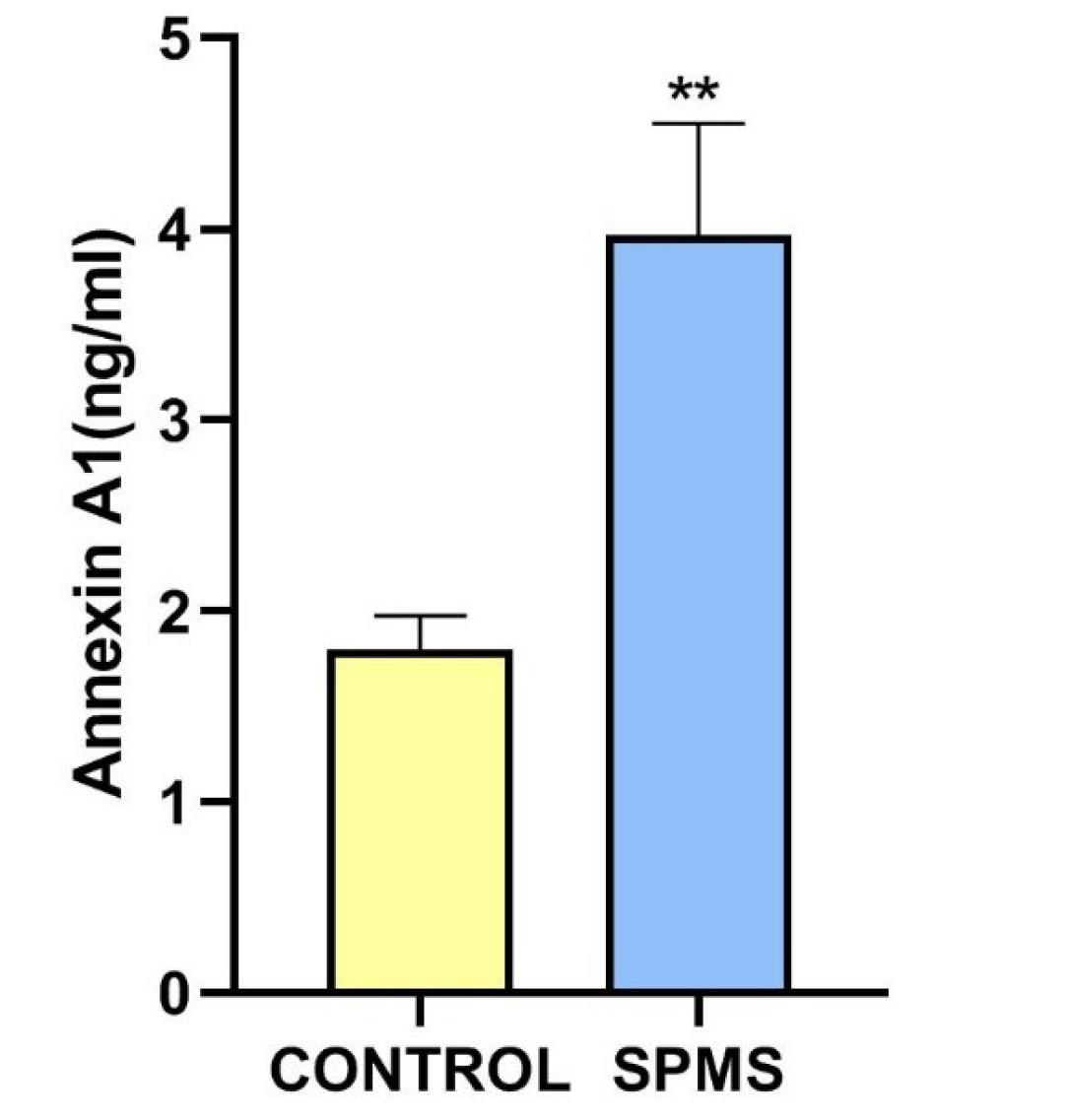
Fig. 1.
