Bioimpacts. 11(1):15-22.
doi: 10.34172/bi.2021.03
Original Research
Presence of pyrroloquinazoline alkaloid in Adhatoda vasica attenuates inflammatory response through the downregulation of pro-inflammatory mediators in LPS stimulated RAW 264.7 macrophages
Reddy Amala , Sundaresan Sujatha * 
Author information:
Animal Cell Culture Laboratory, Department of Biotechnology, SRMIST, Kattankulathur, Tamilnadu, India
Abstract
Introduction:
Inflammation is the primary response caused due to harmful stimuli which are followed by the increased draining of plasma and immune cells from the body into the site of the injured tissue. A signaling cascade of growth factors and cytokines propagates and eventually matures in the inflammatory site involving the blood vessels and immune markers within the injured tissue in order to promote the renewal of the degenerated tissue. During a chronic disorder like diabetic foot ulcer, there is an obstinate inflammation which may act as a prime factor for limb amputation and upon persistent prevalence may even lead to death.
Methods:
This study focuses on the mode of action of ALK-F (alkaloid fraction) isolated from Adhatoda vasica in attenuating the nitric oxide production which was estimated by Griess assay, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) expression was analyzed by ELISA and expression of COX-2 and iNOS by RT-PCR and western blotting in LPS stimulated RAW 264.7 macrophages. Total intracellular ROS was analyzed by DCFH-DA probing and the presence of quinazoline alkaloid (vasicine) in the ALK-F was evidenced by high performance liquid chromatography (HPLC).
Results:
The ALK-F of A. vasica exhibited a significant inhibitory effect on LPS elicited nitrite production (13.2 ± 1.06 µM), iNOS, and COX-2 (2.6 and 3.3 fold) in a dose-dependent manner. There was a significant decrease in the generation of these pro-inflammatory cytokines TNF-α (1102 ± 1.02 pg/mL) and IL-6 (18 ± 0.87 ng/mL) and total intracellular ROS in the highest tested concentrations (1 µg and 10 µg) of ALK-F of A. vasica. HPLC analysis by the gradient elution method revealed the presence of 12% of quinazoline alkaloid vasicine in the crude alkaloid fraction.
Conclusion:
Thus this study communally suggests that attenuation of nitric oxide and the dysregulation of genes responsible for inflammation which deliberates A. vasica to conflict against inflammation and provide remedial benefits in diabetic wound care.
Keywords: Inducible nitric oxide synthase, Cyclooxygenases-2, Lipopolysaccharide, RAW 264.7 macrophages, Quinazoline alkaloids, Diabetic foot ulcer, Limb amputation
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/
). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Inflammation is an acute or chronic condition encountered by pain, redness, heat and swelling causing an imbalance in the culmination of humoral and cellular responses. An obstinate and long-term inflammation may act as fundamental for a large number of chronic illnesses such as diabetic foot ulcer, Alzheimer's, and rheumatoid arthritis. Prostaglandins (PGs) and nitric oxide (NO) are important intermediates in the inflammatory responses.
1
The elevation of pro-inflammatory mediators like NO may slow down the process of inflammation and cause degeneration of tissues which is otherwise a regular process. Their secretion is induced by various stimuli like a bacterial cell wall component (lipopolysaccharides, or LPS) in RAW 264.7 macrophages.
2
Inducible NO synthase (iNOS) plays a role in the conversion of L-arginine to NO.
3
At lower concentrations, NO was added to the anti-microbial role of macrophage against various pathogenic microorganism and benefit in the removal of debris.
4
However, NO in higher concentrations is converted to peroxynitrite which contributes to inflammation and thereby elicits a cascade of pro-inflammatory mediators.
PGE2 is one of the key PGs which is secreted by macrophages that regulates their activity in an autocrine manner.
5
Cyclooxygenases (COXs) are responsible for the conversion of arachidonic acid to PGs.
6
COX-1 is basically expressed in most cell types and it is liable for regulating normal physiological functions but COX-2 is inducible in cell types like macrophages, fibroblasts, and endothelial cells to play a focal role in inflammation.
7
Many commercially available steroids and non-steroidal anti-inflammatory drugs have a boundless influence in the cure for inflammatory diseases, but due to their indeterminate side effects there arises a need for lead targets with the minimal adverse effect.
8,9
Folklore medicinal plants with traditional medicinal values provide enormous health care benefits with new therapies and fewer side effects. The World Health Organization (WHO) estimated that 80% of the world population depends on these customary herbal plants for their health essentials due to their abundance and varied bioactivities compared to other commercial available non-steroidal anti-inflammatory drugs.
10
Active leads from plant-derived alkaloids and phenolic compounds (flavonoids and tannins) were found to possess antioxidant and anti-inflammatory activities both in vitroand in vivo.
11
AdhatodavasicaNees is an evergreen shrub belonging to the family Acanthaceae, found in many regions of India and throughout the world, with expanded use in traditional medicine. The aerial parts and flowers are active lead principles of the plant which possess a number of pharmacological properties and are used as antitussive and anti-asthmatic.
12,13
The aqueous extract from the leaves is used to treat rheumatic pain and urinary tract infections. The chief principle being quinazoline alkaloid, vasicine, and vasicinone, vasicinolone, and vasicol which are conjugated with an acid named adhatoda acid.
14
Vasicine is an alkaloid present in the leaves and it has respiratory restorative activity, protection against histamine-induced bronchospasm in guinea pigs, and uterine stimulant effect.
15
In this report, we investigated the possible action of the alkaloid fraction of Adhatodavasica (ALK-F of A. vasica) in regulating the genes responsible for the synthesis of pro-inflammatory mediators.
Materials and Methods
Plant material
The plant specimen was collected from the botanical garden, Chennai, India. Specimen authentication was carried out by Prof. P. Jayaraman, Director, Plant Anatomy Research Centre, Chennai, India (Ref. no. PARC/2017/3360). An authenticated certificate for the specimen has been submitted for future reference.
Preparation of plant extract
The dried whole plant powder (100 g) of A. vasica was extracted using a sequential extraction method by using various solvents in the increasing order of polarity (hexane, dichloromethane, ethyl acetate, and methanol). About 50 g of dried plant powder was taken and sequentially extracted with 200 mL of solvent.
16
This was then concentrated using reduced pressure under vacuum and the yield was calculated using the formula:
Percentage yield = Weight of the sample extract obtained (g)/ The weight of the powdered sampled used (g)× 100
Acid-base extraction of ALK-F of Adhatodavasica
The methanolic extract was added with 15 mL 2% sulphuric acid (pH=1-2), then the contents were transferred to a separating funnel and extracted twice using chloroform. The liquid portion was separated into two distinct layers, the bottom layer is a chloroform layer and the upper layer is the aqueous layer. Then about 60 mL of liquid ammonium hydroxide was then added to the aqueous layer (pH=9). This was then extracted again in a separating funnel with a mixture of chloroform: methanol in a ratio of 3:1 two times (60:30 mL) and once with chloroform alone (30 mL). The solution was once again separated into two layers and the upper aqueous layer was evaporated in a rotary evaporator and given for high performance liquid chromatography (HPLC) for identification of alkaloids with vasicine as a standard.
17
Drugs and chemicals
Classical media (Dulbecco’s modified essential medium), dimethyl sulphoxide (DMSO), fetal bovine serum (FBS), L-nitro-arginine methyl ester (L-NAME) and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT), RIPA Buffer (10X), sulphuric acid, chloroform liquid ammonium hydroxide, and methanol was procured from HiMedia Laboratories (Mumbai, Maharashtra, India). Vasicine was purchased from Natural remedies (Bangalore, Karnataka, India). Sulfanilamide and naphthylethylenediamine were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture studies
RAW 264.7 macrophages were cultured in DMEM provided with 10% FBS and 5% antibiotic (100X Liquid in 10,000 U Penicillin and 10mg Streptomycin per ml in 0.9% normal saline, purchased from HiMedia, Mumbai, Maharashtra, India) in a 5% CO2 environment.
