Bioimpacts. 2025;15:30707.
doi: 10.34172/bi.30707
Original Article
Nanobody-functionalized liposomal doxorubicin: A novel strategy for angiogenesis suppression via VEGFR2 targeting
Aezam Akbari Conceptualization, Data curation, Formal analysis, Project administration, Visualization, Writing – original draft, 1 
Azadeh Ghaffari Funding acquisition, Project administration, Supervision, Validation, 1
Fahimeh Haji-Ahmadi Formal analysis, 2
Vahideh Farzam Rad Formal analysis, 3
Mahdi Behdani Methodology, Resources, 4
Hamidreza Kheiri-Manjili Investigation, 1, 5
Cobra Moradian Writing – review & editing, 6
Davoud Ahmadvand Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing, 6, * 
Author information:
1Faculty of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran
2University of California San Francisco, Cellular Molecular Pharmacology School, School of Medicine, San Francisco CA, USA
3Department of Physics, Institute for Advanced Studies in Basic Sciences, university of Zanjan, Zanjan, Iran
4Venom and Biotherapeutics Molecules Laboratory, Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
5Cancer Gene Therapy Research Center, Zanjan University of Medical Science, Zanjan, Iran
6Department of Molecular Imaging, Faculty of Advanced Technology in Medicine, Iran University of Medical Sciences, Tehran, Iran
Abstract
Introduction:
Doxorubicin (DOX) is a widely used first-line treatment for various cancers but causes toxicity. Targeted drug delivery systems, particularly DOX-encapsulated liposomes, show clinical success and lower toxicity. The abnormal angiogenesis in high-grade tumors, making it crucial to develop strategies that target this process in conjunction with chemotherapy. This study presents an innovative formulation of anti-VEGFR2-functionalized liposomal DOX, designed to reduce systemic drug release, enhance drug release and bioavailability at tumor sites, and reducing adverse effects, representing a promising advancement in targeted cancer therapy.
Methods:
Liposome formulations including liposome (Lip), DOX loaded liposome (Lip-DOX), anti VEGFR2 Nanobody-conjugated liposome (Lip-Nb), and anti VEGFR2 Nanobody- conjugated DOX-loaded liposome (Lip-DOX-Nb) were prepared by film hydration method and then fully characterized. The cellular uptake of these nanocarriers were assessed by flow cytometry analysis in human umbilical vein endothelial cells (HUVECs). Further, the ability of the different liposomal formulations to suppress angiogenesis were assessed by performing tube formation assay on HUVECs. In addition, the inhibitory impact of low dose consumption of the formulations to inhibit the migratory capacity of glioma cells were assessed by scratch migration assay on U87 cells.
Results:
The prepared liposomal formulations displayed optimal size range of 120-131 nm, with slightly negative charge about -2.4 mv, spherical morphology and effective encapsulation of about 91% of the total DOX and high conjugation efficiency of about 87% of total anti VEGFR2 Nb that are acceptable for nano sized targeted drug delivery systems. In vitro experiments; flow cytometry results verified cellular uptake of DOX loaded liposomes to HUVEC cell line and more cellular uptake was observed for Lip-DOX-Nb liposomes demonstrated that the anti-VEGFR2-conjugated liposomes enhance cellular uptake. Lip-DOX-Nb liposomes also showed more cytotoxicity effect against VEGFR2-positive HUVEC cells in compare with non-conjugated liposomes; effectively induced apoptosis to HUVEC cells and reduced the migratory capacity on U87 cancer cells. Analysis of the treated cells using DHM revealed that Lip-DOX-Nb enhanced nuclear integrity of U87 cancer cells while inducing cell death.
Conclusion:
This designed drug delivery system worked as strong anticancer and angiogenesis suppression agent ex-vitro angiogenesis model via VEGFR2 targeting.
Keywords: Tumor angiogenesis, Anti VEGFR2 nanobody, Liposome, Doxorubicin, DHM
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This work is part of the Ph.D. project and was supported by a grant awarded by the Zanjan University of Medical Sciences (Grant no. A-12-614-15) and Iran University of Medical Sciences(96-04-31-32613).
Introduction
Chemotherapy is a standard cancer treatment regimen using cytotoxic anticancer drugs.1 One of the most important chemo-agents with potent anticancer activity is doxorubicin (DOX), which is widely used in clinical settings for the treatment of both solid and hematologic neoplasms. However, a significant concern regarding DOX is its off-target effect caused by their nonspecific distribution throughout the body, can result in various adverse side effects such as cardiotoxicity leading to congestive heart failure.2-4
Nano-carriers can be more effective than free drugs in delivering and accumulation of anticancer agents in tumor cells that exploit the enhanced permeability and retention (EPR) effect for localized tumor accumulation and providing sustained release.5 Doxil and Caelyx are the first FDA-approved biocompatible liposomal nanoparticle formulations of DOX for clinical use introduced in 1995 for passive tumor targeting.6,7 Although Liposomal DOX strongly reduced the cardiotoxicity of DOX in clinical trials, several patients suffered from mucositis and hand or foot syndrome due to the localization of the liposomes in the capillaries of skin and mucosa.8-12 Therefore, to enhance the targeting of DOX to the tumor sites, liposomes were functionalized with a targeting moiety or antibodies to generate targeted liposomes. This approach improves site-specific accumulation and efficacy of the liposomal drugs. The ligands can target the tumor itself or its surrounding microenvironment, such as the extracellular matrix or the tumor-associated vasculature.13-16
A hallmark characteristic of cancer cells is angiogenesis, which involves the formation of new blood vessels from angiogenetic growths, leading to metastasis of tumors and vascular proliferation. One of the most promising targets for anti-angiogenic therapy are vascular endothelial growth factor receptors (VEGFR1, VEGFR2 and VEGFR3) which are transmembrane tyrosine kinases. Under hypoxic conditions in the tumor microenvironment, vascular endothelial growth factors (VEGFs) and VEGFRs are upregulated in tumoral vascular Endothelial Cells due to hypoxic conditions; which ultimately leads to neo-vascularization in the tumoral tissues. This process is mediated through the binding interaction of VEGF with VEGF receptors. Accumulating evidence suggests targeted anti-angiogenic therapy using monoclonal antibodies (mAbs) could improve patients’ outcomes in a vast variety of cancers (e.g., colorectal cancer, brain neoplasms, and high-grade recurrent ovarian carcinoma).17 However, because of the large molecular sizes of mAbs, their low tumor penetration in solid neoplasms is still a major challenge.18 Compared to intact mAbs, single-domain antibodies (nanobodies), possess distinct characteristics: they are smaller in size, exhibit enhanced tissue penetration, and display high stability under challenging conditions. Additionally, nanobodies have shown superior affinity, sensitivity, and specificity toward target sites.19
In this study, as presents in Fig. 1, we aimed to test if the conjugation of anti- VEGFR2 Nanobody, which was isolated from an immunized dromedaries Camelidae library to the surface of DOX encapsulated liposome, can result in higher therapeutic and anti-angiogenic efficacy against cancer cells.20 The design of these nano-carriers is based on the benefits associated with the use of nanoparticles and anti-VEGFR2 nanobody, overexpress in tumor-associated endothelial, as a novel multidisciplinary VEGFR2 targeted drug delivery system of therapeutics to the tumor vasculaturefor cancer anti-angiogenic therapy and chemotherapy.21
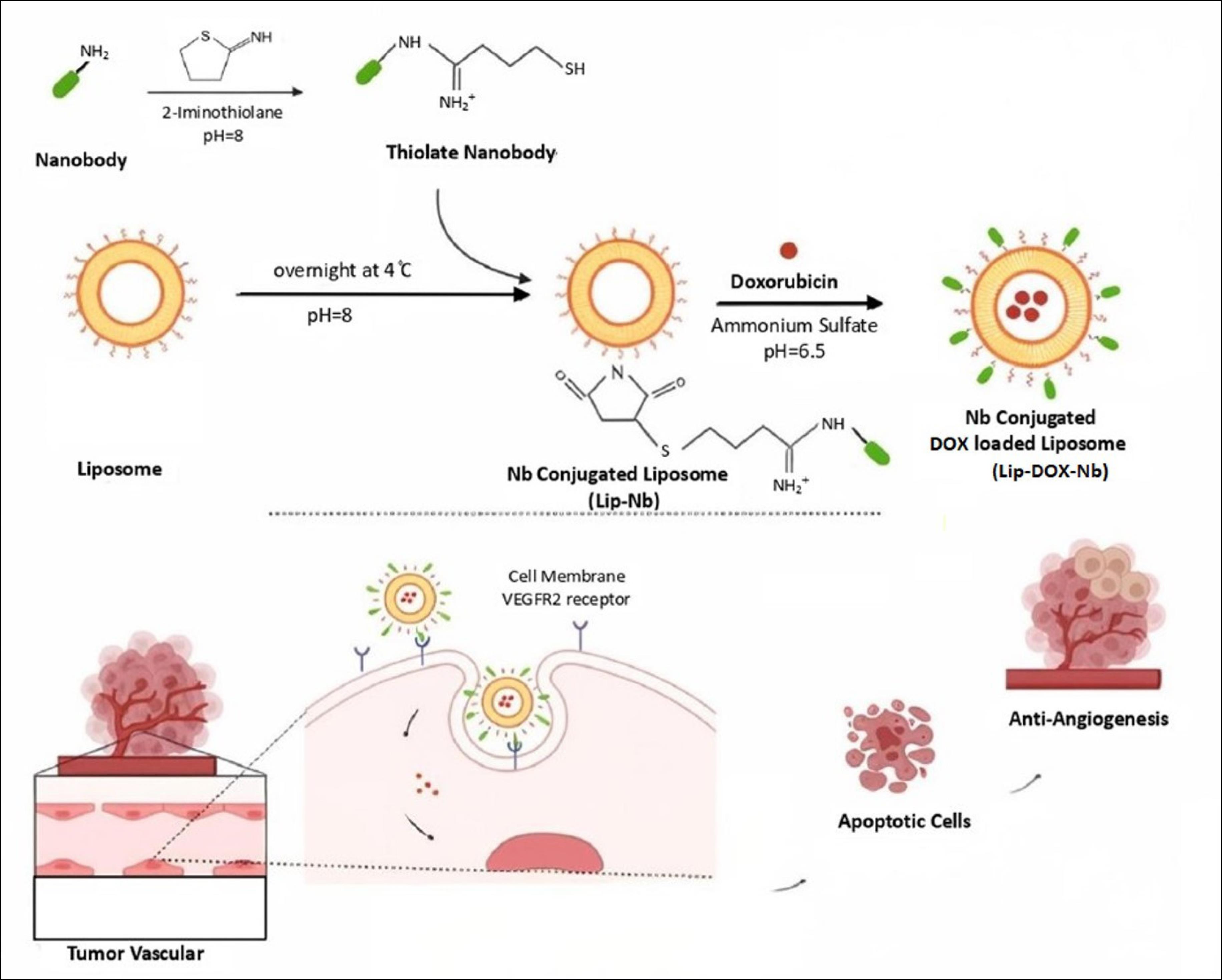
Fig. 1.
