Bioimpacts. 11(3):181-185.
doi: 10.34172/bi.2021.26
Original Research
New description of vagal nerve commanted intrapancreatic taste buds and blood glucose level: An experimental analysis
Mehmet Dumlu Aydin 1, *  , Aybike Aydin 2, Ozgur Caglar 3
, Aybike Aydin 2, Ozgur Caglar 3  , Muhammed Enes Aydin 4
, Muhammed Enes Aydin 4  , Erdem Karadeniz 5
, Erdem Karadeniz 5  , Kemal Alp Nalci 6, Rabia Demirtas 7
, Kemal Alp Nalci 6, Rabia Demirtas 7
Author information:
1Department of Neurosurgery, Medical Faculty of Ataturk University, Erzurum, Turkey
2Medical Faculty of Cerrapasa, Istanbul University, Istanbul, Turkey
3Department of Pediatric Surgery, Medical Faculty of Ataturk University, Erzurum, Turkey
4Department of Anesthesiology and Reanimation, Medical Faculty of Ataturk University, Erzurum, Turkey
5Department of General Surgery, Medical Faculty of Ataturk University, Erzurum, Turkey
6Department of Pharmacology, Medical Faculty of Ataturk University, Erzurum, Turkey
7Department of Pathology, Medical Faculty of Ataturk University, Erzurum, Turkey
Abstract
Introduction:
There have been thousands of neurochemical mechanism about blood glucose level regulation, but intrapancreatic taste buds and their roles in blood glucose level has not been described. We aimed to investigate if there are taste buds cored neural networks in the pancreas, and there is any relationship between blood glucose levels.
Methods:
This examination was done on 32 chosen rats with their glucose levels. Animals are divided into owned blood glucose levels. If mean glucose levels were equal to 105 ± 10 mg/dL accepted as euglycemic (G-I; n = 14), 142 ± 18 mg/dL values accepted as hyperglycemic (G-II; n = 9) and 89 ± 9 mg/dL accepted as hypoglycemic (G-III; n = 9). After the experiment, animals were sacrificed under general anesthesia. Their pancreatic tissues were examined histological methods and numbers of newly described taste bud networks analyzed by Stereological methods. Results compared with Mann-Whitney U test P < 0.005 considered as significant.
Results:
The mean normal blood glucose level (mg/dL) and taste bud network densities of per cm3 were: 105 ± 10 mg/dL; 156±21 in G-I; 142 ± 18 mg/dL and 95 ± 14 in G-II and 89 ± 9 mg/dL and 232 ± 34 in G-III. P values as follows: P < 0.001 of G-II/G-I; P < 0.005 of G-III/G-I and P < 0.0001 of G-III/G-II. We detected periarterial located taste buds like cell clusters and peripherally located ganglia connected with Langerhans cells via thin nerve fibers. There was an inverse relationship between the number of taste buds networks and blood glucose level.
Conclusion:
Newly described intrapancreatic taste buds may have an important role in the regulation of blood glucose level.
Keywords: Taste buds, Pancreas, Blood glucose level
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Taste buds are well defined in the tongue, urethra, but not in the pancreas. Blood glucose level should be regulated via the intrapancreatic glucose-sensing neural web, which stimulated vagal fibers linked with that web. Because increased glucose level is a primary stimulator of glucose regulating vagopancreatic complex.
1
All taste information carry with facial, glossopharyngeal, vagal nerves are required for nutrition,
2
orgasmic taste sensations conveyed with pudendal nerves required for propagation,
3
mammarian taste buds are essential for lactation to baby nutrition
4
and all of them are necessary to avoid toxic materials. All taste information reaches the solitary nucleus, limbic forebrain,
5
reticular formations
6
insular cortex,
7
and endocrine organs to organize all metabolic cascades. Vagal nerve stimulation (VNS) via higher glucose levels increases pancreatic islet blood flow and insulin secretion.
8
An intrapancreatic ganglia-islet cell linked dense neural plexus forming vagal nerve
9
should include newly described glucose-sensing taste bud-like structures of the pancreas by ourselves. Probably intrapancreatic unmyelinated ganglion neurons around to blood vessels and islet cells
10,11
could innervate that glucose-sensing taste buds of the pancreas to secrete insulin. Although taste buds receptors
12
and glucose regulating neural mechanism has been described,
13
taste buds-like structures have not been defined in the pancreas. We examined if there is a link between blood glucose level and taste bud like structures in pancreas.
Materials and Methods
Animals
This examination was studied on 32 rats which were chosen according to blood glucose levels. Blood glucose levels were measured twice a day/consecutive two days. If blood glucose levels were 105 ± 10 mg/dL accepted as euglycemic (G-I; n = 14), 142 ± 18 mg/dL accepted as hyperglycemic (G-II; n = 9) and 89 ± 9 mg/dLhypoglycemic (G-III; n = 9). At the end of the experiment, all rats were sacrificed with general anesthesia following intracardiac formalin injection. Their pancreatic tissues were examined by Stereological methods. The numbers of pancreatic taste buds cored neural network and blood glucose levels were compared with the Mann-Whitney U test.
Tissue preparation
The pancreatic tissues with vagal and sympathetic nerves were gently removed and fixed in 10% formalin solution. The samples were embedded in the paraffin block and sectioned via Leica RM2125RT microtome (Leica Microsystems, Wetzlar, Germany). The pancreas tissues cut into 5-µm thick and consecutive 20 sections using a microtome. To determine histological architectures of the pancreatic taste buds, intrapancreatic ganglia, Langerhans cells, and connecting nerve fibers were examined. The sections were stained with H&E, aldehyde-fuchsin, S100, and gustducin and examined with a light microscope. To estimate the numbers of taste bud networks per cubic, millimeter, stereological, and Cavalieri's methods were used. Taste bud network numbers and glucose levels were analyzed statistically and P< 0.005 accepted as meaningful.
