Bioimpacts. 11(1):59-64.
doi: 10.34172/bi.2021.08
Original Research
Pluronic® F127-mediated control of insulin release rates from NPH microcrystals and blood glucose depression in STZ-induced diabetic rats
Muhammad H. Sultan 1  , Wael A. Mahdi 2, Young M. Kwon 3, *
, Wael A. Mahdi 2, Young M. Kwon 3, * 
Author information:
1Department of Pharmaceutics, College of Pharmacy, Jazan University, Jazan, Kingdom of Saudi Arabia
2Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, Kingdom of Saudi Arabia
3Department of Pharmaceutical Sciences, College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL, USA
Abstract
Introduction:
Neutral protamine Hagedorn (NPH) insulin is an intermediate-acting basal insulin with a long history of clinical use, consisting native human insulin. Its rather undesirable action profile, characterized by a peak release within a few hours, followed by insufficient insulin delivery upon a single subcutaneous (s.c.) dose, is well-documented. This may have been caused by the inherent microcrystal structure involving the basic peptide protamine, as well as the presence of tissue enzyme activities that readily act on protamine at the injection site. This issue may be circumvented by utilizing thermosensitive, erodible Pluronic F127 (PF127) to modulate the kinetics of insulin release from NPH over a period of 24 hours in which the hydrogel is completely eroded.
Methods:
Previously, we have shown that insulin release rates in vitro from NPH/PF127 formulations (0-25% PF127) markedly reduced the initial insulin release, especially in the presence of enzyme activity that selectively degraded protamine at 1-5 U/mL. Insulin release over the course of 20 hours was better modulated in the presence of increasing PF127 content. In this study, the insulin formulations (0, 20, and 25% PF127) were administered s.c. (4 U/kg) to streptozotocin (STZ)-induced diabetic rats and blood glucose levels were monitored over 24 hours.
Results: In vivo
blood glucose depression profiles in STZ-induced diabetic rats exhibited a similar pattern of control to in vitro data at the single s.c. dose of 4 U/kg, apparently extending the duration of action of NPH over a 24-hour period in the presence of PF127.
Conclusion:
Our findings suggest that the undesirable kinetics of insulin release from NPH is significantly influenced by tissue enzyme activity and that the presence of PF127 provided a timely modulation of insulin release from NPH microcrystals in the STZ-induced diabetic rat model.
Keywords: Basal insulin, Drug release, thermosenstive polymerThermosensitive polymer, Blood glucose
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/
). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Diabetes is recognized as a global epidemic and the challenge of the 21st century with its associated physical, emotional, and financial devastation.
1
Insulin therapy is among the major ways to manage diabetes, and in many cases, daily subcutaneous administration of basal insulin is highly desired for managing the condition,
2
as basal insulin represents 50% of the total daily insulin requirement. Therefore, appropriate basal insulin delivery is crucial in managing complications associated with diabetes.
3
Whereas neutral protamine Hagedorn (NPH) insulin has widely been used clinically for many decades as an intermediate basal insulin, there are several newer basal insulin products in the market, including insulin glargine, with more desirable basal insulin action profiles which are compared to NPH.
4
Despite the pharmacological advantages of the newer basal insulin analogs, there are findings in the literature that insulins with an altered amino acid sequence may carry the risk of unintentionally enhancing insulin-like growth factor signaling, which has been proposed as a potential cause of an increased carcinogenicity in rodents.
5
In addition, compared to NPH, insulin glargine was reported to be clinically associated with an increased risk of breast cancer for prior insulin users under the study constraint.
6
However, the long-term safety of the basal insulins with an altered amino acid sequence in man, at this point, is still controversial.
7
Nevertheless, NPH insulin still presents itself as a clinically valuable and a more economical alternative to the newer basal insulins. It also consists of the native form of human insulin with well-known clinical profile.
4
The drawback of subcutaneously administered NPH insulin is associated with its nonideal dissolution and release patterns at the injection site, which may cause hyperinsulinemia leading to the increased risk of hypoglycemia.
8,9
This undesirable insulin action profile of NPH is thought to be facilitated by the presence of tissue enzymatic or fibrinolytic activities that can easily access and break down the protamine moiety in the NPH microcrystals, leading to a relatively rapid insulin release with a peak within a few hours postdose.
10
Previously, to simulate such tissue enzyme activity relevant to insulin release from NPH, we have chosen trypsin as a model enzyme.
11
Trypsin, due to its suitable active site structure, degrades protamine rapidly.
12
On the other hand, it was reported that insulin is relatively resistant to degradation by trypsin whereas insulin digestion by chymotrypsin occurred at a high enzyme:insulin ratio.
13
Thus, trypsin can serve as a model enzyme to study in vitro dissolution of NPH microcrystal since the enzyme acts preferentially on protamine in vitro without significantly degrading insulin.Enzymatic degradation of protamine would accelerate the dissolution of NPH. Therefore, by choosing appropriate concentrations of trypsin in the release media, we were able to assimilate the in vitrorelease profile consistent with the reported NPH action profile with burst release followed by lack of release after 12-16 hours.
4
Pluronic F127 (PF127) is a well-known polymer for its thermogelling properties in aqueous medium and has been extensively studied for biomedical applications, such as drug delivery and tissue engineering.