18
Cell viability assessment of ALK-F of A. vasica in RAW 264.7 macrophage
MTT reduction was used to check the cell viability. Cell seeding was done in a 96-well plate and incubated to achieve 80% confluency. The cells were then treated with ALK-F of A. vasica(1 ng/mL -10 µg/mL; denoted ad ng and µg in the figure) and were incubated for 24 hours roughly about 10 µL of MTT (i.e, 5 mg/mL in PBS) solution was added to each of the wells and incubated for 4 hours.
19
Later, the medium was aspirated without disturbing the purple formazan crystals and a solubilizing agent (DMSO) was added in order to dissolve these insoluble crystals. The absorbance was measured at 570 nm.
20
The absorbance of the ALK-F treated cells and the control cells were used to determine the percentage of cell viability.
Cell viability (%) = (O.D. of test sample / O.D. of control sample) × 100
LPS induced nitrite production in RAW 264.7 macrophage
LPS stimulation (1 µg/mL) was used to analyze the inhibitory effect of ALK-F of A. vasicaon NO production using RAW 264.7 macrophage. Cells were seeded in a 96-well plate and kept under incubation to achieve 80% confluency. Later, the cells were co-incubated with LPS (1 µg/mL) and ALK-F (1 ng – 10 µg) for 24 h. NO synthase inhibitor L-NAME (200 µM) was used as a positive control. After incubation, the supernatant was used for the estimation of nitrite using Griess nitrite assay.
21
Griess nitrite assay
An equal volume of the supernatant and Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamine in 5% phosphoric acid) was mixed well and incubated for 10 minutes in room temperature. The absorbance was measured at 540 nm.
21
Sodium nitrite was used as a standard.
Estimation of intracellular ROS by DCFH-DA fluorescence method
The action of ALK-F of A. vasicaon ROS generation was estimated using DCFH-DA fluorescence method. Cells were seeded in a 24-well plate and kept under incubation for 24 hours. Later, the cells were and treated with LPS (1 µg/mL) for 30 minutes in order to stimulate ROS production and then co-incubated with ALK-F of A. vasica(10 µg) for 24 hours. Cells were then washed with PBS and incubated with DCFH-DA probe (20 µM) for 30 minutes and washed with PBS. The photomicrographs were obtained using a fluorescence microscope CKS-HI-TR (Olympus, Waltham, MA, USA).
23
ELISA analysis
LPS induced expression of pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) and the role of ALK-F of A. vasica in the regulation of expression was estimated using Standard ELISA Kit protocol (Sigma-Aldrich, St. Louis, MO, USA) and ELISA Mutiskan GO reader (Thermofisher Scientific, Waltham, MA, USA).
RNA isolation and qPCR
Total RNA was isolated from RAW 264.7 macrophage using Trizol (HiMedia, Mumbai, Maharashtra, India) method. The concentration and the purity of the isolated RNA were spectrophotometrically determined at 260 nm and 280 nm respectively. Two micrograms of total RNA was transcribed to a single-stranded cDNA using the reverse transcription method and the qPCR analysis was performed using aliquots of the cDNA samples and SYBR Green PCR Master Mix.
23
The gene expression of iNOS, COX-2, and GAPDH (standard) was analyzed using a BIO-RAD CFX96 Real-Time PCR system (C1000 Touch thermal cycler). The 2−ΔΔCT method was employed to analyze the fold increase. All reactions were carried out in triplicates.
Preparation of total cell lysate from RAW 264.7 macrophage
About 5 × 105 cells were seeded in each of the wells of a six-well plate and incubated to achieve 80% confluency. Later, the cells were treated with 1 µg/mL LPS and ALK-F of A. vasica(10 µg) and incubated for 24 hours.
25
After incubation, cells were washed with ice-cold PBS and lysed with 1 mL of lysis buffer (RIPA buffer, pH 7.4). Then the total cell lysate of the homogenate was obtained after centrifugation at 12000 rpm for 20 minutes at 4ºC.
26
SDS-PAGE and immunoblotting
The protein concentration was determined using Bradford’s method and about 40 µg of the protein was subjected to electrophoresis on a 12% SDS-PAGE and transferred to nitrocellulose membrane. The monoclonal antibody for iNOS/COX-2 raised in mouse was used as a primary antibody and the secondary antibody as anti-mouse IgG conjugated with horseradish peroxidase. After washing with PBS-Tween twice, the immunoblot was exposed to luminol and then visualized using chemidoc XRS+ imaging system (Biorad, USA). Quantification of the protein was done by densitometric scanning using Image Studio Lite version 5.2.
27,28
HPLC analysis of ALK-F of A. vasica
ALK-F of A. vasicawas subjected to HPLC (Agilent 1260 Infinity Series) with autosampler and was eluted at a constant flow rate of 1 mL/min. The mobile phase used was buffer consisting of two mobile phases A and B at a flow rate of 1.0 mL/min. Mobile phase A consisted of water and mobile phase B was methanol. The separation was carried out at room temperature. A gradient starting at 95% water and 5% Methanol to 10% water and 90% methanol linearly in 10 minutes was employed to elute vasicine from ALK-F of A. vasica. The detection was performed using a diode array detector (DAD) at 280 nm.
29,30
Results
The yield of plant extract and ALK-F
The extracts obtained by the sequential method of extraction have shown considerable differences in the amount of final product. Maximum yield was obtained for methanolic extract (12.5%) followed by ethyl acetate (7.7 %) and hexane (1.4 %). DCM did not show any considerable yield (0.6 %). About 12% of vasicine was present in ALK-F, revealed from HPLC data.
Assessment of cell viability using MTT assay
MTT reduction was used to access the cytotoxicity effect of ALK-F of A. vasicain RAW 264.7 macrophages. The cell viability in the presence of the ALK-F is represented (Fig. 1). The results showed that ALK-F of A. vasicadid not alter the cell viability even at the highest concentration (10 µg) and were taken for further studies.
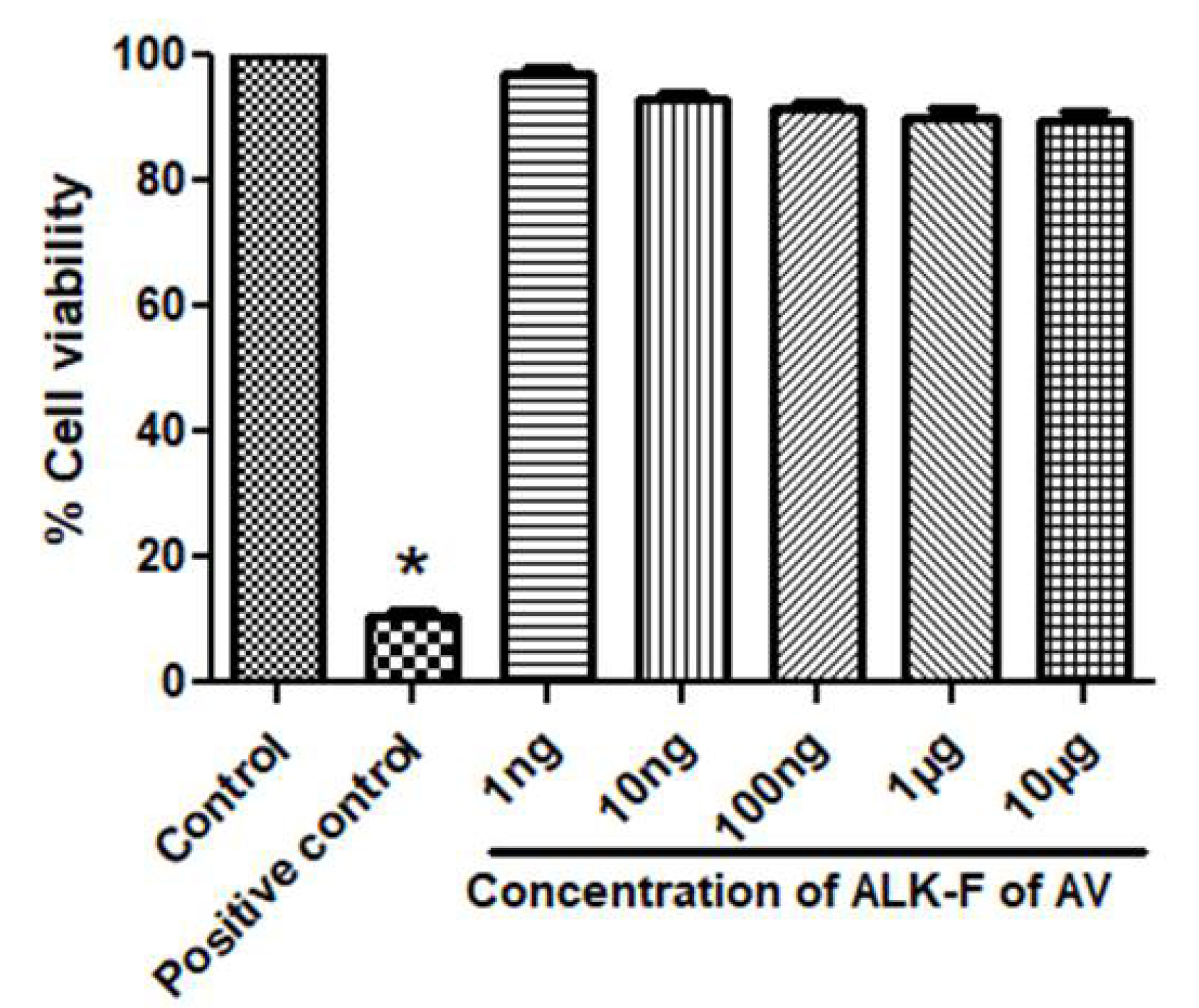
Fig. 1.
