Bioimpacts. 2025;15:30996.
doi: 10.34172/bi.30996
Review
Revolutionizing cancer therapy: Monoclonal antibodies in radiosensitization
Abolfazl Bemidinezhad Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing, 1, 2, 3
Yasaman Abolhassani Methodology, Resources, Visualization, Writing – original draft, 4
Mojgan Noroozi-Karimabad Writing – review & editing, 1
Arman Abroumand Gholami Writing – review & editing, 5
Abbas Alalikhan Writing – review & editing, 3, 6, 7
Ramin Roshani Visualization, Writing – review & editing, 8
Mohammad Parsa-kondelaji Writing – review & editing, 9
Fatemeh Gheybi Investigation, Resources, Supervision, Writing – review & editing, 4, 10, * 
Author information:
1Molecular Medicine Research Center, Research Institute of Basic Medical Sciences, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
2Pharmacological Research Center of Medicinal Plants, Mashhad University of Medical Sciences, Mashhad, Iran
3Department of Clinical Biochemistry, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
4Department of Medical Biotechnology and Nanotechnology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
5Department of Neuroscience, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
6Department of Chemistry, Faculty of Education, Al-Ayen Iraqi University, Thi-Qar, Iraq
7Department of Laboratory Medicine, Nasiriyah Heart Hospital, Thi-Qar Health Directorate, Nasiriyah, Iraq
8Department of Pharmaceutical Biotechnology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
9Basic Science Department, Neyshabur University of Medical Science, Neyshabur, Iran
10Nanotechnology Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran
Abstract
Cancer treatment has advanced significantly, yet traditional modalities such as radiotherapy still encounter challenges, including damage to healthy tissues and limited tumor specificity. Monoclonal antibodies (mAbs) have emerged as powerful tools in oncology, offering particular therapeutic options with reduced toxicity. Their capacity to enhance the efficacy of radiotherapy through radiosensitization presents a promising strategy for improving cancer outcomes. This review synthesizes findings from the past decade, providing an in-depth analysis of the diverse roles of mAbs in radiosensitization. Key mechanisms are discussed, including targeting molecular pathways, modulation of immune responses, and integration with novel platforms such as nanoparticles and antibody-drug conjugates (ADCs). The review also highlights the successes of preclinical and clinical studies while addressing ongoing challenges like delivery inefficiencies, tumor resistance, and antigen heterogeneity. Additionally, emerging alternatives including aptamers, nanobodies, and engineered proteins are explored as potential solutions to these barriers. Advancements in mAb-based delivery systems and combination therapies remain crucial for achieving more personalized and effective cancer treatments.
Keywords: Monoclonal antibodies, Radiosensitization, Cancer therapy, Tumor targeting, Emerging technologies
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
No funding was received for this study.
Introduction
Cancer remains one of the leading causes of death globally, with the World Health Organization reporting nearly 10 million cancer-related deaths annually.1-5 The high incidence and mortality rates associated with cancer highlight the urgent need for better and more effective treatment strategies.6-9 Traditional cancer treatments such as surgery, chemotherapy, and radiotherapy have been the cornerstone of cancer therapy for decades.10-13 However, these methods often have significant limitations, particularly in targeting cancer cells while sparing healthy tissues.14-17 Chemotherapy and radiotherapy, for instance, not only target cancer cells but can also harm surrounding healthy tissues, leading to unwanted side effects.18
Radiotherapy, despite its effectiveness in treating certain cancers, also has the downside of damaging normal, healthy cells, which can result in various adverse effects.19 The inability of radiotherapy to discriminate between cancerous and healthy cells creates a pressing need for more targeted therapies.20 One promising solution to this issue using monoclonal antibodies (mAbs), which offer a more precise approach by targeting cancer cells while minimizing harm to normal cells.21
Monoclonal antibodies are laboratory-engineered molecules designed to bind to specific antigens found on the surface of cancer cells.22 By recognizing and binding to these targets, mAbs can directly interfere with cancer cell growth and survival.23 Additionally, monoclonal antibodies can be combined with other therapeutic agents, such as chemotherapy, nanoparticles, and bacteria, to enhance their effects.24 This combination approach enables more effective treatment strategies and reduces the adverse side effects associated with traditional therapies.25 Furthermore, monoclonal antibodies can sensitize cancer cells to radiotherapy, known as radiosensitization.26
Radiosensitization refers to the process by which cancer cells become more sensitive to the damaging effects of radiation.27,28 This sensitivity can be achieved using antibodies targeting specific cell surface markers.29 When monoclonal antibodies are used in conjunction with radiotherapy, they can enhance the effects of radiation by improving the delivery of radiation directly to the cancer cells while protecting healthy cells from damage.30 This combination approach is auspicious in overcoming the limitations of radiotherapy, as it ensures that the radiation is more effectively absorbed by the cancer cells, increasing the likelihood of tumor regression and improving survival rates.31
Monoclonal antibodies have already shown great potential in preclinical and clinical studies, and their clinical translation has been one of the most successful in cancer therapy.32 Monoclonal antibodies have proven more effective in clinical settings33 than other experimental treatments like aptamers and nanoparticles. They have been widely used in a various cancer treatments, providing positive outcomes and becoming a standard in modern cancer immunotherapy.34,35 In addition to their direct action on cancer cells, monoclonal antibodies can influence various cellular pathways, further enhancing their therapeutic effects.32
This review focuses, on using monoclonal antibodies in clinical and preclinical studies over the past decade. We examine how monoclonal antibodies have been combined with other therapeutic agents, such as nanoparticles and immune checkpoint inhibitors, to improve treatment efficacy. We also explore the molecular and cellular pathways affected by monoclonal antibodies, which contribute to their therapeutic effects in cancer treatment. Finally, we address the current challenges in monoclonal antibody therapies, including tumor heterogeneity, resistance mechanisms, and delivery strategies. We conclude by discussing the prospects of monoclonal antibodies in cancer therapy and their potential to overcome the limitations of traditional treatments, offering more personalized and targeted approaches for cancer patients.
Monoclonal antibodies in cancer therapy
Antibodies are vital immune systems that identify and neutralize foreign substances, including pathogens and abnormal cells.36-39 These Y-shaped proteins are produced by B lymphocytes and are classified into five major isotypes: IgG, IgA, IgM, IgE, and IgD.40-43 Each isotype has unique structural and functional properties, enabling diverse roles in immune defense.41,44-46 Among these, IgG antibodies are the most abundant and are predominantly used in therapeutic applications due to their stability, prolonged half-life, and ability to mediate immune responses effectively.41,47,48
Therapeutic antibodies can be further categorized based on their development and application. Polyclonal antibodies are mixtures that recognize multiple epitopes on an antigen, commonly used for passive immunization or diagnostic purposes.49,50 Monoclonal antibodies (mAbs), on the other hand, are engineered to recognize a single, specific epitope, providing high precision in targeting.51 Advances in genetic engineering have expanded the scope of mAbs, allowing for the development of chimeric, humanized, and fully human antibodies to minimize immunogenicity while enhancing therapeutic efficacy.52 Additionally, antibody-drug conjugates (ADCs), bispecific antibodies, and immune checkpoint inhibitors have emerged as innovative platforms in antibody engineering, further diversifying their clinical utility.53,54
mAbs have emerged as a significant class of therapeutics, with notable applications in treating cancer.55 These antibodies are engineered to target specific antigens present in cancer cells, facilitating tumor cell destruction through several mechanisms, including direct apoptosis induction, immune system activation, and enhanced chemotherapy sensitivity.56 As of 2018, the FDA had approved over 80 mAbs, with a substantial portion of these approvals aimed at treating oncological conditions.57 Approving 12 new mAbs that year alone highlighted their growing importance in cancer therapy.57 The expanding therapeutic pipeline, with over 100 mAbs in development, further underscores their potential in clinical settings.56
The development of mAbs has its roots in the hybridoma technique of the 1970s, which led to the creation of immortal B cell clones capable of producing specific antibodies.58 However, early mAbs faced issues like immunogenicity, particularly when derived from murine sources.59 Over time, advances in genetic engineering have resulted in chimeric, humanized, and fully human mAbs, significantly improving their safety and efficacy profiles.60 These therapeutic antibodies can be directed against tumor-associated antigens, such as the HER2 receptor in breast cancer, where trastuzumab (Herceptin) has proven effective in targeting overexpressed HER2, promoting immune-mediated tumor cell destruction through antibody-dependent cellular cytotoxicity (ADCC).61,62
mAbs' ability to selectively target cancer cells while sparing healthy tissues represents a significant advantage over traditional chemotherapy, which often results in widespread toxicity.63 Furthermore, recent innovations in mAb engineering have aimed to reduce off-target effects and enhance pharmacokinetics, ensuring more efficient delivery to the tumor site and improved clinical outcomes.64 This efficient delivery has led to the development of bispecific antibodies and ADCs, which combine the specificity of mAbs with the cytotoxic power of chemotherapeutic agents, offering a promising avenue for improving cancer treatment.64,65
In addition to their use in cancer therapy, mAbs have demonstrated potential in enhancing radiotherapy, particularly through radiosensitization.66 Radiosensitization makes tumor cells more susceptible to radiation, which is crucial in overcoming resistance to radiotherapy.67 Recent research has shown that mAbs can be engineered to bind to specific receptors on tumor cells, not only inhibiting their growth but also increasing their sensitivity to radiation.68 This synergistic effect has been observed with mAbs targeting growth factor receptors and immune checkpoint inhibitors.68 By improving the efficacy of radiotherapy, mAbs (Fig. 1) may offer an important strategy for treating tumors that are otherwise resistant to conventional radiation-based therapies, thus broadening the therapeutic options available for cancer patients.68
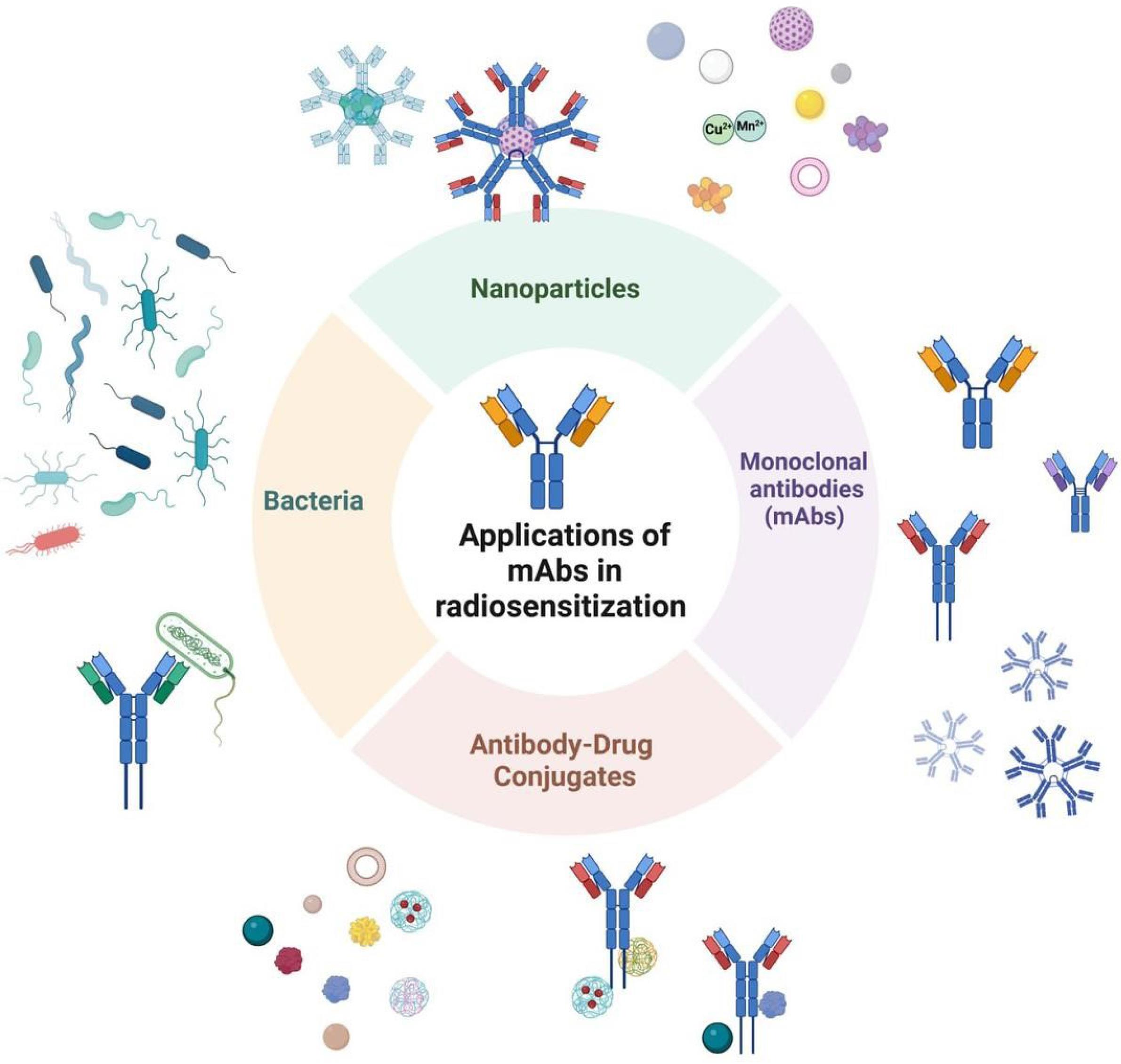
Fig. 1.
