Bioimpacts. 14(6):30136.
doi: 10.34172/bi.2024.30136
Review
A comprehensive review on alpha-lipoic acid delivery by nanoparticles
Navid Mosallaei Data curation, Investigation, Resources, Writing – original draft, Writing – review & editing, 1, # 
Amirhossein Malaekeh-Nikouei Data curation, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft, 2, # 
Setayesh Sarraf Shirazi Data curation, Investigation, Resources, Validation, Writing – original draft, 2
Javad Behmadi Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing, 2
Bizhan Malaekeh-Nikouei Conceptualization, Methodology, Project administration, Writing – review & editing, 1, 3, * 
Author information:
1Department of Pharmaceutics, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
2Student research committee, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
3Nanotechnology Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran
#These authors share the co-first authorships.
Abstract
Alpha-lipoic acid (ALA) has garnered significant attention for its potential therapeutic benefits across a wide spectrum of health conditions. Despite its remarkable antioxidant properties, ALA is hindered by challenges such as low bioavailability, short half-life, and unpleasant odor. To overcome these limitations and enhance ALA's therapeutic efficacy, various nanoparticulate drug delivery systems have been explored. This comprehensive review evaluates the application of different nanoparticulate carriers, including lipid-based nanoparticles (solid lipid nanoparticles, niosomes, liposomes, nanostructured lipid carriers (NLCs), and micelles), nanoemulsions, polymeric nanoparticles (nanocapsules, PEGylated nanoparticles, and polycaprolactone nanoparticles), films, nanofibers, and gold nanoparticles, for ALA delivery. Each nanoparticulate system offers unique advantages, such as improved stability, sustained release, enhanced bioavailability, and targeted delivery. For example, ALA-loaded SLNs demonstrated benefits for skin care products and skin rejuvenation. ALA encapsulated in niosomes showed potential for treating cerebral ischemia, a condition largely linked to stroke. ALA-loaded cationic nanoemulsions showed promise for ophthalmic applications, reducing vascular injuries, and corneal disorders. Coating liposomes with chitosan further enhanced stability and performance, promoting drug absorption through the skin. This review provides a comprehensive overview of the advancements in nanoparticulate delivery systems for ALA, highlighting their potential to overcome the limitations of ALA administration and significantly enhance its therapeutic effectiveness. These innovative approaches hold promise for the development of improved ALA-based treatments across a broad spectrum of health conditions.
Keywords: Alpha-lipoic acid, Drug delivery, Nanoparticles, Bioavailability, Drug release
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
The authors have no funding to disclose.
Introduction
Alpha-lipoic acid (1, 2-dithiolane-3-pentanoic acid) (ALA) also known as thioctic acid is a strong antioxidant that has attracted considerable attention to itself. ALA known as "The universal antioxidant", has turned into a typical component of multivitamin formulations.1-3 ALA comes in two different isomers: R- and S-α-lipoic acid (Fig. 1). R-α-lipoic acid is found in the mitochondria of animals and plants, where it functions as a cofactor for the complexes of pyruvate dehydrogenase (PDH) and other substances such as α-ketoglutarate dehydrogenase.2,4 There is no S-α-lipoic acid in the nature.4 Dihydrolipoate, a reduced version of lipoate, interacts with singlet oxygen, peroxyl radicals, hydroxyl radicals, superoxide radicals, and other various oxygen radicals and it has advantageous roles in recycling vitamin E and safeguarding cell membranes.1 Numerous additional effects of ALA such as chelating metals, improving the handling of glucose and ascorbate, raising endothelial nitric oxide synthase (eNOS) activity, activating phase II detoxification through the transcription factor Nrf2, reestablishing intracellular glutathione levels, and reducing MMP-9 and VCAM-1 expression through repression of NF-kappa-B have been documented.3 Due to the mentioned positive effects, ALA has been demonstrated to be helpful in ischemia-reperfusion injury.5 It prevents diabetic polyneuropathies,6 cataract development, HIV activation, radiation injury,1 obesity, abnormalities in pregnancy,2 neuropathic pain,7 neurodegeneration, autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis, and primary vasculitis, as well as multiple sclerosis,7,8 and has anti-inflammatory and anticancer activities.10
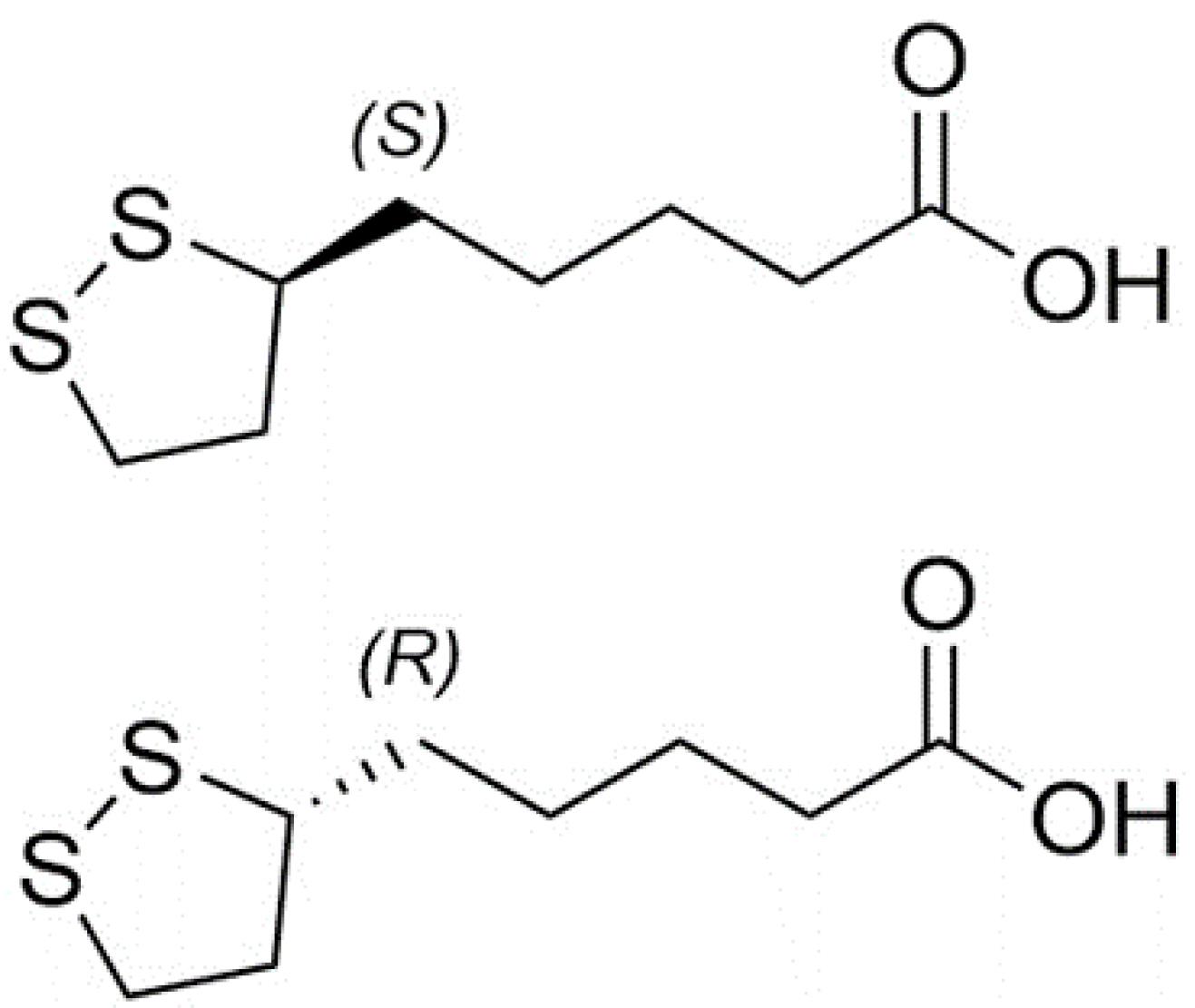
Fig. 1.
Chemical structures of R and S isomers of α-lipoic acid.
.
Chemical structures of R and S isomers of α-lipoic acid.
ALA provides a variety of advantages; however, pharmacokinetic limitations reduce its therapeutic effectiveness. Actually, ALA has a 30% bioavailability.9 In addition to its poor bioavailability, it is also characterized by its unpleasant odor, poor stability, and hepatic degradation which lead to a short half-life.9,10 Better pharmacokinetic parameters are shown by R-α-lipoic acid.2 Nanoparticles are some of the most adaptable and promising drug delivery systems to improve bioavailability, effectiveness, and access to difficult-to-reach areas like the brain.10,11 Docetaxel (DTX) and ALA were co-administered to 4T1 (murine mammary cancer) and MCF-7 (human breast adenocarcinoma) in an in vitro study by Kothari et al to treat breast cancer. The developed solid lipid nanoparticles (SLNs) were used as an example of how effective this combination is in cytotoxic and apoptotic potential as well as absorption efficiency.12 Alvarez-Rivera et al set out to create ALA formulations based on micelles with the goal of boosting apparent solubility by more than ten times when compared to commercially available eye drops. The nanomicelle formulations passed the hen's egg-chorioallantoic membrane (HET-CAM) test effectively and significantly improved ALA flow through the cornea and decreased lag time.13 Besides, nanospheres exhibited exceptional levels of physical and chemical stability in order to serve as a therapeutic agent with antioxidant properties and encapsulation of ALA into these particles, offering a promising approach for the prevention and treatment of diseases associated with oxidative stress.14
Given all of the aforementioned information, it is imperative to develop nanoparticulate delivery technologies to lessen restrictions and increase the effectiveness of ALA (Table 1). Therefore, the goal of this review was to describe several nanoparticulate systems that have been used to increase the therapeutic efficacy of ALA (Fig. 2).
Table 1.