Schematic representation of the Nanobody-conjugated DOX Loaded liposomes preparation and interaction of this DDS with tumoral ECs. As proposed, the DOX-containing liposomes attach to tumoral ECs by VEGFR2 Nanobodies as targeting moieties and cause apoptotic and anti-angiogenesis effects.
.
Schematic representation of the Nanobody-conjugated DOX Loaded liposomes preparation and interaction of this DDS with tumoral ECs. As proposed, the DOX-containing liposomes attach to tumoral ECs by VEGFR2 Nanobodies as targeting moieties and cause apoptotic and anti-angiogenesis effects.
Materials and Methods
Materials
DOX–HCl was obtained from Sigma-Aldrich (St. Louis, MO). 1,2-Distearoyl-snglycero-3-phos-phoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) Cholesterol, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (Mal-PEG2000-DSPE), and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) were obtained from Avanti Polar Lipids (Alabaster, AL, USA). Triton X-100, 2-Iminothiolane (Traut's Reagent) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent were supplied by Sigma Aldrich Co. (St. Louis, MO, USA). Sodium chloride Ammonium sulfate, and Cysteine chloride were obtained from Sigma Aldrich (MO, USA). Fetal bovine serum (FBS) and Trypsin were supplied by Thermo Fisher Scientific Inc. (Waltham, MA, US).
Different liposomal formulations preparation
Thiolation of nanobodies
To conjugate anti VEGFR2 Nanobodies onto the surface of the liposome, amine terminal group on Nanobody molecules must be thiolated to produce linkable sulfhydryl group. Thiolation of Nanobodies was done by Traut's Reagent (2-iminothiolane) at a Traut's Reagent/Nanobody molar ratio of 1:10 in Borate buffer (1ml, 1 mM, pH = 8.3) and then stirred overnight at 4 ℃.21. Then, the borate buffer was exchanged with PBS (1mM, pH = 7.4) by using an Amicon Ultra centrifugal 15 mL filter MWCO 10 kDa Ultracel membrane (Merck, Germany). In this step, extra molecules of Traut's Reagent were eliminated.
DOX-free and DOX-loaded liposomes preparation
Liposomes preparation step was performed along with Nanobody thiolation process.20 All Liposomes were prepared by thin-film hydration method.22 Lipid molecules including: DPPC, cholesterol, DSPE-PEG and DSPE-PEG-Maleimide (65:30:4:1 molar ratio). To prepare unconjugated liposomes (Lip & Lip-Dox); the lipid thin film was made of those lipids with no DSPE-PEG-Maleimide. Lipids were dissolved in a 9:1 (v/v) of chloroform and methanol mixture, and then a lipid thin film was made after removing organic solvents using rotary evaporation (Heidolph, Laborota 4000) in a water bath at 60 ℃ for 1 hour.23 Formulating DOX-loaded liposomes (Lip-DOX), needed to hydrate lipid film by prewarmed (NH4)2SO4 (1 mL, 250 mM, pH = 6.5) at 65 ℃. To prepare DOX-free liposome (Lip), the thin lipid film was hydrated by prewarmed PBS (1 mL, 1 mM, pH = 7.4) at the same temperature. The resulting liposome suspensions were further dispersed by using a probe sonicator (LABTECH, Luc 410) with a cycle of 30 seconds on, 10 seconds off, and power delivery of 20% for 2 minutes. Then, the suspension was extruded (21x at 56 ℃) through two stacked 100 nm pore-sized polycarbonate membranes (Nuclepore, Pleasanton, CA/ Liposofast R, Avastin) to form small unilamellar liposomal vesicles (SUVs). The unilamellar liposomes formulated in 250 mM (NH4)2SO4 were separated from the sulfate solution after a wash-out step with PBS (1mM, pH = 7.4) by an MWCO 50 kDa Ultracel Amicon filter. After buffer exchanging, DOX solution (in PBS 1 mL, 1 mM, pH = 7.4) was added into liposome suspension in PBS at free DOX to total lipid ratio of 2:10 (w/w) followed by stirring for 1 hour at 60 ℃. Afterward, unencapsulated DOX was separated via Gel Filtration using Sepharose CL-4B (GE Healthcare, Bucks) by PBS (1mM, pH = 7.4) as elution buffer.
Anti-VEGFR2 conjugated liposomes; (Lip-Nb and Lip-DOX-Nb) were prepared at the same method mentioned above but by adding prepared thiolated Nanobodies to attach onto the liposomes surface. Briefly; the thiolated Nanobodies were mixed with prepared liposomes (containing maleimide-terminated linker on DSPE-PEG-Maleimide lipid) and were incubated for 1 hour at room temperature with slow shaking and in a dark foil-covered container. Then Cysteine Chloride solution (1 mL, 5 mM) was added to the reaction medium under Nitrogen gas to bloke unreacted maleimide groups. Then the extra cysteine molecules were removed by filtration and free unconjugated Nanobodies were collected by using an Amicon centrifugal filter device with a 10 kDa MWCO Ultracel membrane (Merck, Germany). The mixture was diluted with 2 mL PBS (pH 7.4) in an Eppendorf tube to determine Nanobody Conjugation Efficiency (CE %).20
To prepare Lip-DOX-Nb; Lip-Nb formulation was used to encapsulate DOX in the same method as mentioned above. All prepared liposomes sterilized by passing through a 0.22 mm sterile carbonate filter and then stored at 4 ℃.
Characterization of liposomes
Size distribution, zeta potential and morphology
Liposomes were diluted with PBS (1mM, pH = 7.4) and then Particle size distribution, Poly Dispersity Index (PDI), Mean diameter, and zeta potential of different liposomal formulations were measured by Zeta sizer instrument (ZS90, Malvern) at a fixed angle of 90° at 25 °C. Transmission Electron Microscopy (TEM) was performed to image the liposomes and investigate morphological characteristics. Prior to observation, the liposomes (approximately 100 µg/mL) were positioned as a thin liquid film on a carbon-coated copper grid and then air-dried at room temperature. Subsequently, the images were visualized under a Field-Emission TEM (LEO, Leo906).
Assessment of conjugation efficacy of anti-VEGFR2 nanobody
The amount of all collected unconjugated (free) Nanobodies was measured by Bradford protein assay to determine Nanobody Conjugation Efficiency (CE %) by equation 1:
Eq. (1)
Where Ctotal indicates the total amount of Nanobody initially added into the lipid mixture; Cunlinked is the amount of unlinked anti VEGFR2 Nanobodies separated via filtration.
Encapsulation efficiency of DOX
To determine the DOX encapsulation efficiency (EE%), 100 μL of the Lip-DOX / Lip-DOX-Nb were placed in a 5 mL volumetric flask, 10 μL of 10% Triton X-100 was added and incubated at 37 °C for 10 minutes while vertexing. After filtration by 0.22 μm polycarbonate membrane, the filtrate was subjected to HPLC analysis to determine DOX content according to the DOX standard curve. EE % was calculated by equation 2:
Eq. (2)
Where Ctotal is the total DOX amount initially added into the liposome solution to be entrapped into liposomes; Cfree is the amount of isolated DOX.
SDS-PAGE
Covalent conjugation of anti VEGFR2 Nanobody to liposome was qualitatively assessed by SDS-PAGE. Proper amounts of Lip-Nb, and free Nb were loaded onto a 4-12% SDS-PAGE gel, the resulting gel was stained using Novex Silver Xpress Staining Kit, Thermo-Fisher Scientific (MA, USA), according to manufacturer’s instruction.
In vitro DOX release assay
In vitro release of DOX from prepared Lip-DOX-Nb and Lip-DOX formulations was investigated through dialysis in human plasma matrix. About 1ml of Lip-DOX-Nb or Lip-DOX solution was sealed inside a dialysis bag (MWCO of 10 kDa) containing human plasma and then immersed into 10 mL of release medium (PBS, 1mM, pH = 7.4) under agitation of 120 rpm at 37 °C for 48 hours. Release medium was sampled at scheduled time points (1, 2, 3, 19, 21, 24, and 48 hours), and were replaced with fresh release medium Quantitative analysis was performed on all samples by the HPLC method to determine the release profile of DOX from the two different liposomal formulations. DOX molecules are separated on a C18 column with a mobile phase consisting of 0.05 M sodium acetate (pH 4.0) and acetonitrile (72:28). The column eluate was monitored by UV-visible detection at 487 nm.24
Cells culture
The human umbilical vein endothelial cell line (HUVEC) was reported to have specific binding sites (VEGFR2, with 500 sites/cell) and the dissociation constant was reported as low as 9 Pm.25 So, HUVEC cell line was employed as a VEGFR2 overexpressing positive cell model. Cells were cultured by the protocol previously set up.26 DMEM high-glucose medium (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS, streptomycin (100 µg /mL), and penicillin (100 µg /mL); was used and cells were cultured in T-25 Poly-L-lysine flasks in 37 ℃ in a humidified incubator containing 5% CO2.
Cell toxicity assay
In vitro cytotoxicity of free DOX, Lip-DOX and Lip-DOX-Nb was investigated through MTT assay. HUVECs were seeded into 96-well plates at the density of 5 × 106 cells /well and were incubated for 48 hours, then each well was treated with different concentrations of free DOX between 12-600 nM, in triplicate and was incubated again, with untreated cells considered as a control group. After 24 hours incubation, the upper medium was discarded and cells were washed 3 times with PBS (1mM, pH = 7.4), then150 μL of fresh medium with MTT solution (50 μL, 5 mg/mL) were added to each well except in one that served as blank. Then, the plate was incubated at 37 ℃ for 4 h. Finally, the MTT solution was discarded and Formazan salt crystals were dissolved by adding 150 µL of dimethyl sulfoxide (DMSO) to each well, and cells were incubated at 37 °C with gentle shaking for 30 minutes. After that, samples were analyzed by an ELISA reader (Biorad, Minisubcell-GT) at 570 nm to determine the IC50 of DOX.