Overview of strategies using mAbs for radiosensitization. Nanoparticles: Antibody-functionalized nanoparticles that deliver therapeutic agents or enhance the radiation response, making tumor cells more sensitive to radiation. mAbs: mAbs targeting tumor-specific antigens to block critical signaling pathways, thereby increasing the tumor cells' susceptibility to radiation therapy. ADCs: mAbs conjugated with cytotoxic drugs to target tumor cells. This combination sensitizes the cells to radiation by directly delivering toxic agents to the tumor. Bacteria-based systems: Engineered bacteria expressing or carrying antibody-based therapeutics that facilitate radiosensitization, improving the efficacy of radiation treatment against tumors.
.
Overview of strategies using mAbs for radiosensitization. Nanoparticles: Antibody-functionalized nanoparticles that deliver therapeutic agents or enhance the radiation response, making tumor cells more sensitive to radiation. mAbs: mAbs targeting tumor-specific antigens to block critical signaling pathways, thereby increasing the tumor cells' susceptibility to radiation therapy. ADCs: mAbs conjugated with cytotoxic drugs to target tumor cells. This combination sensitizes the cells to radiation by directly delivering toxic agents to the tumor. Bacteria-based systems: Engineered bacteria expressing or carrying antibody-based therapeutics that facilitate radiosensitization, improving the efficacy of radiation treatment against tumors.
Various applications of mAbs in radiosensitization
Only antibodies
Monoclonal antibodies (mAbs) as standalone radiosensitizers represent a targeted approach to overcoming tumor radiation resistance. While promising, their clinical efficacy varies across studies. This section critically examines recent evidence, emphasizing preclinical mechanisms and clinical findings, while highlighting limitations and future directions.
Hypoxia-induced radioresistance is a significant challenge in radiotherapy, and while SPINK1 has been identified as a key player in this process, its efficacy and limitations across different tumor types and stages remain underexplored. SPINK1 is secreted in response to severe hypoxia and can protect neighboring, oxygenated cells from radiation by activating EGFR- and Nrf2-dependent survival pathways. However, although the role of SPINK1 in radiosensitization has been demonstrated in preclinical models, its therapeutic potential is likely to vary across cancer types due to differences in the tumor microenvironment, the degree of hypoxia, and the expression of SPINK1 across different malignancies. For instance, in some cancers like prostate and colon carcinoma, where SPINK1 is overexpressed even under normoxic conditions, its role as a predictive marker for tumor hypoxia may be less reliable. This unreliability highlights the necessity of evaluating SPINK1's function and predictive value in a broader range of cancer types to fully understand its utility as both a biomarker for tumor hypoxia and a target for radiosensitization.69 Moreover, while anti-SPINK1 neutralizing antibodies have shown promise in radiosensitizing tumors in specific models, translating these findings to clinical settings is still uncertain, particularly considering the potential for off-target effects. For example, systematic inhibition of SPINK1 could increase the risk of pancreatitis, an issue requiring careful consideration in the design of future therapeutic strategies. Therefore, in addition to optimizing SPINK1-targeting treatments, it is crucial to explore more selective delivery methods, such as nanoparticle-based drug delivery systems, to minimize systemic toxicity. These challenges underline the need for additional research to refine our understanding of SPINK1's role in cancer therapy and to develop more effective strategies for exploiting its radiosensitizing properties.69
Similarly, a preclinical study on head and neck squamous cell carcinoma (HNSCC) showed enhanced radiosensitization by simultaneously targeting β1 integrin and EGFR using mAbs. The treatment inhibited FAK- and Erk1-mediated survival signaling, reducing clonogenic survival and improving tumor control in eight of ten cell lines. In vivo, xenografts treated with combined mAbs and radiotherapy showed better tumor control than single-agent therapies.70 However, the study involved small sample sizes (n = 12-16 per group), and potential systemic toxicities were not fully explored. Future investigations should evaluate this combination in models mimicking human immune and stromal components.70
The phase II trial assessing panitumumab (anti-EGFR) combined with radiotherapy in 19 patients with KRAS wild-type locally advanced rectal cancer demonstrated mixed results. Although the treatment achieved a 41% grade 3 pathological tumor regression rate and a 95% sphincter-preservation rate, no complete pathological response (pCR) cases were observed.71 These results indicate partial efficacy but suggest that KRAS wild-type status alone is an insufficient biomarker for response prediction. When comparing these results to other similar studies, such as those investigating cetuximab in combination with radiotherapy, it becomes clear that while anti-EGFR monoclonal antibodies have shown some promise, their efficacy is limited. Cetuximab, for instance, has been associated with higher pCR rates in some studies, though not consistently across all cohorts. This discrepancy could be attributed to factors such as the treatment regimen, the sequencing of therapies, and the patient population. The significant changes in plasma TGF-α and EGF levels observed in responders emphasize the need for multi-biomarker approaches to refine patient selection and optimize therapeutic strategies. Interestingly, plasma TGF-α was significantly elevated in patients with a good response (grade 3 regression), which aligns with findings from other studies suggesting that increased TGF-α levels may correlate with enhanced tumor response in specific settings. However, the concurrent decrease in plasma EGF in these patients challenges the straightforward interpretation of EGFR pathway modulation. This dual biomarker trend underscores the complexity of EGFR inhibition in rectal cancer, where the dynamics between various EGFR ligands and other growth factor receptors, such as MET, may contribute to resistance mechanisms.71
A particularly notable case study involved the use of pembrolizumab (anti-PD1) followed by radiotherapy in a patient with non-resectable relapsed oral cavity carcinoma. This approach resulted in excellent local tumor control, attributed to T-cell activation mediated by checkpoint inhibition.72 Although promising, this report is based on a single patient, limiting its broader applicability. Large-scale clinical trials with defined endpoints, including immune response and survival rates, are critical to validate these findings. Moreover, the potential for immune-related toxicities in combined regimens warrants careful monitoring.72
Using monoclonal antibodies as standalone radiosensitizers demonstrates significant promise, especially in preclinical settings and small-scale clinical studies. However, variability in outcomes and limited patient cohorts underscore the need for refined patient selection, larger clinical trials, and exploration of combination strategies. By addressing these challenges, the full potential of mAbs in radiotherapy can be realized, paving the way for more effective and personalized cancer treatments.
In contrast, ADCs offer a more potent strategy for radiosensitization by not only targeting tumor antigens but also delivering cytotoxic agents directly to the tumor cells.71,73 This method combines the precision of monoclonal antibodies with the tumor-killing potential of chemotherapy, thus providing a more robust approach to overcoming radiation resistance. ADCs, such as those targeting EGFR or other tumor-specific antigens, are designed to maximize the local concentration of toxic drugs within tumor cells, which may significantly enhance radiosensitivity.71,72,74 However, while ADCs offer the advantage of delivering targeted chemotherapy, their efficacy can be affected by the tumor's ability to internalize the conjugated drug and its heterogeneity. Furthermore, ADCs often face challenges related to off-target toxicity and the complex pharmacokinetics of drug release. The advantage of ADCs over standalone mAbs lies in their ability to sensitize tumors to radiation and induce direct tumor cell killing, a key benefit when targeting highly resistant tumors.72-74
On the other hand, mAbs are generally less toxic and more specific in their targeting mechanism, but their efficacy is sometimes limited by the tumor's resistance mechanisms, such as the downregulation of target antigens or activation of alternative survival pathways.74 For example, while EGFR-targeted mAbs like cetuximab have shown some efficacy in radiosensitization, their success can be limited by the tumor's ability to bypass EGFR signaling through other compensatory mechanisms.72,74 By delivering a cytotoxic payload, ADCs can potentially overcome this issue by directly killing tumor cells that would otherwise evade mAb-mediated inhibition. Therefore, combining of ADCs with radiotherapy may offer a synergistic approach, exploiting both the radiosensitizing effects and the direct cytotoxicity of the conjugated drug, making them a compelling alternative to standalone mAbs in specific contexts.72,73
Antibody-drug conjugates
ADCs represent a promising strategy for improving the efficacy of radiotherapy by selectively delivering cytotoxic agents to tumor cells.73,75 This approach leverages the specificity of antibodies to target tumor-associated antigens, thus enabling the precise delivery of potent drugs to malignant cells while minimizing systemic toxicity. However, while ADCs show great potential, several factors must be optimized to enhance their therapeutic impact in radiotherapy.73
Lewis and his colleagues explored using an ADC targeting the radiation-inducible antigen TIP1 on non-small cell lung cancer (NSCLC). A summary of key findings from this and other relevant studies is provided in Supplementary file 1 (Table S1). The study demonstrated that the ADC, composed of the anti-TIP1 antibody 7H5 conjugated to the cytotoxic drug MMAE, significantly enhanced the radiosensitization of cancer cells.74 This conjugate exhibited prolonged circulation times in the bloodstream, allowing continuous drug delivery to the tumor during radiotherapy. Combining 7H5-VcMMAE with radiation resulted in a 70% reduction in viable cells, delayed tumor growth, and improved survival in NSCLC tumor models. These findings underscore the potential of ADCs in improving tumor response to radiotherapy, but they also highlight the need for further optimization of ADCs for specific tumor types and radiation protocols. The cytotoxic agent's antigen specificity and effectiveness, such as MMAE, must be carefully tailored to the tumor's molecular profile.74
In contrast, Guster et al combined radiotherapy with EGFR-targeting antibodies like cetuximab in HNSCC, which showed less favorable results. Although cetuximab is widely used as a radiosensitizer, the study revealed that it failed to enhance the radiosensitivity of HPV-positive HNSCC cell lines significantly.105 This outcome suggests that the effectiveness of ADCs in combination with radiotherapy may vary across cancer types and patient populations.105 A critical takeaway is the necessity for individualized treatment approaches considering tumor heterogeneity and molecular characteristics. Furthermore, this study highlighted those alternative approaches, such as PARP inhibition, might provide more effective radiosensitization in some cancers, indicating that ADCs may not always be the optimal choice in all contexts.105
Another study on histone deacetylase (HDAC) inhibition in bladder cancer (BC) radiosensitivity revealed a potential synergy between ADCs and radiation. The selective inhibition of HDAC6, when combined with radiotherapy, increased radiosensitivity by inducing gene expression changes that counteracted the radiation-induced effects on tumor migration and metastasis.78 These findings suggest using ADCs as standalone therapies and in conjunction with epigenetic modulators or other therapeutic agents to enhance their impact further. This result suggests that multi-pronged treatment strategies involving ADCs could be particularly beneficial in overcoming the tumor's adaptive responses to radiation and limiting metastasis.78
In pancreatic cancer, another research study conducted by Azad et al95 has shown that combining radiotherapy with anti-PD-L1 antibodies can enhance the tumor response, particularly at higher radiation doses. The addition of PD-L1 blockade promoted CD8+ T cell infiltration into the tumor, improving overall radiotherapy efficacy. This effect points to the emerging role of ADCs that can also modulate the immune microenvironment to enhance the immune response alongside traditional therapies. The challenge lies in selecting ADCs that can effectively influence the tumor's immune landscape while sensitizing it to radiation.95
A study (Fig. 2), which was conducted by Hingorani, focusing on a trimodal approach integrating chemotherapy, radiotherapy, and immunotherapy using auristatin-based ADCs showed that MMAE-conjugated antibodies could sensitize tumors to radiation and boost immune responses (Table S1).81 The combination led to durable tumor control and the development of immunologic memory, emphasizing the growing importance of combining ADCs with immunotherapies to amplify therapeutic effects. However, these strategies' success will require thorough clinical trials testing to evaluate long-term outcomes and identify any potential off-target effects or resistance mechanisms that could undermine their efficacy.81


Fig. 2.
