Bioimpacts. 2025;15:30867.
doi: 10.34172/bi.30867
Original Article
Anticancer impacts of the unicellular cyanobacterium Chroococcus turgidus bioactive compounds in colorectal adenocarcinoma
Hamieh Goshtasbi Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing, 1, 2, 3 
Azam Safary Investigation, Methodology, Validation, Visualization, Writing – original draft, 4 
Ali Movafeghi Conceptualization, Resources, Visualization, 1 
Jaleh Barar Conceptualization, Visualization, 5 
Mostafa Akbarzadeh-Khiavi Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing, 6, * 
Yadollah Omidi Conceptualization, Supervision, Writing – review & editing, 5, * 
Author information:
1Department of Plant, Cell and Molecular Biology, Faculty of Natural Sciences, University of Tabriz, Tabriz, Iran
2Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Mechanics and Aerospace Engineering, Southern University of Science and Technology, Shenzhen, Guangdong 518055, China
4Connective Tissue Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Pharmaceutical Sciences, Barry and Judy Silverman College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL 33328, USA
6Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Microalgae and cyanobacteria are promising sources of bioactive compounds with antioxidant and anticancer properties. The cyanobacterium Chroococcus turgidus has been studied for its potential antioxidant, anti-inflammatory, antibacterial, antiviral, and anticancer effects. This study investigates its anticancer effects on colorectal cancer (CRC) at the cellular and molecular levels.
Methods:
The metabolites of C. turgidus were screened using the Folin–Ciocalteu reagent and GC-MS. Antioxidant activity was assessed using the DPPH assay. The biological effects of methanolic extract (ME) were evaluated using MTT assay, Annexin V/PI staining, DAPI staining, and western blotting. Cells were treated with ME at concentrations ranging from 5 to 500 µg/mL for 24 and 48 hours, with the IC50 values determined at 373 µg/mL and 291 µg/mL, respectively.
Results:
ME contained bioactive compounds such as phenols, flavonoids, and anthocyanins. Identified fatty acids included palmitic acid ethyl ester (15.53%), 1-bromo-11-iodoundecane (2.31%), undecanoic acid 2,8-dimethyl methyl ester (6.62%), oleic acid (6.47%), and 7-dehydrocholesterol (7.97%). ME inhibited SW480 cell proliferation in a dose- and time-dependent manner and induced nuclear fragmentation, chromatin remodeling, and apoptosis. Annexin V/PI staining confirmed apoptosis as the dominant mode of cell death. Western blot analysis showed increased Bax and decreased Bcl2 expression, supporting its pro-apoptotic activity.
Conclusion:
C. turgidus may serve as a potential therapeutic agent for gastrointestinal cancers through its ability to modulate the Bax/Bcl2 pathway and promote apoptosis. These findings highlight its novel anticancer effects and support further preclinical investigations.
Keywords: Antioxidant, Chroococcus turgidus, Methanolic extract, Colorectal cancer
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
As a part of a Ph.D. thesis, this work was carried out at TUOMS Biomedicine Institute, Research Center for Pharmaceutical Nanotechnology (# 60572).
Introduction
In recent years, cancer has emerged as one of the most significant global health challenges, consistently ranking as the second leading cause of death across many nations. The World Health Organization (WHO) anticipates a rise in cancer-related mortality rates, particularly in low- and middle-income nations, where access to early detection, treatment, and healthcare resources remains limited. This growing burden underscores the urgent need for comprehensive prevention strategies, improved healthcare infrastructure, and equitable access to cancer care worldwide.1 Globally, colorectal cancer (CRC) ranks as the second most common cancer among women and the third among men.2 The mortality rate of CRC may vary by as much as tenfold, highlighting the widening disparities and increasing disease burden in countries undergoing economic and healthcare transitions. While significant progress has been made in improving CRC outcomes in more developed nations, there persists a significant clinical gap, particularly in regions with limited resources, where access to early detection, advanced treatments, and comprehensive care is often restricted. Addressing these gaps is crucial for reducing the global burden of CRC and ensuring equitable healthcare outcomes.3,4 Different approaches are routinely used in the treatment of various cancers, including chemotherapy, radiotherapy, surgery, cryosurgery, radiation therapy, and immunotherapy.5 However, these treatment modalities are often associated with expensive processes and may result in severe side effects.6,7 Moreover, these combined treatment approaches are not without flaws, often leading to various secondary health threats, unspecific outcomes, and unavoidable toxicity.8,9 In light of these challenges, there is an urgent demand for cost-effective treatment strategies that exhibit reduced side effects and are suitable for a broad range of cancers. The use of traditional natural product-based strategies is becoming more widely recognized for their potential in pharmaceutical innovation, offering promising solutions. The successful isolation and development of natural biomaterials (e.g., carbohydrates, lipids, proteins, enzymes, and secondary metabolites) have opened new avenues for the creation of therapeutic compounds and pharmacophores. This progress has invigorated the scientific community, driving renewed efforts to harness these natural products in the drug discovery and evaluation process with greater enthusiasm and focus.8,10-12 Significant efforts have been dedicated to isolating such compounds, leading to the identification of over 10,000 natural products that may have biotechnological applications. However, the overwhelming abundance of metabolites and their vast dynamic range have limited access to many bioactive natural products, hindering further investigation. Additionally, complex challenges (e.g., evaluating pharmacokinetics, pharmacodynamics, and safety parameters) have emerged as major concerns in the study of these natural products.13-15 The unique ecological, chemical, and biological properties of marine environments have endowed microalgae and cyanobacteria with the potential to produce a variety of bioactive chemical compounds. These compounds, such as phenols, flavonoids, and anthocyanins, have various health benefits and find applications in therapeutics.16 Notably, cyanobacteria, traditionally known for containing chlorophyll a and phycobiliproteins (i.e., phycocyanin and phycoerythrin), have been found to exhibit greater pigment diversity. Recent research suggests the presence of chlorophyll b in some cyanobacteria, challenging previous notions and indicating an evolutionary complexity in their photosynthetic apparatus.17 This includes potential ancestral traits and adaptations allowing efficient light capture across different environments. Such findings reveal that cyanobacteria can utilize various chlorophylls (including chlorophylls d and f), enhancing our understanding of their photosynthetic mechanisms and evolutionary history.18,19 Microalgae and cyanobacteria-derived phytochemicals have distinct and high potential biological actions compared to the phytochemical constituents of terrestrial origin (plant phytochemicals).16,20,21 Of cyanobacteria, some species have been approved as safe for human consumption, such as Spirulina or Chlorella, in large part due to their useful secondary metabolites. Notably, lower organisms elaborate numerous secondary metabolites or natural products as signaling molecules for "offense and defense". Recently, these metabolites have been extracted, their actions were checked in various bioassays, and their potential as a remedy for human diseases was evaluated.22-24 This bioprospecting study aimed to develop novel anticancer agents with enhanced efficacy, focusing on the unicellular cyanobacterium Chroococcus turgidus, which was isolated from the KANI Barazan International Wetland, located to the south of Lake Urmia. The methanolic extract (ME) of C. turgidus was evaluated for its potential to induce apoptosis in the SW480 colon cancer cell line. The antioxidant activity of the ME was assessed using the diphenyl picryl hydrazyl (DPPH) assay. In addition, its ability to inhibit cancer cell growth was thoroughly investigated through multiple techniques, including flow cytometry, DAPI (4′,6-diamidino-2-phenylindole) staining, and western blot analysis. The combination of these methods facilitated a comprehensive evaluation of the ME's anticancer properties.
Materials and Methods
Materials
Ascorbic acid, Folin-Ciocalteu reagent, and Quercetin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Gallic acid, Methanol, and DPPH were obtained from Merck (Kenilworth, NJ, USA). The human colorectal carcinoma SW-480 cell line was obtained from the National Cell Bank of Iran, Pasteur Institute (Tehran, Iran). RPMI 1640 medium, fetal bovine serum (FBS), and trypsin-EDTA (0.02–0.05%) were acquired from Gibco (Paisley, UK). Phosphate-buffered saline (PBS) was purchased from Sigma-Aldrich Company (Munich, Germany). Mouse β-Actin (sc-47778) and Bax (sc-7480) monoclonal antibodies, rabbit Bcl2 (sc-492) antibody, and mouse anti-rabbit IgG-HRP (sc-2357) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The reverse transcriptase reagent and FITC-labeled annexin V- apoptosis detection kits were purchased from TAKARA Co. (Tokyo, Japan) and Applied Biosystems (Foster City, CA, USA), respectively.
Isolation of C. turgidus
The water samples were collected from the regional KANI Barazan International Wetland, which located 30 km from Mahabad city in the West Azerbaijan province (NW Iran) with the geographical position of N 36° 59ˊ 34 ̋ and E 45° 46ˊ 34 ̋. The identification of C. turgidus was accomplished by using the algal flora keys. For the purification of C. turgidus, single colonies were picked and transferred into the 50 mL flasks containing 1M modified BG 11’s medium composed of NaNO3 (. 15 g/L), K2HPO4 (4.0 g/L), MgSO4.7H2O (7.5 g/L), CaCl2.2H2O (3.6 g/L), C6H8O7 (0.6 g/L), (NH4)5[Fe(C6H4O7)2 (0. 6 g/L), EDTANa2 (0.1 g/L), Na2CO3 (2.0 g/L), H3BO3 (2.86 g/L), MnCl2.4H2O (. 181 g/L), ZnSO4.7H2O (0.22 g/L), Na2MoO4.2H2O (0.39 g/L), CuSO4.5H2O (0.08 g/L), Co (NO3) 2.6H2O (0.05 g/L). Moreover, the culture media of Chroococcus turgidus was kept under the required condition (i.e., 26 ˚C temperature with 16:8 light: dark photoperiod, and 80 μmol photon m-2 s-1 irradiance).
Molecular characterization of C. turgidus
After morphological identification, molecular characterizations using16S rRNA gene and 16S-23S ITS region were applied for the accurate and reliable identification of the isolated cyanobacteria, identified as C. turgidus strain KANI.25
Preparation of methanolic extracts of C. turgidus
The cyanobacteria cells were harvested from 500 mL of BG 11’s medium (O.D. 630¼. 15–2.0) by centrifugation (3000g for 5 minutes, at 4 °C) and then freeze-dried for the next process. The soxhlet apparatus extracted freeze-dried cyanobacteria biomass (5 g) with 125 mL of solvent for 7 hours. The extracts were concentrated in a Buchi rotary evaporator at 120 rpm and 60 °C for 2 hours, and then traces of solvent were removed using a desiccator. After filtration of 3 mL ME through the Whatman paper (grade 42), the gas chromatography-mass spectrometry (GC-MS) analysis was performed. The samples were dried under a laminar flow hood, and absolute alcohol and sodium sulfate were added to remove residual water. A quality control sample (blank solvent) was included to ensure the absence of contamination. The GC-MS analysis was conducted using an Agilent 6890 equipped with an HP-5MS (5% diphenyl/95% dimethyl polysiloxane) fused silica capillary column (30 m × 0.25 mm i.d., film thickness 0.25 µm). The compounds were identified by comparing the obtained mass spectra with those available in the National Institute of Standards and Technology (NIST) library, which contains over 62,000 reference patterns. For further validation, an internal standard (n-alkane series, C10 – C40) was used to calibrate retention times, and the spectra of unknown compounds were matched with authenticated reference spectra from the NIST database. Additionally, retention indices (RI) were calculated and compared with literature data to enhance the reliability of compound identification.