Effect of ALK-F of A. vasica(ALK-F of AV)on Cell viability in RAW 264.7 macrophages using MTT assay. Cells at a density of 5 x103/well in a 96 well plate were pre-incubated with indicated concentrations of ALK-F of A. vasica for 24 h. Untreated cells served as control. Data represents mean±SD from three individual experiments. * P < 0.05, #P < 0.01 represents significant difference compared with control.
.
Effect of ALK-F of A. vasica(ALK-F of AV)on Cell viability in RAW 264.7 macrophages using MTT assay. Cells at a density of 5 x103/well in a 96 well plate were pre-incubated with indicated concentrations of ALK-F of A. vasica for 24 h. Untreated cells served as control. Data represents mean±SD from three individual experiments. * P < 0.05, #P < 0.01 represents significant difference compared with control.
Effect of ALK-F of A. vasica in nitrite inhibition
The effect of ALK-F of A. vasica on LPS-induced nitrite inhibition was assessed in RAW 264.7 macrophages. Griess reagent was used to determine nitrite released in the cell culture supernatant. As shown in Fig. 2, the untreated cells revealed a low level of nitrate production (5.2 ± 0.016 μM), while cells treated by LPS promoted nitrite production (27.1 ± 0.110 μM). The ALK-F of A. vasicaexhibited a significant inhibitory effect on nitrite production in a dose-dependent manner (P < 0.05). The positive control, L-NAME significantly decreased nitrite production at 200 µM (P < 0.05) in comparison with cells treated with LPS alone.
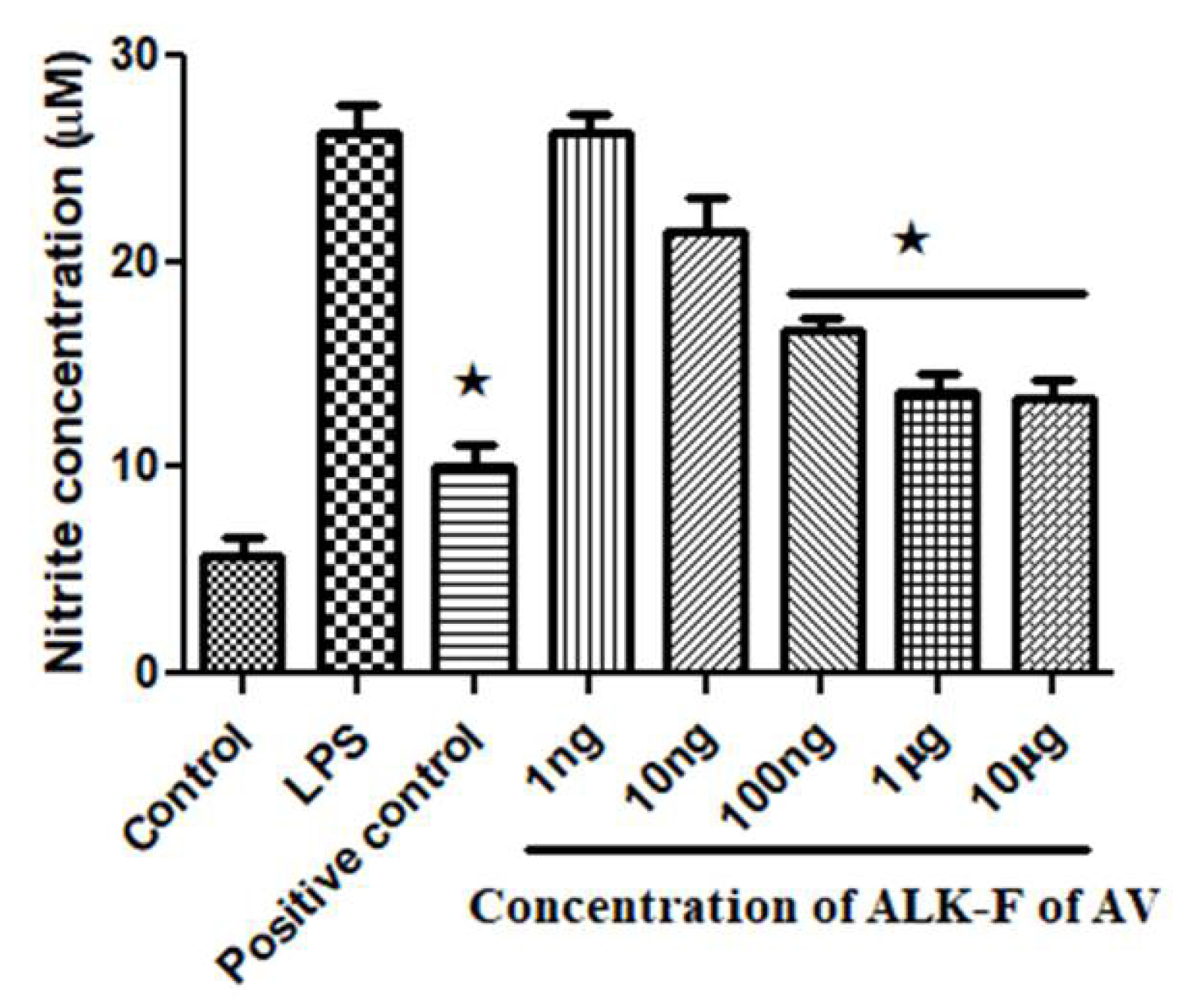
Fig. 2.
Effect of ALK-F of A. vasica on nitrite inhibition in LPS-stimulated RAW 264.7 macrophages. Cells at a density of 5 × 103/well in a 96 well plate were pre-incubated with LPS and with or without the indicated concentrations of ALK-F of A. vasica for 24 h. Untreated cells served as control. Data represents mean±SD from three individual experiments. * P < 0.05 , ** P < 0.01 represents significant difference compared with cells treated with LPS alone, # P < 0.01 represents significant difference compared with control.
.
Effect of ALK-F of A. vasica on nitrite inhibition in LPS-stimulated RAW 264.7 macrophages. Cells at a density of 5 × 103/well in a 96 well plate were pre-incubated with LPS and with or without the indicated concentrations of ALK-F of A. vasica for 24 h. Untreated cells served as control. Data represents mean±SD from three individual experiments. * P < 0.05 , ** P < 0.01 represents significant difference compared with cells treated with LPS alone, # P < 0.01 represents significant difference compared with control.