MMAE-based conjugates enhance cancer therapy via targeted delivery, radiosensitization, and immune modulation. (A) Schematic of MMAE conjugated to antibodies and cell-penetrating peptides using MC-VC-PABC linkers. (B) Treatment plan for tumor-bearing mice showing improved survival with ACPP-MMAE and IR. (C) Reduced tumor size in CAL27-resistant models after ACPP-MMAE treatment. (D) Dose-dependent decrease in B16 cell survival after MMAE treatment. (E) Combined MMAE and radiation increases sensitivity and reduces survival of tumor cells. (F) Enhanced immune response with increased CD8+ T cells and PD-L1/PD-1 modulation. (G) Triple therapy with MMAE, radiation, and anti-PD-1 antibody prolongs survival in MC38 tumors. Statistical analysis: Mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001. These results demonstrate the synergistic therapeutic effects of MMAE-based conjugates with radiation and immunotherapy in targeting cancer cells.Abbreviations: ACPP, activatable cell-penetrating peptide. This figure is reproduced from81 under the CC BY license.
.
MMAE-based conjugates enhance cancer therapy via targeted delivery, radiosensitization, and immune modulation. (A) Schematic of MMAE conjugated to antibodies and cell-penetrating peptides using MC-VC-PABC linkers. (B) Treatment plan for tumor-bearing mice showing improved survival with ACPP-MMAE and IR. (C) Reduced tumor size in CAL27-resistant models after ACPP-MMAE treatment. (D) Dose-dependent decrease in B16 cell survival after MMAE treatment. (E) Combined MMAE and radiation increases sensitivity and reduces survival of tumor cells. (F) Enhanced immune response with increased CD8+ T cells and PD-L1/PD-1 modulation. (G) Triple therapy with MMAE, radiation, and anti-PD-1 antibody prolongs survival in MC38 tumors. Statistical analysis: Mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001. These results demonstrate the synergistic therapeutic effects of MMAE-based conjugates with radiation and immunotherapy in targeting cancer cells.Abbreviations: ACPP, activatable cell-penetrating peptide. This figure is reproduced from81 under the CC BY license.
In conclusion, while ADCs offer a promising avenue for enhancing the efficacy of radiotherapy, several challenges remain in optimizing their use. Personalized treatment regimens that account for tumor heterogeneity, specific antigen profiles, and immune responses are essential to maximize the therapeutic benefits of ADCs. Additionally, the development of combination therapies, incorporating ADCs with other modalities such as immunotherapy or epigenetic modifiers, holds significant promise in overcoming the limitations of single-agent therapies. Ongoing research and clinical trials will be critical in refining these strategies and establishing ADCs as a mainstay in radiotherapy.
Nanoparticles
Nanotechnology offers state of the art approaches for combating cancer.106-110 Integrating nanoparticles with monoclonal antibodies presents an innovative strategy to enhance radiotherapy outcomes by combining radiosensitization, targeted therapy, imaging capabilities, and immune modulation.106,111,112 This multifaceted approach addresses critical limitations in traditional cancer treatments, such as resistance to therapy and lack of specificity, offering promising advancements in glioblastoma and other malignancies.112-114
Nanoparticles provide a unique platform for transporting monoclonal antibodies across biological barriers, exemplified by a study utilizing gold nanoparticles coated with insulin to traverse the blood-brain barrier (BBB) and deliver cetuximab to glioblastoma cells.80 This study demonstrated that combining this targeted nanoparticle delivery system with temozolomide (TMZ) and radiotherapy significantly inhibited tumor growth and prolonged survival in a murine glioblastoma model. Histological analyses further revealed reduced tumor vascularization and enhanced radiosensitization. While this approach effectively addressed the challenge of BBB penetration, its reliance on gold nanoparticles, which may exhibit long-term bioaccumulation, necessitates exploring biodegradable alternatives for clinical translation.80
Building on the implementation of gold nanoparticles, multifunctional magnetic nanoparticles conjugated with cetuximab were employed to target EGFRvIII-overexpressing glioblastoma cells.103 These nanoparticles provided dual functionality as MRI contrast agents and radiosensitizers. When used with ionizing radiation (IR), they significantly enhanced radiosensitivity by increasing DNA double-strand breaks and reactive oxygen species (ROS) formation. The in vivo results showed a marked increase in survival among treated mice, emphasizing the therapeutic potential of combining imaging, targeting, and radiosensitization. However, the study highlights the need for optimizing delivery methods, as convection-enhanced delivery (CED) is invasive and may limit broader clinical applications.103
A novel nanoplatforms incorporating gold and superparamagnetic iron oxide nanoparticles (SPIOs) targeted PD-L1 expression in tumors to enhance therapeutic efficacy further.82 This platform improved imaging with superior T2-weighted MRI contrast and served as a radiosensitizer by increasing ROS production and inhibiting DNA damage repair. Beyond radiosensitivity, the platform exhibited immunomodulatory properties by shifting the tumor microenvironment from immunosuppressive to immunoreactive through TAM polarization and PD-L1/PD-1 pathway blockade. Adding these immune-modulating effects highlights a critical advancement, bridging the gap between localized radiotherapy and systemic immune responses. Despite its promising results, further studies are needed to explore the long-term effects of immune activation and potential off-target impacts in heterogeneous tumor microenvironments.82
Lastly, a study addressing the depth-dependent effectiveness of gold nanoparticles under clinically relevant megavoltage (MV) radiation beams revealed significant radiosensitization in prostate cancer models.83 The nanoparticles, functionalized with PSMA-targeting antibodies, demonstrated active targeting verified through confocal microscopy and transmission electron microscopy (TEM). The therapeutic efficacy was depth-dependent, with increased radiosensitization ratios observed at greater depths due to enhanced low-energy photon interactions. Monte Carlo (MC) microdosimetry and the local effect model (LEM) accurately predicted survival fractions, providing robust evidence for the clinical feasibility of gold nanoparticle-assisted radiotherapy. However, the variability in radiosensitization across depths underscores the need for patient-specific treatment planning and developing nanoparticles capable of maintaining consistent efficacy under diverse clinical conditions.83
Collectively, these studies illustrate the transformative potential of combining monoclonal antibodies with nanoparticles in radiotherapy. They address critical challenges such as BBB penetration, imaging-guided therapy, and immune modulation while demonstrating depth-dependent adaptability. Future efforts should focus on improving delivery methods, minimizing long-term toxicity, and tailoring treatment strategies to patient-specific tumor characteristics to maximize clinical translation, future. This integrated approach enhances therapeutic efficacy and opens new avenues for combining radiotherapy with emerging immunotherapies, paving the way for more personalized and effective cancer treatments.
Bacteria
Integrating of mAbs with anaerobic bacteria as radiosensitizers represents a groundbreaking approach to overcoming challenges in treating hypoxic tumors, which are often resistant to conventional therapies. Recent studies by JingBo Wu's team85 have provided a compelling foundation for understanding how Bifidobacterium infantis (Bi), in conjunction with its specific mAb, can enhance radiotherapy efficacy and modulate the tumor microenvironment. These studies offer complementary perspectives, addressing this innovative strategy’s mechanistic and therapeutic dimensions.76,85
In the study by Yang et al,85 the authors explored the combination of Bi-mAb and radiotherapy in a Lewis lung carcinoma mouse model. This research leveraged advanced imaging techniques, including 18F-FDG and 18F-FMISO PET/CT, to monitor tumor metabolism and hypoxia. The results demonstrated that the combination therapy significantly reduced tumor hypoxia and glucose metabolism, as evidenced by decreased uptake of FDG and FMISO in the treated group.85 Additionally, immunohistochemical analyses revealed a reduction in key markers such as HIF-1α, Glut-1, and Ki-67, indicating suppressed hypoxic signaling and tumor proliferation. Conversely, increased levels of γ-H2AX and TNF-α suggested enhanced DNA damage and a pro-inflammatory response. Tumor growth was significantly slowed, and survival times were markedly prolonged, highlighting the potential of this approach to overcome the limitations of conventional radiosensitizers, which often suffer from poor specificity and significant side effects. However, this study primarily focused on a single tumor model and lacked long-term evaluations, limiting its of its finding's broader applicability.85
Building on these results, Wang et al (Table S1) (Fig. 3) expanded the therapeutic framework by incorporating immune checkpoint inhibitors (αPD-1) into the Bi-mAb and radiotherapy regimen.76 This quadruple therapy addressed limitations in the earlier study, particularly the lack of a robust abscopal effect. Using 4T1 breast and CT26 colon cancer models, the authors demonstrated that Bi-mAb alleviated tumor hypoxia and transformed the tumor microenvironment, converting "cold" tumors into "hot" ones. This localized inflammation, induced by Bi colonization and the transient "infection" it caused, activated innate immune responses, including complement activation and ADCC.76 The therapy synergistically stimulated adaptive immune responses, amplified systemic antitumor immunity, and prolonged survival in mice, combined with αPD-1 and radiotherapy. As illustrated in Fig. 3, the study evaluated the therapeutic efficacy using primary (irradiated) and secondary (non-irradiated) tumors to assess the abscopal effect. Tumor growth curves (Fig. 3b) demonstrated significantly reduced tumor sizes, especially in the quadruple therapy group. PET/CT imaging (Fig. 3c) further revealed tumor metabolic suppression, corroborated by quantitative SUV analysis (Fig. 3d). Immune profiling (Fig. 3e) showed increased CD4+ and CD8+ T cell infiltration, highlighting robust immune activation. Additionally, the study highlighted the ability of PET/CT imaging to visualize the dynamic changes in tumor metabolism and hypoxia, reinforcing the translational potential of this approach.76


Fig. 3.
Antibody Targeting of Anaerobic Bacteria Enhances Radiotherapy and Triggers Abscopal Responses in Cold Tumors. (A) Schematic of the 4T1 tumor model: localized radiotherapy was applied to primary tumors (1°), while secondary tumors (2°) remained untreated to assess systemic abscopal effects. (B) Tumor growth curves for six treatment groups demonstrate the strongest inhibition in the quadruple therapy group (Bi + mAb + RT + αPD-1), confirmed by tumor images at day 25. (C) Representative ^18F-FDG PET/CT scans show reduced metabolic activity following combination therapy. (D) Quantification of SUVmax and SUVmean in both tumor sites shows significantly decreased uptake in treated groups. Together, these findings indicate that combining bacterial targeting with radiotherapy and immune checkpoint blockade enhances both local and systemic anti-tumor responses. Abbreviations: Bi, anaerobic bacteria; αPD-1, anti-programmed cell death protein 1 antibody; ^18F-FDG PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; SUV, standardized uptake value. This figure is reproduced from76 under the CC BY license.
.