Results
Taste buds descriptions in pancreas
We detected taste buds-like structures arranged around pancreatic periarteriolar spaces similar to pine slices. Each taste bud of the pancreas opened in periarteriolar soft tissue compartments with their orifices within the microvilli of the adventitial surface. Taste bud cells have many apical microvilli, a dominant central nucleus in a dense cytoplasmic extension covered by epithelial cells connected with nerve fibers in the basal lamina. A pancreatic taste bud consisted of 5-7 spindle-shaped, modified epithelial cells. Each taste bud has a small orifice on the surface of the arteriolar regions. The base of the taste bud was surrounded by many thin myelinated nerve fibers. The basolateral membrane of taste cells formed a neural connection with many nerve fibers. The nerve fibers entered the base of the taste bud and probably transmit taste information to the brain. Some extensions of small nerve fibers arising from intrapancreatic ganglia ended in inside or bottom of the taste buds and the other extensions connected with Langerhans cells and named as taste buds cored network.
Microphotographic documentations as follows:histological view of the pancreas with pancreatic artery (PA), pancreas innervating vagal nerve branches, Langerhans cells, and thin brown colored nerve fibers (Fig. 1). Histological appearances of taste bud-like structures in the pancreas, and description of taste bud-like (TB) with a specific immunohistochemical method summarized in Fig. 2. Intrapancreatic ganglia, their neural extensions, and taste bud-like structures are seen in Fig. 3. Fig. 4 shows a histological view of the pancreas, pancreatic arteries, and appearances of taste bud-like structures around PA, Langerhans cells. Intrapancreatic taste bud/ganglia/Langerhans cells network, the discriminative appearances of periarterial located taste bud just near of intrapancreatic ganglia in a magnified form, and, TB/PG/L centers connecting nerve fibers are seen in Fig. 5.
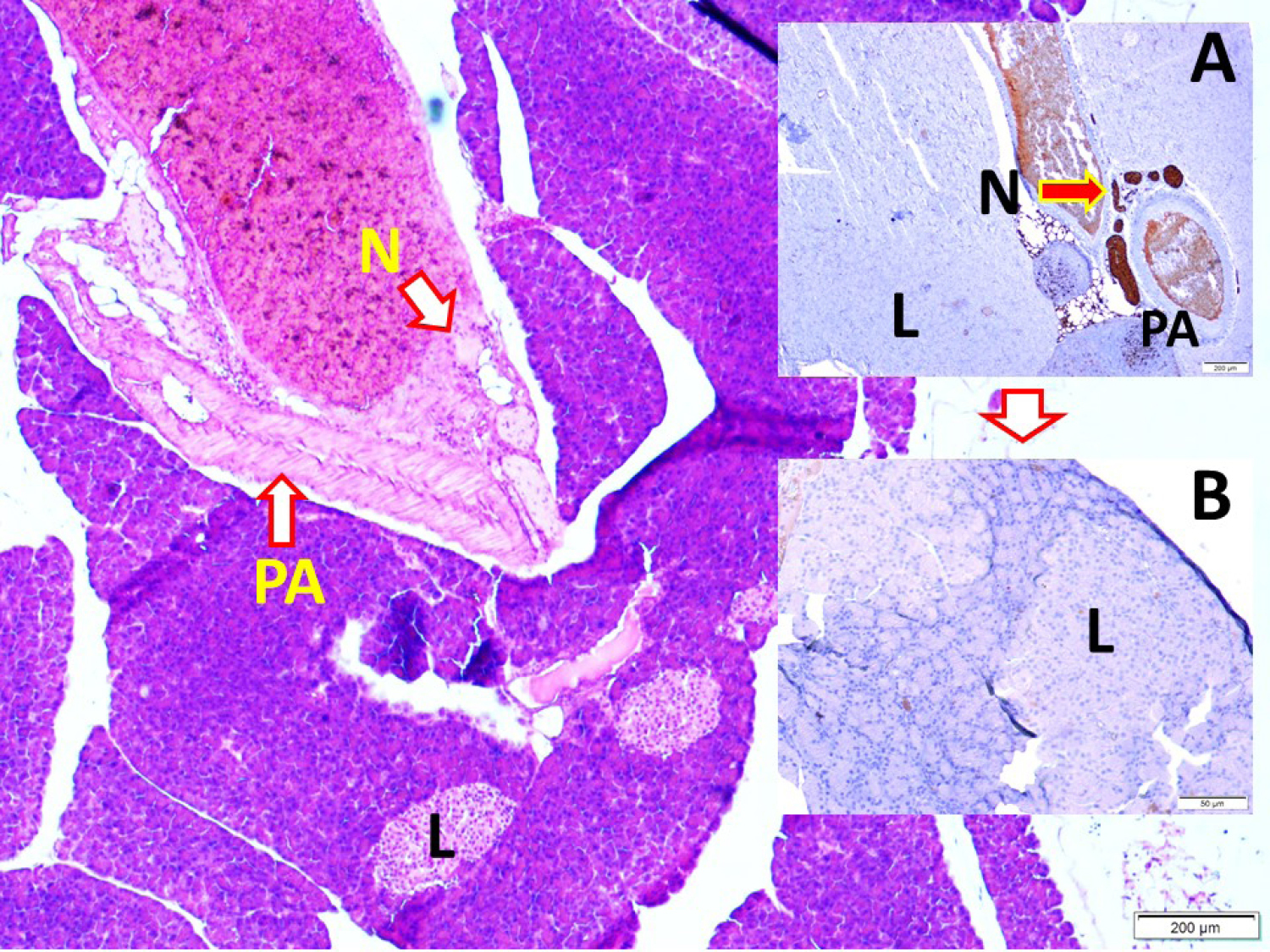
Fig. 1.
Histological view of the pancreas with the pancreatic artery (PA), pancreas innervating vagal nerve branches (N), Langerhans cells (L), (LM, H&E, x4/Base; LM, S100, x4/A) and magnified form of Langerhans cells and thin brown colored nerve fibers (LM, S100, x20/B).
.