ANXA1 level in CSF sample of control group and SPMS patient. Bar graph related to ANXA1 measurement using standard ELISA assay that represents means ± SD measured in each group. A comparison between the results from control group and SPMS patient was done using a t-test. ELISA experiments were repeated at least three times. ** indicates: P < 0.01.
.
ANXA1 level in CSF sample of control group and SPMS patient. Bar graph related to ANXA1 measurement using standard ELISA assay that represents means ± SD measured in each group. A comparison between the results from control group and SPMS patient was done using a t-test. ELISA experiments were repeated at least three times. ** indicates: P < 0.01.
We also identified a significant increase in the level of CRT in the CSF of the patient compared to the levels observed in the control group (Fig. 2). CRT, a chaperone protein located in the endoplasmic reticulum (ER), is crucial for proper protein folding, and disruptions in this process can lead to ER stress, implicated in neurodegenerative disorders like Alzheimer's and Parkinson's diseases.30 CRT is linked to apoptotic pathways and influences cell death in neurodegenerative conditions. Consistent with our findings, externalized CRT signals phagocytic cells to clear damaged cells, contributing to the inflammation observed in various neurological disorders and some autoimmune diseases.20,30-33 CRT, a multifunctional protein, serves as an "eat-me" signal when exposed on dying cells, aiding in phagocytic clearance and providing insights into ICD-related molecular events.27-29 Consistent with our findings, earlier studies have documented an upregulation of molecules associated with the endoplasmic reticulum stress-related signaling pathway, including CRT, in MS lesions.34 As a multifunctional protein involved in various cellular processes, CRT has also been implicated in ICD and immune responses.35 Accordingly, the observed elevated CRT levels may reflect ongoing immunogenic cellular stress or damage associated with neurodegenerative processes in the CNS in MS. Further exploration of the specific implications and mechanisms related to increased CRT levels could deepen our understanding of disease progression and potentially inform targeted therapeutic strategies.