A summary of different studies in ALA nanoformulations and drug delivery systems
|
Type of particle
|
Study model
|
Type of delivery
|
Results
|
Ref
|
| Eudragit E100 films with ALA-loaded nanoparticles |
In vivo
|
Ocular injection |
Concentrations of ALA in cornea ↑
Mucoadhesivity ↑ |
15
|
| Niosomes |
In vivo
|
IP injection |
Brain edema after traumatic injury ↓
Cerebral ischemia ↓ |
16
|
| Self-Nanoemulsifying delivery systems |
In vivo
|
Stomach ulcer injection |
Gastroprotective effects of ALA against indomethacin gastric ulcer ↑ |
17
|
| Liposomes |
In vitro
|
- |
Cisplatin-induced ototoxicity ↓ |
18
|
| Pluronic F127-based nanoparticles |
In vivo
|
Intratympanic injection |
Acute hearing loss ↓
Membrane penetration of ALA ↑
Levels of antioxidant proteins like HO1, Nrf2, SOD1, and SOD2 ↑ |
19
|
| SLNs |
In vitro
|
- |
Cellular uptake of ALA and controlled release of ALA ↑
Apoptosis and cytotoxicity of 4T1and MCF-7 breast cancer cells ↑ |
20
|
| Cationic nano-emulsions |
In vivo
|
Ocular injection |
Corneal epithelium, endothelium and stroma edema, and inflammatory cells ↓ |
21
|
| Gold nanoparticles |
In vitro
|
- |
Expression levels of Runx-2, BMP-2, and OCN ↑
Osteoblast differentiation ↑ |
22
|
| Nanofibers |
In vivo
|
Local implantation |
Regeneration process of the injured sciatic nerve in PNI mouse ↑ |
23
|
| Nanofibrous patches |
In vitro
|
- |
ROS damage ↓
Heart function after myocardial injury ↑ |
24
|
| Lipid nanocapsules |
In vivo
|
Percutaneous injection |
ALA has the potential to be utilized as a formulation for topical administration |
25
|
| NLCs |
In vitro
|
- |
Stability, solubility, and release of ALA ↑ |
26
|
| Nano-capsules |
In vitro
|
- |
Stability, absorptive efficiency, and bioavailability of ALA ↑ |
27
|
| VNP nanomicelles |
In vitro
|
- |
Stabilization and ALA loading in polymeric micelles ↑ |
28
|
| PEGylated nanoparticles |
In vivo
|
Tail vein injection |
DOX-loaded PEG-PαLA NPs displayed effective antitumor activities
PEG-PαLA NPs exhibited reduced side toxicities of DOX |
29
|
| Self-nanoemulsions |
In vivo
|
Transdermal patch |
Transdermal patch could be a successful strategy for the treatment of ED in diabetic patients. |
30
|
| Polycaprolactone nanoparticles |
In vitro
|
- |
Loading of GN with ALA on PCL-NPs could be a successful strategy to inhibit GN nephrotoxicity and extend GN release, which enhances its safety and dose frequency profiles |
31
|
| SLNs |
In vivo
|
- |
Activity of antioxidant enzymes ↑
Hepatotoxicity of tamoxifen ↓ |
32
|
| Soluplus nano micelles |
In vivo
|
Ocular injection |
Performance of ALA compared to currently available eye drop solutions ↑ |
33
|
| Chitosan-coated liposomes |
In vitro
|
- |
Encapsulation efficiency of ALA also increased by interacting with the chitosan shell antioxidant defense mechanisms ↑ |
34
|
| Electrospray system |
In vitro
|
- |
Anti-inflammatory ability of ALA↑ |
35
|
| SLNs |
In vitro
|
- |
Efficiency and effectiveness of ALA topical drug delivery ↑ |
36
|
Abbreviation: IP: intraperitoneal, ALA: Alpha Lipoic Acid, NLC: Nanostructured lipid carrier, DOX: Doxorubicin, PEG: Pegylated, PαLA: Poly α-lipoic acid, GN: Gentamicin, NPs: Nanoparticles, SLN: Solid lipid nanoparticle, VNP: Vinpocetine, PCL: Polycaprolactone
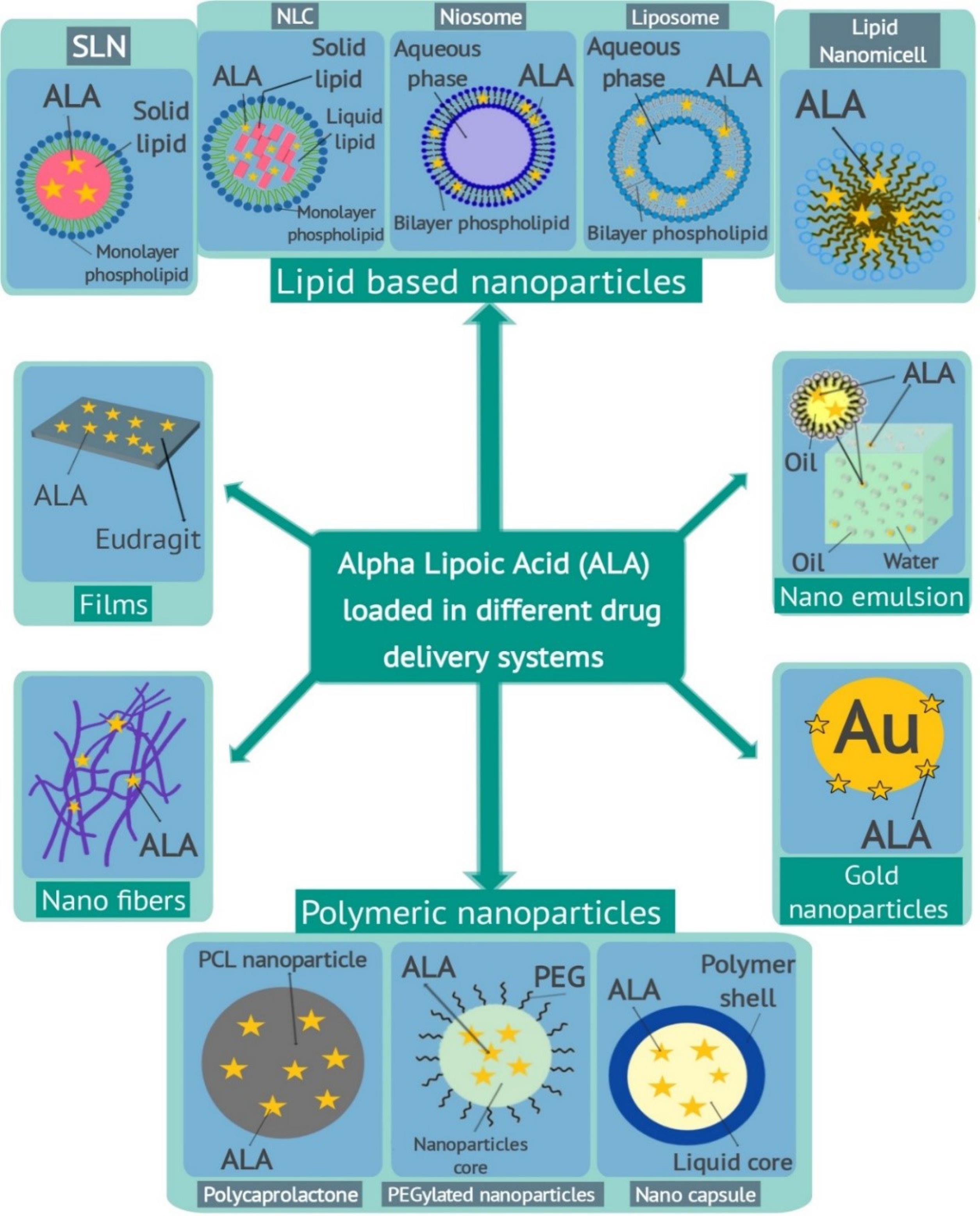
Fig. 2.
A summary of various α-lipoic acid loaded nanoparticles for targeted drug delivery.
.
A summary of various α-lipoic acid loaded nanoparticles for targeted drug delivery.
Search Strategy
A comprehensive search was conducted by using some online databases including PubMed, Scopus, Web of Science, and Google Scholar without date limitation from inception to August 30, 2023, to identify studies on “alpha lipoic acid- nanoparticles” and its diverse therapeutic effects. In this study, all in vitro, in vivo, and clinical studies were considered. In addition, the following medical keywords were investigated and included: "Alpha-Lipoic Acid," "Acid, alpha-Lipoic," "Lipoic Acid," "ALA," "1, 2-dithiolane-3-pentanoic acid," "drug delivery," “nanoparticle,” “nanocarrier,” “lipid nanoparticle,” “dendrimer,” “liposome,” “nanoemulsion,” “nanofibers,” “micelles,” “polymeric nanoparticle,” “quantum dots,” “nanogel,” “nanosphere,” and “nanocapsule.” Then, all relevant original articles were retrieved and based on their contents classified in the body of our article and summarized in Table 1.
Lipid-based nanoparticles carriers
Lipid-based drug delivery systems can be mainly classified into vesicular systems and lipid particulate systems. Here, some of these nanoparticles used as ALA delivery systems are presented and are briefly listed in Table 1.
SLNs
Among the nanoparticles with submicron sizes ranging from 50 to 1000 nm, the significant potential of SLNs as oral drug delivery formulations take them into the center of attention.37,38 Due to their unique property, lymphatic pathways facilitate the transportation of SLNs into the systemic circulation. This mechanism prevents enzymatic degradation in the liver, leading to enhanced bioavailability of the encapsulated drug and a reduction in dose-dependent toxicity.39 As mentioned before, ALA has a low stability, therefore ALA as an antioxidant agent has a great potential to be encapsulated in SLNs. Dhaundiyal et al investigated entrapped ALA into SLNs in SD rats via oral administration and while tamoxifen served as a drug model. Prepared SLNs exhibited a spherical morphology with a smooth surface. The average size of nanoparticles was 261.08 ± 2.13 nm, with an entrapment efficiency of 40.73 ± 2.83%. The release of the drug in tests was slow and pH-dependent, resulting in a 1.59-fold increase in bioavailability compared to the regular suspension. Results proved that the ALA-loaded SLNs reduce the activity of ALT and AST enzymes in the liver. Furthermore, the levels of TBARS, GSH, GPx, CAT, and SOD as antioxidant enzymes were significantly higher than the normal range. So, these results suggest that ALA has hepatoprotective action against tamoxifen hepatotoxicity and besides that, ALA-SLN shows promise in improving the oral bioavailability of tamoxifen but more studies are recommended for depicting the molecular mechanisms that may provide deeper insight toward improved therapeutic outcomes.40
Due to their special properties, SLNs represent great potential for topical drug delivery and cosmeceuticals.41 In fact, SLNs provide UV-resistant properties which may lead to better skin hydration.42 ALA is an antioxidant that inhibits chemical bonds between proteins and other macromolecules which are responsible for the aging process by causing wrinkling of skin and stiffening of joints.43 So the interest in ALA has been growing significantly for skin care products, and this study reached the conclusion that application of ALA with SLNs represents a magnificent formulation for skin rejuvenation and treating its disorders.36
Niosomes
Vesicular systems represent innovative approaches for the controlled delivery of drugs in order to improve their bioavailability and extend their therapeutic effects over a longer period of time.44 Niosomes are an important class of lipid-based vesicular systems and with their special and unique benefits for targeting drug delivery, they have earned much attention over the past 35 years.44-46 The physicochemical stability of antioxidants in lipid-based vesicular systems was investigated to enhance their bioavailability.47 It has been declared that niosomes have great potential for delivering antioxidants through various administration routes, such as intravenous injections, topical applications, and oral ingestion due to their unique properties.47,48 In a study by Raeisi Estabragh et al, the application of ALA in niosomal form to treat cerebral ischemia has been declared.16 Cerebral ischemia is a neuronal disorder, to which eighty percent of strokes are related.49 Free radicals such as hydroxyl and peroxide have a major role in the incidence of cerebral ischemia.50 So, antioxidants like ALA may have some therapeutic effect on it.51 In the mentioned study, encapsulation of ALA into the niosomes increased ALA's bioavailability and penetration into the rats' CNS and controlled its therapeutic activity. In fact, ALA is a lipid-soluble antioxidant.16 However, it is not suitable for intravenous injection to provide a proper concentration in damaged brain tissues.52 The film hydration technique is used to produce niosomes containing ALA. The encapsulation efficiency percentage was 94.5 ± 0.2%. In vitro release studies showed 59.27 ± 5.61% release during 240 minutes, suggesting potential applications in treating brain injuries and neurodegenerative diseases caused by oxidative stress and free radical generation. The ability of surfactants to create bilayers and the presence of cholesterol, as the major sterol in biological membranes, in the niosomal structure allows for the drug carrier to pass through the blood-brain barrier, making it an effective targeted drug delivery system to CNS.53,54 So the administration of ALA niosomes decreased shrunken neurons and significantly increased viable cells, and ischemia could be treated afterward.16
Nanostructured lipid carriers (NLCs)
Multiple studies have demonstrated that lipid nanoparticles can serve as carriers to address challenges associated with delivering lipophilic compounds to the intended site.55 These particles, called NLCs, are composed of lipids, surfactants, and phospholipids that are both biodegradable and biocompatible.56 NLCs are drug delivery systems that consist of both solid and liquid lipids.26 They have the ability to enhance the absorption of topical medications, reinforce their protective properties, and minimize adverse effects.57 Common solid components include glyceryl behenate, glyceryl palmitostearate, waxes, fatty acids, and steroids. The liquid oils used are typically digestible oils obtained from natural sources. In a study by Wang et al, NLCs containing ALA were prepared and their stability, solubility, and compatibility with HaCaT cells during co-incubation were investigated. HaCaT cells are a type of permanent epithelial cell line derived from adult human skin.58 These cells were typically cultured in a 90% minimum essential medium. The study also investigated the sustained release of ALA in aqueous media using nanoparticle-mediated delivery techniques. By addressing challenges such as poor solubility, sensitivity, unfavorable bioavailability, and physicochemical instability, the NLC formulation showed promise in enhancing the efficacy of active ingredients like ALA in biomedical and industrial applications.26 ALA is suitable for transdermal administration due to its favorable physicochemical properties. However, the hydrophobic nature of the material and its susceptibility to degradation from sunlight restrict its application.59,60 Nano lipid carriers can efficiently encapsulate drugs that are both hydrophilic and hydrophobic. Due to the presence of a solid matrix, these lipid nanocarriers can also immobilize the drug and prevent the accumulation of nanoparticles over a prolonged period.61 However, research indicates that when nanoparticles are applied topically, they are rapidly eliminated by the skin's defense mechanisms due to their low viscosity. To address these limitations, a semi-solid system, such as a hydrogel, has been developed as a carrier for NLCs. This system has the appropriate viscosity and mechanical strength to prolong the retention of nanoparticles in the targeted area and enhance the duration of the drug's efficacy.62-65 Hydrogel comprises a combination of natural and synthetic components and as an important biomaterial, it has found extensive utilization in pharmaceuticals.66,67 In a study by Li et al, a hydrogel of ALA-NLC was prepared for topical drug delivery to the skin.68 The researchers produced ALA-NLC, a lipid nanoparticle with a mean particle diameter of 149.7 ± 5.4 nm, through the process of hot high-pressure homogenization. The stability of ALA-NLC is influenced by its zeta potential which exhibited a negative charge. The formulation demonstrates practicality, efficiency, and exceptional biocompatibility with HaCaT cells. The research investigated the release kinetics of ALA from a dialysis bag and found that the ALA-NLC formulation exhibited a significant delay in release compared to the free ALA. The ALA-NLC formulation demonstrated a prolonged release profile lasting up to 72 hours, with more pronounced sustained release characteristics. Additionally, the ALA-NLC formulation effectively prevented an initial burst release of 30.3% of free ALA (within the first 30 minutes), in contrast to the significantly reduced burst release of only 6.9% in the first 30 minutes. This hydrogel has a positive effect on induced edema and erythema in animal models.