Histological view of the pancreas with the pancreatic artery (PA), pancreas innervating vagal nerve branches (N), Langerhans cells (L), (LM, H&E, x4/Base; LM, S100, x4/A) and magnified form of Langerhans cells and thin brown colored nerve fibers (LM, S100, x20/B).
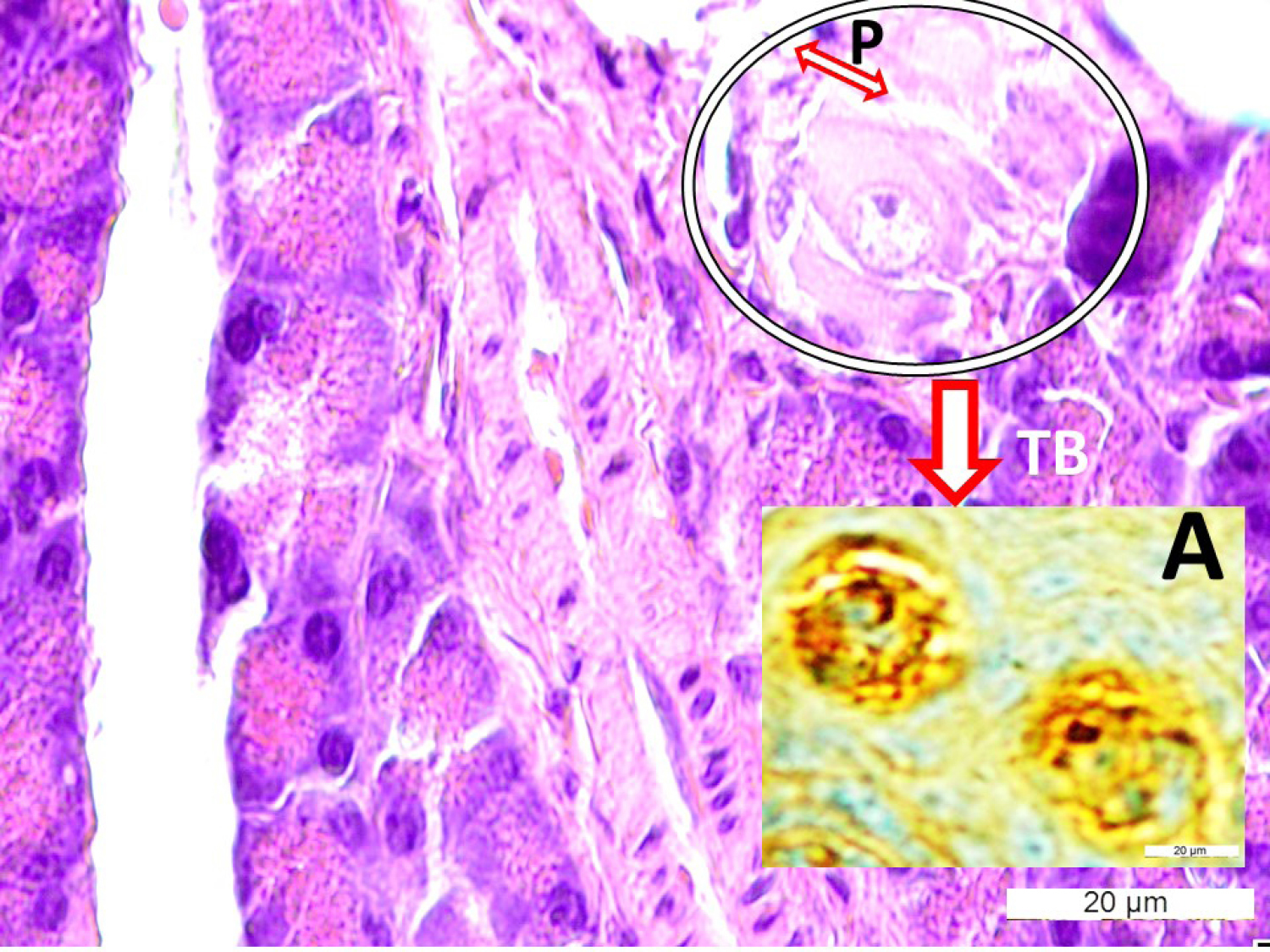
Fig. 2.
Histological appearances of taste bud-like structures (TB in the circle) with consisting cells and apical pores (P) in the pancreas, (LM, H&E, x40/Base) and description of TB with specific immunohistochemical method (LM, Gustducine x40/A).
.
Histological appearances of taste bud-like structures (TB in the circle) with consisting cells and apical pores (P) in the pancreas, (LM, H&E, x40/Base) and description of TB with specific immunohistochemical method (LM, Gustducine x40/A).
