Bioimpacts. 2025;15:30671.
doi: 10.34172/bi.30671
Review Article
Overview of dendritic cells subsets and their involvement in immune-related pathological disease
Mohsen Abbaszadeh Investigation, Writing – original draft, 1 
Bahar Naseri Writing – review & editing, 2
Mohammad Taghizadeh-Teymorloei Investigation, Writing – review & editing, 1 
Amirhossein Mardi Investigation, 2, 3
Mohammad Reza Javan Investigation, 4
Javad Masoumi Conceptualization, Formal analysis, Investigation, Methodology, 2
Farid Ghorbaninezhad Investigation, 5
Amirhossein Hatami‐Sadr Investigation, 2
Şengül Tural Investigation, 6
Behzad Baradaran Conceptualization, Writing – review & editing, 2, *
Mohammad Reza Sadeghi Funding acquisition, Project administration, Supervision, Writing – review & editing, 1, * 
Author information:
1Molecular Medicine Department, Faculty of advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
2Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Immunology, Faculty of Medicine, Zabol University of Medical Sciences, Zabol, Iran
5Department of Immunology, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
6Mayis University, Faculty of Medicine, Department of Medical Biology, Samsun, Turkey
Abstract
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) in linking innate and adaptive immune responses. In addition to presenting antigens to T cells, DCs must also provide co-stimulatory signals along with cytokines for T cells to induce an appropriate cellular immune response. Tolerance is also established and maintained by DCs under homeostatic circumstances. There is remarkable phenotypic heterogeneity in DCs, each with different functional flexibility and specific expression of various markers. The three primary categories of DCs comprise conventional DCs (cDCs), plasmacytoid DCs (pDCs), and monocyte-derived DCs (moDCs). Langerhans cells (LCs) are another type of DCs, which are found in the skin's epidermal layer. DCs may be positioned or triggered inappropriately as a result of dysregulation of DC. This phenomenon can cause an imbalance in immune responses and even immune-related pathological disorders, i.e., autoimmune diseases and malignancies. Herein, by reviewing the ontogeny, biology, characteristics, and function of DCs subsets in immune system, we discuss the contribution of these cells in the mentioned immune-related disorders.
Keywords: Conventional DCs, Plasmacytoid DCs, Monocyte-derived DCs, Langerhans cells, Immune-related disorders
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This work is part of the Ph.D. project and was supported by a grant awarded by the Tabriz University of Medical Sciences (Grant no. 72844).
Introduction
Dendritic cells (DCs), a subset of antigen-presenting cells (APCs), serve as a critical interface between the innate and adaptive immune systems, enabling the initiation and modulation of specific immune responses to antigens.1,2 Additionally, DCs are pivotal in promoting and sustaining self-tolerance.1 These cells are a complex and diverse population, characterized by considerable phenotypic variability and functional adaptability.3 In terms of their development, DCs originate from common myeloid progenitors (CMPs) within the bone marrow. CMPs can follow one of two pathways: with the expression of the Nur77 transcription factor, they differentiate into monocytes, which may then become monocyte-derived DCs (moDCs) during inflammatory conditions.4-7 Conversely, in the absence of Nur77 expression, CMPs give rise to plasmacytoid DCs (pDCs) and conventional DCs (cDCs).5,8,9 A growth factor termed FMS-like tyrosine kinase 3 (FLT3) ligand is often responsible for the differentiation of DCs from their bone marrow precursors.10,11
The FLT3L is a crucial cytokine involved in the differentiation of DCs from bone marrow progenitors. Recent research has provided deeper insights into the mechanisms by which FLT3L influences DC development and the diversification of DC subsets. FLT3L is essential for the expansion of DC progenitors in the bone marrow.12 Studies have shown that FLT3L signaling promotes the proliferation of these progenitors, leading to an increased number of DCs. This is particularly important for maintaining a steady supply of DCs in the body. FLT3L plays a significant role in the differentiation of DC progenitors into various DC subsets, including cDCs and pDCs.13,14 FLT3L signaling is critical for the development of both cDCs and pDCs, with distinct roles for each subset in immune responses. The presence of FLT3L influences the functional properties of DCs, affecting their ability to present antigens and activate T cells. For example, cDCs are efficient in antigen presentation and T cell activation, while pDCs are specialized in producing type I interferons during viral infections. Mutations in the FLT3 gene, such as FLT3-ITD (internal tandem duplication), are associated with certain hematological malignancies, including acute myeloid leukemia (AML).15
DCs can survey their local environment and then convey antigens, activating signals, and cytokines to immune cells, facilitating the activation and coordination of adaptive immune responses.16 In the steady state, DCs are frequently immature and weak APCs, exhibiting characteristics including a high antigen-capture capacity, limited cytokine production, and low co-stimulatory molecule expression.16 The process of DC maturation and activation relies heavily on the interaction between DC receptors and molecular signals, including pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and various cytokines, which collectively facilitate the induction of a robust immune response.17,18 During DC maturation and activation, these cells undergo a significant shift in their molecular expression profile, characteristically featuring increased levels of major histocompatibility complex (MHC) molecules, co-stimulatory molecules (CD80/86), cytokines, chemokines, and C-C chemokine receptor type 7 (CCR7), which collectively enable the DCs to effectively interact with and orchestrate the immune response.19 Following antigen capture, DCs embark on a journey from peripheral tissues to the draining lymph nodes, where they undergo a crucial transformation, presenting intracellular and extracellular antigens to naïve CD8+ and CD4+T cells, respectivel, thereby triggering the activation of distinct effector T cell responses.20,21 Upon activation, T cells exhibit increased expression of CD40 ligand (CD40L), a key molecule that facilitates the maturation of DCs, and hence enhances the immune response.22 The activation of different types of effector cells, including T helper (Th) 1 cells, Th2 cells, Th17 cells, T follicular helper (TFH) cells, regulatory T (Treg) cells, and CD8+ cytotoxic T-lymphocytes (CTLs), is a result of DCs' ability to present diverse antigens.19,23,24 Therefore, DCs have been recognized as pivotal players in the development of antigen-specific immune responses.25 The main subsets of DCs include cDCs, pDCs, and moDCs.25-27 cDCs are further categorized into cDC1 and cDC2 subtypes.25 Each of these DC subsets exhibits unique characteristics and regulatory profiles, characterized by distinct transcriptional factors that will be explored in more depth in their corresponding sections.
The aim of this review is to comprehensively examine the ontogeny, biology, and functional characteristics of DC subsets, including cDCs, pDCs, moDCs), and Langerhans cells (LCs). We seek to elucidate the critical roles these cells play in linking innate and adaptive immune responses, maintaining immune tolerance, and their involvement in immune-related disorders such as autoimmune diseases and malignancies. By synthesizing current knowledge on DC functionality and dysregulation, this review aims to highlight the significance of DCs in immune system dynamics and their potential implications for therapeutic interventions.
DC subsets
cDCs
cDCs have been recognized as potent APCs within the innate immune compartment, playing a crucial role in the initiation of immune responses.28 Despite their short lifespan of approximately 3-6 days, cDCs are continually generated from the bone marrow through the FLT-3-dependent pathway.29 Human cDCs are commonly characterized by the markers CD123-HLADR+ CD11c+, low levels of CD14 and CD16, and are negative for lineage markers including CD3, CD19, CD20, and CD56.30,31 Another defining marker for human cDC populations is CD26.32 Research indicates that significant populations of cDCs in the gut, as well as potentially in other organs, express CD56.32 cDC population is often divided into two distinct subgroups, referred to as cDC1 and cDC2, based on their unique characteristics and functional profiles.
cDC1s
Human cDC1s have been identified in various locations, including the blood, lymphoid tissues, and non-lymphoid tissues.33 These cells are characterized by several markers: DEC-205 (dendritic and epithelial cell-205), CLEC9A (C-type lectin domain family 9 member A), CADM1 (cell adhesion molecule 1), and XCR1 (XC chemokine receptor 1).34,35 Additionally, the blood dendritic cell antigen (BDCA)-3 (CD141) marker is specifically used to distinguish the cDC1 subgroup in humans.36 While migratory cDC1s and those in non-lymphoid tissues express the CD103 marker, cDC1s in lymphoid tissues express CD8α.22 CDC1s contain Toll-like receptors 3 and 9 in their endosomes, which enable the cells to recognize and respond to specific patterns of double-stranded RNA and DNA, respectively.1,37,38 TLR-11, a crucial receptor involved in detecting intracellular pathogens, is also found in cDC1s, highlighting their potential role in recognizing and responding to parasitic infections.39 The development of myeloid cDC1s involves several transcription factors, such as GATA2, PU.1, GFI1, ID2 (inhibitor of DNA binding 2), IRF8 (interferon regulatory factor 8), and BATF3 (basic leucine zipper transcriptional factor ATF-like 3).40-46 The ability of cDC1s to cross-present external antigens to CD8+T cells via MHC-I is well established.36,47 In addition, cDC1s play a role in inducing CD8 + T cell responses by promoting Th1-mediated immunity. Research by Eickhoff et al has shown that cDC1s are essential for enabling CD4 + T cells to collaborate with CD8 + T cells, and their absence can result in impaired CD8 + T cell memory differentiation during viral infections.48 additionally, intracellular parasite infections induce Th1 immunity, with BATF3-dependent CD103 + or CD8+ cDC1s serving as the primary producers of IL-12 during such infections.39,49 Furthermore, TLR-9 agonists have consistently been shown to elicit antigen-specific CD8+T cell responses in humans.50 cDC1s recognize and respond to internalized pathogens by producing IL-12, which triggers a Th1 immune response.51 In cancer immunotherapy, researchers have engineered cDC1s to display tumor-associated antigens (TAAs), aiming to boost the presentation of these antigens to T-cells and stimulate a Th1-mediated immune response.50 In addition to their role in T-cell activation, human cDC1s also play a crucial part in the elimination of skin infections caused by bacteria and fungi by mobilizing neutrophils to the site of infection52,53 (Table 1).
Table 1.