Liposomes
The initial study on enlarged phospholipid systems was conducted by Alec Bingham and his colleagues.69 Following that, the terms "bangosome" and "liposome" were used to describe any structure consisting of a phospholipid bilayer with two monolayers.70,71 Later on, Gregory Grigoriadis suggested that liposomes could serve as vehicles for drug delivery.72 Liposomes were subsequently employed as a technique to enhance drug absorption through the skin.73 Liposomes have been extensively used for their diverse characteristics. Some studies focus on the effects of skin protective agents containing chitosan (CH), coenzyme Q10 (CoQ10), and ALA on the skin.74-76 CoQ10 is essential for cellular functions and has antioxidant characteristics that prevent reactive oxygen species from forming. ALA directly neutralizes free radicals and safeguards cells from oxidative damage. Both CoQ10 and ALA have significant roles in cellular processes. The combination of CoQ10 and ALA can boost the body's antioxidant defense mechanisms. However, both CoQ10 and ALA have low solubility in water and are unstable. CH has a positive charge. The liposome is coated with an adhesive material that can modify the surface charge of the liposomes and penetrate the skin barrier. In this study, CH-coated liposomes incorporating both CoQ10 and ALA were developed as a potential system for transdermal drug delivery. It was discovered that CH can create a polymer shell around the liposome, significantly improving its stability in terms of both chemical and physical properties. The dynamic light scattering technique was employed to measure the size, polydispersity index, and zeta potential of vesicles. The liposomes containing both CoQ10 and ALA exhibited a mean size of 183.4 ± 2.1 nm and a polydispersity index (PDI) of 0.28±0.03. Upon coating the CoQ10 and ALA vesicles with CH, it was observed that increasing the CH concentration from 0.1 to 0.3 (w/v %) led to a significant increase in both the size and polydispersity index compared to CoQ10 and ALA. Specifically, at a CH content of 0.5 (w/v %), the vesicle size increased to 279.9±2.3 nm with a PDI of 0.28±0.04. However, further increasing the CH concentration to 0.7 (w/v %) and 1 (w/v %) resulted in an upward trend in both PDI and size of the CH-coated CoQ10 and ALA vesicles. The study showed that liposomes containing both CoQ10 and ALA improved the accumulation and absorption of CoQ10 and ALA in rabbit skin and could enhance antioxidant defense mechanisms. This indicates that liposomes can be highly efficient in the delivery of these two substances and improve their pharmacological effect in the body, but authors of this study also suggest that further in vivo experiments will need to evaluate the therapeutic performance of the designed liposomes by determining the pharmacokinetics profile and the protective activity.34
Lipid nanomicelles
Nanomicelles are considered some of the most promising strategies for topical drug delivery.77 These nanoparticles enable controlled release at the targeted site and besides, they can protect the drug from degradation and ensure a consistent therapeutic concentration within the tissue.78 In a study by Rivera et al, ALA was encapsulated by nanomicelles, and its therapeutic effect on diabetes-associated corneal disease was evaluated.13 Nanomicelles, as some of the most promising strategies for ophthalmic formulation, can be considered due to their ability to enhance the solubility of hydrophobic agents in aqueous environments, extend retention time on the ocular surface, and improve their ability to penetrate and be absorbed by tissues.79,80 In the eyes, ALA attenuates vascular injuries and subretinal space and rod alterations that happen by oxidative stress.81 Besides, ALA can reduce vascular damage and proliferation in the membrane of the corneal nerve fiber82 So, it can be considered that ALA probably has some benefits for eye disorders. In the mentioned study, formulation of ALA into the Soluplus nanomicelles is considered non-irritating for the eye, and its application may be an advantageous choice to current formulation strategies of ALA for the treatment of retinal and corneal diseases, especially those related to diabetes. In the above study, the formulation of ALA into Soluplus nanomicelles significantly increased the solubility of ALA by more than 10 times. Furthermore, these nanomicelles exhibit temperature-dependent rheological properties, transitioning from a free-flowing liquid state to weak gels at the temperature of the ocular surface. This characteristic may have positive implications for the duration of drug residence in the eye. The use of Soluplus nanomicelles also leads to a notable improvement in the permeation of ALA through the cornea, reducing the lag time. Additionally, these nanomicelles demonstrate stability when subjected to strong dilution, filtration through sterilizing membranes, freeze-drying, and application of it may be an advantageous choice to current formulation strategies of ALA for the treatment of retinal and corneal diseases, especially those related to diabetes.13 Schematic application of ALA nanoparticles in ophthalmic cases has been summarized in Fig. 3.
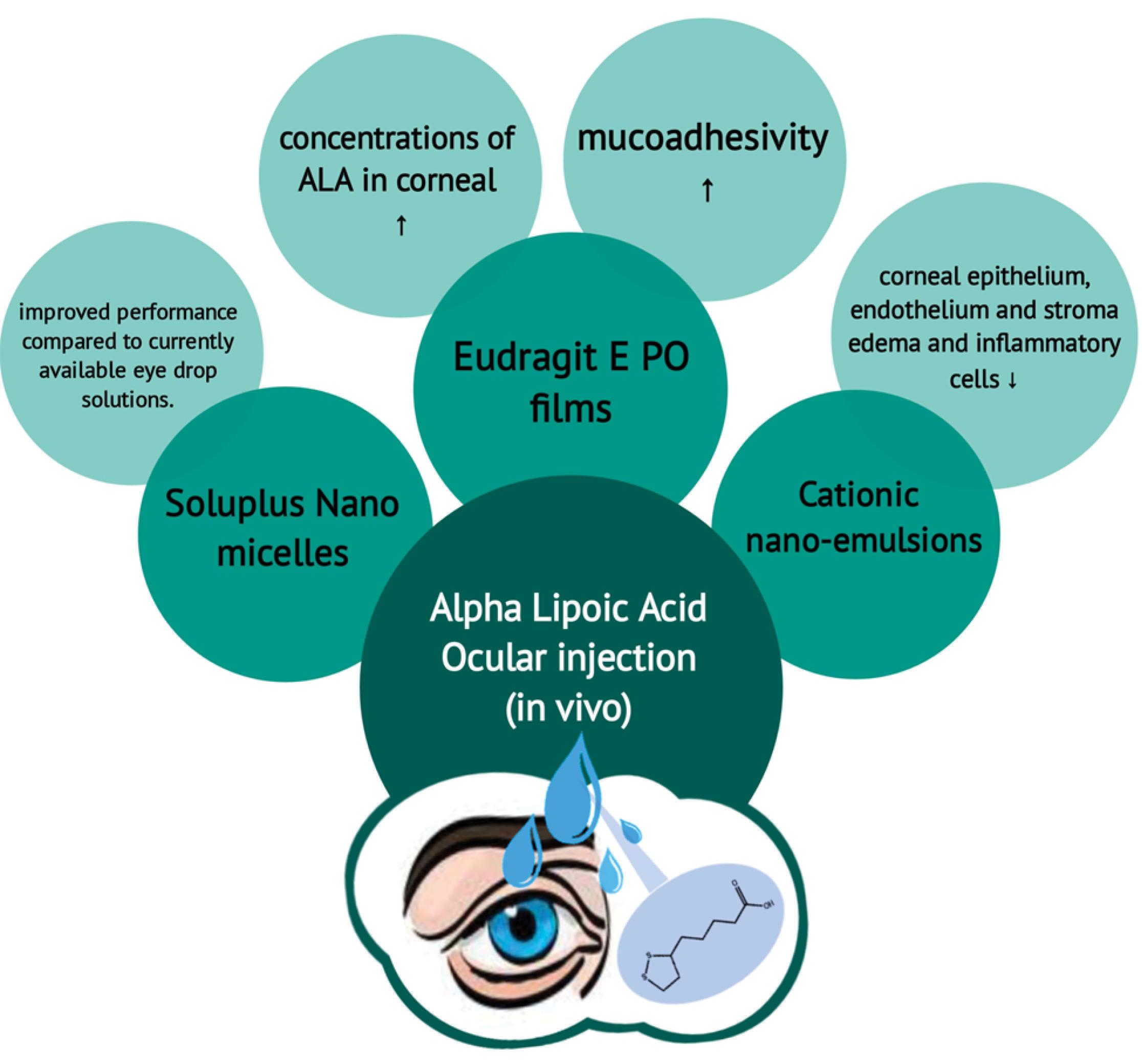
Fig. 3.
Overview of numerous ocular drug delivery systems of α-lipoic acid.
.
Overview of numerous ocular drug delivery systems of α-lipoic acid.
