Bioimpacts. 11(3):227-233.
doi: 10.34172/bi.2021.29
Mini Review
Transformative dynamism in pharmaceutical and biomedical research: Complexity of integration of innovative R & D hubs
Yadollah Omidi *  , Hossein Omidian
, Hossein Omidian
Author information:
Department of Pharmaceutical Sciences, College of Pharmacy, Nova Southeastern University, Fort Lauderdale, Florida, USA
Abstract
Introduction:
To be fully functional, pharmaceutical, and biomedical research centers need to be transformed to become innovative research and development (R & D) hubs. Such transformation, however, is a dynamic complex matter.
Methods:
To establish an innovative R & D hub, a simple and concise manifesto is conceptualized for the nonlinear dynamic transformation towards an innovative research hub to reinforce the transition of the 2nd generation R & D centers.
Results:
Interdisciplinary research is the most demanded field of research to overcome various multi-sided health issues. To become an innovative R & D hub, pharmaceutical centers must function as a small-scale physical infrastructure to support the inter-communication of scientists and provide specific technological needs to promote the related innovation and entrepreneurship with advanced business plans and prototypes. Given that a success paradigm within an unorderly surrounding setting has already been condemned to fail, the orderly integration of nested systems and groups should be carefully implemented towards a shared vision with formal and tacit agreements among all parties, including academia, industry, and finance team.
Conclusion:
To achieve a fully functional innovative R & D hub, a "know-how" approach with the systems thinking mindset within all the parties is of paramount necessity. The healthier the order of the whole working system is, the more effective will be the encompassed entitles and players. However, systems should have several checkpoints to enhance clarity and evade discrepancy and divergence. Since the medication is a highly trusted and needed public enterprise, the drug discovery and development paradigm should be practiced at the highest speed with maximum transparency and accountability.
Keywords: Dynamic transformation, Pharmaceutical sciences, Complexity research, Innovative R & D hubs, Decision making, Drug discovery and development
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
In any pharmaceutical and biomedical research and development (R&D) platform, the dynamic productive transformation concept towards bench-to-bedside (B2B) and bench-to-industry (B2I) approaches demands not only a written content on a shared vision but also a tacit agreement of all partakers and the certainty of the industry and financing bodies regarding the impacts of the invention. We often fail to conceptualize the full range of feedbacks/responses, and hence, face policy resistance in a nonlinear operating system. Regardless of the nature of feedbacks (i.e., positive self-reinforcing or negative self-correcting feedback loops), the whole system might then suffer the consequences. Further, feedback information leads to new decisions, which in turn give rise to new feedbacks. This is the behavior of a complex system with dynamic capabilities.
1,2
A well-intentioned attempt to solve the existing problems might often lead to policy resistance if the policies are not defined well enough to meet the needs of the society or community. Thus, to reach a “tacit agreement” within a complex system, the system dynamics should be fully perceived. It is of great necessity to rationally and scientifically learn how to catalyze sustained changes within dynamic organizations with complex feedbacks, in which yesterday’s solutions might become today’s problems. Organized dynamic systems may confront complexity because they are intrinsically (i) dynamic and nonlinear, (ii) composed of different entities with distinct objectives, (iii) tightly coupled, (iv) feedback-oriented, (v) self-organized and co-adaptive, (vi) history- and path-dependent, (vii) counter-intuitive, (viii) policy-resistance, and (ix) responsive to trade-offs.
3
In the case of drug discovery and development (DDD), transformative approaches are a necessity. Based on decades of experience in academia, we have observed high entropy and chaotic research ideas and works with no specific societal goal that eventually filed and stored indefinitely in the libraries. This not only pictures a flawed and futile approach by academia but also incurs undesired burdens. In modern days, a research center in academia is entitled to fail if it is not oriented towards a bigger goal of contemplating the societal and community needs. Often young generations question the rationale for a research activity if the outcome of such research is not going to be constructive and beneficial. What needs to be done to reinforce the orientation of research towards positive constructivism? If designed rationally and performed purposefully, the research activities in academia not only reform the face of the world but also change it to a better one. Nevertheless, it is a complex matter with dynamic capability. Like any other dynamic product-oriented activity, the success of DDD against any given disease demands the partnership of different related players with a deep understanding and constructive collaboration based on a lawful settlement. This review aims to address some missing parts of such an endeavor in the field of DDD.