Antibody Targeting of Anaerobic Bacteria Enhances Radiotherapy and Triggers Abscopal Responses in Cold Tumors. (A) Schematic of the 4T1 tumor model: localized radiotherapy was applied to primary tumors (1°), while secondary tumors (2°) remained untreated to assess systemic abscopal effects. (B) Tumor growth curves for six treatment groups demonstrate the strongest inhibition in the quadruple therapy group (Bi + mAb + RT + αPD-1), confirmed by tumor images at day 25. (C) Representative ^18F-FDG PET/CT scans show reduced metabolic activity following combination therapy. (D) Quantification of SUVmax and SUVmean in both tumor sites shows significantly decreased uptake in treated groups. Together, these findings indicate that combining bacterial targeting with radiotherapy and immune checkpoint blockade enhances both local and systemic anti-tumor responses. Abbreviations: Bi, anaerobic bacteria; αPD-1, anti-programmed cell death protein 1 antibody; ^18F-FDG PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; SUV, standardized uptake value. This figure is reproduced from76 under the CC BY license.
its complexity and the absence of clinical validation. The lack of detailed mechanistic insights into how Bi-mAb modulates the interplay between hypoxia and immune responses leaves room for further investigation. Moreover, both studies relied heavily on preclinical models, which may not fully capture the complexities of human tumors.76
These studies illustrate the potential of using Bi-mAb as a dual-function agent for radiosensitization and immune modulation. Addressing hypoxia and leveraging immune mechanisms, provide a multifaceted strategy for enhancing radiotherapy outcomes. Future research should aim to validate these findings in diverse tumor models and clinical settings while exploring strategies to simplify the therapeutic regimen for practical application. This innovative paradigm holds promise for improving localized tumor control and offers a pathway to systemic antitumor responses, particularly for metastatic cancers.76,85,115
From a clinical perspective, bacteria-based micro-robotic systems, such as Bi-mAb, offer several unique advantages, including tumor-targeting specificity, modulation of the tumor microenvironment, and the potential to synergize with existing immunotherapies and radiotherapy.76,85 Their ability to thrive in hypoxic cores of tumors gives them a distinct edge over conventional delivery systems. However, significant challenges remain before clinical translation can be realized. These include concerns regarding biosafety, potential immunogenicity, reproducibility of bacterial colonization across patients, and regulatory hurdles associated with using of genetically modified organisms or live bacteria.76,85 Moreover, the complexity of multi-component therapies like Bi-mAb + RT + αPD-1 may limit scalability and clinical implementation without further simplification. Therefore, while the therapeutic potential of bacteria-based systems is considerable, advancing toward clinical application will require robust safety evaluations, standardized manufacturing protocols, and early-phase clinical trials to establish efficacy and safety in human patients.76,85
Molecular targets in radiosensitization
Epidermal growth factor receptor (EFGR) and its family members
EFGR
The EGFR plays a crucial role in cellular proliferation, survival, and repair mechanisms, making it a significant target in cancer therapy.116,117 Overexpression or mutation of EGFR, such as EGFRvIII, is often associated with tumor aggressiveness and resistance to standard treatments, including radiotherapy.118 Monoclonal antibodies targeting EGFR, like cetuximab and panitumumab, have been explored as potential radiosensitizers to enhance the therapeutic efficacy of radiotherapy. However, their success has varied across tumor types and settings, reflecting promise and challenges (Fig. 4).118
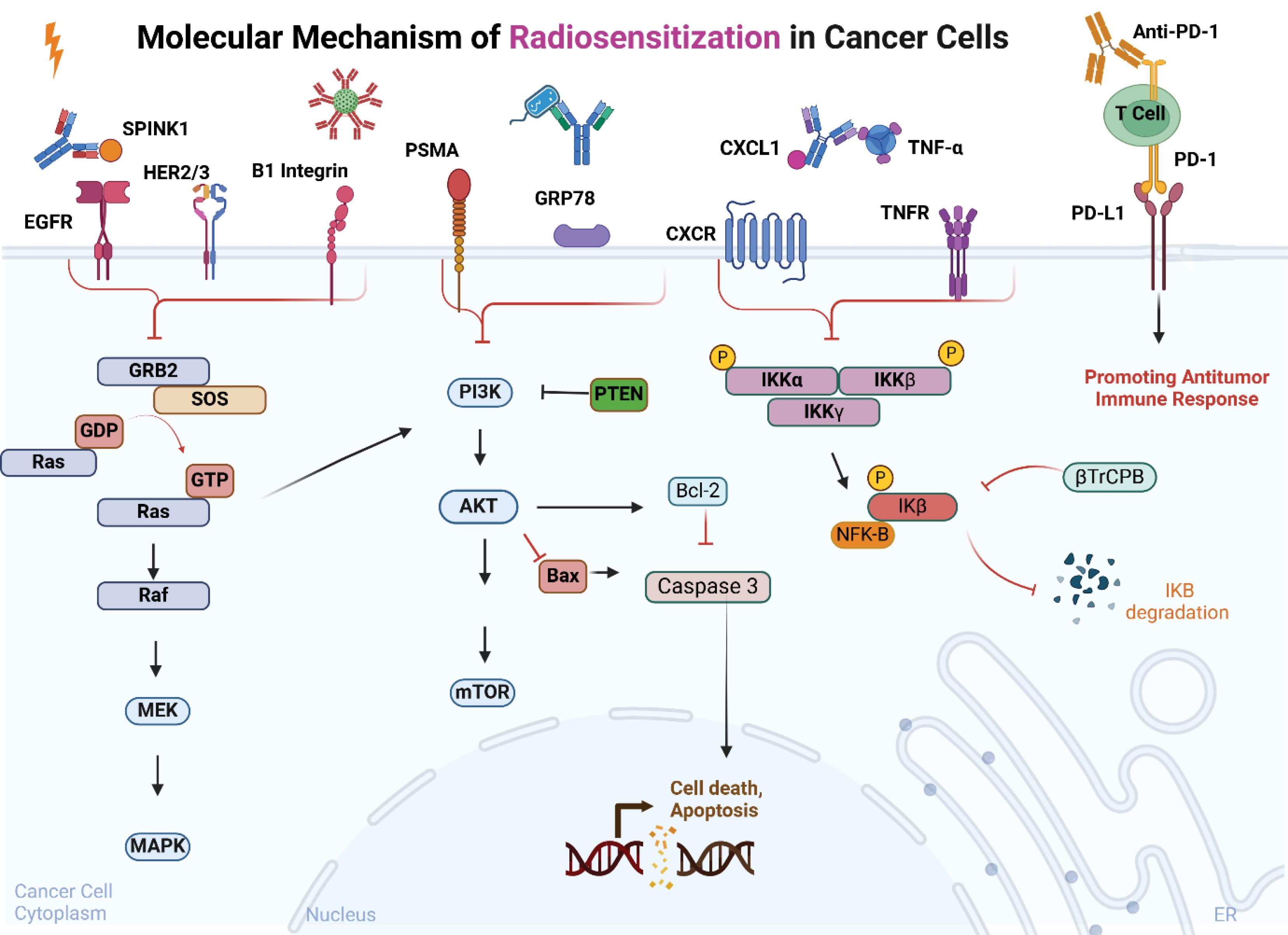
Fig. 4.
Molecular Mechanism of Radiosensitization in Cancer Cells. A schematic representation of radiosensitization mechanisms in cancer cells, emphasizing the role of therapeutic antibodies. These antibodies block key signaling pathways, such as PI3K/AKT/mTOR, MAPK, and NF-κB, targeting molecules like PSMA, GRP78, CXCL1, and TNF-α. This inhibition leads to reduced cell survival and increased activation of apoptotic pathways (e.g., Bax, Caspase 3). Additionally, anti-PD-1 therapy enhances the antitumor immune response, further contributing to radiosensitization and tumor control. Created with BioRender.com.
.
Molecular Mechanism of Radiosensitization in Cancer Cells. A schematic representation of radiosensitization mechanisms in cancer cells, emphasizing the role of therapeutic antibodies. These antibodies block key signaling pathways, such as PI3K/AKT/mTOR, MAPK, and NF-κB, targeting molecules like PSMA, GRP78, CXCL1, and TNF-α. This inhibition leads to reduced cell survival and increased activation of apoptotic pathways (e.g., Bax, Caspase 3). Additionally, anti-PD-1 therapy enhances the antitumor immune response, further contributing to radiosensitization and tumor control. Created with BioRender.com.
EGFR contributes to radioresistance by activating downstream signaling pathways, including the PI3K/AKT and MAPK pathways, which promote DNA repair, cell survival, and anti-apoptotic responses post-irradiation.119 By blocking EGFR signaling, monoclonal antibodies can theoretically enhance radiosensitivity by impeding these repair and survival mechanisms, increasing DNA damage, and inducing apoptosis.120
Recent studies highlight the potential of EGFR-targeted approaches. For example, in glioblastoma, cetuximab bioconjugated to iron oxide nanoparticles (IONPs) significantly enhanced radiosensitivity when combined with ionizing radiation.103 The radiosensitivity emhancement was attributed to increased DNA double-strand breaks and elevated ROS levels, culminating in greater tumor cell death and prolonged survival in animal models. These findings underline the promise of EGFR inhibition in improving radiotherapy outcomes.103
Despite the encouraging articles, some articles have shown its drawbacks. A study by Guster and her colleagues,105 demonstrated that cetuximab failed to radiosensitize HPV-positive HNSCC cell lines.105 This ineffectiveness may be attributed to the distinct biological characteristics of HPV-positive tumors, where alternative pathways such as PARP and Chk1 signaling may dominate in mediating radioresistance. This highlights the need for tailored approaches based on tumor biology. Exploring combination therapies, such as PARP and Chk1 inhibition alongside EGFR targeting, could address these limitations and enhance therapeutic efficacy.105
In a clinical trial setting, the radiosensitizing potential of panitumumab in locally advanced rectal cancer (LARC). Although some pathological tumor regression was observed, the complete pCR rate remained modest.71 The study revealed dynamic changes in plasma levels of EGFR ligands during treatment, suggesting potential biomarker-driven strategies for patient stratification. However, the trial's inability to meet its primary endpoint indicates that EGFR inhibition alone may not suffice in specific contexts and underscores the complexity of integrating such therapies into routine clinical practice.71
The inconsistent outcomes from these studies underscore the necessity for more precise patient selection and a deeper understanding of EGFR's role in specific cancer subtypes. Strategies such as combination therapies, advanced drug delivery systems (e.g., nanoparticle-based approaches), and biomarker-driven patient stratification could enhance the clinical utility of EGFR-targeted radiosensitizers.71,103,105
In conclusion, while EGFR-targeting monoclonal antibodies show potential as radiosensitizers, their efficacy is context-dependent and influenced by tumor-specific biology. Ongoing research into combinatorial approaches and adaptive clinical trial designs will be pivotal in maximizing their therapeutic benefits.
HER2 and HER3
HER2 (human epidermal growth factor receptor 2) and HER3 (human epidermal growth factor receptor 3) are critical members of the EGFR family, playing pivotal roles in tumor growth, survival, and resistance to therapies, including radiotherapy.121 These receptors, often overexpressed or dysregulated in various cancers such as breast and cervical cancers, have emerged as potential targets for enhancing radiosensitivity.122 Their involvement in key signaling pathways such as PI3K/AKT and MAPK contributes to tumor progression and radioresistance.122 Modulate their activity through monoclonal antibodies or ADCs has demonstrated promise in improving radiotherapy outcomes.
HER2 is known to drive aggressive tumor behavior and resistance to radiation through its role in DNA damage repair and cell survival pathways. Targeting HER2 with ADCs, such as trastuzumab emtansine (T-DM1), has shown enhanced radiosensitization by restricting cytotoxic agents specifically to HER2-expressing cells, minimizing off-target effects.88,98 Moreover, preclinical studies have demonstrated that trastuzumab conjugated with cytotoxic agents like monomethyl auristatin F (MMAF) effectively radiosensitizes HER2-positive tumor cells while reducing toxicity to normal tissues.88
Although lacking intrinsic kinase activity, HER3 heterodimerizes with other EGFR family members, particularly HER2, to activate downstream signaling pathways. This interaction promotes cell survival and proliferation, contributing to radiation resistance. Dual targeting of HER2 and HER3, as explored with antibodies like MEHD7945A, has demonstrated synergistic effects with ionizing radiation, enhancing tumor control through increased DNA damage and apoptosis.89
The therapeutic potential of HER2 and HER3 targeting in radiosensitization underscores the importance of integrating molecularly guided therapies with radiotherapy, paving the way for precision oncology approaches.89
SPINK1
Serine protease inhibitor Kazal type I (SPINK1) has recently emerged as a critical player in tumor biology, particularly in modulating radiosensitivity. Its expression is tightly regulated under hypoxic conditions, a hallmark of the tumor microenvironment contributing to radioresistance. Hypoxia-induced SPINK1 expression occurs at the transcriptional level through a HIF-dependent pathway, underscoring its role in adapting to oxygen-deprived conditions within tumors.69
SPINK1 demonstrates a dual role in tumor survival and progression under radiotherapeutic stress. Secreted SPINK1 proteins enhance the radioresistance of cancer cells, even in normoxic environments, by leveraging pathways dependent on EGFR and nuclear factor erythroid 2–related factor 2 (Nrf2). This paracrine mechanism protects hypoxic and relatively oxygenated tumor cells from radiation-induced damage. Furthermore, SPINK1 secretion has been linked to accelerated tumor regrowth post-radiotherapy, presenting a significant barrier to effective cancer treatment.69
Interestingly, therapeutic interventions targeting SPINK1 have shown promise. Using neutralizing antibodies against SPINK1 exhibits a radiosensitizing effect, making it a compelling candidate for combination therapies. Additionally, SPINK1's presence in plasma offers potential as a biomarker for tumor hypoxia, enabling personalized radiotherapy approaches aimed at overcoming hypoxia-associated resistance.69
In summary, SPINK1 is a marker of hypoxia and a facilitator of tumor radioresistance through its paracrine effects and signaling pathway activation (Fig. 5). Its inhibition may enhance the efficacy of radiotherapy and provide a path toward improved cancer treatment outcomes.69


Fig. 5.