Analysis of photosynthetic pigments
The photosynthetic pigments, including chlorophyll a, b, and total carotenoids, were assessed based on the previously described method.26 Briefly, cyanobacteria cells were harvested from 5 mL of media (O.D. 630¼. 15–2.0) by centrifugation (at 26 °C, 3000 g for 5 minutes). The collected biomass (equivalent to approximately 15 mg dry weight) was homogenized in 5 mL of 100% methanol at 4 °C for 24 hours in the dark. Then, the homogenates were centrifuged at 10,000 × g for 10 minutes at 4 °C to remove cell debris. For quantitative determination of pigments, the supernatants were analyzed using a UV/V spectrophotometer at 470, 665, and 653 nm. Methanol was used as a blank to correct baseline absorbance, and potential spectral interferences were minimized by ensuring complete extraction of pigments and avoiding contamination from cellular debris. The content of the pigments was determined based on standard equations. The content of the pigments was determined,26,27 as follows:
Ca = 15.65 A665 – 7.340 A653
Cb = 27.05 A653 – 1.121 A665
Cx + c = (1000 A470 – 2.860 Ca – 129.2 Cb)/245
Where, A denotes the absorbance in the presence of the sample, Ca and Cb represent the chlorophyll a, b and Cx + c denotes the total carotenoids in the presence of the sample.
Analysis of total phenol, flavonoid contents
Total phenols and flavonoids of the cyanobacteria cells were isolated using 100% methanol. The total phenol quantity of the extracts was measured using the Folin-Ciocalteu procedure according to the previous method.28,29 In brief, 100 μL of cyanobacterial extract was mixed with 2.8 mL of deionized water, 100 μL of Folin-Ciocalteu reagent, and 2 mL of sodium carbonate aqueous solution (2%, final concentration 0.4%). The sodium carbonate solution was prepared by first dissolving 2 g of NaOH in 500 mL of distilled water to obtain a 0.1 M NaOH solution. Then, 2 g of Na₂CO₃ was dissolved in 100 mL of this NaOH solution. The reaction mixture was incubated in the dark at room temperature for 30 minutes. Absorbance was measured at 720 nm using a UV/Vis spectrophotometer, with methanol as the blank. A calibration curve was prepared using gallic acid as the standard, and results were expressed as mg gallic acid equivalent (GAE) per gram of fresh weight (mg GAE/g F.W.).
The total flavonoid content was measured using an aluminum chloride colorimetric assay. For each reaction, 500 μL of cyanobacterial extract was mixed with 15 mL of 100% methanol, 100 μL of freshly prepared aluminum chloride solution (10%) (prepared by dissolving 10 g of AlCl₃ in 90 mL methanol), 100 μL of potassium acetate (1 M), and 2.8 mL of distilled water. The mixture was incubated at 25 °C for 40 minutes. The absorbance was recorded at 415 nm using a spectrophotometer, with methanol as the blank. The flavonoid content was calculated as mg quercetin equivalent per gram of fresh weight (mg QE/g F.W.).
For anthocyanin quantification, 500 μL of the extract was mixed with 4.5 mL of acidified methanol (1% HCl in methanol, v/v), and the mixture was incubated at 4 °C for 24 hours in the dark to prevent pigment degradation and enhance extraction efficiency. After incubation, the sample was centrifuged at 10,000 × g for 10 minutes at 4 °C. Absorbance was measured at 530 nm and 657 nm, with methanol as the blank, and the total anthocyanin content was calculated using the equation:
Total Anthocyanin (mg/L) = (A657 × 0.25 - A530)
Where A657 and A530 correspond to the absorbance at 530 and 657 nm, respectively. Cyanidin-3-glucoside was used as the standard for quantification.
Analysis of anthocyanin content
A 20 mg cyanobacteria cells sample was crushed in a porcelain mortar with 4 mL of hydrochloric acid containing 1% methanol to determine the total anthocyanin content. The mixture was kept in the refrigerator for 24 hours and then centrifuged at 13000 × g for 10 minutes. After collecting the supernatant, the absorbance (A) of the solution was determined at 530 and 657 nm and normalized against the control sample of hydrochloric acid containing 1 % methanol. The absorbance used for quantifying the anthocyanin content was calculated using the following equation.30
A = A530 – (0.25 × A657)
Where A denotes the absorbance in the presence of the sample.
Free radical DPPH scavenging capacity
The DPPH radical scavenging assay was carried out based on a method described previously by Ozturk and Tuncel.31 The DPPH assay is a free radical method based on the radical scavenging activity of antioxidants towards the purple-colored DPPH in methanol. The hydrogen donors of antioxidants can reduce the free radical DPPH to the corresponding stable diamagnetic molecule hydrazine (yellow-colored).31,32 Reaction mixtures of samples were prepared by mixing appropriate amounts of extract with different concentrations (100, 200, 300, 400, and 500 μg/mL), 2 ml of DPPH (0.1 mM in methanol 96%) to a total volume of 4 ml. All samples vortexed (1 min) and incubated in the dark for 60 min at 37 °C. All the experiments were done in triplicate. DPPH (0.1mM) was taken as control and ascorbic acid as standard. The decrease in absorbance of each sample was measured against methanol as blank on a spectrophotometer, Ultraspec 2000 (Pharmacia Biotech Co., Garden City, England) at 517 nm. The percentage of DPPH was calculated using the following equation:
Where, Ac and As denote the absorbance of the control reaction and the absorbance in the presence of the sample, respectively.
Cytotoxicity assays
The SW-480 cells were cultivated at a seeding density of 10 × 10⁴ cells/well in 96-well plates. After 24 h, the cells were exposed to 200 μL of fresh medium containing various concentrations (5, 10, 50, 100, 200, 300, 400, and 500 μg/mL) of the extracts. These concentrations were selected based on preliminary dose-response studies. After 24 and 48 hours, the medium of each well was replaced with 200 μL of fresh MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] reagent (2 mg/mL) for 4 hours. Next, the MTT reagent was removed, and then 200 μL dimethyl sulfoxide (DMSO) was added to stop the reaction, and the cells were incubated at 37 °C for an additional 15 min. The optical density was determined using a microplate reader, ELx808 (BioTeck, Winooski, VT), at 570 nm wavelength. The viability of cells was evaluated relative to the absorbance of untreated control cells. All experiments were carried out in triplicates.
Apoptosis assay by Annexin-V
A flow cytometry assay was performed to determine the induction of apoptosis in the treated SW480 cells with microalgae extract. Briefly, the SW480 cells were treated with ME (IC50 Dose) and incubated at 37°C for 24 hours. The final concentration of cells before staining was (10 × 105 cells/well in 12-well plates). The cells were then resuspended in 200 μL of annexin V-binding buffer (from EXBIO) and incubated at room temperature in the dark for 10 min. Afterward, the cells were washed with ice-cold PBS and subjected to flow cytometry analysis using FACSCaliburTM (Becton Dickinson Co., Franklin Lakes, NJ, USA) with an emission filter of 600 nm for P.I. and 515–545 nm for FITC.33 To ensure the validity of the analysis, untreated cells were used as a control group.
Apoptosis assay by DAPI staining
Given that nuclear fragmentation and chromatin condensation and remodeling are the typical markers of apoptosis, the DAPI staining assay was employed to analyze the occurrence of such phenomena in the treated cells. Briefly, the SW480 cells were treated with ME (IC50 Dose) and incubated at 37°C for 24 hours. Next, the cells were fixed with the freshly prepared ice-cold paraformaldehyde 4%. Then, to permeabilize the cells, they were exposed to 0.1% Triton X-100 in PBS (NaCl, KCl, KH2 PO4, and Na2HPO4, pH 7.4) for 5 minutes. The cells were stained with DAPI (1 μg/mL in PBS) in the dark for 3 min. Afterward, the cells were washed ( × 3) with 0.1% Triton X-100 in PBS. Next, they were assessed using a live imaging system, CytationTM 5 (BioTek, Winooski, USA).
Western blotting assay
The SW480 cells were cultivated at a seeding density of 2.0 × 105 cells/well in 6-well plates. Briefly, the SW480 cells were treated with the IC50 dose of ME and incubated at 37 °C for 24 hours. The treated and untreated cells were centrifuged to extract proteins using radioimmunoprecipitation assay (RIPA) lysis buffer containing a protease inhibitor cocktail (PMSF, leupeptin, and aprotinin). The homogenate was centrifuged at 5000 × g, 4 °C for 5 minutes. The concentration of protein samples was determined using the Bradford assay with BSA as the standard. Equal amounts of total protein were loaded per well of a 12% polyacrylamide gel (SDS-PAGE). The proteins were then transferred onto a polyvinylidene fluoride (PVDF) membrane at 100 V for 1 hour. The membranes were incubated in 5% bovine serum albumin (BSA) dissolved in 20 mM Tris–HCl, containing 150 mM NaCl and 0.05% Tween-20 at 4 °C overnight. After three washes, the membranes were incubated with specific primary antibodies (1:4000; Bax, Bcl2, and β-actin) followed by the secondary antibody (1:8,000; horseradish peroxidase-conjugated) in 3% BSA at room temperature for 2 hours and 1 hour, respectively. Protein bands were detected using the PierceTM ECL western blotting substrate chemiluminescent kit. The expression level of β-actin was used to normalize the protein levels. The quantification of Bax and Bcl2 expression was compared with the expression of the housekeeping protein and analyzed using ImageJ software.
Statistical analyses
The data were analyzed to determine statistical differences using one-way ANOVA followed by Tukey’s post hoc test. Statistical analyses were performed with SPSS software version 19.0, and a P value of ≤ 0.05 was considered statistically significant. All data are presented as mean ± standard deviation (SD).
Results
Morphological and molecular traits of isolated cyanobacteria
To identify the collected cyanobacteria, a valid authentication key, algal flora, and some recent related references were carefully used.34,35 C. turgidus strain KANI was identified based on some morphological characteristics, including shape and isolated two- or four-celled groups together with an amorphous mucilage sheath (Fig. 1). For the confirmation of morphologic findings, the 16S rRNA gene and 16S-23S ITS region from the cyanobacteria genome were amplified. Consequently, the samples were sequenced and blasted by means of the National Center for Biotechnology Information (NCBI) database. The blasting data confirmed the morphologic results where the isolated cyanobacteria showed a high genetic similarity to C. turgidus.