The complexity of dynamic systems
The behavior of a dynamic system is largely dependent upon its composition and structure, which consisting of various entities such as feedback loops, stocks and flows, and complex nonlinearities generated via intricate interactions of the physical and institutional settings with the decision-making processes. A well-designed dynamic system shows the exponential growth based on the positive self-reinforcing loop towards its desired goals with harmonized oscillation in terms of proof-of-concept and proof-of-technology (POC/POT). There would be checkpoints at which one can realize the negative or positive loops and fluctuations/oscillations – a heuristic approach for scientific criticism. Given that any exponential growth may show the maximum flux, there would be a stasis/equilibrium condition with the various variables that varies randomly. If not properly controlled by scientific leaders with the desired content among all players, a dynamic system may face chaotic situations such as damped pendulums. To succeed in a complex health care setting, the 21st-century leaders might consider key aspects such as learning with aims, reverence with strategy, service with honesty, authenticity with realism, and rationality with vision.
4-7
Fig. 1 represents a schematic illustration of leadership impacts on the transformation of the DDD process. This can help to have a much better relevant understanding of the complexity in terms of the structure and process.
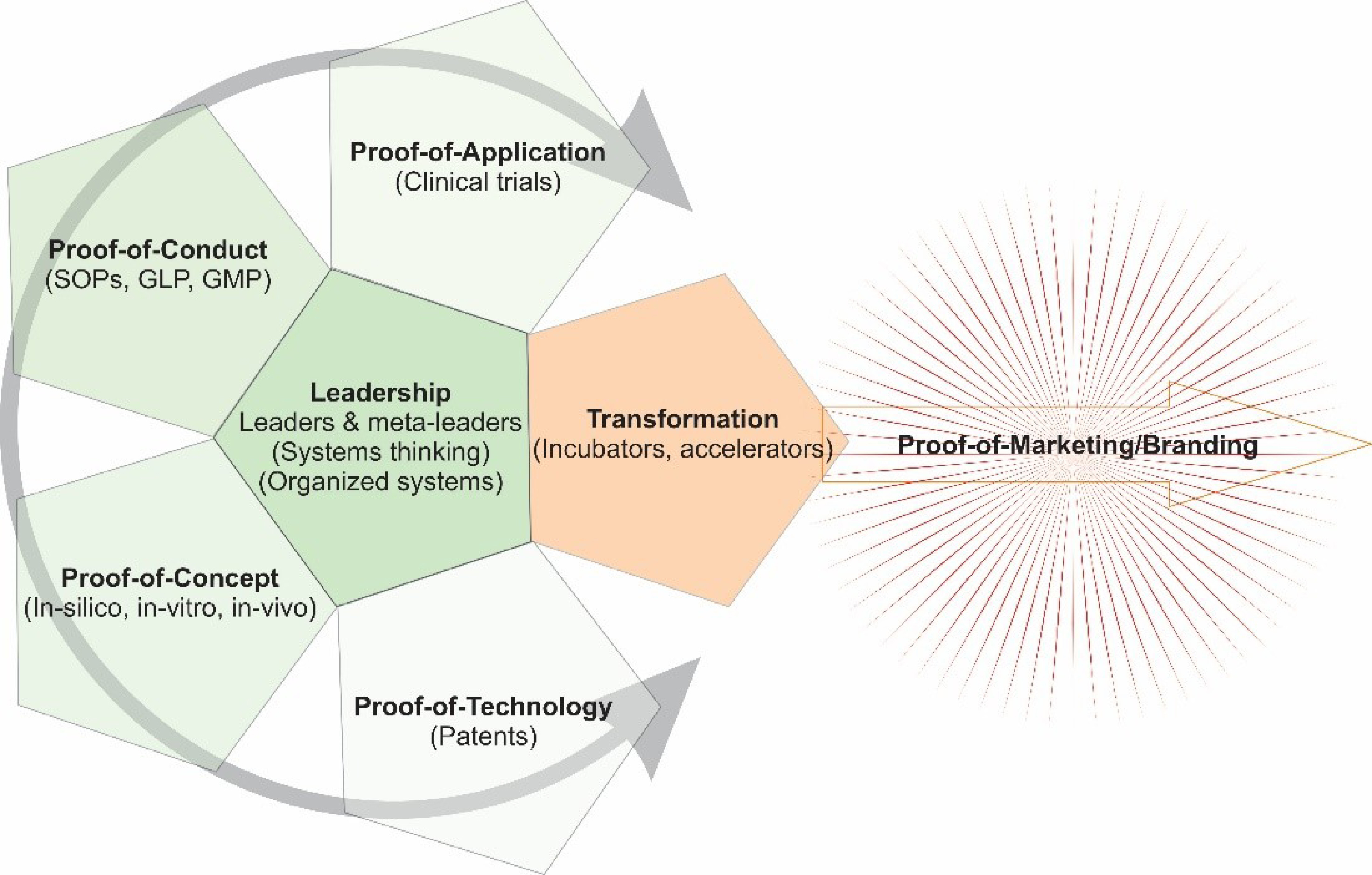
Fig. 1.
Schematic illustration of leadership impacts on the transformation of a drug discovery and development process. Leaders play a key central role in the synchronization and harmonization of different related processes.
.
