Professor Khosro Adibkia (Pharm.D., Ph.D.) is a Professor of Pharmaceutics in Tabriz University of Medical Sciences. He has received Ph.D. from Tabriz University of Medical Sciences in Pharmaceutics. He spent a six months (2007-2008) research fellow at Gent University in the field of Pharmaceutical Nanobiotechnology. He has authored over 100 publications in various areas of nanotechnology and pharmaceutics. He has also achieved outstanding research awards from the Iranian Ministry of Health and Education. He is the winner of the Rhazes Research prize (one of the most valuable prizes in Iran) in 2008. He has been selected as a Member of the Iranian National Elites Organization since 2008. Recently, he has focused on the preparation and physicochemical evaluation of novel drug delivery systems.
Professor Jaleh Barar obtained her Ph.D. degree (2004) in Pharmaceutical Cell Biology from Cardiff University, UK. Since then, she has worked at the Faculty of Pharmacy, Tabriz University of Medical Sciences (Iran), and the Perelman School of Medicine at the University of Pennsylvania (USA), teaching and conducting researches on various aspects of molecular pharmaceutics. Her main research interest is cancer drug delivery and targeting through exploitation of advanced novel multifunctional nanosystems for simultaneous diagnosis and therapy in different malignancies.
Abstract
Summary
Trafficking of macromolecular immunotherapy agent into the tumor microenvironment (TME) is a challenging issue. In the TME, cancer cells exploit indoleamine 2, 3-dioxygenase (IDO), as a cytosolic enzyme that catalyzes the L-tryptophan (Trp) through the kynurenine (Kyn) pathway, which could negatively regulate the activity of T cells. Thus, Trp/Kyn pathway, can be targeted with novel treatment modalities such as IDO1 inhibitor to benefit patients with aggressive solid tumors.
Keywords: Indoleamine 2, 3-dioxygenase, Kynurenine, Immunotherapy, IDO inhibitor, Solid tumors, Cancer therapy
Copyright and License Information
© 2019 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Over the last decades, due to the clinical benefits observed, immunotherapy has gained tremendous attention for cancer treatment when combined with a routine chemotherapy regimen. Traditional immunotherapy in cancer is frequently associated with modest success, mostly due to multiple resistance and escape mechanisms. The emergence of nanotechnology provided a practical solution for fabricating biocompatible/degradable and targeted carrier to transfer immunotherapy agent to the tumor microenvironment (TME). Immune checkpoints play an important role in regulating the physiological response to tumor invasion/tolerance. They can be considered as a promising candidate for the development of targeted immunotherapeutic nanomedicines.
1-4
Cancer immunoediting is a term used to define, both host-protecting and tumor-sculpting responsibility of the immune system. It involves three processes; first, the elimination process, that links to immunosurveillance and describes as interactions which take place between the immune system and tumor cells. Several reports indicate that endogenously produced interferon-γ (IFN-γ) can cause main protection in the healthy cells in this stage. Equilibrium phase, as the second process, consists of host immune cells and also tumor cells that are survived from the elimination process while the tumor is still under control. The last phase is introduced as the escape process. It happens when immunoevasive strategies in tumor cells trigger resistance to both innate and adaptive immune detection and/or elimination. This might allow tumor cells to become clinically detectable. There are various reports elucidating that different immunosuppressive mechanisms occur in escape phase.