Description of the characteristics of DC subtypes
|
Subset
|
Markers
|
Location
|
Transcription factor
|
Main function
|
| CDC1s |
DEC-205, CLEC9A, CADM1, XCR1, and BDCA-3 |
Blood, lymphoid and non-lymphoid tissues |
GATA2, PU.1, GFI1, ID2, IRF-8, and BATF3 |
Cross-presentation of exogenous antigens to CD8+T cells |
| CDC2s |
CD1c, CD11c, SIRPα, and BDCA-1 |
Blood, lymphoid and non-lymphoid tissues |
IRF-4 and IRF-8 |
Presentation of antigens to CD4+T cells |
| pDCs |
CD123, CD303, CD45RA, CD304, and BDCA-2 |
Lymphoid organs and peripheral blood |
E2.2 |
Anti-viral defense |
| moDCs |
BDCA-1, CD1a, CD11c, CD14, and CD172a |
Inflammatory settings |
IRF-4 |
Production of numerous cytokines,
antigens presentation to CD4+ and CD8+T lymphocytes |
| LCs |
Langerin, CD1a, and EpCAM |
Epidermal layer of the skin |
? |
Potent cross-presenting DC in humans, and presentation of glycolipid antigens to CD8+T cells |
Abbreviations: DCs = dendritic cells, DEC-205 = dendritic and epithelial cell-205, CLEC9A = C-type lectin domain family 9 member A, CADM1 = cell adhesion molecule 1, and XCR1 = XC chemokine receptor 1, BDCA = blood dendritic cell antigen, GATA2 = GATA binding protein 2, ID2 = inhibitor of DNA binding 2, IRF = interferon regulatory factor, BATF3 = basic leucine zipper transcriptional factor ATF-like 3, SIRPα = signal regulatory protein alpha, EpCAM = epithelial cell adhesion molecule.
CDC2s
Similar to cDC1s, cDC2s are present in blood, as well as in lymphoid and non-lymphoid tissues.33 Human cDC2s are characterized by the expression of specific surface markers, including CD1c, CD11c, and signal regulatory protein alpha (SIRPα), which has led to their designation as BDCA-1 DCs or CD1c-positive dendritic cells.37,54,55 In blood or tissues, cDC2s may exhibit low levels of CD14, CD123, and CD26, and they are generally negative for CD209 and the B and T lymphocyte attenuator (BTLA).37,54,55 Depending on their location, cDC2s also express additional markers, such as CD1a in the skin’s dermal layer and CD103 in the gut.56,57 IRF-4 is believed to be the key transcription factor in cDC2 differentiation, although other factors are also necessary.58,59 In human cells, the transcription factor IRF-8 has been found to have a crucial function in the development of cDC2s, influencing their differentiation and maturation process.60 The primary function of these DCs is to process and present antigens to naive CD4 + T cells through the MHC class II pathway, facilitating their activation and subsequent immune response.61 Research has shown that cDC2s can stimulate a wide variety of effector T cell subsets, including Th1, Th2, Th17, Tregs, and TFH cells.62-66 Additionally, cDC2s can activate CD8+T lymphocytes, although they are less efficient at priming these cells.67 It has been observed that type I IFN-responsive cDC2s, also known as ISG + DCs, can stimulate CD8+T lymphocytes by externally presenting tumor-derived MHC class I molecules, a process referred to as "MHC-I dressing".68 Furthermore, cDC2s express various TLRs, including TLR-2, TLR-4, TLR-5, TLR-6, TLR-8, and TLR-9.1 Upon activation by TLR signaling, cDC2s release a broad range of pro-inflammatory cytokines, including TNF-α, IL-1, IL-6, IL-8, IL-12, and IL-18.37 Beyond their conventional functions, cDC2s have also been found to play a role in shaping immune responses in specific tissues, such as inducing Tregs in the intestinal mucosa,56 and promoting tolerance in the liver.69 As cDC2s represent a more heterogeneous population compared to cDC1s, various cDC2 subgroups have been identified in both mice and humans during inflammation or in a steady state. In line with this, research has distinguished two distinct subpopulations within the CD1c+ subgroup: which have been designated as DC2 and DC3.19,37 The CD1c + DC subset has been found to comprise two distinct populations, which can be differentiated by their surface expression patterns, with DC2 and DC3 exhibiting distinct profiles of CD32B and CD163/CD36 molecule expression.19,37 The two CD2c DC subtypes demonstrate distinct capabilities in stimulating naive T cell proliferation, while also exhibiting distinct patterns of cytokine production in response to various TLR agonists.19,37 Other identified subtypes include CD301b+ and CD301b- cDC2s,70,71 Notch2-dependent CX3CR1low Esamhigh cDC2,72 CLEC12A- Esam+ Tbet+ cDC2A,71 CLEC12A+ Esam- Tbet- cDC2B,73 and IRF8+ CD64+ inflammatory cDC2s74 (Table 1).
pDCs
pDCs are typically found in lymphoid organs and can be detected in peripheral blood circulation. Their migration from the bloodstream to lymph nodes, facilitated by high endothelial venules, is influenced by chemokine receptors such as CCR7,75 ChemR23,76 and CXCR3,77 as well as adhesion molecules like L-selectin and E-selectin.78 pDCs represent a unique subset of DCs that play a vital role in antiviral immune responses.79 pDCs can be identified by their distinct immunophenotype, which is marked by the lack of CD11c expression and the presence of various surface markers, including CD123, CD303, CD45RA, and CD304.80 Furthermore, pDCs display a unique set of surface markers, including CD4, BDCA-2, HLA-DR, and immunoglobulin-like receptors ILT-3 and ILT-7. Additionally, they possess specific TLRs within their endosomes, namely TLR-7 and TLR-9.81 The transcription factor E2.2 and the FLT-3 ligand are critical for the differentiation of human pDCs.82,83 pDCs are characterized by their capacity to produce copious amounts of IFN-α/β and other pro-inflammatory cytokines, including IL-6 and TNF-alpha, in response to TLR-7/9 activation. This characteristic highlights their key role in antiviral defense.79 Although pDCs display co-stimulatory molecules and MHC-II, they are found to have a restricted ability to engage in antigen presentation.84 Although pDCs can cross-present antigens to activate CD8 + T cells, they are less effective in this function compared to cDCs.85 In the context of cancer, pDCs have a dual role. On one hand, their production of IFN-α has been shown to hinder cancer cell growth, metastasis, and angiogenesis.86 In addition, pDCs have been shown to possess a direct cytotoxic capacity, which involves the production of granzyme B and TNF-related apoptosis-inducing ligand (TRAIL), leading to the elimination of malignant cells.86 The production of type I interferons (primarily IFN-α) by pDCs is crucial for the local activation of CTLs and NK cells, and for supporting the maturation of cDC1s.87,88 In melanoma patients, research has shown that CpG B-type oligodeoxynucleotide (ODN) PF-3512676 can activate specific immune cells in the sentinel lymph nodes through a specific signaling pathway, leading to the production of IFN-α production and and strengthening the body's response against cancer.50 However, pDCs associated with tumors may also contribute to immune suppression and tolerance. For example, Studies have indicated that the binding of LAG-3 to MHC-II on pDCs enhances IL-6 production and reduces IFN-α release through a mechanism that does not involve TLR activation. Additionally, this interaction stimulates CCL2 production in monocytes and facilitates the development of Tregs from allogeneic CD4 + CD25- T cells, contributing to immunosuppression in cancer.89,90 Moreover, pDCs secrete indoleamine 2,3-dioxygenase (IDO), which significantly enhances Treg activation, leading to immune tolerance and allowing tumor cells to evade immune detection91 (Table 1).
moDCs
During inflammatory responses, a distinct subset of dendritic cells, known as moDCs, emerges from the differentiation of monocytes that have migrated into tissues.92 Monocytes can transform into moDCs under both in vitro and in vivo conditions. Research has shown that moDCs are present within the microenvironments of lung, breast, and colorectal cancers.67,93,94 Apart from their role in inflammatory settings, these cells have also been observed in a steady-state within the intestines and in non-diseased lungs.95,96 In the bone marrow, the generation of moDCs is heavily influenced by IRF-4, a transcription factor that is similarly essential for the development of cDC2s.97 In humans, moDCs develop from CD14high monocytes.98,99 Human moDCs express a variety of surface markers, including BDCA-1, CD1a, CD11c, CD14, and CD172a.1 Functionally, these cells are capable of expressing co-stimulatory molecules, producing a range of cytokines, and presenting antigens to CD4+ and CD8+T lymphocytes.100,101 Consequently, moDCs play a crucial role in guiding the differentiation of CD4+ naïve T cells into Th1,102 Th2,103 and Th17 cells,104 as well as in promoting the differentiation of CD8+ naïve T cells into CTLs.105 In vitro, the development of moDCs from monocytes is supported by granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 through an IRF4-dependent pathway.106 Through the development and clinical testing of moDC vaccines, this culture method has been instrumental in shaping the field of moDC-based immunotherapy. Notably, clinical trials have demonstrated the efficacy and benefits of moDC vaccines in treating AML.107 In a recent study, researchers utilized a novel approach to stimulate immune responses in patients who relapsed after autologous hematopoietic stem cell transplantation (HSCT). By using moDCs loaded with tumor lysates from AML patients, the team was able to enhance the ability of autologous T cells to activate DCs and mount an effective immune response108 (Table 1). Recent studies have highlighted the cross-talk between moDCs and pDCs during inflammatory responses, revealing its significant impact on orchestrating immune responses. One study demonstrated that under inflammatory conditions, moDCs and pDCs interact closely to enhance the efficacy of immune responses.109,110 These interactions involve the recognition and response to various DAMPs, which are released during tissue damage or infection. moDCs play a crucial role in cross-presentation, where they process and present antigens to CD8 + T cells, leading to the activation of CTLs.111 This process is essential for initiating strong antiviral and antitumor immune responses. On the other hand, pDCs are specialized in producing large amounts of type I IFNs in response to viral infections. These IFNs have potent antiviral properties and can also modulate the activity of other immune cells, including moDCs. The cross-talk between moDCs and pDCs is facilitated by the exchange of cytokines and other signaling molecules. For example, pDC-derived IFNs can enhance the antigen-presenting capacity of moDCs, leading to more effective T cell activation.110 Additionally, moDCs can influence the function of pDCs by providing co-stimulatory signals and cytokines that promote their maturation and activation. This synergistic interaction ensures a coordinated and robust immune response during inflammation.112
Langerhans cells (LCs)
LCs, a type of dendritic cell, reside in the outer layer of the skin, where they play a crucial role in immune surveillance.113 First identified by Paul Langerhans in the 19th century, these cells are now recognized as part of the DC family.114 LCs are characterized by a high expression of markers such as langerin, CD1a, and epithelial cell adhesion molecule (EpCAM), while exhibiting low levels of CD11c, CD11b, and CD13.115,116 Predominantly located in the epidermis, LCs maintain close interactions with keratinocytes but are also found in stratified epithelia.117 The differentiation of LCs is regulated by transforming growth factor-β (TGF-β), a process that is independent of FLT-3 and FLT-3 ligand-mediated pathways.118 LCs are resistant to radiation and have a lifespan of approximately two months, in contrast to other DCs.118 When skin inflammation occurs, a cascade of cytokines including TNF-α and IL-1β is released, triggering Langerhans cells to break away from their epithelial attachment and migrate through the basement membrane into the lymphatic vessels, ultimately facilitating their entry into the lymphatic system.119,120 Despite their long-standing reputation as the paradigmatic example of myeloid DCs, the distinctive contributions of LCs to immune function have proven surprisingly difficult to fully elucidate. In humans, LCs can develop into highly efficient cross-presenting DCs, producing significant amounts of IL-15, presenting mycobacterial glycolipid antigens, and stimulating CD8+T cells119,120 (Table 1).