In vitro cellular uptake
Flow cytometry was adopted to analyze the uptake of Lip-DOX-Nb toward HUVEC (VEGFR2 + ) cell lines. HUVECs were seeded onto 24-well plates at a density of 2 × 105 cells /well in triplicate and were incubated for 24 hours. The next day, the medium was changed with fresh medium and treated with Lip-DOX-Nb formulation (of 25 nM final concentration of DOX). After 2 and 4 h incubation at 37 °C, the medium of each well was removed and the cells were washed with PBS (1mM, pH = 7.4), three times. Treated cells were trypsinized and pelleted by centrifugation (1200 rpm for 5 minutes). Finally, the cells were harvested and resuspended in 1 mL of PBS (1 mM, pH = 7.4). FACS CALIBUR was applied to measure the Mean Fluorescence Intensity (MFI). Cell-associated DOX was excited with an Argon laser (488 nm) and the fluorescent emission was detected at 585 nm (FL2 filter). The data, obtained from flow cytometry were analyzed by using the FlowJo software. In addition, the cells were double-strained using annexin V-FITC and propidium iodide (PI), and apoptosis rate was investigated by flow cytometry.
Tube formation assay
In order to investigate the potency of different liposomal formulations to reduce the tube-forming capacity of HUVECs, we first set a 0.1% Hyaluronic acid gel using a complete culture medium of ECs containing DMEM / F12 with 20% FBS and 1% penicillin-streptomycin and 200 µL of this gel was set in each well of a 24-well culture plate and was incubated in a CO2 incubator at 37 °C for 1 day. The next day, 2 × 104 cells were seeded in each well and the tube formation was evaluated up to 36 hours after treatment with a 2 × IC50 dose for each formulation. The number of tubes, and the diameter of each tube was examined by ImageJ software. Tubulation 2d parameters were analyzed by ImageJ Angiogenesis plugin and also manual interpretation of the data for tube diameter and total number of tubes.
Cell migration assay
To investigate the effects of various formulations on migration of tumor cells, wound healing assay (Cell Migration assay) was performed. U87 cells, a human glioblastoma GBM cell line, were plated in 24-well culture plates at a density of 1 × 104 cells/well and cultured overnight to form a confluent monolayer. The following day, a vertical scratch wound was generated using a 100 mL pipette and then washed with PBS three times to remove exfoliated cells. Cells were incubated with the fresh medium, followed by adding prepared formulations; free DOX, Lip, Lip-DOX, Lip-Nb and Lip-DOX-Nb (DOX equivalent concentration of IC50) for 24 hours. Then the image of each sample was captured using a microscope (Olympus, Japan) at the beginning and the ending to monitor the wound status. Migration was quantified as the percent decrease in mean migration zone diameter.
DHM analysis of cellular nuclear damage induced by DOX
Digital holographic microscopy (DHM) has unique properties in assessment of cells 3D morphology. In the current study, we used digital holographic microscopy to investigate the impact of each treatment on U87 cells morphology and nuclear integrity. To do so we setup a Mach-Zehnder interferometer. After illuminating the samples by a He-Ne 632.8 nm laser, the holograms were recorded by a CCD camera (Thorlabs Sciences,). The.tiff hologram image was then reconstructed using the ASP in MATLAB software by the following steps: (1) Fourier transformation (2) phase image visualization (3) numerical focusing (4) reference phase subtraction and production of the filtered phase difference image (5) unwrapping the phase difference image by ASP (6) production of the 3D profile and then smoothening the obtained image to optimize the visualization procedure.
Statistical analysis
GraphPad Prism version 9 was used for data analysis and visualization of the data presented in the current work. The independent t-test and analysis of variance (ANOVA) were used to determine Statistically significant difference among groups (P < 0.05). Mean ± standard deviation (SD) was used to report the continuous measures.
Results
Characterization of liposomes
Conjugation of VEGFR2 Nanobody to liposomes, were confirmed by 4-12% SDS-PAGE and silver nitrate staining method as increasing in molecular weight of Lip-Nb liposome compared to the free Nb (Fig. 2).
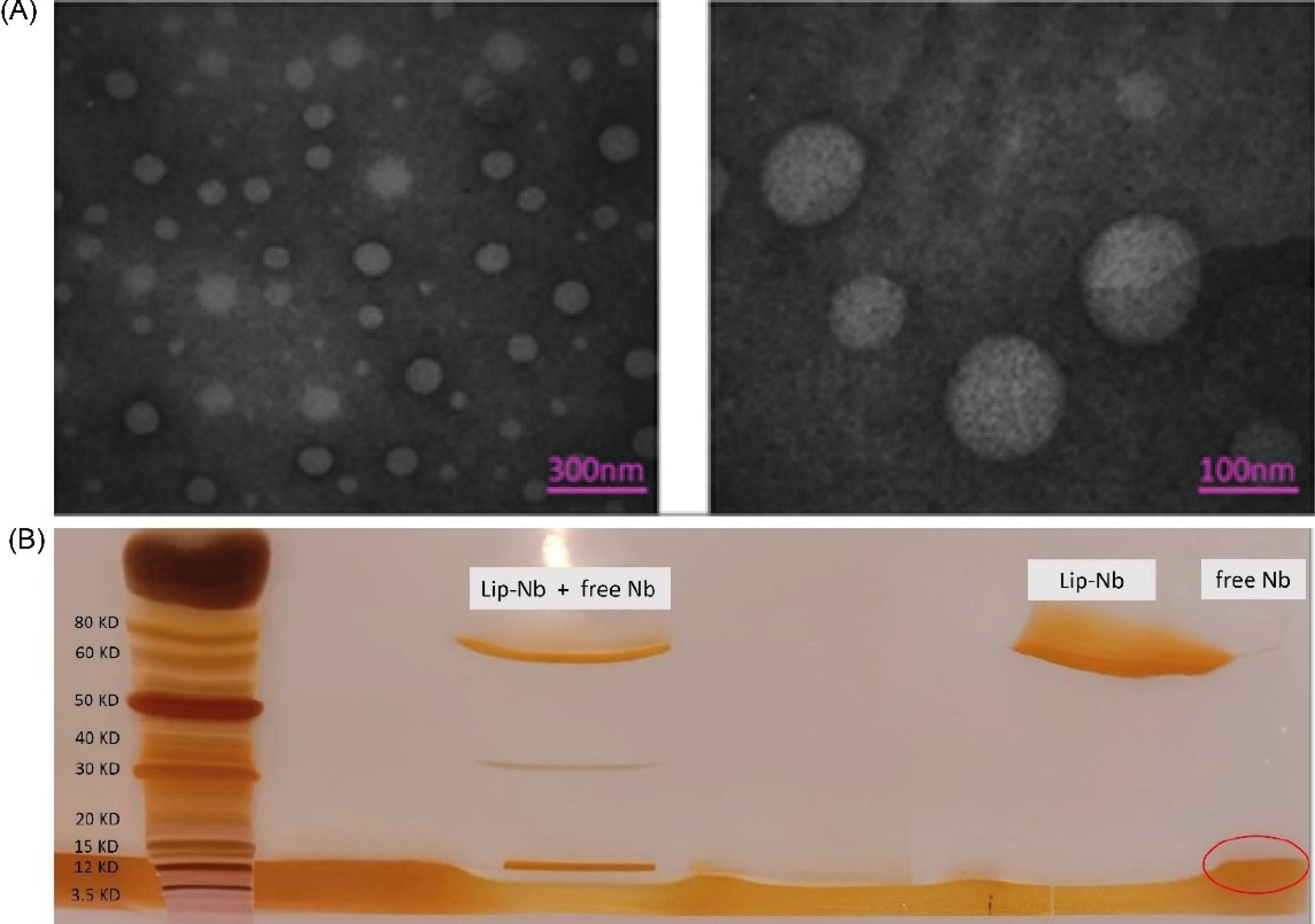
Fig. 2.
Characterizations of liposomes by A) TEM and B) DLS measurements. The prepared liposomes showed nanostructured spherical morphology and homogenous size. SDS-PAGE results showed coupling of Nb to liposomes.
.
Characterizations of liposomes by A) TEM and B) DLS measurements. The prepared liposomes showed nanostructured spherical morphology and homogenous size. SDS-PAGE results showed coupling of Nb to liposomes.
DOX encapsulation efficacy of the Lip-DOX and the Lip-DOX-Nb were 91.7 ± 0.6% and 89.56 ± 1.4%; respectively (Table 1), implying that the DOX was efficiently loaded.
Table 1.
Particle size, PDI, EE%, CE%, and ZP of liposomes formulated
|
Formulations
|
Particle size (intensity)
|
PDI
|
EE%
|
CE%
|
ZP (mv)
|
| lip |
120.76 ± 0.35 |
0.11 ± 0.06 |
- |
- |
0.56 ± 0.07 |
| Lip-DOX |
125.72 ± 2.3 |
0.16 ± 0.1 |
91.7 ± 0.6 |
- |
0.46 ± 1.81 |
| Lip-Nb |
128.06 ± 1.65 |
0.12 ± 0.02 |
- |
87 ± 1.1 |
-2.4 ± 0.26 |
| Lip-DOX-Nb |
131.8 ± 2.63 |
0.14 ± 0.09 |
89.56 ± 1.4 |
86 ± 0.8 |
-2.4 ± 0.53 |
The size and zeta potential of the liposomal formulations were measured by Zeta sizer. The results showed that the mean particle size of Lip and Lip-DOX preparations were 120.76 ± 0.35 nm and 125.72 ± 2.3 nm and PDI of 0.11 ± 0.06 and 0.16 ± 0.1; respectively. The prepared Lip-DOX-Nb had a processed size of 131.8 ± 2.63 nm and PDI of 0.14 ± 0.09 and after conjugating with anti VEGFR2, exhibited a slight increase in size compared to the Lip-DOX with the detected size of 125.7 ± 2.3 nm and PDI of 0.16 ± 0.1 (Table 1) implying that the liposomal formulations exerted acceptable size homogeneity.27 Besides, the zeta potential of Lip and Lip-DOX; were 0.56 ± 0.07 mV and 0.46 ± 1.81 mV; respectively while that of Lip-Nb and Lip-DOX-Nb; were -2.4 ± 0.26 mV and -2.4 ± 0.53 mV; respectively, showing acceptable stability.