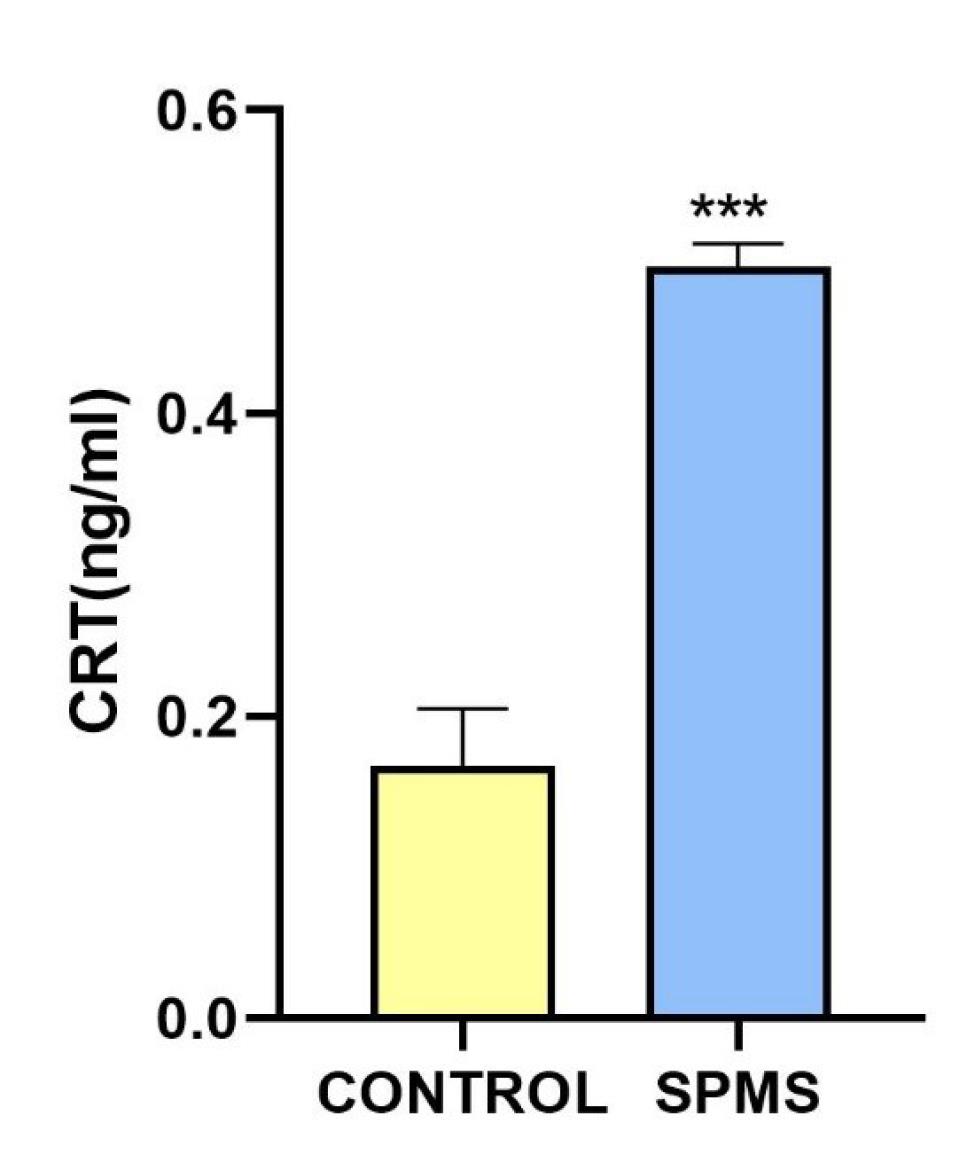
Fig. 2.
CSF concentration of Calreticulin in control group and SPMS patient. Calreticulin levels were measured using ELISA assay. The bar graph indicates the level of calreticulin in healthy controls and SPMS patient. Results showed a statistically significant difference between control group and SPMS patient (P > 0.001). ELISA experiments were repeated at least three times. *** indicates: P < 0.001.
.
CSF concentration of Calreticulin in control group and SPMS patient. Calreticulin levels were measured using ELISA assay. The bar graph indicates the level of calreticulin in healthy controls and SPMS patient. Results showed a statistically significant difference between control group and SPMS patient (P > 0.001). ELISA experiments were repeated at least three times. *** indicates: P < 0.001.
As shown in Fig. 3. the level of HMGB1 was also significantly elevated in the CSF of the SPMS patient compared to the levels observed in the control group. HMGB1 is a protein crucial in regulating inflammation and immune responses, released during cell injury to signal the immune system. It interacts with receptors like Toll-like receptor 4 (TLR4) on DCs resulting in efficient antigen processing and presentation.36 Reduction of HMGB1 and ANXA1 by malignant cells is one of the mechanisms, by which these cells reduce the immune infiltration.37,38 Previous studies have noted the involvement of HMGB1 in neuroinflammation, contributing to immune responses in neurodegenerative diseases, stroke, traumatic brain injury, epilepsy, and neurodegenerative disorders like Alzheimer's, Parkinson's diseases,39-41 and antiphospholipid syndrome.42 Our finding underscores the potential involvement of HMGB1 in the pathogenesis of MS. HMGB1, a nuclear protein with diverse functions, is known for its role in inflammatory responses and cell death processes.43,44 The observed heightened levels of HMGB1 in CSF of the SPMS patient may reflect ongoing cellular stress and inflammation in the CNS, contributing to the neuroinflammatory milieu characteristic of MS. Further investigation into the specific mechanisms and consequences of elevated HMGB1 levels could enhance our understanding of disease dynamics and possibly unveil novel therapeutic targets.