DCs and their association with immune-related pathological disorders
DCs play a pivotal role in orchestrating the initiation and modulation of immune responses, functioning as highly specialized APCs that facilitate the interaction between the immune system and foreign antigens.121 DCs are capable of inducing robust immune responses by engaging in a multistep process, which involves the uptake of antigens, processing and presentation of antigenic peptides alongside co-stimulatory signals (CD80 and CD86) and cytokines, ultimately activating both CD4 + and CD8 + T cells.122 In contrast, DCs play a crucial role in promoting self-tolerance by regulating the immune response through mechanisms that include suppressing the activation and proliferation of effector T cells, generating and activating Tregs, and inducing clonal deletion or anergy in autoreactive T cells.121 These are among the characteristic features of tolerogenic DCs. Hence, two states can be imagined for DCs: immunogenic versus tolerogenic. In the context of autoimmune disease, while DCs may contribute to tolerance induction, their potent antigen-presenting capabilities can also inadvertently amplify the activation and differentiation of autoreactive T cells, potentially due to faulty signaling or compromised negative regulation within the DC population.123 Consequently, abnormal immunogenic DCs triggering may contribute to human autoimmune disorders. Conversely, cancer cells employ regulatory mechanisms to subvert the function of DCs, inducing a tolerogenic or defective phenotype that ultimately enables tumors to evade immune surveillance and promote their growth and progression.124 Several mechanisms dysregulate DCs. In this section, the association between dysregulated DCs and immune-related pathological diseases will be discussed.
Autoimmunity
The organization and function of DCs are altered in autoimmune disorders, making them important players in these diseases. Research has demonstrated that autoimmune patients exhibit distinct changes in the distribution of DC subtypes, with a decrease in their presence in peripheral circulation and an increase in their accumulation in affected tissues, compared to healthy individuals.125-127 Elevated migration to the lymphoid organs or inflammatory tissues, as well as reduced generation or release from the bone marrow, are some of the mechanisms that contribute to this phenomenon.125-127 For example, systemic lupus erythematosus (SLE) patients have reduced quantities of pDCs in their blood, however, these cells are mostly prevalent in skin lesions, indicating that pDCs are preferentially attracted to the inflamed skin128,129 via chemerin76 and CCL19130 chemokines. Increased auto-reactive T cell priming and SLE pathogenesis may result from pDC accumulation in lymph node T cell regions as a result of increased CCL19 reactivity.131
Notably, rheumatoid arthritis (RA) patients exhibit altered circulating levels of conventional DCs (cDCs) and pDCs, with lower numbers in peripheral blood and higher numbers in synovial fluid, suggesting a possible shift in DC distribution.127 Myeloid DCs isolated from RA synovial fluid show an active state, with up-regulated expression of co-stimulatory and HLA-DR molecules. In the context of inflammatory processes, these cells activate T cells that recognize self-antigens, leading to immune responses in the affected tissues.132 Due to their capacity to release pro-inflammatory mediators, these myeloid DCs potentially contribute to promoting synovial inflammation.132,133 Because of their capacity to release pro-inflammatory mediators, these myeloid DCs potentially contribute to the promotion of synovial inflammation.131 Moreover, myeloid DCs are more frequently found in the CNS in the multiple sclerosis (MS) early stages, and they are present within demyelinating lesions, indicating their involvement in the activation of autoreactive T lymphocytes to myelin.134 In addition to dysregulation in the organization of DCs, their functions, i.e., phagocytosis and cytokine secretion are also dysregulated in autoimmune diseases. DCs play a role in SLE-associated defects in apoptotic cell clearance. It has been shown that SLE-related genetic alterations increase the exposure of DCs to nuclear autoantigens by impairing immune complex absorption.135-137 Notably, mice that lack the Mer tyrosine kinase gene, which plays a crucial role in the clearance of apoptotic material by macrophages, develop a systemic autoimmune response and produce antibodies against nuclear antigens, underscoring the importance of this gene in maintaining immune tolerance.135 The abnormal exposure to autoantigens caused by a defect in the removal of apoptotic cells may be the cause of SLE. This change in turn causes DCs to continue to be activated, which in turn causes type I interferons to be produced.138 Besides, apoptotic cells that are not immediately cleared generate blebs that contain SLE auto-antigens and cause DC to mature. Such DC can trigger T cells to produce IL-2, IFN-γ, and particularly IL-17, which support autoimmune reactions.139 In murine studies, the deletion of MFGE8 leads to the development of autoimmune symptoms reminiscent of lupus, which is characterized by an accumulation of apoptotic cells and an infiltration of CD8 + T cells at affected sites. This phenomenon may be attributed to an augmentation of cross-presentation by DCs, resulting in amplified CD8 + T-cell activation.140 In addition, alterations in the cytokine secretion pattern of DCs can also occur in autoimmune diseases. In line with this, assessments of myeloid DCs in MS patients' peripheral blood showed that these cells have a greater capacity to release pro-inflammatory cytokines compared with DC from healthy donors.141 These stimulated myeloid DCs can directly differentiate naïve CD4+T cells into effector cells that secret IFN-γ.141 As a result, myeloid DCs in MS patients are vastly immunogenic and associated with the initiation and development of the disease. Furthermore, myeloid DCs present in psoriatic lesions are a significant source of pro-inflammatory cytokines, including TNF-alpha, IL-12, and IL-23, which contribute to the inflammatory response in the affected tissue.142,143 These mediators cause keratinocytes and fibroblasts to release IL-1 and IL-6, which stimulate effector Th1 and Th17 lymphocytes, causing epidermal hyperplasia and the dermal inflammation that is a psoriasis hallmark.144,145 Studies have revealed that plasmacytoid dendritic cells (pDCs) obtained from the peripheral blood of patients with ulcerative colitis and Crohn's disease during active flare-ups demonstrate elevated levels of CD40 and CD86 surface molecules, as well as increased TNF-α production, when compared to pDCs from healthy individuals (Fig. 1).
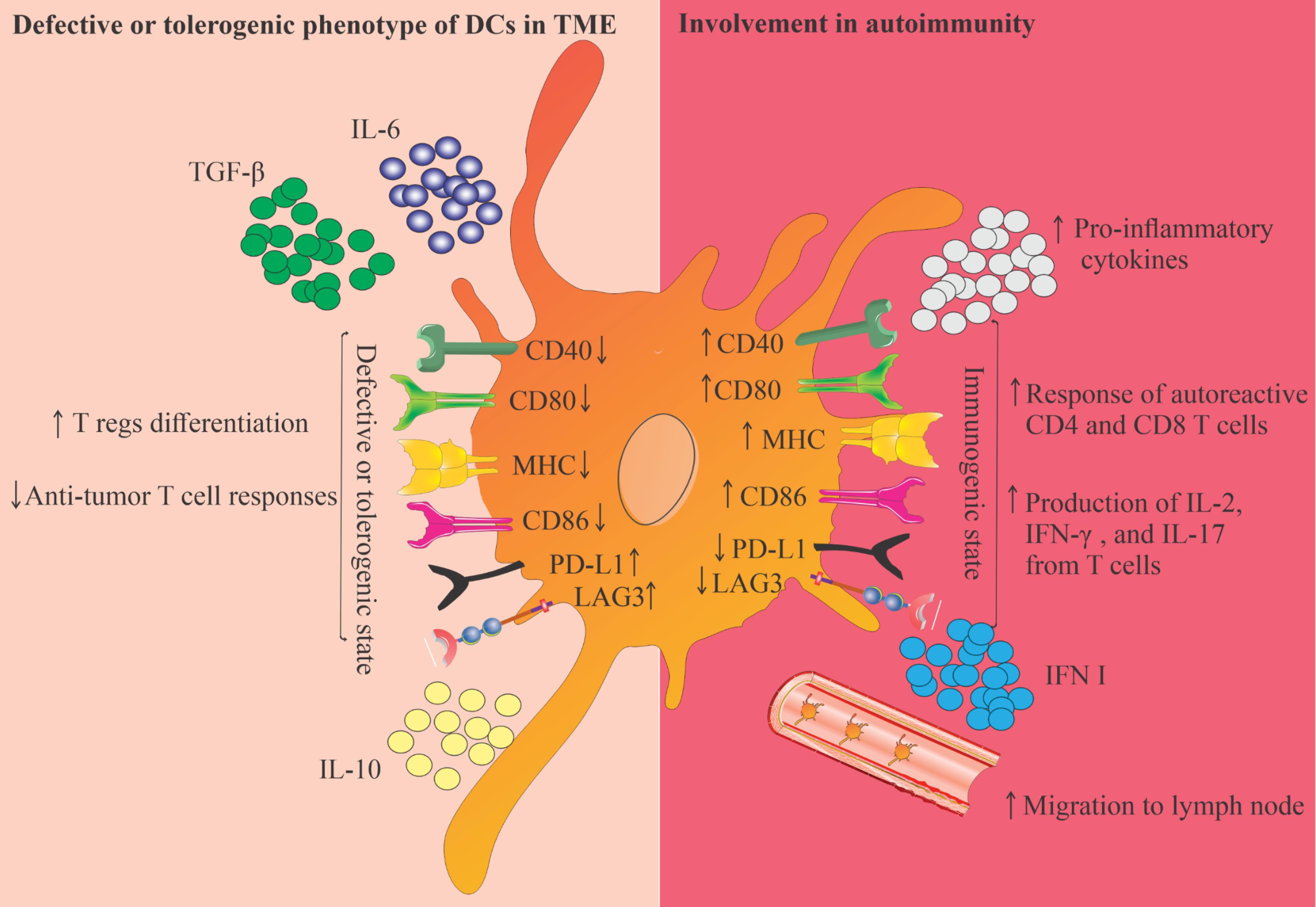
Fig. 1.