5,6
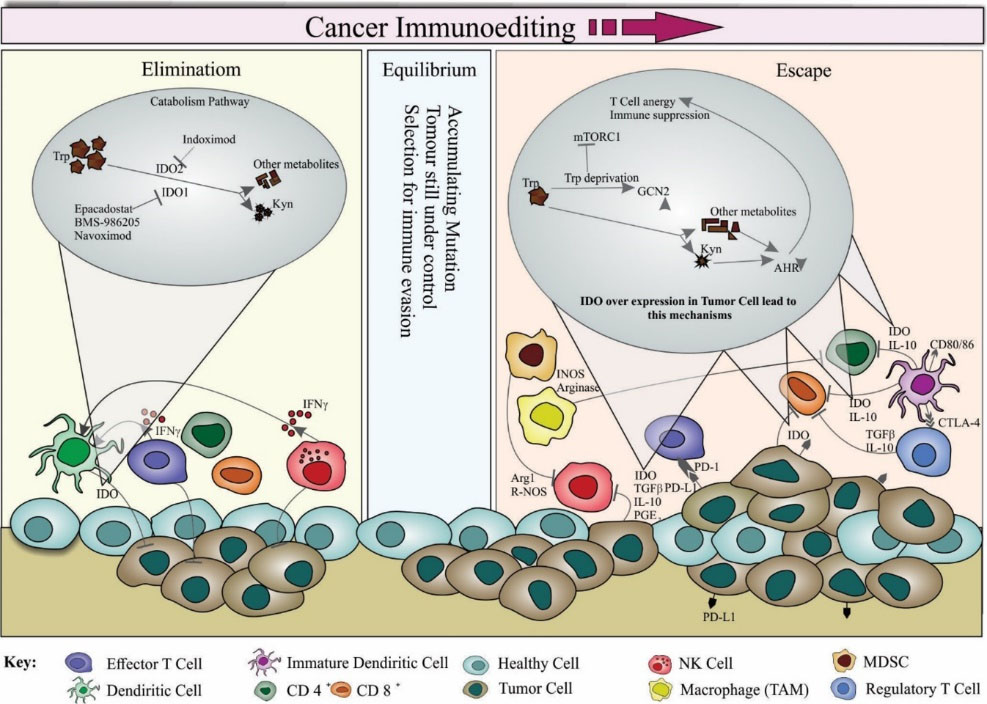

Figure 1.
Schematic representation of indoleamine 2, 3-dioxygenase (IDO) enzyme mechanism in healthy and tumor cells. For therapeutic interventions, the cancer immunoediting involves three processes, including (i) elimination, (ii) equilibrium and (iii) escape. In the elimination process, the immune system cells can recognize and eradicate tumor cells through an immunosurveillance and elimination phase. Various innate and adaptive immune cells are present in this phase such as dendritic cells (DCs), natural killer (NK) cells, CD4+, CD8+, and effector T cells. NK cells and T cells are stimulated to produce interferon gamma (IFNγ), which in turn activates the DCs and induces tumor demise. In this process, IDO as a cytosolic heme enzyme is secreted by cancer cells and DCs and is involved in the catabolism of L-tryptophan (Trp) through kynurenine (Kyn) pathway, resulting in the immune escape of tumor cells. There are some known inhibitors for different IDO isomers. Indoximod blocks IDO2 whereas Navoximod, BMS986205, and Epacadostat obstruct IDO1. Effector T cells and NK cells also destroy tumor cells. Consequently, most of the tumor cells are eliminated in this phase, but some of them survive and ultimately reach an equilibrium phase. In the equilibrium process, the tumor cells are still under control, while numerous mutations may be accumulated. In the escape process, the escaped tumor cells are resistant to elimination and detection, resulting in uncontrolled mutations, metastasis, and invasion. There are various mechanisms that lead to the escape of malignant cells from the immune system. Various cytokines (e.g., IL10, TGFβ, PGF2, etc.) play vital roles in the modulation of some of these mechanisms. Myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells can respectively inhibit NK and CD8+ cells by the secretion of cytokines. Besides, the upregulation of programmed death ligand-1 (PD-L1) by the tumor cell can result in the suppression of T cells due to the release of prostaglandin E2 (PGE2), arginase, and IDO. During this phase, IDO enzyme is produced by numerous immune cell types and induce tolerance leading to T-cell anergy, increased T cells apoptosis and decreased T-cell proliferation. Once tumor microenvironment is formed, tumor cells can produce IDO and tryptophan 2,3-dioxygenase (TDO) to further escape from the immunosurveillance functions. Readers are directed to see the following citations.
6,12,14,19
.