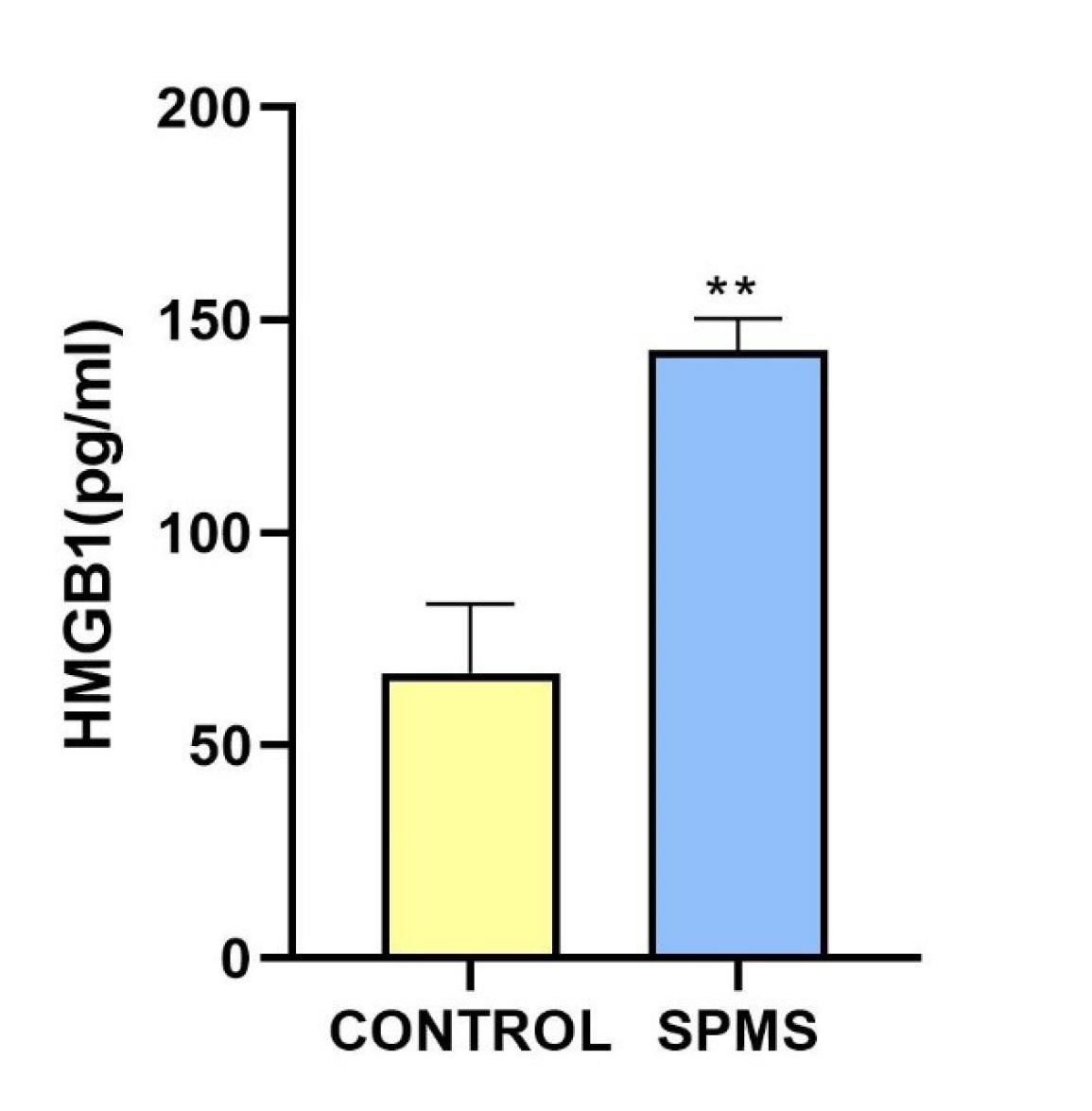
Fig. 3.
ELISA measurement of HMGB1 levels in CSF of control group and SPMS patient.ELISA assay measured HMGB1 concentrations in CSF samples of control group and SPMS patient. Each sample was analyzed in triplicate. Data were reported as mean ± SD. Significant differences between groups are indicated (**: shows values significantly different from the control group by Student’s t-test, P < 0.01).
.
ELISA measurement of HMGB1 levels in CSF of control group and SPMS patient.ELISA assay measured HMGB1 concentrations in CSF samples of control group and SPMS patient. Each sample was analyzed in triplicate. Data were reported as mean ± SD. Significant differences between groups are indicated (**: shows values significantly different from the control group by Student’s t-test, P < 0.01).