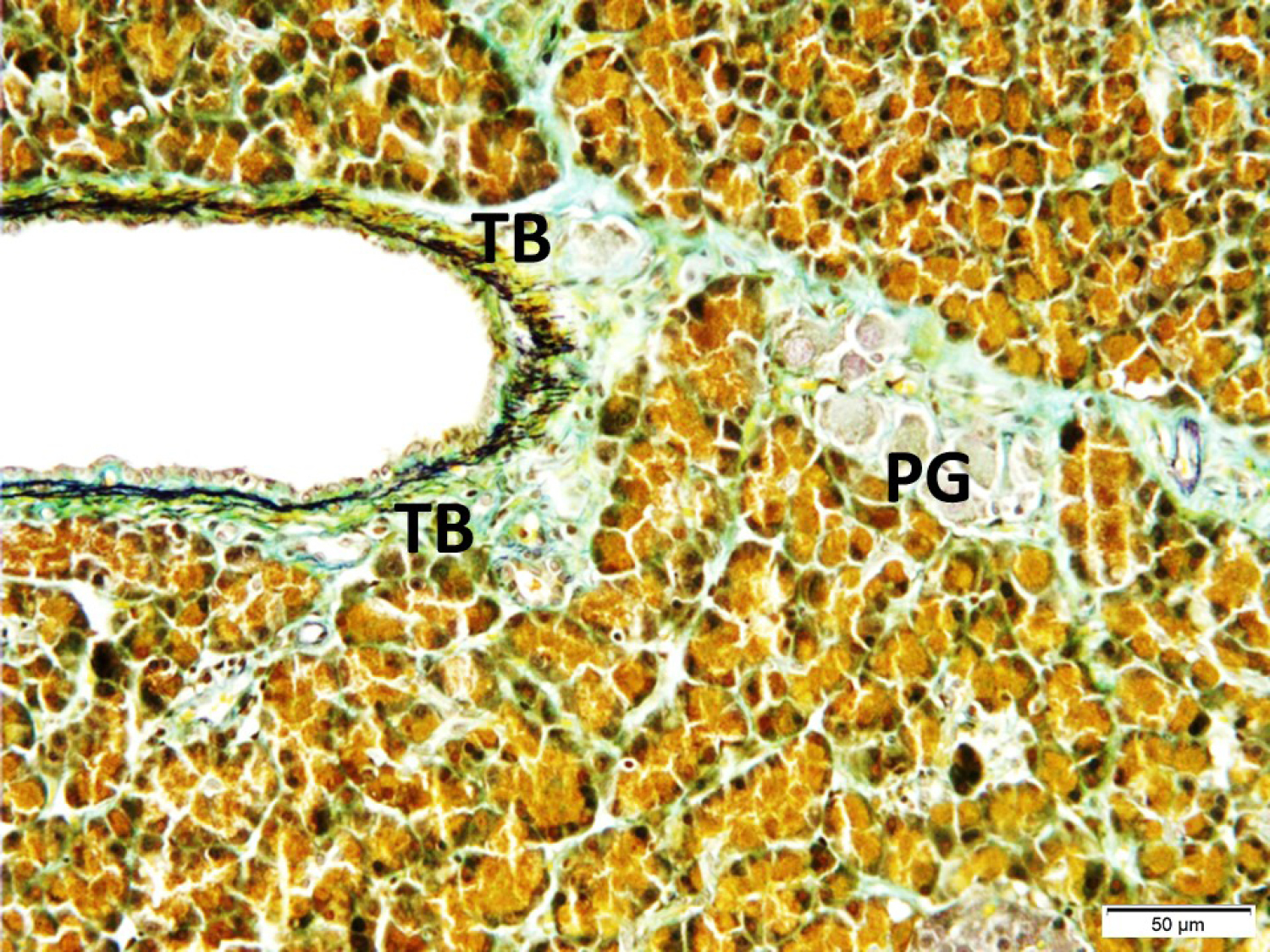
Fig. 3.
Intrapancreatic ganglia (PG), their neural extensions, and taste bud-like structures (TB) are seen (LM, Aldehyde-fuchsin, x20).
.
Intrapancreatic ganglia (PG), their neural extensions, and taste bud-like structures (TB) are seen (LM, Aldehyde-fuchsin, x20).
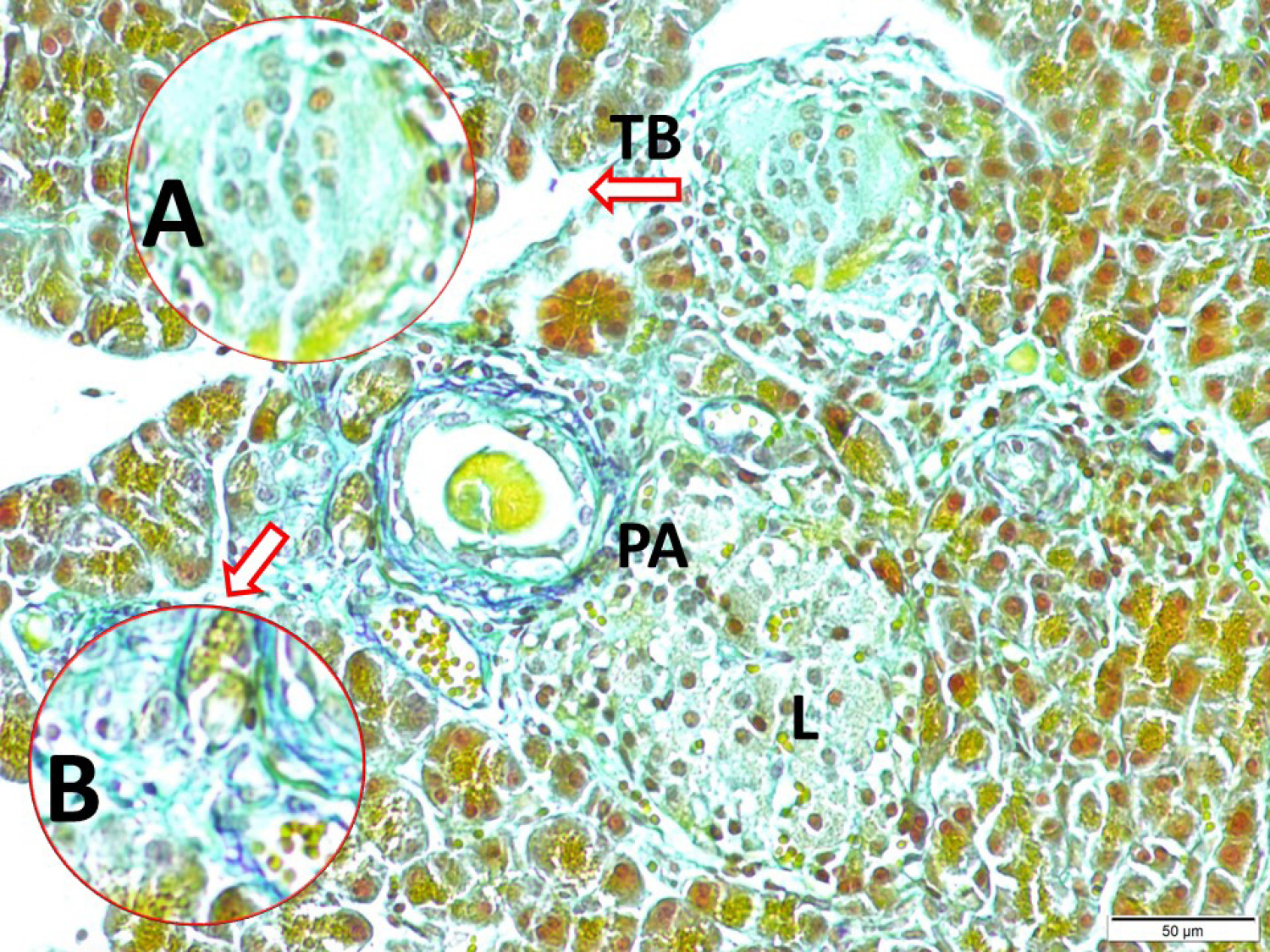
Fig. 4.