Estimation of intracellular ROS by DCFH-DA fluorescence method
Results showed that LPS significantly increased the intracellular ROS production (Fig. 3A); whereas treatment with ALK-F of A. vasicaattenuated the process (Fig. 3D); Only cells (Fig. 3B); and ALK-F of A. vasica alone (Fig. 3C); did not affect ROS production (absence of LPS). These results collectively proved that ALK-F of A. vasicacould significantly reduce LPS stimulated ROS generation in macrophages.
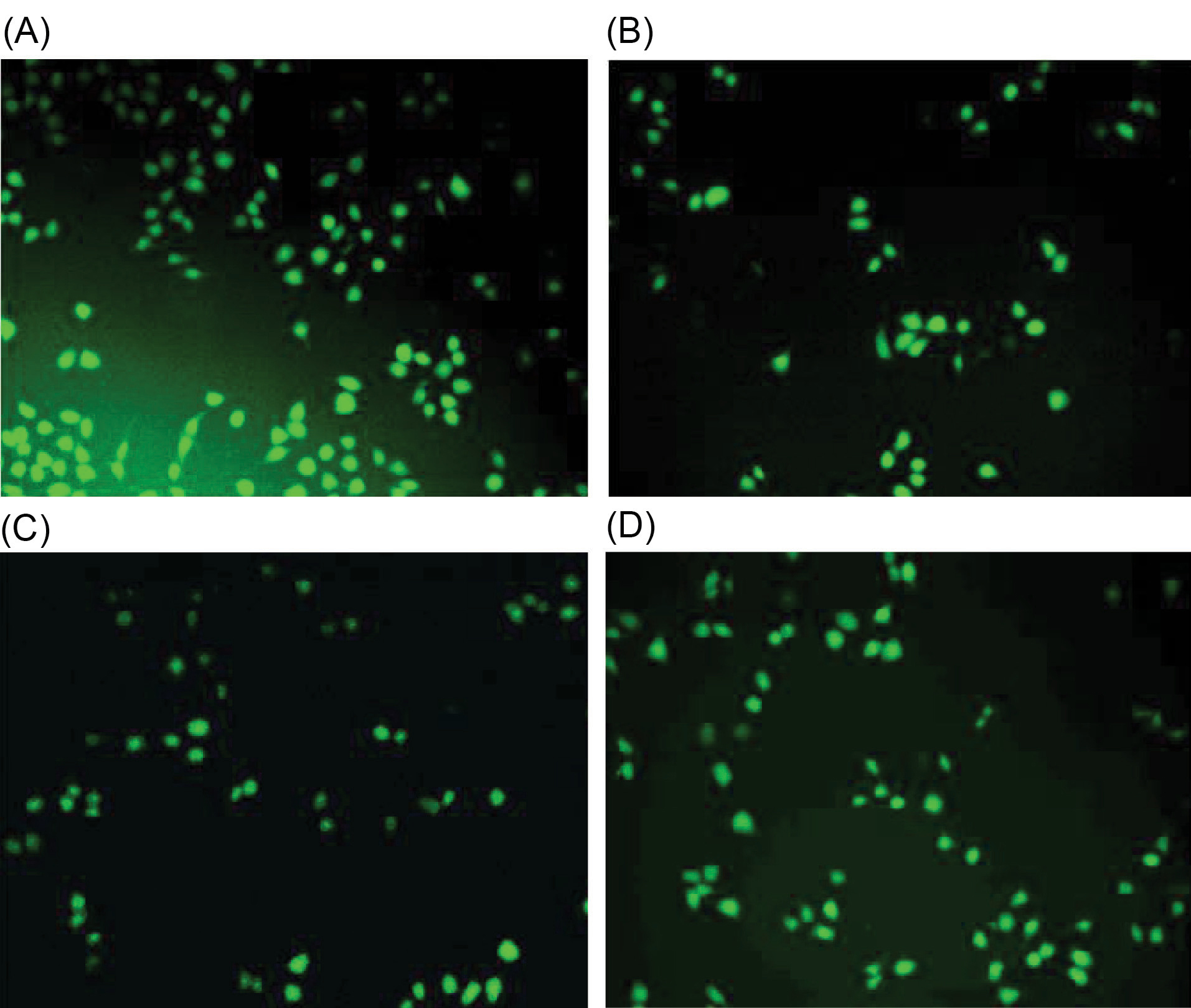
Fig. 3.
Effect of ALK-F of A. vasicaon ROS generation in RAW 264.7 macrophages using DCFH-DA probing. Cells were seeded in a 24-well plate and kept under incubation for 24 h. Later, the cells were and treated with LPS (1 µg) to stimulate ROS production and the effect of later ALK-F of A. vasica(10 µg) was tested. Probing was done with non-fluorescent DCFH-DA (20µM) for 30 min and washed with PBS. (A) LPS alone; (B) Only cells; (C) ALK-F of A. vasica alone and D) LPS with ALK-F of A. vasica.These findings collectively prove that ALK-F of A. vasicacould significantly reduce LPS stimulated intracellular ROS generation in macrophages.
.
Effect of ALK-F of A. vasicaon ROS generation in RAW 264.7 macrophages using DCFH-DA probing. Cells were seeded in a 24-well plate and kept under incubation for 24 h. Later, the cells were and treated with LPS (1 µg) to stimulate ROS production and the effect of later ALK-F of A. vasica(10 µg) was tested. Probing was done with non-fluorescent DCFH-DA (20µM) for 30 min and washed with PBS. (A) LPS alone; (B) Only cells; (C) ALK-F of A. vasica alone and D) LPS with ALK-F of A. vasica.These findings collectively prove that ALK-F of A. vasicacould significantly reduce LPS stimulated intracellular ROS generation in macrophages.
Effect of ALK-F of A. vasica in the expression of pro-inflammatory cytokines using ELISA
LPS stimulation release of pro-inflammatory cytokines TNF-α and IL-6 was down-regulated by ALK-F of A. vasica(Fig. 4). There was a significant decrease in the generation of these pro-inflammatory cytokines in the highest tested concentrations (1 µg and 10 µg) of ALK-F of A. vasica.
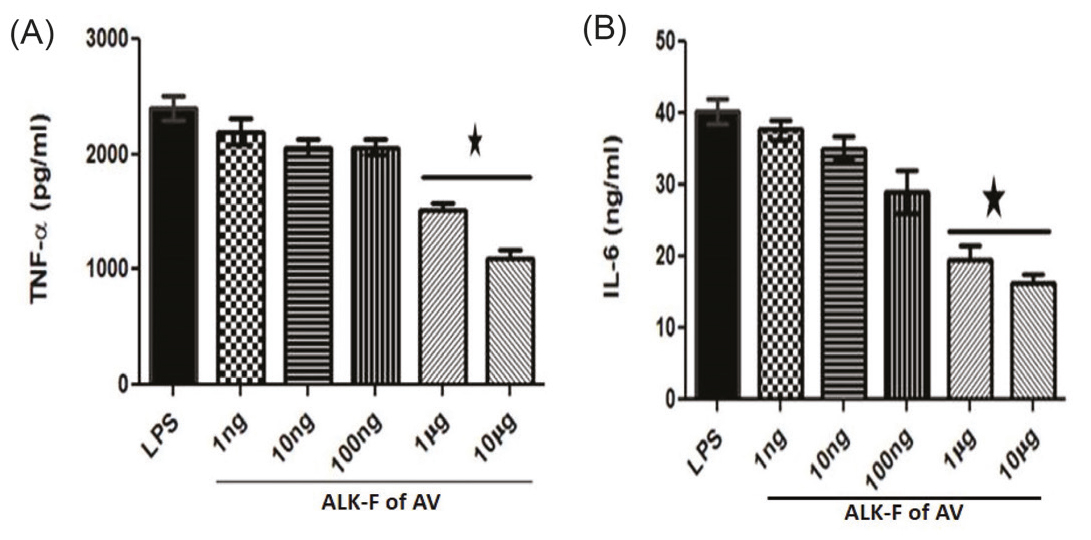
Fig. 4.