14
Pluronic F127 has also been explored for insulin delivery applications: in combination with insulin-loaded nanoparticles,
15
buccal delivery,
16,17
methylcellulose/PF127 composites,
18
or in combination with liposomal insulin.
19
However, these systems either explored non-parental route of short-acting insulins or basal insulins involving complex fabrication processes. None of those studies examined PF127 as a potential modulator of insulin release from NPH microcrystals.
We have prepared NPH-loaded PF127 formulations under a mild mixing condition in which predetermined amount of PF127 is directly dissolved into commercial NPH insulin microcrystal suspension.
11
The in vitrorelease rates from three formulations (NPH, NPH/PF127 20% (w/w), and NPH/PF127 25% (w/w), 75 U insulin/mL) were evaluated at 37°C using 1-mL dialysis cartridges.
The in vitro release rate versus time profiles in these three formulations (NPH insulin in 0, 20, and 25% of PF127) are shown in Fig. 1.
11
As can be seen in Fig. 1A, a significant initial burst effect (~30% of loading, 75 U) was observed during the first hour even without the presence of trypsin. In the presence of 5 U/mL trypsin, the initial release rate of insulin from NPH microcrystals were further increased. In the subsequent hours (especially during 2-6 hours from commencing release), the presence of enzyme activities (both 1 and 5 U/mL) significantly enhanced the rate of insulin release compared to that without trypsin (Fig. 1A).
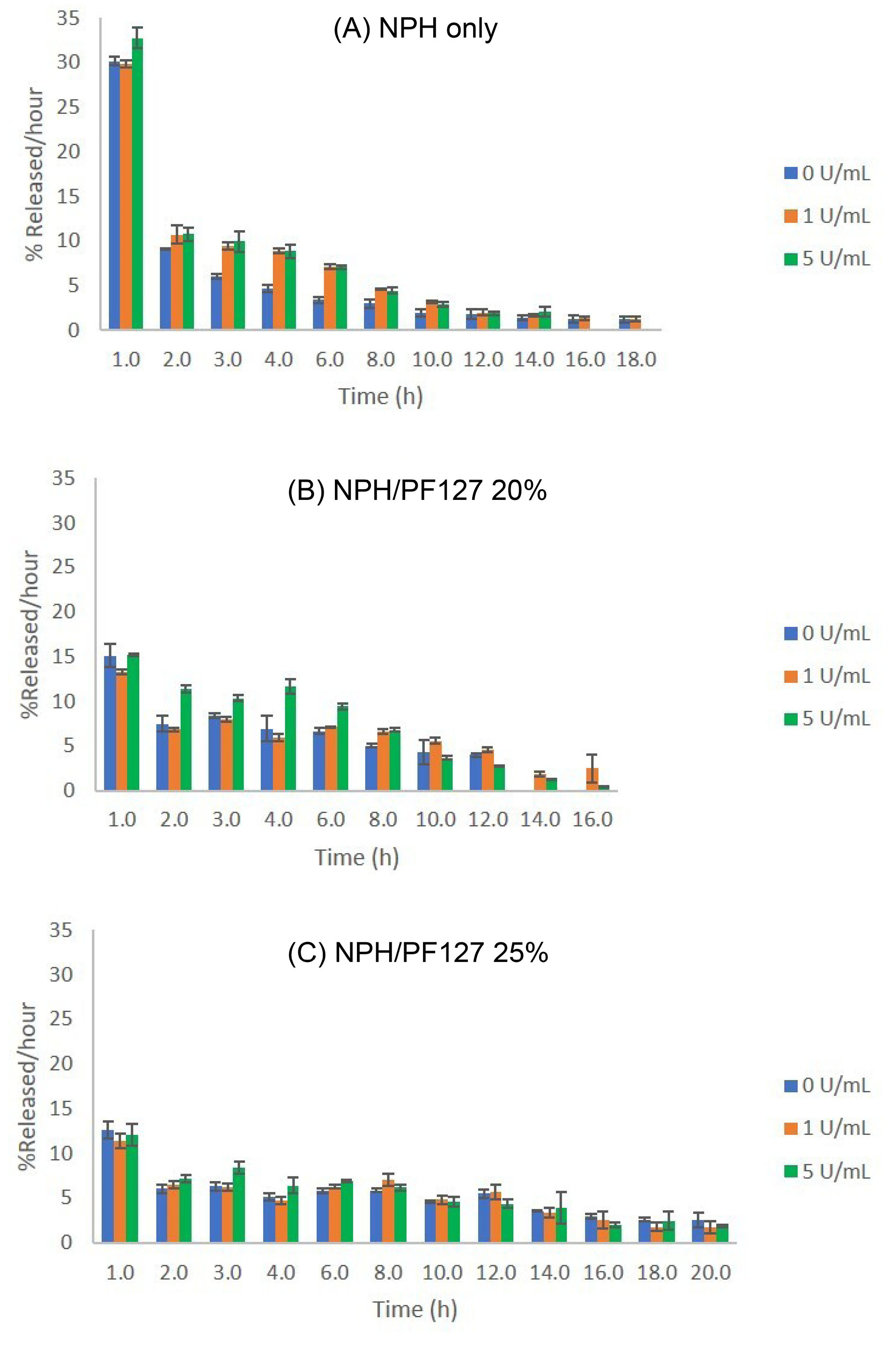
Fig. 1.