Histological view of the pancreas, pancreatic arteries (PA), and appearances of taste bud-like structures (arrows-A/B) around PA, Langerhans (L) cells (LM, Aldehyde-fuchsin x20).
.
Histological view of the pancreas, pancreatic arteries (PA), and appearances of taste bud-like structures (arrows-A/B) around PA, Langerhans (L) cells (LM, Aldehyde-fuchsin x20).
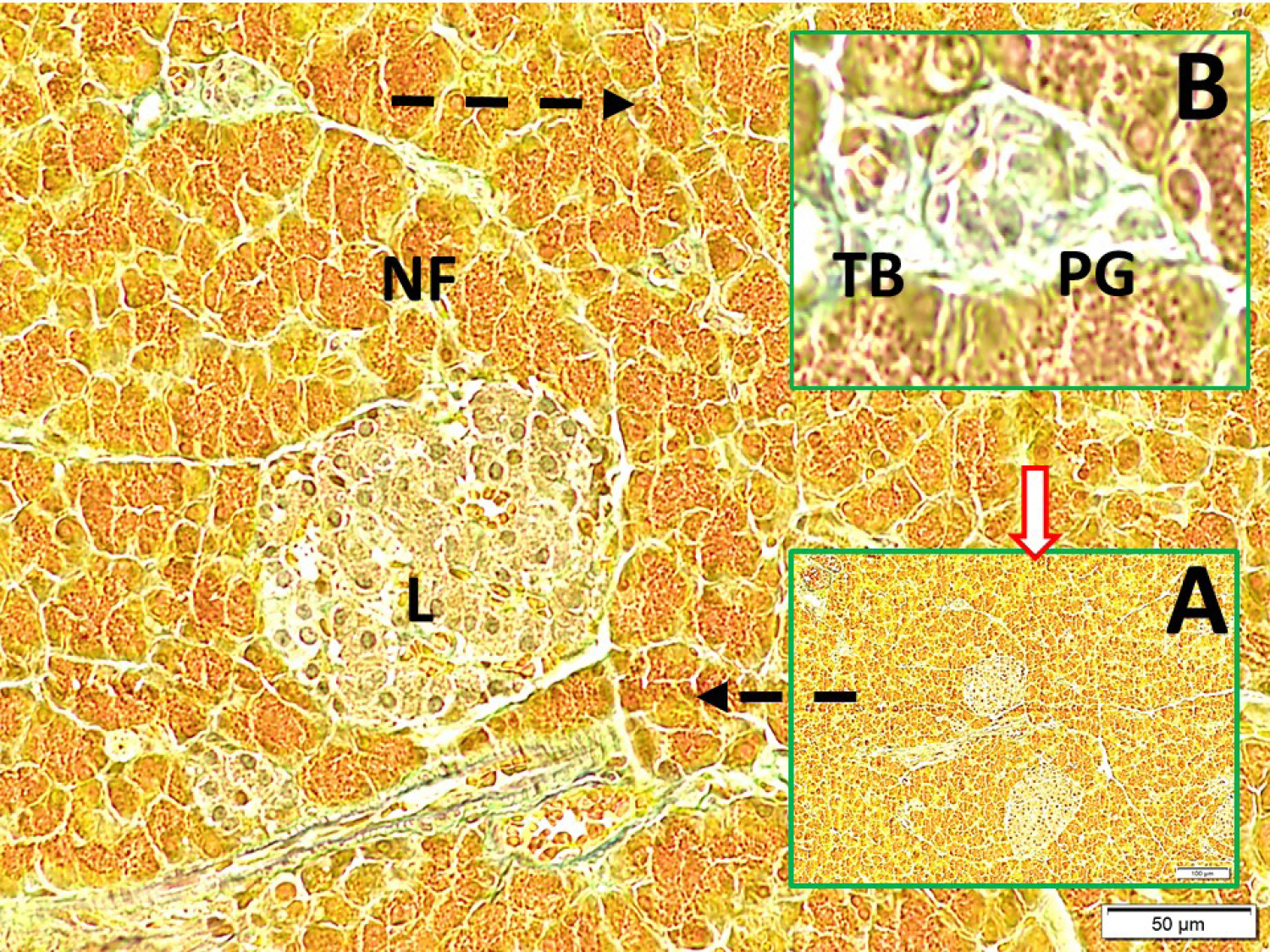
Fig. 5.
Glucose level regulating intrapancreatic taste bud/ganglia/Langerhans cells network (LM, Aldehyde-fuchsin, x10/A), the discriminative appearances of a periarterial located taste bud (TB) just near of intrapancreatic ganglia (PG) in a magnified form (LM, Aldehyde-fuchsin, x40/B); and, TB/PG/L centers connecting nerve fibers are seen (LM, Aldehyde-fuchsin, x20/Base).
.
Glucose level regulating intrapancreatic taste bud/ganglia/Langerhans cells network (LM, Aldehyde-fuchsin, x10/A), the discriminative appearances of a periarterial located taste bud (TB) just near of intrapancreatic ganglia (PG) in a magnified form (LM, Aldehyde-fuchsin, x40/B); and, TB/PG/L centers connecting nerve fibers are seen (LM, Aldehyde-fuchsin, x20/Base).