The Lip-DOX-Nb was also examined by TEM imaging for morphology depicted in Fig. 2, where demonstrated that the prepared liposome had roughly spheroid shapes with average sizes of less than 200 nm which is favorable for nanoliposomes to accumulate efficiently in tumor tissues.28
In vitro drug release
The in vitro release patterns of the DOX preparations were measured in equilibrium solutions of PBS and human plasma. DOX was released continuously and slowly from the DOX-loaded liposomes, without a primary burst release effect, which suggests sustained release that the drug is released by diffusion through the lipid bilayer29 in Also, DOX release from Lip-DOX-Nb was slightly slower than that from the Lip-DOX (Fig. 3). This slightly slower release rate may be due to the higher zeta potential (ZP) of the conjugated Lip-DOX-Nb (ZP: −2.4 mV) compared to that of Lip-DOX (ZP: −0.46 mV). As a rule, colloidal nano-systems such as liposomes with higher ZP, are more stable morphologically and structurally in aqueous solutions.30 this would keep drugs into the liposomes tightly in the blood circulation. Additionally; the linking of anti VEGFR2 molecules on the surface of liposomes formed a sterically barrier that alters the diffusion rate among bilayers. These results indicated that both liposomal preparations were preventing an initial burst release and hence better suited for a sustained release of the drug, making increased drug accumulation in tumor tissues over time possible. Studies on the Dipalmitoyl Phosphatidyl Choline (DPPC) involved liposomes showed strong interactions between the amin (NH3+) group in DOX and the phosphate (PO2-) group in the polar head of zwitterionic DPPC. It would cause a decrease in transition temperature and plastic viscosity and also an increase in membrane fluidity of the DPPC liposomes.31 This phenomenon causes more controlled releasing process. Interestingly, DPPC (Tm = 41℃) with two saturated alkyl chains, shows different phase behavior at 37 ℃ in its gel phase. This unique property of DPPC involved liposome allows the release of DOX from liposomes at tumoral vessels where the temperature is slightly higher than systemic circulation due to excessive proliferation and metabolism in tumoral tissues. This is in good agreement with the slow-release rate of the drug from liposomal formulation.32
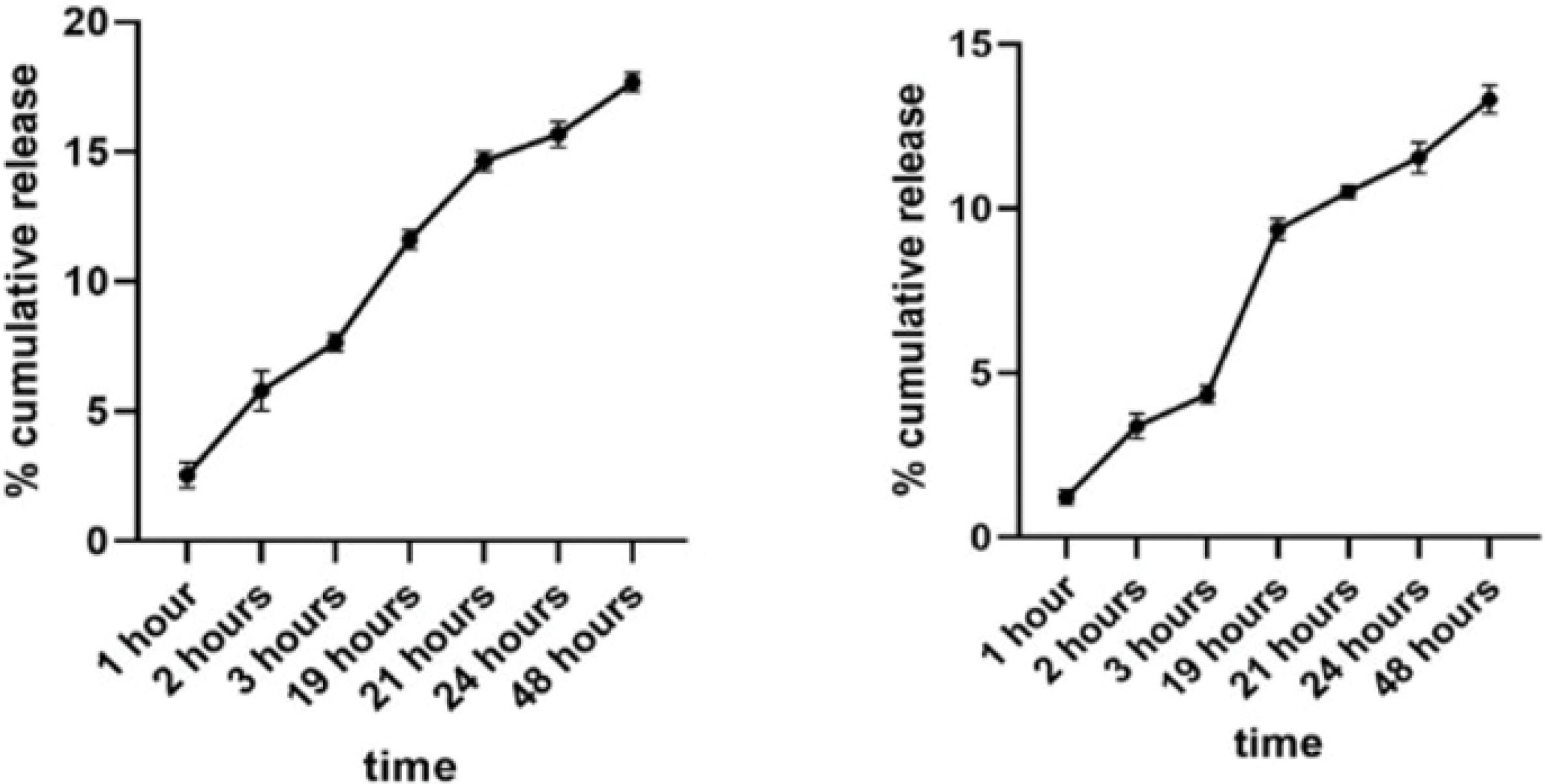
Fig. 3.
Assessment of drug release from the liposomal formulations. % cumulative release of Lip-DOX (Left) and of Lip-DOX-Nb (Right).
.
Assessment of drug release from the liposomal formulations. % cumulative release of Lip-DOX (Left) and of Lip-DOX-Nb (Right).
Cell toxicity
The cell toxicity test for DOX in different formulations was checked on HUVEC (VEGFR2 + ) cells using the MTT test. To this end, the IC50 value was first determined from different amounts of free DOX on the cells (18 µg /mL) and then the toxicity test was performed considering this fixed value for all forms of the drug. Upon direct exposure to free DOX; DOX molecules were able to rapidly enter the cells through simple diffusion, but without any release process from the liposomal preparations, and moved toward the nuclei favored by its high nucleophilicity.33 Then initiated toxic and apoptosis-inducing effect on cells. Free DOX caused high levels of cell death through DOX inducing DNA damage response. In contrast, the cell viability% of HUVECs of the Lip treated group after 4 hours; was approximately 98% near to 100% which proved the non-toxic effect of unloaded Liposome (Lip) on cells. Lip-Nb appeared more toxic effect than Lip formulation on cells (cell viability of 85%; P < 0.05) which could be referred to inhibiting effect of anti VEGFR2 moiety on liposomes. The Lip-DOX formulation showed a significantly more toxic effect than Lip-Nb (cell viability% of 44.8%; P < 0.05). Comparatively; Lip-DOX-Nb formulation toxic effects with cell viability% of 32.5% was significantly the highest one among other groups (P < 0.05) unless compared to the free DOX treated group; which could be described by the slow release of DOX from the liposomes which is in line with the results of our release assay. Conjugating DOX-loaded liposomes with anti VEGFR2 Nanobody enhanced HUVEC cell targeting and also improved cellular attachment with more efficient cellular internalization of the nano-liposomal carrier that cause more drug accumulation in the cells which in turn caused more toxic effects on cells (Supplementary file 1, Fig. S1).
Cellular uptake
The cellular uptake efficiencies of liposomal DOX formulations were investigated by the HUVECs FCM. The Flow Cytometry fluorescent histogram analysis indicated that the cellular uptake for the Lip-DOX-Nb formulation was much higher than the non-conjugated Lip-DOX formulation as depicted in Fig. 4. Further, in order to quantify the comparison between the two DOX-loaded drug delivery systems; the amounts of mean fluorescent Index (MFI) were analyzed. Lip-DOX-Nb with an average MFI of 152 indicated significantly higher cellular uptake efficiency than lip-DOX (P < 0.05).
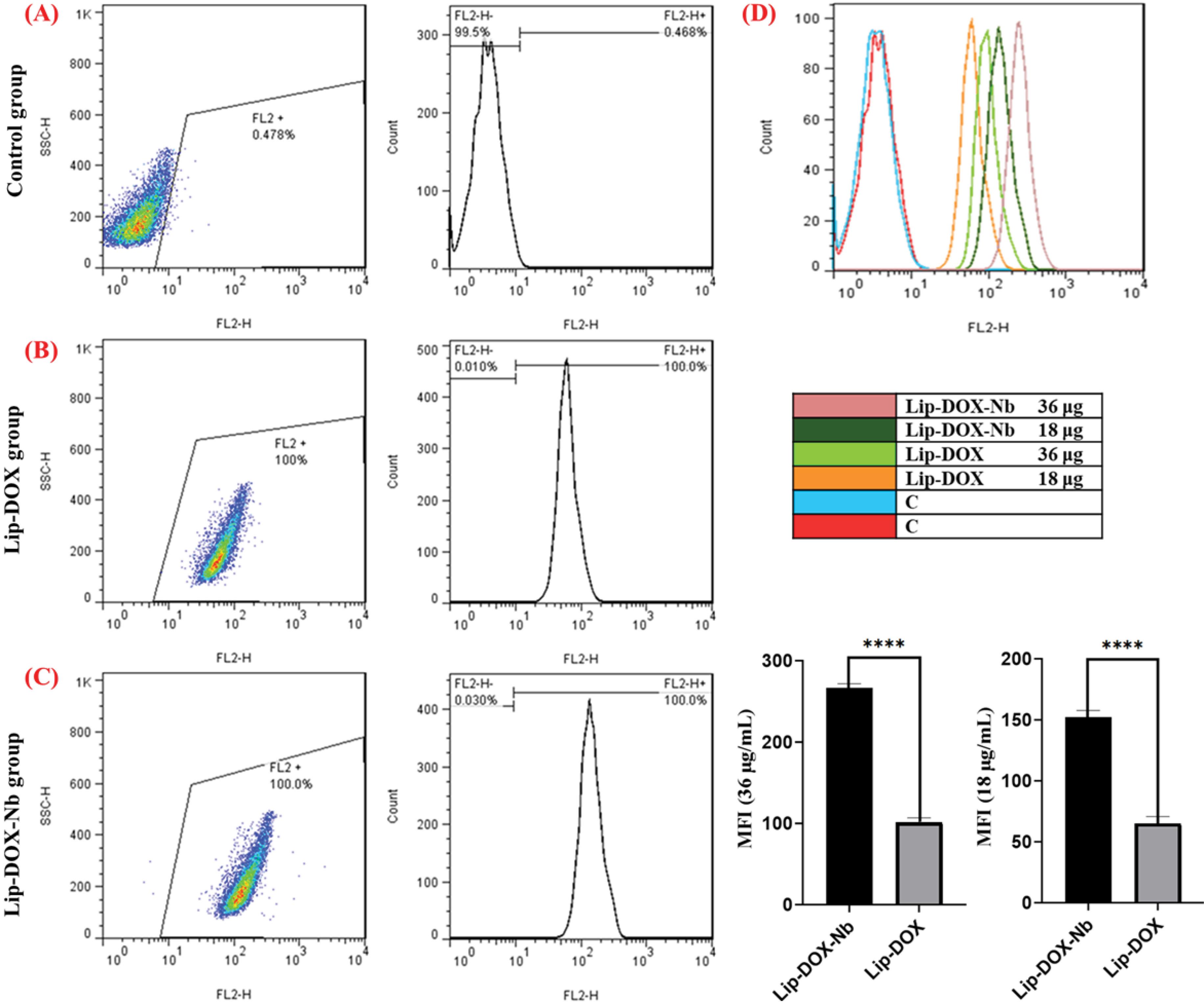
Fig. 4.
