Bioimpacts. 2025;15:30989.
doi: 10.34172/bi.30989
Systematic Review
microRNAs shuttled by mesenchymal stromal cell-derived exosomes in coronary artery disease: A systematic review of preclinical studies
Soroush Mostafavi Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing, 1 
Amin Arasteh Data curation, Investigation, Methodology, Resources, 2, 3 
Seyedeh Mina Mostafavi Montazeri Data curation, Formal analysis, Investigation, 3 
Seyyedeh Mina Hejazian Resources, Writing – review & editing, 2, 4 
Farahnoosh Farnood Visualization, Writing – review & editing, 4 
Sima Abediazar Resources, Visualization, Writing – review & editing, 4 
Abolfazl Barzegari Conceptualization, Project administration, Writing – original draft, Writing – review & editing, 5, 6, 7, * 
Sepideh Zununi Vahed Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing, 4, * 
Author information:
1Department of Cardiology, Hazrat-e-Rasool General Hospital, School of Medicine, Iran University of Medical Sciences (IUMS), Tehran, Iran
2Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
3Clinical Research Development Center of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4Kidney Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Medical Biotechnology, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
6Research Center for Pharmaceutical Nanotechnology (RCPN), Tabriz University of Medical Sciences, Tabriz, Iran
7Université Sorbonne Paris Nord, INSERM U1148, Laboratory for Vascular Translational Science, Nanotechnologies for Vascular Medicine and Imaging Team, 99 Av. Jean-Baptiste Clément 93430 Villetaneuse, France
Abstract
Introduction:
Coronary artery disease (CAD) is a life-threatening cardiac condition with high morbidity and mortality worldwide. This systematic review article highlighted the therapeutic roles of mesenchymal stromal cells (MSCs)-derived exosomal microRNAs (exo-miRs) in preclinical models of CAD.
Methods:
A comprehensive search was conducted on PubMed, Web of Science, Scopus, and Google Scholar to identify relevant publications until 04 Apr 2025. The literature review focuses on the origin of MSCs, the technique employed for exosome extraction and identification, the route and frequency of exosomal administration, the mechanisms through which exo-miRs regulate paracrine activity, and their impact on cardiac outcome.
Results:
After meticulous evaluation, fifty-six studies were deemed eligible for inclusion in this systematic review. Bone marrow-derived MSCs were the most commonly utilized cell type in the preclinical studies. The majority of studies employed the ultracentrifugation method for exosome isolation from MSCs. The administration of exosomes was primarily achieved through a single intramyocardial injection, utilizing a wide range of exosome concentrations (ranging from 0.02-400 μg/μL).
Conclusion:
The included studies predominantly have reported the anti-inflammatory, anti-apoptotic, angiogenic, antifibrotic, and reparative effects of MSC-exo-miRs, especially under hypoxic conditions. These findings support the capacity of MSC-exo-miRs to regulate the immune system and facilitate cardiac recovery following an injury.
Keywords: Ischemic heart disease, Myocardial infarction, Coronary heart disease, microRNA, Mesenchymal stem cells, Exosomes
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This study was supported by Tabriz University of Medical Sciences, Tabriz, Iran (Grant No.: 73046).
Introduction
Coronary artery disease (CAD), also called ischemic heart disease, is a life-threatening cardiac condition characterized by impaired blood flow in the coronary arteries, primarily due to atherosclerosis. This process leads to the narrowing and stiffening of the arteries, limiting oxygen-rich blood supply to the heart muscle and potentially causing myocardial ischemia, angina, myocardial infarction (MI), or ultimately heart failure. Despite advances in medical and surgical therapies, the burden of CAD continues to rise,1 necessitating the exploration of new therapeutic strategies and biomarkers for both early diagnosis and effective treatment.
The role of multipotent mesenchymal stromal cells (MSCs) in preventing and treating CAD has become more prominent. MSCs can self-renew and differentiate into various types of cells thanks to their multipotency. Clinical studies have shown that MSC therapy can compensate for the limitation of the repair ability of myocardial cells. These cells promote angiogenesis and neovascularization, limit the infarcted area, regulate the immune system, and prevent fibrosis. Moreover, these cells can differentiate into smooth muscle cells, endothelial cells, and pericytes, generally improving heart function.2-5
MSCs secrete many paracrine factors, including growth factors, cytokines, chemokines, and extracellular vesicles (EVs), mainly exosomes, having multiple implications in regulating key biological processes. These effects include the modulation of immune responses, migration and proliferation of effector cells, and inhibition of apoptosis. Various components, such as proteins, nucleic acids [DNA, mRNAs, non-coding microRNAs (miRs/miRNAs), long non-coding RNAs (LncRNAs)], lipids, and enzymes are encapsulated within exosomes and contribute to preserving and mediating the functional effects of their parent cells.6 MSCs-derived exosomes are advantageous over parental MSCs due to lower immunogenicity, better crossing of membrane barriers, not being trapped in capillary beds, a higher safety profile, and less possibility of ectopic tumor formation. By transferring the contents of stromal cells to nearby or distant cells, exosomes present their biological effects and play a vital role in disease processes. These properties make MSC exosomes more favorable compared to the original cells.7
microRNAs have crucial functions in regulating gene expression.8 Dysregulation of miR expression has been implicated in the pathophysiology of CAD,9,10 causing inflammation, vascular remodeling, and endothelial dysfunction. As a result, miRs have garnered significant interest as potential diagnostic and therapeutic targets in cardiovascular diseases. Integrating findings from the study on human epicardial adipose tissue-exos-miRs profiles provides deeper insights into their roles in CAD pathophysiology and highlights their diagnostic and therapeutic potentials,11 which can complement preclinical studies on MSC-exos-miRs. The shuttling of miRs via MSC-derived exosomes represents a critical mechanism through which these stem cells exert their beneficial effects, particularly in the context of tissue repair and regeneration in CAD. This systematic review article highlights the therapeutic roles of MSC-exosomal miRs in CAD. Primary outcomes were cardiac function, apoptosis, inflammation, and fibrosis.
Methods
Search strategy and selection of papers
This systematic review was conducted on studies reporting the impact of exosomal miRs derived from MSCs (MSC-exos-miRs) on in vivo and in vitro cardiovascular models. This study was designed following PRISMA. A librarian searched PubMed, Web of Science, Scopus, and Google Scholar until 04 April 2025. Table S1 (Supplementary file 1) presents the terms and MeSH-based keywords used for the search strategy.
Inclusion and exclusion criteria
Original research articles in English describing preclinical in vitro and animal models with CAD using exosomes of MSCs as experimental intervention were included. MSC-derived exosomal miRs used for the regeneration and treatment of cardiovascular injuries, such as myocardial ischemia-reperfusion (I/R) damage, MI, acute coronary syndromes (ACS), vascular calcification, and atherosclerosis were included since they are either causes, complications, or related conditions to CAD. Coronary artery disease is the overarching term that includes the long-term pathological process and risk factors, while ACS is a critical and acute manifestation of CAD. Therefore, CAD is the most appropriate term to encompass the entire spectrum of related conditions. Review articles, abstracts, and articles written in a language other than English were excluded. Moreover, retracted articles and the ones with low quality were excluded. Two separate researchers (S.M, A.A) reviewed all the records from the primary search of the databases. The included studies by two researchers were matched and compared, and the third researcher controlled the controversial studies.
Extracting data
Two researchers (S.M and S.M.M.M) independently reviewed the included studies. They extracted the articles’ data regarding the type of study, animal/cellular CAD models, sample size, the source and origin of MSCs, the technique employed for exosome extraction and identification, the route, frequency, and dosage of exosomal administration, the mechanisms through which exo-miRs regulate paracrine activity, and their impact on cardiac outcome, and therapeutic potential. These data were controlled by the other researchers. The detailed extracted data of the included studies are presented in Tables 1 and 2.
Table 1.
Therapeutic role of MSC-derived exosomal microRNAs in CAD models
|
Authors
|
Model
|
CAD
|
Animals
|
Cells
|
Sample size & groups
|
MSC donor
organism
|
Source of MSCs
|
MSCs preconditioning
|
Outcome
|
Therapeutic potential
|
| Yang et al (2021)14 |
In vitro, In vivo
|
MI |
SD male rats |
Rat primary CMECs under hypoxia (0.3% O2) |
Sham/ MI/ MI + hMSCs- Exo |
Human |
American Type Culture Collection (ATCC, USA |
Transfection with miR-543 inhibitor, pCDNA3.1-COL4A1, and their negative controls |
↑LVEF
↓LVEDD
↓Infarct size |
Diminished infarction size
attenuated MI-induced injuries
↑Ki-67 expression |
| Wang et al (2023)15 |
In vitro, In vivo
|
MI |
C57BL/6J mice |
HUVECs |
30: Sham/ MI/ MI + ADSC-Exo |
Mice |
AD-MSCs |
Transfected with a miRNA-205 inhibitor |
↑EF and FS
↓Cardiomyocyte apoptosis
↑Number of neo-vessels |
↑Angiogenesis
↑cardiac function
↓Fibrosis |
| Sun et al (2022)16 |
In vitro, In vivo
|
MI |
C57BL/6J male mice |
Neonatal mouse ventricle myocytes |
30: Sham/ MI/ MI + exosome |
Male C57BL/6J mice |
BM-MSCs |
Transfection with miR-182-5p mimics and control |
↓LVEDD and LVESD
↑LVEF and LVFS |
Anti-inflammatory on MI |
| Pu et al (2021)17 |
In vivo
|
MI |
Male SD rats |
H9c2 cells |
36: PBS/ Exo/ Exo-NC/ Exo-miR-30e |
Tibia and femur of healthy rats |
BM-MSCs |
Infected with LV-miR-30e-5p or empty vector with a MOI of 20 |
↑LVEF and LVFS
↓LVEDD, LVESD, LVVs, and LVVd |
↓Heart failure in rats with MI |
| Wang (2017)18 |
In vitro, In vivo
|
MI |
Male C57BL/6J mice |
Human fibroblasts and HUVECs |
Sham/ control/ fibroblast-EV/ MSC-EV/MSC- scramble-EV/ MSC-siR210-EV |
C57BL/6 mice |
BM-MSCs |
- |
↑LVEF and LVFS
↑Capillaries in peri-infarct regions |
Angiogenesis
on MI |
| Xiao et al (2018)19 |
In vitro, In vivo
|
MI |
Male C57BL/6J mice |
Neonatal mouse ventricle myocytes |
27: Sham/MI/MI + MSC |
- |
BM-MSCs |
- |
↓Autophagic Flux |
Cardioprotective effects: ↓Inflammation in the myocardial repair process |
| Peng et al (2020)20 |
In vitro, In vivo
|
MI |
BALB/c mice |
Primary cardiomyocytes from adult BALB/c mice |
50: sham, I/R I/, I/R + EXO, I/R + EXO/inhibitor NC,I/R + EXO/miR-25 inhibitor |
BALB/c mice |
BM-MSCs |
- |
↓Infarct area
↓Upregulation of IL-1β, IL-6, and TNF-α |
Cardioprotective effects: ↓inflammation in the myocardial repair process |
| Zheng et al (2022)21 |
In vivo
|
MI |
SD rats |
- |
100: sham/ MI/ Blank-Ex/ mimic-NC-Ex/miR-29b-3p mimic-Ex/ oe-NC / oe-ADAMTS16/ miR-29b-3p mimic-Ex + oe-NC/ miR-29b-3p mimic-Ex + oe- ADAMTS16 |
Femur and lumen bone of rats |
BM-MSCs |
- |
↑Cardiac hemodynamic function |
↑Angiogenesis and ventricular remodeling
MI |
| Xiong et al (2022)22 |
In vitro, In vivo
|
MI |
SD rats |
H9c2 cells |
Sham/AMI/ MSCs-Exo/ MSCTXL-Exo |
Tibia and femur of male SD rats |
BM-MSCs |
Transfected with miR-146a-5p inhibitors or its negative control |
↓Apoptotic cardiomyocytes
↓Levels of pro-apoptotic Bax and cleaved-Caspase 3 and inflammatory cytokines
↑LVEF
↓Infarct size |
Cardiac repair after MI |
| Wang et al (2021)23 |
In vitro, In vivo
|
MI |
C57BL/ 6JNifdc mice |
Cardiomyocytes |
32: Sham/ MI group/ Exo-NC/ Exo-inhibitor |
Mouse |
AD-MSCs |
Transfected with NC inhibitor or miR-671 inhibitor |
↓Myocardial fibrosis
↓Concentrations of IL-6 and TNF-α in the MI model mice |
MI |
| Li et al (2019)24 |