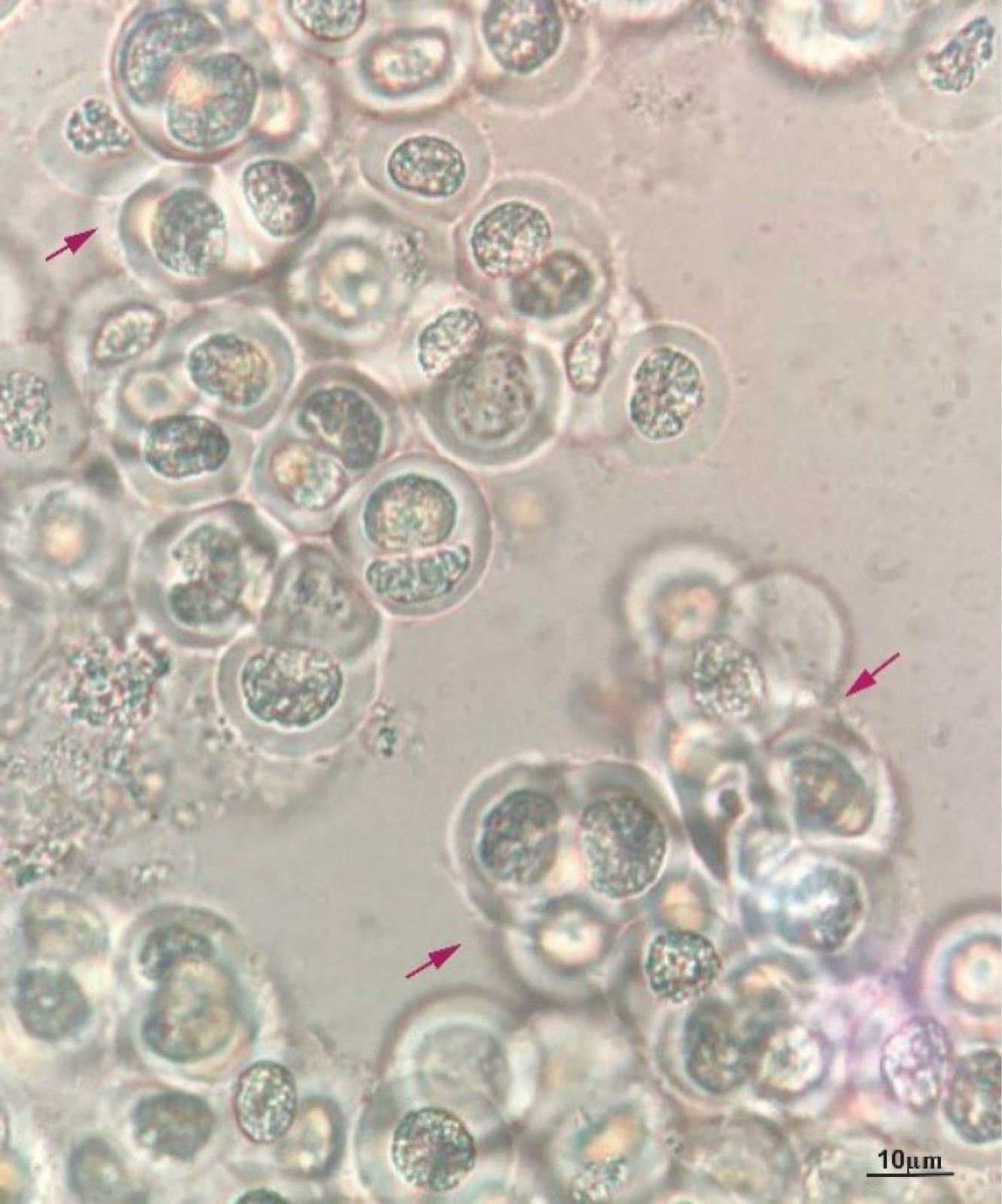
Fig. 1.
The morphology of the isolated C. turgidus strain KANI. Red arrows depict two, four, and eight-celled colonies enclosed with an amorphous (Scale bar = 10 µm).
.
The morphology of the isolated C. turgidus strain KANI. Red arrows depict two, four, and eight-celled colonies enclosed with an amorphous (Scale bar = 10 µm).
Fatty acid identification by GC-MS analysis
GC-MS analysis indicated the occurrence of different compounds, especially fatty acids, including fatty acid esters (FAEs), oleic acid, palmitic acids, and provitamin D3 in C. turgidus strainKANI ME (Table 1).
Table 1.
The identified fatty acids in Chroococcus turgidus strain KANI by GC-MS
|
Compound analyzed
|
Retention Time
|
Molecular
Formula
|
Molecular weight
|
Peak
area%
|
Nature of compound
|
Bioactivity
|
| 1-Bromo-11-iodoundecane |
24.54 |
C11H22BrI |
36. 11 |
2.31 |
Halogenated fatty acid |
Antimicrobial, Antifungal36 |
| Palmitic acid, ethyl ester |
24.70 |
C18H36O2 |
284.5 |
15.53 |
Fatty acid ester |
Antioxidant, Nematicide, Insecticide, Lubricant, Hemolytic, Hypocholesterolemic, Pesticide, Antiandrogenic, Flavor, Hemolytic,5-Alpha reductase inhibitor37-39 |
| Undecanoic acid, 2,8-dimethyl-, methyl ester |
24.86 |
C14H28O2 |
228.37 |
6.62 |
Fatty acid ester |
Antibacterial, Antitumor40 |
| Oleic acid |
27.04 |
C18H34O2 |
282.5 |
6.47 |
Fatty acid |
Antitumor, Lubricant Antibacterial, Antitumor, antioxidant and Anticancer Antimicrobial, Antiandrogenic 41,42 |
| 7-Dehydrocholesterol |
29.46 |
C27H44O |
384.6 |
7.97 |
Fatty acid |
Provitamin D343 |
Spectrophotometric analysis of photosynthetic pigments microalgae
Chl a, Chl b and carotenoid levels were determined with a UV-Vis spectrophotometer measuring absorbencies at 665, 649 and 470 nm, respectively, as shown in Fig. 2a. Besides, the level of photosynthetic pigments in the extract was measured for chl a and b, and carotenoids were 18.424, 12.864, and 9.428 µg/g FW, respectively (Fig. 2b).
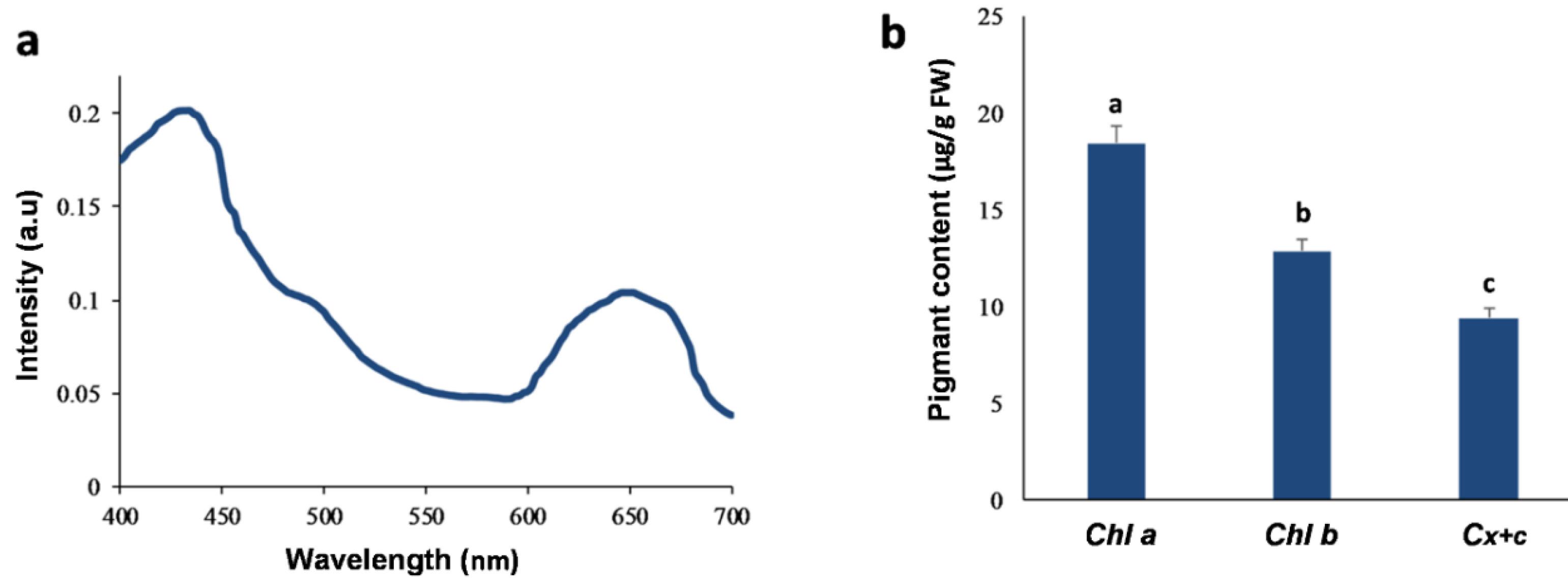
Fig. 2.
Photosynthetic pigments from the methanolic extract (ME) of C. turgidus strain KANI. (a) visible absorption spectra of ME (b) Chlorophylls (Chl a and b) and total Carotenoids (Cx + c ) contents. Columns labeled with different letters are significantly different (P < 0.05). Data were expressed as mean values of independent triplicates (mean ± SD).
.
Photosynthetic pigments from the methanolic extract (ME) of C. turgidus strain KANI. (a) visible absorption spectra of ME (b) Chlorophylls (Chl a and b) and total Carotenoids (Cx + c ) contents. Columns labeled with different letters are significantly different (P < 0.05). Data were expressed as mean values of independent triplicates (mean ± SD).
Estimation of phenol, flavonoid, and anthocyanin contents
As shown in Fig. 3, the ME 's phenols, flavonoids, and anthocyanins content were measured at a concentration of 7.428, 1. 1864, and 4.424 mg/g FW, respectively. Based on the results, flavonoids are the dominating phenolic compounds in C. turgidus strain KANI.
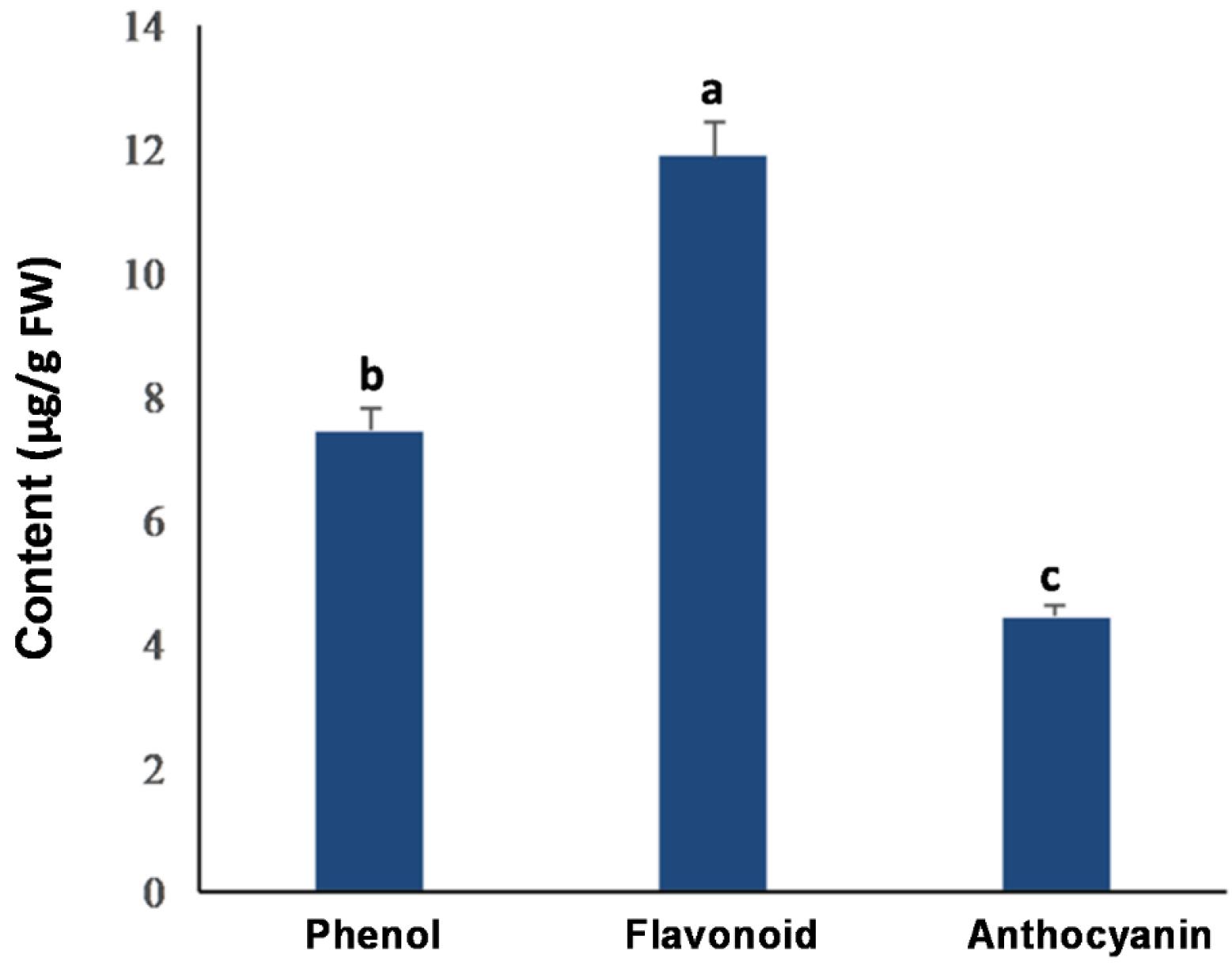
Fig. 3.