Effect of ALK-F of A. vasica in the production of pro-inflammatory cytokines estimated using ELISA. Cells were treated with 1 µg/mL LPS and ALK-F of A. vasica(10 µg). The cell lysis was done with RIPA Buffer (pH 7.4) after centrifugation at 12000 rpm for 20 min at 4ºC. The total cell lysate of the homogenate was used for the estimation of pro-inflammatory cytokines using the standard ELISA kit protocol (A) TNF-α and (B) IL-6 in RAW 264.7 macrophages.
.
Effect of ALK-F of A. vasica in the production of pro-inflammatory cytokines estimated using ELISA. Cells were treated with 1 µg/mL LPS and ALK-F of A. vasica(10 µg). The cell lysis was done with RIPA Buffer (pH 7.4) after centrifugation at 12000 rpm for 20 min at 4ºC. The total cell lysate of the homogenate was used for the estimation of pro-inflammatory cytokines using the standard ELISA kit protocol (A) TNF-α and (B) IL-6 in RAW 264.7 macrophages.
Effect of ALK-F on LPS induced COX-2 and iNOS using Real-time PCR
LPS elicited a response of iNOS and COX-2 was restrained by ALK-F of A. vasicain a dose-dependent manner. There was attenuation in iNOS and COX-2 mRNA expression levels when compared to LPS treated cells at 10 μg concentration of ALK-F of A. vasica(2.5 and 2.6 fold decrease). These results suggest that transcription of LPS induced gene expression of COX-2 and iNOS can be down-regulated by ALK-F of A. vasica(Figs. 5A and 5B).
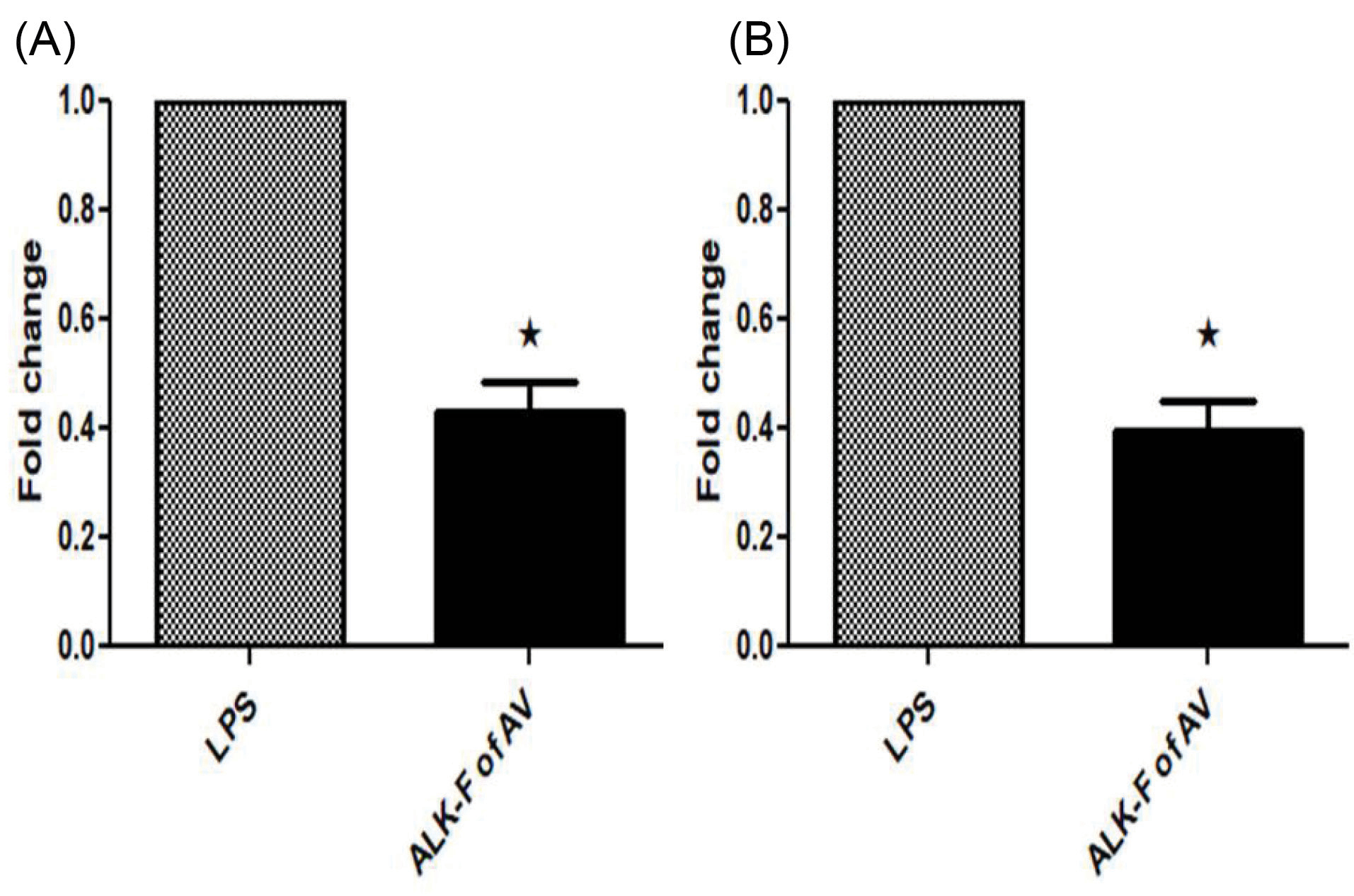
Fig. 5.
Effect of ALK-F of A. vasica on LPS-stimulated iNOS and COX-2 gene expression in RAW 264.7 macrophages. The cells were treated with 1 µg/mL of LPS only or with the indicated concentrations of ALK-F of A. vasica for 24 h and total RNA were isolated. (A) mRNA levels of iNOS. (B) mRNA levels of COX-2, quantified using qPCR analysis with SYBR Green PCR Master Mix. The gene expression of iNOS, COX-2, and GAPDH (an internal standard) was analyzed using BIO-RAD CFX96 Real-Time PCR system (C1000 Touch thermal cycler). The 2−ΔΔCT method was utilized to analyze the fold increase. Data represent mean±SD from three individual experiments. * P < 0.05 represents a significant difference compared with cells treated with LPS alone.
.
Effect of ALK-F of A. vasica on LPS-stimulated iNOS and COX-2 gene expression in RAW 264.7 macrophages. The cells were treated with 1 µg/mL of LPS only or with the indicated concentrations of ALK-F of A. vasica for 24 h and total RNA were isolated. (A) mRNA levels of iNOS. (B) mRNA levels of COX-2, quantified using qPCR analysis with SYBR Green PCR Master Mix. The gene expression of iNOS, COX-2, and GAPDH (an internal standard) was analyzed using BIO-RAD CFX96 Real-Time PCR system (C1000 Touch thermal cycler). The 2−ΔΔCT method was utilized to analyze the fold increase. Data represent mean±SD from three individual experiments. * P < 0.05 represents a significant difference compared with cells treated with LPS alone.
Effect of ALK-F of A. vasica on LPS induced COX-2 and iNOS protein expressions
In the presence and absence of ALK-F of A. vasicatreatment, the protein expression of the two major pro-inflammatory enzymes COX-2 and iNOS in LPS exposed cells were evaluated by densitometric analysis of the Western blots. LPS induction increased the expression levels of COX-2 and iNOS in RAW 264.7 macrophages (Figs. 6A and 6B). A significant reduction (P < .05) in these protein expressions were observed upon treatment with ALK-F of A. vasica(2.6 and 3.3 fold decrease) when compared with the LPS treated cells alone.