SPINK1 as a Plasma Biomarker for Tumor Hypoxia and a Radiosensitization Target. (A) Immunofluorescence staining of HeLa tumor xenografts showing SPINK1 (red) in hypoxic areas marked by pimonidazole (green). Blue: counterstaining with Hoechst 33342. The dotted line indicates the outer edge of pimonidazole-positive regions. Scale bar: 50 μm. (B) SPINK1 overexpression in DU145/EGFP-53BP1-M cells reduces DSBs after irradiation (0 or 4 Gy), shown by EGFP-53BP1 foci (green). (C–E) Quantification of EGFP-53BP1 and γH2AX foci confirms that SPINK1 reduces irradiation-induced DSBs, and this effect is reversed by EGFR inhibition. (F–G) Cell viability assays reveal that rSPINK1 promotes cancer cell survival after radiation, while EGFR inhibition or cetuximab treatment restores radiosensitivity. (H) Clonogenic assay confirms that SPINK1’s protective effect is EGFR-dependent. These findings suggest that SPINK1 promotes EGFR-dependent radioresistance in cancer cells, highlighting its potential as a therapeutic target and supporting the use of monoclonal antibodies like cetuximab to restore radiosensitivity. Abbreviations: EV, empty vector; EGFR-I III, EGFR inhibitor. This figure is reproduced from69 under the CC BY license.
.
SPINK1 as a Plasma Biomarker for Tumor Hypoxia and a Radiosensitization Target. (A) Immunofluorescence staining of HeLa tumor xenografts showing SPINK1 (red) in hypoxic areas marked by pimonidazole (green). Blue: counterstaining with Hoechst 33342. The dotted line indicates the outer edge of pimonidazole-positive regions. Scale bar: 50 μm. (B) SPINK1 overexpression in DU145/EGFP-53BP1-M cells reduces DSBs after irradiation (0 or 4 Gy), shown by EGFP-53BP1 foci (green). (C–E) Quantification of EGFP-53BP1 and γH2AX foci confirms that SPINK1 reduces irradiation-induced DSBs, and this effect is reversed by EGFR inhibition. (F–G) Cell viability assays reveal that rSPINK1 promotes cancer cell survival after radiation, while EGFR inhibition or cetuximab treatment restores radiosensitivity. (H) Clonogenic assay confirms that SPINK1’s protective effect is EGFR-dependent. These findings suggest that SPINK1 promotes EGFR-dependent radioresistance in cancer cells, highlighting its potential as a therapeutic target and supporting the use of monoclonal antibodies like cetuximab to restore radiosensitivity. Abbreviations: EV, empty vector; EGFR-I III, EGFR inhibitor. This figure is reproduced from69 under the CC BY license.
Key signaling pathways in radiosensitization
Radiosensitization is a promising approach to enhance the therapeutic efficacy of radiotherapy, especially in targeting critical molecular pathways (Fig. 4). Among these, downstream signaling pathways, cytokine-related pathways, and cancer-specific pathways play pivotal roles in modulating tumor radiosensitivity.
PI3K/AKT and NF-κB/MAPK
The PI3K/AKT pathway has been extensively studied for its role in promoting tumor survival and resistance to radiotherapy.123 In a study investigating erlotinib-induced radiosensitization in lung adenocarcinoma cells, the blockade of the c-MET-PI3K-AKT pathway significantly enhanced the radiosensitizing effect of erlotinib. Combined treatment with erlotinib and radiation increased apoptosis and reduced colony formation, while inhibition of c-MET further decreased the activation of PI3K and AKT, demonstrating the pathway's central role in radioprotection.104
The NF-κB and MAPK pathways are also crucial in radiation-induced cellular responses, including inflammation, survival, and proliferation.124 Their inhibition has been implicated in reducing tumor resilience to radiotherapy, though specific monoclonal antibodies targeting these pathways warrant further exploration for enhanced clinical outcomes.124
CXCL1 and TNF-α
CXCL1 signaling contributes to tumor invasion, angiogenesis, and resistance to radiotherapy. A study on bladder cancer revealed that selective inhibition of HDAC6 suppressed radiation-induced CXCL1 expression, effectively reducing tumor migration and malignancy. This suggests that targeting CXCL1 can enhance radiosensitization while mitigating radiation-induced oncogenic signaling.78
TNF-α, a key pro-inflammatory cytokine, has been identified as a critical mediator of apoptosis in radiosensitization. The novel SMAC-mimetic Debio 1143 significantly enhanced radiosensitivity in HNSCC models by activating caspase-3 and increasing TNF-α expression. Neutralizing TNF-α or inhibiting caspase activity reversed this effect, confirming their synergistic role in enhancing tumor cell death under radiotherapy.101
PSMA
In prostate cancer, the prostate-specific membrane antigen (PSMA) is a valuable target for radiosensitization. PSMA-targeted gold nanoparticles (PSMA-AuNPs) demonstrated significant radiosensitization under clinical megavoltage radiation beams. The efficacy increased with tumor depth, attributed to enhanced low-energy photon interactions, which boosted dose enhancement ratios. Monte Carlo-based microdosimetry confirmed the distribution and cytoplasmic localization of PSMA-AuNPs, highlighting their potential in depth-dependent radiosensitization strategies.83
These findings underscore the significance of targeting key signaling, cytokine-related pathways, and cancer-specific markers like PSMA in advancing radiosensitization. Integrating molecular insights with targeted monoclonal antibody therapies could revolutionize radiotherapy by overcoming tumor resistance and improving patient outcomes.
Surface proteins
β1 Integrin
β1 integrin is pivotal in mediating cancer cell interactions with the extracellular matrix (ECM), facilitating survival and therapy resistance.125,126 Multiple studies highlight its significance in repairing radiation-induced DNA double-strand breaks (DSBs) through classical non-homologous end joining (NHEJ). Targeting β1 integrin with monoclonal antibodies, such as AIIB2, impairs the repair of radiogenic DSBs, reduces the expression of DNA repair proteins (e.g., Ku70 and Rad50), and enhances radiosensitivity. These effects are evident in HNSCC models, both in vitro and in vivo.97
Furthermore, β1 integrin inhibition demonstrates robust radiosensitizing effects in pancreatic ductal adenocarcinoma (PDAC) by disrupting kinase activity and impairing ECM-mediated resistance. This effect extends to therapy-naïve and radioresistant cell lines, suggesting its potential to address tumor heterogeneity and improve patient survival. Additionally, combined inhibition of β1 integrin and other targets, such as EGFR or PARP, amplifies radiosensitization, indicating its suitability for synergistic treatment approaches.70,94
GRP78
Glucose-regulated protein 78 (GRP78) is overexpressed in several aggressive cancers, including NSCLC and glioblastoma multiforme (GBM). GRP78 is implicated in radioresistance by regulating the PI3K/Akt/mTOR pathway. Anti-GRP78 antibodies have demonstrated significant antitumor activity by reducing proliferation, enhancing apoptosis, and suppressing PI3K/Akt/mTOR signaling in both NSCLC and GBM cell lines. Notably, combining anti-GRP78 antibodies with ionizing radiation (XRT) further delays tumor growth in xenograft models, positioning GRP78 as a promising target for radiosensitization.96
Immune-related pathways: PDL1 in radiosensitization
Programmed death ligand-1 (PD-L1) plays a pivotal role in modulating the immune response in the tumor microenvironment, and recent studies have demonstrated its potential in radiosensitization.127 PD-L1, through its interaction with the PD-1 receptor on T cells, contributes to immune suppression in tumors, allowing them to evade immune surveillance.128 However, targeting the PD-1/PD-L1 axis has emerged as a promising strategy to enhance radiotherapy (RT) effectiveness by reversing immune suppression and promoting antitumor immune responses.128
Several studies have explored how PD-L1 blockade can synergize with radiotherapy to improve therapeutic outcomes. For instance, in a study by Yin et al77 (Table S1), combining PD-1 blockades with radiotherapy and Wee1 inhibition showed enhanced radiosensitivity in hepatoma models. This synergistic effect was attributed to the reactivation of CD8+ T cells, which are crucial for effective immune responses. Specifically, anti-PD-1 therapy increased the proliferation of cytotoxic T cells and reduced T cell depletion, ultimately improving the tumor response to radiation.77
Similarly, a study by Zhou et al (Table S1) discusses the role of immunoradiotherapy, which combines radiotherapy with immune checkpoint inhibition, including anti-PD-L1 antibodies. This study highlighted that anti-PD-L1 treatment, when combined with ultrasmall polyoxotungstate nanoclusters, not only enhanced local tumor destruction but activated a systemic antitumor immune response, further improving the overall efficacy of radiotherapy. This was achieved by generating oxidative stress in the tumor and simultaneously depleting GSH to activate the immune system, thereby overcoming radiation-induced immunosuppression.87
In another study, Azad et al demonstrated that PD-L1 expression in PDAC cells was upregulated after radiotherapy, and blocking PD-L1 enhanced the response to high doses of radiation. This radiosensitizing effect was linked to a reduced immunosuppressive myeloid cell and an increased activated CD8+ T cells within the tumor. The combination of RT and anti-PD-L1 therapy significantly improved tumor control, emphasizing the critical role of PD-L1 blockade in enhancing RT outcomes, especially at higher radiation doses.95
A case report (Fig. 6) also highlights the clinical benefits of combining anti-PD-1 therapy with radiation in advanced oral cavity cancer. This sequential approach led to significant tumor shrinkage and symptom relief after a period of stable disease on pembrolizumab. Although radiation following PD-1 inhibition can increase PD-L1 expression and generate tumor-specific antigens, which may improve the efficacy of checkpoint inhibitors, careful timing and sequencing are crucial to avoid increased toxicity.72
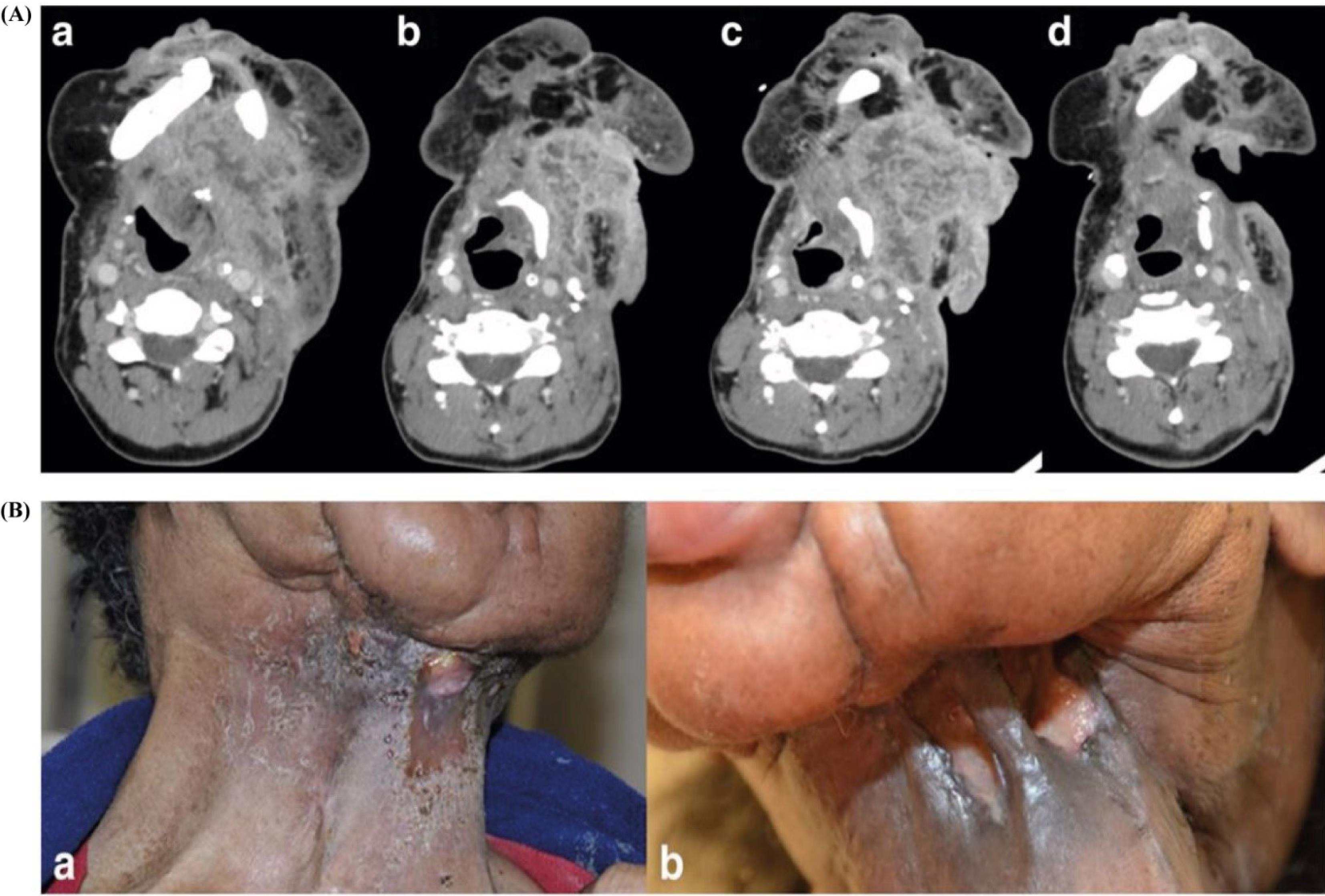
Fig. 6.