Total phenol, flavonoid, and anthocyanin contents from the methanolic extract (ME) of C. turgidus strain KANI. Columns labeled with different letters are significantly different (P < 0.05). Data were expressed as mean values of independent triplicates (mean ± SD).
.
Total phenol, flavonoid, and anthocyanin contents from the methanolic extract (ME) of C. turgidus strain KANI. Columns labeled with different letters are significantly different (P < 0.05). Data were expressed as mean values of independent triplicates (mean ± SD).
Determination of antioxidant activity
For the analysis of the antioxidant activity, the EC50 is determined from the dependence between the DPPH concentration remaining after its reaction with the antioxidant and different antioxidant concentrations. As a result, the EC50value was estimated at around 400 μg/mL of the extract (Fig. 4).
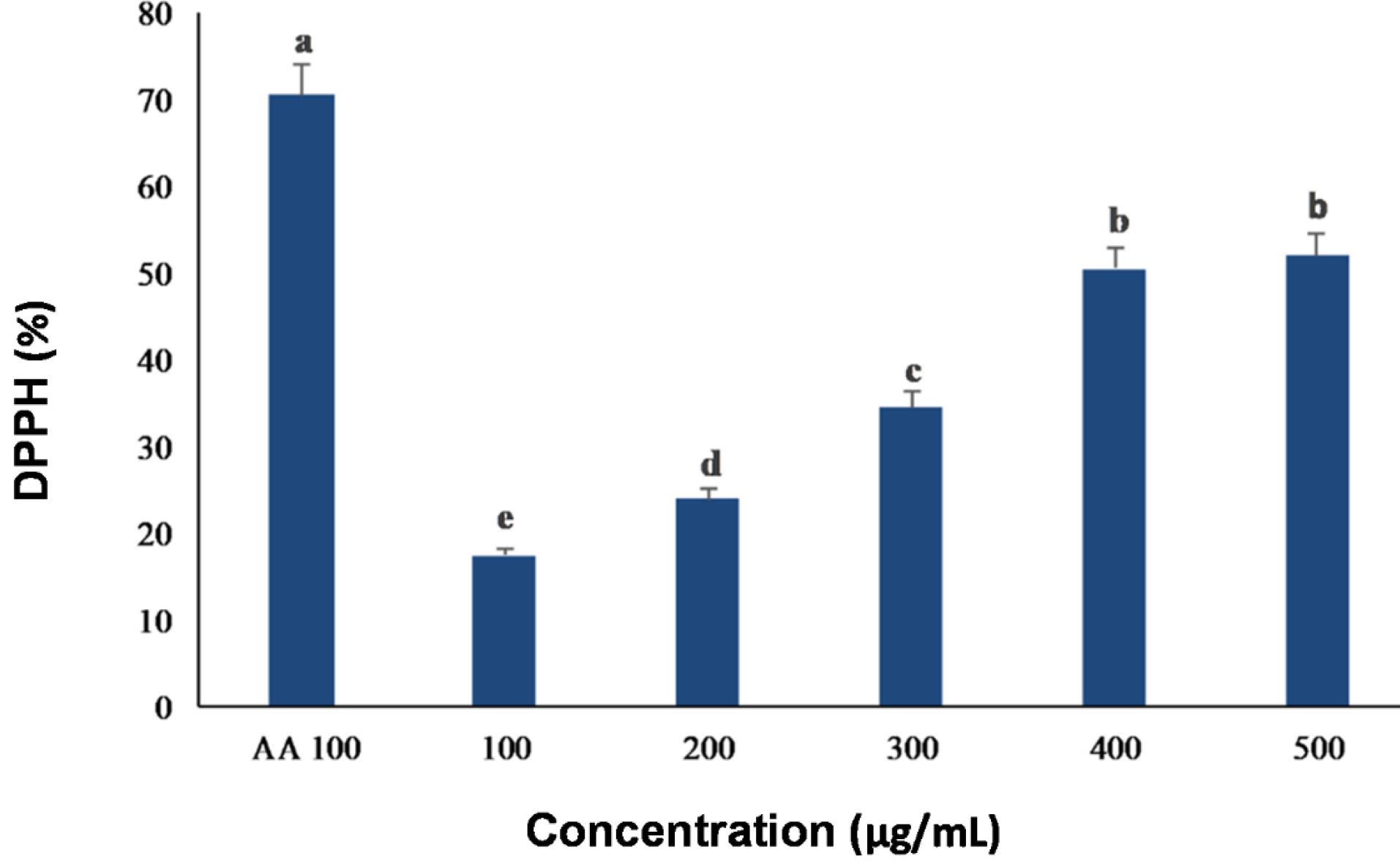
Fig. 4.
DPPH radical scavenging activity from the methanolic extract (ME) of C. turgidus strain KANI compared with standard (AA; ascorbic acid). Data were expressed as mean values of independent triplicates (mean ± SD). Different subscripts in small letters (a, b, c, d, and e) above the columns indicate significant differences at P ≤ 0.05.
.
DPPH radical scavenging activity from the methanolic extract (ME) of C. turgidus strain KANI compared with standard (AA; ascorbic acid). Data were expressed as mean values of independent triplicates (mean ± SD). Different subscripts in small letters (a, b, c, d, and e) above the columns indicate significant differences at P ≤ 0.05.
Evaluation of the cytotoxic effect of ME
The ME concentrations from C. turgidus strain KANI were evaluated for their cytotoxic effects on SW480 cancer cells using the MTT assay. As shown in Fig. 5, viable cell numbers were significantly reduced (P < 0.05) in a dose- and time-dependent manner, with the greatest effect observed at 48 hours. The statistical analysis confirmed that both dose and time had a significant impact on cell viability. The IC50 values for ME after 24 and 48 hours were 373 μg/mL and 291 μg/mL, respectively. Thus, it can be deduced that the cyanobacterial extract affects SW480 cells through a dose- and time-dependent mechanism (Fig. 5).
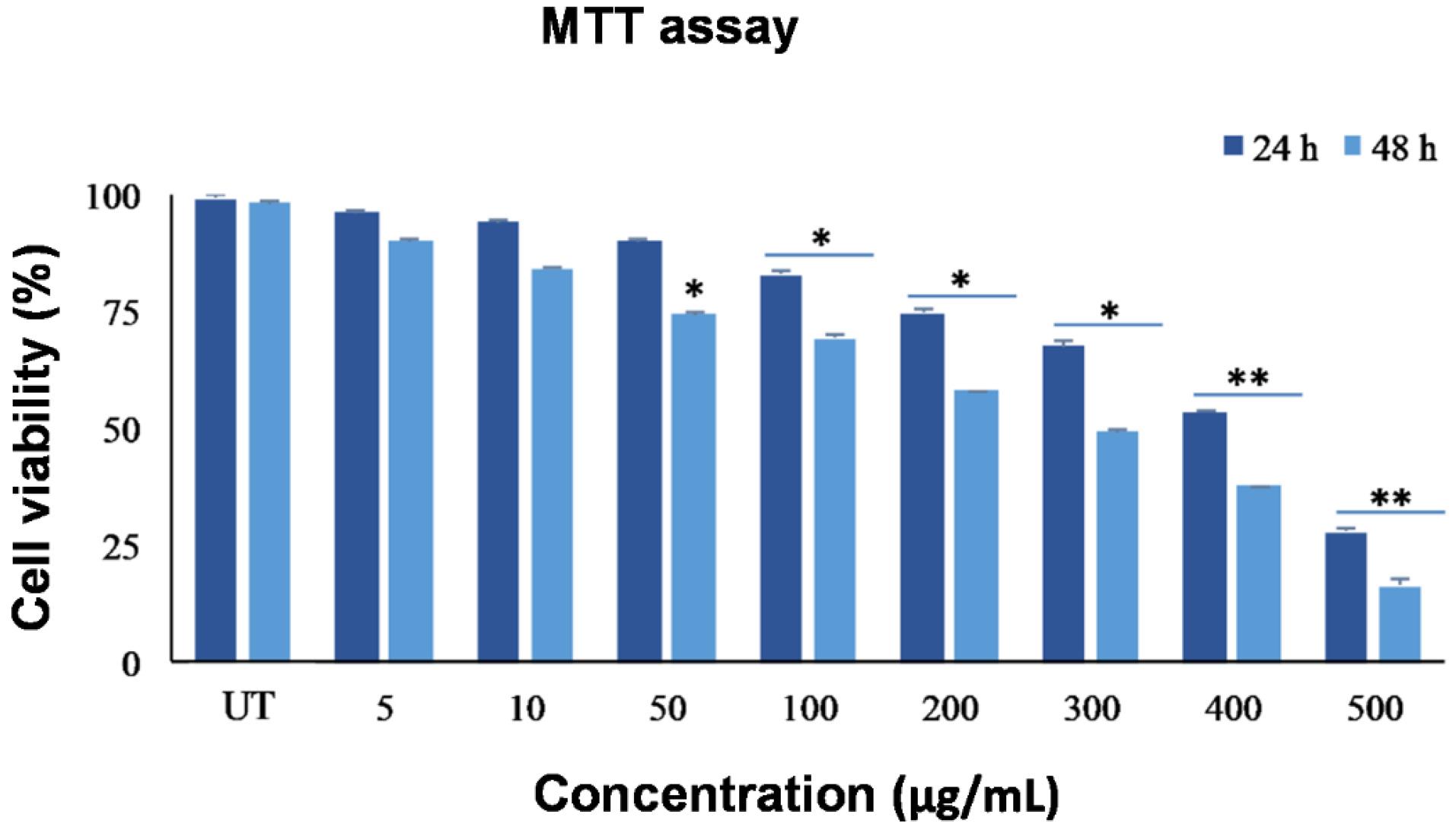
Fig. 5.