Nanoemulsions
In recent years, application of the nano-sized emulsions has been studied.83 The advantages of this system include: sub-micrometer droplet size range, natural biodegradability, sterilizability, and improved bioavailability.84,85 Due to these advantages, the nano-sized emulsion has gained recognition as a promising drug delivery vehicle or carrier, especially for topical and ocular forms.86 As a powerful antioxidant, ALA can be used for ophthalmic complications, and it leads to capillary injury relief and corneal epithelium reparation, and it reduces vascular proliferation through the regulation of vascular endothelial growth factor levels.87,88 In a study by Mahmoudi et al, encapsulation of ALA into cationic nanoemulsions for the preparation of an efficient ophthalmic formulation has been studied.21 Cationic nanoemulsions as a kind of cationic carrier engage in electrostatic interactions with the negatively charged cell membrane that lead to prolonged residence time and better penetration efficiency and bioavailability.89,90 The average size of the nanoparticles in the ALA-containing cationic nanoemulsion was found to be 289.13 ± 4.55 nm, while the zeta potential was measured to be 40.5 ± 1.10 mV. It was observed that the entrapment of ALA in the nanoparticles resulted in an increase in their size by approximately 150 nm. However, this entrapment did not have any effect on the surface charge of the nanoparticles, which was expected due to the presence of cetalkonium chloride as a cationic preservative and agent. The water solubility of ALA was significantly enhanced by cationic nanoemulsion, resulting in a 7-fold increase with a loading efficiency of 61%. An assessment of rheological parameters revealed that the entire tear film demonstrates non-Newtonian shear-thinning characteristics, implying that the viscosity of tears decreases when subjected to the shear rate during eyelid motion. The osmolality of the formulated solution was measured to be 595.66 ± 2.30 mOsm/kg, indicating that it falls within a non-irritant range and is therefore suitable for use. The release study was carried out at a controlled temperature of 34 ± 0.5 °C, specifically targeting the ocular surface. During the experiment, it was observed that up to 10% of the entrapped ALA was released within the specified time frame. After 6 hours, the release of ALA reached a plateau, indicating that the nanoemulsions functioned as a reservoir and facilitated a sustained release pattern. Application of ALA in rabbits’ eyes showed a reduction in corneal epithelial, endothelial, and stromal edema, as well as inflammatory cells.21 In another research by Jung et al, the usage of ALA for treating acute hearing loss has been surveyed. During this study, ALA was encapsulated by Pluronic F-127 nanoparticles which were fabricated by an oil-in-water emulsion method.91 These nanoparticles were 87.3 ± 5.3 nm in diameter and had a spherical shape which leads to a slower and prolonged release of ALA. As an antioxidant, ALA can preserve ototoxicity by increasing the level of antioxidant proteins HO1, Nrf2, SOD1, and SOD2.92,93 But as we mentioned before, systematic administration of ALA has several drawbacks as the drug is only minimally absorbed by the cochlea, due to the blood–labyrinthine barrier.94 But usage of nanoparticles in the inner ear in several cases showed that we can overcome these obstacles.95,96 This study concluded that ALA has great potential for treating hearing loss by loading in emulsion-made nanoparticles. Although the authors of this study mentioned that they were unable to measure the ALA concentration in the inner ear tissue, further studies are needed to find a proper therapeutic dose for the injection of ALA nanoparticles into the inner ear.91
Polymeric nanoparticles
In recent years, there has been significant interest in polymeric nanoparticles due to their small size and unique characteristics.97-99 In order to safeguard pharmaceuticals and other biologically active compounds from potential environmental degradation, actions have been taken. Polymeric nanoparticles can improve bioavailability and therapeutic effectiveness.97,100 The term "nanoparticle" encompasses both nanospheres and nanocapsules, which have distinct differences in terms of their shapes. Nanocapsules are composed of a central oily core that contains the drug, enclosed by a polymer shell that controls the drug's release. On the other hand, nanospheres are composed of a continuous polymer network where the drug can either be encapsulated within or adsorbed onto their surface.101-103 Among the types of polymeric nanoparticles, three types of nanocapsules, PEGs, and polycaprolactone can be mentioned.
Nanocapsules
A nanocapsule is a minuscule shell made of a biocompatible polymer material at the nanoscale. Vesicular systems consist of a polymeric membrane that surrounds a small liquid core at the nanoscale. Nanocapsules have various applications, such as potential medical uses for drug delivery, fortifying food, delivering nutrients, and creating self-healing materials. The benefits of using encapsulation methods to protect these materials in harsh conditions, incorporation of controlled release mechanisms, and precise targeting strategies is an essential aspect in various fields of research and application.104 The utilization of therapeutic products based on nanoparticles has risen in recent times. Nanomaterials possess unique physical and chemical characteristics, such as their small size, high surface-to-mass ratio, and strong reactivity, which distinguish them from bulk materials. Nishiura et al have developed a novel nanoparticle to enhance the effectiveness of ALA and promote its application in pharmaceuticals, supplements, and cosmetics. They have also developed a new nanocapsule that contains ALA through a process of self-assembly. The physicochemical characteristics of nanocapsules and the impact of nanoencapsulation contribute to the stability of ALA.105 The degradation of ALA is undesirable because it does not only lead to a loss of its content but also results in the release of sulfur compounds that have an unpleasant odor. Additionally, direct administration of ALA can irritate the skin and mucous membranes due to its acidic nature. These issues make it challenging to administer ALA orally or topically. Therefore, researchers are seeking assistance from a carrier in the form of a nanocapsule. In one of recent studies, the nanocapsule had a spherical shape with a size of 10 nm, and nano-encapsulation of ALA by freeze-drying improved its stability up to 60 °C. As an amphiphilic particle, ALA has the ability to form micelles with a nonionic surfactant like sucrose laurate, polyoxymethylene, stearyl ether, polyoxymethylene, and hydrogenated castor oil that maintain its stable dispersion in an ionic solution. The nanocapsule exhibited satisfactory dispersion in water without aggregation and greatly improved the stability of ALA in both liquid and solid states. The researchers emphasize that this enhanced stability is achieved through their encapsulation technology which can lead to improved absorptive efficiency and bioavailability.27
PEGylated nanoparticles
Polyethylene glycol (PEG) is a water-soluble polymer that is highly compatible with biological systems and has low toxicity. It is commonly used for bioconjugation due to its non-immunogenic properties. PEG has been approved by the Food and Drug Administration (FDA) for drug delivery applications and has been successfully used in lipid-based nanoparticle formulations in clinical settings. Various types of nanoparticles, including liposomes, metallic nanoparticles, and synthetic polymers, have been modified with PEG. The ALA nanoparticles which are PEG-modified have shown improved physicochemical properties, biocompatibility, and serum stability. The addition of PEG to the surface of nanoparticles can result in a "stealth" behavior in vivo, but the extent of this behavior depends on factors such as the material being encapsulated.106,107 The primary function of PEG is to prevent surface-surface interactions that can cause particle aggregation, as mentioned by Dos Santos et al.29 In the mentioned study, by increasing ALA's temperature over its melting point, it can self-polymerize into poly-ALA. Then PEG conjugates onto the poly-ALA backbone via esterification reaction and after freeze-drying, the PEG-poly-ALA was obtained in the form of a white powder. The obtained PEG-poly-ALA could self-assemble into nanoparticles in an aqueous environment due to the presence of both hydrophilic PEG chains and hydrophobic poly-ALA backbone in the polymer structure. These nanoparticles were spherical which showed a 3 times longer half-life and more accumulation of ALA in the blood.
Polycaprolactone nanoparticles
Polycaprolactone (PCL) is a biodegradable and bioresorbable polymer. It can stimulate collagen production and is classified as an aliphatic polyester and is widely recognized in the field of polymers.108 A study investigated the development of a biodegradable PCL nanoparticle formulation using ALA and gentamicin (GN).31 GN is a member of the aminoglycoside class of antibiotics that exerts its antimicrobial activity by irreversibly inhibiting bacterial protein synthesis, binding with the 30S subunit, resulting in bacterial death. It is commonly used to treat infections caused by Gram-negative bacteria in the urinary tract, soft tissues, and upper respiratory tract. However, the use of GN is associated with various adverse effects, including neuromuscular problems, and nerve and kidney damage.109 GN has the potential to impact the cells in the proximal tubules. It is easily filtered by the glomeruli and then transported to the proximal tubular epithelial cells. This occurs after the initial interaction with phospholipids present on the brush border membranes. GN disrupts the metabolism of phospholipids in tubular cells. Additionally, GN leads to vasoconstriction in the kidneys.110,111 Previous research has indicated that ALA can protect against the harmful effects of gentamicin on the kidneys. Sandhya and Varalakshmi found that ALA can protect the basal and brush border membranes from damage caused by gentamicin. However, no previous studies have examined the overall kidney function to determine the extent of ALA's protective effect against gentamicin-induced nephrotoxicity. Additionally, to date, there are no reports investigating the combined use of ALA and gentamicin. Furthermore, the delayed release of GN offers the advantage of reducing the frequency of administration and enhancing convenience for patients. The objective of this research was to examine the kidney protective properties of GN-ALA. ALA was encapsulated with GN onto PCL-NPs by the solvent evaporation technique, and the size of GN-ALA-PCL NPs was in the range of 540.4±40.4 nm, with a zeta potential about 21.2±2.1 mV. These NP formulations result in spherical particles with regular edges and a sustained release pattern with no significant change in particle size or zeta potential. GN-ALA is also used in the treatment of various conditions, including neurological dysfunction and cancer. The study affirms the protective impact of ALA on GN-induced kidney damage. Additionally, the formulation of GN-ALA-PCL nanoparticles demonstrated satisfactory and high encapsulation efficiency percentages. It also exhibited a prolonged release pattern and maintained a stable composition.112 This is because the small size of the PCL enables it to trap, bind, and stabilize proteins within cells, as well as escape from lysosomes after treatment is terminated. Consequently, this leads to improved effectiveness and availability of the formulated drug.113-115
Films
In recent years, films as oral, vaginal, and ocular drug delivery systems gained a lot of attention.116-118 Due to their versatility and the combined potential of using permeation enhancers and mucoadhesive polymers, films are widely used to formulate biological substances using alternative routes.119 In new research, Eudragit films loaded with nanoparticles were used as carriers for ALA ocular drug delivery.15 Eudragit shows highly adaptable mechanical characteristics and excellent conditions for its use in drug-controlled release and has favorable behaviors in the formulation of various active ingredients.120 The application of these polymers in ophthalmic drug delivery has great potential due to their non-toxic nature, as well as their notable mucoadhesive properties and ability to facilitate the controlled release of drugs.121,122 As we mentioned before, ALA has different characterizations that we can use against eye disorders.87,88 In this study, PEGylated Eudragit films loaded with nanoparticles had different sizes ranging from 110 to 745 nm with a negative zeta potential value. The in-situ release of particles suggests that there is a good interaction between the nanometric structures of ALA and Eudragit. Also, it was mentioned that higher ALA concentration increased the degree of films’ swelling and may act as a plasticizer for Eudragit. After ALA was added to films it was found to have a plasticizing effect. Higher concentrations of ALA component result in increased flexibility of the films, accelerating mucoadhesivity, and improved film disintegration time, diffusion, and corneal permeability. All of the mentioned changes led to an increase in ALA.15
Nanofibers
Drug delivery systems are typically nanostructures that can be filled with small or large molecules. They act as carriers for specific compounds used in pharmaceutical administration. Currently, they are considered highly promising areas for advancing biomedical research.123 These materials can transport a chemotherapy drug to a specific location, leading to increased drug concentrations that can be released in a controlled manner. Electrospinning is a simple technique for producing nanofibers with interconnected pores in the nanoscale range.124 Electrospinning produces nanofibers that have a high surface area to volume ratio and a porous structure between fibers, facilitate easy mass transfer, provide flexibility in handling, can be customized in shape, and exhibit strong mechanical properties. Nanofibers possess characteristics that make them suitable for a variety of biomedical applications, including therapeutic patches or mats125,126 Nonwoven fibers are commonly used in the production of fibers with sizes ranging from nanometers to microns. Electrospinning technology has become a versatile method for creating films that are several microns thick and contain nanofibers at the sub-micron scale. In this process, polymers such as poly lactic-co-glycolic acid (PLGA) are used.127 PLGA copolymers result from polymerization of lactic acid and glycolic acid. PLGA possesses desirable characteristics such as appropriate degradability and excellent biocompatibility, making it a popular choice for manufacturing products that can undergo controlled release and degradation.128 PLGA is known for its ability to form films and act as a carrier for vesicles. It is widely used in the manufacturing of environmentally friendly composites, such as synthetic channels, controlled-release drug carriers, and scaffolds for tissue engineering.129 ALA was incorporated into PLGA nanofibers to enhance rapid action in the initial stage of peripheral nerve injury (PNI). Conversely, CH nanoparticles loaded with atorvastatin were incorporated into PLGA nanofibers to achieve a sustained release pattern after PNI.130 In this study, composite nanofibers were fabricated by embedding atorvastatin-loaded CH nanoparticles into PLGA nanofibers wherein ALA was encapsulated. These nanoparticles were prepared by spray drying. After conducting the necessary tests to determine cytotoxicity, the effectiveness of the composite nanofiber formulations was examined. PNI is a common issue that impacts both humans and animals, resulting in the impairment of functional and sensory capabilities. PNI can cause dysfunction in motor, sensory, and autonomic functions in related organs. The clinical outcomes of PNI have a significant impact on quality of life and impose a financial burden on healthcare systems. PNI can be caused by various types of traumas, including accidents or surgical procedures. PNI accounts for approximately 2.8% to 5% of all traumatic cases.131 The estimates suggest that over half a million individuals are impacted by PNI each year.132,133 The use of electrospun composite nanofibers showed potential for promoting the regeneration of the sciatic nerve by treating areas of axonal loss and transection. The mentioned study demonstrated the efficacy of the collective impacts of various neuroprotective medications with distinct release profiles on the process of nerve regeneration. However, the selection of a particular method to investigate the neurofunctional recovery after neuronal intervention in rat sciatic nerve injury model remains a challenge for researchers and the authors indicated that in the longer treatment window of ALA-nanofibers, there was no considerable difference in the results of tissue recovery. So, utilization of any particular method to evaluate nerve regeneration is suggested in future studies 134
Gold nanoparticles
Gold nanoparticles (GNPs) were first discovered by Michael Faraday in the 19th century.135 These particles have unique properties, including surface plasmon resonance, surface modification, and the ability to bind amine and thiol groups. GNPs can be utilized as carriers for drugs, contrast agents, and photochemical agents. A study by Pan et al demonstrated promising results in the treatment of osteoporosis using ALA-GNP. Osteoporosis is a common condition that affects older individuals. It is estimated to affect 200 million women and men worldwide, especially individuals over the age of 60. According to the International Osteoporosis Foundation, approximately 33% of women and 20% of men aged 50 and above will encounter fractures associated with osteoporosis during their lifespan.136 The development of osteoporosis is influenced by oxidative stress, which occurs due to the excessive buildup of reactive oxygen species (ROS).