This Figure illustrates the dual roles of DCs in the TME and their involvement in autoimmunity. On the left, it highlights the defective or tolerogenic phenotype of DCs, characterized by decreased expression of co-stimulatory molecules (CD40, CD80, MHC, CD86) and increased levels of immunosuppressive factors (TGF-β, IL-6, IL-10). This state promotes regulatory T cell differentiation and inhibits anti-tumor T cell responses. Conversely, the right side depicts the immunogenic state of DCs, which enhances the activation of autoreactive CD4 and CD8 T cells, leading to increased production of pro-inflammatory cytokines and migration to lymph nodes. This figure emphasizes the complex role of DCs in balancing immunity and tolerance within the TME and autoimmune contexts.
.
This Figure illustrates the dual roles of DCs in the TME and their involvement in autoimmunity. On the left, it highlights the defective or tolerogenic phenotype of DCs, characterized by decreased expression of co-stimulatory molecules (CD40, CD80, MHC, CD86) and increased levels of immunosuppressive factors (TGF-β, IL-6, IL-10). This state promotes regulatory T cell differentiation and inhibits anti-tumor T cell responses. Conversely, the right side depicts the immunogenic state of DCs, which enhances the activation of autoreactive CD4 and CD8 T cells, leading to increased production of pro-inflammatory cytokines and migration to lymph nodes. This figure emphasizes the complex role of DCs in balancing immunity and tolerance within the TME and autoimmune contexts.
Cancer
DCs that infiltrate the tumor microenvironment (TME) frequently exhibit defective or tolerogenic characteristics and aid in the development of tumors. These characteristics in the TME are caused by several mechanisms.124 For instance, according to research on pancreatic cancer, DCs that have infiltrated the TME directly interact with Tregs, which causes these DCs to adopt a tolerogenic state. They've also shown that reducing Tregs causes tumor-infiltrating DCs to exhibit an immunogenic phenotype rather than a tolerogenic one, promoting the activation of CTLs.146 They've also shown that reducing Treg causes the tumor-infiltrating DCs to have an immunogenic phenotype rather than a tolerogenic one, which promotes the activation of CTLs.146 Myeloid-derived suppressor cells (MDSCs) have been shown to impair the function of dendritic cells through various mechanisms, including diminishing the ability of DCs to internalize antigens, inhibiting the migration of immature and mature DCs, reducing the capacity of DCs to stimulate T cells to produce IFN-γ, and altering the pattern of cytokine production by DCs.147,148 Studies conducted by Challier et al have found that adenosine and cAMP-mediated signaling pathways can induce human DCs to differentiate into a tolerogenic phenotype, characterized by a reduced ability to stimulate CD8 + T cell priming and activation. Since the TME contains a significant amount of adenosine, this process may contribute to the development of DCs with a tolerogenic phenotype in cancer.149 Research by Bekeredjian-Ding et al has demonstrated that the administration of prostaglandin E2 (PGE2) and transforming growth factor beta (TGF-β) to pDCs induces a tolerogenic phenotype, characterized by a reduction in CD40 expression and an increase in CD86 expression.150 Additionally, IL-10 can produce tolerogenic DCs with limited expression of co-stimulatory and MHC molecules and the capacity to release a significant quantity of IL-10. It has also been demonstrated that TGF-β stimulates the release of immunosuppressive cytokines from DCs, resulting in the generation of tolerogenic DCs that inhibit the growth of anti-tumor T cells and support the differentiation of T cells into Tregs.151 It has also been demonstrated that TGF-β stimulates the release of immunosuppressive cytokines from DCs, which results in the generation of tolerogenic DCs, which inhibit the growth of anti-tumor T cells and support the differentiation of T cells into Tregs.152,153 In cancer patients, it has been demonstrated that functionally deficient DCs are related to excessive IL-6 production.154 Dendritic cells are pivotal in orchestrating immune responses, particularly in the TME. Various inhibitory immune checkpoints, such as PD-1, PD-L1, and LAG3, significantly impact the functional state of DCs. These checkpoints can induce a tolerogenic phenotype in DCs, which supports the growth of malignant cells.155 The interaction between PD-1 on T cells and PD-L1 on DCs inhibits T cell activation and promotes immune tolerance. LAG3 engagement further inhibits DC-mediated T cell activation, enhancing the tolerogenic signals from DCs. Tolerogenic DCs promote the differentiation of Tregs, which suppress anti-tumor immune responses. This leads to a decreased ability of the immune system to recognize and eliminate malignant cells.156 Immunosuppressive cytokines like TGF-β and IL-10 in the TME reinforce the tolerogenic state of DCs. These cytokines contribute to Treg differentiation and inhibit CTL activation. Understanding how inhibitory immune checkpoints induce a tolerogenic phenotype in DCs is crucial for developing effective immunotherapies. Targeting these pathways could enhance the efficacy of treatments by reversing the immunosuppressive TME.157 Various inhibitory immune checkpoints can induce the tolerogenic phenotype in DCs and support the growth of malignant cells17 (Fig. 1). Recent clinical trials targeting DCs for therapeutic purposes have identified several potential side effects and risks, particularly concerning immune overactivation. One of the primary concerns is immune overactivation, where the immune system becomes excessively active, leading to autoimmune responses and inflammation.158 This can result in conditions such as cytokine release syndrome (CRS), where a large number of cytokines are released into the bloodstream, causing fever, fatigue, and severe inflammation. Targeting DCs can sometimes trigger autoimmune reactions, where the immune system attacks healthy tissues and organs. This can lead to a range of autoimmune diseases, depending on the tissues affected. Combining DC-based therapies with other treatments, such as immune checkpoint inhibitors, can enhance the efficacy of the treatment but also increases the risk of adverse effects. These combination therapies can lead to more pronounced immune overactivation and other side effects.159 Patients receiving DC-based therapies may experience infusion-related reactions, such as chills, fever, and allergic reactions. These reactions are typically mild to moderate but can be concerning for some patients. There is a potential risk of long-term immune dysregulation, where the immune system remains in a state of heightened activity even after the treatment has ended. This can lead to chronic inflammation and other immune-related issues. In cancer patients, DC-based therapies can sometimes lead to tumor lysis syndrome, where the rapid destruction of tumor cells releases large amounts of cellular debris into the bloodstream, causing metabolic disturbances. Despite these potential side effects, DC-based therapies hold significant promise for treating various diseases, including cancer. Ongoing research aims to optimize these therapies to minimize risks while maximizing therapeutic benefits.
Other diseases
Dendritic cells play a crucial role in the development and regulation of hypersensitivity reactions, particularly in allergic responses. In allergic reactions, DCs capture and process allergens, presenting them to naïve T cells. This can lead to the differentiation of T cells into Th2 cells, which produce cytokines like IL-4, IL-5, and IL-13. These cytokines promote IgE class switching in B cells, leading to the production of allergen-specific IgE antibodies. DCs also release pro-inflammatory cytokines and chemokines that recruit other immune cells, such as eosinophils and mast cells, exacerbating the allergic response. In terms of hypersensitivity types, type I (Immediate hypersensitivity) involves DCs in the sensitization phase and the subsequent allergic response, while type IV (Delayed-type hypersensitivity) sees DCs presenting antigens to CD4 + T helper cells, leading to a delayed inflammatory response, as seen in contact dermatitis.160,161
Dendritic cells are pivotal in the immune response to various infections, acting as a bridge between innate and adaptive immunity. They express PRRs that detect pathogens through PAMPs.162 This recognition triggers DC activation and maturation. Once activated, DCs migrate to lymph nodes, where they present processed antigens to T cells. This is essential for initiating adaptive immune responses, including the activation of CD4 + helper T cells and CD8 + cytotoxic T cells. DCs produce various cytokines that shape the immune response, such as IL-12, which promotes Th1 responses critical for combating intracellular pathogens, and IL-10, which can have immunosuppressive effects.163
Dendritic cells have a dual role in transplantation, influencing both graft acceptance and rejection. They can present donor antigens to recipient T cells, leading to an immune response against the transplanted tissue, which is a critical factor in acute rejection episodes. Conversely, DCs can also promote tolerance to transplanted organs. Regulatory DCs can induce the differentiation of regulatory Tregs, which help suppress the immune response against the graft. In hematopoietic stem cell transplantation, donor DCs can present recipient antigens to T cells, leading to graft-versus-host disease (GVHD). Understanding the role of DCs in this context is crucial for developing strategies to minimize GVHD while preserving graft-versus-tumor effects.164,165
Conclusion
DCs play a crucial role in the immune system as a type of APC, serving as an indispensable link between the innate and adaptive immune responses. By modulating the balance between immune activation and tolerance, DCs help maintain homeostasis. However, in immune-related disorders, the normal function of DCs is disrupted through various mechanisms, which has been previously described. Understanding the underlying mechanisms that contribute to the dysfunction of DCs in immune-related disorders may provide a promising avenue for therapeutic intervention. By targeting and modulating these mechanisms, it is possible to develop effective treatments that can help restore the normal function of DCs and alleviate the symptoms of these disorders. There is also an alternative perspective on this scenario. In order to treat autoimmune disorders and cancer, it may be beneficial to investigate strategies for suppressing and boosting the immune system, respectively. Since immunogenic DCs are contributed to developing anti-cancer immunity by triggering antigen-specific T cell responses, therapeutic implications of these DCs have received great interest among other cancer immunotherapies. Furthermore, a potential benefit of tolerogenic DCs is their ability to induce tolerance and hence therapeutic applications in autoimmune diseases. Hence, due to the special capacity to either activate or suppress immune responses, both states of DCs have emerged as very potential immunotherapy approaches for immune-related disorders. DCs occupy a complex and nuanced position in the immune system, as they can both contribute to the development of immune-related disorders and be leveraged as therapeutic agents to combat these same diseases. This dual functionality highlights the potential for DCs to be both a driving force behind disease pathology and a valuable tool for treatment.