Conclusion
In conclusion, this study provides valuable insights into the complex interplay between ICD and MS. Our investigation of ICD biomarkers, particularly ANXA1, CRT, and HMGB1, in the CSF of the SPMS patient reveals significant elevations in ANXA1, CRT, and HMGB1 levels compared to those without MS. The heightened levels of these biomarkers suggest their potential roles in the neuroinflammatory responses characteristic of MS. These findings contribute to our understanding of MS pathophysiology and may pave the way for novel diagnostic and therapeutic avenues. Although continued exploration of the specific mechanisms underlying ICD biomarkers’ variations is crucial for advancing our comprehension of the disease dynamics and identifying potential targets for more targeted therapeutic interventions in the realm of MS. Meanwhile, the elevated levels of DAMPs could imply ICD occurrence leading to adaptive autoimmunity in MS. Therefore, prescribing cell-damage and cell-stress-interfering compounds in this disease is suggested.
Research Highlights
What is the current knowledge?
√ DAMPs are considered as danger signals that elicit the innate and adaptive immune responses to enhance overt autoimmune disorder
What is new here?
√ The levels of ANXA1, CRT, and HMGB1, as DAMPs, significantly elevated in CSF sample of SPMS patient compared to those without MS.
√ The study provides valuable insights into the complex interplay between immunogenic cell death and multiple sclerosis.
Competing Interests
The authors declare that there are no conflicts of interest associated with this study.
Ethical Statement
Ethical consent was approved by an ethics committee at Tabriz University of Medical Sciences, Tabriz, Iran. Ethic Code No: IR.TBZMED.REC.1403.218.
Acknowledgements
The authors acknowledge personnel of Women Ward, Razi Hospital, Tabriz, Iran. The authors also acknowledge Mr Tabrizi and Clinical Laboratory of Nomouneh, Tabriz, Iran for providing MS samples data. Unfortunately, these data were not match with this study and were not included in this paper.
References
- Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol 2019; 26:27-40. doi: 10.1111/ene.13819 [Crossref] [ Google Scholar]
- Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022; 375:296-301. doi: 10.1126/science.abj8222 [Crossref] [ Google Scholar]
- Robinson WH, Steinman L. Epstein-Barr virus and multiple sclerosis. Science 2022; 375:264-5. doi: 10.1126/science.abm7930 [Crossref] [ Google Scholar]
- Goldenberg MM. Multiple sclerosis review. P T 2012; 37:175-84. [ Google Scholar]
- Katz Sand I. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr Opin Neurol 2015; 28:193-205. doi: 10.1097/wco.0000000000000206 [Crossref] [ Google Scholar]
- Sen MK, Almuslehi MSM, Shortland PJ, Coorssen JR, Mahns DA. Revisiting the pathoetiology of multiple sclerosis: has the tail been wagging the mouse?. Front Immunol 2020; 11:572186. doi: 10.3389/fimmu.2020.572186 [Crossref] [ Google Scholar]
- Titus HE, Chen Y, Podojil JR, Robinson AP, Balabanov R, Popko B. Pre-clinical and clinical implications of "inside-out" vs "outside-in" paradigms in multiple sclerosis etiopathogenesis. Front Cell Neurosci 2020; 14:599717. doi: 10.3389/fncel.2020.599717 [Crossref] [ Google Scholar]