ALA, a natural antioxidant found in the body, has the ability to relieve the presence of ROS and counteract the detrimental effects of oxidative stress, it is imperative to implement measures that result in reduction of ROS levels. The impact of ALA on osteoblasts is also a topic of interest because of its potent antioxidant properties. ALA can reduce intracellular oxidative stress and promote the growth and differentiation of osteoblasts. These nanoparticles have minimal toxicity, can be easily produced, and are compatible with living organisms, making them commonly utilized in medical fields such as disease detection and cellular imaging. Researchers found that there are limited nanoparticles available for the treatment of osteoporosis in the field of orthopedics.137 However, GNPs show promise in the field of intracellular oxidative stress and tissue engineering regenerative medicine.138 GNPs have a high photothermal conversion efficiency due to their localized surface plasmon resonance property, making them suitable for phototherapy.139 The particle size of GNPs was about 54.6 nm with a PDI of 0.059. Besides, the morphology of GNPs was spherical and hollow leading to higher drug loading capacity and sustained-release effect of ALA. The average zeta potential for GNPs was about 20.62 mV, and the maximum absorption wavelength was 777 nm. This study suggests that PEGylated ALA-loaded hollow GNPs could be a potential treatment for osteoporosis.22
Perspectives and Future Directions
According to the research conducted on the impact of ALA nanoparticles and the subsequent findings, several nanoformulations have been investigated. Various types of nanoparticles, such as NLCs, SLNs, liposomes, lipid nanomicelles, nanoemulsions, nanofibers, some polymeric nanoparticles, etc., have been developed. These nanoparticles have shown increased effectiveness of ALA in the human body. The utilization of these nanoparticles has demonstrated the significant impact of ALA on the body, leading to improved bioavailability and enhanced biological effects. Therefore, it is recommended that future articles and studies undertake additional research to investigate the potential benefits of other nanoparticles that contain ALA. This review shows that ALA is a good candidate for safe and efficient ophthalmic drug delivery systems to cure numerous eye diseases due to its great ability as an antioxidant. So, we recommend more studies on these systems and trying other nanoparticles and their method of synthesis to reach the best performance. This research aims to validate the positive effects of ALA nanoparticles and emphasizes the need for further clinical and laboratory investigations in the human body due to the absence of clinical studies. The conducted research demonstrates potential benefits for future studies that aim to address the existing gaps and enhance the performance of ALA nanoparticles. This will facilitate the observation of the optimal performance of the nanoparticles in future investigations.
Concluding remarks
Although ALA possesses numerous advantageous characteristics, there exist certain limitations pertaining to its absorption, bio-distribution, and dissolution. However, these issues can potentially be addressed through the utilization of various drug delivery systems. This comprehensive review evaluated these methods and classified their respective advantages and disadvantages. These systems improved the controlled/sustained release of ALA and gained better therapeutic efficiency. The encapsulation of ALA into different forms of polymeric nanoparticles like PEG, nanocapsule, and polycaprolactone led to a stable release pattern and enhanced its absorptive efficiency. ALA-loaded lipid-based nanocarriers (SLNs, niosomes, liposomes, NLCs, and micelles) can be used for better topical and systematic use of ALA against different sets of disorders like CNS and skin. Besides, the application of ALA in nano-emulsions has improved properties such as the enhanced ability to penetrate and bioavailability which can be used for acute hearing loss and ocular disorders. ALA nanofilms enhanced the adhesive properties of mucosal surfaces and reduced film disintegration time and permeability across the cornea. The utilization of ALA nanofibers has been found to significantly expedite the initial phase of PNI due to their enhanced rapid action. ALA-GNP has been found to enhance the proliferation and maturation of osteoblasts while exhibiting low levels of toxicity, making it a potential therapeutic option for individuals with osteoporosis. Based on the existing literature, the utilization of nanoformulations has been demonstrated to enhance the effectiveness of ALA in the human body, leading to improved bioavailability and biological effects. Consequently, it is recommended and imperative that further preclinical and clinical investigations be undertaken to validate the beneficial effects of ALA nanoparticles.
Review Highlights
What is the current knowledge?
√ ALA is an antioxidant with many different therapeutic effects in the body that leads to consideration as a potential target to treat various disorders.
√ Application of nanomedicine for the delivery of ALA to the body may improve its beneficial effects, but there was no study to classify the cons and pros.
What is new here?
√ Names of the diseases that can be treated by ALA-nanoparticles with their brief mechanism.
√ Various targeted drug delivery systems of ALA and description of their advantages and disadvantages over others.
√ Direction to future clinical studies and ophthalmic drug delivery due to the ability of ALA.
Acknowledgments
The authors would like to appreciate Mashhad University of Medical Sciences, Iran, for their supportive role during this study.
Competing Interests
There are no conflicts of interest in this study.
Ethical Statement
None to be declared.
References
- Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med 1995; 19:227-50. doi: 10.1016/0891-5849(95)00017-r [Crossref] [ Google Scholar]
- Salehi B, Berkay Yılmaz Y, Antika G, Boyunegmez Tumer T, Fawzi Mahomoodally M, Lobine D. Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules 2019; 9:356. doi: 10.3390/biom9080356 [Crossref] [ Google Scholar]
- Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta 2009; 1790:1149-60. doi: 10.1016/j.bbagen.2009.07.026 [Crossref] [ Google Scholar]
- Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic Acid. Front Pharmacol 2011; 2:69. doi: 10.3389/fphar.2011.00069 [Crossref] [ Google Scholar]
- Ghibu S, Richard C, Vergely C, Zeller M, Cottin Y, Rochette L. Antioxidant properties of an endogenous thiol: Alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J Cardiovasc Pharmacol 2009; 54:391-8. doi: 10.1097/fjc.0b013e3181be7554 [Crossref] [ Google Scholar]
- Capece U, Moffa S, Improta I, Di Giuseppe G, Nista EC, Cefalo CMA. Alpha-Lipoic Acid and Glucose Metabolism: A Comprehensive Update on Biochemical and Therapeutic Features. Nutrients 2022; 15:18. doi: 10.3390/nu15010018 [Crossref] [ Google Scholar]
- Liu W, Shi LJ, Li SG. The Immunomodulatory Effect of Alpha-Lipoic Acid in Autoimmune Diseases. Biomed Res Int 2019; 2019:8086257. doi: 10.1155/2019/8086257 [Crossref] [ Google Scholar]
- Xie H, Yang X, Cao Y, Long X, Shang H, Jia Z. Role of lipoic acid in multiple sclerosis. CNS Neurosci Ther 2022; 28:319-31. doi: 10.1111/cns.13793 [Crossref] [ Google Scholar]
- Brufani M, Figliola R. (R)-α-lipoic acid oral liquid formulation: pharmacokinetic parameters and therapeutic efficacy. Acta Biomed 2014; 85:108-15. [ Google Scholar]
- Metwaly HH, Fathy SA, Abdel Moneim MM, Emam MA, Soliman AF, El-Naggar ME. Chitosan and solid lipid nanoparticles enhance the efficiency of alpha-lipoic acid against experimental neurotoxicity. Toxicol Mech Methods 2022; 32:268-79. doi: 10.1080/15376516.2021.1998275 [Crossref] [ Google Scholar]
- Abdelkader NF, El-Batal AI, Amin YM, Hawas AM, Hassan SHM, Eid NI. Neuroprotective Effect of Gold Nanoparticles and Alpha-Lipoic Acid Mixture against Radiation-Induced Brain Damage in Rats. Int J Mol Sci 2022; 23:9640. doi: 10.3390/ijms23179640 [Crossref] [ Google Scholar]
- Kothari IR, Mazumdar S, Sharma S, Italiya K, Mittal A, Chitkara D. Docetaxel and alpha-lipoic acid co-loaded nanoparticles for cancer therapy. Ther Deliv 2019; 10:227-40. doi: 10.4155/tde-2018-0074 [Crossref] [ Google Scholar]
- Alvarez-Rivera F, Fernández-Villanueva D, Concheiro A, Alvarez-Lorenzo C. α-Lipoic Acid in Soluplus(®) Polymeric Nanomicelles for Ocular Treatment of Diabetes-Associated Corneal Diseases. J Pharm Sci 2016; 105:2855-63. doi: 10.1016/j.xphs.2016.03.006 [Crossref] [ Google Scholar]
- Lee BS, Yuan X, Xu Q, McLafferty FS, Petersen BA, Collette JC. Preparation and characterization of antioxidant nanospheres from multiple alpha-lipoic acid-containing compounds. Bioorg Med Chem Lett 2009; 19:1678-81. doi: 10.1016/j.bmcl.2009.01.102 [Crossref] [ Google Scholar]
- Bierbrauer KL, Comini LR, Leonhard V, Escobar Manzanelli MA, Castelli G, Farfán S. Eudragit Films as Carriers of Lipoic Acid for Transcorneal Permeability. Polymers (Basel) 2023; 15:1793. doi: 10.3390/polym15071793 [Crossref] [ Google Scholar]
- Raeisi Estabragh MA, Pardakhty A, Ahmadzadeh S, Dabiri S, Malekpour Afshar R, Farajli Abbasi M. Successful Application of Alpha Lipoic Acid Niosomal Formulation in Cerebral Ischemic Reperfusion Injury in Rat Model. Adv Pharm Bull 2022; 12:541-9. doi: 10.34172/apb.2022.058 [Crossref] [ Google Scholar]