Numerical results of the experiment
The mean normal blood glucose level and taste bud densities per cm3 of control animals were: 105 ± 10 mg/dL; 156 ± 21 in G-I; 142 ± 18 mg/dL and 95 ± 14 in G-II and 89 ± 9 mg/dL and 232 ± 34 in G-III. P values between that groups: P < 0.001 of G-II/G-I; P < 0.005 of G-III/G-I and P < 0.0001 of G-III/G-II. There was a linear relationship between the numbers of taste roseas and neuron density of geniculate ganglion neurons (P < 0.005). But, there was an inverse relationship between taste buds-neuron density of geniculate ganglia and blood glucose levels (P < 0.0001). The numbers of taste buds and glucose levels are summarized in Table 1.
Table 1.
Numerical values of study
|
|
Group I (n=14)
|
Group II (n=9)
|
Group III (n=9)
|
|
Taste Bud/cm3
|
156±21a
|
95±14c
|
232±34b
|
| Blood glucose mg/dL |
105±10a
|
142±18c
|
89±9 b
|
All values given mean ± standard deviation, Group I: Normal, Group II: Hyperglicemic, Group III: Hypoglicemic.
a
P < 0.001 between groups I/II; P < 0.005 between groups I/III; cP < 0.0001 between groups II/III.
Discussion
Blood glucose level and regulation are essential for life because glucose is an important energy source for animals and a primary stimulator for insulin secretion in the pancreas. Tongue taste information conveys with facial, glossopharyngeal, vagal nerves
2
; genital-mammarial taste bud information carry with pudendal nerves in animals.
3,4
All of those networks may be responsible for body glucose regulation. Our previous studies have shown that blood glucose regulating mechanisms should include mammarial and genital taste buds in addition to oral taste buds and pancreas.
The pancreas has been accepted as an essential organ and the vagal nerve as a primary nerve for glucose regulation. A combination of pancreatic innervation by nodose ganglia, vagal dorsal motor nucleus, nucleus ambiguus, T6-L2 spinal ganglia, and coeliac ganglion
14
is essential for glucose regulation.
15,16
Glucose-recognizing pancreatic paraneurons are required for insulin release.
17
All taste information of tongue, mammary gland and genitalia reach to caudal regions of the solitary tract nucleus, limbic forebrain, reticular formation,
6
and insular cortex.
7
In the brain, primarily mediodorsal prefrontal cortex,
18
insular cortex,
19
lateral hypothalamus, amygdala and globus pallidus have also glucose-sensitive and glucose level monitoring neurons
20
to manage body glucose modulation. The sympathetic nervous system also mediates catabolic responses to glucose metabolism.
21
Our previous studies and publications proved that pleasure-creating hedonic sensations originating from taste bud including organs such as tongue, mammary gland and genitalia reach to the spinal cord and mentioned higher brain centers to regulate all body-organ glucose levels.
3,4
How the pancreas feels blood sugar, which cell group firstly informs glucose regulation requirement, and how the first information is created in the pancreas has not yet been clearly defined. According to our hypotheses, there should be glucose-sensing cells, which co-working with the vagal nerve in the pancreas. Because vagal nerve determines to some extent sugar sense in tongue and probably informs pancreas to secrete insulin with facial nerves. Without a taste bud-vagal nerve complex as described in the pancreas, it will be difficult to understand the blood sugar-regulating mechanisms by the pancreas with the available information. Wherever the glucose-sensitive receptors of β-cells have been described for explaining insulin secretion
1
this theory doesn’t explain the rationale mechanism of glucose regulation. Insulin-producing cell surfaces have sweet taste receptors
22
like in the stomach, pancreas, gut, liver, and brain.
12
Intra and extrapancreatic ganglionar unmyelinated axons usually located adjacent to exocrine epithelial ducts, interlobular space, and blood vessels in the pancreas regulate glucose level.
10
That pancreatic ganglia receive visceral information from pancreatic exocrine and endocrine glands.
23
Endocrine pancreas innervated by intrapancreatic ganglia.
24
The intrapancreatic ganglia fibers and vagal fibers could innervate the taste receptors of the pancreas just as the tongue.
11
Taste sensing receptor deficiency might result in glucose absorption and metabolism disorders.
25
Bilateral vagotomy abolished insulin response to glucose.
26
Bilateral gustatory cortex lesions rely on diabetes mellitus.
27
Hyperglycemia is common after ischemic lesions of the vagus nerve nuclei.
28
The second-generation antipsychotic drugs have the efficacy to treat schizophrenia but can cause diabetes mellitus.
29
If many drugs could cause autonomic ganglia/islet cells toxication induced diabetes mellitus, the deficiency of newly described intrapancreatic taste bud may rely on diabetes mellitus.
Clinical implications and importance of the study
The vagus nerve has a key function to regulate blood glucose level and neuromodulation techniques are more likely to succeed in type II diabetes treatment.
30
VNS has been used to reduce body blood glucose levels in diabetic rats by enhancing vagal efferent activity and the release of GLP-1.
31
Afferent VNS inhibits pancreatic insulin secretion. In contrast, efferent VNS stimulates pancreatic insulin secretion in treating type II diabetes.
32
The pathological insults of the vagus nerve cause diabetes in chronic alcoholism.
33
Altered insulin sensitivity/secretion causes diabetic cardiovascular autonomic neuropathy in recent-onset type 1 and type 2 diabetes.
34
Acute vagal stimulation alters glucose and insulin metabolism but chronic vagal stimulation improves insulin sensitivity substantially in diet-induced obesity by both peripheral and central mechanisms.
35
In that circumstances, we easily hypothesized that afferent fibers of vagal nerves may sense information of glucose sensing pancreatic taste buds, and efferent fibers of vagal nerves could be stimulated by glucose-sensing intrapancreatic taste buds/vagus circuitry to secrete insulin from the pancreas.
New ones in this study
The firstly described pancreatic taste buds are similar to the tongue mammarian gland or genitalia. That differentiation may reflect the innervating nerve differentiates described by previous studies.