Schematic illustration of leadership impacts on the transformation of a drug discovery and development process. Leaders play a key central role in the synchronization and harmonization of different related processes.
Modeling dynamic systems
To model a dynamic system, one may capitalize on the causal loop diagrams (CLDs) for different reasons, including (i) to study the cause of dynamics, (ii) to capture the mental models of individuals/team members, and (iii) to investigate the influential feedbacks. However, CLDs may face several limitations, including their incapability to gain and control the stock and flow structure that are two key hallmarks of systems as accumulations showing the state of the systems. One may use the logistic growth model to analyze the growth, while the growth can also be modeled based on the stock accumulations. Further, Richards' model is another method, in which the fractional growth rate of growth is also nonlinear. It should be stated that the logistic model cannot explain the innovation diffusion such as the startup problem, thus the Bass model, which does not specify the feedbacks at the operational level and is analogous to the simple SI model, can be used as a tool to forecast the sales of new products.
3
Further, unpredicted incidences can generate unprecedented errors that cannot be modeled or even explained.
Size and nature of systems
The size of the system plays a key role, the larger the firm is, the greater the clouts will be with all the customers, workers, and suppliers. However, it should be noted that once a monopoly is formed, the market power can expand, and workers and customers may earn more or fewer benefits depending on the mindset of the decision-makers and social powers. Indeed, the outcomes in the open and closed societies are somewhat different in terms of the quality of the workforce and products as well as services. By the growth of the firm, the expectations for future earnings growth can be expected as well. Moreover, the greater market value with higher stock price can generally reduce the cost of raising new capital through the equity market. Collectively, to give meaningful growth to a dynamic system, rational positive loops are necessary to preempt vicious cycles that may result in markedly lower returns. While this is a general concept, the pharmaceutical centers and also industries may face similar pattern.
Pharmaceutical/biomedical R&D hub characteristics
A pharmaceutical/biomedical R&D hub needs to be energized, first with the virtuous cycles of technology development yet with little path-dependency, and second with the content involvement of all players based on a tacit agreement upon a shared vision. The first may happen, yet the second demands very careful mitigative approaches to earn the trust of most, if not all, by providing righteous shares. According to Best et al, because of possible cultural changes and differences, it is paramount to implement working strategies such as front-line ownership in the framework of macro-level social forces, in which very significant challenge seems to be the scaling-up of the labor-intensive change strategies and implementation of "simple rules" based on systems thinking.
7
Further, the higher the transparency of the process with simple rules is, the greater the outcome of the system with compliance will be.
The spin-off of start-ups can simply be arranged around a shared vision of creating incorporation with the shared path, yet it needs to build a long-run trust. In this line, healthy competitions can encourage the contenders of the incorporation to establish success paradigms, which brings more success to the system. The most successful party of the incorporation should become the leading dominant one, while such a path-dependent system may rapidly lock into a stable equilibrium with possible depression if the future of the market is not carefully predicted. As result, such a system needs to make sure to utilize potential visionary leaders. Policies need to be set without negative implications while the network effects should be studied and addressed by the leaders to direct the system and keep it on the right track with no prejudice or bullying.
8-10
It should be noted that, in locked-in path-dependent incorporation, different parties are game players holding on to the strategic plans of the holding while making their benefits. Thereby, the better the accountability is, the better the responses of the system will be.
Translational research for transformation
Any collaborative pharmaceutical research center with a state-of-the-art setting can play the R&D hub role, in which start-up networks can be formed by the trained postgraduates and scientists with developed protocols for translational research.
11
Such a network can be expanded using a shared vision of the system based on a self-fulfilling prophecy–systems thinking network with path-dependency yet as a function-independent system. Such policies can be analyzed in terms of duration and delays in processes (e.g., information delay, transit delay, pipeline delay), delay rate, supply chain, process validations, inventory, and production progression, and so forth. The pricing of the product prototypes can be set based on demand analysis and forecasting. Further, the inflows, coflows, and outflows can be modeled and processed, which can result in key concepts in terms of the aging chain. All these can lead to a decision while the decision making can also be modeled. To become a successful paradigm, the R&D setting must stay fully functional, which demands the inflow of finance. The latter needs the outflow of technologies and product prototypes with a high revenue rate. All these explain the essence of the transformation manifesto. An innovative R&D hub in the field of pharmaceutical sciences must act accordingly to achieve maximum alignment by showing the inevitability and necessity of such innovations and harmonization of all parties, as illustrated in Fig. 2.