Effect of protamine-degrading enzyme (trypsin) activities on the hourly in vitro release rates of insulin (% of initial loading/hour) from: (A) NPH only; (B) NPH/PF127 20% (w/w); (C) NPH/PF127 25% (w/w). Data are expressed as mean ± SD (n=3). Reproduced from Sultan et al
11
under Creative Commons Attribution 4.0 International license (CC BY 4.0).
.
Effect of protamine-degrading enzyme (trypsin) activities on the hourly in vitro release rates of insulin (% of initial loading/hour) from: (A) NPH only; (B) NPH/PF127 20% (w/w); (C) NPH/PF127 25% (w/w). Data are expressed as mean ± SD (n=3). Reproduced from Sultan et al
11
under Creative Commons Attribution 4.0 International license (CC BY 4.0).
On the other hand, the initial burst release was markedly reduced in the presence of PF127 (both 20% and 25%) as shown in Fig. 1B and 1C regardless of the presence of tryptic activity. As the enzyme concentration was increased to 5 U/mL, the hourly insulin release rates for NPH/PF127 20% began showing notable increases during the 2-hour to 6-hour period compared to those in NPH/PF127 25%, where the release rates did not change as significantly as those at lower polymer concentrations.
Based on the aforementioned in vitro findings, it is anticipated that PF127 can serve as a potential modifier of NPH release in vivo, especially during the early phase of insulin release, in the presence of protamine-degrading enzymatic activity. In this paper, we reported the effect of PF127 incorporation on the in vitro release rates in an enzymatic environment and on the blood glucose depression profiles after administration of single doses of NPH or NPH in PF127 in a streptozotocin (STZ)- induced type 1 diabetic rat model.
Materials and Methods
In vivo study
Male Wistar rats (225 ± 25 g) were obtained from Charles River Laboratories, Inc. (Wilmington, MA). Rats were housed in ventilated cages (1 rat per cage) in the animal facility in the temperature range of 20-25°C and were allowed access to standard food and water ad libitum.
STZ-Induction of high blood glucose in rats
After one week of acclimatization, diabetes was induced in Wistar rats via single intraperitoneal (i.p.) injection of 70 mg/kg STZ, dissolved in a sterile 0.1 M citrate buffer at pH 4.5.
20
Fasting blood glucose levels were monitored every two days using Alphatrak®2 glucometer (Zoetis - Parsippany, New Jersey, USA). Seven days after the STZ administration, the rats with blood glucose concentrations greater than 300 mg/dL were included in the experiment.
Time course of blood glucose concentrations upon subcutaneous administration of single doses of NPH insulins
STZ-induced diabetic rats were randomly divided into the groups of six animals. Prior to administrating treatments, STZ-induced diabetic rats were fasted for 12 hours with access to water ad libitum. Initial blood glucose level was recorded for all experimental rats. Subcutaneous injections were performed on the back of the neck area, which were shaved and cleansed with alcohol swipe prior to injections. NPH insulin formulations were administered to the corresponding group of rats (groups 3-5 in Table 1) at an insulin dose of 4 U/kg and the PF127 concentrations of 0, 20, and 25 %. As control groups, 200 μL of insulin free PF127 (25% w/w) was injected to group 2 and no injection was provided to the STZ diabetic control (group 1). Blood glucose levels were determined at 1, 3, 6, 12, and 24 hours postdose. Blood glucose levels was expressed as percentage of the initial levels before treatments.
Table 1.
Control and treatment groups in the in vivo glucose depression experiment in rats
|
Group
|
Group name
|
Insulin dose U/kg
|
Sample size (n)
|
| 1 |
STZ- induced diabetic control |
0 |
6 |
| 2 |
PF127 vehicle |
0 |
6 |
| 3 |
NPH alone (0% PF127) |
4 |
6 |
| 4 |
NPH/PF127 (20%) |
4 |
6 |
| 5 |
NPH/PF127 (25%) |
4 |
6 |
| 6 |
Normal rats |
0 |
6 |
Statistical analysis
Values were shown as mean +/- standard deviation (SD). One-way analysis of variance (ANOVA) was employed to compare multiple groups. Post hoc analyses were conducted using Tukey’s comparison to detect statistically significant differences between the groups. All statistical analyses were conducted using GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla, CA). The level of significance (P) was set at 0.05.
Results
Glucose depression patterns in STZ-induced rats
STZ-induced blood glucose levels in Wistar rats
Following i.p. administration of 70 mg/kg of STZ in rats, the time course of blood glucose was followed. As can be seen in Fig. 2A, blood glucose levels reached >300 mg/dL in 7 days in the majority of the diabetic rats after STZ injections. Prior to administering treatments or vehicle, blood glucose concentrations in the qualified rats were monitored over a 24-hour period and we confirmed that the blood glucose in STZ-diabetic rats were stabilized, despite the substantial variations in the starting blood glucose levels among those rats prior to treatments (Fig. 2B).