In vivo
|
MI |
Male SD rats |
- |
20: Sham/ Model/ BMSC-Exos/ BMSC-301- Exos |
Male SD rats |
BM-MSCs |
Transfection with miR-301 mimics |
↑LVEF and LVFS
↓LVESD and LVEDD |
↓Myocardial autophagy protects against MI |
| Zhu et al (2022)25 |
In vitro, In vivo
|
MI |
Male
C57BL/6 mice |
HMVEC transduction with Lenti/F1H1 and a corresponding control |
66: PBS, Exo, Exo/ anti-miR-Con, Exo/ anti miR-31 |
Human |
UC-MSC |
Transduced with a lentiviral antimiR-31 |
↑Cardiac function |
↓MI |
| Wan et al (2022)26 |
In vivo
|
MI |
c57BL/6 mice |
- |
198: PBS, MSCs-EVs, EVs-NC, EVs-miR-200b-3p, MSCs-EVs + lentivirus-expressing control short hairpin RNA, MSCs-EVs + lentivirus-expressing BCL2L11 short hairpin RNA, MSCs-EVs + overexpressed-negative lentivirus, MSCs-EVs + BCL2L11-overexpressing lentivirus, EVs-miR-200b-3p + Neg, EVs-miR-200b-3p + BCL2L11 |
- |
MSCs (Shanghai Zhongqiao Xinzhou Biotechnology Co.) |
Transfected with miRNA mimic NC or miR-200b-3p mimic |
↓LVEDD and LVESD
↑LVEF and LVFS |
↓MI-induced apoptosis of cardiomyocytes and inflammation |
| Xuan et al (2019)27 |
In vitro, In vivo
|
MI |
NOD/SCID mice
C57/B6 mice |
Human dermal fibroblast cell line
(CC-2511) and lung fibroblast cell line (CC-2512) |
9: PBS, EV-hiPSC, or EV-CPCISX-9 |
Human |
iPSC cell line |
Transfected with 25 nM miR-373 mimic, anti-miR-373, negative controls, and RNAiMAX |
↑CM Proliferation and angiogenesis
Reversed Ventricular Remodeling in Mice Post MI |
↓Fibrosis
↑Angiogenesis in Infarcted Heart |
| Fu et al (2020)28 |
In vitro, In vivo
|
MI |
Female SD rats |
H9c2 cells |
40: Sham/ PBS/ EXO-control/ EXO-338 mimic |
Rat femur and tibia |
BM-MSCs |
Transfection of miR-338 mimics or negative control |
↓LVESD and LVEDD
↑EF and FS |
↓Cardiomyocyte apoptosis in MI |
| Ma et al (2018)29 |
In vitro, In vivo
|
MI |
Female C57BL/6J mice |
HUVECs |
Saline control/ miR-132/ Exo-null/ Exo-132 |
Bone cavity of mouse femurs and tibias |
BM-MSCs |
- |
↑LVEF |
↑Angiogenesis in MI |
| Huang et al (2020)30 |
In vitro, In vivo
|
MI |
Male SD rats |
H9C2 cells under hypoxia |
105: AMI + PBS, AMI + hucMSC-exo, AMI + in-NC/hucMSC-exo, AMI + in-miR-19a/hucMSC-exo, AMI + NC/hucMSC-exo, and AMI + miR-19a/hucMSC-exo |
Human |
hUC-MSCs |
Transfected with miR-19a mimic, miR-19a inhibitor, and NC vectors |
The cardiomyocytes are arranged regularly |
↓Acute MI |
| Yang et al (2022)31 |
In vitro, In vivo
|
MI |
SD rats |
Rat cardiomyocyte H9c2 cells |
60: Sham /MI + PBS/ MI + Vs-NC/MI + EVs-miR-223/ MI + EVs-miR-223 + pcDNA3.1-P53/ MI + EVs-miR-223 + pcDNA3.1-S100A9 |
Human |
hUC-MSCs |
Transfection with miR-223 mimic or NC mimic |
↑Cardiac function |
↓Fibrosis and inflammation of cardiomyocytes
↑Angiogenesis |
| Pu et al (2023)32 |
In vitro, In vivo
|
MI |
SD rats |
HUVECs |
20: Sham/MI/M-EVs/N-EVs |
Human |
hUC-MSCs |
-Incubation with 2.25 μM NMN for 48 h
-Transfected with miR-210-3p inhibitor or negative control |
↓Fibrosis size and cell apoptosis in infarcted hearts |
↑Angiogenesis
MI |
| Ji et al (2024)33 |
In vitro, In vivo
|
MI |
Male SD rats |
H9c2 cells |
40: Sham/ AMI/ Control-Exo/ miR-21-5p-Exo |
Rat |
MSCs (CP-R131) |
Transfected with miR-21-5p inhibitor or NC inhibitor |
↓Myocyte apoptosis and fibroblast proliferation
Reverse ventricular remodeling |
RNA-based therapies in cardiovascular disease |
| Wang et al (2024)34 |
In vitro, In vivo
|
MI |
Mice |
Cardiac muscle cells |
- |
- |
BM-MSCs |
- |
↓Expression of inflammatory cytokines |
↑Cardiac function
↓Expression of inflammatory cytokines |
| You et al (2024)35 |
In vivo, in vitro
|
MI |
C57B/6 male mice |
H9C2 cells under hypoxia (1% O2) |
Sham/MI/ MI + exos/ MI + exos + miR-let-7i-5p inhibitor/ MI + exos + miR-let-7i-5p inhibitor NC |
SD mice |
BM-MSCs |
Transfected with MiRNA-let-7i-5p mimic, miRNA-let-7i-5p mimic NC, miRNA-let-7i-5p inhibitor, or miRNA-let- 7i-5p inhibitor NC |
↓Myocardial apoptosis ↓Infarction progression |
↓Myocardial apoptosis
↓MI progression
↓MI |
| Zhu et al (2022)36 |
In vitro, In vivo
|
MI |
Male C57BL/6 J mice |
HUVECs |
10: PBS, Exo, Exo/ antimiR-Cont, Exo/ antimiR-31 |
Human |
hAD-MSC |
Transduction with a lentiviral antimiR-31 or antimiR-control |
↑Cardiac function
↓Infarct size
↑Angiogenesis |
Angiogenesis
on MI |
| Zhao et al (2019)37 |
In vivo
|
I/R |
C57BL/6 mice |
RAW264.7 cells or peritoneal macrophages |
Untreated/ LPS/ LPS + NC-mimic/ LPS + miR-182-mimic |
Mouse |
BM-MSCs |
Transfection with miR-182 inhibitor NC inhibitor |
↑EF and FS |
Anti-inflammatory on MI |
| Chen et al (2020)38 |
In vitro, In vivo
|
I/R |
Male SD rats |
I/R myocardium cells |
20: Sham, I/R, Exo-67, Exo-125b |
Femur and tibia of 2 male SD rats |
BM-MSCs |
Transfected with Lv-cel-miR-67 or Lv-miR-125b |
↑LVEF, LVFS, and LVSP
↓LVESD, LVEDD, and LVEDP |
Protects against myocardial I/R |
| Mao et al (2022)39 |
In vivo
|
I/R |
Male SD rats |
- |
Sham, I/R, I/R ± Exo, I/R ± NC-Exo, I/R ± miR-183-5p-Exo, I/R ± anti-miR-183-5p-Exo |
6 male SD rats |
BM-MSCs |
Transduction with LV-miR-183-5p, LVanti-miR-183-5p, or NC |
↓MI size |
↓Apoptosis and oxidative stress in I/R cardiomyocytes
↑Cardiac function
Protecting against MI/R injury |
| Chen et al (2021)40 |
In vitro, In vivo
|
I/R |
SD rats |
H9c2 cells |
Control, I/R, I/R + exosome, I/R + miR-143-3p KD exosome, I/R + miR-143-3p OE |
Femur and tibia of SD rats |
BM-MSCs |
Transfection with NC mimic, miR-145-5p mimic, NC inhibitor, or miR-145-5p inhibitor |
↓Apoptosis and autophagy of rat cardiomyocytes |
Myocardial I/R injury
regulating autophagy |
| Wang et al (2022)41 |
In vitro, In vivo
|
I/R |
Male SD rats |
H9c2 cells under H/R or transfection |
30: Sham group, Exo-miR-455-3p, I/R, I/R + Exo-miR-455-3p |
Rats |
BM-MSCs |
-Stimulated with H/R (O2 < 1%, hypoxia 48 h, reoxygenation 24 h)
-Transfection with MEKK1 overexpression plasmid, miR-455-3p mimics, or miR-455-3p inhibitor |
↓Myocardial cell apoptosis |
Myocardial I/R damage |
| Zhang et al (2021)42 |
In vivo
|
I/R |
SD rats |
- |
120: Sham, model,
normal BMSC-exos, Hypoxic exos, exos-miR-98-5p Antagomir, miR-98-5p Agomir, miR-98-5p Agomir NC,
miR-98-5p Agomir + oeTLR4 |
6 male SD rats |
BM-MSCs |
-Stimulated by hypoxia (1% O2 for 24 h)
-Transfected with miR-98-5p antagomir |
↓LVEDP
↑LVSP |
↓Myocardial I/R injury |
| Li et al (2020)43 |
In vitro, In vivo
|
I/R |
Male C57BL/6 N mice |
Neonatal rat cardiomyocyte transfected with miR-NC or miR-29c mimic |
I/R + PBS, I/R + Nor-exo, and I/R + Hypo-exo |
Mouse |
BM-MSCs |
H/R (O2 < 1%, hypoxia 48 h, reoxygenation 24 h) |
↓Infarcted size |
↓Excessive Autophagy ↓Cardiac I/R Injury |
| Gao et al (2023)44 |
In vitro, In vivo
|
I/R |
Female C57BL/6 mice |
-Murine macrophage RAW 264.7 cells stimulated with 100 ng/mL LPS
-Primary neonatal mouse cardiomyocytes, cardiac fibroblasts, and endothelial cells isolated from C57BL/6 |
18: Sham, I/R control, MSC, MSC-Exo, NC agomir, miR-125-5p |
Mouse |
BM-MSCs |
Transfected with mmu-miR-125a-5p antagomir and NC antagomir for 24 h |
↑Cardiac function on day 28 post-myocardial I/R |
↑Recovery from myocardial I/R injury |
| Zou et al (2020)45 |
In vitro
|
I/R |
- |
H9c2 cells transfected with 10 pmol/mL miR-149, let-7c, or control mimics |
Control, H/R, H/R + Exo, H/R + NC,H/R + mimics-149, H/R + mimics-7c |
Rat |
BM-MSCs |
H/R (Hypoxic atmosphere for 4 h followed by reoxygenation for another 24 h) |
↓H/R-induced apoptosis |
Cardiomyoblast H/R injury |
| Wei et al (2019)46 |
In vitro, In vivo
|
I/R |
C57BL/6 male mice |
PBMCs transfected with the miRNA-181a precursor, NC, mimic inhibitor, or inhibitor NC |
12: Sham /PBS/ WT-EXO, miRNA- 181a-EXO |
Human |
UC blood-MSCs |
Transduction with GV309-neg-EGFP-LV or GV309-miRNA-181a-EGFP-LV |
↑EF and FS |
Influenced the inflammatory response after myocardial I/R injury |
| Yue et al (2022)47 |
In vitro, In vivo
|
I/R |
Male C57BL/6 mice |
-H/R-exposed myocardial cells co-incubated with Exo-mimic-NC, exo-miR-182-5p mimic, Exoinhibitor-NC, or exo-miR-182-5p inhibitor
-HUVECs |
30: Sham, I /R, I/ R + Exo-mimic-NC, I/ R + Exo- miR-182-5p mimic |
Well-grown C57BL/6 mice |
BM-MSCs |
Treated with 10% GW4869 or 0.005% DMSO |
↓MI size and recovery of cardiac function |
↓I/R-evoked inflammation, apoptosis, and injury |
| Ou et al (2020)48 |
In vitro, In vivo
|
I/R |
Male SD rats |
Neonatal cardiomyocytes under hypoxia (1% O2) and transducted with 1000 ng/ mL EVagomir-NC, EVmiR-150-5p-agomir, EVantagomir-NC, or EVmiR-150-5p-antagomir |
72: I/R,I/R + sh-NC, I /R + sh-TXNIP, I/ R + EVmiR-150-5pagomir, I/ R + EVagomir-NC, I/ R + EVmiR- 150-5p-agomir + oeTXNIP |
Healthy SD rats |
MSCs |
Transduction with agomir-NC (50 nM), miR-150-5p-agomir (50 nM), antagomir-NC (100 nM), or miR-150-5p-antagomir (100 nM) |
↓LVEDV, LVEDD, LVESV, and LVESD
↑LVEEF and LVEFS |
↓Apoptosis and myocardial I/R injury |
| Tang et al (2020)49 |
In vitro, In vivo
|
I/R |
Male SD rats |
Primary cardiomyocytes under H/R for 18 h |
Sham, I/R, I/R + PBS, I/R + exosome |
Human |
MSCs |
miR-320b mimic and its control |
- |
Anti-pyroptosis
↓I/R Injury |
| Chen et al (2024)50 |
In vivo, in vitro
|
I/R |
SD rats |
H9c2 cells under H/R (95% N2 and 5% CO2 for 3 h) and 100 nM miRNA/NC inhibitor |
Control /model/ BMSC exo/BMSC exo + anti-miR-93-5p /BMSC exo + DSD/BMSC exo + DSD + anti-miR-93-5p |
Rat |
BM-MSCs |
H/R
Pretreated with DSD or miRNA inhibitor |
↓Cardiac damage |
↓Activation of the TXNIP/NLRP3/Caspase-1 signaling pathway and cardiomyocyte pyroptosis |
| Du et al (2024)51 |
In vivo, in vitro
|
I/R |
SD male rats |
H9C2 cells under hypoxia (95% N2 and 5% CO2) |
40: Sham, I/R + PBS, I/R + BMSC- Exo, I/R + BMSC- Exo-25-3p |
20 male rats |
BM-MSCs |
- |
↓Cardiac infarct size
↓Incidence of malignant arrhythmias
↓Myocardial enzyme activity |
↓Inflammatory response
↓Myocardial I/R injury |
| Gu et al (2024)52 |
In vivo, in vitro
|
I/R |
Male C57BL/6 mice |
H9c2 cells under hypoxia (5% CO2 and 95% N2 for 6 h) pretreated with DSPE-PEG-CMP, DSPE-PEG-CMP-EXO, DSPE-PEG-CMP-miR302-EXO, or miR302 |
30: Control / model/ DSPE- PEG-CMP/ DSPE- PEG- CMP- EXO/ DSPE- PEG-P- miR302- EXO/ miR302 |
Tibia and femur of C57BL/6 mice |
BM-MSCs |
- |
↓Myocardial I/R injury |
↓Cell apoptosis, inflammation
↑Cardiac function |
| Lee et al (2025)53 |
In vivo, in vitro
|
I/R |
C57BL/6J mice |
Embryonic rat cardiomyocyte-derived H9c2 cardiac myoblasts exposed to PM (10 µg/ mL or 50 µg/mL) for 6 h, H/R (hypoxia (1% O2) for 6 h followed by reoxygenation for 12 h), and transfected with miR-221 and miR-222 mimics or inhibitors (100 nM/well) |
Control, PM + I/ R, PM + I/ R + ADSC-Exo, PM + I/ R + miR-221 miR-222 mimics |
Human |
AD-MSCs |
- |
↓Cardiomyocyte mitophagy and apoptosis |
↓Cardiac damage caused by PM + I/R |
| Du et al (2017)54 |
In vitro, In vivo
|
Ischemia |
Transgenic mice expressing VEGFR2-Luc |
HUVECs with NO stimulation (with chitosan NO-releasing polymer and β-galactosidase) |
60: PBS/EXO/NO EXO |
Human |
P-MSCs |
Transfected with 100 nmol/L miR-126 inhibitor and a NC inhibitor |
↓PIK3R2/↑Level of AKT phosphorylation
↑Angiogenic processes |
↑Angiogenesis |
| Feng et al (2014)55 |
In vivo
|
Ischemia |
C57BL/6J mice |
- |
48: microRNA Scramble / siRNA-Mecp2/miR-22-mimic/Exonon-IPC, ExoIPC/ ExoIPC + miR-22Inhibitor |
C57Black -6 mice |
BM-MSCs |
-Starved overnight of glucose followed by ischemia (Repeated cycles of anoxia (30 min) with intermittent reoxygenation (10 min) for two cycles in an anoxic chamber)
-Transfected with miR-22 mimics FOR 24 h |
↓Fibrotic area |
Anti-fibrotic |
| Sánchez-Sánchez et al (2021)57 |
In vitro, In vivo
|
Ischemia |
Nude rats |
Neonatal rat cardiomyocytes and HUVECs transfected with 20 nM miR-4732-3p for 6 h |
70: Control /glucose deprivation/ glucose deprivation + EVs /glucose deprivation + miR-NC/glucose deprivation + miR-4732 |
- |
Immortal MSC-TERT line |
- |
Recovery of systolic function |
↑Angiogenic and cardioprotective responses |
| Luo et al (2017)56 |
In vitro, In vivo
|
Ischemia |
Male SD rats |
H9c2 cells under hypoxia (93% N2, 2% O2 and 5% CO2) for 24 h |
Normal/AMI + PBS/ AMI + Exosome /AMI + miR-126-Exosome |
- |
AD-MSCs |
Transfection of miR-126 mimics or miR-126 NC for 48 h |
↓Cardiac fibrosis
↑Cell proliferation in the border zone |
Protecting myocardial cells: ↓Apoptosis, inflammation, fibrosis, and ↑angiogenesis |
| Yu et al (2015)58 |
In vitro, In vivo
|
Hypoxia |
Female SD rats |
Primary rat neonatal cardiomyocytes under hypoxia (1% O2, 5% CO2, and 94% N2) |