- Zirngibl M, Assinck P, Sizov A, Caprariello AV, Plemel JR. Oligodendrocyte death and myelin loss in the cuprizone model: an updated overview of the intrinsic and extrinsic causes of cuprizone demyelination. Mol Neurodegener 2022; 17:34. doi: 10.1186/s13024-022-00538-8 [Crossref] [ Google Scholar]
- Land WG. Role of DAMPs and cell death in autoimmune diseases: the example of multiple sclerosis. Genes Immun 2023; 24:57-70. doi: 10.1038/s41435-023-00198-8 [Crossref] [ Google Scholar]
- Legrand AJ, Konstantinou M, Goode EF, Meier P. The diversification of cell death and immunity: memento mori. Mol Cell 2019; 76:232-42. doi: 10.1016/j.molcel.2019.09.006 [Crossref] [ Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 2018; 25:486-541. doi: 10.1038/s41418-017-0012-4 [Crossref] [ Google Scholar]
- Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol 2022; 23:487-500. doi: 10.1038/s41590-022-01132-2 [Crossref] [ Google Scholar]
- Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res 2019; 29:347-64. doi: 10.1038/s41422-019-0164-5 [Crossref] [ Google Scholar]
- Cui J, Zhao S, Li Y, Zhang D, Wang B, Xie J. Regulated cell death: discovery, features and implications for neurodegenerative diseases. Cell Commun Signal 2021; 19:120. doi: 10.1186/s12964-021-00799-8 [Crossref] [ Google Scholar]
- Linkermann A, Stockwell BR, Krautwald S, Anders HJ. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol 2014; 14:759-67. doi: 10.1038/nri3743 [Crossref] [ Google Scholar]
- Onyango IG, Jauregui GV, Čarná M, Bennett JP Jr, Stokin GB. Neuroinflammation in Alzheimer's disease. Biomedicines 2021; 9:524. doi: 10.3390/biomedicines9050524 [Crossref] [ Google Scholar]
- Lin MM, Liu N, Qin ZH, Wang Y. Mitochondrial-derived damage-associated molecular patterns amplify neuroinflammation in neurodegenerative diseases. Acta Pharmacol Sin 2022; 43:2439-47. doi: 10.1038/s41401-022-00879-6 [Crossref] [ Google Scholar]
- Mansilla MJ, Comabella M, Río J, Castilló J, Castillo M, Martin R. Up-regulation of inducible heat shock protein-70 expression in multiple sclerosis patients. Autoimmunity 2014; 47:127-33. doi: 10.3109/08916934.2013.866104 [Crossref] [ Google Scholar]
- Shi J, Xiao Y, Zhang N, Jiao M, Tang X, Dai C. HMGB1 from astrocytes promotes EAE by influencing the immune cell infiltration-associated functions of BMECs in mice. Neurosci Bull 2022; 38:1303-14. doi: 10.1007/s12264-022-00890-1 [Crossref] [ Google Scholar]
- Ní Fhlathartaigh M, McMahon J, Reynolds R, Connolly D, Higgins E, Counihan T, et al. Calreticulin and other components of endoplasmic reticulum stress in rat and human inflammatory demyelination. Acta Neuropathol Commun 2013. 1: 37. 10.1186/2051-5960-1-37.
- Tobore TO. Oxidative/nitroxidative stress and multiple sclerosis. J Mol Neurosci 2021; 71:506-14. doi: 10.1007/s12031-020-01672-y [Crossref] [ Google Scholar]
- Mohammadinasr M, Montazersaheb S, Hosseini V, Kahroba H, Talebi M, Molavi O. Epstein-Barr virus-encoded BART9 and BART15 miRNAs are elevated in exosomes of cerebrospinal fluid from relapsing-remitting multiple sclerosis patients. Cytokine 2024; 179:156624. doi: 10.1016/j.cyto.2024.156624 [Crossref] [ Google Scholar]
- Mohammadinasr M, Montazersaheb S, Hosseini V, Kahroba H, Talebi M, Molavi O, et al. Epstein-Barr virus-encoded BART9 and BART15 miRNAs are elevated in exosomes of cerebrospinal fluid from relapsing-remitting multiple sclerosis patients. bioRxiv [Preprint]. November 21, 2023. Available from: https://www.biorxiv.org/content/10.1101/2023.11.21.568021v1.