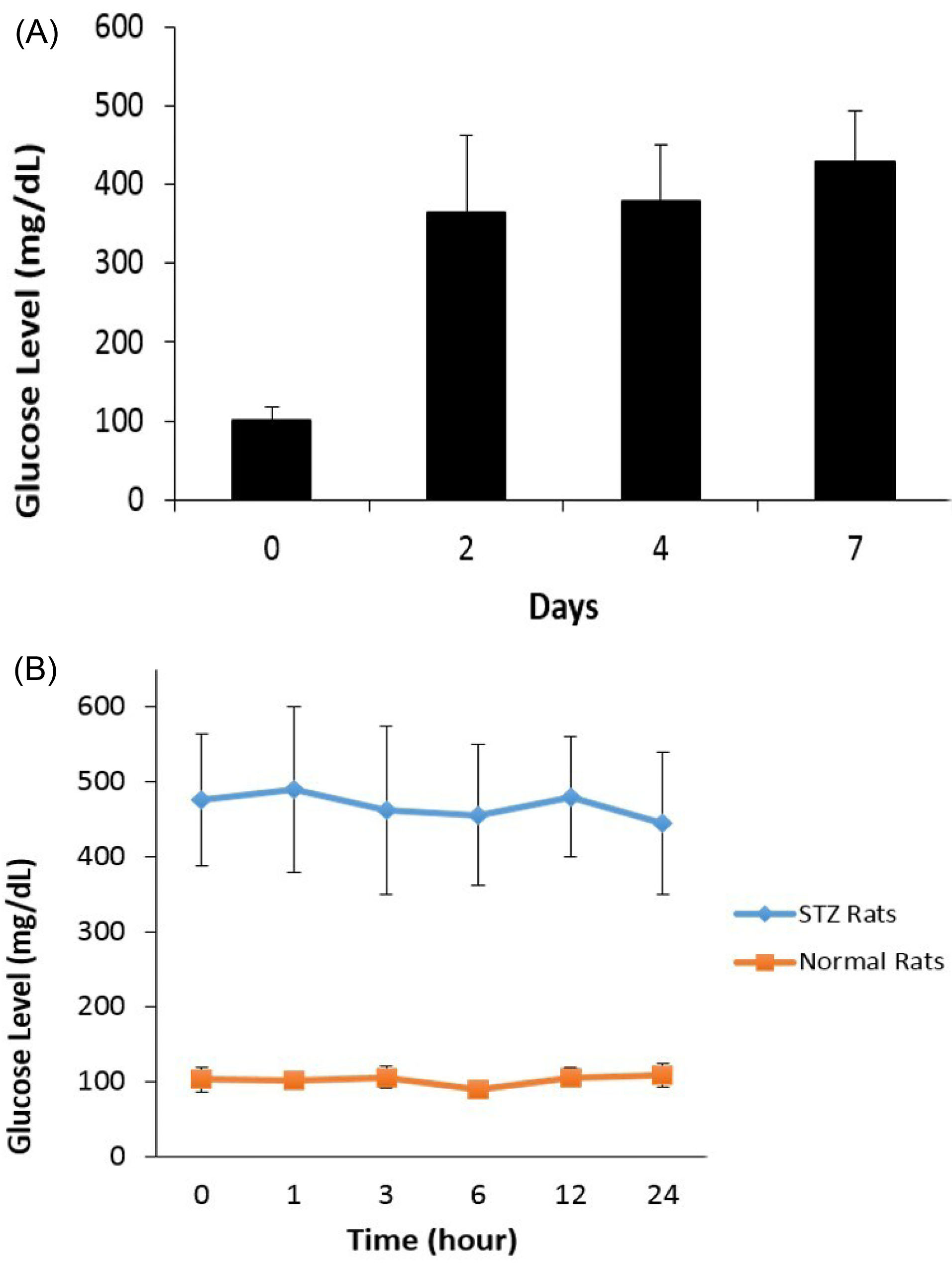
Fig. 2.
(A) Time course of blood glucose change in STZ-treated Wistar rats; (B) Blood glucose concentrations in STZ-diabetic rats vs. normal rats over the course of 24 hours prior to treatments.
.
(A) Time course of blood glucose change in STZ-treated Wistar rats; (B) Blood glucose concentrations in STZ-diabetic rats vs. normal rats over the course of 24 hours prior to treatments.
Blood glucose depression patterns in NPH and NPH/PF127 formulations
As described in Table 1, the STZ-induced rats (>300 mg/dL in blood glucose) were divided into groups 1-5. As can be seen from Fig. 3, over the 24-hour period, the rats in group 1 (STZ control) and group 2 (PF127 vehicle) did not show a significant change in blood glucose relative to the initial blood glucose level measured right before the time of s.c. administration, suggesting insignificant contribution from the PF127 to blood glucose by itself.