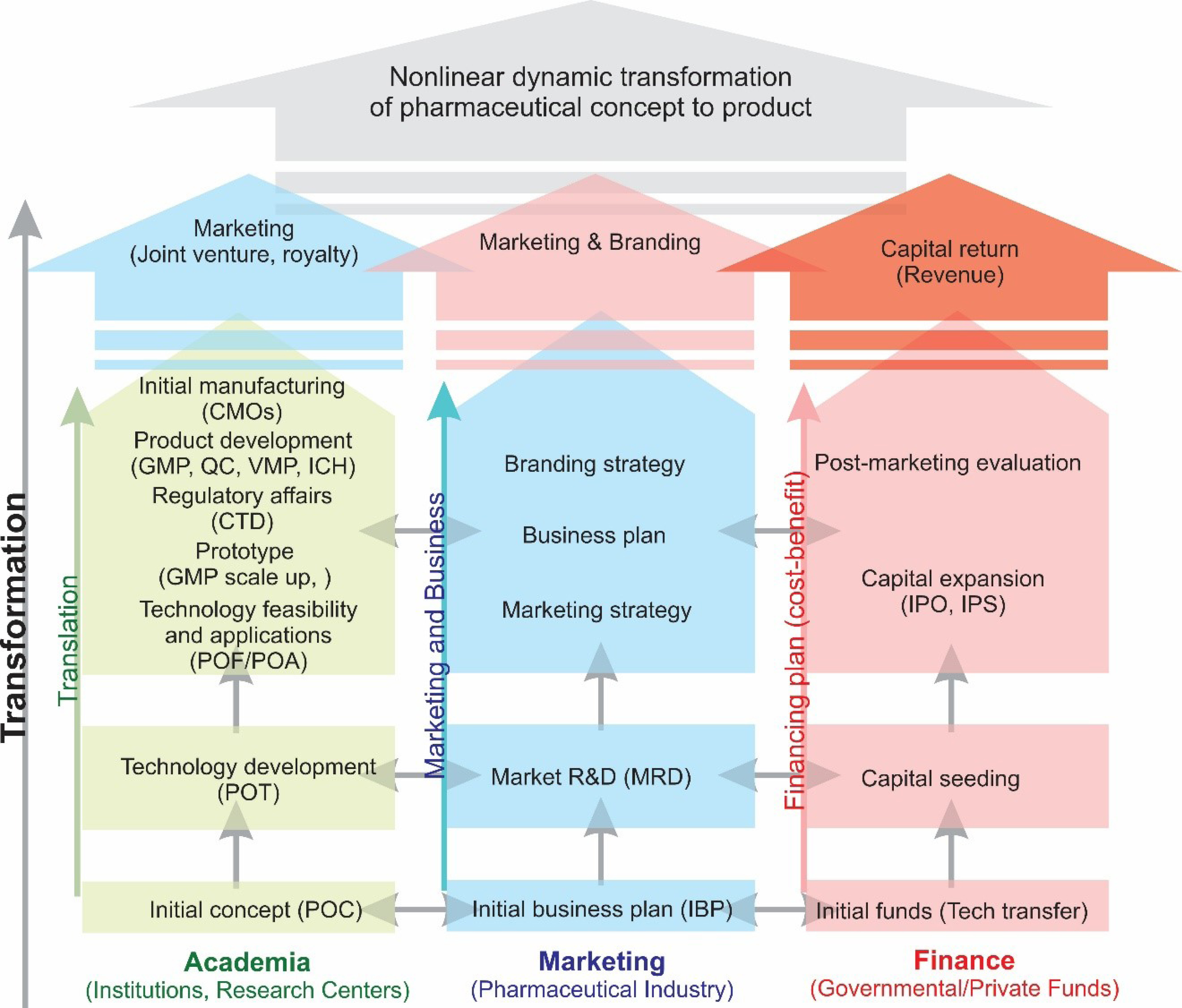
Fig. 2.
The schematic illustration of the nonlinear dynamic manifesto for the transformation of concepts in the pharmaceutical field. The academic team is responsible for the developments of new concepts and technologies as well as applications such as proofs of concept, technology, and application (POC, POT, and POA, respectively) in a close association with the governmental/private sectors which provide funds. Once the developed technologies entered the incubator and accelerator, the marketing team performs market and business strategy and plans in a cost-benefit manner in close cooperation with the finance team who provides funds and presents the product for the IPOs and capital expansions. Such a joint venture can be set based upon a legal agreement considering an agreed royalty, for which a tacit agreement around a shared vision is a must. IPO: initial public offering. IPS: initial public stock.
.
The schematic illustration of the nonlinear dynamic manifesto for the transformation of concepts in the pharmaceutical field. The academic team is responsible for the developments of new concepts and technologies as well as applications such as proofs of concept, technology, and application (POC, POT, and POA, respectively) in a close association with the governmental/private sectors which provide funds. Once the developed technologies entered the incubator and accelerator, the marketing team performs market and business strategy and plans in a cost-benefit manner in close cooperation with the finance team who provides funds and presents the product for the IPOs and capital expansions. Such a joint venture can be set based upon a legal agreement considering an agreed royalty, for which a tacit agreement around a shared vision is a must. IPO: initial public offering. IPS: initial public stock.