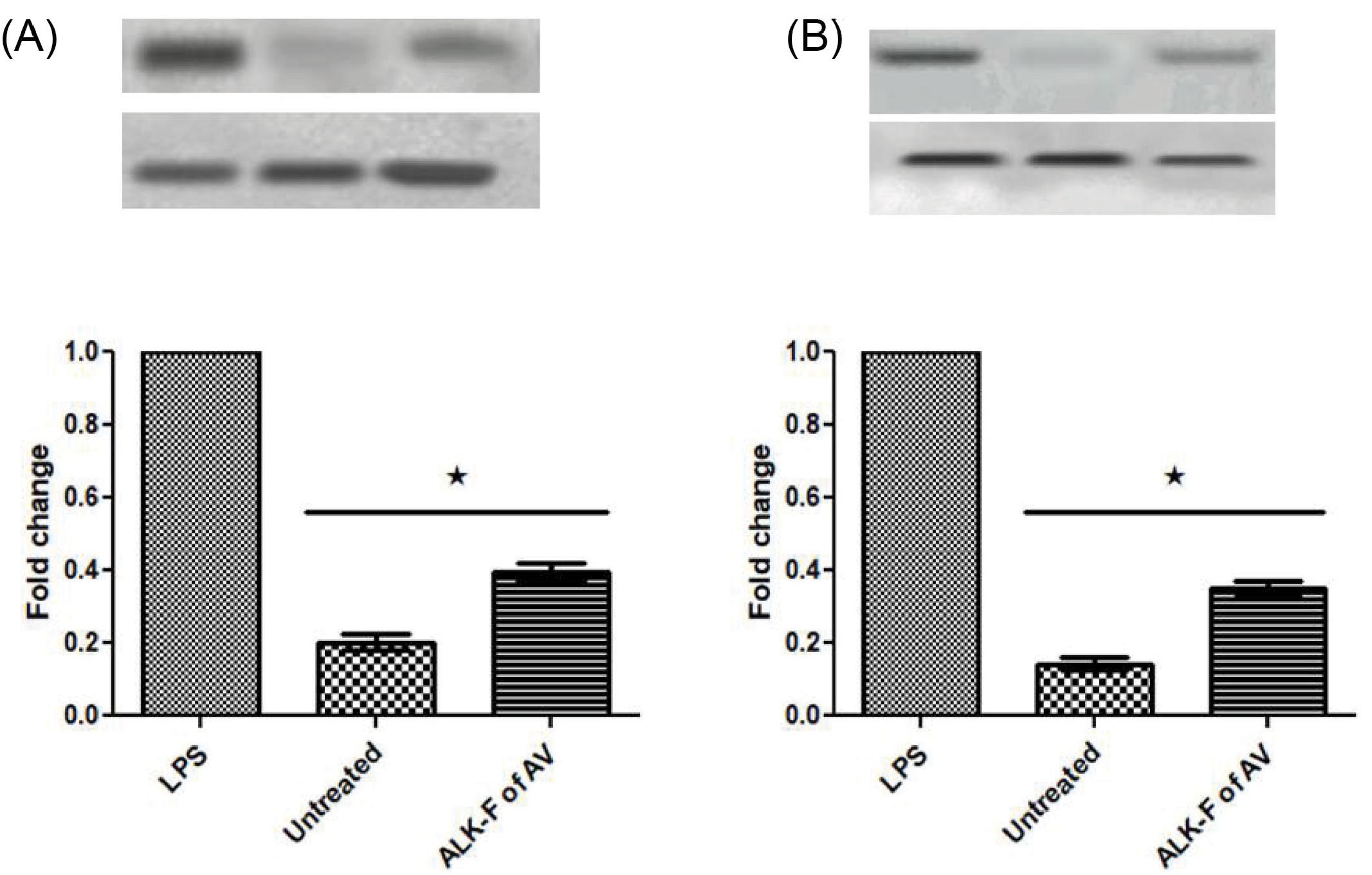
Fig. 6.
Effect of ALK-F of A. vasicaon LPS-stimulated iNOS and COX-2 protein expression in RAW 264.7 macrophages. The cells were treated with 1μg of LPS only or with the indicated concentrations of ALK-F of A. vasicafor 24 hours and the whole protein was isolated. β-actin was used as an internal control for normalizing the protein expression. The Blot was analyzed using BIO-RAD Chemi-Doc system. (A) Densitometric analysis of iNOS and (B) COX-2 upon treatment with ALK-F of A. vasica. Data were expressed as mean ± SD of three independent experiments in triplicates. * P<0.05 vs LPS treated cells.
.
Effect of ALK-F of A. vasicaon LPS-stimulated iNOS and COX-2 protein expression in RAW 264.7 macrophages. The cells were treated with 1μg of LPS only or with the indicated concentrations of ALK-F of A. vasicafor 24 hours and the whole protein was isolated. β-actin was used as an internal control for normalizing the protein expression. The Blot was analyzed using BIO-RAD Chemi-Doc system. (A) Densitometric analysis of iNOS and (B) COX-2 upon treatment with ALK-F of A. vasica. Data were expressed as mean ± SD of three independent experiments in triplicates. * P<0.05 vs LPS treated cells.
HPLC analysis of ALK-F of A. vasica
In context with the chromatographic conditions mentioned above the retention time of standard vasicine was around 4 min. HPLC analysis of ALK-F of A. vasica revealed the presence of vasicine (12%) as a major fraction which was in agreement with the values reported for the standard (Figs. 7A and 7B).
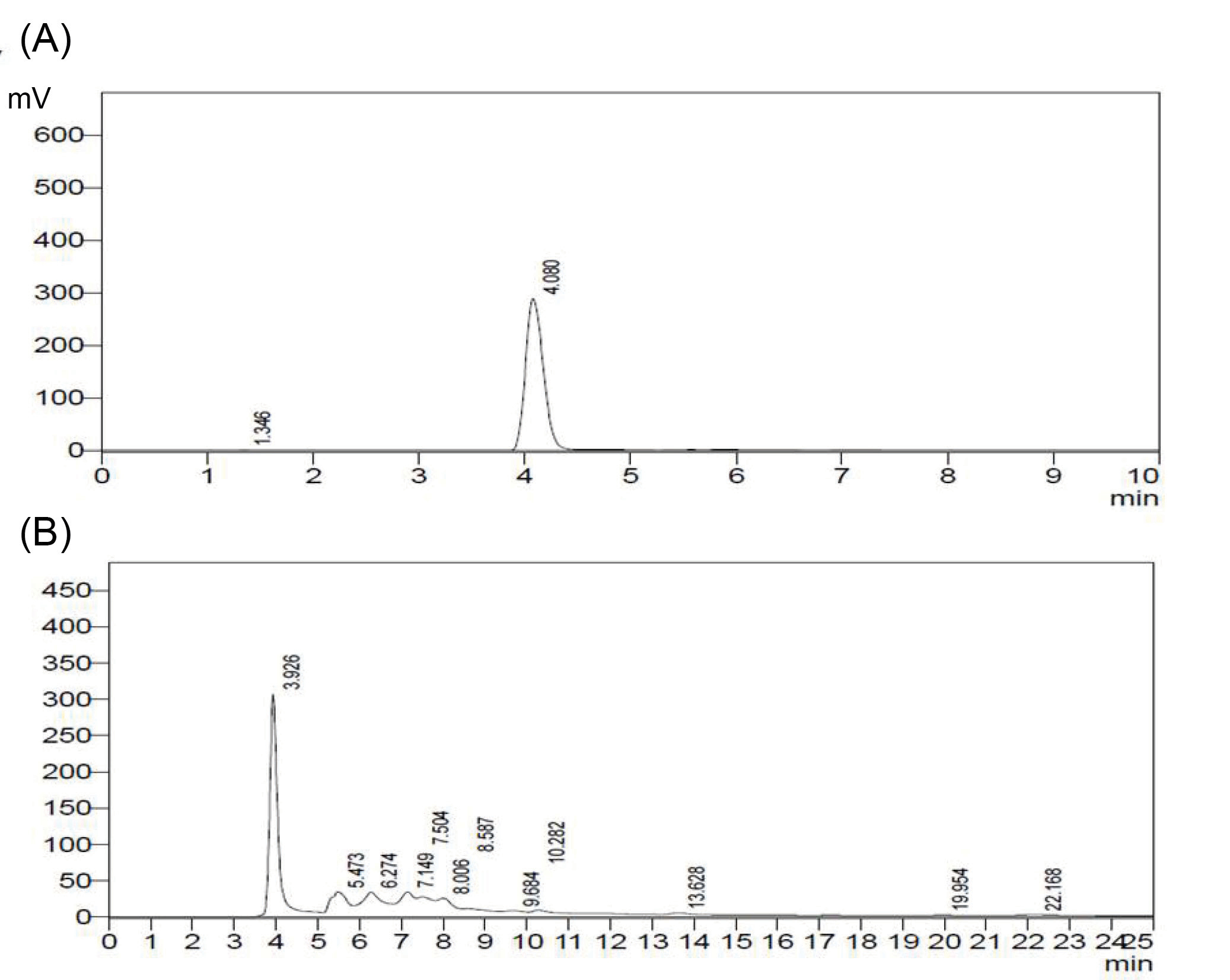
Fig. 7.