Sham / Saline control / ExoGATA-4/ ExoNull |
Femurs and tibias of SD rats |
BM-MSCs |
Transduction with recombinant GATA-4 |
↓Infarct size in heart tissue |
Anti-apoptotic
MI |
| Zhao et al (2024)59 |
In vitro
|
Hypoxia/reperfusion |
- |
H9c2 rat cardiomyocytes under H/R (incubated in 3.3 mmol/L H2O2 for 10 min followed by reoxygenation for 30 min) |
- |
Femur and tibia of SD rats |
BM-MSCs |
- |
↑Cardiomyocyte
apoptosis |
↓Pik3c3 expression and
phosphorylation of AKT/mTOR |
| Sun et al (2019)60 |
In vitro, In vivo
|
Ischemia, Hypoxia |
Male SD rats |
H9C2 cells under H/R (hypoxia (95% N2 and 5% CO2 for 16 h, followed by reoxygenation for 3 h) and transfection with pre-miRNA of miR-468-5p andanti-miR-486-5p |
28: H/R, BMSC-exo, exo-miR-486-5p, exo- anti-miR-486-5p |
Femur and tibia of SD rats |
BM-MSCs |
- |
↓Area of MI |
↓Myocardial I/R injury |
| Yang et al (2021)61 |
In vitro, In vivo
|
Atherosclerosis |
ApoE−/− female C57BL/6J mice |
HUVECs |
Blank, AS model, AS model + miR- 145 exosome |
Human |
hUC-MSCs |
Transfected with 10 nM Cy3-labeled miR-145 mimic |
↓Atherosclerotic plaques |
↓Atherosclerosis |
| Ma et al (2021)62 |
In vitro, In vivo
|
Atherosclerosis |
ApoE–/– mice |
RAW264.7 cells transfected with miR-21a-5p inhibitor or inhibitor NC |
20: PBS/MSC-exo |
Male C57BL/6 J mice |
BM-MSCs |
Transfection with miR-21a-5p mimic, mimic NC, miR-21a-5p inhibitor, or inhibitor NC |
↓Plaque area and macrophage infiltration in AS mice |
↑M2 macrophage polarization
↓Macrophage infiltration |
| Wang et al (2015)63 |
In vitro, In vivo
|
Sepsis |
Male WT C57BL/6 mice |
RAW264.7 cells
Primary cardiomyocytes isolated from adult rat hearts |
40: sham, CLP + PBS control, CLP + PBS, WT- MSC, miR- 223- KO-MSC |
Mouse tibia and femur |
BM-MSCs |
- |
↑Survival rate
↑Values of left ventricular EF and FS |
Anti-inflammation, Cardioprotection in polymicrobial sepsis |
| Pei et al (2021)64 |
In vitro, In vivo
|
Sepsis |
Male KM mice |
Mouse primary cardiomyocytes transfected with miR-141 mimic or miR-141 inhibitor |
31: control, CLP, exo, exo- NC, exo- knockout, PBS |
Mouse |
BM-MSCs |
Transfection with miR-141 inhibitor |
↓Number of apoptotic cells in mouse myocardial tissues |
↓Myocardial injury in septic mice |
| Luo et al (2022)65 |
In vitro
|
Calcification |
- |
Human aortic vascular smooth muscle cells |
- |
Human |
BM-MSCs |
Transfection with hsa-miR-15a-5p mimics/inhibitors, hsa-miR-15b-5p mimics/inhibitors, hsa-miR-16-5p mimics/inhibitors, or mimics NC/inhibitors NC |
↓HA-VSMCs osteogenic transdifferentiation |
↓Atherosclerosis |
| Chen et al (2021)66 |
In vitro
|
Endothelial dysfunction |
- |
Primary EC cells from male C57BL/6 mice transfected with Keap1 overexpressed and knockdown plasmids and NC |
Untreated /ox-LDL/ ox-LDL + EXO- miR-NC/ ox-LDL + EXO- miR-512-3p |
Bilateral leg bones of mice |
BM-MSCs |
Transfection with miR-512-3p mimics and miR-NC |
↓EC cell apoptosis and inflammatory
Response
↑Proliferation |
↓Apoptosis and inflammatory response
↓Atherosclerosis |
| Lei et al (2021)67 |
In vitro, In vivo
|
Myocardial toxicity |
Healthy SPF SD female rats |
H9c2 cells |
85: normal/ doxorubicin/ Exo/ Exo + mimic NC/ Exo + miR-96 mimic/ Exo + inhibitor NC/ Exo + miR-96 inhibitor |
Rats |
BM-MSCs |
Transfected with miR-96 mimic, mimic NC, miR-96 inhibitor or inhibitor NC |
↓Proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and collagen fibers |
↓Doxorubicin-Induced Myocardial Toxicity |
| Wang et al (2021)68 |
In vitro, In vivo
|
Chronic heart failure |
Male SD rats |
H9C2 cells and HUVECs treated with OGD/R for simulating myocardial and under H/ R (hypoxia (5% CO2 and 95% N2 for 3 h), followed by reoxygenation (5% CO2 for 48 h) injury |
20: Sham/LAD /LAD + PBS/LAD + hucMSC-Exo |
Human |
UC-MSCs |
Transfected with miR-1246 inhibitor or the corresponding NC |
↓LVSD, IVSS, LVIDD, and LVIDS
↑EF |
Hypoxia-induced myocardial tissue damage in chronic heart failure |
| Yan et al (2022)69 |
In vitro, In vivo
|
Heart failure |
Male C57BL/6 J mice |
Mouse cardiomyocytes HL-1 |
24: Sham/HF /HF + PBS/HF + MSC-Exos |
Mouse |
BM-MSCs |
Transfection with miR-129-5p inhibitor, small interfering RNA (si)-TRAF3, or negative controls for 24 h |
↑Level of stroke volume |
↓Apoptosis and Oxidative Stress in Heart Failure |
ADAM19: A Disintegrin and metalloproteinase 19, ADAMTS16: A disintegrin and metalloproteinase with thrombospondin motifs 16, AD-MSCs: Adipose derived mesenchymal stem cells, AKT: protein kinase, B, BCL2L11: Bcl-2-like protein 11, BM-MSCs: Bone marrow derived MSCs, Bnip3: B-cell lymphoma 2–interacting protein 3, DAAM1: Disheveled-associated activator of morphogenesis 1, DSD: Danshen decoction, EMT: Epithelial–myofibroblast transdifferentiation, ESRK1/2: Extracellular signal-related kinases 1 and 2, Faslg: Fas ligand gene, FG: Fractional shortening, FIH: Factor-inhibiting HIF, GDF11: Growth differentiation factor 11, GSDMD: Gasdermin D, HDAC2: Histone deacetylase 2, HIF: Hypoxia-inducible factor, H/R: Hypoxia-reperfusion, HUVECs: human umbilical vein endothelial cells, IMI: Intramyocardial injection, I/R: Ischemia-reperfusion, IRAK: Interleukin-1 receptor-associated kinase, IV: Intravenously injection, JAM-A: Junctional adhesion molecule A, JNK: c-Jun NH2-terminal kinase, Keap1: Keleh-like ECH-associated protein 1, KLF-6: Kruppel-like factor 6, LVD: LV diastolic dimension, LVEDD: Left ventricular end-diastolic diameter, LVEDV: LV enddiastolic volume, LVEF: LV ejection fraction, LVESD: LV end-systolic diameter, LVESV: LV end-systolic volume, MAPK: Mitogen-activated protein kinase, Mecp2: Methyl CpG binding protein 2, MI: Myocardial infarction, NAT1: N-Acetyltransferase 1, NFAT: Nuclear factor of activated T cells, NLRP3: NLR Family pyrin domain containing 3, NO: Nitric oxide, Nrf2: NF-E2-related factor 2, P53: Tumor protein 53, PDK4: Pyruvate dehydrogenase kinase 4, PI3K: Phosphatidylinositol 3 kinase, PM: Particulate matter, P-MSCs: Placenta-derived MSCs, PRSS23: Protein serine protease 23, PTEN: Phosphatase and tensin homolog, RASA1: RAS P21 protein activator 1, ROCK2: Rho associated coiled-coil containing protein kinase 2, SD: Sprague-dawley, SMAD: Suppressor of mothers against decapentaplegic, SNRK: Sucrose non-fermenting-1 related kinase, S100A9: S100 calcium-binding protein A9, SOX6: Sry-related high-mobility group box6, TERT: Telomerase reverse transcriptase, TGF-β: Transforming Growth Factor beta, TGF-βR: TGF-β receptor, TLR4: Toll-like receptor 4, TRAF6: Tumor necrosis factor receptor-associated factor 6, TXNIP: Thioredoxin interacting protein, UC-MSCs: Umbilical cord derived MSCs, VEGFA: Vascular endothelial growth factor A, YAP1: Yes-associated protein 1.
Table 2.
Characteristics of MSCs and their exosomal microRNAs in CAD models
|
Authors
|
Exosome isolation
|
Characterization
|
Exosome markers
|
Exosome concentration
|
miRs
|
Deliver target miRs into exosomes/ cell lines
|
Administration routes
|
Treatment route/time
|
miRNA Targets
|
| Yang et al (2021)14 |
Exosome extraction kit (Sigma-Aldrich, Merck KgaA, Darmstadt, Germany) |
Particle size analysis
TEM
Western blotting |
TSG101, HSP70, and CD63 |
0.13 μg/μl |
miR-543 |
|
IV |
40 μg protein in 300 μl PBS per rat |
Downregulating COL4A1 expression |
| Wang et al (2023)15 |
ExoQuick-TCTM kit |
TEM
NTA
Bradford assay
Western blotting |
CD63 and CD9 |
100 μg protein, 50 μL |
miR-205 |
|
Intramuscularly / IV |
Five locations along the anterior wall of the left ventricle’s border zone |
Cardiac function and HIF-1a and VEGF increased expression |
| Sun et al (2022)16 |
Ultracentrifugation method |
TEM
NTA
Western blotting |
CD9, CD63 and Alix |
5 μg |
miR-182-5p |
|
IMI |
several sites around the infarct region |
TLR4/NF-κB signaling pathway |
| Pu et al (2021)17 |
Ultracentrifugation method |
TEM
NTA
Western blotting |
TSG101, Alix, and CD81 |
20 μg/mL |
miR-30e |
|
IV |
Exosomes were injected via the tail vein for 3 consecutive days 7 days after MI surgery |
LOX1/ NF-κB p65/ Caspase-9 axis |
| Wang (2017)18 |
Ultracentrifugation method |
TEM
Western blotting |
LAMP-1, CD44,
CD105, and TSG 101 |
- |
miR-210 |
|
IV |
MSC-EVs in mice subjected with MI injury |
Efna3 |
| Xiao et al (2018)19 |
Ultracentrifugation method |
Electron microscopy and immunoblotting |
CD63, CD9, and Alix |
0.2 μg/μl |
miR-125b |
|
IMI |
Injection of 5 μl exosomes into 5 sites at the border of the infarct 30 min after ligation |
p53-Bnip3 signaling |
| Peng et al (2020)20 |
Total Exosome Isolation Reagent |
TEM
NanoSight NS500
Western blotting |
HSP70, CD63, and CD9 |
0.05 μg/μl |
miR-25-3p |
|
IMI |
5 μg in 100 μL PBS injected into the border zone of the infarcted heart at three sites 30 min after ligation |
Pro- apoptotic genes FASL and PTEN |
| Zheng et al (2022)21 |
Ultracentrifugation method |
Western blotting
TEM
NTA |
CD81 and
TSG101 |
- |
miR-29b-3p |
|
Injection |
|
ADAMTS16 |
| Xiong et al (2022)22 |
Ultracentrifugation method |
Micro BCA protein assay
TEM
NTA
Western blotting |
CD63, TSG101, and Alix |
0.2 μg/μl |
miR-146a-5p |
|
IMI |
20 μg exosomes in 100 μL PBS injected into the border zone of the infarcted heart at three sites |
IRAK1/ NF-κB p65 pathway |
| Wang et al (2021)23 |
Total Exosome Isolation Reagent |
TEM
NTA
Western blotting |
CD63 and CD81 |
100 μg |
miR-671 |
|
IMI |
100 μg Exo-NC or Exo-inhibitor was dissolved in PBS and injected in the boundary area of the infarcted cardiac near the ligation site |
TGFBR2/ Smad2 Axis |
| Li et al (2019)24 |
Exosomes isolation kit |
TEM
Western blotting |
CD81, CD63, and CD9 |
- |
miR-301 |
|
IMI |
30 min after LAD artery ligation, BMSCExos were injected at 5 points in the peripheral area of the MI |
- |
| Zhu et al (2022)25 |
Ultracentrifugation method |
|
|
2 μg/μl |
miR-24-3p |
|
IMI |
|
Plcb3 and NF-κB pathway
↑M2 Macrophage Polarization |
| Wan et al (2022)26 |
Ultracentrifugation method |
TEM
NTA
Western blotting |
CD9, CD81, and GRP94 |
6.66 μg/μl |
miR-200b-3p |
|
IMI |
EVs (100 μg) or recombinant lentivirus (5 × 107 viral genome particles per mouse heart) injection around the infarct area (anterior wall, lateral wall, and apical area) after LAD artery ligation |
BCL2L11 |
| Xuan et al (2019)27 |
Ultracentrifugation method |
TEM
TRPS
Western blotting |
Tsg101, CD9, Hsp70, and flotillin-1 |
1012 particles/ml |
miR-373 |
|
IMI |
10 mins after LAD ligation, EVs (1 × 1012/ml) were injected
into the myocardium along the border zone with a total of 20 μl |
GDF-11 and ROCK-2 |
| Fu et al (2020)28 |
Exosome Isolation Reagent |
TEM
Western blotting |
CD9,CD63, and CD81 |
- |
miR-338 |
|
IMI |
50 μL of exosome were injected before the chest was closed |
Regulate the JNK pathway via targeting MAP3K2 |
| Ma et al (2018)29 |
Total exosome isolation reagent |
TEM
Western blotting |
CD63 and CD9 |
30 μg/μl |
miR-132 |
Loading miR-132 via electroporation |
IMI |
Injection of exosome (600 μg) after LAD ligation |
↓The expression level of its target gene RASA1 |
| Huang et al (2020)30 |
Ultracentrifugation method |
TEM
NTA
Western blotting |
CD9, CD63, Alix 3, and GM130 |
400 μg/g |
miR-19a |
- |
IV |
400 μg/g exosome injection |
SOX6 |
| Yang et al (2022)31 |
Ultracentrifugation method |
BCA assay
TEM
Western blotting |
CD9, CD81, and CD63 |
50 μg/mL |
miR-223 |
Transfection of EVs with miR-223 mimic or NC mimic |
IMI |
EV injection (50 μg/mL) after the ligation at three different sites around the infarcted area |
Modulate the P53/S100A9 axis |
| Pu et al (2023)32 |
Ultracentrifugation method |
TEM
NTA
Western blotting |
TSG101and CD63 |
4 × 107 particles/ μl |
miR-210-3p |
|
IMI |
EVs (50 uL or 2 × 109 particles) were injected in the border zone of the infarction area 30 min after ligation |
EphrinA3 |
| Ji et al (2024)33 |
Ultracentrifugation method |
TEM
NTA
Western blotting |
CD81 and TSG101 |
100 µg |
miR-21-5p |
|
IV |
100 µg of exosomes suspended in 100 µL sterile PBS via tail vein injection |
YAP1 signaling pathway |
| Wang et al (2024)34 |
- |
|
|
|
miR-223-3p |
|
IMI |
Peri-infarct myocardial region was injected |
↓NLRP3 |
| You et al (2024)35 |
Ultracentrifugation method |
TEM
Western blotting
BCA assay |
CD63, CD81, and TSG101 |
0 or 50 µg/ml |
miR-let-7i-5p |
|
IMI |
Immediately after ligation, the peri-infarct myocardial region was injected at three different points with a total of 10 uL of exosomes |
Bcl-2 |
| Zhu et al (2022)36 |
Ultracentrifugation method |
NTA
TEM