Schematic representation of indoleamine 2, 3-dioxygenase (IDO) enzyme mechanism in healthy and tumor cells. For therapeutic interventions, the cancer immunoediting involves three processes, including (i) elimination, (ii) equilibrium and (iii) escape. In the elimination process, the immune system cells can recognize and eradicate tumor cells through an immunosurveillance and elimination phase. Various innate and adaptive immune cells are present in this phase such as dendritic cells (DCs), natural killer (NK) cells, CD4+, CD8+, and effector T cells. NK cells and T cells are stimulated to produce interferon gamma (IFNγ), which in turn activates the DCs and induces tumor demise. In this process, IDO as a cytosolic heme enzyme is secreted by cancer cells and DCs and is involved in the catabolism of L-tryptophan (Trp) through kynurenine (Kyn) pathway, resulting in the immune escape of tumor cells. There are some known inhibitors for different IDO isomers. Indoximod blocks IDO2 whereas Navoximod, BMS986205, and Epacadostat obstruct IDO1. Effector T cells and NK cells also destroy tumor cells. Consequently, most of the tumor cells are eliminated in this phase, but some of them survive and ultimately reach an equilibrium phase. In the equilibrium process, the tumor cells are still under control, while numerous mutations may be accumulated. In the escape process, the escaped tumor cells are resistant to elimination and detection, resulting in uncontrolled mutations, metastasis, and invasion. There are various mechanisms that lead to the escape of malignant cells from the immune system. Various cytokines (e.g., IL10, TGFβ, PGF2, etc.) play vital roles in the modulation of some of these mechanisms. Myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells can respectively inhibit NK and CD8+ cells by the secretion of cytokines. Besides, the upregulation of programmed death ligand-1 (PD-L1) by the tumor cell can result in the suppression of T cells due to the release of prostaglandin E2 (PGE2), arginase, and IDO. During this phase, IDO enzyme is produced by numerous immune cell types and induce tolerance leading to T-cell anergy, increased T cells apoptosis and decreased T-cell proliferation. Once tumor microenvironment is formed, tumor cells can produce IDO and tryptophan 2,3-dioxygenase (TDO) to further escape from the immunosurveillance functions. Readers are directed to see the following citations.
6,12,14,19
One of the important immunosuppressive pathways in TME which greatly considered is Tryptophan (Trp) catabolism. Trp is an essential amino acid metabolized using two independent pathways. Indoleamine 2, 3-dioxygenase (IDO) is a cytosolic haem enzyme that catalyzes the destruction of Trp through the kynurenine (Kyn) pathway.
7-10
IDO, also known as checkpoint protein, can negatively regulate the activity of T cell. It exerts this robust immunosuppressive effect by different mechanisms. Both of these mechanisms can be explained by its principal effect in Trp depletion. As mentioned, IDO catalyzes the conversion of essential amino acid L-Trp into the L-Kyn, and subsequently, the inhibition of this enzyme could initiate several events. First, a drop in the level of Trp might consequently lead to growth in uncharged tRNA in T cells. This result in cell cycle arrest and inhibition of T cell proliferation. Second, the accumulation of downstream metabolites (e.g., Kyn, kynurenic acid, 3-hydroxykynurenine, and 3-3-hydroxyanthranilic acid) might be involved in such a phenomenon. These molecules mediate growth arrest and apoptosis of the effector T cells; they also induce the differentiation of naive CD4+ T cells into the Treg cells with immune suppressive effect.
4,7,11-15
IDO exists in two isoforms, IDO1 and IDO2. Both regulate the degradation of Trp to Kyn but IDO1 display 20-30-fold more enzymatic activity than IDO2. Recent studies have discovered that IDO1 is expressed on cancer cells and also on its surroundings, such as antigen presenting cells (APCs), dendritic cells (DCs), macrophages, etc. But IDO2 is mainly expressed in liver, kidney and APC and DCs without considerable enzymatic activity.
11,12,14
It is believed that the inhibition of IDO effectively enhances the anti-tumor immune response. A detailed schematic image of represented mechanisms is illustrated based on a dozen recent studies (Fig. 1).
Previous reports have demonstrated strong anti-cancer effects by the combination of an IDO inhibitor with a cytotoxic agent. To date, at least 12 IDO inhibitors are in development for the clinical setting, mostly in combination with other immunotherapies including therapeutic vaccines and checkpoint blockade agents, or with standard chemotherapies, but only a few of them are settled for breast cancer therapy.
Based on the isoforms’ classifications, these inhibitors could be categorized into three groups, including (i) IDO1 specific inhibitors such as Epacadostat (INCB024360) and its analogs, BMS-986205, (ii) indirect IDO2 inhibitors such as Indoximod, and (iii) inhibitors that block both, IDO1 and IDO2.
1,2,10,12,14,16-18
Interestingly, a number of studies have shown a strong correlation between IDO expression and poor prognosis in breast cancer, where its expression is higher in triple negative breast carcinomas than low grade, hormone responsive cancers.
16
Therefore, this would be desirable to target such patients (with aggressive cancer type) with novel treatment modalities such as IDO1 inhibitor. This can suggest further studies to focus on these types of breast cancer using IDO molecule as a target to suppress. Also, a report which published in May 2018, is wondering that three companies have canceled, suspended or downsized their trials due to large failure,
2
that may raise some questions upon safety/effectiveness of such compounds.