HPLC analysis of ALK-F of A. vasica. Detection performed using a diode array detector (DAD) at 280 nm. (A) Chromatograph of standard vasicine eluted at 4 min; (B) Chromatograph of ALK-F of A. vasica eluted at 3.92 min.
.
HPLC analysis of ALK-F of A. vasica. Detection performed using a diode array detector (DAD) at 280 nm. (A) Chromatograph of standard vasicine eluted at 4 min; (B) Chromatograph of ALK-F of A. vasica eluted at 3.92 min.
Discussion
The cell viability was assessed using MTT reduction by mitochondrial dehydrogenases, which could be possible only by metabolically active cells. It was found that there was no significant difference in the ALK-F treated groups in comparison with the control, which proved the non-toxic effect imposed by ALK-F of A. vasica even at the highest concentration tested.
A potent stimulus like LPS, interacting with TLR4, can lead to the production of NO, which can participate in the signaling cascade leading to acute and chronic inflammatory imbalances. Therefore drugs that decrease the expression of NO by gene regulation of iNOS have therapeutic importance in various inflammatory disorders including diabetic wound and Alzheimer’s disease. The ALK-F of A. vasica significantly inhibited NO production in LPS stimulated macrophages in a dose-dependent manner. Moreover, the inhibitory activity of this alkaloid fraction with LPS activation of macrophages was found to be associated with the down-regulation of iNOS expression.
31
A similar effect was found with COX-2, an inducible enzyme that contributes to a cascade of inflammatory responses and catalysis of PGs. Our results proved that ALK-F of A. vasica can inhibit NO production, which could be possibly due to the suppression in the expression of iNOS which is useful for the conversion of arginine to NO, apparently down-regulating COX-2 through NO suppression.
The antioxidant activity of ALK-F of A. vasicawas assessed by a cell-based assay, using an intracellular binding probe called DCFH-DA. When this non-fluorescent dye is taken inside the cells, the diacetate moiety is hydrolyzed by the cellular esterase which produces an oxidized fluorescent product called DCF. NO rapidly reacts with superoxide anion (O2•−) to form peroxynitrite (ONOO−), a highly potent oxidant molecule that diffuses across phospholipid membranes, resulting in substrate nitrosylation. ALK-F of A. vasicawas found to exert a strong inhibition in ROS generation thereby preventing the coupling with NO and the underlying inflammatory response.
TNF-α and IL-6 induced by LPS in RAW 264.7 macrophages are pro-inflammatory and play a key role in the systemic inflammatory cascade. Based on the results the pharmacological suppression of these cytokines is needed for the prevention of septic shock and inflammation. TNF-α and IL-6 itself being an inducer for iNOS, needs to be regulated in order to obstinate the sequential response in the cascade of expression of pro-inflammatory mediators leading to inflammation. Our data collectively prove that ALK-F of A. vasicawas found to inhibit TNF-α and IL-6 in a dose-dependent manner in LPS induced RAW 264.7 macrophages.
HPLC analysis evidenced that ALK-F of A. vasica mainly comprised of quinazoline alkaloid vasicine.
32-34
Significant levels of this compound in the ALK-F furnish further approaches for clinical utility and therapeutics. The results revealed the sequential suppression efficacy of more than one inflammatory cytokine by ALK-F of A. vasica. Therefore, the presence of vasicine in ALK-F of A. vasica has proven for its modulatory role in the regulation of inflammatory mediators using the LPS-induced model, which proves it to be a potential therapeutic agent for various inflammatory mediated diseases.
Conclusion
HPLC results revealed the presence of Pyrroloquinazoline alkaloid vasicine in ALK-F of A. vasica, which was found to exert anti-inflammatory activity by the inhibition of NO production, and downregulation of mRNA and protein expression of pro-inflammatory cytokines, namely, iNOS and COX-2. The results also revealed that the production of more than one inflammatory cytokine can be suppressed by ALK-F of A. vasica. Therefore, the presence of vasicine in ALK-F of A. vasica does a regulatory function in inflammatory mediators using the LPS-induced model. This implies that vasicine in ALK-F of A. vasica acts as a potential therapeutic agent for various inflammatory mediated diseases. Thus, it can be concluded that ALK-F of A. vasica does a potential anti-inflammatory activity that need to be further analyzed for future approaches in clinical therapeutics.
Acknowledgments
We thank Department of Biotechnology, SRMIST for providing the chemicals and other consumables for the study. We also convey our heartfelt gratitude to the Biotechnology students who worked in the animal cell and tissue culture lab for assisting us in this research work.
Funding sources
This research did not receive any grant from funding agencies.
Ethical statement
Not applicable.
Competing interests
No potential conflict of interests.
Authors’ contribution
AR has contributed towards conducting experiments, draft preparation, study consultation, writing, data handling. SS has contributed towards consolidating study materials, experiment design, study validation, supervision, and data analysis.
Research Highlights
What is the current knowledge?
simple
-
√ The crude extract of Adhatodavasica is used as an in vivomodel for carrageenan-induced inflammation studies.
What is new here?
simple
-
√ Isolation of alkaloid fraction from Adhatodavasicacontaining vasicine as the main constituent.
-
√ Deciphering the regulation of key pro-inflammatory mediators using alkaloid fraction in in vitro.