Cell viability assay of the SW480 cell line in different concentrations (0-500 µg/mL) from the ME of C. turgidus strain KANI. Data were expressed as mean values of independent triplicates (mean ± SD). Indicates significant difference in compared to control group (*P < 0.05 and **P < 0.01). ME: methanolic extract, UT: untreated control.
.
Cell viability assay of the SW480 cell line in different concentrations (0-500 µg/mL) from the ME of C. turgidus strain KANI. Data were expressed as mean values of independent triplicates (mean ± SD). Indicates significant difference in compared to control group (*P < 0.05 and **P < 0.01). ME: methanolic extract, UT: untreated control.
Evaluation of apoptosis by Annexin v
To analyze the apoptosis in SW480 cells treated with ME (IC50 Dose) using Annexin-V, PI was removed due to its spectrum overlap (620 nm) with photosynthetic pigments spectrum in the ME (600-700 nm). In comparison with the untreated control cells, after 24 hours, 38.97% apoptosis was induced in the SW480 cells treated with the IC50 concentration of the ME (Fig. 6).
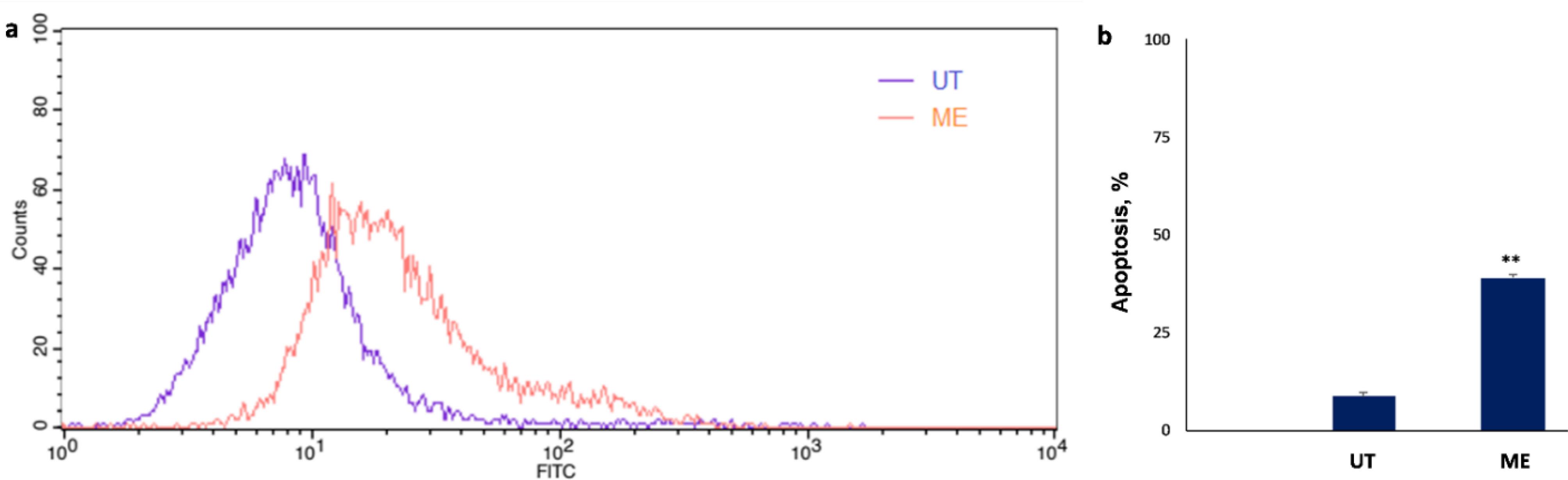
Fig. 6.
Apoptosis assay using Annexin-V flow cytometry analysis on SW480 cancer cells. (a) Treated cells with ME comparison with untreated control cells. (b) The rate of apoptosis in the treated cells with ME data was expressed as mean values of independent triplicates (mean ± SD). Indicates a significant difference compared to the control group (**P < 0.01). ME: methanolic extract, UT: untreated control cells.
.
Apoptosis assay using Annexin-V flow cytometry analysis on SW480 cancer cells. (a) Treated cells with ME comparison with untreated control cells. (b) The rate of apoptosis in the treated cells with ME data was expressed as mean values of independent triplicates (mean ± SD). Indicates a significant difference compared to the control group (**P < 0.01). ME: methanolic extract, UT: untreated control cells.
Apoptosis evaluation by DAPI staining
DAPI is a cell-permeable fluorescent compound that stains DNA by binding with high affinity to the minor groove at A–T-rich regions. As shown in Fig. 7, compared to untreated cells, a notable proportion of treated SW480 cells exhibited apoptotic nuclear features, including chromatin condensation and nuclear fragmentation, following 24-hour exposure to ME at the IC50 dose. These morphological changes are characteristic of apoptosis and further support the cytotoxic effects of the extract.
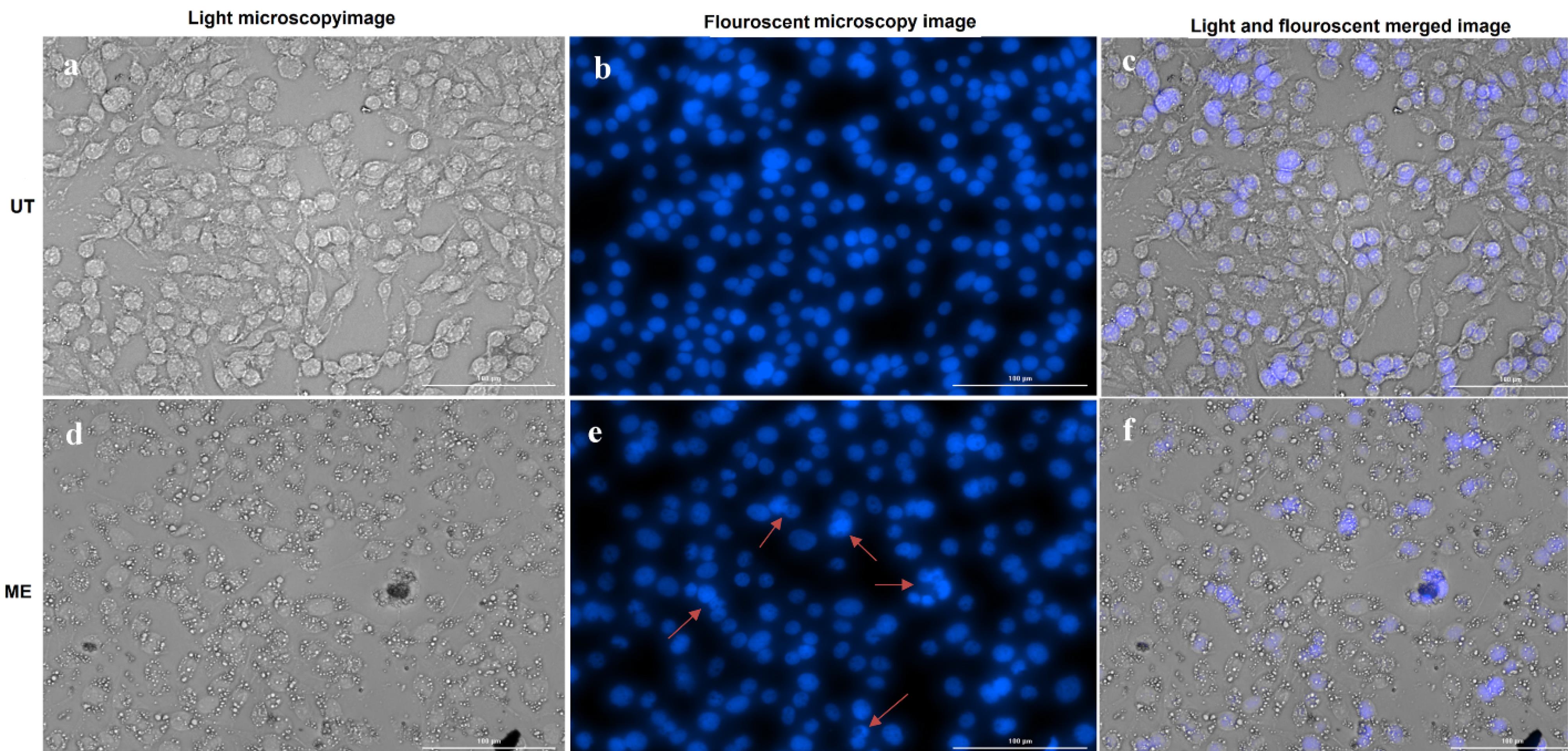
Fig. 7.
Apoptosis assay by DAPI staining of SW480 cells. (a, b and c) UT group and (d, e, and f) treated with the ME. Red arrows depict chromatin condensation and fragmented nuclei. ME: methanolic extract, UT: untreated control cells.
.
Apoptosis assay by DAPI staining of SW480 cells. (a, b and c) UT group and (d, e, and f) treated with the ME. Red arrows depict chromatin condensation and fragmented nuclei. ME: methanolic extract, UT: untreated control cells.
Apoptosis induction by protein expression assay in Western-blotting
As shown in Fig. 8, treatment with ME (IC50 Dose) has reduced the expression of Bcl2 proteins and increased the Bax expression in the SW480 cell line. In addition, the Bax/Bcl2 ratio was significantly different between the treated and control groups, indicating the relative protein expression of Bcl2 and Bax compared to the control group.