Flow cytometry analysis to investigate cellular uptake of liposomal formulations. (A) Control group (B) Lip-DOX group (C) Lip-DOX-Nb group (D) Florescent intensity of DOX in Different formulation. The results indicated that the mean fluorescence intensity (MFI) in the Lip-DOX-Nb formulation was the highest among all the groups examined.
.
Flow cytometry analysis to investigate cellular uptake of liposomal formulations. (A) Control group (B) Lip-DOX group (C) Lip-DOX-Nb group (D) Florescent intensity of DOX in Different formulation. The results indicated that the mean fluorescence intensity (MFI) in the Lip-DOX-Nb formulation was the highest among all the groups examined.
In order to investigate the occurrence of apoptosis and necrosis, Annexin‐V‐FITC/PI staining assays was performed. Results indicated that treating HUVECs with Lip-DOX-Nb for 4 hours resulted in a reduced necrosis rate and an increased apoptosis rate compared to a 2-hour incubation. This suggests that prolonged exposure to Lip-DOX-Nb promotes a greater occurrence of apoptosis (Fig. 5).
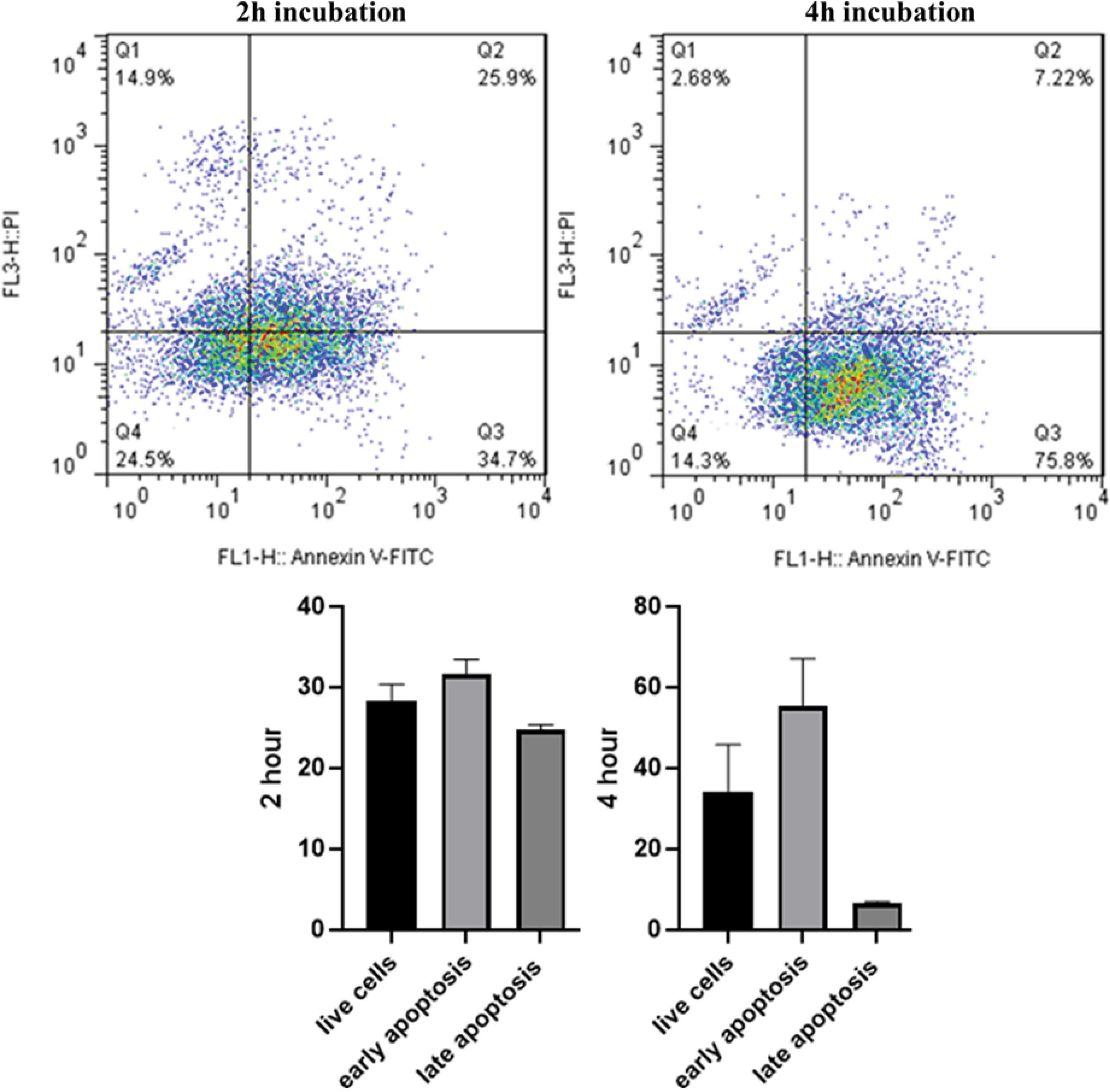
Fig. 5.
Flow cytometry analysis of apoptosis assay. The results showed a decrease in the number of live cells after 4 hours, with a significant percentage of cells undergoing apoptosis following treatment with Lip-DOX-Nb.
.
Flow cytometry analysis of apoptosis assay. The results showed a decrease in the number of live cells after 4 hours, with a significant percentage of cells undergoing apoptosis following treatment with Lip-DOX-Nb.
Scratch migration
As depicted in Fig. 6, the mean scratch diameter was significantly higher in the Lip-DOX-Nb group compared to the control group (40 ± 6.24 µm vs. 18 ± 3.60; respectively, P = 0.0056) suggesting that the migratory capacity of U87 cells were significantly decreased by Lip-DOX-Nb treatment. Furthermore, the mean scratch diameter in the Lip-Nb group was also more than the control group (30.33 ± 3.51 µm; P = 0.025) showing that the Nanobody-conjugated liposomes significantly reduced migration of the U87 cells. This data suggests that the multidisciplinary Liposome is capable of reducing the migration and invasive properties of cancer cells in a successful manner.
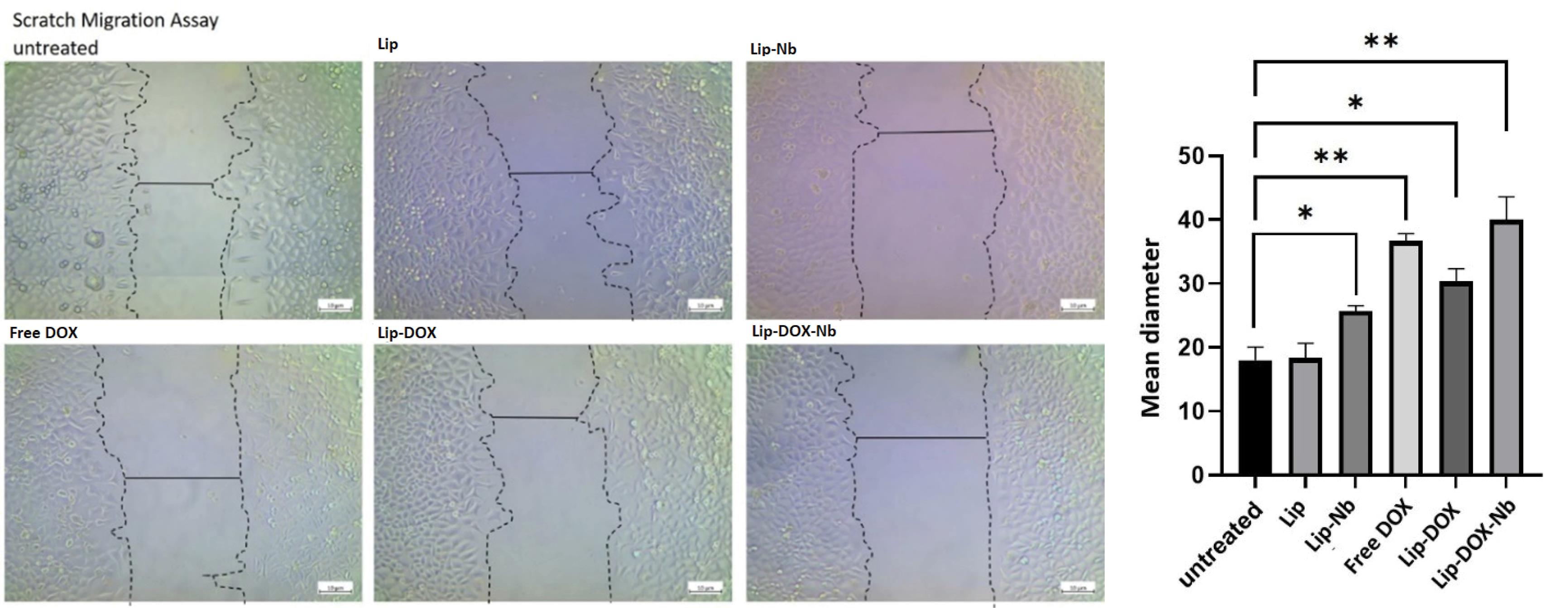
Fig. 6.
Scratch migration assay on U87 cells.
.
Scratch migration assay on U87 cells.
As depicted, the mean diameter of the scratch was significantly increased in Lip-DOX-Nb and Lip-Nb treatments suggesting that the formulated liposomes were able to reduce the migratory capacity of U87 cells successfully.