References
- Kim SF. The role of nitric oxide in prostaglandin biology; update. Nitric Oxide 2011; 25:255-64. doi: 10.1016/j.niox.2011.07.002 [Crossref] [ Google Scholar]
- Gaekwad J, Zhang Y, Zhang W, Reeves J, Wolfert MA, Boons GJ. Differential induction of innate immune responses by synthetic lipid a derivatives. J Biol Chem 2010; 285:29375-86. doi: 10.1074/jbc.M110.115204 [Crossref] [ Google Scholar]
- Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 2012; 33:829-37. doi: 10.1093/eurheartj/ehr304 [Crossref] [ Google Scholar]
- Silva MT. Macrophage phagocytosis of neutrophils at inflammatory/infectious foci: a cooperative mechanism in the control of infection and infectious inflammation. J Leukoc Biol 2011; 89:675-83. doi: 10.1189/jlb.0910536 [Crossref] [ Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol 2011; 31:986-1000. doi: 10.1161/ATVBAHA.110.207449 [Crossref] [ Google Scholar]
- Furse KE, Pratt DA, Porter NA, Lybrand TP. Molecular dynamics simulations of arachidonic acid complexes with COX-1 and COX-2: insights into equilibrium behavior. Biochemistry 2006; 45:3189-205. [ Google Scholar]
- Chen L, Teng H, Fang T, Xiao J. Agrimonolide from Agrimoniapilosa suppresses inflammatory responses through down-regulation of COX-2/iNOS and inactivation of NF-κB in lipopolysaccharide-stimulated macrophages. Phytomedicine 2016; 23:846-55. doi: 10.1016/j.phymed.2016.03.016 [Crossref] [ Google Scholar]
- Adhikary R, Majhi A, Mahanti S, Bishayi B. Protective effects of methanolic extract of AdhatodavasicaNees leaf in collagen-induced arthritis by modulation of synovial toll-like receptor-2 expression and release of pro-inflammatory mediators. J Nutr Intermed Metab 2016; 3:1-11. doi: 10.1016/j.jnim.2015.11.001 [Crossref] [ Google Scholar]
- Kuo YC, Lai CS, Wang JM, Badmaev V, Nagabhushanam K. Differential inhibitory effects of inotilone on inflammatory mediators, inducible nitric oxide synthase and cyclooxygenase-2, in LPS-stimulated murine macrophage. Mol Nutr Food Res 2009; 53:1386-95. doi: 10.1002/mnfr.200800583 [Crossref] [ Google Scholar]
- Reddy A, Sundaresan S. Phytochemical analysis and suppression of inflammatory targets by Adathoda vasica. Asian J Pharm Clin Res 2018; 11:162-6. doi: 10.22159/ajpcr.2018.v11i5.24243 [Crossref] [ Google Scholar]
- Bhatti HN, Khan SS, Khan A, Rani M, Ahmad VU, Choudhary MI. Biotransformation of monoterpenoids and their antimicrobial activities. Phytomedicine 2014; 21:1597-626. doi: 10.1016/j.phymed.2014.05.011 [Crossref] [ Google Scholar]
- Latha M, Priyanka M, Rajasekar P, Manikandan R, Prabhu NM. Biocompatibility and antibacterial activity of the AdathodavasicaLinn extract mediated silver nanoparticles. MicrobPathog 2016; 93:88-94. doi: 10.1016/j.micpath.2016.01.013 [Crossref] [ Google Scholar]
- Mulla WA, More SD, Jamge SB, Pawar AM, Kazi MS, Varde MR. Evaluation of antiinflammatory and analgesic activities of ethanolic extract of roots Adhatodavasica Linn. Int J Pharm Tech Res 2010; 2:1364-8. doi: 10.1155/2016/8247014 [Crossref] [ Google Scholar]
- Rayees S, Mabalirajan U, Bhat WW, Rasool S, Rather RA. Therapeutic effects of R8, a semi-synthetic analogue of Vasicine, on murine model of allergic airway inflammation via STAT6 inhibition. Int Immunopharmacol 2015; 26:246-56. doi: 10.1016/j.intimp.2015.03.035 [Crossref] [ Google Scholar]
- Vijayanandraj S, Brinda R, Kannan K, Adhithya R, Vinothini S, Senthil K. Detoxification of aflatoxin B1 by an aqueous extract from leaves of AdhatodavasicaNees. Microbiol Res 2014; 169:294-300. doi: 10.1016/j.micres.2013.07.008 [Crossref] [ Google Scholar]
- Nemudzivhadi V, Masoko P. In vitro assessment of cytotoxicity, antioxidant, and anti-inflammatory activities of Ricinus communis(Euphorbiaceae) leaf extracts. Evid Based Complementary Altern Med 2014; 2014:625961. doi: 10.1155/2014/625961 [Crossref] [ Google Scholar]
- Das C, Poi R, Chowdhury A. HPTLC determination of vasicine and vasicinone in Adhatodavasica. Phytochem Anal 2005; 16:90-2. doi: 10.1002/pca.817 [Crossref] [ Google Scholar]
- Oskoueian E, Abdullah N, Saad WZ, Omar AR, Ahmad S, Kuan WB. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Jatropha curcas Linn. J Med Plants Res 2011; 5:49-57. [ Google Scholar]
- Verma N, Tripathi SK, Sahu D, Das HR, Das RH. Evaluation of inhibitory activities of plant extracts on production of LPS-stimulated pro-inflammatory mediators in J774 murine macrophages. Mol Cell Biochem 2010; 336:127-35. doi: 10.1007/s11010-009-0263-6 [Crossref] [ Google Scholar]
- Pa R, Mathew L. Antimicrobial activity of leaf extracts of Justicia adhatoda L in comparison with vasicine. Asian Pac J Trop Biomed 2012; 2:S1556-60. doi: 10.1016/S2221-1691(12)60452-3 [Crossref] [ Google Scholar]
- Prasannabalaji N, Muralitharan G, Sivanandan RN, Kumaran S, Pugazhvendan SR. Antibacterial activities of some Indian traditional plant extracts. Asian Pac J Trop Dis 2012; 2:S291-5. doi: 10.1016/S2222-1808(12)60168-6 [Crossref] [ Google Scholar]
- Roja G, Vikrant BH, Sandur SK, Sharma A, Pushpa KK. Accumulation of vasicine and vasicinone in tissue cultures of Adhatodavasica and evaluation of the free radical-scavenging activities of the various crude extracts. Food Chem 2011; 126:1033-8. doi: 10.1016/j.foodchem.2010.11.115 [Crossref] [ Google Scholar]
- Wang X, Roper MG. Measurement of DCF fluorescence as a measure of reactive oxygen species in murine islets of Langerhans. Anal Methods 2014; 6:3019-24. doi: 10.1039/C4AY00288A [Crossref] [ Google Scholar]
- Kapewangolo P, Omolo JJ, Bruwer R, Fonteh P, Meyer D. Antioxidant and anti-inflammatory activity of Ocimumlabiatumextract and isolated labdane diterpenoid. J Inflamm 2015; 12:4. doi: 10.1186/s12950-015-0049-4 [Crossref] [ Google Scholar]
- Khursheed A, Devender P, Ansari SH. Phytochemical and pharmacological investigations on Adhatodazeylanica(medic): A review. Pharmacogn J 2010; 2:513-9. doi: 10.1016/S0975-3575(10)80041-0 [Crossref] [ Google Scholar]
- Hyun TK, Kim HC, Ko YJ, Kim JS. Antioxidant, α-glucosidase inhibitory and anti-inflammatory effects of aerial parts extract from Korean crowberry (Empetrum nigrum var japonicum). Saudi J Biol Sci 2016; 23:181-8. doi: 10.1016/j.sjbs.2015.02.008 [Crossref] [ Google Scholar]
- Venkatayappa G, Reddy A. Free radical quenching efficacy of various extracts of Costuspictus to combat oxidative damage/stress: an in vitro study. Asian J Pharm Clin Res 2017; 10:178-81. doi: 10.22159/ajpcr.2017.v10i2.15011 [Crossref] [ Google Scholar]
- Sujatha S, Anand S, Sangeetha KN, Shilpa K, Lakshmi J, Balakrishnan A. Biological evaluation of (3β)-STIGMAST-5-EN-3-OL as potent anti-diabetic agent in regulating glucose transport using in vitro model. Int J Diabetes Mellit 2010; 2:101-9. doi: 10.1016/j.ijdm.2009.12.013 [Crossref] [ Google Scholar]
- Boakye-Gyasi E, Woode E, Ainooson GK, Obiri DD, Ansah C, Duwejua M. Anti-Inflammatory and antipyretic effects of an ethanolic extract of Palisotahirsuta K Schum roots. Afr J Pharm 2008; 2:191-9. doi: 10.5897/AJPP.9000033 [Crossref] [ Google Scholar]
- Yadav AK, Tangpu V. Anticestodal activity of Adhatodavasicaextract against Hymenolepisdiminuta infections in rats. J Ethnopharmacol 2008; 119:322-4. doi: 10.1016/j.jep.2008.07.012 [Crossref] [ Google Scholar]
- Bhattacharyya D, Pandit S, Jana U, Sen S, Sur TK. Hepatoprotective activity of Adhatodavasica aqueous leaf extract on D-galactosamine-induced liver damage in rats. Fitoterapia 2005; 76:223-5. doi: 10.1016/j.fitote.2004.10.014 [Crossref] [ Google Scholar]
- Ala AA, Olotu BB, Ohia CM. Assessment of cytotoxicity of leaf extracts of Andrographis paniculataand Aspiliaafricanaon murine cells in vitro. Arch Basic Appl Med 2018; 6:61-5. [ Google Scholar]
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018; 9:7204-18. doi: 10.18632/oncotarget.23208 [Crossref] [ Google Scholar]
- Trendowski M, Yu G, Wong V, Acquafondata C, Christen T, Fondy TP. The real deal: using cytochalasin B in sonodynamic therapy to preferentially damage leukemia cells. Anticancer Res 2014; 34:2195-202. doi: 10.1016/S1387-2656(05)11004-7 [Crossref] [ Google Scholar]