Review Highlights
What is the current knowledge?
-
DCs link innate and adaptive immune responses.
-
DCs present antigens, provide co-stimulatory signals, and secrete cytokines.
-
DCs show phenotypic heterogeneity and functional flexibility.
-
cDCs, pDCs, and moDCs are main types.
-
Dysregulated DCs cause immune imbalances, autoimmune diseases, and malignancies.
What is new here?
-
Comprehensive review of DC subsets' ontogeny, biology, and function.
-
Analysis of DCs' role in immune-related disorders.
Competing Interests
The authors declare no conflict of interest.
Ethical Approval
All experiments and procedures were performed following the ethical principles of Tabriz University of Medical Science, Tabriz, Iran, and were approved by the regional ethical committee for medical research (Ethical code: IR.TBZMED.REC.1403.199).
Acknowledgements
This work is part of the Ph.D. project and was supported by a grant awarded by the Tabriz University of Medical Sciences (Grant no. 72844).
References
- Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology 2018; 154:3-20. doi: 10.1111/imm.12888 [Crossref] [ Google Scholar]
- Del Prete A, Salvi V, Soriani A, Laffranchi M, Sozio F, Bosisio D. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol 2023; 20:432-47. doi: 10.1038/s41423-023-00990-6 [Crossref] [ Google Scholar]
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 2012; 30:1-22. doi: 10.1146/annurev-immunol-100311-102839 [Crossref] [ Google Scholar]
- Domínguez PM, Ardavín C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev 2010; 234:90-104. doi: 10.1111/j.0105-2896.2009.00876.x [Crossref] [ Google Scholar]
- Gardner A, Ruffell B. Dendritic cells and cancer immunity. Trends Immunol 2016; 37:855-65. doi: 10.1016/j.it.2016.09.006 [Crossref] [ Google Scholar]
- León B, Ardavín C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol Cell Biol 2008; 86:320-4. doi: 10.1038/icb.2008.14 [Crossref] [ Google Scholar]
- Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol 2017; 45:43-51. doi: 10.1016/j.coi.2017.01.002 [Crossref] [ Google Scholar]
- Kang SJ, Park KJ, Jin HM, Cho YN, Oh TH, Kim SE. Circulating plasmacytoid and conventional dendritic cells are numerically and functionally deficient in patients with scrub typhus. Front Immunol 2021; 12:700755. doi: 10.3389/fimmu.2021.700755 [Crossref] [ Google Scholar]
- Liu H, Lu Y, Zong J, Zhang B, Li X, Qi H. Engineering dendritic cell biomimetic membrane as a delivery system for tumor targeted therapy. J Nanobiotechnology 2024; 22:663. doi: 10.1186/s12951-024-02913-7 [Crossref] [ Google Scholar]
- Balan S, Saxena M, Bhardwaj N. Dendritic cell subsets and locations. In: Lhuillier C, Galluzzi L, eds. International Review of Cell and Molecular Biology. Academic Press; 2019. p. 1-68. 10.1016/bs.ircmb.2019.07.004.
- Curtin JF, King GD, Barcia C, Liu C, Hubert FX, Guillonneau C. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol 2006; 176:3566-77. doi: 10.4049/jimmunol.176.6.3566 [Crossref] [ Google Scholar]
- Cueto FJ, Sancho D. The Flt3L/Flt3 axis in dendritic cell biology and cancer immunotherapy. Cancers (Basel) 2021; 13:1525. doi: 10.3390/cancers13071525 [Crossref] [ Google Scholar]
- Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3 + lymphoid and myeloid-committed progenitors to Flt3 + dendritic cells in vivo. J Exp Med 2003; 198:305-13. doi: 10.1084/jem.20030323 [Crossref] [ Google Scholar]
- Cheng H, Chen W, Lin Y, Zhang J, Song X, Zhang D. Signaling pathways involved in the biological functions of dendritic cells and their implications for disease treatment. Mol Biomed 2023; 4:15. doi: 10.1186/s43556-023-00125-3 [Crossref] [ Google Scholar]
- Régnier P, Vetillard M, Bansard A, Pierre E, Li X, Cagnard N. FLT3L-dependent dendritic cells control tumor immunity by modulating Treg and NK cell homeostasis. Cell Rep Med 2023; 4:101256. doi: 10.1016/j.xcrm.2023.101256 [Crossref] [ Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 2007; 7:19-30. doi: 10.1038/nri1996 [Crossref] [ Google Scholar]
- Ghorbaninezhad F, Asadzadeh Z, Masoumi J, Mokhtarzadeh A, Kazemi T, Aghebati-Maleki L. Dendritic cell-based cancer immunotherapy in the era of immune checkpoint inhibitors: from bench to bedside. Life Sci 2022; 297:120466. doi: 10.1016/j.lfs.2022.120466 [Crossref] [ Google Scholar]
- Alipour S, Kazemi T, Sadeghi MR, Heris JA, Masoumi J, Naseri B. Glyburide-treated human monocyte-derived dendritic cells loaded with insulin represent tolerogenic features with anti-inflammatory responses and modulate autologous T cell responses in vitro. Int Immunopharmacol 2024; 126:111230. doi: 10.1016/j.intimp.2023.111230 [Crossref] [ Google Scholar]
- Patente TA, Pinho MP, Oliveira AA, Evangelista GC, Bergami-Santos PC, Barbuto JA. Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy. Front Immunol 2018; 9:3176. doi: 10.3389/fimmu.2018.03176 [Crossref] [ Google Scholar]
- Colbert JD, Cruz FM, Rock KL. Cross-presentation of exogenous antigens on MHC-I molecules. Curr Opin Immunol 2020; 64:1-8. doi: 10.1016/j.coi.2019.12.005 [Crossref] [ Google Scholar]
- Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 2020; 20:7-24. doi: 10.1038/s41577-019-0210-z [Crossref] [ Google Scholar]
- Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity 2014; 40:642-56. doi: 10.1016/j.immuni.2014.04.016 [Crossref] [ Google Scholar]
- Hilligan KL, Ronchese F. Antigen presentation by dendritic cells and their instruction of CD4 + T helper cell responses. Cell Mol Immunol 2020; 17:587-99. doi: 10.1038/s41423-020-0465-0 [Crossref] [ Google Scholar]
- Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol 2002; 20:621-67. doi: 10.1146/annurev.immunol.20.100301.064828 [Crossref] [ Google Scholar]
- Gerhard GM, Bill R, Messemaker M, Klein AM, Pittet MJ. Tumor-infiltrating dendritic cell states are conserved across solid human cancers. J Exp Med 2021; 218. 10.1084/jem.20200264.