Tube formation
As depicted in Fig. 7, the untreated group and Lip treated group; contained densely packed tube-like structures with a nearly homogenous spatial distribution of the “tip” cells suggesting that the HUVECs showed marked angiogenic properties under normal circumstances. Vascular endothelial sprouting is a critical step during angiogenesis. The key step is differentiation of ECs into “tip” cells which are located at the growing ends of sprouting vessels. EC-specific growth factors, VEGF, and its receptor VEGFR2, drive EC differentiation into the tip cell. Tip cells display long filopodia. They sense proangiogenic directional cues in their environment through cell surface receptors and integrate downstream signaling to migrate in a specific direction thereby facilitating ECs migration and mediate capillary extension to form blood vessel networks.27,34
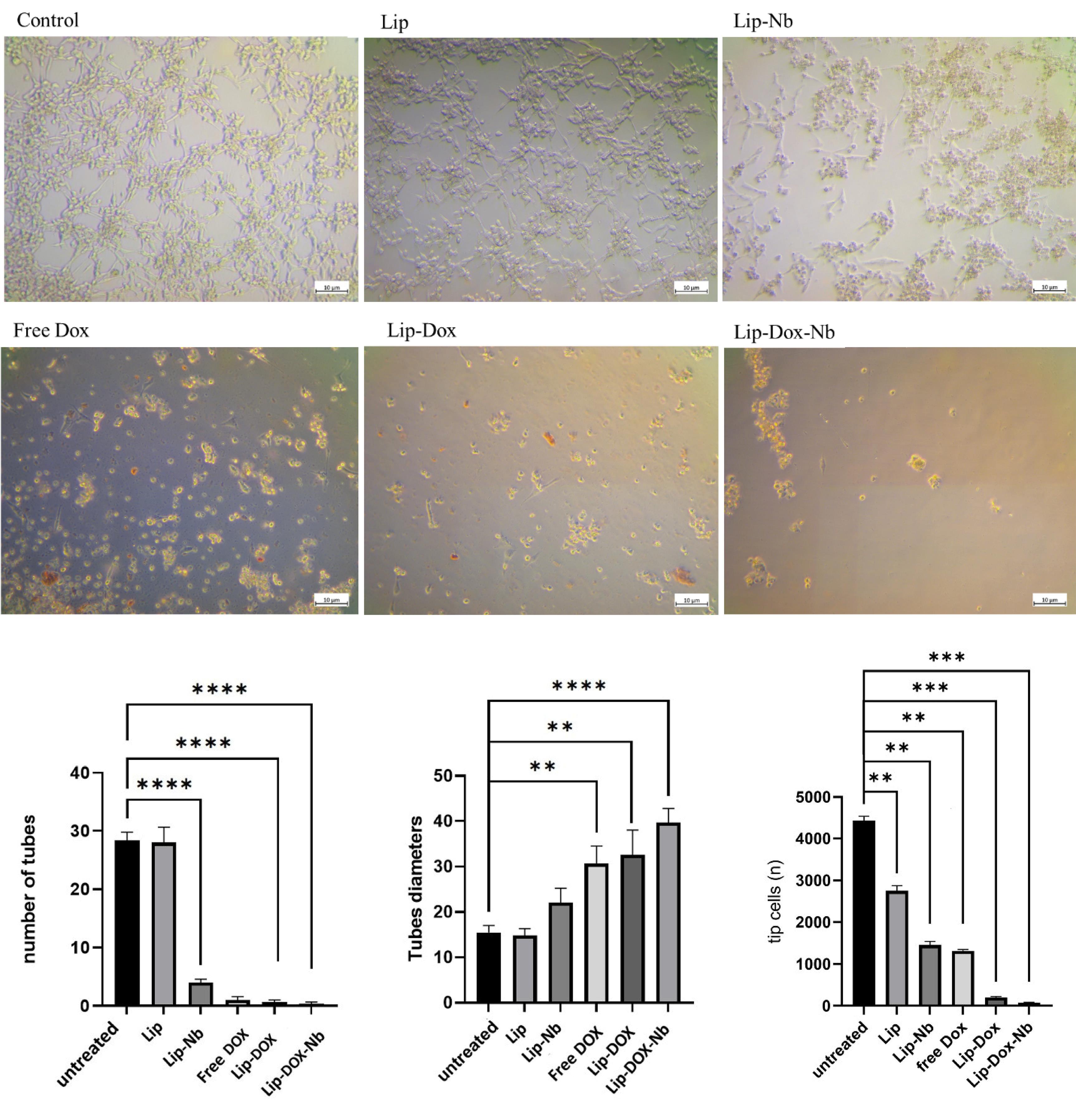
Fig. 7.
Tube Formation Assay on HUVEC cells.
.
Tube Formation Assay on HUVEC cells.
As depicted in Fig. 7, In the untreated group and Lip group, prominent tube formation and intense accumulation of tip cells with a normal spatial distribution were observed. However, in the Lip-Nb group, the number of tubes was significantly reduced (P < 0.05). Additionally, the mean tube diameters were notably increased, suggesting that the migration of endothelial cells (ECs) was markedly diminished. In the Lip-Nb treated group, there was a marked decrease in the number of the completed tube-like structures and also the number of tip cells that initiate angiogenesis (P < 0.05). Moreover, the mean tube diameters were significantly higher in the Lip-Nb treated group compared to the untreated group (P < 0.05). In the free DOX treated group, the formation of complete tube-like structures was totally impaired. In the Lip-DOX treated group also nearly comparable results in line with the results of free DOX were obtained (P < 0.05), indicating cell proliferation suppression through DOX inducing DNA damage. In the Lip-DOX-Nb treated group also, both tip cells and tube-like structures formation was totally inhibited and only cellular debris was observed,indicating both cell proliferation suppression through DOX and tube formation inhibition through anti VEGFR2 Nanobody blocking performance of Lip-DOX-Nb.
DHM analysis
In the current study, we also performed a single cell DHM analysis of the cells incubated with each formulation to assess cells nuclear integrity. Cells had intact nuclei in the untreated or liposome-treated group however the nuclear integrity was completely lost in the lip-DOX group which suggests that DOX has successfully caused cell death. Similarly, in the Lip-DOX-Nb and free DOX group only cell debris were present suggesting that cells have undergone apoptosis. Moreover, in the Lip-Nb group cells were swollen suggesting that cells were undergoing necroptotic changes however this claim needs further credence by future studies as a future goal (Fig. 8). Quantifying data obtained from cells Volume Changes (∆ Volume) after treating cells with free DOX, Lip-DOX and specially Lip-DOX-Nb formulations showed necrosis and apoptosis occurring, which is favorable for destruction of cancerous cells.
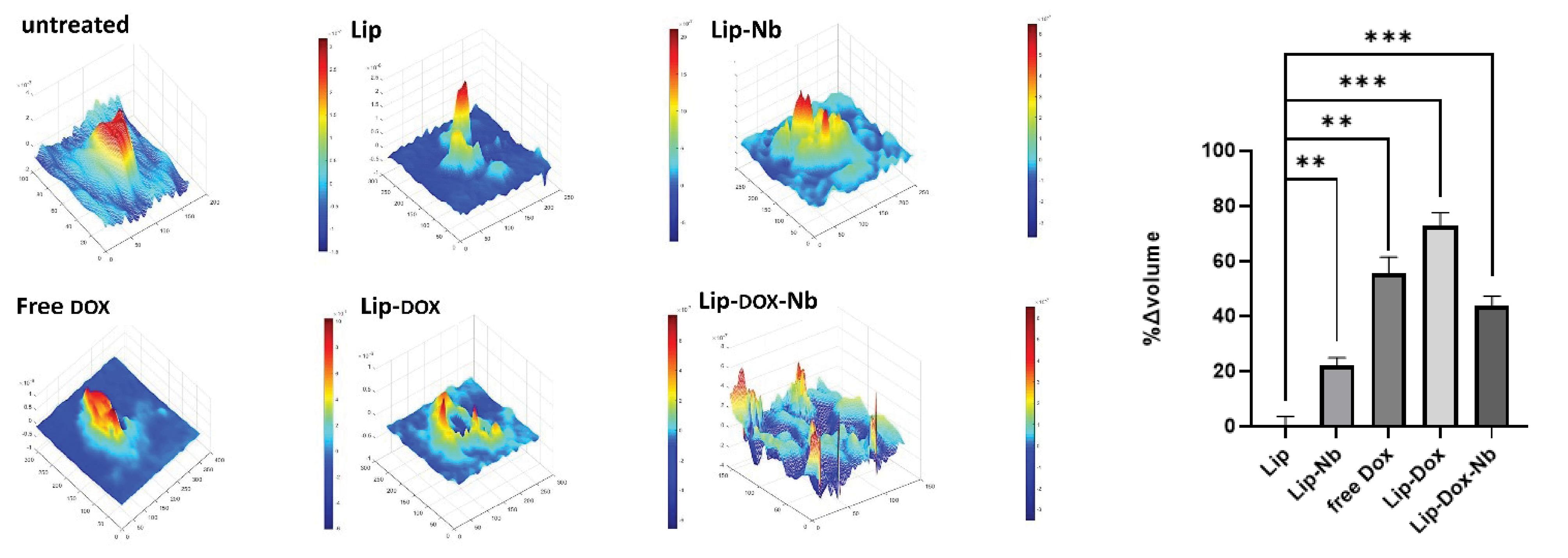
Fig. 8.
DHM analysis of the U87 cells after treatment.
.
DHM analysis of the U87 cells after treatment.
As shown, untreated cells and those treated with liposomes exhibited a spindle-shaped morphology with intact nuclei. In contrast, cells treated with Lip-Nb appeared swollen, indicating that they were undergoing the necroptosis process. In the free DOX treatment group, only cellular debris was observed. Additionally, in the Lip-DOX treatment group, cell nuclei were damaged, reflecting the direct action of DOX on the nuclei. In the Lip-DOX-Nb group, the cells were completely dead, with only small fragments of cellular debris remaining.
Discussion
Tumor-associated angiogenesis is one of the significant aspects of cancer development and metastasis.35 Angiogenesis is the formation of new blood vessels which is necessary to maintain sufficient blood supply; oxygen and nutrients. Tumor-induced angiogenesis involves the release of various angiogenic factors such as VEGF; which play crucial role in promoting the growth of tumor vessels and causes morphological changes in vascular endothelial cells and surrounding extracellular matrix.37 The VEGF family includes VEGFA, VEGFB, VEGFC, VEGFD, and placenta growth factor.36 These ligands bind to three endothelial receptor tyrosine kinases (RTKs), such as VEGFR family, and resulting downstream signaling pathways to angiogenesis and lymph angiogenesis.