A harmonized parallel cooperation between all parties of the transformation process from scratch seems to profoundly accelerate the whole process (Fig. 2). The analysis of primary POCs/POTs by the marketing/financing R&D can support all the parties to agree upon a shared vision and act based on a system thinking approach. Subsequently, a decision can be made based on the information provided to perform a successful approach in terms of the production, marketing, and branding of the initial concept. The common mistake, which often occurs in most R&D settings, is that the science and technology team works linearly on its own with trivial/no purposeful alignment and protocols to attain the main goal (POC/POT), after which the marketing team is informed concomitantly.
11
This mindset and strategy can not only elongate the transformation process but may also result in several hurdles/obstacles that may segregate the teams. Therefore, to fasten the entire transformation process with a greater rate of success, the synchronized parallel alignment with the association of all contributors is a “must” not a “should” tactic. The veracity and robustness of this strategy can be intensified with the harmonization of all parties upon a clear shared vision – a “know-how” approach. All these synchronized alignments can occur in an open system and society, in which the transparency of the vindicated policies can play a key role. Taken all, constitutional policies need to be set to visit future needs and people affluence and wealth and gratification. Based on these days' needs for the business dynamics and complexity in pharmaceutical fields, the upstream constitutional policies, legislations, and acts are the key parameters that must be set because the dynamic world demands constructive decisions and policies with no rant corruption. Given that groups of international scientists may work together towards a shared vision, the work ecosystem must be set to meet all requirements with no prejudice/discrimination, which appears not to be the best motivations of systems and decision-makers, particularly in closed systems. The transformative activity in most R&D settings and similar national settings can be shattered by the wrong policies, collapsing the entire system. Above all, the fiscal and marketing monopolies of various running settings reliant on powerful politicians can waste away all the endeavors both by disappointing the scientists and failing the marketing of the knowledge-based products. This latter phenomenon can be seen not only in the undeveloped systems but may also among corrupted corporations in the developed systems.
Pharmaceutical R&D settings
As shown in Fig. 3, the success of pharmaceutical R&D settings within academia depends on many parameters that are beyond the circle of control of research owners. This means that all parties of the triangle need to see each other interests and come to tacit content for written win-win contracts.
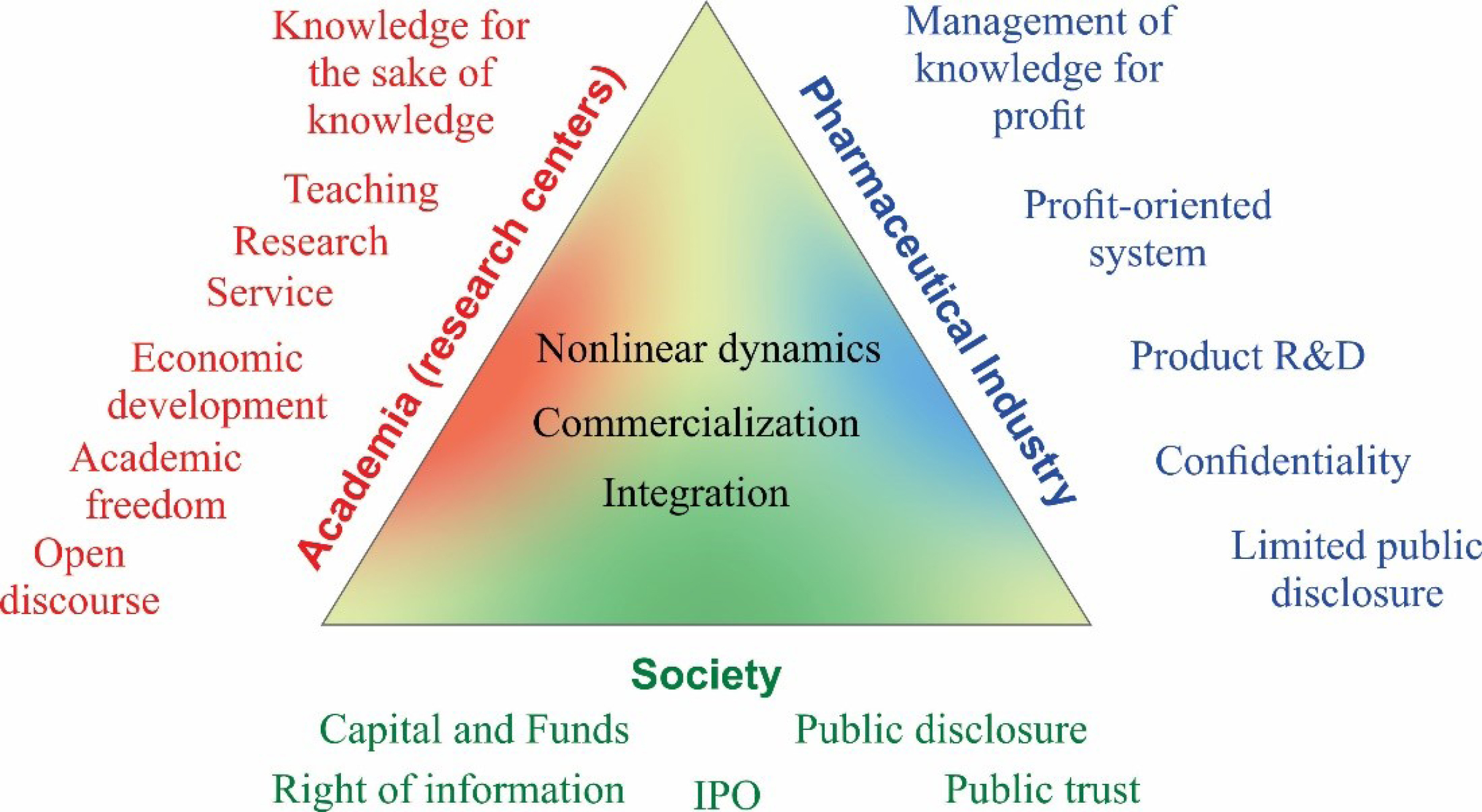
Fig. 3.