36-38
According to our observations, glucose molecules stimulate taste buds in the pancreas and created information carry with afferent fibers of vagal nerves to brain centers and integrated pancreatic signals probably stimulate efferent fibers of vagal nerves to secrete insulin from the pancreas.
Limitation of the study
Insulin, HbA1c, glucagon, and required biochemical or radiological studies should be done.
Conclusion
The newly described taste buds cored network of the pancreas may have functioned as a pre-determinative role on the blood glucose level regulation, and starts new hardware and software examinations to understand secret mechanisms of diabetes mellitus
Future insights
We hope that this study will open new horizons in the diagnosis and treatment of type-2 diabetes mellitus. Tissue implantation or future neural networks can help insulin-dependent patients.
Funding sources
There was no funding for this study.
Ethical statement
The study protocol and permissions were reviewed and approved by the Ethics Committee for Animal Experiments, Faculty of Medicine, Ataturk University. The animals were managed, and the experiments were conducted according to the guidelines prescribed by the Committee.
Competing interests
None.
Authors’ contribution
MDA and AA conceptualized and refined research ideas. MDA, OC, and MEA performed the literature search. EK, KAN, and RD conducted the experiments. MEA, OC, and EK performed collection and preparation of datas. MDA, OC, MEA performed the writing and editing manuscript. All authors read and approved the final manuscript.
Research Highlights
What is the current knowledge?
simple
-
√ Diabetes is a disease with a history as old as medical history. Although many in-depth studies have been conducted to enlighten its etiopathogenesis, there has not been enough focus on these taste buds located in the pancreas.
What is new here?
simple
-
√ Stimulation of nerves or ganglia responsible for pancreatic innervation by means of mini neurostimulators may shed light on the future electrophysiological treatment of diabetes, which has until now been pharmacologically treated.
References
- Kojima I, Medina J, Nakagawa Y. Role of the glucose-sensing receptor in insulin secretion. Diabetes Obes Metab 2017; 19 Suppl 1:54-62. doi: 10.1111/dom.13013 [Crossref] [ Google Scholar]
- Sweazey RD, Bradley RM. Responses of lamb nucleus of the solitary tract neurons to chemical stimulation of the epiglottis. Brain Res 1988; 439:195-210. doi: 10.1016/0006-8993(88)91476-x [Crossref] [ Google Scholar]
- Aydın MD, Aydın N, Dane Ş, Gündoğdu C, Gürsan N, Akçay F. Taste bud-like structures in penile tissues and a predictive neural mechanism of male orgasm: A preliminary hypothesis based on histological evidence. Neurology, Psychiatry and Brain Research 2014; 20:55-62. doi: 10.1016/j.npbr.2014.06.003 [Crossref] [ Google Scholar]
- Demirci T, Ozmen S, Aydin N, Aydin MD, Caglar O, Ahiskalioglu A. Toward the First Definition of Taste Rosea in Female Breasts: Histological Analysis. Int J Morphol 2020; 38:565-9. [ Google Scholar]
- Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J Auton Nerv Syst 1982; 6:303-22. [ Google Scholar]
- Hanamori T, Smith DV. Central projections of the hamster superior laryngeal nerve. Brain Res Bull 1986; 16:271-9. doi: 10.1016/0361-9230(86)90042-0 [Crossref] [ Google Scholar]
- Dalenberg JR, Hoogeveen HR, Renken RJ, Langers DR, ter Horst GJ. Functional specialization of the male insula during taste perception. Neuroimage 2015; 119:210-20. doi: 10.1016/j.neuroimage.2015.06.062 [Crossref] [ Google Scholar]
- Iwase M, Sandler S, Carlsson PO, Hellerstrom C, Jansson L. The pancreatic islets in spontaneously hypertensive rats: islet blood flow and insulin production. Eur J Endocrinol 2001; 144:169-78. [ Google Scholar]
- Sun N, Yi H, Cassell MD. Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. J Comp Neurol 1994; 340:43-64. doi: 10.1002/cne.903400105 [Crossref] [ Google Scholar]
- Bosco C, Diaz E, Gutierrez R, Gonzalez J, Perez J. Ganglionar nervous cells and telocytes in the pancreas of Octodon degus: extra and intrapancreatic ganglionar cells and telocytes in the degus. Auton Neurosci 2013; 177:224-30. doi: 10.1016/j.autneu.2013.05.002 [Crossref] [ Google Scholar]
- Zaidi FN, Whitehead MC. Discrete innervation of murine taste buds by peripheral taste neurons. J Neurosci 2006; 26:8243-53. doi: 10.1523/jneurosci.5142-05.2006 [Crossref] [ Google Scholar]
- Neiers F, Canivenc-Lavier MC, Briand L. What Does Diabetes "Taste" Like?. Curr Diab Rep 2016; 16:49. doi: 10.1007/s11892-016-0746-2 [Crossref] [ Google Scholar]
- Niijima A. An electrophysiological study on the regulatory mechanism of blood sugar level in the rabbit. Brain Res 1975; 87:195-9. doi: 10.1016/0006-8993(75)90416-3 [Crossref] [ Google Scholar]
- Sharkey KA, Williams RG. Extrinsic innervation of the rat pancreas: demonstration of vagal sensory neurones in the rat by retrograde tracing. Neurosci Lett 1983; 42:131-5. doi: 10.1016/0304-3940(83)90395-6 [Crossref] [ Google Scholar]