In recent years, targeted therapy has emerged as an effective treatment for tumor angiogenesis VEGF/VEGFR downstream signaling pathways are promising targets in anti-angiogenic therapy.38 Molecules blocking the interaction of VEGF and VEGFR can prevent the activation of VEGFR signaling, resulting in anti-angiogenic affects. “Bevacizumab” and “ramucirumab”, act as VEGF/VEGFR interaction inhibitors and are approved as anti-angiogenic drugs to treat cancers.38 VEGFR2 is the main signaling VEGFR in blood vascular endothelial cells and is a suitable target for cancer therapy.39
One of the most commonly used drugs for chemotherapy is DOX which is sold under the name adriamycin.40 But the major concern for DOX treatment is the cardiotoxicity which makes it dosage dependent.4 Apart from cardiotoxicity, DOX also shows lipid peroxidation, DNA damage and build-up of tumor suppressor protein. One approach to overcome these, is using DOX encapsulated liposomes; represents the most successful strategy to date for increasing the therapeutic index of DOX. The encapsulation of DOX into nanosized liposomes significantly enhances the pharmacokinetic profile of liposomal DOX compared to free DOX. Doxil®, liposomal formulation of DOX, is approved by US the Food and Drug Administration.6 A further advancement in this line of treatment is targeting drug delivery.
In the present study, as depicted in Figure 1, an anti-VEGFR2 Nb targeting on the VEGFR2 antigen was conjugated on DOX liposomes. The resulting nano liposome were loaded with DOX and we investigated their effect on tumor angiogenesis. All liposomes prepared by thin-film hydration followed by extrusion method and homogenous unilamellar vesicles are formed. Extrusion is the technique to control vesicle size.41
The physicochemical characteristics of liposomal nanoparticles play a critical role in their behavior in the biological matrix and their ultimate fate, thereby affecting the biodistribution and biological activity of the drug.42
In this study, the size of all prepared nano liposomes was less than 200 nm (Table 1) with a smooth spherical shape as displayed in TEM imaging, and PDI less than 0.2 (Fig. 2) which can target antibodies to tumors by enhanced permeation and retention (EPR) mechanisms and induce higher cellular binding/uptake by tumor cells.43 The size of the Lip-Nb and Lip-DOX-Nb were more than Lip and Lip-DOX formulas. The increased size was attributed to the conjugated Nbs on their surface. The surface charge of Lip-Nb and Lip-DOX-Nb is slightly negative confirming successful Nb conjugation on Liposomes surface and cause to sterically stabilized liposomes. Conjugation of Nb to lipid was observed as a band shift due to increased molecular weight, in comparison with unconjugated liposome, in SDS-PAGE. The conjugation efficiency of Nanobody was significantly higher (CE%⁓ 87), owing to the high bonding efficiency between the thiol group and maleimide via the Michael addition reaction. In addition, the encapsulation efficiency of DOX was notably high (EE%⁓90), attributed to the gradient loading method.
The in vitro release profile of DOX from liposomes (Fig. 3) demonstrated sustained slow-release rate in plasma matrix (pH 7.4) with no obvious burst release and more than 85% of the encapsulated DOX was retained in nanoliposomes at 37 ℃, after 48 hours of incubation. This formulation is ideal to prevents systemic drug release and enhances drug accumulation at tumor sites.23 This phenomenon was corroborated by the cytotoxicity levels of DOX-loaded liposomes, which exhibited lower cell viability percentage in HUVECs after exposure (Supplementary file 1, Fig. S1). This is attributed to the prolonged release of DOX from liposomes in extracellular matrix but improves cellular uptake and intracellular DOX accumulation, leading to a more potent inhibitory effect of the anti-VEGFR2 moiety bonding and greater toxic drug accumulation within the cells (viability% of 32.5%).
To achieve targeted delivery of DOX, uptake or binding of Nb to VEGFR2 antigen on target cell is needed. Flow cytometry results were proved efficiently uptake of Lip-DOX-Nb to VEGFR2-positive HUVEC cell in compare with Lip-DOX with lower fluorescent intensity, causes high levels of intracellular DOX delivering, accumulation23 and also increased cellular apoptosis rate, an ideal cytotoxicity effect on tumor cells, by prolonged exposure (Figs. 4-5). These results are in line with DHM analysis that confirmed apoptosis effect of Nb-conjugated liposomal dox with completely nuclear integrity loss of U87 cancer cells (Fig. 8). On the other hand, attachment of anti-VEGFR2 moiety of Lip-DOX-Nb to VEGF receptor 2 on endothelial cells, blocking angiogenesis downstream signaling pathways, promotes cell senescence and apoptosis.
The conjugation of anti-VEGFR2 Nbs to liposomes were designed to inhibit or reduce the formation of new neovascular tubes through binding to VEGFR 2 on endothelial cell (EC) membranes and blocking their function. This objective was successfully demonstrated in tube formation assays using normal HUVEC cells (Fig. 7). Notably, the formation of tip cells and tube-like structures in HUVECs was completely inhibited following exposure to the Lip-DOX-Nb formulation, cellular debris and apoptotic bodies were observed, indicating a high rate of cell death due to the potent and effective targeting of this nano drug delivery system.20
Endothelial cell migration is an essential to support angiogenic activity.44 GBM, which is one of the most well-known and highly vascularized solid tumors, exhibits an overextension of both VEGFs and VEGFRs in their extent to neovascularization.45,46 Scratch assay on U87 cell lines (Fig. 6) revealed the superior efficacy of Lip-DOX-Nb in inhibiting cells migration resulting VEGFR2 inhibition.20
Our results are consistent with previous literature, indicating that tumor vasculature-targeting liposomes hold promise as a future prospect for delivering chemotherapeutic agents to tumor sites while minimizing adverse events.47-49
Conclusion
In this study, a novel anti-VEGFR2-conjugated liposomal DOX drug delivery system was designed and developed for angiogenesis inhibition in chemotherapy. A series of in vitro experiments confirmed the targeting ability of the anti-VEGFR2 Nanobody used in this system. The high cellular uptake demonstrated that the Lip-DOX-Nb formulation exhibited a strong affinity towards HUVEC cells, which highly express VEGFR2.
Research Highlights
What is the current knowledge?
What is new here?
-
Targeting the DOX loaded Liposomes as combinatorial therapeutic can improve the treatment outcomes.
-
Targeting the VEGFR2 can help to block proliferation of cancer cells and thereby can be used as antiangiogenics agent.
Conflict of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Statement
Not applicable.
Supplementary files
Supplementary file 1 contains Fig. S1.
(pdf)
Acknowledgements
This work was supported by research grants from the Iran University of Medical Sciences (grant/award number: 32613), Tehran, Iran and Zanjan University of Medical Sciences, Zanjan, Iran. The authors would like to express their deep gratitude to all who provided support during the course of this research, especially Kamran Mansuri in KUM university. We also thank the Razi Drug Research Center, Iran University of Medical Sciences, for providing analytical instruments.
References
- Chabner BA, Roberts TG Jr. Timeline: chemotherapy and the war on cancer. Nat Rev Cancer 2005; 5:65-72. doi: 10.1038/nrc1529 [Crossref] [ Google Scholar]
- Najafi M, Hooshangi Shayesteh MR, Mortezaee K, Farhood B, Haghi-Aminjan H. The role of melatonin on doxorubicin-induced cardiotoxicity: a systematic review. Life Sci 2020; 241:117173. doi: 10.1016/j.lfs.2019.117173 [Crossref] [ Google Scholar]
- Pugazhendhi A, Edison T, Velmurugan BK, Jacob JA, Karuppusamy I. Toxicity of doxorubicin (DOX) to different experimental organ systems. Life Sci 2018; 200:26-30. doi: 10.1016/j.lfs.2018.03.023 [Crossref] [ Google Scholar]
- Mohan UP, Tirupathi Pichiah PB, Iqbal ST, Arunachalam S. Mechanisms of doxorubicin-mediated reproductive toxicity - a review. Reprod Toxicol 2021; 102:80-9. doi: 10.1016/j.reprotox.2021.04.003 [Crossref] [ Google Scholar]
- Nakamura Y, Mochida A, Choyke PL, Kobayashi H. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer?. Bioconjug Chem 2016; 27:2225-38. doi: 10.1021/acs.bioconjchem.6b00437 [Crossref] [ Google Scholar]
- Barenholz YC. Doxil®—the first FDA-approved nano-drug: from an idea to a product. In: Handbook of Harnessing Biomaterials in Nanomedicine. Jenny Stanford Publishing; 2021. p. 463-528.