Acknowledgments
The authors like to acknowledge the financial support provided by the Research Vice-Chancellor (No. 129), and technical support provided by the Research Center for Pharmaceutical Nanotechnology at Tabriz University of Medical Sciences on the microfluidics project. The authors are also thankful to Prof. Omidi for his constructive remarks and valuable inputs.
Funding sources
None to be declared.
Ethical statement
There is none to be declared.
Competing interests
No competing interests to be disclosed.
Authors contribution
NH, KA, and JB gathered the data and drafted the manuscript. JB finalized the manuscript.
References
- Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 2017; 17:286. doi: 10.1038/nrc.2017.17 [Crossref] [ Google Scholar]
- Garber K. A new cancer immunotherapy suffers a setback. Science 2018; 360:588. doi: 10.1126/science.360.6389.588 [Crossref] [ Google Scholar]
- Colombo MP, Piconese S. Regulatory T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer 2007; 7:880. doi: 10.1038/nrc2250 [Crossref] [ Google Scholar]
- Löb S, Königsrainer A, Rammensee H-G, Opelz G, Terness P. Inhibitors of indoleamine-2, 3-dioxygenase for cancer therapy: can we see the wood for the trees?. Nat Rev Cancer 2009; 9:445. doi: 10.1038/nrc2639 [Crossref] [ Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3:991. doi: 10.1038/ni1102-991 [Crossref] [ Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004; 22:329-60. doi: 10.1146/annurev.immunol.22.012703.104803 [Crossref] [ Google Scholar]
- Basu GD, Tinder TL, Bradley JM, Tu T, Hattrup CL, Pockaj BA. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol 2006; 177:2391-402. doi: 10.4049/jimmunol.177.4.2391 [Crossref] [ Google Scholar]
- Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res 2012; 72:5435-40. doi: 10.1158/0008-5472.CAN-12-0569 [Crossref] [ Google Scholar]
- Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2, 3-dioxygenase inhibition for cancer therapy. Eur J Cancer 2017; 76:167-82. doi: 10.1016/j.ejca.2017.01.011 [Crossref] [ Google Scholar]
-
Röhrig UF, Zoete V, Michielin O. Inhibitors of the Kynurenine Pathway. 2017.
- Vacchelli E, Aranda F, Eggermont A, Sautes-Fridman C, Tartour E, Kennedy EP. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology 2014; 3:e957994. doi: 10.4161/21624011.2014.957994 [Crossref] [ Google Scholar]
- Bilir C, Sarisozen C. Indoleamine 2, 3-dioxygenase (IDO): Only an enzyme or a checkpoint controller?. J Oncol Sci 2017; 3:52-6. doi: 10.1016/j.jons.2017.04.001 [Crossref] [ Google Scholar]
- Chen W. IDO: more than an enzyme. Nat Immunol 2011; 12:809-11. doi: 10.1038/ni.2088 [Crossref] [ Google Scholar]
- Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res 2017; 77:6795-811. doi: 10.1158/0008-5472.CAN-17-2285 [Crossref] [ Google Scholar]
- Jiang T, Sun Y, Yin Z, Feng S, Sun L, Li Z. Research progress of indoleamine 2, 3-dioxygenase inhibitors. Future Med Chem 2015; 7:185-201. doi: 10.4155/fmc.14.151 [Crossref] [ Google Scholar]
- Dill EA, Dillon PM, Bullock TN, Mills AM. IDO expression in breast cancer: an assessment of 281 primary and metastatic cases with comparison to PD-L1. Mod Pathol 2018:1. doi: 10.1038/s41379-018-0061-3 [Crossref]
- Cheong JE, Ekkati A, Sun L. A patent review of IDO1 inhibitors for cancer. Expert Opin Ther Pat 2018; 28:317-30. doi: 10.1080/13543776.2018.1441290 [Crossref] [ Google Scholar]
- Moon YW, Hajjar J, Hwu P, Naing A. Targeting the indoleamine 2, 3-dioxygenase pathway in cancer. J Immunother Cancer 2015; 3:51. doi: 10.1186/s40425-015-0094-9 [Crossref] [ Google Scholar]
- Monjazeb AM, Zamora AE, Grossenbacher SK, Mirsoian A, Sckisel GD, Murphy WJ. Immunoediting and antigen loss: overcoming the achilles heel of immunotherapy with antigen non-specific therapies. Front Oncol 2013; 3:197. doi: 10.3389/fonc.2013.00197 [Crossref] [ Google Scholar]