- Anderson DA 3rd, Dutertre CA, Ginhoux F, Murphy KM. Genetic models of human and mouse dendritic cell development and function. Nat Rev Immunol 2021; 21:101-15. doi: 10.1038/s41577-020-00413-x [Crossref] [ Google Scholar]
- Murphy TL, Murphy KM. Dendritic cells in cancer immunology. Cell Mol Immunol 2022; 19:3-13. doi: 10.1038/s41423-021-00741-5 [Crossref] [ Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013; 31:563-604. doi: 10.1146/annurev-immunol-020711-074950 [Crossref] [ Google Scholar]
- McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E. Mice lacking Flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000; 95:3489-97. [ Google Scholar]
- O'Keeffe M, Mok WH, Radford KJ. Human dendritic cell subsets and function in health and disease. Cell Mol Life Sci 2015; 72:4309-25. doi: 10.1007/s00018-015-2005-0 [Crossref] [ Google Scholar]
- Samadi M, Kamrani A, Nasiri H, Shomali N, Ahmadian J, Shahabi P. Cancer immunotherapy focusing on the role of interleukins; A comprehensive and updated study. Pathol Res Pract 2023; 249:154732. doi: 10.1016/j.prp.2023.154732 [Crossref] [ Google Scholar]
- Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 2016; 45:669-84. doi: 10.1016/j.immuni.2016.08.015 [Crossref] [ Google Scholar]
- Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103 + nonlymphoid dendritic cells. Immunity 2012; 37:60-73. doi: 10.1016/j.immuni.2012.04.012 [Crossref] [ Google Scholar]
- Patente TA, Pinho MP, Oliveira AA, Evangelista GC, Bergami-Santos PC, Barbuto JAM. Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy. Front Immunol 2018; 9:3176. doi: 10.3389/fimmu.2018.03176 [Crossref] [ Google Scholar]
- Villadangos JA, Shortman K. Found in translation: the human equivalent of mouse CD8 + dendritic cells. J Exp Med 2010; 207:1131-4. doi: 10.1084/jem.20100985 [Crossref] [ Google Scholar]
- Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE. Human CD141 + (BDCA-3) + dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 2010; 207:1247-60. doi: 10.1084/jem.20092140 [Crossref] [ Google Scholar]
- Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017; 356:eaah4573. doi: 10.1126/science.aah4573 [Crossref] [ Google Scholar]
- Lafaille FG, Pessach IM, Zhang SY, Ciancanelli MJ, Herman M, Abhyankar A. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 2012; 491:769-73. doi: 10.1038/nature11583 [Crossref] [ Google Scholar]
- Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS. CD8α + dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity 2011; 35:249-59. doi: 10.1016/j.immuni.2011.08.008 [Crossref] [ Google Scholar]
- Collin M, Dickinson R, Bigley V. Haematopoietic and immune defects associated with GATA2 mutation. Br J Haematol 2015; 169:173-87. doi: 10.1111/bjh.13317 [Crossref] [ Google Scholar]
- Onodera K, Fujiwara T, Onishi Y, Itoh-Nakadai A, Okitsu Y, Fukuhara N. GATA2 regulates dendritic cell differentiation. Blood 2016; 128:508-18. doi: 10.1182/blood-2016-02-698118 [Crossref] [ Google Scholar]
- Carotta S, Dakic A, D'Amico A, Pang SH, Greig KT, Nutt SL. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity 2010; 32:628-41. doi: 10.1016/j.immuni.2010.05.005 [Crossref] [ Google Scholar]
- Rathinam C, Geffers R, Yücel R, Buer J, Welte K, Möröy T. The transcriptional repressor Gfi1 controls STAT3-dependent dendritic cell development and function. Immunity 2005; 22:717-28. doi: 10.1016/j.immuni.2005.04.007 [Crossref] [ Google Scholar]
- Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol 2003; 4:380-6. doi: 10.1038/ni903 [Crossref] [ Google Scholar]
- Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J Immunol 2003; 170:1131-5. doi: 10.4049/jimmunol.170.3.1131 [Crossref] [ Google Scholar]
- Grajales-Reyes GE, Iwata A, Albring J, Wu X, Tussiwand R, Kc W. Batf3 maintains autoactivation of Irf8 for commitment of a CD8α + conventional DC clonogenic progenitor. Nat Immunol 2015; 16:708-17. doi: 10.1038/ni.3197 [Crossref] [ Google Scholar]
- Kretzer NM, Theisen DJ, Tussiwand R, Briseño CG, Grajales-Reyes GE, Wu X. RAB43 facilitates cross-presentation of cell-associated antigens by CD8α + dendritic cells. J Exp Med 2016; 213:2871-83. doi: 10.1084/jem.20160597 [Crossref] [ Google Scholar]
- Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell 2015; 162:1322-37. doi: 10.1016/j.cell.2015.08.004 [Crossref] [ Google Scholar]
- Martínez-López M, Iborra S, Conde-Garrosa R, Sancho D. Batf3-dependent CD103 + dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice. Eur J Immunol 2015; 45:119-29. doi: 10.1002/eji.201444651 [Crossref] [ Google Scholar]
- Molenkamp BG, van Leeuwen PA, Meijer S, Sluijter BJ, Wijnands PG, Baars A. Intradermal CpG-B activates both plasmacytoid and myeloid dendritic cells in the sentinel lymph node of melanoma patients. Clin Cancer Res 2007; 13:2961-9. doi: 10.1158/1078-0432.Ccr-07-0050 [Crossref] [ Google Scholar]
- Del Prete A, Sozio F, Barbazza I, Salvi V, Tiberio L, Laffranchi M. Functional role of dendritic cell subsets in cancer progression and clinical implications. Int J Mol Sci 2020; 21:3930. doi: 10.3390/ijms21113930 [Crossref] [ Google Scholar]
- Janela B, Patel AA, Lau MC, Goh CC, Msallam R, Kong WT, et al. A subset of type I conventional dendritic cells controls cutaneous bacterial infections through VEGFα-mediated recruitment of neutrophils. Immunity 2019; 50: 1069-83.e8. 10.1016/j.immuni.2019.03.001.
- Del Fresno C, Saz-Leal P, Enamorado M, Wculek SK, Martínez-Cano S, Blanco-Menéndez N. DNGR-1 in dendritic cells limits tissue damage by dampening neutrophil recruitment. Science 2018; 362:351-6. doi: 10.1126/science.aan8423 [Crossref] [ Google Scholar]
- Heger L, Balk S, Lühr JJ, Heidkamp GF, Lehmann CHK, Hatscher L. CLEC10A is a specific marker for human CD1c + dendritic cells and enhances their toll-like receptor 7/8-induced cytokine secretion. Front Immunol 2018; 9:744. doi: 10.3389/fimmu.2018.00744 [Crossref] [ Google Scholar]
- Shin JS, Greer AM. The role of FcεRI expressed in dendritic cells and monocytes. Cell Mol Life Sci 2015; 72:2349-60. doi: 10.1007/s00018-015-1870-x [Crossref] [ Google Scholar]
- Watchmaker PB, Lahl K, Lee M, Baumjohann D, Morton J, Kim SJ. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat Immunol 2014; 15:98-108. doi: 10.1038/ni.2768 [Crossref] [ Google Scholar]
- Lang M, Krump C, Meshcheryakova A, Tam-Amersdorfer C, Schwarzenberger E, Passegger C. Microenvironmental and cell intrinsic factors governing human cDC2 differentiation and monocyte reprogramming. Front Immunol 2023; 14:1216352. doi: 10.3389/fimmu.2023.1216352 [Crossref] [ Google Scholar]
- Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D. IRF4 transcription factor-dependent CD11b + dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 2013; 38:970-83. doi: 10.1016/j.immuni.2013.04.011 [Crossref] [ Google Scholar]
- Camacho DF, Velez TE, Hollinger MK, Wang E, Howard CL, Darnell EP, et al. IRF4 expression by lung dendritic cells drives acute but not Trm cell-dependent memory Th2 responses. JCI Insight 2022; 7. 10.1172/jci.insight.140384.
- Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med 2011; 365:127-38. doi: 10.1056/NEJMoa1100066 [Crossref] [ Google Scholar]
- Matsuo K, Yoshie O, Kitahata K, Kamei M, Hara Y, Nakayama T. Recent progress in dendritic cell-based cancer immunotherapy. Cancers (Basel) 2021; 13:2495. doi: 10.3390/cancers13102495 [Crossref] [ Google Scholar]
- Tussiwand R, Everts B, Grajales-Reyes GE, Kretzer NM, Iwata A, Bagaitkar J. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 2015; 42:916-28. doi: 10.1016/j.immuni.2015.04.017 [Crossref] [ Google Scholar]
- Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hägerbrand K, Marsal J. IRF4 transcription-factor-dependent CD103 + CD11b + dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 2013; 38:958-69. doi: 10.1016/j.immuni.2013.03.009 [Crossref] [ Google Scholar]
- Shin C, Han JA, Choi B, Cho YK, Do Y, Ryu S. Intrinsic features of the CD8α- dendritic cell subset in inducing functional T follicular helper cells. Immunol Lett 2016; 172:21-8. doi: 10.1016/j.imlet.2016.01.009 [Crossref] [ Google Scholar]
- Sittig SP, Bakdash G, Weiden J, Sköld AE, Tel J, Figdor CG. A comparative study of the T cell stimulatory and polarizing capacity of human primary blood dendritic cell subsets. Mediators Inflamm 2016; 2016:3605643. doi: 10.1155/2016/3605643 [Crossref] [ Google Scholar]
- Nizzoli G, Larghi P, Paroni M, Crosti MC, Moro M, Neddermann P. IL-10 promotes homeostatic proliferation of human CD8 + memory T cells and, when produced by CD1c + DCs, shapes naive CD8 + T-cell priming. Eur J Immunol 2016; 46:1622-32. doi: 10.1002/eji.201546136 [Crossref] [ Google Scholar]
- Laoui D, Keirsse J, Morias Y, Van Overmeire E, Geeraerts X, Elkrim Y. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat Commun 2016; 7:13720. doi: 10.1038/ncomms13720 [Crossref] [ Google Scholar]
- Duong E, Fessenden TB, Lutz E, Dinter T, Yim L, Blatt S, et al. Type I interferon activates MHC class I-dressed CD11b + conventional dendritic cells to promote protective anti-tumor CD8 + T cell immunity. Immunity 2022; 55: 308-23.e9. 10.1016/j.immuni.2021.10.020.
- Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol 2009; 182:1901-11. doi: 10.4049/jimmunol.0803404 [Crossref] [ Google Scholar]
- Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, et al. Unleashing type-2 dendritic cells to drive protective antitumor CD4 + T cell immunity. Cell 2019; 177: 556-71.e16. 10.1016/j.cell.2019.02.005.
- Kumamoto Y, Linehan M, Weinstein JS, Laidlaw BJ, Craft JE, Iwasaki A. CD301b + dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 2013; 39:733-43. doi: 10.1016/j.immuni.2013.08.029 [Crossref] [ Google Scholar]
- Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity 2011; 35:780-91. doi: 10.1016/j.immuni.2011.08.013 [Crossref] [ Google Scholar]
- Brown CC, Gudjonson H, Pritykin Y, Deep D, Lavallée VP, Mendoza A, et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell 2019; 179: 846-63.e24. 10.1016/j.cell.2019.09.035.
- Bosteels C, Neyt K, Vanheerswynghels M, van Helden MJ, Sichien D, Debeuf N, et al. Inflammatory type 2 cDCs acquire features of cDC1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity 2020; 52: 1039-56.e9. 10.1016/j.immuni.2020.04.005.