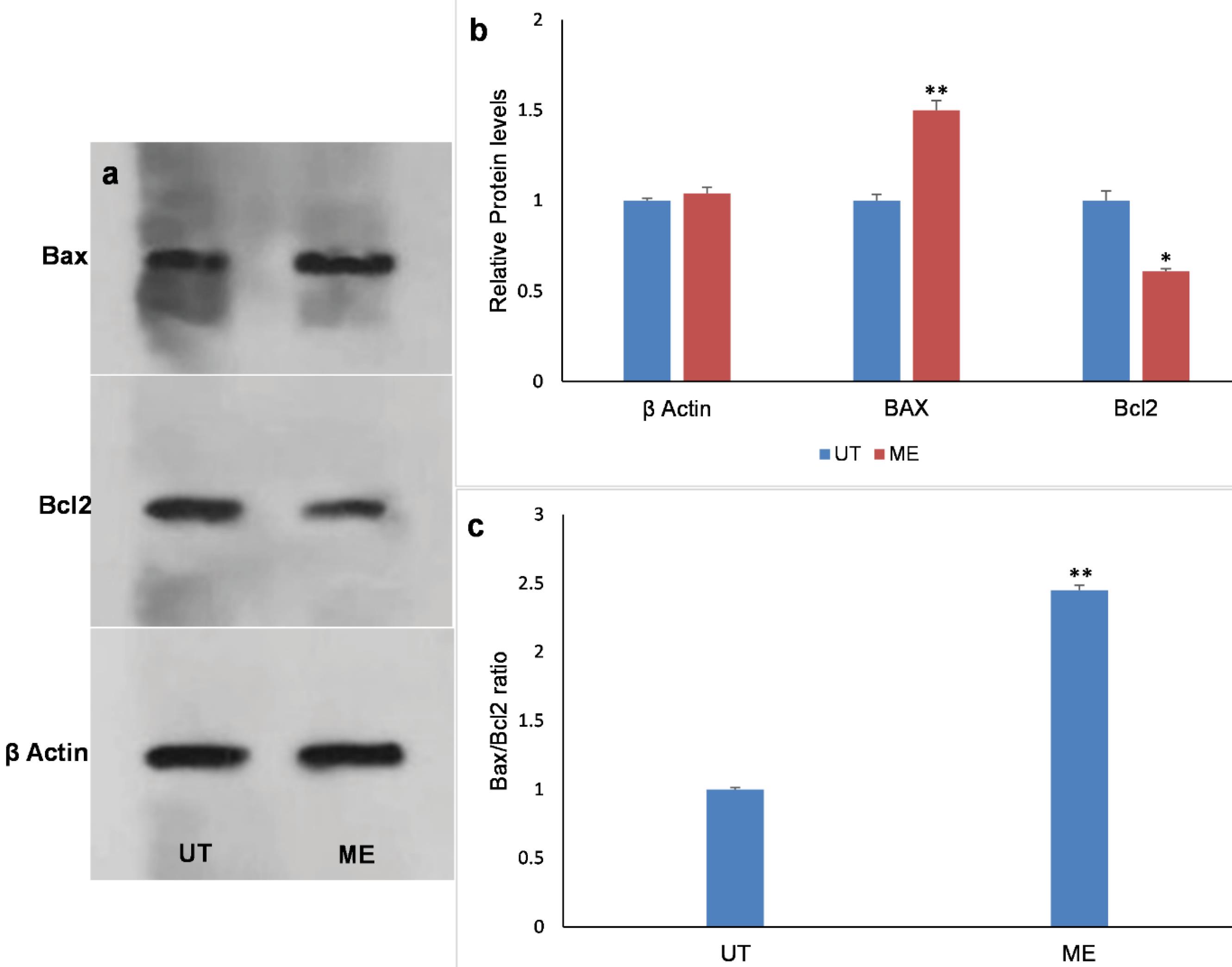
Fig. 8.
Western blot analysis of Bcl2 and Bax protein expression in SW480 cells after treatment by ME. (a) Bax, Bcl2, and β-actin protein bands. (b)The relative protein expression of Bcl2 and Bax compared with that in the control group (UT). (c) The Bax: Bcl2 ratio. Data were expressed as mean values of independent triplicates (mean ± SD). Indicates a significant difference compared to the control group (*P < 0.05 and **P < 0.01). ME: methanolic extract, UT: untreated control cells.
.
Western blot analysis of Bcl2 and Bax protein expression in SW480 cells after treatment by ME. (a) Bax, Bcl2, and β-actin protein bands. (b)The relative protein expression of Bcl2 and Bax compared with that in the control group (UT). (c) The Bax: Bcl2 ratio. Data were expressed as mean values of independent triplicates (mean ± SD). Indicates a significant difference compared to the control group (*P < 0.05 and **P < 0.01). ME: methanolic extract, UT: untreated control cells.
Discussion
Over the past few decades, more than 50 000 natural products have been discovered from marine microorganisms, many of them with biomedical applications.44-46 Research on molecules produced by aquatic organisms reveals that microalgae and cyanobacteria synthesize a vast array of compounds with promising biotechnological applications. Notably, these compounds exhibit significant therapeutic potential against cancer cells due to their diverse biological functions. These include antioxidant activity, anti-inflammatory properties, anti-mutagenic effects, inhibition of cell proliferation, promotion of cell cycle arrest, induction of apoptosis or autophagy, potential anti-invasion and anti-metastasis effects, suppression of drug resistance mechanisms, and enhancement of chemotherapy sensitivity.47-54 The growing interest in marine-derived natural products has led to the identification of bioactive flavonoids, polyphenols, and sterols with potent anticancer activity. Notably, compounds such as quercetin and resveratrol, widely recognized for their anti-tumor properties, exert their effects through oxidative stress modulation, PI3K/AKT inhibition, and apoptotic signaling activation.55,56 To contextualize our findings, we have expanded our discussion by comparing the anticancer effects of C. turgidus metabolites with these well-characterized plant-derived compounds, highlighting the unique mechanistic aspects of our study.
Fucosterol, a sterol commonly found in marine algae, has demonstrated anti-inflammatory, antioxidant, and anticancer properties, particularly against hematologic malignancies.57 Moreover, hexadecanoic acid methyl esters were highlighted for use as anti-inflammatory agents, cancer preventive, hepatoprotective, antiarthritic, and anti-coronary attributes.58-60 In our study, we identified 7-dehydrocholesterol (provitamin D3) as one of the major components of C. turgidus extract. Given the established link between vitamin D deficiency and increased CRC risk, this sterol suggests an additional potential mechanism of action, warranting further investigation into its role in modulating cancer cell behavior.61 To explore new anticancer molecules with potentially fewer side effects and reduced resistance to existing drugs, this study focused on isolating and identifying compounds from cyanobacteria in the Kani Barazan International Wetland, located to the south of Lake Urmia. The cyanobacterium C. turgidus strain KANI was successfully isolated from the wetland, with its microscopic morphology aligning with the Chroococcaceae family. The molecular identification was performed using the 16S rRNA gene and the 16S-23S ITS region from its genome. Based on NCBI database recommendations, the organism was given a Barcode of Life identifier (MW040530.1, 2021)_ENREF_49.25 The amount of natural compounds in C. turgidus strain KANI is very impressive, which increases the importance of this cyanobacterium for the cultivation and extraction of these compounds for medicinal purposes. Other related investigations have reported the therapeutic effects of the identified fatty acids in this study (Table 1).36-43
The cytotoxicity assay results demonstrated that the methanolic ME of C. turgidus strain KANI exerted a dose- and time-dependent inhibitory effect on the proliferation of SW480 CRC cells, consistent with previous reports on the pro-apoptotic effects of microalgal metabolites. The cytotoxic effects suggest that C. turgidus metabolites primarily exert their anticancer activity through apoptosis induction.62,63 However, the therapeutic potential of these compounds extends beyond their in vitro cytotoxicity and is influenced by their bioavailability and metabolic stability. Several studies have highlighted that the pharmacokinetics of marine-derived bioactive compounds can significantly impact their therapeutic applicability.64,65 While this study focused on in vitro cytotoxic effects, further in vivo validation is necessary to evaluate these metabolites' absorption, distribution, metabolism, and excretion properties. Structurally similar compounds, such as polyphenols and sterols, exhibit variable bioavailability due to metabolic modifications; therefore, future research should focus on optimizing formulation strategies that enhance the stability and systemic delivery of compounds originated by C. turgidus, potentially through nanoscale delivery systems.
Mechanistically, natural compounds can exert cytotoxic effects through multiple pathways. For instance, curcumin and curcumol induce apoptosis via p53 activation, oxidative stress modulation, and NF-κB suppression, while kaempferol inhibits angiogenesis through the ERK/NF-κB/c-Myc axis. Moreover, zerumbone, a naturally occurring sesquiterpene compound found primarily in the rhizomes of Zingiber zerumbet, promotes apoptosis by enhancing the expression of pro-apoptotic proteins such as Bax via cytochrome c release and activating caspase cascades, while simultaneously reducing the levels of anti-apoptotic proteins like Bcl2.66-69 There is increasing evidence to prove the prognostic and predictive role of apoptosis-related markers such as Bax and Bcl2. The Bax/Bcl2 ratio can behave as a rheostat that regulates cell sensitivity to apoptosis. Decreased levels of this ratio may result in cancer cells' resistance to apoptosis. Hence, the Bax/Bcl2 ratio can impact tumor progression and aggressiveness.70-72 Our findings indicate that C. turgidus extract similarly modulates apoptotic pathways, as evidenced by increased Bax expression and reduced Bcl2 levels. Given the established role of Bax/Bcl2 as a key determinant of apoptotic sensitivity,73 these results suggest that C. turgidus metabolites may act via the mitochondrial apoptotic pathway. To improve the clarity and robustness of our apoptosis assay, we have refined our Annexin V flow cytometry data presentation by explicitly reporting the percentages of early apoptosis, late apoptosis, and necrosis. This adjustment ensures a more precise interpretation of apoptotic responses following treatment with C. turgidus extract.
Beyond apoptosis induction, drug delivery remains a critical aspect of CRC therapy. Given the increasing use of nanoformulations to enhance the stability and bioavailability of natural compounds,74 metabolites of C. turgidus can be formulated and used as nanosized drug delivery systems. Lipid-based carriers, polymeric nanoparticles, and liposomal formulations have demonstrated the ability to improve the pharmacokinetics and targeted delivery of marine bioactive compounds.75,76 Although our study did not specifically investigate delivery strategies, these insights provide a foundation for future formulation-based studies.
The bioactivity of C. turgidus extract is likely attributable to different types of metabolites, including (i) lipopeptides (e.g., cyclic depsipeptides micropeptins, and linear peptides aeruginosins) that interfere with proteolytic enzymes and cellular signaling pathways and used in inflammatory diseases and certain types of cancer, (ii) polyketides (e.g., curacin, and apratoxin) that inhibit microtubule polymerization with potential therapeutic applications in oncology, (iii) indole alkaloids, which disrupt DNA replication and interfere with cell cycle progression, (iv) cyclic peptide toxins (e.g., microcystins, and nodularins) that inhibit protein phosphatases and dysregulate oncogenic pathways, (v) mycosporine-like amino acids, which exhibit photoprotective and antioxidative properties.
Although our study provides compelling evidence supporting the anticancer potential of C. turgidus, additional studies are warranted to elucidate the precise molecular interactions underlying its cytotoxic activity. Future research should focus on metabolomic profiling, pharmacokinetic studies, and in vivo validation to further explore the therapeutic applicability of C. turgidus bioactive compounds in CRC treatment.
Conclusion
Microalgae and cyanobacteria are recognized as crucial organisms across diverse ecosystems, largely due to their varied structures and capacity to synthesize a wide range of bioactive compounds. While extensive information exists regarding the compounds produced by microalgae and their biological properties, ongoing research continues to explore their potential applications in drug development and the production of innovative industrial materials. Consequently, there is a pressing need to investigate the therapeutic potentials and applications of various species of microalgae and cyanobacteria. A recent study focusing on the ME from the C. turgidus strain KANI revealed significant anticancer activity in vitro. This activity may be positively correlated with the presence of bioactive compounds such as fatty acids, polyphenolic compounds, and anthocyanins. These metabolites are well-known for their antioxidant properties, which allow them to combat free radical oxidation, exhibit therapeutic benefits, and perform essential physiological functions. This research offers valuable insights into the in vitro effects of the ME from C. turgidus strain KANI. In summary, the identification and characterization of algal metabolites hold great promise for the development of new antitumor agents and could pave the way for innovative approaches to cancer therapy in the future.
Research Highlights
What is the current knowledge?
-
Microalgae and cyanobacteria produce bioactive compounds with antioxidant and anticancer effects.
-
Chroococcus turgidus has demonstrated antioxidant, anti-inflammatory, antibacterial, and antiviral properties.
-
The Bax/Bcl2 pathway plays a key role in apoptosis induction in colorectal cancer cells.
What is new here?