- Mussa BM, Verberne AJ. Activation of the dorsal vagal nucleus increases pancreatic exocrine secretion in the rat. Neurosci Lett 2008; 433:71-6. doi: 10.1016/j.neulet.2007.12.048 [Crossref] [ Google Scholar]
- Sha L, Szurszewski JH. Leptin modulates fast synaptic transmission in dog pancreatic ganglia. Neurosci Lett 1999; 263:93-6. doi: 10.1016/s0304-3940(99)00122-6 [Crossref] [ Google Scholar]
- Niki A, Niki H, Hashioka T. Receptors of paraneurons, with special reference to glucoreceptors. Arch Histol Cytol 1989; 52 Suppl:33-8. [ Google Scholar]
- Nagy B, Takacs G, Szabo I, Lenard L, Karadi Z. Taste reactivity alterations after streptozotocin microinjection into the mediodorsal prefrontal cortex. Behav Brain Res 2012; 234:228-32. doi: 10.1016/j.bbr.2012.06.029 [Crossref] [ Google Scholar]
- Aydin MD, Kanat A, Aydin N, Kantarci A, Ayvaz MA, Rakici H. New Evidence for Causal Central Mechanism of Hyperglycemia in Subarachnoid Hemorrhage Secondary to Ischemic Degenerative Disruption of Circuitry Among Insular Cortex, Nodose Ganglion, and Pancreas: Experimental Study. World Neurosurg 2017; 106:570-7. doi: 10.1016/j.wneu.2017.06.176 [Crossref] [ Google Scholar]
- Karadi Z, Faludi B, Hernadi I, Lenard L. Role of forebrain glucose-monitoring neurons in the central control of feeding: II Complex functional attributes. Neurobiology (Bp) 1995; 3:241-56. [ Google Scholar]
- Teff KL. Visceral nerves: vagal and sympathetic innervation. JPEN J Parenter Enteral Nutr 2008; 32:569-71. doi: 10.1177/0148607108321705 [Crossref] [ Google Scholar]
- Malaisse WJ. Insulin release: the receptor hypothesis. Diabetologia 2014; 57:1287-90. doi: 10.1007/s00125-014-3221-0 [Crossref] [ Google Scholar]
- Love JA. Electrical properties and synaptic potentials of rabbit pancreatic neurons. Auton Neurosci 2000; 84:68-77. doi: 10.1016/s1566-0702(00)00187-9 [Crossref] [ Google Scholar]
- Purwar RS, Petkov P, Prakash R. Innervation of the endocrine part of the pancreas of the Bulgarian rabbit as revealed by the cholinesterase technique. Folia Morphol (Praha) 1985; 33:139-42. [ Google Scholar]
- Murovets VO, Bachmanov AA, Zolotarev VA. Impaired Glucose Metabolism in Mice Lacking the Tas1r3 Taste Receptor Gene. PLoS One 2015; 10:e0130997. doi: 10.1371/journal.pone.0130997 [Crossref] [ Google Scholar]
- Carlsson PO, Iwase M, Jansson L. Intraportal glucose infusion and pancreatic islet blood flow in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 2000; 279:R1224-9. doi: 10.1152/ajpregu.2000.279.4.R1224 [Crossref] [ Google Scholar]
- Bales MB, Schier LA, Blonde GD, Spector AC. Extensive Gustatory Cortex Lesions Significantly Impair Taste Sensitivity to KCl and Quinine but Not to Sucrose in Rats. PLoS One 2015; 10:e0143419. doi: 10.1371/journal.pone.0143419 [Crossref] [ Google Scholar]
- Ruano L, Alves I, Barreto R, Araujo I, Veira C, Cruz VT. Ischemic vagus nuclei lesions and hyperglycemia: a study in 26 patients with lateral medullary infarction and matched controls. Cerebrovasc Dis 2012; 34:406-10. doi: 10.1159/000343654 [Crossref] [ Google Scholar]
- Mourad FH, Saade NE. Neural regulation of intestinal nutrient absorption. Prog Neurobiol 2011; 95:149-62. doi: 10.1016/j.pneurobio.2011.07.010 [Crossref] [ Google Scholar]
- Berthoud HR, Neuhuber WL. Vagal mechanisms as neuromodulatory targets for the treatment of metabolic disease. Ann N Y Acad Sci 2019; 1454:42-55. doi: 10.1111/nyas.14182 [Crossref] [ Google Scholar]
- Yin J, Ji F, Gharibani P, Chen JD. Vagal Nerve Stimulation for Glycemic Control in a Rodent Model of Type 2 Diabetes. Obes Surg 2019; 29:2869-77. doi: 10.1007/s11695-019-03901-9 [Crossref] [ Google Scholar]
- Meyers EE, Kronemberger A, Lira V, Rahmouni K, Stauss HM. Contrasting effects of afferent and efferent vagal nerve stimulation on insulin secretion and blood glucose regulation. Physiol Rep 2016; 4. doi: 10.14814/phy2.12718 [Crossref]
- Guo YP, McLeod JG, Baverstock J. Pathological changes in the vagus nerve in diabetes and chronic alcoholism. J Neurol Neurosurg Psychiatry 1987; 50:1449-53. doi: 10.1136/jnnp.50.11.1449 [Crossref] [ Google Scholar]
- Ziegler D, Strom A, Bönhof G, Püttgen S, Bódis K, Burkart V. Differential associations of lower cardiac vagal tone with insulin resistance and insulin secretion in recently diagnosed type 1 and type 2 diabetes. Metabolism 2018; 79:1-9. doi: 10.1016/j.metabol.2017.10.013 [Crossref] [ Google Scholar]
- Malbert CH, Picq C, Divoux JL, Henry C, Horowitz M. Obesity-Associated Alterations in Glucose Metabolism Are Reversed by Chronic Bilateral Stimulation of the Abdominal Vagus Nerve. Diabetes 2017; 66:848-57. doi: 10.2337/db16-0847 [Crossref] [ Google Scholar]
- Kinnamon JC, Sherman TA, Roper SD. Ultrastructure of mouse vallate taste buds: III Patterns of synaptic connectivity. J Comp Neurol 1988; 270:1-10, 56. doi: 10.1002/cne.902700102 [Crossref] [ Google Scholar]
- Miller RL, Chaudhry AP. Comparative ultrastructure of vallate, foliate and fungiform taste buds of golden Syrian hamster. Acta Anat (Basel) 1976; 95:75-92. doi: 10.1159/000144604 [Crossref] [ Google Scholar]
- Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol 1984; 222:560-77. doi: 10.1002/cne.902220408 [Crossref] [ Google Scholar]