Western blotting
BCA assay |
CD9 and TSG101 |
5 μg (IM)
100 μg (IV) |
miR-31 |
|
IMI, IV |
IMI: Exosomes (5 μg, 2.2 × 107 particles) were injected in the infarct border area two times on each side of the ligation
IV: Exosomes (100 μg, 4.3 × 108 particles) were injected at the tail vein at 7, 14, and 21 days post-surgery |
FIH1/ HIF-1α pathway |
| Zhao et al (2019)37 |
Ultracentrifugation method |
NTA
TEM
Western blotting
BCA assay |
CD9, CD63 TSG101, and Alix |
- |
miR-182 |
|
IMI |
Exosomes (5, 30, or 50 ug) 3 days following myocardial I/R injury |
TLR4 signal |
| Chen et al (2020)38 |
ExoQuick-TC kit |
TEM
Western blotting |
CD9 and CD63 |
50 µg |
miR-125b |
|
IMI |
After LAD, exosomes (50 µg) were injected into the ligation zone adjacent to the left anterior free wall after left ventricle exposure |
Sirtuin7 |
| Mao et al (2022)39 |
Ultracentrifugation method |
NTA
TEM
Western blotting |
CD9, CD63, and CD81 |
2 μg/μl |
miR-183-5p |
|
IV |
Exosomes (400 µg in 200 µL PBS) were injected via the tail vein within 5 min of the beginning of reperfusion |
FOXO1 |
| Chen et al (2021)40 |
Hieff TM Quick exosome isolation kit |
NTA
TEM
Western blotting |
CD63 and CD81 |
0.5 μg/μl |
miR-143-3p |
|
IMI |
Exosomes (200 μg suspended in 400 μl PBS) were injected into the myocardium |
CHK2- Beclin2 pathway |
| Wang et al (2022)41 |
Ultracentrifugation method |
TEM
Western blotting |
CD63, CD9, and Alix |
10 μg |
miR-455-3p |
|
IMI |
Exosomes (10 μg) were transfused into the left ventricular wall of rats |
MEKK1- MKK4-JNK signaling pathway |
| Zhang et al (2021)42 |
Ultracentrifugation method |
TEM
BCA assay
Western blotting |
CD63 and CD9 |
1 μg/μl |
miR-98-5p |
|
IMI |
100 μL exosomes (1 μg/μL) were injected into 4 different sites of the anterior wall of the left ventricle |
↓TLR4 and activating the PI3K/ Akt signaling pathway |
| Li et al (2020)43 |
Ultracentrifugation method |
Western blotting |
CD9, CD63, and Alix |
1 μg/μl |
miR-29c |
|
IMI |
Exosomes (20 μg resuspended in 20μL PBS) were injected in 2 sides of the border zones right after LAD coronary ligation |
PTEN/ Akt/ mTOR Signaling Pathway |
| Gao et al (2023)44 |
Ultracentrifugation method |
NTA
TEM
BCA assay
Western blotting |
CD63, CD9, TSG101, and Alix |
0.66 μg/μl |
miR-125a-5p |
|
IMI |
10 μg exosomes or 20 nmol miR-125a-5p agomir into the border zone at the onset of reperfusion |
Klf13, Tgfbr1, and DAAM1 |
| Zou et al (2020)45 |
Ultracentrifugation method |
TEM
Western blotting |
CD63, ALIX, and TSG101 |
- |
miR-149 |
- |
- |
- |
Faslg and w/β- catenin signaling pathway |
| Wei et al (2019)46 |
Ultracentrifugation method |
NTA
TEM
Western blotting |
CD9, CD63, TSH, and ALIX-101 |
200 μg |
miR-181 |
|
IMI |
200 μg of exosomes suspended in PBS were injected before the chest was closed |
T-cell receptor signaling and TGF-β signaling |
| Yue et al (2022)47 |
Ultracentrifugation method |
NTA
TEM
Western blotting |
CD63, HSP70, TSG 101, Alix, and Calnexin |
1 μg/μl |
miR-182-5p |
|
IMI |
10 μg exosomes dissolved in 10 μL PBS injected at the front and outside of the visible injury area |
GSDMD |
| Ou et al (2020)48 |
ExoQuick-TC EV purify reagent |
NTA
TEM
BCA assay
Western blotting |
CD9, CD63, Alix, and GRP94 |
107 U/μl |
miR-150-5p |
|
IMI |
EVs (10 μL per injection, 5.8 × 1012 particles) were injected 5 times 10 min before perfusion |
TXNIP |
| Tang et al (2020)49 |
Ultracentrifugation method |
NTA
TEM
Western blotting |
CD9, CD81 and TSG101 |
- |
miR-320b |
|
- |
Exosomes (50μg/25μL PBS) |
NLRP3 protein |
| Chen et al (2024)50 |
Ultracentrifugation method |
TEM
NTA
PKH67 staining
miRNA sequencing |
TSG101, CD63, and calnexin |
20 μg |
miR-93-5p |
|
IMI |
20 μl of exosomes (50 µg) were injected in situ into the original location of the infarcted myocardium |
TXNIP/ NLRP3/ Caspase-1 |
| Du et al (2024)51 |
Ultracentrifugation method |
NTA
TEM
Western blotting |
CD63 and CD9 |
- |
miR-25-3p |
Electroporation of 100 µg of miR-25-3p in 500 µl BMSC-Exo (250 µM) |
IV |
Exosomes (100 µg/kg) were injected through the tail vein 2 h before I/R surgery |
JAK2 / STAT3 signaling pathway |
| Gu et al (2024)52 |
Exosome isolation reagent kit |
NTA
TEM
Flow cytometry |
CD29 and CD44 |
2.5 to 40 μM |
miR-302 |
-4 μL ethanolic solution (100 nM) including DSPE-PEG-CMP was incubated with the exosomes (200 μL, 1 × 1010 particles)
-electroporation of exosomes via miR302 mimic |
IV |
Engineered exosomes were injected via the tail vein (0.25 μg/100 μL PBS/mouse) 12 h after reperfusion after coronary artery ligation every 2 days for 4 weeks |
Cardiomyocyte specific peptide |
| Lee et al (2025)53 |
ExoQuick-TC Exosome Precipitation Solution |
NTA
TEM
BCA assay
Western blotting |
CD9 and CD63 |
- |
miR-221- and miR-222 |
|
Intratracheally, intraperitoneally, and intramuscularly injection |
Exosomes (100 μg of protein in 50 μL) were uniformly injected into the left ventricular marginal zone |
BNIP3-MAP1LC3B-BBC3/ PUMA pathway |
| Du et al (2017)54 |
Ultracentrifugation method |
ELISA
TEM
BCA assay |
- |
- |
miR-126 |
- |
IMI |
Exosomes (100 µg in 100 μl PBS) were immediately injected post-ischemia at 3 sites in the right adductor muscle adjacent to and within 1 mm proximal or distal to the ligation site |
VEGF |
| Feng et al (2014)55 |
ExoQuick + Ultracentrifugation |
TEM
Western blotting
Bioanalyzer for RNA |
CD63 |
1 μg |
miR-22 |
- |
IMI |
1 mg exosomes
were injected along the border between the infarct zone and normal myocardium after LAD |
MeCP2 |
| Sánchez-Sánchez et al (2021)57 |
Ultracentrifugation method |
NTA
TEM
BCA assay
Western blotting
EVs Small RNA Sequencing |
ALIX, HSP70, TSG101, and CD9 |
3.5 × 109 EVs/ μl |
miR-4732-3p |
Electroporation of miR- 4732-3p (40 nM) into EVs |
IMI |
EVs (3.5 × 1010 per animal) were transplanted immediately after permanent LAD artery ligation in two injections of 10 mL, at two discrete locations of the infarct border zone |
SMAD2 and SMAD4 components of the TGF-β pathway |
| Luo et al (2017)56 |
ExoQuick-TC |
NTA
TEM
Western blotting |
CD63, CD9, and TSG101 |
2 μg/μl |
miR-126 |
- |
IV |
Exosomes (400 μg of protein suspended in 200 μl PBS) were injected at the tail vein immediately after the ligation operation |
- |
| Yu et al (2015)58 |
ExoQuick-TC kit |
TEM
Western blotting |
CD9, CD63, and HSP70 |
- |
miR-19a |
- |
IMI |
Exosomes (harvested from 4 × 106 MSCs in 50 μl saline) were injected after LAD coronary artery |
PTEN/ Akt/ ERK signaling pathways |
| Zhao et al (2024)59 |
Exo Quick-TC kit |
NTA
TEM
Western blotting |
CD63, CD9, and CD81 |
- |
miR-101a-3p |
Transfected with miR- 101a- 3p inhibitor (2 μg/ mL) |
- |
- |
PIK3-Akt signaling
pathway |
| Sun et al (2019)60 |
Total Exosome Isolation kit |
NTA
TEM
Western blotting |
CD9, CD63, ALIX, and TSG101 |
2 μg/μl |
miR-486-5p |
- |
IV |
rats with exosomes (400 µg in 200 µL PBS) were injected into the tail vein at the beginning of the reperfusion and 3 h later coronary artery was re-ligated |
PTEN/ PI3K/ AKT signaling pathway |
| Yang et al (2021)61 |
Total exosome isolation reagent |
NTA
TEM
Western blotting |
CD63 and CD9 |
0.932 × 103 copies/μL |
miR-145 |
- |
IV |
Exosomes (80 mg) were injected every week, one week after carotid atherosclerotic plaque induction in the right common carotid artery |
JAM-A |
| Ma et al (2021)62 |
Total Exosome Isolation Reagent Kit |
NTA
TEM |
- |
0.5 mg/ml |
miR-21a-5p |
- |
IV |
Exosomes (200 µl, 0.5 mg/ml) were injected into the caudal vein once a day for 2 weeks |
KLF6 and ERK1 /2 signaling pathways |
| Wang et al (2015)63 |
Ultracentrifugation method |
NTA
TEM
BCA assay
Western blotting |
CD63 and CD81 |
- |
miR-223miR-233SEMA3A; STAT3 |
- |
IV |
Exosomes (2μg/g body weight in 150 μl of incomplete culture medium) were injected through the tail or jugular vein, 1 hour after CLP surgery |
SEMA3A, STAT3 |
| Pei et al (2021)64 |
Ultracentrifugation method |
NTA
TEM
Western blotting |
CD63 and CD9 |
- |
miR-141 |
- |
IV |
Exosomes (2 μg/g) were injected through caudal veins 1 hour after CLP |
PTEN and activates β-catenin |
| Luo et al (2022)65 |
Complete exosome isolation kit |
NTA
TEM
Western blotting |
CD9, CD81, Tsg101, and Histone H3 |
- |
miR-15a/15b/16 |
- |
- |
- |
NFAT-3 |
| Chen et al (2021)66 |
Ultracentrifugation method |
TEM
Western blotting |
CD63, CD81, and CD9 |
- |
miR-512-3p |
- |
- |
- |
Keap1/ Nrf2 signaling pathway |
| Lei et al (2021)67 |
Ultracentrifugation method |
BCA assay
NTA
TEM
Western blotting |
CD63 and CD81 |
3 × 1011 particles/ml |
miR-96 |
- |
IV |
2 doses of exosomes (3 × 1010 particles suspended in 0.1 mL PBS) were injected into the tail vein of the rats on days 5 and 11 |
Inhibiting the Rac1/Nuclear Factor-κB Signaling Pathway |
| Wang et al (2021)68 |
Gradient centrifugation |
NTA
TEM
Western blotting |
CD63, PDCD6IP, TSG101, and LC3A |
1 μg/μl |
miR-1246 |
- |
IMI |
Exosomes (20 μg in 20 μl PBS) were directly injected into two lesions of the infarcted myocardial boundary area |
Targeting PRSS23
↓Activation of the Snail/alpha-smooth muscle actin signaling |
| Yan et al (2022)69 |
Ultracentrifugation method |
BCA assay
DLS
TEM
Western blotting |
CD81 and TSG101 |
0.1 μg/μl |
miR-129-5p |
- |
IV |
Exosomes (50 μL, 100 μg/mL) were postoperatively injected through the tail vein once a week for 3 times |
TRAF3, NF-κB signaling |
ADAM19: A Disintegrin and metalloproteinase 19, ADAMTS16: A disintegrin and metalloproteinase with thrombospondin motifs 16, AKT: protein kinase, B, BCL2L11: Bcl-2-like protein 11, Bnip3: B-cell lymphoma 2–interacting protein 3, DAAM1: Disheveled-associated activator of morphogenesis 1, DSPE-PEG-NHS: 1,2-distearoyl-sn-glycero-3-phosphoethanolamineN-[hydroxysuccinimidyl polyethylene glycol-2000], EMT: Epithelial–myofibroblast transdifferentiation, ESRK1/2: Extracellular signal-related kinases 1 and 2, Faslg: Fas ligand gene, FG: Fractional shortening, FIH: Factor-inhibiting HIF, GDF11: Growth differentiation factor 11, GSDMD: Gasdermin D, HDAC2: Histone deacetylase 2, HIF: Hypoxia-inducible factor, H/R: Hypoxia-reperfusion, HUVECs: human umbilical vein endothelial cells, IMI: Intramyocardial injection, I/R: Ischemia-reperfusion, IRAK: Interleukin-1 receptor-associated kinase, IV: Intravenously injection, JAM-A: Junctional adhesion molecule A, JNK: c-Jun NH2-terminal kinase, Keap1: Keleh-like ECH-associated protein 1, KLF-6: Kruppel-like factor 6, LVD: LV diastolic dimension, LVEDD: Left ventricular end-diastolic diameter, LVEDV: LV enddiastolic volume, LVEF: LV ejection fraction, LVESD: LV end-systolic diameter, LVESV: LV end-systolic volume, MAPK: Mitogen-activated protein kinase, Mecp2: Methyl CpG binding protein 2, MI: Myocardial infarction, NAT1: N-Acetyltransferase 1, NC: Negative control, NFAT: Nuclear factor of activated T cells, NLRP3: NLR Family pyrin domain containing 3, NMN: Nicotinamide mononucleotide, Nrf2: NF-E2-related factor 2, P53: Tumor protein 53, PDK4: Pyruvate dehydrogenase kinase 4, PI3K: Phosphatidylinositol 3 kinase, PRSS23: Protein serine protease 23, PTEN: Phosphatase and tensin homolog, RASA1: RAS P21 protein activator 1, ROCK2: Rho associated coiled-coil containing protein kinase 2, SD: Sprague-dawley, SMAD: Suppressor of mothers against decapentaplegic, SNRK: Sucrose non-fermenting-1 related kinase, S100A9: S100 calcium-binding protein A9, SOX6: Sry-related high-mobility group box6, TERT: Telomerase reverse transcriptase, TGF-β: Transforming Growth Factor beta, TGF-βR: TGF-β receptor, TLR4: Toll-like receptor 4, TRAF6: Tumor necrosis factor receptor-associated factor 6, TRPS: Tunable resistive pulse sensing, TXNIP: Thioredoxin interacting protein, VEGFA: Vascular endothelial growth factor A, YAP1: Yes-associated protein 1
Assessment of the quality of studies
The quality of the included studies was assessed based on the ARRIVE guidelines 2.0.12 Besides, the risk of bias in studies was evaluated by SYRCLE's risk of bias tool.13
Results
Included studies
The primary database search resulted in 1300 articles, out of which 578 were eliminated due to duplicate records. Some studies (n = 565) were excluded based on the subject and abstract screening. Forty-seven studies were excluded due to the unavailability of the full text. One hundred and ten full texts were reviewed and screened to finalize the included studies. A total of 56 studies were included in the review. Fig. 1 shows the detailed PRISMA flow diagram of the literature search.