- Badr-Eldin SM, Fahmy UA, Aldawsari HM, Ahmed OAA, Alhakamy NA, Okbazghi SZ. Optimized self-nanoemulsifying delivery system based on plant-derived oil augments alpha-lipoic acid protective effects against experimentally induced gastric lesions. Dose Response 2021; 19:15593258211001259. doi: 10.1177/15593258211001259 [Crossref] [ Google Scholar]
- Curcio M, Cirillo G, Amato R, Guidotti L, Amantea D, De Luca M. Encapsulation of Alpha-Lipoic Acid in Functional Hybrid Liposomes: Promising Tool for the Reduction of Cisplatin-Induced Ototoxicity. Pharmaceuticals (Basel) 2022; 15:394. doi: 10.3390/ph15040394 [Crossref] [ Google Scholar]
- Jung SY, Yoo J, Yang KJ, Jang SY, Yi G, Kim DK, Koo H. Intratympanic administration of alpha-lipoic acid-loaded pluronic F-127 nanoparticles ameliorates acute hearing loss. Nanotechnology, Biology, and Medicine 2021; 32:Nanotechnology, Biology, and Medicine 2021; 32. doi: 10.1016/j.nano.2020.102329 [Crossref] [ Google Scholar]
- Kothari IR, Mazumdar S, Sharma S, Italiya K, Mittal A, Chitkara D. Docetaxel and alpha-lipoic acid co-loaded nanoparticles for cancer therapy. Therapeutic Delivery 2019; 10:227-40. doi: 10.4155/tde-2018-0074 [Crossref] [ Google Scholar]
- Mahmoudi A, Jaafari MR, Malaekeh-Nikouei B. Preparation, characterization and preliminary in vivo safety evaluation of cationic nano-emulsions containing α-lipoic acid after ocular administration in NZW rabbits. Nanomedicine Journal 2023; 10:33-40. doi: 10.22038/NMJ.2022.69767.1746 [Crossref] [ Google Scholar]
- Xi Y, Pan W, Liu Y, Liu J, Xu G, Su Y. α-Lipoic acid loaded hollow gold nanoparticles designed for osteoporosis treatment: preparation, characterization and in vitro evaluation. Artif Cells Nanomed Biotechnol 2023; 51:131-8. doi: 10.1080/21691401.2022.2149542 [Crossref] [ Google Scholar]
- Haidar MK, Timur SS, Kazanci A, Turkoglu OF, Gürsoy RN, Nemutlu E. Composite nanofibers incorporating alpha lipoic acid and atorvastatin provide neuroprotection after peripheral nerve injury in rats. Eur J Pharm Biopharm 2020; 153:1-13. doi: 10.1016/j.ejpb.2020.05.032 [Crossref] [ Google Scholar]
- Xie DM, Zhong Q, Xu X, Li Y, Chen S, Li M, Peng C. Alpha lipoic acid–loaded electrospun fibrous patch films protect heart in acute myocardial infarction mice by inhibiting oxidative stress. Int J Pharm 2023; 632:122581. doi: 10.1016/j.ijpharm.2023.122581 [Crossref] [ Google Scholar]
- Xia N, Liu T, Wang Q, Xia Q, Bian X. In vitro evaluation of α-lipoic acid-loaded lipid nanocapsules for topical delivery. J Microencapsul 2017; 34:571-81. doi: 10.1080/02652048.2017.1367852 [Crossref] [ Google Scholar]
- Wang J, Xia Q. Alpha-lipoic acid-loaded nanostructured lipid carrier: sustained release and biocompatibility to HaCaT cells in vitro. Drug Deliv 2014; 21:328-41. doi: 10.3109/10717544.2013.846435 [Crossref] [ Google Scholar]
- Nishiura H, Sugimoto K, Akiyama K, Musashi M, Kubota Y, Yokoyama T. A novel nano-capsule of α-lipoic acid as a template of core-shell structure constructed by self-assembly. J Nanomed Nanotechnol 2013; 4:2. doi: 10.4172/2157-7439.1000155 [Crossref] [ Google Scholar]
- Ahmed OAA, El-Say KM, Aljaeid BM, Badr-Eldin SM, Ahmed TA. Optimized vinpocetine-loaded vitamin E D-α-tocopherol polyethylene glycol 1000 succinate-alpha lipoic acid micelles as a potential transdermal drug delivery system: In vitro and ex vivo studies. Int J Nanomedicine 2019; 14:33-43. doi: 10.2147/IJN.S187470 [Crossref] [ Google Scholar]
- Yang H, Shen W, Liu W, Chen L, Zhang P, Xiao C, Chen X. PEGylated Poly(α-lipoic acid) Loaded with Doxorubicin as a pH and Reduction Dual Responsive Nanomedicine for Breast Cancer Therapy. Biomacromolecules 2018; 19:4492-503. doi: 10.1021/acs.biomac.8b01394 [Crossref] [ Google Scholar]
- Fahmy UA, Aljaeid BM. Tadalafil transdermal delivery with alpha-lipoic acid self nanoemulsion for treatment of erectile dysfunction by diabetes mellitus. Int J Pharmacol 2018; 14:945-51. doi: 10.3923/ijp.2018.945.951 [Crossref] [ Google Scholar]
- Aljaeid BM, El-Moselhy MA. Loading of gentamicin and alpha lipoic acid on a biodegradable polymer for more effective and less nephrotoxic formula. Int J Pharmacol 2018; 14:796-801. doi: 10.3923/ijp.2018.796.801 [Crossref] [ Google Scholar]
- Dhaundiyal A, Jena SK, Samal SK, Sonvane B, Chand M, Sangamwar AT. Alpha-lipoic acid–stearylamine conjugate-based solid lipid nanoparticles for tamoxifen delivery: formulation, optimization, in-vivo pharmacokinetic and hepatotoxicity study. J Pharm Pharmacol 2016; 68:1535-50. doi: 10.1111/jphp.12644 [Crossref] [ Google Scholar]
- Alvarez-Rivera F, Fernández-Villanueva D, Concheiro A, Alvarez-Lorenzo C. α-Lipoic Acid in Soluplus® Polymeric Nanomicelles for Ocular Treatment of Diabetes-Associated Corneal Diseases. Journal of Pharmaceutical Sciences 2016; 105:2855-63. doi: 10.1016/j.xphs.2016.03.006 [Crossref] [ Google Scholar]
- Zhao GD, Sun R, Ni SL, Xia Q. Development and characterisation of a novel chitosan-coated antioxidant liposome containing both coenzyme Q10 and alpha-lipoic acid. J Microencapsul 2015; 32:157-65. doi: 10.3109/02652048.2014.973072 [Crossref] [ Google Scholar]
- Bai MY, Hu YM. Development of alpha-lipoic acid encapsulated chitosan monodispersed particles using an electrospray system: Synthesis, characterisations and anti-inflammatory evaluations. J Microencapsul 2014; 31:373-81. doi: 10.3109/02652048.2013.863395 [Crossref] [ Google Scholar]
- Souto EB, Müller RH, Gohla S. A novel approach based on lipid nanoparticles (SLN) for topical delivery of alpha-lipoic acid. J Microencapsul 2005; 22:581-92. doi: 10.1080/02652040500162378 [Crossref] [ Google Scholar]
- Das S, Chaudhury A. Recent Advances in Lipid Nanoparticle Formulations with Solid Matrix for Oral Drug Delivery. AAPS PharmSciTech 2011; 12:62-76. doi: 10.1208/s12249-010-9563-0 [Crossref] [ Google Scholar]
- Mendoza-Muñoz N, Urbán-Morlán Z, Leyva-Gómez G, Zambrano-Zaragoza ML, Piñón-Segundo E, Quintanar-Guerrero D. Solid Lipid Nanoparticles: An Approach to Improve Oral Drug Delivery. J Pharm Pharm Sci 2021; 24:509-32. doi: 10.18433/jpps31788 [Crossref] [ Google Scholar]
- Aji Alex MR, Chacko AJ, Jose S, Souto EB. Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur J Pharm Sci 2011; 42:11-8. doi: 10.1016/j.ejps.2010.10.002 [Crossref] [ Google Scholar]
- Dhaundiyal A, Jena SK, Samal SK, Sonvane B, Chand M, Sangamwar AT. Alpha-lipoic acid-stearylamine conjugate-based solid lipid nanoparticles for tamoxifen delivery: formulation, optimization, in-vivo pharmacokinetic and hepatotoxicity study. J Pharm Pharmacol 2016; 68:1535-50. doi: 10.1111/jphp.12644 [Crossref] [ Google Scholar]
- Liu M, Wen J, Sharma M. Solid Lipid Nanoparticles for Topical Drug Delivery: Mechanisms, Dosage Form Perspectives, and Translational Status. Curr Pharm Des 2020; 26:3203-17. doi: 10.2174/1381612826666200526145706 [Crossref] [ Google Scholar]
- Wissing SA, Müller RH. Cosmetic applications for solid lipid nanoparticles (SLN). Int J Pharm 2003; 254:65-8. doi: 10.1016/S0378-5173(02)00684-1 [Crossref] [ Google Scholar]
- Kim K, Kim J, Kim H, Sung GY. Effect of α-Lipoic Acid on the Development of Human Skin Equivalents Using a Pumpless Skin-on-a-Chip Model. Int J Mol Sci 2021; 22:2160. doi: 10.3390/ijms22042160 [Crossref] [ Google Scholar]
- Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: novel sustained release nonionic stable vesicular systems--an overview. Adv Colloid Interface Sci 2012; 183-184:46-54. doi: 10.1016/j.cis.2012.08.002 [Crossref] [ Google Scholar]
- Muzzalupo R, Mazzotta E. Do niosomes have a place in the field of drug delivery?. Expert Opin Drug Deliv 2019; 16:1145-7. doi: 10.1080/17425247.2019.1663821 [Crossref] [ Google Scholar]
- Puras G, Mashal M, Zárate J, Agirre M, Ojeda E, Grijalvo S. A novel cationic niosome formulation for gene delivery to the retina. J Control Release 2014; 174:27-36. doi: 10.1016/j.jconrel.2013.11.004 [Crossref] [ Google Scholar]
- Junyaprasert VB, Singhsa P, Suksiriworapong J, Chantasart D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int J Pharm 2012; 423:303-11. doi: 10.1016/j.ijpharm.2011.11.032 [Crossref] [ Google Scholar]
- Ge X, Wei M, He S, Yuan WE. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019; 11:55. doi: 10.3390/pharmaceutics11020055 [Crossref] [ Google Scholar]
- Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45:2160-236. doi: 10.1161/str.0000000000000024 [Crossref] [ Google Scholar]
- Sinha J, Das N, Basu MK. Liposomal antioxidants in combating ischemia-reperfusion injury in rat brain. Biomed Pharmacother 2001; 55:264-71. doi: 10.1016/s0753-3322(01)00060-9 [Crossref] [ Google Scholar]
- Lapchak PA. A critical assessment of edaravone acute ischemic stroke efficacy trials: is edaravone an effective neuroprotective therapy?. Expert Opin Pharmacother 2010; 11:1753-63. doi: 10.1517/14656566.2010.493558 [Crossref] [ Google Scholar]
- Salinthone S, Yadav V, Bourdette DN, Carr DW. Lipoic acid: a novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the CNS. Endocr Metab Immune Disord Drug Targets 2008; 8:132-42. doi: 10.2174/187153008784534303 [Crossref] [ Google Scholar]
- Ingallina C, Rinaldi F, Bogni A, Ponti J, Passeri D, Reggente M. Niosomal approach to brain delivery: Development, characterization and in vitro toxicological studies. Int J Pharm 2016; 511:969-82. doi: 10.1016/j.ijpharm.2016.08.002 [Crossref] [ Google Scholar]