PD1/PD-L1 inhibition as a potential radiosensitizer in head and neck squamous cell carcinoma: a case report. (A) Change in largest dimensions of neck mass on CT scans over the treatment period. a) Prior to pembrolizumab. 8.8 × 5.9 cm. b) Best response to pembrolizumab. 6 × 4 cm. c) Progression on pembrolizumab. 7.1 × 7.2 cm. d) Post radiation 5.9 × 3.4 cm. (B) Appearance of neck mass post pembrolizumab and radiation therapy. a) Local tumor control was achieved after six cycles of pembrolizumab monotherapy. b) Bleeding from the tumor mass resolved completely after subsequent radiation therapy, highlighting the combined therapeutic effect. This figure is reproduced from Nagasaka et al72 under the CC BY license.
.
PD1/PD-L1 inhibition as a potential radiosensitizer in head and neck squamous cell carcinoma: a case report. (A) Change in largest dimensions of neck mass on CT scans over the treatment period. a) Prior to pembrolizumab. 8.8 × 5.9 cm. b) Best response to pembrolizumab. 6 × 4 cm. c) Progression on pembrolizumab. 7.1 × 7.2 cm. d) Post radiation 5.9 × 3.4 cm. (B) Appearance of neck mass post pembrolizumab and radiation therapy. a) Local tumor control was achieved after six cycles of pembrolizumab monotherapy. b) Bleeding from the tumor mass resolved completely after subsequent radiation therapy, highlighting the combined therapeutic effect. This figure is reproduced from Nagasaka et al72 under the CC BY license.
Ongoing clinical investigations further support the synergistic potential of combining immunotherapy with radiation. For instance, several clinical trials are currently evaluating the efficacy and safety of combining checkpoint inhibitors with radiotherapy in HNSCC, such as a phase Ib study of cetuximab, ipilimumab, and intensity-modulated radiation therapy (IMRT) in stage III–IVa HPV-positive oropharyngeal SCC (NCT01935921), and a phase II study comparing concurrent versus sequential administration of pembrolizumab, cisplatin, and IMRT in stage III–IVb HNSCC (NCT0277385). These trials underscore the growing clinical interest in harnessing the immunomodulatory effects of radiation in combination with immune checkpoint blockade.72
Lastly, Zhai et al investigated PD-L1-targeted nanoplatforms (Table S1), combining gold nanoparticles and superparamagnetic iron oxide nanoparticles (antiPD-L1-SPIOs@PLGA@Au), which was found to enhance radiosensitivity. This platform blocked the PD-L1/PD-1 axis and reversed the immunosuppressive microenvironment caused by tumor-associated macrophages (TAMs). The nanoplatform, combined with radiation, increased reactive oxygen species (ROS) production, attenuated DNA repair, and promoted tumor-associated macrophage polarization towards an M1 phenotype, thus activating the anti-tumor immune response.82
In summary, targeting PD-L1 with radiotherapy has shown promising results in enhancing radiosensitization. The blockade of PD-L1 helps reverse immune suppression in the tumor microenvironment, activates cytotoxic T cells, and enhances the overall immune response, making it a valuable strategy for improving radiotherapy efficacy.
Challenges, emerging alternatives, and future perspectives
Challenges
Despite their immense potential, mAbs face several significant challenges in serving as effective radiosensitizers. These challenges stem from biological, pharmacological, and technical limitations, as demonstrated by various studies.
One major challenge is the inability of mAbs to cross the BBB, which severely limits their application in treating brain malignancies like glioblastoma. Glioblastoma is notoriously challenging to treat due to its highly invasive nature and resistance to conventional therapies. In a study, researchers attempted to overcome this limitation by combining radiosensitizer nanoparticles coated with insulin and tumor-targeting antibodies (cetuximab). While this approach showed promise in a mouse model, effectively reducing tumor growth and improving survival, it highlights the inherent barrier posed by the BBB.80,129 Without innovative delivery methods, such as nanoparticles, the therapeutic potential of mAbs in brain cancers remains restricted.
Several studies have shown that not all mAbs function effectively as radiosensitizers. Despite reducing microvessel density and metastasis in some cases, for instance Song et al (Table S1) reported that the anti-PDGFRα antibody 1E10Fc failed to significantly enhance the radiosensitivity of soft tissue sarcoma (STS) models.93 This lack of efficacy raises questions about the mechanisms underlying radiosensitization and suggests several possibilities for why specific mAbs may not succeed:
One important factor is the tumor microenvironment. Tumors often exhibit high interstitial pressure and poor vascularization, which can impair the adequate penetration of therapeutic antibodies. As a result, even when the antibodies are present systemically, their concentration within the tumor may be insufficient to achieve effective radiosensitization.
Another contributing factor is variability in target expression. The therapeutic efficacy of monoclonal antibodies largely depends on the expression level of their target antigen. In the case of PDGFRα, heterogeneous or low-level expression across tumor cells can reduce the overall effectiveness of the treatment, limiting its ability to sensitize tumors to radiation.
Additionally, the activation of compensatory signaling pathways by tumor cells represents a major challenge. Even when an mAb successfully binds to its intended target, cancer cells may evade its effects by engaging alternative pathways that maintain survival and proliferation. This adaptability can undermine the radiosensitizing effect of the treatment.
In a study belonging to Guster et al,105 cetuximab, an EGFR inhibitor, failed to enhance the radiosensitivity of HPV-positive HNSCC cells.105 This suggests that EGFR inhibition alone may not sufficiently impact the cellular mechanisms involved in radiosensitivity in these specific tumor subtypes.105 One possible explanation for this underperformance is the complex role of EGFR signaling in the tumor microenvironment, particularly under hypoxic conditions. As demonstrated by Suwa et al69 secreted SPINK1 proteins—induced by hypoxia in a HIF-dependent manner—can activate EGFR and its downstream antioxidant pathways (e.g., Nrf2), promoting radioresistance even in oxygenated tumor regions. This implies that blocking EGFR without addressing hypoxia-induced compensatory mechanisms, such as SPINK1-mediated signaling, may be inadequate to reverse radioresistance.Interestingly, PARP inhibitors, particularly when combined with Chk1 inhibition, demonstrated significant radiosensitization, highlighting the importance of selecting the right molecular targets and combination therapies for effective outcomes.105
Mignot et al evaluated T-DM1, a HER2-targeted ADC, in HER2-positive breast cancer cell lines. While T-DM1 effectively induced cell death and G2/M cell cycle arrest, it did not act as a radiosensitizer under the experimental conditions.86 A potential explanation lies in the intrinsic radioresistance of high HER2-expressing cell lines, which may overshadow the radiosensitizing effects of T-DM1. Additionally, the in vitro nature of the study might not fully replicate the complex in vivo tumor environment where T-DM1 could potentially exhibit better radiosensitizing properties.86
Emerging alternatives
The emergence of alternative technologies, such as aptamers, nanobodies, engineered proteins, and other innovative approaches, poses significant challenges to the widespread use of mAbs.130-133 Aptamers, short nucleic acid sequences that bind to specific targets, offer distinct advantages, including ease of chemical synthesis at lower costs with greater reproducibility, reduced immunogenicity that minimizes the risk of eliciting immune responses, and superior tumor penetration due to their smaller size, which enhances their potential for radiosensitization.130,134-136 Similarly, nanobodies, derived from camelid antibodies, are smaller and more stable than traditional mAbs, allowing them to access difficult-to-reach targets, penetrate tissues more effectively, and retain high binding affinities even under harsh conditions.134
Engineered proteins, such as DARPins and scaffold proteins, broaden the spectrum of alternatives by offering exceptional stability, precise target binding, and ease of production, making them versatile for therapeutic applications. With their minimal size and straightforward synthesis, Short peptides are gaining traction in specific therapies, providing efficient targeting capabilities, particularly in radiosensitization.137,138 Additionally, oncolytic viruses selectively infect and destroy tumor cells, act as direct antitumor agents, and stimulate the immune system to target residual cancer cells, offering a multifaceted therapeutic approach.139 RNA-based therapeutics, including siRNA and mRNA technologies, also present promising alternatives by enabling precise gene modulation and protein expression, making them powerful tools for immunotherapy and tumor radiosensitization.140-143
These advancements collectively highlight the dynamic evolution of alternatives to mAbs, reflecting ongoing efforts to optimize therapeutic outcomes across diverse medical applications.