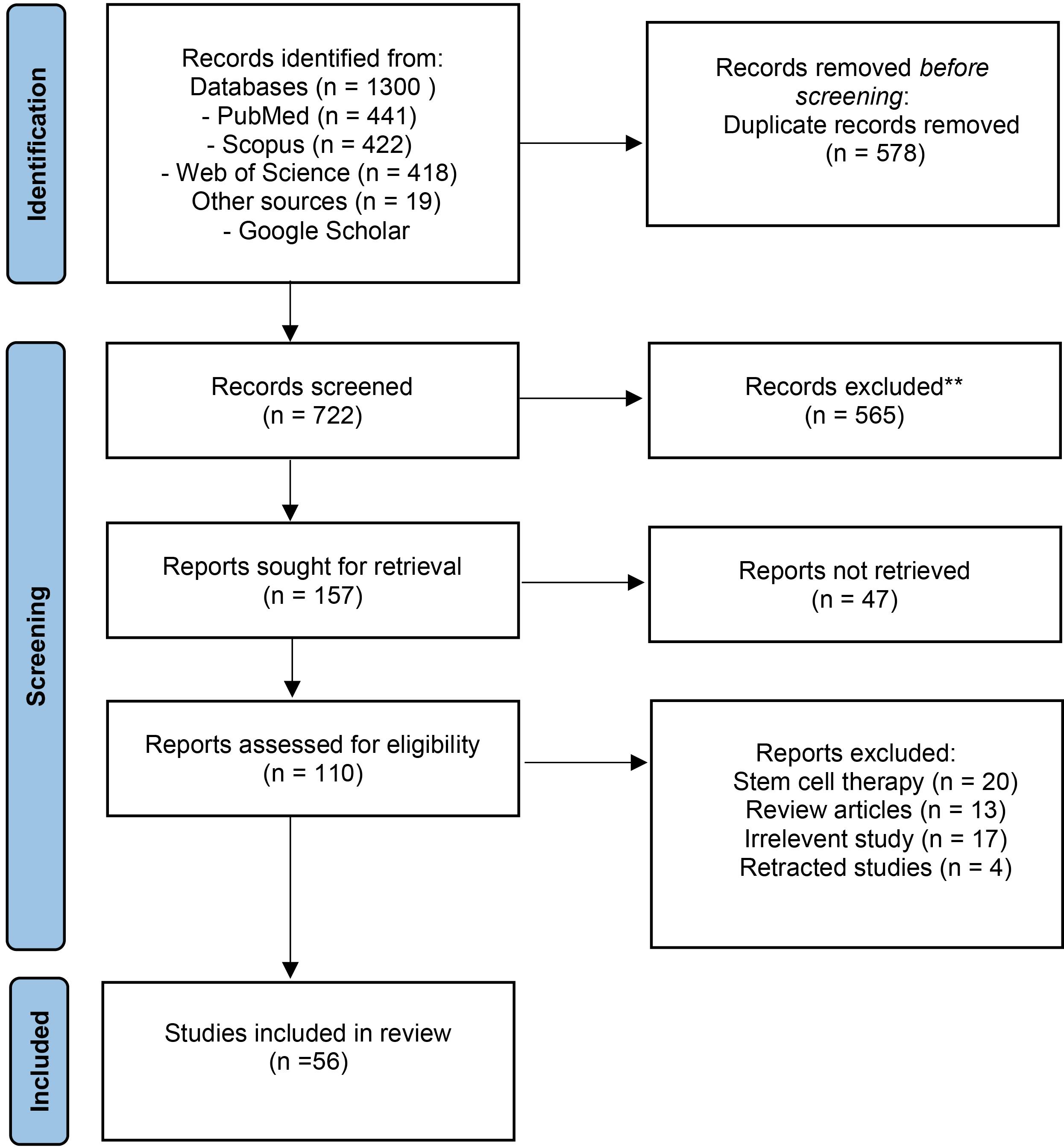
Fig. 1.
PRISMA flow diagram of literature search and selection process.
.
PRISMA flow diagram of literature search and selection process.
Characteristics of the included studies
Preclinical models of CAD were MI (n = 23),14-36 I/R (n = 17),37-53 ischemia (n = 4),54-57 hypoxia (n = 1),58 hypoxia/reperfusion,59 ischemia/hypoxia,60 atherosclerosis (n = 2),61,62 sepsis (n = 2),63,64 calcification (n = 1),65 endothelial dysfunction,66 myocardial toxicity,67 and heart failure.68,69
Of the 56 included studies, 44 articles had a mixed methodology (in vitro studies were followed by in vivo studies),14-16,18-20,22,23,25,27-36,38,40-54,56-58,60-64,67-69 8 were in vivo,17,21,24,26,37,39,42,55 and 4 were in vitro45,59,65,66 studies. Mice were the model animals in 18 in vivo studies,15,16,18-20,23,25,26,29,33-37,43,44,46,47,52,53,55,61-64,66,69 and the rest of the articles used rats as their study models. Most in vitro studies utilized animal model cardiomyocytes [neonatal rat cardiomyocytes or H9C2 cells (a rat cardiomyoblast cell line)]; however, 9 studies examined human umbilical vein endothelial cells (HUVECs)15,29,32,36,47,54,57,61,68 and 1 article studied both human fibroblasts and HUVEC.18
Sources of MSCs and their preconditioning methods
The included studies utilized a diverse range of MSCs, including bone marrow-derived MSCs (BM-MSCs), umbilical cord MSCs (UC-MSCs), placenta-derived MSCs (P-MSCs), and adipose-derived MSCs (AD-MSCs). Specifically, BM-MSCs were the most prevalent source being used in 37 studies (66.07%), followed by UC-MSCs in 6 studies (10.71%),25,30-32,61,68 AD-MSCs in 5 studies (8.92%),15,23,36,53,56 and human placenta-derived MSCs (hP-MSCs) in one study.54 Cell lines (Shanghai Zhongqiao Xinzhou Biotechnology Co.),26 immortal MSC-TERT lines,57 human MSC (American Type Culture Collection),14,49 and rat MSCs (CP-R131)33 along with induced pluripotent stem cell (iPS)27 were also applied. The tissue of origin was unspecified in a minority of studies.48 Nineteen, eighteen, and fourteen articles used rats,17,21,22,24,28,33,38-42,45,48,50,51,58-60,67 mice,15,16,18,20,23,29,35,37,43,44,47,52,55,62-64,66,69 and human-derived tissue,14,25,27,30-32,36,46,49,53,54,61,65,68 respectively, as a donor of MSC organism. The donor organism of MSCs was not defined in 5 studies19,26,34,56,57 (Table 1). Regarding preconditioning methods, the majority of studies did not employ specific techniques. However, 6 studies (10.71%)41-43,45,50,55 utilized a hypoxic environment to precondition MSCs before exosome isolation, and 16 studies (28.57%)14,30,35,41,47-53,56,58-60,68 used preconditioning for H9c2, HUVEC, myocardial, and cardiomyocyte cells (Table 1).
For exosome isolation, ultracentrifugation was the dominant method used in 34 out of 56 studies (60.71%). Other methods included the use of exosome extraction kits such as ExoQuick (used in 7 studies, 12.5%),15,38,48,53,56,58,59 the Total/complete Exosome Isolation Reagent Kit (10 studies, 17.85%),20,23,24,28,29,52,60-62,65 Hieff Quick (used in 1 study, 1.78%),40 Sigma (1 study, 1.78%),14 and Gradient centrifugation (1 study, 1.78%).68 ExoQuick and ultracentrifugation were employed in one study.55 The isolation method of one study was not defined.34
The identification of exosomes primarily relied on transmission electron microscopy (TEM) to examine their morphology. Western blotting and Flow cytometry were used to detect specific biomarkers. Other techniques, such as dynamic light scattering (DLS), and nanoparticle tracking analysis (NTA) were employed to characterize and analyze the size, distribution, and concentration of exosomes. In terms of miRNA insertion, transfection into MSC cells was used in 31 out of 56 studies (55.35%)14,16,22-24,26-28,30-33,35,37,38,40-42,44,49,54-56,61,62,64-69 and 5 studies of 56 studies used transduction (8.92%).25,36,39,46,48 On the other hand, 2 studies used transfection to insert microRNAs into exosomes (3.57%) and 4 of them used electroporation for this approach (7.14%)29,51,52,57 to deliver target miRs into exosomes and cell lines. For more details, see Table 2.
Route and frequency of MSC-exosomal miR administration
In 31 in vivo studies,16,19,20,22-29,31,32,34,35,37,38,40-44,46-48,50,54,55,57,58,68 the exosomes were injected intramyocardially around the infarct area, and in 16 studies,14,17,18,30,33,39,51,52,56,60-64,67,69 the intravenous route was used for exosome administration. Interestingly, one study used both intramyocardial and intravenous routes for their experiment,36 one study used both intramuscular and intravenous routes,15 and another study used simultaneous intratracheally, intraperitoneally, and intramuscularly injection.53 Two studies did not explain the exact route of administration in the methodology.21,49 Most studies used a single intramyocardial injection of exosomes after surgical I/R induction. Studies used a broad spectrum of exosome concentrations varying from 0.02 μg/μL to 400 μg/μL. In some studies, the exosome concentration was not declared, and the total amount of injected exosomes was just mentioned.
Improvement/outcomes
In vitro studies focused on the anti-inflammatory and anti-apoptotic effects of exosomes under hypoxic conditions.14,30,35,41-43,45,47-53,56,58-60,68 On the other hand, the in vivo studies mainly focused on cardiac function improvement (increased left ventricular ejection fraction and fractional shortening (LVEF and LVFS), decreased end-diastolic and systolic diameter of the left ventricle (LVEDD and LVESD), and the reduction in the infarct area size and fibrosis.14-18,22,24,26,28,29,37,38,42,46,48,50-52,58,63,68,69 Exosomal miRNAs derived from various MSC sources have been shown to play beneficial roles in targeting dysregulated signaling in CAD. These roles include anti-apoptotic effects,26,28,35,39,47,48,52,56,58,66,69 anti-inflammatory actions,16,19,20,26,31,37,46,47,51,52,56,63,66 promotion of differentiation, anti-fibrotic activity,15,27,31,55,56 pro-angiogenic effects.15,18,21,27,29,31,32,36,54,56,57 To a lesser extent, MSC-exos-miRs were involved in reducing calcification,65 suppressing autophagy,24,40,43 and enhancing cell viability. The detailed results of each study are summarized in Table 1 and Fig. 2.
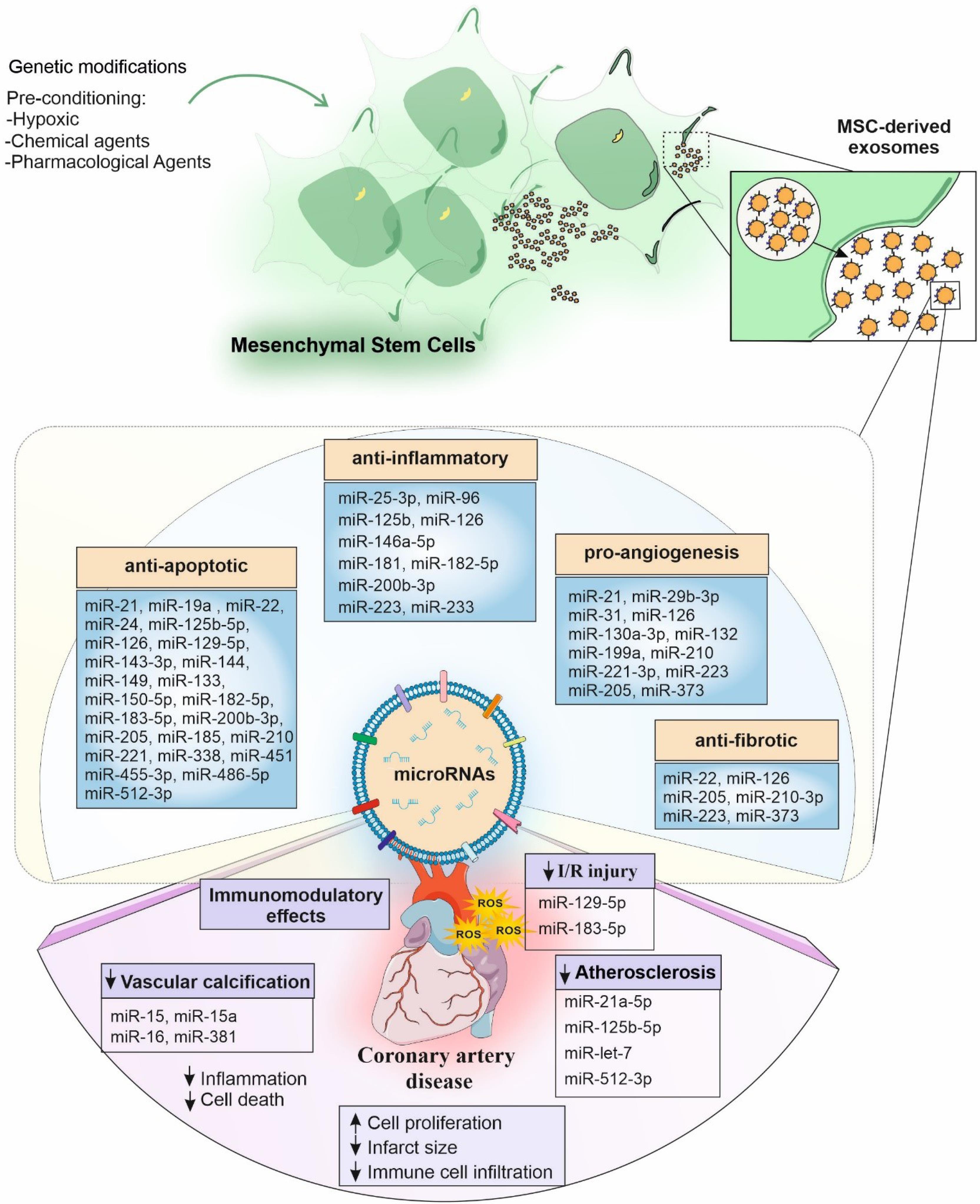
Fig. 2.
The effect of exosomal miRs derived from mesenchymal stromal cells on coronary artery disease.Exosomal miRs derived fromMesenchymal stromal cells (MSCs) could have anti-inflammatory, anti-apoptotic, pro-angiogenesis, and anti-fibrotic effects on patients with coronary artery disease. Besides, some miRs could have immunomodulatory effects, and reduce ischemia-reperfusion (I/R) injury, vascular calcification, atherosclerosis, infarct size, and immune cell infiltration.
.
The effect of exosomal miRs derived from mesenchymal stromal cells on coronary artery disease.Exosomal miRs derived fromMesenchymal stromal cells (MSCs) could have anti-inflammatory, anti-apoptotic, pro-angiogenesis, and anti-fibrotic effects on patients with coronary artery disease. Besides, some miRs could have immunomodulatory effects, and reduce ischemia-reperfusion (I/R) injury, vascular calcification, atherosclerosis, infarct size, and immune cell infiltration.
Quality and risk of bias assessment
According to the ARRIVE guidelines 2.0 evaluations, the selected studies have had an appropriate quality to be included in the review. The main risk of bias in the included studies was the lack of clear information about the blinding and randomization process in the performance and detection phases. However, due to the small sample size of most of these animal studies, researchers assessed all the target animals instead of randomization. There was no significant risk of bias in the included studies regarding the SYRCLE's risk of bias tool (Figs. 3 and 4).