The triangle of players in pharmaceutical R&D settings. Diversity of the merits, desires, and interests of the players may intervene with the inter-collaboration policies and create conflicts. The complex nature of the interrelation of different parties demands full understanding, aligned inline education, and lawful rights.
.
The triangle of players in pharmaceutical R&D settings. Diversity of the merits, desires, and interests of the players may intervene with the inter-collaboration policies and create conflicts. The complex nature of the interrelation of different parties demands full understanding, aligned inline education, and lawful rights.
Nonetheless, as a complex system, the success trajectory of a small setting even within a degraded system can substantially influence the entire capacity of the whole system. That is why the power of transformation is deemed to be reinforced by the younger well-trained scientists as a proven manifesto in many scholastic communities. While an R&D setting has great potential for national and international collaborations, this strategy will be inaccessible unless by (i) national willingness and preparedness, (ii) establishment of on-purpose and on-demand infrastructures, (iii) universal constructivism in all aspects, and finally (iv) cross-border educating with the leading scientists around the world for the sake of knowledge transfer.
The partnership is a key factor for the success of DDD
Surprisingly, the academia R&D today is rich in knowledge with minimal orientation on how to utilize it. Due to its relative freedom and flexibility, the academia R&D today is faster than the industry in creating and exposing itself to emerging technologies and utilizing them on a short scale. The academia R&D today, however, is very limited in conducting meaningful and deep research aimed at generating and securing intellectual properties that is crucial for its sustainability for the years to come. The academia R&D today also needs to be concerned about the fate of its graduates as more and more pharmaceutical and biomedical sponsors are now leaning towards using the Contract Research Organizations (CROs) in drug discovery, pre-formulation, formulation, development, and trials.
12
In short, the academia R&D, today, can only survive if it is successful in securing funding and using it wisely for a real problem that threatens the health of society. On the other hand, while the industry, today, enjoys the wealth and experience as well as realistic goals and objectives, it needs to embrace new and emerging technologies to (i) enhance the life cycle management (LCM) of its blockbuster products, (ii) extend the shelf-life of its expiring albeit successful patents, new technologies for faster and more profitable manufacturing, and (iii) compete with the emerging generics and biosimilar products. The timing cannot be better for both to experience and enjoy the mixed wealth of funding, knowledge, and expertise that both can offer only within the context of real but fruitful collaboration. In this regard, the CROs, for instance, have been far ahead of the academia R&D in feeling the urge of joining efforts with industry and vice versa. The emergence of many CROs and CRO start-ups and their consolidation in a short time and being vastly accepted and received by pharmaceutical and biomedical sponsors highlights the importance of dynamic living transformation that both the sponsors and the CROs have embraced for further growth. To achieve the same success and most importantly to survive, the academia R&D has no choice but to follow the same dynamic pattern as the CROs to engage themselves in a win-win strategic partnership with the pharmaceutical and biomedical sponsors. This is evidenced by the recent partnership of GSK–Harvard, AstraZeneca–Columbia University, AstraZeneca-Oxford University, Pfizer–University of California, Monsanto–University of Washington, and Hoechst–Massachusetts General Hospital.