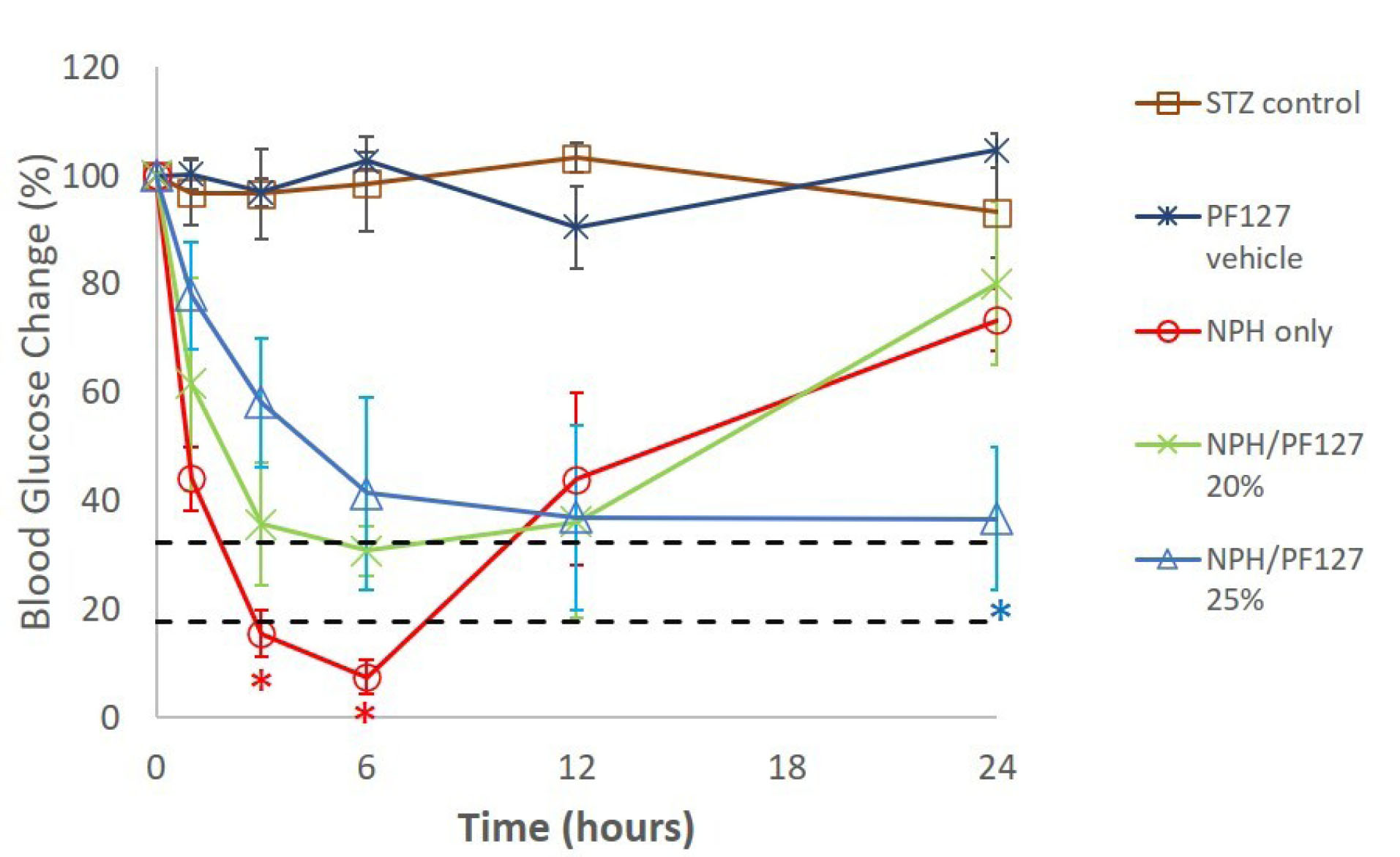
Fig. 3.
Blood glucose levels (% of initial) vs. time (hours) in STZ-induced diabetic rats (mean ± SD, n = 6/group) following single-dose s.c administration of different treatments. The dotted lines represent the window (95% confidence interval) of normal blood glucose (~100 mg/dL) relative to the initial blood glucose levels of the STZ-diabetic rats prior to treatments. *P<0.05.
.
Blood glucose levels (% of initial) vs. time (hours) in STZ-induced diabetic rats (mean ± SD, n = 6/group) following single-dose s.c administration of different treatments. The dotted lines represent the window (95% confidence interval) of normal blood glucose (~100 mg/dL) relative to the initial blood glucose levels of the STZ-diabetic rats prior to treatments. *P<0.05.
Groups 3, 4, and 5 received 4 U/kg of NPH only, NPH/PF127 20%, and NPH/PF127 25%, respectively. As can be seen from Fig. 3, at hour 3 and hour 6, the NPH suspensions at the 4 U/kg dose created blood glucose levels significantly below the values from NPH/PF127 (both concentrations), and for hour 6, the blood glucose of NPH was even lower than the lower limit of the 95% confidence region (dotted lines) of normal glucose (~100 mg/dL relative to the initial blood glucose concentrations prior to treatments). On the other hand, initial drop in relative blood glucose levels were attenuated in NPH/PF127 formulations (4 U/kg dose) in a polymer concentration-dependent manner. The NPH/PF127 20% exhibited blood glucose levels close to the normal range from 3 hours to 12 hours post treatment. However, its blood glucose concentration at hour 24 was elevated to the level comparable to that of NPH alone at hour 24. On the other hand, the administration of NPH/PF127 25% (4 U/kg) resulted in a slower change in blood glucose during the initial hours compared to the other two groups, followed by sustained blood glucose depression up to 24 hours postdose, approaching the normal range.