- Seth S, Oberdörfer L, Hyde R, Hoff K, Thies V, Worbs T. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J Immunol 2011; 186:3364-72. doi: 10.4049/jimmunol.1002598 [Crossref] [ Google Scholar]
- Vermi W, Riboldi E, Wittamer V, Gentili F, Luini W, Marrelli S. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med 2005; 201:509-15. doi: 10.1084/jem.20041310 [Crossref] [ Google Scholar]
- Vanbervliet B, Bendriss-Vermare N, Massacrier C, Homey B, de Bouteiller O, Brière F. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J Exp Med 2003; 198:823-30. doi: 10.1084/jem.20020437 [Crossref] [ Google Scholar]
- Diacovo TG, Blasius AL, Mak TW, Cella M, Colonna M. Adhesive mechanisms governing interferon-producing cell recruitment into lymph nodes. J Exp Med 2005; 202:687-96. doi: 10.1084/jem.20051035 [Crossref] [ Google Scholar]
- Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol 2011; 29:163-83. doi: 10.1146/annurev-immunol-031210-101345 [Crossref] [ Google Scholar]
- Reynolds G, Haniffa M. Human and mouse mononuclear phagocyte networks: a tale of two species?. Front Immunol 2015; 6:330. doi: 10.3389/fimmu.2015.00330 [Crossref] [ Google Scholar]
- Cao W. Molecular characterization of human plasmacytoid dendritic cells. J Clin Immunol 2009; 29:257-64. doi: 10.1007/s10875-009-9284-x [Crossref] [ Google Scholar]
- Reizis B. Plasmacytoid dendritic cells: development, regulation, and function. Immunity 2019; 50:37-50. doi: 10.1016/j.immuni.2018.12.027 [Crossref] [ Google Scholar]
- Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 2015; 15:471-85. doi: 10.1038/nri3865 [Crossref] [ Google Scholar]
- Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 2008; 29:352-61. doi: 10.1016/j.immuni.2008.09.002 [Crossref] [ Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S. Differential antigen processing by dendritic cell subsets in vivo. Science 2007; 315:107-11. doi: 10.1126/science.1136080 [Crossref] [ Google Scholar]
- Caro AA, Deschoemaeker S, Allonsius L, Coosemans A, Laoui D. Dendritic cell vaccines: a promising approach in the fight against ovarian cancer. Cancers (Basel) 2022; 14:4037. doi: 10.3390/cancers14164037 [Crossref] [ Google Scholar]
- Brewitz A, Eickhoff S, Dähling S, Quast T, Bedoui S, Kroczek RA. CD8 + T cells orchestrate pDC-XCR1 + dendritic cell spatial and functional cooperativity to optimize priming. Immunity 2017; 46:205-19. doi: 10.1016/j.immuni.2017.01.003 [Crossref] [ Google Scholar]
- Persson CM, Chambers BJ. Plasmacytoid dendritic cell-induced migration and activation of NK cells in vivo. Eur J Immunol 2010; 40:2155-64. doi: 10.1002/eji.200940098 [Crossref] [ Google Scholar]
- Camisaschi C, De Filippo A, Beretta V, Vergani B, Villa A, Vergani E. Alternative activation of human plasmacytoid DCs in vitro and in melanoma lesions: involvement of LAG-3. J Invest Dermatol 2014; 134:1893-902. doi: 10.1038/jid.2014.29 [Crossref] [ Google Scholar]
- Castelli C, Triebel F, Rivoltini L, Camisaschi C. Lymphocyte activation gene-3 (LAG-3, CD223) in plasmacytoid dendritic cells (pDCs): a molecular target for the restoration of active antitumor immunity. Oncoimmunology 2014; 3:e967146. doi: 10.4161/21624011.2014.967146 [Crossref] [ Google Scholar]
- Gerlini G, Urso C, Mariotti G, Di Gennaro P, Palli D, Brandani P. Plasmacytoid dendritic cells represent a major dendritic cell subset in sentinel lymph nodes of melanoma patients and accumulate in metastatic nodes. Clin Immunol 2007; 125:184-93. doi: 10.1016/j.clim.2007.07.018 [Crossref] [ Google Scholar]
- Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Whynot J, Novitskaya I, Cardinale I. Major differences in inflammatory dendritic cells and their products distinguish atopic dermatitis from psoriasis. J Allergy Clin Immunol 2007; 119:1210-7. doi: 10.1016/j.jaci.2007.03.006 [Crossref] [ Google Scholar]
- Michea P, Noël F, Zakine E, Czerwinska U, Sirven P, Abouzid O. Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat Immunol 2018; 19:885-97. doi: 10.1038/s41590-018-0145-8 [Crossref] [ Google Scholar]
- Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell 2017; 169: 750-65.e17. 10.1016/j.cell.2017.04.014.
- Patel VI, Booth JL, Duggan ES, Cate S, White VL, Hutchings D. Transcriptional classification and functional characterization of human airway macrophage and dendritic cell subsets. J Immunol 2017; 198:1183-201. doi: 10.4049/jimmunol.1600777 [Crossref] [ Google Scholar]
- Richter L, Landsverk OJ, Atlasy N, Bujko A, Yaqub S, Horneland R. Transcriptional profiling reveals monocyte-related macrophages phenotypically resembling DC in human intestine. Mucosal Immunol 2018; 11:1512-23. doi: 10.1038/s41385-018-0060-1 [Crossref] [ Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992; 176:1693-702. doi: 10.1084/jem.176.6.1693 [Crossref] [ Google Scholar]
- León B, Ardavín C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol Cell Biol 2008; 86:320-4. doi: 10.1038/icb.2008.14 [Crossref] [ Google Scholar]
- Domínguez PM, Ardavín C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev 2010; 234:90-104. doi: 10.1111/j.0105-2896.2009.00876.x [Crossref] [ Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994; 179:1109-18. doi: 10.1084/jem.179.4.1109 [Crossref] [ Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003; 19:59-70. doi: 10.1016/s1074-7613(03)00171-7 [Crossref] [ Google Scholar]
- León B, López-Bravo M, Ardavín C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007; 26:519-31. doi: 10.1016/j.immuni.2007.01.017 [Crossref] [ Google Scholar]
- Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W. Conventional and monocyte-derived CD11b + dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013; 38:322-35. doi: 10.1016/j.immuni.2012.10.016 [Crossref] [ Google Scholar]
- Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013; 38:336-48. doi: 10.1016/j.immuni.2012.10.018 [Crossref] [ Google Scholar]
- Shin KS, Jeon I, Kim BS, Kim IK, Park YJ, Koh CH. Monocyte-derived dendritic cells dictate the memory differentiation of CD8 + T cells during acute infection. Front Immunol 2019; 10:1887. doi: 10.3389/fimmu.2019.01887 [Crossref] [ Google Scholar]
- Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c + MHCII + macrophages and dendritic cells. Immunity 2015; 42:1197-211. doi: 10.1016/j.immuni.2015.05.018 [Crossref] [ Google Scholar]
- Anguille S, Willemen Y, Lion E, Smits EL, Berneman ZN. Dendritic cell vaccination in acute myeloid leukemia. Cytotherapy 2012; 14:647-56. doi: 10.3109/14653249.2012.693744 [Crossref] [ Google Scholar]
- Lee JJ, Kook H, Park MS, Nam JH, Choi BH, Song WH. Immunotherapy using autologous monocyte-derived dendritic cells pulsed with leukemic cell lysates for acute myeloid leukemia relapse after autologous peripheral blood stem cell transplantation. J Clin Apher 2004; 19:66-70. doi: 10.1002/jca.10080 [Crossref] [ Google Scholar]
- Chen MY, Zhang F, Goedegebuure SP, Gillanders WE. Dendritic cell subsets and implications for cancer immunotherapy. Front Immunol 2024; 15:1393451. doi: 10.3389/fimmu.2024.1393451 [Crossref] [ Google Scholar]
- Medrano RF, Hunger A, Mendonça SA, Barbuto JA, Strauss BE. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget 2017; 8:71249-84. doi: 10.18632/oncotarget.19531 [Crossref] [ Google Scholar]
- Marciscano AE, Anandasabapathy N. The role of dendritic cells in cancer and anti-tumor immunity. Semin Immunol 2021; 52:101481. doi: 10.1016/j.smim.2021.101481 [Crossref] [ Google Scholar]
- Yu R, Zhu B, Chen D. Type I interferon-mediated tumor immunity and its role in immunotherapy. Cell Mol Life Sci 2022; 79:191. doi: 10.1007/s00018-022-04219-z [Crossref] [ Google Scholar]
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol 2008; 8:935-47. doi: 10.1038/nri2455 [Crossref] [ Google Scholar]
- Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev 2010; 234:120-41. doi: 10.1111/j.0105-2896.2009.00886.x [Crossref] [ Google Scholar]
- De Monte A, Olivieri CV, Vitale S, Bailleux S, Castillo L, Giordanengo V. CD1c-related DCs that express CD207/langerin, but are distinguishable from langerhans cells, are consistently present in human tonsils. Front Immunol 2016; 7:197. doi: 10.3389/fimmu.2016.00197 [Crossref] [ Google Scholar]
- Bigley V, McGovern N, Milne P, Dickinson R, Pagan S, Cookson S. Langerin-expressing dendritic cells in human tissues are related to CD1c + dendritic cells and distinct from Langerhans cells and CD141high XCR1 + dendritic cells. J Leukoc Biol 2015; 97:627-34. doi: 10.1189/jlb.1HI0714-351R [Crossref] [ Google Scholar]
- Kashem SW, Haniffa M, Kaplan DH. Antigen-presenting cells in the skin. Annu Rev Immunol 2017; 35:469-99. doi: 10.1146/annurev-immunol-051116-052215 [Crossref] [ Google Scholar]
- Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol 2002; 3:1135-41. doi: 10.1038/ni852 [Crossref] [ Google Scholar]
- Romano E, Cotari JW, Barreira da Silva R, Betts BC, Chung DJ, Avogadri F. Human Langerhans cells use an IL-15R-α/IL-15/pSTAT5-dependent mechanism to break T-cell tolerance against the self-differentiation tumor antigen WT1. Blood 2012; 119:5182-90. doi: 10.1182/blood-2011-09-382200 [Crossref] [ Google Scholar]
- Banchereau J, Thompson-Snipes L, Zurawski S, Blanck JP, Cao Y, Clayton S. The differential production of cytokines by human Langerhans cells and dermal CD14 + DCs controls CTL priming. Blood 2012; 119:5742-9. doi: 10.1182/blood-2011-08-371245 [Crossref] [ Google Scholar]