-
Chroococcus turgidus methanolic extract inhibits colorectal cancer cell proliferation in a dose- and time-dependent manner.
-
Bioactive metabolites, including phenols, flavonoids, and fatty acids, contribute to its anticancer effects.
-
Apoptosis induction is confirmed by nuclear fragmentation, Annexin V staining, and Bax/Bcl2 regulation.
Competing Interests
The authors declare that the research was carried out without any commercial or financial relationships that could be perceived as a potential conflict of interest.
Ethical Approval
This is an in vitro exploratory study. The Institutional Research Ethics Committee has confirmed that no ethical approval is required.
Acknowledgements
The authors would like to acknowledge the support provided by Tabriz University of Medical Sciences (TUOMS).
References
- Brennan-Olsen SL, Cook S, Leech MT, Bowe SJ, Kowal P, Naidoo N. Prevalence of arthritis according to age, sex and socioeconomic status in six low- and middle-income countries: analysis of data from the World Health Organization study on global AGEing and adult health (SAGE) wave 1. BMC Musculoskelet Disord 2017; 18:271. doi: 10.1186/s12891-017-1624-z [Crossref] [ Google Scholar]
- Akbarzadeh-Khiavi M, Farzi-Khajeh H, Somi MH, Safary A, Barar J, Ansari R. Eradication of KRAS mutant colorectal adenocarcinoma by PEGylated gold nanoparticles-cetuximab conjugates through ROS-dependent apoptosis. Colloids Surf A Physicochem Eng Asp 2022; 653:129890. doi: 10.1016/j.colsurfa.2022.129890 [Crossref] [ Google Scholar]
- Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg 2016; 68:7-11. doi: 10.1007/s13304-016-0359-y [Crossref] [ Google Scholar]
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66:683-91. doi: 10.1136/gutjnl-2015-310912 [Crossref] [ Google Scholar]
- Yousefi M, Farzi-Khajeh H, Akbarzadeh-Khiavi M, Safary A, Adibkia K. Improved biological impacts of anti-EGFR monoclonal antibody in KRAS-mutant colorectal cancer cells by silica-coated magnetic nanoparticle conjugation. Pharm Sci 2024; 30:444-55. doi: 10.34172/PS.2024.9 [Crossref] [ Google Scholar]
- Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC. New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med 2021; 9:20503121211034366. doi: 10.1177/20503121211034366 [Crossref] [ Google Scholar]
- Zeng L, Gowda BHJ, Ahmed MG, Abourehab MA, Chen ZS, Zhang C. Advancements in nanoparticle-based treatment approaches for skin cancer therapy. Mol Cancer 2023; 22:10. doi: 10.1186/s12943-022-01708-4 [Crossref] [ Google Scholar]
- Zhong Q, Wei B, Wang S, Ke S, Chen J, Zhang H. The antioxidant activity of polysaccharides derived from marine organisms: an overview. Mar Drugs 2019; 17:674. doi: 10.3390/md17120674 [Crossref] [ Google Scholar]
- Macharia JM, Mwangi RW, Rozmann N, Zsolt K, Varjas T, Uchechukwu PO. Medicinal plants with anti-colorectal cancer bioactive compounds: potential game-changers in colorectal cancer management. Biomed Pharmacother 2022; 153:113383. doi: 10.1016/j.biopha.2022.113383 [Crossref] [ Google Scholar]
- Sofi FA, Tabassum N. Natural product inspired leads in the discovery of anticancer agents: an update. J Biomol Struct Dyn 2023; 41:8605-28. doi: 10.1080/07391102.2022.2134212 [Crossref] [ Google Scholar]
- Safary A, Moniri R, Hamzeh-Mivehroud M, Dastmalchi S. Identification and molecular characterization of genes coding pharmaceutically important enzymes from halo-thermo tolerant Bacillus. Adv Pharm Bull 2016; 6:551-61. doi: 10.15171/apb.2016.069 [Crossref] [ Google Scholar]
- Goshtasbi H, Okolodkov YB, Movafeghi A, Awale S, Safary A, Barar J. Harnessing microalgae as sustainable cellular factories for biopharmaceutical production. Algal Res 2023; 74:103237. doi: 10.1016/j.algal.2023.103237 [Crossref] [ Google Scholar]
- Leal MC, Puga J, Serôdio J, Gomes NC, Calado R. Trends in the discovery of new marine natural products from invertebrates over the last two decades--where and what are we bioprospecting?. PLoS One 2012; 7:e30580. doi: 10.1371/journal.pone.0030580 [Crossref] [ Google Scholar]
- Thomford NE, Senthebane DA, Rowe A, Munro D, Seele P, Maroyi A. Natural products for drug discovery in the 21st century: innovations for novel drug discovery. Int J Mol Sci 2018; 19:1578. doi: 10.3390/ijms19061578 [Crossref] [ Google Scholar]
- Izzo AA, Teixeira M, Alexander SP, Cirino G, Docherty JR, George CH. A practical guide for transparent reporting of research on natural products in the British Journal of Pharmacology: reproducibility of natural product research. Br J Pharmacol 2020; 177:2169-78. doi: 10.1111/bph.15054 [Crossref] [ Google Scholar]
- de Jesus Raposo MF, de Morais RM, de Morais AM. Health applications of bioactive compounds from marine microalgae. Life Sci 2013; 93:479-86. doi: 10.1016/j.lfs.2013.08.002 [Crossref] [ Google Scholar]
- Xu H, Vavilin D, Vermaas W. The presence of chlorophyll b in Synechocystis sp PCC 6803 disturbs tetrapyrrole biosynthesis and enhances chlorophyll degradation. J Biol Chem 2002; 277:42726-32. doi: 10.1074/jbc.M205237200 [Crossref] [ Google Scholar]
- Xu H, Vavilin D, Vermaas W. Chlorophyll b can serve as the major pigment in functional photosystem II complexes of cyanobacteria. Proc Natl Acad Sci U S A 2001; 98:14168-73. doi: 10.1073/pnas.251530298 [Crossref] [ Google Scholar]
- Tomitani A, Okada K, Miyashita H, Matthijs HC, Ohno T, Tanaka A. Chlorophyll b and phycobilins in the common ancestor of cyanobacteria and chloroplasts. Nature 1999; 400:159-62. doi: 10.1038/22101 [Crossref] [ Google Scholar]
- Barkia I, Saari N, Manning SR. Microalgae for high-value products towards human health and nutrition. Mar Drugs 2019; 17:304. doi: 10.3390/md17050304 [Crossref] [ Google Scholar]
- Singh DP, Prabha R, Verma S, Meena KK, Yandigeri M. Antioxidant properties and polyphenolic content in terrestrial cyanobacteria. 3 Biotech 2017; 7:134. doi: 10.1007/s13205-017-0786-6 [Crossref] [ Google Scholar]
- Hay ME. Challenges and opportunities in marine chemical ecology. J Chem Ecol 2014; 40:216-7. doi: 10.1007/s10886-014-0393-5 [Crossref] [ Google Scholar]
- Ibrahim T, Feisal NA, Kamaludin NH, Cheah WY, How V, Bhatnagar A. Biological active metabolites from microalgae for healthcare and pharmaceutical industries: a comprehensive review. Bioresour Technol 2023; 372:128661. doi: 10.1016/j.biortech.2023.128661 [Crossref] [ Google Scholar]
-
Goshtasbi H, Dalir Abdolahinia E, Fathi M, Movafeghi A, Omidian H, Barar J, et al. Astaxanthin-loaded alginate-chitosan gel beads activate Nrf2 and pro-apoptotic signalling pathways against oxidative stress. J Microencapsul 2024. 41: 140-56. doi: 10.1080/02652048.2024.2319048.
- Goshtasbi H, Atazadeh E, Movafeghi A. Polyphasic study of three cyanobacteria species from Kani Barazan international wetland in the northwest of Iran using morphological, molecular, biochemical, and bioinformatics approaches. Biologia 2022; 77:503-16. doi: 10.1007/s11756-021-00940-5 [Crossref] [ Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 1987; 147:350-82. doi: 10.1016/0076-6879(87)48036-1 [Crossref] [ Google Scholar]
- Dere Ş, Güneş T, Sivaci R. Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Turk J Bot 1998; 22:13-8. [ Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 2005; 91:571-7. doi: 10.1016/j.foodchem.2004.10.006 [Crossref] [ Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 2002; 10:178-82. doi: 10.38212/2224-6614.2748 [Crossref] [ Google Scholar]
- Mita S, Murano N, Akaike M, Nakamura K. Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for beta-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J 1997; 11:841-51. doi: 10.1046/j.1365-313x.1997.11040841.x [Crossref] [ Google Scholar]
- Öztürk N, Tunçel M. Assessment of phenolic acid content and in vitro antiradical characteristics of hawthorn. J Med Food 2011; 14:664-9. doi: 10.1089/jmf.2010.0063 [Crossref] [ Google Scholar]
- Huang CW, Hung YC, Chen LY, Asatiani M, Klarsfeld G, Melamed D. Antioxidant activities of hot water extracts from mycelial biomass of different combinations of medicinal Agaricomycetes mushrooms. Int J Med Mushrooms 2022; 24:21-30. doi: 10.1615/IntJMedMushrooms.2022044221 [Crossref] [ Google Scholar]
- Akbarzadeh Khiavi M, Safary A, Barar J, Farzi-Khajeh H, Barzegari A, Mousavi R. PEGylated gold nanoparticles-ribonuclease induced oxidative stress and apoptosis in colorectal cancer cells. Bioimpacts 2020; 10:27-36. doi: 10.15171/bi.2020.04 [Crossref] [ Google Scholar]
- Ling HU, Tyler PA. Australian freshwater algae (exclusive of diatoms). Acta Bot Hung 2001; 43:222-3. [ Google Scholar]
- York PV, Johnson LR. The freshwater algal flora of the British Isles: an identification guide to freshwater and terrestrial algae. The freshwater algal flora of the British Isles. Cambridge University Press; 2002.