Future perspectives
Future research should focus on several key areas to address the current challenges in utilizing mAbs as radiosensitizers:
-
Innovative delivery systems: The limited ability of mAbs to cross biological barriers, such as the BBB, necessitates the development of advanced delivery mechanisms.144 Combining mAbs with nanoparticles or other carriers could significantly enhance their penetration and targeting efficiency.145 For example, gold nanoparticles have shown promise in enhancing the transport of therapeutic agents, including antibodies, into hard-to-reach tumor sites, making this a vital avenue for research.146
-
Combination therapies: Tumor resistance remains a significant obstacle to effective radiosensitization. Exploring combination therapies that synergize mAbs with other agents, such as PARP or Chk1 inhibitors, could improve outcomes.147 Such combinations could disrupt compensatory pathways and enhance tumor radiosensitivity, particularly in intrinsically resistant tumor subtypes.147,148
-
Target validation: Identifying and validating new molecular targets is essential for overcoming heterogeneity in tumor response.149-151 Future studies should focus on discovering targets more universally expressed or critical to radiosensitivity. This approach could help expand the applicability of mAbs across diverse cancer types and improve treatment outcomes.149
-
Integration of alternatives: Evaluating novel modalities such as aptamers as replacements or complements to mAbs may offer cost-effective and concrete alternatives for radiosensitization.152,153 Aptamers, with their smaller size and high specificity, could address some limitations of mAbs, including production costs and tumor penetration challenges.152
-
Exploiting tumor hypoxia: Hypoxia in the tumor microenvironment is a significant barrier to effective cancer treatment. Recent studies have demonstrated the potential of utilizing Bifidobacterium infantis engineered to target hypoxic tumor regions. Specific antibodies against B. infantis were developed, allowing the selective eradication of hypoxic tumor areas.76,85 This innovative approach underscores the potential of leveraging hypoxia for targeted therapy, a promising direction for future research (Fig. 3).76,85
-
Immunochemoradiotherapy integration: The combination of immunotherapy, chemotherapy, and radiotherapy—termed immunochemoradiotherapy—holds significant promise.154,155 This approach integrates mAbs into multimodal treatment strategies, enhancing therapeutic efficacy while addressing the limitations of single-modality treatments. For instance, combining mAbs with chemotherapeutic agents and radiation could simultaneously target multiple aspects of tumor biology, creating a more comprehensive and practical treatment framework.154,156-158
By addressing these areas, future research can overcome the limitations of mAbs as radiosensitizers and realize their full therapeutic potential. These advancements would offer more effective and personalized cancer treatment options, significantly improving patient outcomes.
Conclusion
mAbs have revolutionized cancer therapy by offering targeted, effective treatments with reduced systemic toxicity compared to traditional approaches.159 Their ability to selectively bind specific antigens on tumor cells and mediate immune responses has established them invaluable tools in oncology.159 Beyond their standalone therapeutic efficacy, mAbs have demonstrated immense potential in radiosensitization, enhancing tumor sensitivity to radiation while sparing healthy tissues.160 By targeting pathways like EGFR, HER2, and immune checkpoints like PD-L1, mAbs inhibit tumor growth and synergistically amplify radiotherapy's effect.161
However, despite their success, mAbs as radiosensitizers face notable challenges, including limited penetration into hypoxic tumor microenvironments, variability in target expression, and the inability to cross biological barriers like the blood-brain barrier. Furthermore, the emergence of resistance mechanisms in certain tumors underscores the need for combination therapies and novel delivery strategies. Innovations such as ADCs, bispecific antibodies, and engineered nanoparticles pave the way for more precise and practical applications.162
Emerging alternatives, including aptamers, show promise in addressing some limitations of mAbs by offering better tumor penetration and reduced immunogenicity, suggesting a complementary role in future cancer therapies.163 Moreover, integrating mAbs with immunotherapy and leveraging their radiosensitizing properties in combination with immune checkpoint inhibitors could redefine treatment paradigms, particularly for resistant and aggressive cancers.163
To fully harness the potential of mAbs in radiosensitization, future research must focus on optimizing their delivery, validating new molecular targets, and developing integrative approaches that address tumor heterogeneity and microenvironmental challenges. By overcoming these obstacles, mAbs could significantly enhance the efficacy of radiotherapy, offering personalized, effective treatment options that improve outcomes for cancer patients globally.
Review Highlights
What is the current knowledge?
-
Radiotherapy is a cornerstone of cancer treatment but lacks specificity, causing damage to normal tissues.
-
Monoclonal antibodies (mAbs) enhance radiosensitivity by targeting cancer cells, improving radiotherapy efficacy while minimizing collateral damage.
-
mAbs function through diverse mechanisms, including immune activation, growth factor receptor inhibition, and apoptosis induction.
-
Antibody-drug conjugates (ADCs), nanoparticles, and immune checkpoint inhibitors improve radiosensitization.
-
Tumor heterogeneity, resistance mechanisms, and delivery barriers remain key challenges in the clinical application of mAbs.
What is new here?
-
Provides the first integrated analysis of 20 years of clinical and preclinical data on mAb-based radiosensitization.
-
It highlights novel therapeutic combinations that have not been comprehensively reviewed, such as mAbs with HDAC inhibitors and nanoparticles.
-
Covers emerging strategies like bacterial vectors, aptamers, nanobodies, and engineered proteins as next-gen radiosensitizers.
-
Discusses multimodal therapies, especially immunochemoradiotherapy, as a forward-looking solution to resistance mechanisms.
-
Maps key molecular targets to specific antibody-based interventions, offering a practical guide for future therapeutic development.
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
Not applicable to this review.
Supplementary files
Supplementary file 1 contains Table S1.
(pdf)
Acknowledgements
The authors sincerely thank Mashhad University of Medical Sciences for supporting this work.
References
- Deo SV, Sharma J, Kumar S. GLOBOCAN 2020 report on global cancer burden: challenges and opportunities for surgical oncologists. Ann Surg Oncol 2022; 29:6497-500. doi: 10.1245/s10434-022-12151-6 [Crossref] [ Google Scholar]
- Bemidinezhad A, Mirzavi F, Gholamhosseinian H, Gheybi F, Soukhtanloo M. Gold-containing liposomes and glucose-coated gold nanoparticles enhances the radiosensitivity of B16F0 melanoma cells via increasing apoptosis and ROS production. Life Sci 2023; 318:121495. doi: 10.1016/j.lfs.2023.121495 [Crossref] [ Google Scholar]
- Wu B, Wang ZX, Xie H, Xie PL. Dimethyl fumarate augments anticancer activity of ångstrom silver particles in myeloma cells through NRF2 activation. Adv Ther 2025; 8:2400363. doi: 10.1002/adtp.202400363 [Crossref] [ Google Scholar]
- Hosseini FS, Noroozi Karimabad M, Hajizadeh MR, Khoshdel A, Khanamani Falahati-Pour S, Mirzaei MR. Evaluating of induction of apoptosis by Cornus mass L extract in the gastric carcinoma cell line (AGS). Asian Pac J Cancer Prev 2019; 20:123-30. doi: 10.31557/apjcp.2019.20.1.123 [Crossref] [ Google Scholar]
- Mohammad-Sadeghipour M, Mahmoodi M, Noroozi Karimabad M, Mirzaei MR, Hajizadeh MR. Diosgenin and 4-hydroxyisoleucine from fenugreek are regulators of genes involved in lipid metabolism in the human colorectal cancer cell line SW480. Cell J 2021; 22:514-22. doi: 10.22074/cellj.2021.6751 [Crossref] [ Google Scholar]
- Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019; 69:363-85. doi: 10.3322/caac.21565 [Crossref] [ Google Scholar]
- Yang H, Zhou H, Fu M, Xu H, Huang H, Zhong M. TMEM64 aggravates the malignant phenotype of glioma by activating the Wnt/β-catenin signaling pathway. Int J Biol Macromol 2024; 260:129332. doi: 10.1016/j.ijbiomac.2024.129332 [Crossref] [ Google Scholar]
- Lou Y, Cheng M, Cao Q, Li K, Qin H, Bao M. Simultaneous quantification of mirabegron and vibegron in human plasma by HPLC-MS/MS and its application in the clinical determination in patients with tumors associated with overactive bladder. J Pharm Biomed Anal 2024; 240:115937. doi: 10.1016/j.jpba.2023.115937 [Crossref] [ Google Scholar]
- Wang K, Ning S, Zhang S, Jiang M, Huang Y, Pei H. Extracellular matrix stiffness regulates colorectal cancer progression via HSF4. J Exp Clin Cancer Res 2025; 44:30. doi: 10.1186/s13046-025-03297-8 [Crossref] [ Google Scholar]
- Sen A, Kumar K, Khan S, Pathak P, Singh A. Current therapy in cancer: advances, challenges, and future directions. Asian J Nurs Educ Res 2024; 14:77-84. doi: 10.52711/2349-2996.2024.00016 [Crossref] [ Google Scholar]
- Chen S, Long S, Liu Y, Wang S, Hu Q, Fu L. Evaluation of a three-gene methylation model for correlating lymph node metastasis in postoperative early gastric cancer adjacent samples. Front Oncol 2024; 14:1432869. doi: 10.3389/fonc.2024.1432869 [Crossref] [ Google Scholar]
- Duan WW, Yang LT, Liu J, Dai ZY, Wang ZY, Zhang H. A TGF-β signaling-related lncRNA signature for prediction of glioma prognosis, immune microenvironment, and immunotherapy response. CNS Neurosci Ther 2024; 30:e14489. doi: 10.1111/cns.14489 [Crossref] [ Google Scholar]
- Chen L, Wu L, Zhang L, Sun B, Wu W, Lei Y. Effect of metformin on hepatocellular carcinoma patients with type II diabetes receiving transarterial chemoembolization: a multicenter retrospective cohort study. Int J Surg 2025; 111:828-38. doi: 10.1097/js9.0000000000001872 [Crossref] [ Google Scholar]
- Manzari MT, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller DA. Targeted drug delivery strategies for precision medicines. Nat Rev Mater 2021; 6:351-70. doi: 10.1038/s41578-020-00269-6 [Crossref] [ Google Scholar]
- Mobasheri L, Ahadi M, Beheshti Namdar A, Alavi MS, Bemidinezhad A, Moshirian Farahi SM. Pathophysiology of diabetic hepatopathy and molecular mechanisms underlying the hepatoprotective effects of phytochemicals. Biomed Pharmacother 2023; 167:115502. doi: 10.1016/j.biopha.2023.115502 [Crossref] [ Google Scholar]
- Chen Y, Deng Y, Li Y, Qin Y, Zhou Z, Yang H. Oxygen-independent radiodynamic therapy: radiation-boosted chemodynamics for reprogramming the tumor immune environment and enhancing antitumor immune response. ACS Appl Mater Interfaces 2024; 16:21546-56. doi: 10.1021/acsami.4c00793 [Crossref] [ Google Scholar]
- Jiang Z, Chen Z, Xu Y, Li H, Li Y, Peng L. Low-frequency ultrasound sensitive Piezo1 channels regulate keloid-related characteristics of fibroblasts. Adv Sci (Weinh) 2024; 11:e2305489. doi: 10.1002/advs.202305489 [Crossref] [ Google Scholar]
- van den Boogaard WM, Komninos DS, Vermeij WP. Chemotherapy side-effects: not all DNA damage is equal. Cancers 2022; 14:627. doi: 10.3390/cancers14030627 [Crossref] [ Google Scholar]
- De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AW, Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Dis Primers 2019; 5:13. doi: 10.1038/s41572-019-0064-5 [Crossref] [ Google Scholar]
- Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther 2022; 7:258. doi: 10.1038/s41392-022-01102-y [Crossref] [ Google Scholar]
- Cruz E, Kayser V. Monoclonal antibody therapy of solid tumors: clinical limitations and novel strategies to enhance treatment efficacy. Biologics 2019; 13:33-51. doi: 10.2147/btt.S166310 [Crossref] [ Google Scholar]
- Mekala JR, Nalluri HP, Reddy PN, Sainath SB, Sampath Kumar NS, Sai Kiran GV. Emerging trends and therapeutic applications of monoclonal antibodies. Gene 2024; 925:148607. doi: 10.1016/j.gene.2024.148607 [Crossref] [ Google Scholar]
- Marhelava K, Pilch Z, Bajor M, Graczyk-Jarzynka A, Zagozdzon R. Targeting negative and positive immune checkpoints with monoclonal antibodies in therapy of cancer. Cancers (Basel) 2019; 11:1756. doi: 10.3390/cancers11111756 [Crossref] [ Google Scholar]
- Wang H, Chen D, Lu H. Anti-bacterial monoclonal antibodies: next generation therapy against superbugs. Appl Microbiol Biotechnol 2022; 106:3957-72. doi: 10.1007/s00253-022-11989-w [Crossref] [ Google Scholar]
- Lee A, Djamgoz MBA. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat Rev 2018; 62:110-22. doi: 10.1016/j.ctrv.2017.11.003 [Crossref] [ Google Scholar]
- Ali MY, Oliva CR, Noman AS, Allen BG, Goswami PC, Zakharia Y. Radioresistance in glioblastoma and the development of radiosensitizers. Cancers (Basel) 2020; 12:2511. doi: 10.3390/cancers12092511 [Crossref] [ Google Scholar]
- Babaye Abdollahi B, Malekzadeh R, Pournaghi Azar F, Salehnia F, Naseri AR, Ghorbani M. Main approaches to enhance radiosensitization in cancer cells by nanoparticles: a systematic review. Adv Pharm Bull 2021; 11:212-23. doi: 10.34172/apb.2021.025 [Crossref] [ Google Scholar]
- Abolhassani Y, Mirzaei S, Nejabat M, Talebian S, Gholamhosseinian H, Iranshahi M. 7-Geranyloxcycoumarin enhances radio sensitivity in human prostate cancer cells. Mol Biol Rep 2023; 50:5709-17. doi: 10.1007/s11033-023-08439-9 [Crossref] [ Google Scholar]
- Singh S, Kumar NK, Dwiwedi P, Charan J, Kaur R, Sidhu P. Monoclonal antibodies: a review. Curr Clin Pharmacol 2018; 13:85-99. doi: 10.2174/1574884712666170809124728 [Crossref] [ Google Scholar]
- Wang Y, Deng W, Li N, Neri S, Sharma A, Jiang W. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol 2018; 9:185. doi: 10.3389/fphar.2018.00185 [Crossref] [ Google Scholar]
- Gurunathan S, Kang MH, Qasim M, Kim JH. Nanoparticle-mediated combination therapy: two-in-one approach for cancer. Int J Mol Sci 2018; 19:3264. doi: 10.3390/ijms19103264 [Crossref] [ Google Scholar]
- Tsao LC, Force J, Hartman ZC. Mechanisms of therapeutic antitumor monoclonal antibodies. Cancer Res 2021; 81:4641-51. doi: 10.1158/0008-5472.Can-21-1109 [Crossref] [ Google Scholar]
- Perwein MK, Smestad JA, Warrington AE, Heider RM, Kaczor MW, Maher LJ, 3rd 3rd. A comparison of human natural monoclonal antibodies and aptamer conjugates for promotion of CNS remyelination: where are we now and what comes next?. Expert Opin Biol Ther 2018; 18:545-60. doi: 10.1080/14712598.2018.1441284 [Crossref] [ Google Scholar]
- Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol 2020; 17:251-66. doi: 10.1038/s41571-019-0308-z [Crossref] [ Google Scholar]
- Huang H, Huang F, Liang X, Fu Y, Cheng Z, Huang Y. Afatinib reverses EMT via inhibiting CD44-Stat3 axis to promote radiosensitivity in nasopharyngeal carcinoma. Pharmaceuticals (Basel) 2022; 16:37. doi: 10.3390/ph16010037 [Crossref] [ Google Scholar]
- Sompayrac LM. How the Immune System Works. John Wiley & Sons; 2022.