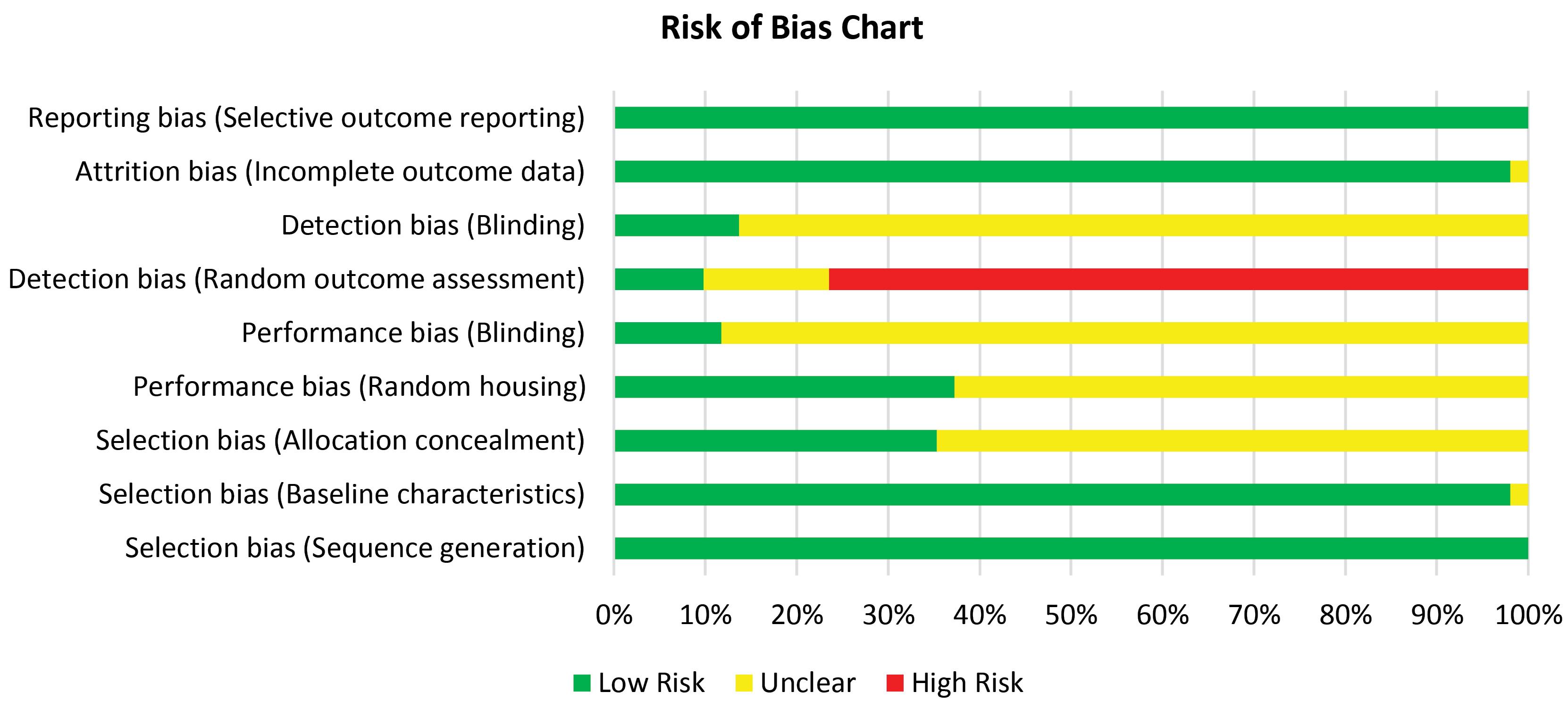
Fig. 3.
Risk of bias chart based on the SYRCLE tool.
.
Risk of bias chart based on the SYRCLE tool.
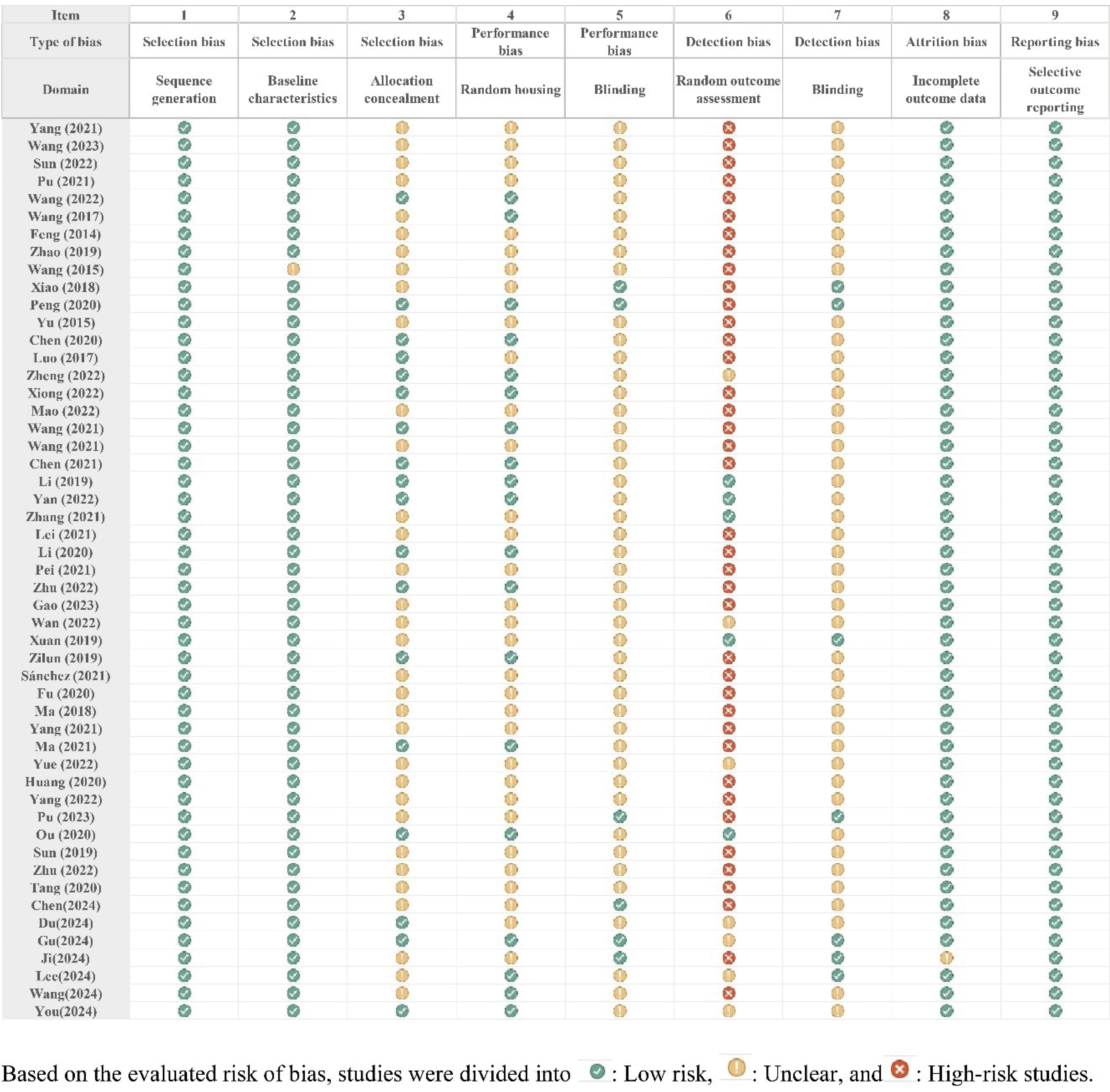
Fig. 4.
Risk of Bias in the included studies.
.
Risk of Bias in the included studies.
Discussion
Recent studies support that MSC-exosomal miRs are the most functional factors regulating the regeneration of the cardiovascular, offering a multifaceted strategy to combat CAD by addressing cellular dysfunction, apoptosis, and inflammation while promoting angiogenesis and tissue repair and suppressing fibrosis.
Both MI and I/R injury are downstream effects of CAD. Myocardial ischemia occurs when the coronary artery is partially or totally occluded, resulting in the functional loss of cardiomyocytes. After an MI and performing reperfusion treatments by fibrinolytic or angioplasty, the re-establishment of blood supply causes cell damage, called I/R injury. Although reperfusion is crucial to preventing more damage, it leads to further injury due to oxidative stress, inflammation, and an overload of calcium. The pathophysiology of maladaptive cardiac remodeling after MI is the early inflammatory response, apoptosis, and the succeeding longer-term scar alteration.
Inflammation plays a central role in the development and progression of CAD by contributing to immune cell recruitment, endothelial dysfunction, and atherosclerotic plaque formation and destabilization. The result of this review indicated that MSC-exos-miRs can decrease inflammation in MI,19,20,23,26,31,34 I/R,47,52,56 and sepsis63 models. MSCs exosomes carrying miR-25-3p,20 miR-125b,19 miR-200b-3p,26 and miR-22331 have anti-inflammatory and cardioprotective effects after MI. Moreover, in I/R models, miR-126,56 miR-182-3p,47 and miR-30252 could diminish inflammation. Surprisingly, as an effective agent, miRs can target the inflammatory signaling pathways through several mechanisms, such as the TLR/NF‐ĸB pathway, NLRP334 inflammasome, PI3K/AKT pathway, and JAK/STAT pathway. Reducing macrophage infiltration and promoting the M2 macrophage phenotype is another anti-inflammatory role of MSC-exos-miRs.62 Likewise, BM-MSCs-Exos diminish the inflammatory response by miR-302d and controling the BCL6/MD2/NF-κB signaling pathway in cardiac regeneration after AMI.70
The main features of acute MI include elevated oxidative stress, loss of NADH and ATP, and cell death, all connected directly to cellular bioenergetics. MSC-exosomal miRs can preserve cardiac cells by suppressing oxidative stress and cell apoptosis and promoting cardiac regeneration and repair. In this review, an important group of MSC-exo-miRNAs was identified in CAD models that present antiapoptotic effects by targeting different pathways. This antiapoptotic miRNA group includes let-7i-5p,35 miR-21-5p,33 miR-101a-3p,59 miR-129-5p,69 miR-143-3p,40 miR-149,45 miR-150-5p,48 miR-182,47 miR-183-5p,39 miR-200b-3p,26 miR-205,15 miR-210-3p,32 miR-221 and miR-222,53 miR-302,52 miR-338,28 miR-455-3p,41 miR-512-3p,66 and miR-4732-3p.56
Shuttled miR-486-5p, miR-144, and miR-2171 from MSC-exos can prevent cardiomyocyte apoptosis by targeting the PTEN/PI3K/AKT pathway.60,72 miR-21a-5p participates in cell survival/death pathways, presenting cardioprotective effects after MI by targeting PTEN, FasL, PDCD4, and Peli1.73 Likewise, in ischemic human cardiomyocytes, it is found that human BM-MSCs-exosomal miR-21-5p could enhance cardiac tissue's calcium handling gene and contractility.74 Moreover, miR-22 (by targeting methyl CpG binding protein 2),55 miR-24,75 miR-125b-5p (by interaction with SMAD4),76 miR-221,77 and miR-451 (by targeting TLR4/NF-κB pathway) hinder apoptosis. AD-MSCs-derived exosomal miR-221/222 and miR-146a can reduce MI-induced myocardial injury by targeting PUMA and EGR1 (early growth response factor 1), respectively.78,79 Similarly, exosomal miR-25-3p alleviates MI by targeting a histone-lysine N-methyltransferase EZH2 (Enhancer of zest homolog 2) that promotes the formation of heterochromatin and thereby represses gene expression.60 Cardiomyocytes are protected after reperfusion with the help of the miR149/let-7c/Faslg pathway.45 Moreover, these exosomes can reduce myocardial cell damage through the HAND2-AS1/miR-17-5p/Mfn2 pathways.45 Some other MSCs-exosomal miRs such as miR-338,28 miR-133,80 miR-210,81 and miR-12682 could improve cardiac function, diminish infarct size, and prevent cardiomyocyte apoptosis by targeting different pathways. Other exosomal miRs, such as miR-132, miR-223, miR-19a, and miR-22 have also been reported to have cardioprotective effects.55,58,83 miR-125b-5p derived from MSC exosomes can regulate the infarct size of mice and cardiac function by reducing autophagy through the p53-BNIP3 signaling pathway.19
A plethora of reports support that the paracrine activity of ischemic preconditioning MSCs can improve their therapeutic effect on MI in primates.84 Hypoxia-conditioned BM-MSCs,76 ischemic pre-conditioned BM-MSCs,55 and GATA-4-overexpressing MSCs that were enriched in exosomal miR-125b, miR-22, and miR-19a,58 respectively, could enhance the apoptosis of cardiomyocytes, decrease cardiac fibrosis, and ease cardiac repair. Besides, MSCs, grown under hypoxic conditions, secret exosomal miR-19a, that acts as anti-apoptotic. These exosomes with a high concentration of miR-19a can reduce apoptosis and increase mitochondrial membrane stability, increasing rat cardiomyocytes' survival rate.58
One of the important complications after MI is cardiac fibrosis and scarring. Therefore, a critical principle following MI is preventing fibrosis and reducing its progression. Scar repair, which includes angiogenesis and activation of myofibroblasts (MFBs), is responsible for cardiac structural recovery at the early stages of MI. On the other hand, insufficient repair can cause thinning and eventual rupture of the heart wall. Therefore, a precise mechanism in scar repair should be established to balance maintaining the heart's function and creating resistance of the walls.85 Based on the results of this systematic review, MSC-exos carrying miR-22, miR-126, miR-205, miR-210-3p, miR-223, and miR-373 exert antifibrotic effects on CAD preclinical models.15,23,27,31,32,55,56 AD-MSC-derived exosomal miR-671 attenuates myocardial fibrosis by hindering the TGFBR2/Smad2 signaling pathway.23
After MI, the angiogenesis of the myocardium is critical for stimulating the function of the ischemic heart.86 MCSs-exosomal miRs can induce the formation of new blood vessels87 by penetrating the endothelial cells to increase cell proliferation and help re-endothelialize blood vessels.88 The proliferative and angiogenic effects of MSC-derived exosomes can be primarily attributed to specific miRs, including miR-199a and miR-130a-3p.71 miR-126 enhanced the VEGF signaling pathway by downregulating the expression of PI3KR2 and SPRED1. Moreover, miR-126 targeting VCAM1, PIK3R2, and SPRED1 presents anti-inflammatory effects.82 miR-210 found in the exosomes derived from BM-MSCs can improve the angiogenesis of the repair process by affecting the EFNA3 gene. Likewise, MSC can preserve myocytes against stress in vitro and in vivo by overexpressing exosomal miR-210. It is important to note that both exogenous and endogenous miR-210 have similar therapeutic effects.81 MiR-21 is also associated with the property of neovascularization and angiogenesis by the PTEN/Akt pathway in preventing MI complications.18,89 It should be noted that inflammatory factors (IL-6 and TNF-α) and miR-dysregulated angiogenesis-related miRs (miR-320, miR-21-3p, miR-146b-5p, miR-17-5p, and 196a-5p) impair the MSCs-exo ability to stimulate angiogenesis. Those proinflammatory cytokines also decrease VEGF, MAPK, and PI3K-AKT signaling pathways related to angiogenesis.90 Nevertheless, alternative results indicate that inflammatory mediators could enhance the capacity of MSC-derived exosomes to facilitate angiogenesis.91
Atherosclerosis is the underlying cause of CAD and is involved in the development of both CAD and ACS. Coronary atherosclerosis, progressively narrows the coronary arteries' lumen, leading to reduced blood flow and myocardial ischemia. Exosomes that contain miR-512-3p have a protective role against oxidized low-density lipoproteins-induced vascular damage. This miR inhibits the destructive effect of Keleh-like ECH-associated protein 1 (Keap1) to cause endothelial damage.66 Vesicles containing miR-21a-5p stimulate M2 macrophage polarization and reduce the infiltration of macrophages through ERK1/2 and KLF6 signaling pathways, thus diminishing atherosclerosis.62 In addition, macrophage accumulation is suppressed through the miR-let7/IGF2BP1/PTEN pathway. MSC-exos also exerts atherosclerosis inhibitory properties by inhibiting miR-342-5p.79 MSC-derived miR-145-rich exosomes can downregulate junction adhesion molecule A, prevent cell migration in vitro, and diminish atherosclerotic plaque.61 On the other hand, exosomes derived from BM-MSCs carrying miR-223 stabilize atherosclerotic plaques by suppressing the expression of NLRP3.92
Differential expression of epicardial adipose tissue-exos-miRs was found in CAD patients compared to patients without CAD, providing hints for further mechanisms of atherosclerosis. Among 53 uniquely identified miRs, 21 miRs were downregulated and 32 miRs were upregulated in CAD patients. Seven differentially expressed miRs (miR-485-3p, miR-382-5p, miR-429, miR-205-5p, miR-200a-5p, miR-183-5p, and miR-141-3p) were involved in cell proliferation, survival, differentiation, and apoptosis.11
Vascular calcification (VC), a common cardiovascular problem in chronic kidney disease (CKD) cases, is caused by irregular inflammation, metabolism of phosphate and calcium, and other factors. Vascular calcification is often associated with atherosclerosis and CAD. Gau et al. found that miRs derived from BM-MSCs-exosomes can diminish calcium deposition in the human aorta's vascular smooth muscle cells (VSMCs) by affecting the central pathways.93 Later, this team found that BM-MSCs-exosomes play a role in inhibiting VC by transferring miR-16/-15a/-15 and hindering nuclear factors of activated T cells 3 (NFAT-3). This target gene can prevent the osteogenic trans-differentiation of VSMCs in the aorta by downregulating the osteocalcin expression.65 Moreover, Liu et al. indicated that BM-MSCs-derived exosomes exert anti-apoptosis and anti-calcification roles in CKD by transferring miR-381. This miR directly downregulates NFAT-5, reducing VSMC apoptosis and VC.94
Conclusion
Cell-free therapy using MSC-exos-miRs demonstrates remarkable potential in cardiology, particularly through its ability to mitigate inflammation, apoptosis, and fibrosis, prevent tissue damage, promote angiogenesis, and protect against I/R injury. Exosomes derived from MSCs play a pivotal role in regulating physiological and pathological processes by transporting bioactive molecules such as miRs to recipient cells. These exosomal miRs contribute significantly to the therapeutic effects of MSCs by influencing cell proliferation, differentiation, and migration. However, challenges remain due to the heterogeneity of MSC sources, preconditioning methods, and exosome extraction protocols. These variations complicate the assessment of therapeutic efficacy and hinder clinical translation. Standardized protocols for preparing and evaluating MSC-exosomal miRs are crucial to ensuring reproducibility in clinical trials. Additionally, optimizing therapeutic parameters such as exosome extraction,95 content, concentration, administration frequency, and delivery routes are vital for enhancing their efficacy in treating CAD. Developing universally accepted methods for isolating and characterizing MSC-derived exosomes is essential to ensure homogeneity and reproducibility in clinical applications. Future studies should focus on identifying optimal therapeutic concentrations, dosing regimens, and delivery routes to maximize the cardioprotective effects of MSC-exosomal miRs.