- Dissanayake D, Perera D, Keerthirathna LR, Heendeniya S, Anderson RJ, Williams DE. Antimicrobial activity of Plumbago indica and ligand screening of plumbagin against methicillin-resistant Staphylococcus aureus. J Biomol Struct Dyn 2022; 40:3273-84. doi: 10.1080/07391102.2020.1846622 [Crossref] [ Google Scholar]
- Li YH, Yang YF, Li K, Jin LL, Yang NY, Kong DY. 5 alpha-reductase and aromatase inhibitory constituents from Brassica rapa L pollen. Chem Pharm Bull (Tokyo) 2009; 57:401-4. doi: 10.1248/cpb.57.401 [Crossref] [ Google Scholar]
- Starlin T, Prabha PS, Thayakumar BK, Gopalakrishnan VK. Screening and GC-MS profiling of ethanolic extract of Tylophora pauciflora. Bioinformation 2019; 15:425-9. doi: 10.6026/97320630015425 [Crossref] [ Google Scholar]
- Pavani P, Naika R. Evaluation of antibacterial activity and GCMS analysis of Zanthoxylum ovalifolium fruit extracts. J Pharm Res Int 2021; 33:7-17. doi: 10.9734/jpri/2021/v33i30B31634 [Crossref] [ Google Scholar]
- Mohamed EM, Farghaly FA. Bioactive compounds of fresh and dried Pleurotus ostreatus mushroom. Int J Biotechnol Wellness Ind 2014; 3:4-14. doi: 10.6000/1927-3037.2014.03.01.2 [Crossref] [ Google Scholar]
- Rabbi F, Zada A, Adhikari A, Nisar A, Khan FU, Sohail M. GC-MS analysis, metal analysis and antimicrobial investigation of Sterculia diversifolia. Pharm Chem J 2020; 54:943-53. doi: 10.1007/s11094-020-02301-z [Crossref] [ Google Scholar]
- Adegoke AS, Jerry OV, Ademola OG. GC-MS analysis of phytochemical constituents in methanol extract of wood bark from Durio zibethinus Murr. Int J Med Plants Nat Prod 2019; 5:1-11. doi: 10.20431/2454-7999.0503001 [Crossref] [ Google Scholar]
- Vasilevskaya AV, Yantsevich AV, Sergeev GV, Lemish AP, Usanov SA, Gilep AA. Identification of Mycobacterium tuberculosis enzyme involved in vitamin D and 7-dehydrocholesterol metabolism. J Steroid Biochem Mol Biol 2017; 169:202-9. doi: 10.1016/j.jsbmb.2016.05.021 [Crossref] [ Google Scholar]
- Tavares-Carreón F, De la Torre-Zavala S, Arocha-Garza HF, Souza V, Galán-Wong LJ, Avilés-Arnaut H. In vitro anticancer activity of methanolic extract of Granulocystopsis sp, a microalgae from an oligotrophic oasis in the Chihuahuan Desert. PeerJ 2020; 8:e8686. doi: 10.7717/peerj.8686 [Crossref] [ Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 2012; 75:311-35. doi: 10.1021/np200906s [Crossref] [ Google Scholar]
- Wiese J, Thiel V, Nagel K, Staufenberger T, Imhoff JF. Diversity of antibiotic-active bacteria associated with the brown alga Laminaria saccharina from the Baltic Sea. Mar Biotechnol (NY) 2009; 11:287-300. doi: 10.1007/s10126-008-9143-4 [Crossref] [ Google Scholar]
- Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett 2008; 269:281-90. doi: 10.1016/j.canlet.2008.05.020 [Crossref] [ Google Scholar]
- Lin BW, Gong CC, Song HF, Cui YY. Effects of anthocyanins on the prevention and treatment of cancer. Br J Pharmacol 2017; 174:1226-43. doi: 10.1111/bph.13627 [Crossref] [ Google Scholar]
-
Hudlikar R, Wu R, Cheng D, Kuo DH, Wang L, Peter R, et al. Anthocyanins and cancer prevention. In: Pezzuto JM, Vang O, eds. Natural Products for Cancer Chemoprevention: Single Compounds and Combinations. Cham: Springer International Publishing; 2020. p. 351-73. doi: 10.1007/978-3-030-39855-2_11.
- Djuric Z, Rifkin S. A new score for quantifying adherence to a cancer-preventive Mediterranean diet. Nutr Cancer 2022; 74:579-91. doi: 10.1080/01635581.2021.1909738 [Crossref] [ Google Scholar]
- Wei L, Wu Z, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids-an update. Cancer Lett 2022; 526:193-204. doi: 10.1016/j.canlet.2021.11.023 [Crossref] [ Google Scholar]
- Sahin D. Effect of oil extract from microalgae (Schizochytrium sp) on the viability and apoptosis of human osteosarcoma cells. Curr Pharm Biotechnol 2021; 22:1099-105. doi: 10.2174/1389201021666200928101029 [Crossref] [ Google Scholar]
- Toshkova-Yotova T, Georgieva A, Iliev I, Alexandrov S, Ivanova A, Pilarski P. Antitumor and antimicrobial activity of fatty acids from green microalga Coelastrella sp BGV. S Afr J Bot 2022; 151:394-402. doi: 10.1016/j.sajb.2022.04.003 [Crossref] [ Google Scholar]
- Kalt W, Cassidy A, Howard LR, Krikorian R, Stull AJ, Tremblay F. Recent research on the health benefits of blueberries and their anthocyanins. Adv Nutr 2020; 11:224-36. doi: 10.1093/advances/nmz065 [Crossref] [ Google Scholar]
- Biswas P, Dey D, Biswas PK, Rahaman TI, Saha S, Parvez A. A comprehensive analysis and anti-cancer activities of quercetin in ROS-mediated cancer and cancer stem cells. Int J Mol Sci 2022; 23:11746. doi: 10.3390/ijms231911746 [Crossref] [ Google Scholar]
- Alavi M, Farkhondeh T, Aschner M, Samarghandian S. Resveratrol mediates its anti-cancer effects by Nrf2 signaling pathway activation. Cancer Cell Int 2021; 21:579. doi: 10.1186/s12935-021-02280-5 [Crossref] [ Google Scholar]
- Banni S, Angioni E, Murru E, Carta G, Melis MP, Bauman D. Vaccenic acid feeding increases tissue levels of conjugated linoleic acid and suppresses development of premalignant lesions in rat mammary gland. Nutr Cancer 2001; 41:91-7. doi: 10.1080/01635581.2001.9680617 [Crossref] [ Google Scholar]
- Teixeira TR, Santos GS, Turatti IC, Paziani MH, von Zeska Kress MR, Colepicolo P. Characterization of the lipid profile of Antarctic brown seaweeds and their endophytic fungi by gas chromatography–mass spectrometry (GC–MS). Polar Biol 2019; 42:1431-44. doi: 10.1007/s00300-019-02529-w [Crossref] [ Google Scholar]
- Kumari M, Acharya GC, Naresh P, Bhanja PK, Kumar Y. Solena amplexicaullis (Lam) Gandhi: an underutilized cucurbit in India. Genet Resour Crop Evol 2021; 68:79-85. doi: 10.1007/s10722-020-01043-x [Crossref] [ Google Scholar]
- Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M. Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol Drug Des 2012; 80:434-9. doi: 10.1111/j.1747-0285.2012.01418.x [Crossref] [ Google Scholar]
- Na SY, Kim KB, Lim YJ, Song HJ. Vitamin D and colorectal cancer: current perspectives and future directions. J Cancer Prev 2022; 27:147-56. doi: 10.15430/jcp.2022.27.3.147 [Crossref] [ Google Scholar]
- Gupta SP, Siddiqi NJ, Khan HA, Alrokayan SH, Alhomida AS, Singh RK. Phytochemical profiling of microalgae Euglena tuba and its anticancer activity in Dalton's lymphoma cells. Front Biosci (Landmark Ed) 2022; 27:120. doi: 10.31083/j.fbl2704120 [Crossref] [ Google Scholar]
- Ferdous UT, Nurdin A, Ismail S, Balia Yusof ZN. Evaluation of the antioxidant and cytotoxic activities of crude extracts from marine Chlorella sp. Biocatal Agric Biotechnol 2023; 47:102551. doi: 10.1016/j.bcab.2022.102551 [Crossref] [ Google Scholar]
- Singh R, Chauhan N, Kuddus M. Exploring the therapeutic potential of marine-derived bioactive compounds against COVID-19. Environ Sci Pollut Res Int 2021; 28:52798-809. doi: 10.1007/s11356-021-16104-6 [Crossref] [ Google Scholar]
- Karthikeyan A, Joseph A, Nair BG. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J Genet Eng Biotechnol 2022; 20:14. doi: 10.1186/s43141-021-00290-4 [Crossref] [ Google Scholar]
- Chen XY, Chen Y, Qu CJ, Pan ZH, Qin Y, Zhang X. Vitamin C induces human melanoma A375 cell apoptosis via Bax- and Bcl-2-mediated mitochondrial pathways. Oncol Lett 2019; 18:3880-6. doi: 10.3892/ol.2019.10686 [Crossref] [ Google Scholar]
- Helaly NA, Esheba NE, Abou Ammo DE, Elwan NM, Elkholy RA. High Bax/Bcl-2 ratio is associated with good prognosis and better survival in patients with B cell chronic lymphocytic leukemia. Leuk Res 2021; 107:106604. doi: 10.1016/j.leukres.2021.106604 [Crossref] [ Google Scholar]
-
Akram R, Anwar H, Rasul A, Malik SA, Maqbool J, Sajid F, et al. Natural compounds as versatile potential therapeutic agents of lung cancer. In: Dua K, Nammi S, Chang D, Chellappan DK, Gupta G, Collet T, eds. Medicinal Plants for Lung Diseases: A Pharmacological and Immunological Perspective. Singapore: Springer; 2021. p. 229-56. doi: 10.1007/978-981-33-6850-7_10.
-
Manna I, Das D, Mondal S, Bandyopadhyay M. Potential pharmacotherapeutic phytochemicals from Zingiberaceae for cancer prevention. In: Kumar M, Sharma A, Kumar P, eds. Pharmacotherapeutic Botanicals for Cancer Chemoprevention. Singapore: Springer; 2020. p. 221-81. doi: 10.1007/978-981-15-5999-0_10.
- Mbaveng AT, Chi GF, Bonsou IN, Ombito JO, Yeboah SO, Kuete V. Cytotoxic phytochemicals from the crude extract of Tetrapleura tetraptera fruits towards multi-factorial drug resistant cancer cells. J Ethnopharmacol 2021; 267:113632. doi: 10.1016/j.jep.2020.113632 [Crossref] [ Google Scholar]
- Ansil PN, Wills PJ, Varun R, Latha MS. Cytotoxic and apoptotic activities of Amorphophallus campanulatus (Roxb) Bl tuber extracts against human colon carcinoma cell line HCT-15. Saudi J Biol Sci 2014; 21:524-31. doi: 10.1016/j.sjbs.2014.01.004 [Crossref] [ Google Scholar]
- Saha SK, Sikdar S, Mukherjee A, Bhadra K, Boujedaini N, Khuda-Bukhsh AR. Ethanolic extract of the goldenseal, Hydrastis canadensis, has demonstrable chemopreventive effects on HeLa cells in vitro: Drug-DNA interaction with calf thymus DNA as target. Environ Toxicol Pharmacol 2013; 36:202-14. doi: 10.1016/j.etap.2013.03.023 [Crossref] [ Google Scholar]
- Qian S, Wei Z, Yang W, Huang J, Yang Y, Wang J. The role of Bcl-2 family proteins in regulating apoptosis and cancer therapy. Front Oncol 2022; 12:985363. doi: 10.3389/fonc.2022.985363 [Crossref] [ Google Scholar]
- Akbarzadeh-Khiavi M, Torabi M, Olfati AH, Rahbarnia L, Safary A. Bio-nano scale modifications of melittin for improving therapeutic efficacy. Expert Opin Biol Ther 2022; 22:895-909. doi: 10.1080/14712598.2022.2088277 [Crossref] [ Google Scholar]
- Jeong GJ, Khan S, Tabassum N, Khan F, Kim YM. Marine-bioinspired nanoparticles as potential drugs for multiple biological roles. Mar Drugs 2022; 20:527. doi: 10.3390/md20080527 [Crossref] [ Google Scholar]
- Favas R, Almeida H, Peixoto AF, Ferreira D, Silva AC. Advances in encapsulating marine bioactive compounds using nanostructured lipid carriers (NLCs) and solid lipid nanoparticles (SLNs) for health applications. Pharmaceutics 2024; 16:1517. doi: 10.3390/pharmaceutics16121517 [Crossref] [ Google Scholar]