- Domogalla MP, Rostan PV, Raker VK, Steinbrink K. Tolerance through education: how tolerogenic dendritic cells shape immunity. Front Immunol 2017; 8:1764. doi: 10.3389/fimmu.2017.01764 [Crossref] [ Google Scholar]
- Alvarez-Dominguez C, Calderón-Gonzalez R, Terán-Navarro H, Salcines-Cuevas D, Garcia-Castaño A, Freire J. Dendritic cell therapy in melanoma. Ann Transl Med 2017; 5:386. doi: 10.21037/atm.2017.06.13 [Crossref] [ Google Scholar]
- Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol 2013; 13:566-77. doi: 10.1038/nri3477 [Crossref] [ Google Scholar]
- Shurin GV, Ma Y, Shurin MR. Immunosuppressive mechanisms of regulatory dendritic cells in cancer. Cancer Microenviron 2013; 6:159-67. doi: 10.1007/s12307-013-0133-3 [Crossref] [ Google Scholar]
- Gill MA, Blanco P, Arce E, Pascual V, Banchereau J, Palucka AK. Blood dendritic cells and DC-poietins in systemic lupus erythematosus. Hum Immunol 2002; 63:1172-80. doi: 10.1016/s0198-8859(02)00756-5 [Crossref] [ Google Scholar]
- Jin O, Kavikondala S, Sun L, Fu R, Mok MY, Chan A. Systemic lupus erythematosus patients have increased number of circulating plasmacytoid dendritic cells, but decreased myeloid dendritic cells with deficient CD83 expression. Lupus 2008; 17:654-62. doi: 10.1177/0961203308089410 [Crossref] [ Google Scholar]
- Jongbloed SL, Lebre MC, Fraser AR, Gracie JA, Sturrock RD, Tak PP. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis Res Ther 2006; 8:R15. doi: 10.1186/ar1864 [Crossref] [ Google Scholar]
- Cederblad B, Blomberg S, Vallin H, Perers A, Alm GV, Rönnblom L. Patients with systemic lupus erythematosus have reduced numbers of circulating natural interferon-alpha- producing cells. J Autoimmun 1998; 11:465-70. doi: 10.1006/jaut.1998.0215 [Crossref] [ Google Scholar]
- Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol 2001; 159:237-43. doi: 10.1016/s0002-9440(10)61689-6 [Crossref] [ Google Scholar]
- Gerl V, Lischka A, Panne D, Grossmann P, Berthold R, Hoyer BF. Blood dendritic cells in systemic lupus erythematosus exhibit altered activation state and chemokine receptor function. Ann Rheum Dis 2010; 69:1370-7. doi: 10.1136/ard.2009.111021 [Crossref] [ Google Scholar]
- Lebre MC, Jongbloed SL, Tas SW, Smeets TJ, McInnes IB, Tak PP. Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP- dendritic cells with distinct cytokine profiles. Am J Pathol 2008; 172:940-50. doi: 10.2353/ajpath.2008.070703 [Crossref] [ Google Scholar]
- Santiago-Schwarz F. Dendritic cells: friend or foe in autoimmunity?. Rheum Dis Clin North Am 2004; 30:115-34. doi: 10.1016/s0889-857x(03)00108-x [Crossref] [ Google Scholar]
- Canavan M, Marzaioli V, Bhargava V, Nagpal S, Gallagher P, Hurson C. Functionally mature CD1c + dendritic cells preferentially accumulate in the inflammatory arthritis synovium. Front Immunol 2021; 12:745226. doi: 10.3389/fimmu.2021.745226 [Crossref] [ Google Scholar]
- Wu GF, Laufer TM. The role of dendritic cells in multiple sclerosis. Curr Neurol Neurosci Rep 2007; 7:245-52. doi: 10.1007/s11910-007-0037-z [Crossref] [ Google Scholar]
- Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-Mer membrane tyrosine kinase. J Exp Med 2002; 196:135-40. doi: 10.1084/jem.20012094 [Crossref] [ Google Scholar]
- Leffler J, Bengtsson AA, Blom AM. The complement system in systemic lupus erythematosus: an update. Ann Rheum Dis 2014; 73:1601-6. doi: 10.1136/annrheumdis-2014-205287 [Crossref] [ Google Scholar]
- Hu CY, Wu CS, Tsai HF, Chang SK, Tsai WI, Hsu PN. Genetic polymorphism in milk fat globule-EGF factor 8 (MFG-E8) is associated with systemic lupus erythematosus in human. Lupus 2009; 18:676-81. doi: 10.1177/0961203309103027 [Crossref] [ Google Scholar]
- Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S. Nucleic acids of mammalian origin can act as endogenous ligands for toll-like receptors and may promote systemic lupus erythematosus. J Exp Med 2005; 202:1131-9. doi: 10.1084/jem.20050914 [Crossref] [ Google Scholar]
- Fransen JH, Hilbrands LB, Ruben J, Stoffels M, Adema GJ, van der Vlag J. Mouse dendritic cells matured by ingestion of apoptotic blebs induce T cells to produce interleukin-17. Arthritis Rheum 2009; 60:2304-13. doi: 10.1002/art.24719 [Crossref] [ Google Scholar]
- Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med 2004; 200:459-67. doi: 10.1084/jem.20040342 [Crossref] [ Google Scholar]
- Karni A, Abraham M, Monsonego A, Cai G, Freeman GJ, Hafler D. Innate immunity in multiple sclerosis: myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J Immunol 2006; 177:4196-202. doi: 10.4049/jimmunol.177.6.4196 [Crossref] [ Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suárez-Fariñas M, Fuentes-Duculan J. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med 2007; 204:3183-94. doi: 10.1084/jem.20071094 [Crossref] [ Google Scholar]
- Lowes MA, Chamian F, Abello MV, Leonardi C, Dummer W, Papp K. Eruptive papules during efalizumab (anti-CD11a) therapy of psoriasis vulgaris: a case series. BMC Dermatol 2007; 7:2. doi: 10.1186/1471-5945-7-2 [Crossref] [ Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007; 445:648-51. doi: 10.1038/nature05505 [Crossref] [ Google Scholar]
- Islander U, Andersson A, Lindberg E, Adlerberth I, Wold AE, Rudin A. Superantigenic Staphylococcus aureus stimulates production of interleukin-17 from memory but not naive T cells. Infect Immun 2010; 78:381-6. doi: 10.1128/iai.00724-09 [Crossref] [ Google Scholar]
- Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, Bar-Sagi D. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep 2017; 20:558-71. doi: 10.1016/j.celrep.2017.06.062 [Crossref] [ Google Scholar]
- Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother 2012; 61:827-38. doi: 10.1007/s00262-011-1143-y [Crossref] [ Google Scholar]
- Zhang C, Wang S, Yang C, Rong R. The crosstalk between myeloid derived suppressor cells and immune cells: to establish immune tolerance in transplantation. J Immunol Res 2016; 2016:4986797. doi: 10.1155/2016/4986797 [Crossref] [ Google Scholar]
- Challier J, Bruniquel D, Sewell AK, Laugel B. Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8 + T-cell priming capacity. Immunology 2013; 138:402-10. doi: 10.1111/imm.12053 [Crossref] [ Google Scholar]
- Bekeredjian-Ding I, Schäfer M, Hartmann E, Pries R, Parcina M, Schneider P. Tumour-derived prostaglandin E and transforming growth factor-beta synergize to inhibit plasmacytoid dendritic cell-derived interferon-alpha. Immunology 2009; 128:439-50. doi: 10.1111/j.1365-2567.2009.03134.x [Crossref] [ Google Scholar]
- Steinbrink K, Jonuleit H, Müller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8 + T cells resulting in a failure to lyse tumor cells. Blood 1999; 93:1634-42. [ Google Scholar]
- Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med 2001; 193:F5-9. doi: 10.1084/jem.193.2.f5 [Crossref] [ Google Scholar]
- Shurin GV, Ouellette CE, Shurin MR. Regulatory dendritic cells in the tumor immunoenvironment. Cancer Immunol Immunother 2012; 61:223-30. doi: 10.1007/s00262-011-1138-8 [Crossref] [ Google Scholar]
- Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood 2002; 100:230-7. doi: 10.1182/blood.v100.1.230 [Crossref] [ Google Scholar]
- Fu C, Jiang A. Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front Immunol 2018; 9:3059. doi: 10.3389/fimmu.2018.03059 [Crossref] [ Google Scholar]
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 2009; 10:1185-92. doi: 10.1038/ni.1790 [Crossref] [ Google Scholar]
- Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel) 2016; 4:28. doi: 10.3390/vaccines4030028 [Crossref] [ Google Scholar]
- Xiang Y, Zhang M, Jiang D, Su Q, Shi J. The role of inflammation in autoimmune disease: a therapeutic target. Front Immunol 2023; 14:1267091. doi: 10.3389/fimmu.2023.1267091 [Crossref] [ Google Scholar]
- Murthy H, Iqbal M, Chavez JC, Kharfan-Dabaja MA. Cytokine release syndrome: current perspectives. Immunotargets Ther 2019; 8:43-52. doi: 10.2147/itt.S202015 [Crossref] [ Google Scholar]
- Liu P, Kang C, Zhang J, Liu Y, Liu J, Hu T. The role of dendritic cells in allergic diseases. Int Immunopharmacol 2022; 113:109449. doi: 10.1016/j.intimp.2022.109449 [Crossref] [ Google Scholar]
- Üzülmez Ö, Kalic T, Breiteneder H. Advances and novel developments in molecular allergology. Allergy 2020; 75:3027-38. doi: 10.1111/all.14579 [Crossref] [ Google Scholar]
- Soto JA, Gálvez NMS, Andrade CA, Pacheco GA, Bohmwald K, Berrios RV. The role of dendritic cells during infections caused by highly prevalent viruses. Front Immunol 2020; 11:1513. doi: 10.3389/fimmu.2020.01513 [Crossref] [ Google Scholar]
- Lee HK, Iwasaki A. Innate control of adaptive immunity: dendritic cells and beyond. Semin Immunol 2007; 19:48-55. doi: 10.1016/j.smim.2006.12.001 [Crossref] [ Google Scholar]
- Ashraf MI, Mengwasser J, Reutzel-Selke A, Polenz D, Führer K, Lippert S. Depletion of donor dendritic cells ameliorates immunogenicity of both skin and hind limb transplants. Front Immunol 2024; 15:1395945. doi: 10.3389/fimmu.2024.1395945 [Crossref] [ Google Scholar]
- Zhuang Q, Liu Q, Divito SJ, Zeng Q, Yatim KM, Hughes AD. Graft-infiltrating host dendritic cells play a key role in organ transplant rejection. Nat Commun 2016; 7:12623. doi: 10.1038/ncomms12623 [Crossref] [ Google Scholar]