Review Highlights
What is the current knowledge?
What is new here?
-
MSC-exosomal miRs are emerging as key regulators in CAD, influencing atherosclerosis progression, plaque stability, and post-ischemic cardiac repair.
-
MSC-exosomal miRs offer dual diagnostic and therapeutic potential in CAD, modulating inflammation, apoptosis, and tissue repair.
-
The cardioprotective properties MSC-exosomal miRs could play a role in the management of CAD patients.
Competing Interests
The authors declared that there was no conflict of interest in this study.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Data Availability Statement
Data will be made available upon a reasonable request.
Ethical Approval
This study was ethically approved via Tabriz University of Medical Sciences (Ethical code: IR.TBZMED.VCR.REC.1402.231).
Supplementary files
Supplementary file 1 contains Table S1.
(pdf)
References
- Ralapanawa U, Sivakanesan R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: a narrative review. J Epidemiol Glob Health 2021; 11:169-77. doi: 10.2991/jegh.k.201217.001 [Crossref] [ Google Scholar]
- Wöhrle J, Merkle N, Mailänder V, Nusser T, Schauwecker P, von Scheidt F. Results of intracoronary stem cell therapy after acute myocardial infarction. Am J Cardiol 2010; 105:804-12. doi: 10.1016/j.amjcard.2009.10.060 [Crossref] [ Google Scholar]
- Traverse JH, McKenna DH, Harvey K, Jorgenso BC, Olson RE, Bostrom N. Results of a phase 1, randomized, double-blind, placebo-controlled trial of bone marrow mononuclear stem cell administration in patients following ST-elevation myocardial infarction. Am Heart J 2010; 160:428-34. doi: 10.1016/j.ahj.2010.06.009 [Crossref] [ Google Scholar]
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012; 308:2369-79. doi: 10.1001/jama.2012.25321 [Crossref] [ Google Scholar]
- Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL, Wang ZG. Intracoronary infusion of Wharton's jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med 2015; 13:162. doi: 10.1186/s12916-015-0399-z [Crossref] [ Google Scholar]
- Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine 2020; 15:6917-34. doi: 10.2147/ijn.S264498 [Crossref] [ Google Scholar]
- Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 2015; 6:127. doi: 10.1186/s13287-015-0116-z [Crossref] [ Google Scholar]
- Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res 2010; 38:215-24. doi: 10.1093/nar/gkp857 [Crossref] [ Google Scholar]
- Nariman-Saleh-Fam Z, Zununi Vahed S, Aghaee-Bakhtiari SH, Daraei A, Saadatian Z, Samadi Kafil H. Expression pattern of miR-21, miR-25 and PTEN in peripheral blood mononuclear cells of patients with significant or insignificant coronary stenosis. Gene 2019; 698:170-8. doi: 10.1016/j.gene.2019.02.074 [Crossref] [ Google Scholar]
- Saadatian Z, Mansoori Y, Nariman-Saleh-Fam L, Daraei A, Zununi Vahed S, Navid S. Peripheral blood mononuclear cells expression of miR-200c, miR-125b, miR-27b, miR-203, and miR-155 in patients with significant or insignificant coronary artery stenosis. Sci Rep 2023; 13:18438. doi: 10.1038/s41598-023-45146-8 [Crossref] [ Google Scholar]
- Liu J, Gao A, Liu Y, Sun Y, Zhang D, Lin X. MicroRNA expression profiles of epicardial adipose tissue-derived exosomes in patients with coronary atherosclerosis. Rev Cardiovasc Med 2022; 23:206. doi: 10.31083/j.rcm2306206 [Crossref] [ Google Scholar]
- Percie du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 20. PLoS Biol 2020; 18:e3000411. doi: 10.1371/journal.pbio.3000411 [Crossref] [ Google Scholar]
- Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol 2014; 14:43. doi: 10.1186/1471-2288-14-43 [Crossref] [ Google Scholar]
- Yang M, Liu X, Jiang M, Li J, Tang Y, Zhou L. miR-543 in human mesenchymal stem cell-derived exosomes promotes cardiac microvascular endothelial cell angiogenesis after myocardial infarction through COL4A1. IUBMB Life 2021; 73:927-40. doi: 10.1002/iub.2474 [Crossref] [ Google Scholar]
- Wang T, Li T, Niu X, Hu L, Cheng J, Guo D. ADSC-derived exosomes attenuate myocardial infarction injury by promoting miR-205-mediated cardiac angiogenesis. Biol Direct 2023; 18:6. doi: 10.1186/s13062-023-00361-1 [Crossref] [ Google Scholar]
- Sun C, Li W, Li Y, Chen J, An H, Zeng G. MiR-182-5p mediated by exosomes derived from bone marrow mesenchymal stem cell attenuates inflammatory responses by targeting TLR4 in a mouse model of myocardial infraction. Immune Netw 2022; 22:e49. doi: 10.4110/in.2022.22.e49 [Crossref] [ Google Scholar]
- Pu L, Kong X, Li H, He X. Exosomes released from mesenchymal stem cells overexpressing microRNA-30e ameliorate heart failure in rats with myocardial infarction. Am J Transl Res 2021; 13:4007-25. [ Google Scholar]
- Wang N, Chen C, Yang D, Liao Q, Luo H, Wang X. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta Mol Basis Dis 2017; 1863:2085-92. doi: 10.1016/j.bbadis.2017.02.023 [Crossref] [ Google Scholar]
- Xiao C, Wang K, Xu Y, Hu H, Zhang N, Wang Y. Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circ Res 2018; 123:564-78. doi: 10.1161/circresaha.118.312758 [Crossref] [ Google Scholar]
- Peng Y, Zhao JL, Peng ZY, Xu WF, Yu GL. Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis 2020; 11:317. doi: 10.1038/s41419-020-2545-6 [Crossref] [ Google Scholar]
- Zheng J, Zhang X, Cai W, Yang Y, Guo T, Li J. Bone marrow mesenchymal stem cell-derived exosomal microRNA-29b-3p promotes angiogenesis and ventricular remodeling in rats with myocardial infarction by targeting ADAMTS16. Cardiovasc Toxicol 2022; 22:689-700. doi: 10.1007/s12012-022-09745-7 [Crossref] [ Google Scholar]
- Xiong Y, Tang R, Xu J, Jiang W, Gong Z, Zhang L. Tongxinluo-pretreated mesenchymal stem cells facilitate cardiac repair via exosomal transfer of miR-146a-5p targeting IRAK1/NF-κB p65 pathway. Stem Cell Res Ther 2022; 13:289. doi: 10.1186/s13287-022-02969-y [Crossref] [ Google Scholar]
- Wang X, Zhu Y, Wu C, Liu W, He Y, Yang Q. Adipose-derived mesenchymal stem cells-derived exosomes carry microRNA-671 to alleviate myocardial infarction through inactivating the TGFBR2/Smad2 axis. Inflammation 2021; 44:1815-30. doi: 10.1007/s10753-021-01460-9 [Crossref] [ Google Scholar]
- Li Y, Yang R, Guo B, Zhang H, Zhang H, Liu S. Exosomal miR-301 derived from mesenchymal stem cells protects myocardial infarction by inhibiting myocardial autophagy. Biochem Biophys Res Commun 2019; 514:323-8. doi: 10.1016/j.bbrc.2019.04.138 [Crossref] [ Google Scholar]
- Zhu F, Chen Y, Li J, Yang Z, Lin Y, Jiang B. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate myocardial infarction injury via miR-24-3p-promoted M2 macrophage polarization. Adv Biol (Weinh) 2022; 6:e2200074. doi: 10.1002/adbi.202200074 [Crossref] [ Google Scholar]
- Wan J, Lin S, Yu Z, Song Z, Lin X, Xu R. Protective effects of microRNA-200b-3p encapsulated by mesenchymal stem cells-secreted extracellular vesicles in myocardial infarction via regulating BCL2L11. J Am Heart Assoc 2022; 11:e024330. doi: 10.1161/jaha.121.024330 [Crossref] [ Google Scholar]
- Xuan W, Wang L, Xu M, Weintraub NL, Ashraf M. miRNAs in extracellular vesicles from iPS-derived cardiac progenitor cells effectively reduce fibrosis and promote angiogenesis in infarcted heart. Stem Cells Int 2019; 2019:3726392. doi: 10.1155/2019/3726392 [Crossref] [ Google Scholar]
- Fu DL, Jiang H, Li CY, Gao T, Liu MR, Li HW. MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur Rev Med Pharmacol Sci 2020; 24:10107-17. doi: 10.26355/eurrev_202010_23230 [Crossref] [ Google Scholar]
- Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao L. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int 2018; 2018:3290372. doi: 10.1155/2018/3290372 [Crossref] [ Google Scholar]
- Huang L, Yang L, Ding Y, Jiang X, Xia Z, You Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle 2020; 19:339-53. doi: 10.1080/15384101.2019.1711305 [Crossref] [ Google Scholar]
- Yang M, Liao M, Liu R, Zhang Q, Zhang S, He Y. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles loaded with miR-223 ameliorate myocardial infarction through P53/S100A9 axis. Genomics 2022; 114:110319. doi: 10.1016/j.ygeno.2022.110319 [Crossref] [ Google Scholar]
- Pu Y, Li C, Qi X, Xu R, Dong L, Jiang Y. Extracellular vesicles from NMN preconditioned mesenchymal stem cells ameliorated myocardial infarction via miR-210-3p promoted angiogenesis. Stem Cell Rev Rep 2023; 19:1051-66. doi: 10.1007/s12015-022-10499-6 [Crossref] [ Google Scholar]
- Ji Z, Wang C. Mesenchymal stem cell-derived exosomal mir-21-5p inhibits YAP1 expression and improves outcomes in myocardial infarction. BMC Cardiovasc Disord 2024; 24:547. doi: 10.1186/s12872-024-04197-z [Crossref] [ Google Scholar]
- Wang S, Zhang X, Wang Y, Wang S, Li L. MiR-223-3p-loaded exosomes derived from bone marrow mesenchymal stem cells ameliorate mouse myocardial infarction injury through inhibiting NLRP3 inflammasome activation. J Med Biol Eng 2024; 44:655-65. doi: 10.1007/s40846-024-00902-7 [Crossref] [ Google Scholar]
- You F, Shen Y, Hao Y. Exosomal miRNA-let-7i-5p from bone marrow mesenchymal stem cells protects against myocardial infarction by inhibiting myocardial apoptosis. Am J Transl Res 2024; 16:6528-39. doi: 10.62347/vxnd1945 [Crossref] [ Google Scholar]
- Zhu D, Wang Y, Thomas M, McLaughlin K, Oguljahan B, Henderson J. Exosomes from adipose-derived stem cells alleviate myocardial infarction via microRNA-31/FIH1/HIF-1α pathway. J Mol Cell Cardiol 2022; 162:10-9. doi: 10.1016/j.yjmcc.2021.08.010 [Crossref] [ Google Scholar]
- Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res 2019; 115:1205-16. doi: 10.1093/cvr/cvz040 [Crossref] [ Google Scholar]
- Chen Q, Liu Y, Ding X, Li Q, Qiu F, Wang M. Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Mol Cell Biochem 2020; 465:103-14. doi: 10.1007/s11010-019-03671-z [Crossref] [ Google Scholar]
- Mao S, Zhao J, Zhang ZJ, Zhao Q. MiR-183-5p overexpression in bone mesenchymal stem cell-derived exosomes protects against myocardial ischemia/reperfusion injury by targeting FOXO1. Immunobiology 2022; 227:152204. doi: 10.1016/j.imbio.2022.152204 [Crossref] [ Google Scholar]
- Chen G, Wang M, Ruan Z, Zhu L, Tang C. Mesenchymal stem cell-derived exosomal miR-143-3p suppresses myocardial ischemia-reperfusion injury by regulating autophagy. Life Sci 2021; 280:119742. doi: 10.1016/j.lfs.2021.119742 [Crossref] [ Google Scholar]
- Wang Y, Shen Y. Exosomal miR-455-3p from BMMSCs prevents cardiac ischemia-reperfusion injury. Hum Exp Toxicol 2022; 41:9603271221102508. doi: 10.1177/09603271221102508 [Crossref] [ Google Scholar]
- Zhang L, Wei Q, Liu X, Zhang T, Wang S, Zhou L. Exosomal microRNA-98-5p from hypoxic bone marrow mesenchymal stem cells inhibits myocardial ischemia-reperfusion injury by reducing TLR4 and activating the PI3K/Akt signaling pathway. Int Immunopharmacol 2021; 101:107592. doi: 10.1016/j.intimp.2021.107592 [Crossref] [ Google Scholar]
- Li T, Gu J, Yang O, Wang J, Wang Y, Kong J. Bone marrow mesenchymal stem cell-derived exosomal miRNA-29c decreases cardiac ischemia/reperfusion injury through inhibition of excessive autophagy via the PTEN/Akt/mTOR signaling pathway. Circ J 2020; 84:1304-11. doi: 10.1253/circj.CJ-19-1060 [Crossref] [ Google Scholar]
- Gao L, Qiu F, Cao H, Li H, Dai G, Ma T. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics 2023; 13:685-703. doi: 10.7150/thno.73568 [Crossref] [ Google Scholar]
- Zou L, Ma X, Wu B, Chen Y, Xie D, Peng C. Protective effect of bone marrow mesenchymal stem cell-derived exosomes on cardiomyoblast hypoxia-reperfusion injury through the miR-149/let-7c/Faslg axis. Free Radic Res 2020; 54:722-31. doi: 10.1080/10715762.2020.1837793 [Crossref] [ Google Scholar]
- Wei Z, Qiao S, Zhao J, Liu Y, Li Q, Wei Z. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci 2019; 232:116632. doi: 10.1016/j.lfs.2019.116632 [Crossref] [ Google Scholar]
- Yue R, Lu S, Luo Y, Zeng J, Liang H, Qin D. Mesenchymal stem cell-derived exosomal microRNA-182-5p alleviates myocardial ischemia/reperfusion injury by targeting GSDMD in mice. Cell Death Discov 2022; 8:202. doi: 10.1038/s41420-022-00909-6 [Crossref] [ Google Scholar]
- Ou H, Teng H, Qin Y, Luo X, Yang P, Zhang W. Extracellular vesicles derived from microRNA-150-5p-overexpressing mesenchymal stem cells protect rat hearts against ischemia/reperfusion. Aging (Albany NY) 2020; 12:12669-83. doi: 10.18632/aging.102792 [Crossref] [ Google Scholar]
- Tang J, Jin L, Liu Y, Li L, Ma Y, Lu L. Exosomes derived from mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through inhibiting pyroptosis. Drug Des Devel Ther 2020; 14:3765-75. doi: 10.2147/dddt.S239546 [Crossref] [ Google Scholar]
- Chen M, Wang R, Liao L, Li Y, Sun X, Wu H. DanShen decoction targets miR-93-5p to provide protection against MI/RI by regulating the TXNIP/NLRP3/caspase-1 signaling pathway. Phytomedicine 2024; 135:156225. doi: 10.1016/j.phymed.2024.156225 [Crossref] [ Google Scholar]
- Du J, Dong Y, Song J, Shui H, Xiao C, Hu Y, et al. BMSC-derived exosome-mediated miR-25-3p delivery protects against myocardial ischemia/reperfusion injury by constraining M1-like macrophage polarization. Mol Med Rep 2024; 30. doi: 10.3892/mmr.2024.13266.