- Gharbavi M, Amani J, Kheiri-Manjili H, Danafar H, Sharafi A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv Pharmacol Sci 2018; 2018:6847971. doi: 10.1155/2018/6847971 [Crossref] [ Google Scholar]
- Fang YP, Lin YK, Su YH, Fang JY. Tryptanthrin-loaded nanoparticles for delivery into cultured human breast cancer cells, MCF7: the effects of solid lipid/liquid lipid ratios in the inner core. Chem Pharm Bull (Tokyo) 2011; 59:266-71. doi: 10.1248/cpb.59.266 [Crossref] [ Google Scholar]
- Thatipamula R, Palem C, Gannu R, Mudragada S, Yamsani M. Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers. Daru 2011; 19:23-32. [ Google Scholar]
- Rizwanullah M, Amin S, Ahmad J. Improved pharmacokinetics and antihyperlipidemic efficacy of rosuvastatin-loaded nanostructured lipid carriers. J Drug Target 2017; 25:58-74. doi: 10.1080/1061186x.2016.1191080 [Crossref] [ Google Scholar]
- Wilson VG. Growth and differentiation of HaCaT keratinocytes. Methods Mol Biol 2014; 1195:33-41. doi: 10.1007/7651_2013_42 [Crossref] [ Google Scholar]
- Wang J, Tang J, Zhou X, Xia Q. Physicochemical characterization, identification and improved photo-stability of α-lipoic acid-loaded nanostructured lipid carrier. Drug Dev Ind Pharm 2014; 40:201-10. doi: 10.3109/03639045.2012.753901 [Crossref] [ Google Scholar]
- Ruktanonchai U, Bejrapha P, Sakulkhu U, Opanasopit P, Bunyapraphatsara N, Junyaprasert V, Puttipipatkhachorn S. Physicochemical characteristics, cytotoxicity, and antioxidant activity of three lipid nanoparticulate formulations of alpha-lipoic acid. AAPS PharmSciTech 2009; 10:227-34. doi: 10.1208/s12249-009-9193-6 [Crossref] [ Google Scholar]
- Fathi M, Mozafari MR, Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends in Food Science & Technology 2012; 23:13-27. doi: 10.1016/j.tifs.2011.08.003 [Crossref] [ Google Scholar]
- Yu Y, Feng R, Yu S, Li J, Wang Y, Song Y. Nanostructured lipid carrier-based pH and temperature dual-responsive hydrogel composed of carboxymethyl chitosan and poloxamer for drug delivery. Int J Biol Macromol 2018; 114:462-9. doi: 10.1016/j.ijbiomac.2018.03.117 [Crossref] [ Google Scholar]
- Senna JP, Barradas TN, Cardoso S, Castiglione TC, Serpe MJ, Silva K, Mansur CRE. Dual alginate-lipid nanocarriers as oral delivery systems for amphotericin B. Colloids Surf B Biointerfaces 2018; 166:187-94. doi: 10.1016/j.colsurfb.2018.03.015 [Crossref] [ Google Scholar]
- Yu Y, Xu S, Yu S, Li J, Tan G, Li S, Pan W. A hybrid genipin-cross-linked hydrogel/nanostructured lipid carrier for ocular drug delivery: cellular, ex vivo, and in vivo evaluation. ACS Biomater Sci Eng 2020; 6:1543-52. doi: 10.1021/acsbiomaterials.9b01800 [Crossref] [ Google Scholar]
- Tan G, Yu S, Li J, Pan W. Development and characterization of nanostructured lipid carriers based chitosan thermosensitive hydrogel for delivery of dexamethasone. Int J Biol Macromol 2017; 103:941-7. doi: 10.1016/j.ijbiomac.2017.05.132 [Crossref] [ Google Scholar]
- Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev 2001; 53:321-39. doi: 10.1016/s0169-409x(01)00203-4 [Crossref] [ Google Scholar]
- Ito T, Takami T, Uchida Y, Murakami Y. Chitosan gel sheet containing drug carriers with controllable drug-release properties. Colloids Surf B Biointerfaces 2018; 163:257-65. doi: 10.1016/j.colsurfb.2017.12.054 [Crossref] [ Google Scholar]
- Li Q, Gong S, Yao W, Yu Y, Liu C, Wang R. PEG-interpenetrated genipin-crosslinked dual-sensitive hydrogel/nanostructured lipid carrier compound formulation for topical drug administration. Artif Cells Nanomed Biotechnol 2021; 49:345-53. doi: 10.1080/21691401.2021.1879104 [Crossref] [ Google Scholar]
- Dhiman N, Awasthi R, Sharma B, Kharkwal H, Kulkarni GT. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front Chem 2021; 9:580118. doi: 10.3389/fchem.2021.580118 [Crossref] [ Google Scholar]
- Batzri S, Korn ED. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta 1973; 298:1015-9. doi: 10.1016/0005-2736(73)90408-2 [Crossref] [ Google Scholar]
- Deamer DW. From "banghasomes" to liposomes: a memoir of Alec Bangham, 1921-2010. FASEB J 2010; 24:1308-10. doi: 10.1096/fj.10-0503 [Crossref] [ Google Scholar]
- Mirhadi E, Rezaee M, Malaekeh-Nikouei B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed Pharmacother 2018; 104:465-73. doi: 10.1016/j.biopha.2018.05.067 [Crossref] [ Google Scholar]
- Scott BC, Aruoma OI, Evans PJ, O'Neill C, Van der Vliet A, Cross CE. Lipoic and dihydrolipoic acids as antioxidants. A critical evaluation. Free Radic Res 1994; 20:119-33. doi: 10.3109/10715769409147509 [Crossref] [ Google Scholar]
- Wu H, Zhong Z, Lin S, Qiu C, Xie P, Lv S. Coenzyme Q(10) Sunscreen Prevents Progression of Ultraviolet-Induced Skin Damage in Mice. Biomed Res Int 2020; 2020:9039843. doi: 10.1155/2020/9039843 [Crossref] [ Google Scholar]
- Qianqian O, Songzhi K, Yongmei H, Xianghong J, Sidong L, Puwang L, Hui L. Preparation of nano-hydroxyapatite/chitosan/tilapia skin peptides hydrogels and its burn wound treatment. Int J Biol Macromol 2021; 181:369-77. doi: 10.1016/j.ijbiomac.2021.03.085 [Crossref] [ Google Scholar]
- Gómez C, Costela Á, García-Moreno I, Llanes F, Teijón JM, Blanco MD. Skin laser treatments enhancing transdermal delivery of ALA. J Pharm Sci 2011; 100:223-31. doi: 10.1002/jps.22270 [Crossref] [ Google Scholar]
- Jo MJ, Jin IS, Park CW, Hwang BY, Chung YB, Kim JS, Shin DH. Revolutionizing technologies of nanomicelles for combinatorial anticancer drug delivery. Arch Pharm Res 2020; 43:100-9. doi: 10.1007/s12272-020-01215-4 [Crossref] [ Google Scholar]
- Bose A, Roy Burman D, Sikdar B, Patra P. Nanomicelles: Types, properties and applications in drug delivery. IET Nanobiotechnol 2021; 15:19-27. doi: 10.1049/nbt2.12018 [Crossref] [ Google Scholar]
- Gote V, Ansong M, Pal D. Prodrugs and nanomicelles to overcome ocular barriers for drug penetration. Expert Opinion on Drug Metabolism & Toxicology 2020; 16:885-906. doi: 10.1080/17425255.2020.1803278 [Crossref] [ Google Scholar]
- Vadlapudi AD, Mitra AK. Nanomicelles: an emerging platform for drug delivery to the eye. Ther Deliv 2013; 4:1-3. doi: 10.4155/tde.12.122 [Crossref] [ Google Scholar]
- Chen C-L, Cheng W-S, Chen J-L, Chiang C-H. Potential of Nonoral α-Lipoic Acid Aqueous Formulations to Reduce Ocular Microvascular Complications in a Streptozotocin-Induced Diabetic Rat Model. Journal of Ocular Pharmacology and Therapeutics 2013; 29:738-45. doi: 10.1089/jop.2012.0147 [Crossref] [ Google Scholar]
- Koufaki M. Therapeutic applications of lipoic acid: a patent review (2011 - 2014). Expert Opin Ther Pat 2014; 24:993-1005. doi: 10.1517/13543776.2014.937425 [Crossref] [ Google Scholar]
- Wang Y, Wang C. Novel Eye Drop Delivery Systems: Advance on Formulation Design Strategies Targeting Anterior and Posterior Segments of the Eye. Pharmaceutics 2022; 14:1150. doi: 10.3390/pharmaceutics14061150 [Crossref] [ Google Scholar]
- Tamilvanan S. Formulation of multifunctional oil-in-water nanosized emulsions for active and passive targeting of drugs to otherwise inaccessible internal organs of the human body. Int J Pharm 2009; 381:62-76. doi: 10.1016/j.ijpharm.2009.08.001 [Crossref] [ Google Scholar]
- Tamilvanan S. Oil-in-water lipid emulsions: implications for parenteral and ocular delivering systems. Prog Lipid Res 2004; 43:489-533. doi: 10.1016/j.plipres.2004.09.001 [Crossref] [ Google Scholar]
- Tamilvanan S, Benita S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur J Pharm Biopharm 2004; 58:357-68. doi: 10.1016/j.ejpb.2004.03.033 [Crossref] [ Google Scholar]
- Obrosova IG, Minchenko AG, Marinescu V, Fathallah L, Kennedy A, Stockert CM. Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia 2001; 44:1102-10. doi: 10.1007/s001250100631 [Crossref] [ Google Scholar]
- Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr 2014; 6:80. doi: 10.1186/1758-5996-6-80 [Crossref] [ Google Scholar]
- Lallemand F, Daull P, Benita S, Buggage R, Garrigue JS. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. J Drug Deliv 2012; 2012:604204. doi: 10.1155/2012/604204 [Crossref] [ Google Scholar]
- du Toit LC, Pillay V, Choonara YE, Govender T, Carmichael T. Ocular drug delivery - a look towards nanobioadhesives. Expert Opin Drug Deliv 2011; 8:71-94. doi: 10.1517/17425247.2011.542142 [Crossref] [ Google Scholar]
- Jung SY, Yoo J, Yang KJ, Jang SY, Yi G, Kim DK, Koo H. Intratympanic administration of alpha-lipoic acid-loaded pluronic F-127 nanoparticles ameliorates acute hearing loss. Nanomedicine 2021; 32:102329. doi: 10.1016/j.nano.2020.102329 [Crossref] [ Google Scholar]
- Kim KH, Lee B, Kim YR, Kim MA, Ryu N, Jung DJ. Evaluating protective and therapeutic effects of alpha-lipoic acid on cisplatin-induced ototoxicity. Cell Death Dis 2018; 9:827. doi: 10.1038/s41419-018-0888-z [Crossref] [ Google Scholar]
- Cho S, Hong SJ, Kang SH, Park Y, Kim SK. Alpha-Lipoic Acid Attenuates Apoptosis and Ferroptosis in Cisplatin-Induced Ototoxicity via the Reduction of Intracellular Lipid Droplets. Int J Mol Sci 2022; 23:10981. doi: 10.3390/ijms231810981 [Crossref] [ Google Scholar]
- Rybak LP, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Semin Hear 2019; 40:197-204. doi: 10.1055/s-0039-1684048 [Crossref] [ Google Scholar]
- Ramaswamy B, Roy S, Apolo AB, Shapiro B, Depireux DA. Magnetic Nanoparticle Mediated Steroid Delivery Mitigates Cisplatin Induced Hearing Loss. Front Cell Neurosci 2017; 11:268. doi: 10.3389/fncel.2017.00268 [Crossref] [ Google Scholar]