13
New emerging and/or re-emerging infectious diseases highlight the paramount importance of such partnership. Now, the traditional exploratory research behavior of traditional academia is shifting towards B2B/B2I translation and transformation. However, such transition needs investments for the construction of aligned R&D settings to meet emerging demands, for which the vital role of funding mechanisms is the key. Partnerships aimed at getting funds cannot be a long-lasting approach. The academia world needs to develop excellence in leadership with compelling evidence in a given scientific domain to convince the industry to join. On the other hand, the pharmaceutical industry should build a vivid dynamic R&D streamline to act as the main driving force. In a joint milieu, the research-end and the-development and marketing end should be in continual inter-digitation, which can digitize a harmonic fruitful long-lasting partnership.
13,14
Both sides need to minimize the potential risks of breakdown by a realistic assessment of the costs and benefits. In fact, capitalizing on an aligned solid plan with academia can secure real outcomes with the development of the anticipated incentive as a necessary step to produce novel medication.
Conclusions and final remarks
Consolidating the innovative endeavors of academia with the resources of pharmaceutical industries together with the IPOs has always been a striking challenge that depends on (i) proven leadership of academia with the right infrastructure (e.g., a center of excellence with national/international success continuity), (ii) entrepreneurship of pharma industry, (iii) formation of permissive context for both parties, (iv) long-term visionary plans, (v) security in capitalization and return of funds with benefits. The emergence of new technologies can be in favor of the creation of such settings. While translational concepts have evolved the direction of R&D in academia, the use of blockchain technology can guarantee the benefits for both parties with the anticipated settlements.
15-18
Given that the advent and implementation of big data and machine learning technologies have revolutionized the high throughput screening,
19,20
the target identification and validation could be performed in a very short period as seen in the development of vaccines against COVID-19.
21
Drug repurposing is another example of the use of machine learning technologies.
22,23
Having exploited the aforementioned technologies, the dynamic complexity in the transformation of the academic world be substantially simplified. Further, project managers need to be equipped with these new technologies on both sides, and the industry standards should be revisited and amended to accommodate the emerging requirements. Agencies such as the FDA can quickly adapt to the new situation even though the regulatory aspects are very challenging for the governance body to keep the focus on timelines and guidelines. Accelerated programs can be implemented for the clinical trials and the requirements can be refined based on the progress of the project, so are the objectives, decision criteria, responsibilities, and budgeting plans as well as the timelines for promoted success. Here, a simple manifesto is given that can be accomplished in any R&D setting under a respectful endeavor with teamwork, shared vision, hard yet smart work in a harmonized system thinking manner, and “good deed” towards B2B and B2I approaches. Such a great goal can be achieved if the ecosystem of the work within the entire setting is wisely designed. Otherwise, the objectives of the transformation process cannot result in the desired science and technology outburst, and hence, there would be no/trivial positive impacts on the progression and advancement of the society as seen in most institutions of the third world countries. Collectively, for the successful transformation, a working system needs to be dynamically evolved to meet the metaphors and complexities, with needs transparency with constructive communication, openness, and accountability. Both parties from academia and industry should have a tacit agreement upon “integrated marketing communications” for a potential product that can be simply emanated through an initiative from the academic party based on the society needs for coming future. Such an approach can become a strong driving force for a successful development in meeting the need of health system if the policies see all aspects of such transformation from academia to industry.
Funding sources
There is no funding by any agency regarding this study.
Ethical statement
None to be divulged.
Competing interests
Both authors act as the Editors of the journal, Bioimpacts. It is declared that the publication of this article has been carried out following COPE and ICMJE guidelines.
Authors’ contribution
YO developed the initial concept. YO and HO gathered data, drafted, and revised the manuscript.
Review Highlights
What is the current knowledge?
simple
-
√ Pharmaceutical research and development (R&D) are considered a dynamic complex matter.
-
√ The transition of R&D centers towards a production-oriented system demands the integration of infrastructures.
What is new here?
simple
-
√ A “know-how” approach with systems thinking is essential for the success of pharmaceutical R&D.
-
√ To be successful, academia-pharma partnership is vital, which should embrace maximum transparency and accountability.
References
- Eisenhardt KM, Martin JA. Dynamic Capabilities: What Are They?. Strategic Management Journal 2000; 21:1105-21. [ Google Scholar]
- Teece DJ, Pisano G, Shuen A. Dynamic Capabilities and Strategic Management. Strategic Management Journal 1997; 18:509-33. [ Google Scholar]
-
Sterman JD. Business Dynamics: Systems Thinking and Modeling for a Complex World. Boston: Irwin McGraw-Hill; 2000.