Discussion
In the absence of protamine-degrading enzymatic activity, it is expected that the dissolution and release of insulin from NPH microcrystals follow a model based on the Noyes-Whitney type dissolution kinetics,
21
which predicts a substantial initial flux out of the microcrystal surface. As the dissolution proceeds, the surface areas of the crystals are also expected to decline, contributing to the rapid decline in the rate of microcrystal dissolution after the initial release phase. Moreover, at the beginning of the release, the crystal stabilizing auxiliary factors such as zinc ions and phenolic compounds (phenol and m-cresol) are rapidly released in the absence of the polymer, contributing to the large initial release even in the absence of tryptic activity.
It appears that when NPH microcrystals were loaded in PF127 hydrogels, the release rates were more evident (compared to 0% polymer) as the polymer concentration was increased to 25%. The lack of initial burst effects in NPH/PF127 groups in the absence of trypsin (Fig. 1B and 1C) suggests that PF127 hydrogel serves as a diffusion barrier to rapid insulin release. Potentially, the hydrogel may also serve as a modifier of NPH dissolution rates by impacting the nature and the thickness of the boundary layer of NPH microcrystals upon contact between the PF127 polymer and the surface of the NPH crystals, thereby altering the boundary layer parameters and diffusivity in the altered boundary layer. While further studies will be required to elucidate its mechanistic aspect, a similar but exaggerated profile of protracted dissolution and release of insulin from a 4-zinc rhombohedral crystal loaded in a biodegradable and thermosensitive PLGA-PEG-PLGA hydrogel was observed, which cannot solely be explained by confining the role of the hydrogel as a diffusion barrier only.
22
During the 2-6 hours from the beginning of the release, the NPH insulin loaded in 25% demonstrated ‘resistance’ against acceleration of insulin release aided by increasing the enzyme activity that breaks down protamine species. We also prepared NPH/PF127 with the PF127 concentrations of 15% and 30% (w/w). However, the formulation with 15% PF127 was too close to the critical gel concentration of PF127 in PBS and therefore too loose to serve as an effective physical barrier against protein entities (insulin and trypsin enzyme), whereas the 30% PF127 presented an inconvenience in handling due to the lowering of its sol-gel transition temperature at the relatively high polymer concentration, as per our previous observation in its phase behavior.
11
Therefore, these formulations were deemed rather suboptimal for further applications.
The release of insulin from NPH/PF127 (both 20% and 25%) appears to be well correlated with the known erosion behavior of PF127 hydrogel, as evidenced in the literature that PF127 hydrogels are known to undergo a constant rate erosion (under a fixed surface area of the hydrogel) in which the rate of drug release was correlated well with its erosion rates.
23
Fig. 1C shows that the NPH/PF127 25% exhibited more constant, sustained release of insulin at later hours (>12 hours) compared to the samples with lower polymer content (Fig. 1B) in the presence of higher (5 U/mL) tryptic activity, in which the drug release was affected both by the gel erosion and the significant penetration of environmental enzyme molecules into the matrix. The polymer concentration of 25% (w/w) appears to be able to retard such penetration by trypsin so that the drug release was more of the function of the gel erosion, being relatively independent of the external enzymatic activity.
The formulations were tested in a well-established, STZ-induced diabetic rat model. The blood glucose after the administration of STZ was stabilized in a week, as shown in Fig. 2. The three aforementioned formulations were tested in terms of their ability to lower blood glucose in a polymer concentration-dependent fashion. We chose the dose of 4 U/kg of NPH, which, upon a single s.c. dosing, created statistically significant hypoglycemic blood glucose levels at hours 3 and 6, respectively (Fig. 3), followed by rapid elevation in blood glucose afterwards. Whereas the NPH/PF127 20% was able to exhibit less initial burst compared to NPH alone and maintained constant blood glucose levels near the normal range from hour 3 to hour 12, the action did not sustain beyond 12 hours postdose. On the contrary, the dose 4 U/kg delivered via NPH/PF127 25% exhibited a further attenuation of blood glucose depression during the initial hours, followed by sustained blood glucose depression, approaching the upper limit of the normal range, up to 24 hours postdose, which was statistically significant compared to the levels from NPH/PF127 20% or NPH alone at hour 24. For a better control of the blood glucose, further individualization of dosing would be desired based on the magnitude of the difference between the initial blood glucose and the target level of blood glucose, which was highly variable between rats in our study. We used a fixed dose of insulin adjusted for bodyweight only, which left some room for fine-tuning of the insulin dose. Low injection volumes (~less than 0.2 mL from a 1-mL syringe) contributed to the difficulty of fine-control of the individual dose based on the bodyweight of each rat. Nevertheless, we were able to demonstrate the consistent patterns of in vitro release rates and the blood glucose depression dynamics in the experimental animal model within the study constraints.