- Martín-Saldaña S, Palao-Suay R, Trinidad A, Aguilar MR, Ramírez-Camacho R, San Román J. Otoprotective properties of 6α-methylprednisolone-loaded nanoparticles against cisplatin: In vitro and in vivo correlation. Nanomedicine 2016; 12:965-76. doi: 10.1016/j.nano.2015.12.367 [Crossref] [ Google Scholar]
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 2001; 70:1-20. doi: 10.1016/s0168-3659(00)00339-4 [Crossref] [ Google Scholar]
- Cano A, Ettcheto M, Chang JH, Barroso E, Espina M, Kühne BA. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer's disease mice model. J Control Release 2019; 301:62-75. doi: 10.1016/j.jconrel.2019.03.010 [Crossref] [ Google Scholar]
- Cano A, Sánchez-López E, Ettcheto M, López-Machado A, Espina M, Souto EB. Current advances in the development of novel polymeric nanoparticles for the treatment of neurodegenerative diseases. Nanomedicine (Lond) 2020; 15:1239-61. doi: 10.2217/nnm-2019-0443 [Crossref] [ Google Scholar]
- Owens DE, 3rd 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm 2006; 307:93-102. doi: 10.1016/j.ijpharm.2005.10.010 [Crossref] [ Google Scholar]
- Schaffazick SR, Pohlmann AR, Dalla-Costa T, Guterres SS. Freeze-drying polymeric colloidal suspensions: nanocapsules, nanospheres and nanodispersionA comparative study. Eur J Pharm Biopharm 2003; 56:501-5. doi: 10.1016/s0939-6411(03)00139-5 [Crossref] [ Google Scholar]
- Crucho CIC, Barros MT. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater Sci Eng C Mater Biol Appl 2017; 80:771-84. doi: 10.1016/j.msec.2017.06.004 [Crossref] [ Google Scholar]
- Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights 2007; 2:147-57. [ Google Scholar]
- Alfei S, Schito AM, Zuccari G. Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vsStill Undefined Risks. Polymers (Basel) 2021; 13:2262. doi: 10.3390/polym13142262 [Crossref] [ Google Scholar]
- Matsugo S, Han D, Tritschler HJ, Packer L. Decomposition of alpha-lipoic acid derivatives by photoirradiation-formation of dihydrolipoic acid from alpha-lipoic acid. Biochem Mol Biol Int 1996; 38:51-9. [ Google Scholar]
- Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009; 30:2180-98. doi: 10.1016/j.biomaterials.2009.01.026 [Crossref] [ Google Scholar]
- Huo M, Yuan J, Tao L, Wei Y. Redox-responsive polymers for drug delivery: from molecular design to applications. Polymer Chemistry 2014; 5:1519-28. doi: 10.1039/C3PY01192E [Crossref] [ Google Scholar]
- Grossen P, Witzigmann D, Sieber S, Huwyler J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J Control Release 2017; 260:46-60. doi: 10.1016/j.jconrel.2017.05.028 [Crossref] [ Google Scholar]
- Elsayed MG, Elkomy AA, Gaballah MS, Elbadawy M. Nephrotoxicity of cefepime: A new cephalosporin antibiotic in rats. J Pharmacol Pharmacother 2014; 5:33-8. doi: 10.4103/0976-500x.124419 [Crossref] [ Google Scholar]
- Nasri H, Nematbakhsh M, Rafieian-Kopaei M. Ethanolic extract of garlic for attenuation of gentamicin-induced nephrotoxicity in Wistar rats. Iran J Kidney Dis 2013; 7:376-82. [ Google Scholar]
- Tavafi M. Protection of renal tubules against gentamicin induced nephrotoxicity. J Renal Inj Prev 2013; 2:5-6. doi: 10.12861/jrip.2013.03 [Crossref] [ Google Scholar]
- Aljaeid B, El-Moselhy M. Loading of Gentamicin and Alpha Lipoic Acid on a Biodegradable Polymer for More Effective and Less Nephrotoxic Formula. Int J Pharmacol 2018; 14:796-801. doi: 10.3923/ijp.2018.796.801 [Crossref] [ Google Scholar]
- Karagoz B, Esser L, Duong HT, Basuki JS, Boyer C, Davis TP. Polymerization-Induced Self-Assembly (PISA) – control over the morphology of nanoparticles for drug delivery applications. Polymer Chemistry 2014; 5:350-5. doi: 10.1039/C3PY01306E [Crossref] [ Google Scholar]
- Li JH, Ju GX, Jiang JL, Li NS, Peng J, Luo XJ. Lipoic acid protects gastric mucosa from ethanol-induced injury in rat through a mechanism involving aldehyde dehydrogenase 2 activation. Alcohol 2016; 56:21-8. doi: 10.1016/j.alcohol.2016.10.004 [Crossref] [ Google Scholar]
- Monastra G, De Grazia S, Cilaker Micili S, Goker A, Unfer V. Immunomodulatory activities of alpha lipoic acid with a special focus on its efficacy in preventing miscarriage. Expert Opin Drug Deliv 2016; 13:1695-708. doi: 10.1080/17425247.2016.1200556 [Crossref] [ Google Scholar]
- Machado RM, Palmeira-de-Oliveira A, Martinez-De-Oliveira J, Palmeira-de-Oliveira R. Vaginal films for drug delivery. J Pharm Sci 2013; 102:2069-81. doi: 10.1002/jps.23577 [Crossref] [ Google Scholar]
- Wafa HG, Essa EA, El-Sisi AE, El Maghraby GM. Ocular films versus film-forming liquid systems for enhanced ocular drug delivery. Drug Deliv Transl Res 2021; 11:1084-95. doi: 10.1007/s13346-020-00825-1 [Crossref] [ Google Scholar]
- Montenegro-Nicolini M, Morales JO. Overview and Future Potential of Buccal Mucoadhesive Films as Drug Delivery Systems for Biologics. AAPS PharmSciTech 2017; 18:3-14. doi: 10.1208/s12249-016-0525-z [Crossref] [ Google Scholar]
- Morales JO, McConville JT. Manufacture and characterization of mucoadhesive buccal films. Eur J Pharm Biopharm 2011; 77:187-99. doi: 10.1016/j.ejpb.2010.11.023 [Crossref] [ Google Scholar]
- Glaessl B, Siepmann F, Tucker I, Rades T, Siepmann J. Deeper insight into the drug release mechanisms in Eudragit RL-based delivery systems. Int Jf Pharm 2010; 389:139-46. doi: 10.1016/j.ijpharm.2010.01.031 [Crossref] [ Google Scholar]
- Pignatello R, Bucolo C, Ferrara P, Maltese A, Puleo A, Puglisi G. Eudragit RS100® nanosuspensions for the ophthalmic controlled delivery of ibuprofen. European Journal of Pharmaceutical Sciences 2002; 16:53-61. doi: 10.1016/S0928-0987(02)00057-X [Crossref] [ Google Scholar]
- Khin SY, Soe HMSH, Chansriniyom C, Pornputtapong N, Asasutjarit R, Loftsson T, Jansook P. Development of Fenofibrate/Randomly Methylated β-Cyclodextrin-Loaded Eudragit® RL 100 Nanoparticles for Ocular Delivery. Molecules 2022; 27:4755. [ Google Scholar]
- Sunderland S. Rate of regeneration of sensory nerve fibers. Arch Neurol Psychiatry 1947; 58:1-6. doi: 10.1001/archneurpsyc.1947.02300300011001 [Crossref] [ Google Scholar]
- Nachemson AK, Lundborg G, Myrhage R, Rank F. Nerve regeneration and pharmacological suppression of the scar reaction at the suture siteAn experimental study on the effect of estrogen-progesterone, methylprednisolone-acetate and cis-hydroxyproline in rat sciatic nerve. Scand J Plast Reconstr Surg 1985; 19:255-60. doi: 10.3109/02844318509074512 [Crossref] [ Google Scholar]
- Suslu H, Altun M, Erdivanli B, Turan Suslu H. Comparison of the effects of local and systemic dexamethasone on the rat traumatic sciatic nerve model. Turk Neurosurg 2013; 23:623-9. doi: 10.5137/1019-5149.Jtn.7761-13.0 [Crossref] [ Google Scholar]
- Câmara CC, Araújo CV, de Sousa KKO, Brito GAC, Vale ML, Raposo RDS. Gabapentin attenuates neuropathic pain and improves nerve myelination after chronic sciatic constriction in rats. Neurosci Lett 2015; 607:52-8. doi: 10.1016/j.neulet.2015.09.021 [Crossref] [ Google Scholar]
- Luraghi A, Peri F, Moroni L. Electrospinning for drug delivery applications: A review. Journal of Controlled Release 2021; 334:463-84. doi: 10.1016/j.jconrel.2021.03.033 [Crossref] [ Google Scholar]
- Song R, Murphy M, Li C, Ting K, Soo C, Zheng Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des Devel Ther 2018; 12:3117-45. doi: 10.2147/dddt.S165440 [Crossref] [ Google Scholar]
- Xie DM, Zhong Q, Xu X, Li Y, Chen S, Li M, Peng C. Alpha lipoic acid-loaded electrospun fibrous patch films protect heart in acute myocardial infarction mice by inhibiting oxidative stress. Int J Pharm 2023; 632:122581. doi: 10.1016/j.ijpharm.2023.122581 [Crossref] [ Google Scholar]
- Haidar M, Demirbolat G, Timur S, Gürsoy R, Nemutlu E, Ulubayram K. Atorvastatin Loaded Nanosprayed Chitosan Nanoparticles for Peripheral Nerve Injury. Bioinspired, Biomimetic and Nanobiomaterials 2019; 9:1-11. doi: 10.1680/jbibn.19.00006 [Crossref] [ Google Scholar]
- Kemp SW, Cederna PS, Midha R. Comparative outcome measures in peripheral regeneration studies. Exp Neurol 2017; 287:348-57. doi: 10.1016/j.expneurol.2016.04.011 [Crossref] [ Google Scholar]
- Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int 2014; 2014:698256. doi: 10.1155/2014/698256 [Crossref] [ Google Scholar]
- Navarro X. Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models: a critical overview. Eur J Neurosci 2016; 43:271-86. doi: 10.1111/ejn.13033 [Crossref] [ Google Scholar]
- Haidar MK, Timur SS, Kazanci A, Turkoglu OF, Gürsoy RN, Nemutlu E. Composite nanofibers incorporating alpha lipoic acid and atorvastatin provide neuroprotection after peripheral nerve injury in rats. European Journal of Pharmaceutics and Biopharmaceutics 2020; 153:1-13. doi: 10.1016/j.ejpb.2020.05.032 [Crossref] [ Google Scholar]
- Faraday M. X. The Bakerian Lecture.—Experimental relations of gold (and other metals) to light. Philosophical transactions of the Royal Society of London 1857; 145-81.
- Noh JY, Yang Y, Jung H. Molecular Mechanisms and Emerging Therapeutics for Osteoporosis. Int J Mol Sci 2020; 21. 10.3390/ijms21207623.
- You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano 2010; 4:1033-41. doi: 10.1021/nn901181c [Crossref] [ Google Scholar]
- Daraee H, Eatemadi A, Abbasi E, Fekri Aval S, Kouhi M, Akbarzadeh A. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol 2016; 44:410-22. doi: 10.3109/21691401.2014.955107 [Crossref] [ Google Scholar]
- Fairbairn N, Christofidou A, Kanaras AG, Newman TA, Muskens OL. Hyperspectral darkfield microscopy of single hollow gold nanoparticles for biomedical applications. Phys Chem Chem Phys 2013; 15:4163-8. doi: 10.1039/c2cp43162a [Crossref] [ Google Scholar]