- Gu J, You J, Liang H, Zhan J, Gu X, Zhu Y. Engineered bone marrow mesenchymal stem cell-derived exosomes loaded with miR302 through the cardiomyocyte specific peptide can reduce myocardial ischemia and reperfusion (I/R) injury. J Transl Med 2024; 22:168. doi: 10.1186/s12967-024-04981-7 [Crossref] [ Google Scholar]
- Lee TL, Shen WC, Chen YC, Lai TC, Lin SR, Lin SW. Mir221- and Mir222-enriched ADSC-exosomes mitigate PM exposure-exacerbated cardiac ischemia-reperfusion injury through the modulation of the BNIP3-MAP1LC3B-BBC3/PUMA pathway. Autophagy 2025; 21:374-93. doi: 10.1080/15548627.2024.2395799 [Crossref] [ Google Scholar]
- Du W, Zhang K, Zhang S, Wang R, Nie Y, Tao H. Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials 2017; 133:70-81. doi: 10.1016/j.biomaterials.2017.04.030 [Crossref] [ Google Scholar]
- Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One 2014; 9:e88685. doi: 10.1371/journal.pone.0088685 [Crossref] [ Google Scholar]
- Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from miR-126-overexpressing ADSCs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem 2017; 44:2105-16. doi: 10.1159/000485949 [Crossref] [ Google Scholar]
- Sánchez-Sánchez R, Gómez-Ferrer M, Reinal I, Buigues M, Villanueva-Bádenas E, Ontoria-Oviedo I. miR-4732-3p in extracellular vesicles from mesenchymal stromal cells is cardioprotective during myocardial ischemia. Front Cell Dev Biol 2021; 9:734143. doi: 10.3389/fcell.2021.734143 [Crossref] [ Google Scholar]
- Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol 2015; 182:349-60. doi: 10.1016/j.ijcard.2014.12.043 [Crossref] [ Google Scholar]
- Zhao D, Bu Y, Shao H, Wang J, Li W, Li Q. Protective effect of exosomes derived from bone marrow mesenchymal stem cells on hypoxia reperfusion injury of cardiomyocytes. Cell Mol Biol (Noisy-le-grand) 2024; 70:73-80. doi: 10.14715/cmb/2024.70.2.10 [Crossref] [ Google Scholar]
- Sun XH, Wang X, Zhang Y, Hui J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res 2019; 177:23-32. doi: 10.1016/j.thromres.2019.02.002 [Crossref] [ Google Scholar]
- Yang W, Yin R, Zhu X, Yang S, Wang J, Zhou Z. Mesenchymal stem-cell-derived exosomal miR-145 inhibits atherosclerosis by targeting JAM-A. Mol Ther Nucleic Acids 2021; 23:119-31. doi: 10.1016/j.omtn.2020.10.037 [Crossref] [ Google Scholar]
- Ma J, Chen L, Zhu X, Li Q, Hu L, Li H. Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2 macrophage polarization and reduces macrophage infiltration to attenuate atherosclerosis. Acta Biochim Biophys Sin (Shanghai) 2021; 53:1227-36. doi: 10.1093/abbs/gmab102 [Crossref] [ Google Scholar]
- Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K. Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci Rep 2015; 5:13721. doi: 10.1038/srep13721 [Crossref] [ Google Scholar]
- Pei Y, Xie S, Li J, Jia B. Bone marrow-mesenchymal stem cell-derived exosomal microRNA-141 targets PTEN and activates β-catenin to alleviate myocardial injury in septic mice. Immunopharmacol Immunotoxicol 2021; 43:584-93. doi: 10.1080/08923973.2021.1955920 [Crossref] [ Google Scholar]
- Luo F, Guo W, Liu W. Exosomes derived from bone marrow mesenchymal stem cells inhibit human aortic vascular smooth muscle cells calcification via the miR-15a/15b/16/NFATc3/OCN axis. Biochem Biophys Res Commun 2022; 635:65-76. doi: 10.1016/j.bbrc.2022.09.076 [Crossref] [ Google Scholar]
- Chen S, Zhou H, Zhang B, Hu Q. Exosomal miR-512-3p derived from mesenchymal stem cells inhibits oxidized low-density lipoprotein-induced vascular endothelial cells dysfunction via regulating Keap1. J Biochem Mol Toxicol 2021; 35:1-11. doi: 10.1002/jbt.22767 [Crossref] [ Google Scholar]
- Lei B, Wu X, Xia K, Sun H, Wang J. Exosomal micro-RNA-96 derived from bone marrow mesenchymal stem cells inhibits doxorubicin-induced myocardial toxicity by inhibiting the Rac1/nuclear factor-κB signaling pathway. J Am Heart Assoc 2021; 10:e020589. doi: 10.1161/jaha.120.020589 [Crossref] [ Google Scholar]
- Wang Z, Gao D, Wang S, Lin H, Wang Y, Xu W. Exosomal microRNA-1246 from human umbilical cord mesenchymal stem cells potentiates myocardial angiogenesis in chronic heart failure. Cell Biol Int 2021; 45:2211-25. doi: 10.1002/cbin.11664 [Crossref] [ Google Scholar]
- Yan F, Cui W, Chen Z. Mesenchymal stem cell-derived exosome-loaded microRNA-129-5p inhibits TRAF3 expression to alleviate apoptosis and oxidative stress in heart failure. Cardiovasc Toxicol 2022; 22:631-45. doi: 10.1007/s12012-022-09743-9 [Crossref] [ Google Scholar]
- Liu Y, Guan R, Yan J, Zhu Y, Sun S, Qu Y. Mesenchymal stem cell-derived extracellular vesicle-shuttled microRNA-302d-3p represses inflammation and cardiac remodeling following acute myocardial infarction. J Cardiovasc Transl Res 2022; 15:754-71. doi: 10.1007/s12265-021-10200-1 [Crossref] [ Google Scholar]
- Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep 2018; 8:1419. doi: 10.1038/s41598-018-19581-x [Crossref] [ Google Scholar]
- Wen Z, Mai Z, Zhu X, Wu T, Chen Y, Geng D. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther 2020; 11:36. doi: 10.1186/s13287-020-1563-8 [Crossref] [ Google Scholar]
- Luther KM, Haar L, McGuinness M, Wang Y, Lynch Iv TL, Phan A. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J Mol Cell Cardiol 2018; 119:125-37. doi: 10.1016/j.yjmcc.2018.04.012 [Crossref] [ Google Scholar]
- Mayourian J, Ceholski DK, Gorski PA, Mathiyalagan P, Murphy JF, Salazar SI. Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ Res 2018; 122:933-44. doi: 10.1161/circresaha.118.312420 [Crossref] [ Google Scholar]
- Zhang CS, Shao K, Liu CW, Li CJ, Yu BT. Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur Rev Med Pharmacol Sci 2019; 23:6691-9. doi: 10.26355/eurrev_201908_18560 [Crossref] [ Google Scholar]
- Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018; 8:6163-77. doi: 10.7150/thno.28021 [Crossref] [ Google Scholar]
- Yu B, Gong M, Wang Y, Millard RW, Pasha Z, Yang Y. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS One 2013; 8:e73304. doi: 10.1371/journal.pone.0073304 [Crossref] [ Google Scholar]
- Lee TL, Lai TC, Lin SR, Lin SW, Chen YC, Pu CM. Conditioned medium from adipose-derived stem cells attenuates ischemia/reperfusion-induced cardiac injury through the microRNA-221/222/PUMA/ETS-1 pathway. Theranostics 2021; 11:3131-49. doi: 10.7150/thno.52677 [Crossref] [ Google Scholar]
- Pan J, Alimujiang M, Chen Q, Shi H, Luo X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J Cell Biochem 2019; 120:4433-43. doi: 10.1002/jcb.27731 [Crossref] [ Google Scholar]
- Chen Y, Zhao Y, Chen W, Xie L, Zhao ZA, Yang J. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res Ther 2017; 8:268. doi: 10.1186/s13287-017-0722-z [Crossref] [ Google Scholar]
- Cheng H, Chang S, Xu R, Chen L, Song X, Wu J. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res Ther 2020; 11:224. doi: 10.1186/s13287-020-01737-0 [Crossref] [ Google Scholar]
- Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from miR-126-overexpressing ADSCs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem 2017; 44:2105-16. doi: 10.1159/000485949 [Crossref] [ Google Scholar]
- Goradel NH, Hour FG, Negahdari B, Malekshahi ZV, Hashemzehi M, Masoudifar A, Mirzaei H. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J Cell Biochem 2018; 119:95-104. doi: 10.1002/jcb.26169 [Crossref] [ Google Scholar]
- Hu X, Xu Y, Zhong Z, Wu Y, Zhao J, Wang Y. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: paracrine activity without remuscularization. Circ Res 2016; 118:970-83. doi: 10.1161/circresaha.115.307516 [Crossref] [ Google Scholar]
- Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML. Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends Pharmacol Sci 2017; 38:448-58. doi: 10.1016/j.tips.2017.03.001 [Crossref] [ Google Scholar]
- Garikipati VN, Krishnamurthy P, Verma SK, Khan M, Abramova T, Mackie AR. Negative regulation of miR-375 by interleukin-10 enhances bone marrow-derived progenitor cell-mediated myocardial repair and function after myocardial infarction. Stem Cells 2015; 33:3519-29. doi: 10.1002/stem.2121 [Crossref] [ Google Scholar]
- Zhang J, Sun XJ, Chen J, Hu ZW, Wang L, Gu DM. Increasing the miR-126 expression in the peripheral blood of patients with diabetic foot ulcers treated with maggot debridement therapy. J Diabetes Complications 2017; 31:241-4. doi: 10.1016/j.jdiacomp.2016.07.026 [Crossref] [ Google Scholar]
- Wang W, Zhao Y, Li H, Zhang Y, Jia X, Wang C. Exosomes secreted from mesenchymal stem cells mediate the regeneration of endothelial cells treated with rapamycin by delivering pro-angiogenic microRNAs. Exp Cell Res 2021; 399:112449. doi: 10.1016/j.yexcr.2020.112449 [Crossref] [ Google Scholar]
- Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem Cells Transl Med 2017; 6:209-22. doi: 10.5966/sctm.2015-0386 [Crossref] [ Google Scholar]
- Huang C, Luo WF, Ye YF, Lin L, Wang Z, Luo MH. Characterization of inflammatory factor-induced changes in mesenchymal stem cell exosomes and sequencing analysis of exosomal microRNAs. World J Stem Cells 2019; 11:859-90. doi: 10.4252/wjsc.v11.i10.859 [Crossref] [ Google Scholar]
- Beylerli O, Gareev I, Shi H, Ilyasova T. Effect of mesenchymal stem cell-derived exosomes on the inflammatory response after stroke. Brain Hemorrhages 2024; 5:248-56. doi: 10.1016/j.hest.2024.04.003 [Crossref] [ Google Scholar]
- Lin Y, Liu M, Chen E, Jiang W, Shi W, Wang Z. Bone marrow-derived mesenchymal stem cells microvesicles stabilize atherosclerotic plaques by inhibiting NLRP3-mediated macrophage pyroptosis. Cell Biol Int 2021; 45:820-30. doi: 10.1002/cbin.11526 [Crossref] [ Google Scholar]
- Guo Y, Bao S, Guo W, Diao Z, Wang L, Han X. Bone marrow mesenchymal stem cell-derived exosomes alleviate high phosphorus-induced vascular smooth muscle cells calcification by modifying microRNA profiles. Funct Integr Genomics 2019; 19:633-43. doi: 10.1007/s10142-019-00669-0 [Crossref] [ Google Scholar]
- Liu Y, Guo Y, Bao S, Huang H, Liu W, Guo W. Bone marrow mesenchymal stem cell-derived exosomal microRNA-381-3p alleviates vascular calcification in chronic kidney disease by targeting NFAT5. Cell Death Dis 2022; 13:278. doi: 10.1038/s41419-022-04703-1 [Crossref] [ Google Scholar]
- Zununi Vahed S, Hejazian SM, Bakari WN, Landon R, Gueguen V, Meddahi-Pellé A. Milking mesenchymal stem cells: Updated protocols for cell lysate, secretome, and exosome extraction, and comparative analysis of their therapeutic potential. Methods 2025; 238:40-60. doi: 10.1016/j.ymeth.2025.03.004 [Crossref] [ Google Scholar]