- Dawidczyk CM, Kim C, Park JH, Russell LM, Lee KH, Pomper MG. State-of-the-art in design rules for drug delivery platforms: lessons learned from FDA-approved nanomedicines. J Control Release 2014; 187:133-44. doi: 10.1016/j.jconrel.2014.05.036 [Crossref] [ Google Scholar]
- Hamilton A, Biganzoli L, Coleman R, Mauriac L, Hennebert P, Awada A. EORTC 10968: a phase I clinical and pharmacokinetic study of polyethylene glycol liposomal doxorubicin (Caelyx, Doxil) at a 6-week interval in patients with metastatic breast cancer European Organization for Research and Treatment of Cancer. Ann Oncol 2002; 13:910-8. doi: 10.1093/annonc/mdf157 [Crossref] [ Google Scholar]
- Judson I, Radford JA, Harris M, Blay JY, van Hoesel Q, le Cesne A. Randomised phase II trial of pegylated liposomal doxorubicin (Doxil/Caelyx) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2001; 37:870-7. doi: 10.1016/s0959-8049(01)00050-8 [Crossref] [ Google Scholar]
- Marina NM, Cochrane D, Harney E, Zomorodi K, Blaney S, Winick N. Dose escalation and pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in children with solid tumors: a pediatric oncology group study. Clin Cancer Res 2002; 8:413-8. [ Google Scholar]
- Mangili G, Petrone M, Gentile C, De Marzi P, Viganò R, Rabaiotti E. Prevention strategies in palmar-plantar erythrodysesthesia onset: the role of regional cooling. Gynecol Oncol 2008; 108:332-5. doi: 10.1016/j.ygyno.2007.10.021 [Crossref] [ Google Scholar]
- Jung S, Sehouli J, Chekerov R, Kluschke F, Patzelt A, Fuss H. Prevention of palmoplantar erythrodysesthesia in patients treated with pegylated liposomal doxorubicin (Caelyx®). Support Care Cancer 2017; 25:3545-9. doi: 10.1007/s00520-017-3781-x [Crossref] [ Google Scholar]
- Schiffelers RM, Koning GA, ten Hagen TL, Fens MH, Schraa AJ, Janssen AP. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J Control Release 2003; 91:115-22. doi: 10.1016/s0168-3659(03)00240-2 [Crossref] [ Google Scholar]
- Pastorino F, Di Paolo D, Piccardi F, Nico B, Ribatti D, Daga A. Enhanced antitumor efficacy of clinical-grade vasculature-targeted liposomal doxorubicin. Clin Cancer Res 2008; 14:7320-9. doi: 10.1158/1078-0432.Ccr-08-0804 [Crossref] [ Google Scholar]
- Chiang YT, Lo CL. pH-responsive polymer-liposomes for intracellular drug delivery and tumor extracellular matrix switched-on targeted cancer therapy. Biomaterials 2014; 35:5414-24. doi: 10.1016/j.biomaterials.2014.03.046 [Crossref] [ Google Scholar]
- Raavé R, van Kuppevelt TH, Daamen WF. Chemotherapeutic drug delivery by tumoral extracellular matrix targeting. J Control Release 2018; 274:1-8. doi: 10.1016/j.jconrel.2018.01.029 [Crossref] [ Google Scholar]
- Al-Husein B, Abdalla M, Trepte M, Deremer DL, Somanath PR. Antiangiogenic therapy for cancer: an update. Pharmacotherapy 2012; 32:1095-111. doi: 10.1002/phar.1147 [Crossref] [ Google Scholar]
- Cruz E, Kayser V. Monoclonal antibody therapy of solid tumors: clinical limitations and novel strategies to enhance treatment efficacy. Biologics 2019; 13:33-51. doi: 10.2147/btt.S166310 [Crossref] [ Google Scholar]
- Bathula NV, Bommadevara H, Hayes JM. Nanobodies: the future of antibody-based immune therapeutics. Cancer Biother Radiopharm 2021; 36:109-22. doi: 10.1089/cbr.2020.3941 [Crossref] [ Google Scholar]
- Behdani M, Zeinali S, Khanahmad H, Karimipour M, Asadzadeh N, Azadmanesh K. Generation and characterization of a functional nanobody against the vascular endothelial growth factor receptor- 2; angiogenesis cell receptor. Mol Immunol 2012; 50:35-41. doi: 10.1016/j.molimm.2011.11.013 [Crossref] [ Google Scholar]
- Fontanella C, Ongaro E, Bolzonello S, Guardascione M, Fasola G, Aprile G. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med 2014; 2:123. doi: 10.3978/j.issn.2305-5839.2014.08.14 [Crossref] [ Google Scholar]
- Song X, Wan Z, Chen T, Fu Y, Jiang K, Yi X. Development of a multi-target peptide for potentiating chemotherapy by modulating tumor microenvironment. Biomaterials 2016; 108:44-56. doi: 10.1016/j.biomaterials.2016.09.001 [Crossref] [ Google Scholar]
- Farasat A, Rahbarizadeh F, Ahmadvand D, Ranjbar S, Khoshtinat Nikkhoi S. Effective suppression of tumour cells by oligoclonal HER2-targeted delivery of liposomal doxorubicin. J Liposome Res 2019; 29:53-65. doi: 10.1080/08982104.2018.1430829 [Crossref] [ Google Scholar]
- Chin DL, Lum BL, Sikic BI. Rapid determination of PEGylated liposomal doxorubicin and its major metabolite in human plasma by ultraviolet-visible high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 2002; 779:259-69. doi: 10.1016/s1570-0232(02)00395-1 [Crossref] [ Google Scholar]
- Qian Y, Wang W, Wang Z, Han Q, Jia X, Yang S. Switchable probes: pH-triggered and VEGFR2 targeted peptides screening through imprinting microarray. Chem Commun (Camb) 2016; 52:5690-3. doi: 10.1039/c6cc01302c [Crossref] [ Google Scholar]
- Chamani R, Asghari SM, Alizadeh AM, Mansouri K, Doroudi T, Kolivand PH. The antiangiogenic and antitumor activities of the N-terminal fragment of endostatin augmented by Ile/Arg substitution: the overall structure implicated the biological activity. Biochim Biophys Acta 2016; 1864:1765-74. doi: 10.1016/j.bbapap.2016.09.014 [Crossref] [ Google Scholar]
- Badran M, Shalaby K, Al-Omrani A. Influence of the flexible liposomes on the skin deposition of a hydrophilic model drug, carboxyfluorescein: dependency on their composition. ScientificWorldJournal 2012; 2012:134876. doi: 10.1100/2012/134876 [Crossref] [ Google Scholar]
- Fu M, Tang W, Liu JJ, Gong XQ, Kong L, Yao XM. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J Drug Target 2020; 28:245-58. doi: 10.1080/1061186x.2019.1656725 [Crossref] [ Google Scholar]
- Deamer DW, Bramhall J. Permeability of lipid bilayers to water and ionic solutes. Chem Phys Lipids 1986; 40:167-88. doi: 10.1016/0009-3084(86)90069-1 [Crossref] [ Google Scholar]
- Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (part 2). Trop J Pharm Res 2013; 12:265-73. doi: 10.4314/tjpr.v12i2.20 [Crossref] [ Google Scholar]
- Mady MM, Shafaa MW, Abbase ER, Fahium AH. Interaction of doxorubicin and dipalmitoylphosphatidylcholine liposomes. Cell Biochem Biophys 2012. 62: 481-6. doi: 10.1007/s12013-011-9334-x.
- Nele V, Holme MN, Kauscher U, Thomas MR, Doutch JJ, Stevens MM. Effect of formulation method, lipid composition, and PEGylation on vesicle lamellarity: a small-angle neutron scattering study. Langmuir 2019; 35:6064-74. doi: 10.1021/acs.langmuir.8b04256 [Crossref] [ Google Scholar]
- Zhang C, Pan D, Luo K, She W, Guo C, Yang Y. Peptide dendrimer-doxorubicin conjugate-based nanoparticles as an enzyme-responsive drug delivery system for cancer therapy. Adv Healthc Mater 2014; 3:1299-308. doi: 10.1002/adhm.201300601 [Crossref] [ Google Scholar]
- Mazurek R, Dave JM, Chandran RR, Misra A, Sheikh AQ, Greif DM. Vascular cells in blood vessel wall development and disease. Adv Pharmacol 2017; 78:323-50. doi: 10.1016/bs.apha.2016.08.001 [Crossref] [ Google Scholar]
- Hermans D, Rodriguez-Mogeda C, Kemps H, Bronckaers A, Helga E, Broux BJA. Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis 2023; 26:349-62. doi: 10.1007/s10456-023-09876-7 [Crossref] [ Google Scholar]
- Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol 2011; 27:563-84. doi: 10.1146/annurev-cellbio-092910-154002 [Crossref] [ Google Scholar]
- Luo Q, Wang J, Zhao W, Peng Z, Liu X, Li B. Vasculogenic mimicry in carcinogenesis and clinical applications. J Hematol Oncol 2020; 13:19. doi: 10.1186/s13045-020-00858-6 [Crossref] [ Google Scholar]
- Wang L, Liu WQ, Broussy S, Han B, Fang H. Recent advances of anti-angiogenic inhibitors targeting VEGF/VEGFR axis. Front Pharmacol 2023; 14:1307860. doi: 10.3389/fphar.2023.1307860 [Crossref] [ Google Scholar]
- Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res 2003; 93:354-63. doi: 10.1161/01.Res.0000089257.94002.96 [Crossref] [ Google Scholar]
- Tan YY, Yap PK, Xin Lim GL, Mehta M, Chan Y, Ng SW. Perspectives and advancements in the design of nanomaterials for targeted cancer theranostics. Chem Biol Interact 2020; 329:109221. doi: 10.1016/j.cbi.2020.109221 [Crossref] [ Google Scholar]
- Pande S. Liposomes for drug delivery: review of vesicular composition, factors affecting drug release and drug loading in liposomes. Artif Cells Nanomed Biotechnol 2023; 51:428-40. doi: 10.1080/21691401.2023.2247036 [Crossref] [ Google Scholar]
- Wang Y, Wang J, Zhu D, Wang Y, Qing G, Zhang Y. Effect of physicochemical properties on in vivo fate of nanoparticle-based cancer immunotherapies. Acta Pharm Sin B 2021; 11:886-902. doi: 10.1016/j.apsb.2021.03.007 [Crossref] [ Google Scholar]
- Nikpoor AR, Tavakkol-Afshari J, Gholizadeh Z, Sadri K, Babaei MH, Chamani J. Nanoliposome-mediated targeting of antibodies to tumors: IVIG antibodies as a model. Int J Pharm 2015; 495:162-70. doi: 10.1016/j.ijpharm.2015.08.048 [Crossref] [ Google Scholar]
- Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res 2007; 100:782-94. doi: 10.1161/01.RES.0000259593.07661.1e [Crossref] [ Google Scholar]
- Hosseini H, Rajabibazl M, Ebrahimizadeh W, Rafiei Dehbidi G. Inhibiting angiogenesis with human single-chain variable fragment antibody targeting VEGF. Microvasc Res 2015; 97:13-8. doi: 10.1016/j.mvr.2014.09.002 [Crossref] [ Google Scholar]
- Alhagh Charkhat Gorgich E, Kasbiyan H, Shabani R, Mehdizadeh M, Hajiahmadi F, Ajdary M. Smart chlorotoxin-functionalized liposomes for sunitinib targeted delivery into glioblastoma cells. J Drug Deliv Sci Technol 2022; 77:103908. doi: 10.1016/j.jddst.2022.103908 [Crossref] [ Google Scholar]
- Zhang Y, Zhai M, Chen Z, Han X, Yu F, Li Z. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv 2017; 24:1045-55. doi: 10.1080/10717544.2017.1344334 [Crossref] [ Google Scholar]
- Orleth A, Mamot C, Rochlitz C, Ritschard R, Alitalo K, Christofori G. Simultaneous targeting of VEGF-receptors 2 and 3 with immunoliposomes enhances therapeutic efficacy. J Drug Target 2016; 24:80-9. doi: 10.3109/1061186x.2015.1056189 [Crossref] [ Google Scholar]
- Aldughaim MS, Muthana M, Alsaffar F, Barker MD. Specific targeting of PEGylated liposomal doxorubicin (Doxil®) to tumour cells using a novel TIMP3 peptide. Molecules 2020; 26:100. doi: 10.3390/molecules26010100 [Crossref] [ Google Scholar]