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17:162-73. doi: 10.1016/s1474-4422(17)30470-2 [Crossref] [ Google Scholar]
- Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83:278-86. doi: 10.1212/wnl.0000000000000560 [Crossref] [ Google Scholar]
- Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol 2017; 17:262-75. doi: 10.1038/nri.2017.9 [Crossref] [ Google Scholar]
- Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis 2020; 11:1013. doi: 10.1038/s41419-020-03221-2 [Crossref] [ Google Scholar]
- Cruickshank B, Giacomantonio M, Marcato P, McFarland S, Pol J, Gujar S. Dying to be noticed: epigenetic regulation of immunogenic cell death for cancer immunotherapy. Front Immunol 2018; 9:654. doi: 10.3389/fimmu.2018.00654 [Crossref] [ Google Scholar]
- Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 2015; 350:972-8. doi: 10.1126/science.aad0779 [Crossref] [ Google Scholar]
- Luo X, Weber GA, Zheng J, Gendelman HE, Ikezu T. C1q-calreticulin induced oxidative neurotoxicity: relevance for the neuropathogenesis of Alzheimer's disease. J Neuroimmunol 2003; 135:62-71. doi: 10.1016/s0165-5728(02)00444-7 [Crossref] [ Google Scholar]
- Wang Y, Xie J, Liu Z, Fu H, Huo Q, Gu Y. Association of calreticulin expression with disease activity and organ damage in systemic lupus erythematosus patients. Exp Ther Med 2017; 13:2577-83. doi: 10.3892/etm.2017.4235 [Crossref] [ Google Scholar]
- Ni M, Wei W, Wang Y, Zhang N, Ding H, Shen C. Serum levels of calreticulin in correlation with disease activity in patients with rheumatoid arthritis. J Clin Immunol 2013; 33:947-53. doi: 10.1007/s10875-013-9885-2 [Crossref] [ Google Scholar]
- Taguchi J, Fujii A, Fujino Y, Tsujioka Y, Takahashi M, Tsuboi Y. Different expression of calreticulin and immunoglobulin binding protein in Alzheimer's disease brain. Acta Neuropathol 2000; 100:153-60. doi: 10.1007/s004019900165 [Crossref] [ Google Scholar]
- Mháille AN, McQuaid S, Windebank A, Cunnea P, McMahon J, Samali A. Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. J Neuropathol Exp Neurol 2008; 67:200-11. doi: 10.1097/NEN.0b013e318165b239 [Crossref] [ Google Scholar]
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54-61. doi: 10.1038/nm1523 [Crossref] [ Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050-9. doi: 10.1038/nm1622 [Crossref] [ Google Scholar]
- Ladoire S, Enot D, Senovilla L, Ghiringhelli F, Poirier-Colame V, Chaba K. The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy 2016; 12:864-75. doi: 10.1080/15548627.2016.1154244 [Crossref] [ Google Scholar]
- Baracco EE, Stoll G, Van Endert P, Zitvogel L, Vacchelli E, Kroemer G. Contribution of annexin A1 to anticancer immunosurveillance. Oncoimmunology 2019; 8:e1647760. doi: 10.1080/2162402x.2019.1647760 [Crossref] [ Google Scholar]
- Yang Y, Han C, Guo L, Guan Q. High expression of the HMGB1-TLR4 axis and its downstream signaling factors in patients with Parkinson's disease and the relationship of pathological staging. Brain Behav 2018; 8:e00948. doi: 10.1002/brb3.948 [Crossref] [ Google Scholar]
- Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw 2018; 18:e27. doi: 10.4110/in.2018.18.e27 [Crossref] [ Google Scholar]
- Festoff BW, Sajja RK, van Dreden P, Cucullo L. HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer's disease. J Neuroinflammation 2016; 13:194. doi: 10.1186/s12974-016-0670-z [Crossref] [ Google Scholar]
- Manganelli V, Capozzi A, Truglia S, Alessandri C, Lococo E, Garofalo T. Elevated serum level of HMGB1 in patients with the antiphospholipid syndrome. J Immunol Res 2017; 2017:4570715. doi: 10.1155/2017/4570715 [Crossref] [ Google Scholar]
- Bell CW, Jiang W, Reich CF 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 2006; 291:C1318-25. doi: 10.1152/ajpcell.00616.2005 [Crossref] [ Google Scholar]
- Xue J, Suarez JS, Minaai M, Li S, Gaudino G, Pass HI. HMGB1 as a therapeutic target in disease. J Cell Physiol 2021; 236:3406-19. doi: 10.1002/jcp.30125 [Crossref] [ Google Scholar]