With further, potentially clinical applications of PF127-based drug delivery systems in mind, it should be noted that Pluronic F127 (Poloxamer 407), at high doses, were used to create experimental animal models of hyperlipidemia and atherosclerosis. Such a model was created by i.p. injections of 0.5 g/kg of the polymer every 3rd day for 4 months in mice.
24
This would correspond to 0.17 g/kg PF127 per day. Based on the current dosing guideline, daily NPH dose for a 70-kg adult is approximately on the order ~100 U/day with individual fine-tuning of the dose.
25
Since the formulation in our study contains 75 U of insulin/mL (for NPH/PF127 25%), about 1.3 mL would deliver ~100 U of insulin and 0.33 g of the polymer each day. Per kilogram basis (bodyweight), this corresponds to the polymer dose of 0.0047 g/kg/d from NPH/PF127 25%, which is about 36-fold lower than the dose used to create experimental model of hyperlipidemia/ atherosclerosis. However, caution must be exercised, and more data are needed in further interpretation of the said polymer dose regarding any potential long-term effects or lack of effects in vivo. Measurements of key parameters in the potential development of atherosclerosis (such as the levels of relevant pro-inflammatory mediators
24
) may need to be included in in vivo studies involving PF127-based drug delivery systems down the road, in an attempt to better understand the long-term safety of the PF127 at the suggested dose level.
Conclusion
The patterns of blood glucose changes in an STZ-induced diabetic rat model were consistent with the previously observed in vitro insulin release patterns with different polymer contents in the formulations tested in this study, extending the action of NPH in the experimental animal model over a full 24-hour period. The incorporation of NPH microcrystals in the aqueous pluronic F127 polymer hydrogel appears to modulate insulin release both in vitro and in vivo in the presence of enzymes degrading protamine within the NPH microcrystals by limiting the subcutaneous tissue enzyme activities thought to accelerate NPH insulin release.
Acknowledgments
The authors acknowledge NSU College of Pharmacy for equipment and laboratory support.
Funding sources
This research was funded in part by Nova Southeastern University, Health Professions Division Grant and by Jazan University through Saudi Arabian Cultural Mission (SACM) designated to M.H.S.
Ethical statement
All animal experiments were conducted in accordance with the protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Nova Southeastern University (IACUC Control #: 067-468-15-0512).
Competing interests
The authors declare no conflict of interests.
Authors’ contribution
YMK contributed to the conceptualization. YMK and MHS contributed to the experimental design. MHS and WAM contributed to the acquisition, analysis, and interpretation of data. MHS prepared the manuscript draft. YMK finalized the manuscript. All authors read and agreed to the published version of the manuscript.
Research Highlights
What is the current knowledge?
simple
-
√ Despite the prevalence of engineered basal insulins, NPH insulin is a native human insulin with a long history clinical application. However, its time profile of action upon single s.c. administration is rather undesirable, characterized by significantly higher release rates within the first few hours followed by the lack of sufficient insulin release, making it an intermediate basal insulin.
What is new here?
simple
-
√ The drawback of NPH insulin release profile can be positively modulated by combining NPH in the thermosensitive pluronic F127 polymer, which can effectively block tissue enzyme activity that may accelerate insulin release, especially during the first few hours, the control of which is rather critical in preventing hypoglycemia and extending the action after a single subcutaneous dose. This concept of combining polymer hydrogel and polypeptide microcrystal formulation may shed light on the old materials and the concept may be extended to other situations for the controlled delivery of polypeptides involving crystal/polymer interface.
References
- Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol 2014; 2:56-64. doi: 10.1016/s2213-8587(13)70112-8 [Crossref] [ Google Scholar]
- Pouwer F, Hermanns N. Insulin therapy and quality of life A review. Diabetes Metab Res Rev 2009; 25 Suppl 1:S4-s10. doi: 10.1002/dmrr.981 [Crossref] [ Google Scholar]
- Petznick A. Insulin management of type 2 diabetes mellitus. Am Fam Physician 2011; 84:183-90. [ Google Scholar]
- Heise T, Mathieu C. Impact of the mode of protraction of basal insulin therapies on their pharmacokinetic and pharmacodynamic properties and resulting clinical outcomes. Diabetes ObesMetab 2017; 19:3-12. doi: 10.1111/dom.12782 [Crossref] [ Google Scholar]