- O'Connell PK. A simplified framework for 21st century leader development. Leadersh Q 2014; 25:183-203. doi: 10.1016/j.leaqua.2013.06.001 [Crossref] [ Google Scholar]
- Dionne SD, Gupta A, Sotak KL, Shirreffs KA, Serban A, Hao C. A 25-year perspective on levels of analysis in leadership research. Leadersh Q 2014; 25:6-35. doi: 10.1016/j.leaqua.2013.11.002 [Crossref] [ Google Scholar]
- Porter-O'Grady T. Complexity Leadership: Constructing 21st-Century Health Care. Nurs Adm Q 2020; 44:92-100. doi: 10.1097/NAQ.0000000000000405 [Crossref] [ Google Scholar]
- Best A, Saul J, Willis C. Doing the dance of culture change: complexity, evidence and leadership. Healthc Pap 2013; 13:64-8; discussion 78. doi: 10.12927/hcpap.2013.23346 [Crossref] [ Google Scholar]
- Mahmoudi M. Academic bullies leave no trace. Bioimpacts 2019; 9:129-30. doi: 10.15171/bi.2019.17 [Crossref] [ Google Scholar]
- Mahmoudi M, Ameli S, Moss S. The urgent need for modification of scientific ranking indexes to facilitate scientific progress and diminish academic bullying. Bioimpacts 2020; 10:5-7. doi: 10.15171/bi.2019.30 [Crossref] [ Google Scholar]
- Mahmoudi M, Moss S. The absence of legal remedies following academic bullying. Bioimpacts 2020; 10:63-4. doi: 10.34172/bi.2020.08 [Crossref] [ Google Scholar]
- Omidi Y. Translational researches require effective protocols for knowledge and technology transfer and integration. Bioimpacts 2011; 1:71-3. doi: 10.5681/bi.2011.010 [Crossref] [ Google Scholar]
-
Buvailo A. The Evolving Pharma R&D Outsourcing Industry: A Bird’s-eye View. Pharmaceutical Industry [serial on the Internet]. 2020: Available from: https://www.biopharmatrend.com/post/146-the-evolving-pharma-rd-outsourcing-industry-a-birds-eye-view/.
- Palmer M, Chaguturu R. Academia-pharma partnerships for novel drug discovery: essential or nice to have?. Expert Opin Drug Discov 2017; 12:537-40. doi: 10.1080/17460441.2017.1318124 [Crossref] [ Google Scholar]
- Flier JS. Academia and industry: allocating credit for discovery and development of new therapies. J Clin Invest 2019; 129:2172-4. doi: 10.1172/JCI129122 [Crossref] [ Google Scholar]
- Radanovic I, Likic R. Opportunities for Use of Blockchain Technology in Medicine. Appl Health Econ Health Policy 2018; 16:583-90. doi: 10.1007/s40258-018-0412-8 [Crossref] [ Google Scholar]
- Sylim P, Liu F, Marcelo A, Fontelo P. Blockchain Technology for Detecting Falsified and Substandard Drugs in Distribution: Pharmaceutical Supply Chain Intervention. JMIR Res Protoc 2018; 7:e10163. doi: 10.2196/10163 [Crossref] [ Google Scholar]
- Dubovitskaya A, Novotny P, Xu Z, Wang F. Applications of Blockchain Technology for Data-Sharing in Oncology: Results from a Systematic Literature Review. Oncology 2020; 98:403-11. doi: 10.1159/000504325 [Crossref] [ Google Scholar]
- Abu-Elezz I, Hassan A, Nazeemudeen A, Househ M, Abd-Alrazaq A. The benefits and threats of blockchain technology in healthcare: A scoping review. Int J Med Inform 2020; 142:104246. doi: 10.1016/j.ijmedinf.2020.104246 [Crossref] [ Google Scholar]
- Safdari R, Ferdousi R, Aziziheris K, Niakan-Kalhori SR, Omidi Y. Computerized techniques pave the way for drug-drug interaction prediction and interpretation. Bioimpacts 2016; 6:71-8. doi: 10.15171/bi.2016.10 [Crossref] [ Google Scholar]
- Ferdousi R, Safdari R, Omidi Y. Computational prediction of drug-drug interactions based on drugs functional similarities. J Biomed Inform 2017; 70:54-64. doi: 10.1016/j.jbi.2017.04.021 [Crossref] [ Google Scholar]
-
Salemi A, Pourseif MM, Omidi Y. Next-generation vaccines and the impacts of state-of-the-art in-silico technologies. Biologicals 2020. 10.1016/j.biologicals.2020.10.002
- Masoudi-Sobhanzadeh Y, Omidi Y, Amanlou M, Masoudi-Nejad A. DrugR+: A comprehensive relational database for drug repurposing, combination therapy, and replacement therapy. Comput Biol Med 2019; 109:254-62. doi: 10.1016/j.compbiomed.2019.05.006 [Crossref] [ Google Scholar]
- Masoudi-Sobhanzadeh Y, Omidi Y, Amanlou M, Masoudi-Nejad A. Drug databases and their contributions to drug repurposing. Genomics 2020; 112:1087-95. doi: 10.1016/j.ygeno.2019.06.021 [Crossref] [ Google Scholar]