- Redwan EM, Linjawi MH, Uversky VN. Looking at the carcinogenicity of human insulin analogues via the intrinsic disorder prism. Sci Rep 2016; 6:23320. doi: 10.1038/srep23320 [Crossref] [ Google Scholar]
- Wu JW, Azoulay L, Majdan A, Boivin JF, Pollak M, Suissa S. Long-Term Use of Long-Acting Insulin Analogs and Breast Cancer Incidence in Women With Type 2 Diabetes. J Clin Oncol 2017; 35:3647-53. doi: 10.1200/jco.2017.73.4491 [Crossref] [ Google Scholar]
- But A, De Bruin ML, Bazelier MT, Hjellvik V, Andersen M, Auvinen A. Cancer risk among insulin users: comparing analogues with human insulin in the CARING five-country cohort study. Diabetologia 2017; 60:1691-703. doi: 10.1007/s00125-017-4312-5 [Crossref] [ Google Scholar]
- Owens DR. Insulin preparations with prolonged effect. Diabetes Technol Ther 2011; 13 Suppl 1:S5-14. doi: 10.1089/dia.2011.0068 [Crossref] [ Google Scholar]
- Rostami PM, Setoodeh AM, Rabbani AM, Nakhaei-Moghadam MM, Najmi-Varzaneh FM, Rezaei NMP. A Randomized Clinical Trial of Insulin Glargine and Aspart, Compared to NPH and Regular Insulin in Children with Type 1 Diabetes Mellitus. Iran J Pediatr 2014; 24:173-8. [ Google Scholar]
- Lepore M, Pampanelli S, Fanelli C, Porcellati F, Bartocci L, Di Vincenzo A. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000; 49:2142-8. doi: 10.2337/diabetes.49.12.2142 [Crossref] [ Google Scholar]
- Sultan MH, Mahdi WA, Kwon YM. Insulin Release from NPH Insulin-Loaded Pluronic® F127 Hydrogel in the Presence of Simulated Tissue Enzyme Activity. Processes 2020; 8. doi: 10.3390/pr8101320 [Crossref]
- Wang K, Guo DS, Zhao MY, Liu Y. A Supramolecular Vesicle Based on the Complexation of p-Sulfonatocalixarene with Protamine and its Trypsin-Triggered Controllable-Release Properties. Chemistry 2016; 22:1475-83. doi: 10.1002/chem.201303963 [Crossref] [ Google Scholar]
- Schilling RJ, Mitra AK. Degradation of insulin by trypsin and alpha-chymotrypsin. Pharm Res 1991; 8:721-7. doi: 10.1023/a:1015893832222 [Crossref] [ Google Scholar]
- Akash MS, Rehman K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J Control Release 2015; 209:120-38. doi: 10.1016/j.jconrel.2015.04.032 [Crossref] [ Google Scholar]
- Barichello JM, Morishita M, Takayama K, Nagai T. Absorption of insulin from pluronic F-127 gels following subcutaneous administration in rats. Int J Pharm 1999; 184:189-98. doi: 10.1016/s0378-5173(99)00119-2 [Crossref] [ Google Scholar]
- Morishita M, Barichello JM, Takayama K, Chiba Y, Tokiwa S, Nagai T. Pluronic F-127 gels incorporating highly purified unsaturated fatty acids for buccal delivery of insulin. Int J Pharm 2001; 212:289-93. doi: 10.1016/s0378-5173(00)00615-3 [Crossref] [ Google Scholar]
- Das N, Madan P, Lin S. Statistical optimization of insulin-loaded Pluronic F-127 gels for buccal delivery of basal insulin. Pharm Dev Technol 2012; 17:363-74. doi: 10.3109/10837450.2010.542164 [Crossref] [ Google Scholar]
- Nasir F, Iqbal Z, Khan A, Khan JA, Khan A, Khuda F. Development and evaluation of pluronic- and methylcellulose-based thermoreversible drug delivery system for insulin. Drug Dev Ind Pharm 2014; 40:1503-8. doi: 10.3109/03639045.2013.831441 [Crossref] [ Google Scholar]
- Chen X, Wong BCK, Chen H, Zhang S, Bian Z, Zhang G. Long-lasting Insulin Treatment Via a Single Subcutaneous Administration of Liposomes in Thermoreversible Pluronic® F127 Based Hydrogel. Curr Pharm Des 2018; 23:6079-85. doi: 10.2174/1381612823666170509123844 [Crossref] [ Google Scholar]
- Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi S, Farhangi A, Verdi AA. Induction of diabetes by Streptozotocin in rats. Indian J Clin Biochem 2007; 22:60-4. doi: 10.1007/bf02913315 [Crossref] [ Google Scholar]
- Søeborg T, Rasmussen CH, Mosekilde E, Colding-Jørgensen M. Absorption kinetics of insulin after subcutaneous administration. Eur J Pharm Sci 2009; 36:78-90. doi: 10.1016/j.ejps.2008.10.018 [Crossref] [ Google Scholar]
- Kwon YM, Kim SW. Biodegradable triblock copolymer microspheres based on thermosensitive sol-gel transition. Pharm Res 2004; 21:339-43. doi: 10.1023/b:pham.0000016248.30579.2f [Crossref] [ Google Scholar]
- Moore T, Croy S, Mallapragada S, Pandit N. Experimental investigation and mathematical modeling of Pluronic F127 gel dissolution: drug release in stirred systems. J Control Release 2000; 67:191-202. doi: 10.1016/s0168-3659(00)00215-7 [Crossref] [ Google Scholar]
- Johnston TP, Li Y, Jamal AS, Stechschulte DJ, Dileepan KN. Poloxamer 407-induced atherosclerosis in mice appears to be due to lipid derangements and not due to its direct effects on endothelial cells and macrophages. Mediators Inflamm 2003; 12:147-55. doi: 10.1080/0962935031000134860 [Crossref] [ Google Scholar]
- Davidson MB. Insulin Therapy: A Personal Approach. Clin Diabetes 2015; 33:123-35. doi: 10.2337/diaclin.33.3.123 [Crossref] [ Google Scholar]