- Duan JL, Wang CC, Yuan Y, Hui Z, Zhang H, Mao ND. Design, synthesis, and structure-activity relationship of novel pyridazinone-based PARP7/HDACs dual inhibitors for elucidating the relationship between antitumor immunity and HDACs inhibition. J Med Chem 2024; 67:4950-76. doi: 10.1021/acs.jmedchem.4c00090 [Crossref] [ Google Scholar]
- Zhou Y, Li L, Yu Z, Gu X, Pan R, Li Q. Dermatophagoides pteronyssinus allergen Der p 22: cloning, expression, IgE-binding in asthmatic children, and immunogenicity. Pediatr Allergy Immunol 2022; 33:e13835. doi: 10.1111/pai.13835 [Crossref] [ Google Scholar]
- Peng L, Wu Z, Sun W, Wang C. Clinical characteristics, treatment, and outcomes of nivolumab induced immune thrombocytopenia. Invest New Drugs 2024; 42:575-80. doi: 10.1007/s10637-024-01472-w [Crossref] [ Google Scholar]
- Atkinson TP. Immunoglobulins, structure, and function. In: Ragab G, Quartuccio L, Goubran H, eds. Paraproteinemia and Related Disorders. Cham: Springer International Publishing. 2022. p. 27-36. doi: 10.1007/978-3-031-10131-1_3.
- Wadsworth PA, Ho CC, Zhang BM. Immunoglobulin and T‐cell receptor gene assessment. In: Schmitz JL, Detrick B, O'Gorman MR, eds. Manual of Molecular and Clinical Laboratory Immunology. John Wiley & Sons. 2024. p. 23-38. doi: 10.1002/9781683674023.ch3.
- Lin W, Shen C, Li M, Ma S, Liu C, Huang J. Programmable macrophage vesicle based bionic self-adjuvanting vaccine for immunization against monkeypox virus. Adv Sci (Weinh) 2025; 12:e2408608. doi: 10.1002/advs.202408608 [Crossref] [ Google Scholar]
- Sun DY, Hu YJ, Li X, Peng J, Dai ZJ, Wang S. Unlocking the full potential of memory T cells in adoptive T cell therapy for hematologic malignancies. Int Immunopharmacol 2025; 144:113392. doi: 10.1016/j.intimp.2024.113392 [Crossref] [ Google Scholar]
- Hu S, Jiang S, Qi X, Bai R, Ye XY, Xie T. Races of small molecule clinical trials for the treatment of COVID-19: An up-to-date comprehensive review. Drug Dev Res 2022; 83:16-54. doi: 10.1002/ddr.21895 [Crossref] [ Google Scholar]
- Lin PH, Yao HY, Huang L, Fu CC, Yao XL, Lian C. Autoimmune astrocytopathy double negative for AQP4-IgG and GFAP-IgG: retrospective research of clinical practice, biomarkers, and pathology. CNS Neurosci Ther 2024; 30:e70042. doi: 10.1111/cns.70042 [Crossref] [ Google Scholar]
- Yang Z, Liu X, Xu H, Teschendorff AE, Xu L, Li J. Integrative analysis of genomic and epigenomic regulation reveals miRNA mediated tumor heterogeneity and immune evasion in lower grade glioma. Commun Biol 2024; 7:824. doi: 10.1038/s42003-024-06488-9 [Crossref] [ Google Scholar]
- Muhammed Y. The best IgG subclass for the development of therapeutic monoclonal antibody drugs and their commercial production: a review. Immunome Res 2020; 16:173. doi: 10.35248/1745-7580.20.16.173 [Crossref] [ Google Scholar]
- Li YY, Zhou LW, Qian FC, Fang QL, Yu ZM, Cui T. scImmOmics: a manually curated resource of single-cell multi-omics immune data. Nucleic Acids Res 2025; 53:D1162-72. doi: 10.1093/nar/gkae985 [Crossref] [ Google Scholar]
- Demlie T, Balcha E, Fesseha H. Monoclonal antibody and its diagnostic application-review. Biomed J Sci Tech Res 2020; 30:23645-51. doi: 10.26717/bjstr.2020.30.004997 [Crossref] [ Google Scholar]
- Yin D, Zhong Y, Ling S, Lu S, Wang X, Jiang Z. Dendritic-cell-targeting virus-like particles as potent mRNA vaccine carriers. Nat Biomed Eng 2025; 9:185-200. doi: 10.1038/s41551-024-01208-4 [Crossref] [ Google Scholar]
- Aboul-Ella H, Gohar A, Ali AA, Ismail LM, Mahmoud AE, Elkhatib WF. Monoclonal antibodies: from magic bullet to precision weapon. Mol Biomed 2024; 5:47. doi: 10.1186/s43556-024-00210-1 [Crossref] [ Google Scholar]
- Raja A, Kasana A, Verma V. Next-generation therapeutic antibodies for cancer treatment: advancements, applications, and challenges. Mol Biotechnol 2024: 1-21. doi: 10.1007/s12033-024-01270-y.
- Sasso JM, Tenchov R, Bird R, Iyer KA, Ralhan K, Rodriguez Y. The evolving landscape of antibody-drug conjugates: in depth analysis of recent research progress. Bioconjug Chem 2023; 34:1951-2000. doi: 10.1021/acs.bioconjchem.3c00374 [Crossref] [ Google Scholar]
- Jiang Z, Li Y, Wei Z, Yuan B, Wang Y, Akakuru OU. Pressure-induced amorphous zeolitic imidazole frameworks with reduced toxicity and increased tumor accumulation improves therapeutic efficacy In vivo. Bioact Mater 2021; 6:740-8. doi: 10.1016/j.bioactmat.2020.08.036 [Crossref] [ Google Scholar]
- Jin S, Sun Y, Liang X, Gu X, Ning J, Xu Y. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct Target Ther 2022; 7:39. doi: 10.1038/s41392-021-00868-x [Crossref] [ Google Scholar]
- Gun SY, Lee SW, Sieow JL, Wong SC. Targeting immune cells for cancer therapy. Redox Biol 2019; 25:101174. doi: 10.1016/j.redox.2019.101174 [Crossref] [ Google Scholar]
- Castelli MS, McGonigle P, Hornby PJ. The pharmacology and therapeutic applications of monoclonal antibodies. Pharmacol Res Perspect 2019; 7:e00535. doi: 10.1002/prp2.535 [Crossref] [ Google Scholar]
- Parray HA, Shukla S, Samal S, Shrivastava T, Ahmed S, Sharma C. Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. Int Immunopharmacol 2020; 85:106639. doi: 10.1016/j.intimp.2020.106639 [Crossref] [ Google Scholar]
- Harris CT, Cohen S. Reducing immunogenicity by design: approaches to minimize immunogenicity of monoclonal antibodies. BioDrugs 2024; 38:205-26. doi: 10.1007/s40259-023-00641-2 [Crossref] [ Google Scholar]
- Stone CA Jr, Spiller BW, Smith SA. Engineering therapeutic monoclonal antibodies. J Allergy Clin Immunol 2024; 153:539-48. doi: 10.1016/j.jaci.2023.11.018 [Crossref] [ Google Scholar]
- Costa RL, Czerniecki BJ. Clinical development of immunotherapies for HER2+ breast cancer: a review of HER2-directed monoclonal antibodies and beyond. NPJ Breast Cancer 2020; 6:10. doi: 10.1038/s41523-020-0153-3 [Crossref] [ Google Scholar]
- Li F, Liu S. Focusing on NK cells and ADCC: a promising immunotherapy approach in targeted therapy for HER2-positive breast cancer. Front Immunol 2022; 13:1083462. doi: 10.3389/fimmu.2022.1083462 [Crossref] [ Google Scholar]
- Paul S, Konig MF, Pardoll DM, Bettegowda C, Papadopoulos N, Wright KM. Cancer therapy with antibodies. Nat Rev Cancer 2024; 24:399-426. doi: 10.1038/s41568-024-00690-x [Crossref] [ Google Scholar]
- Li M, Mei S, Yang Y, Shen Y, Chen L. Strategies to mitigate the on- and off-target toxicities of recombinant immunotoxins: an antibody engineering perspective. Antib Ther 2022; 5:164-76. doi: 10.1093/abt/tbac014 [Crossref] [ Google Scholar]
- Mukherjee A, Bandyopadhyay D. Targeted therapy in breast cancer: advantages and advancements of antibody-drug conjugates, a type of chemo-biologic hybrid drugs. Cancers (Basel) 2024; 16:3517. doi: 10.3390/cancers16203517 [Crossref] [ Google Scholar]
- Natangelo S, Trapani D, Koukoutzeli C, Boscolo Bielo L, Marvaso G, Jereczek-Fossa BA. Radiation therapy, tissue radiosensitization, and potential synergism in the era of novel antibody-drug conjugates. Crit Rev Oncol Hematol 2024; 195:104270. doi: 10.1016/j.critrevonc.2024.104270 [Crossref] [ Google Scholar]
- Tang L, Wei F, Wu Y, He Y, Shi L, Xiong F. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J Exp Clin Cancer Res 2018; 37:87. doi: 10.1186/s13046-018-0758-7 [Crossref] [ Google Scholar]
- Large DE, Soucy JR, Hebert J, Auguste DT. Advances in receptor-mediated, tumor-targeted drug delivery. Adv Ther (Weinh) 2019; 2:1800091. doi: 10.1002/adtp.201800091 [Crossref] [ Google Scholar]
- Suwa T, Kobayashi M, Shirai Y, Nam JM, Tabuchi Y, Takeda N. SPINK1 as a plasma marker for tumor hypoxia and a therapeutic target for radiosensitization. JCI Insight 2021; 6:e148135. doi: 10.1172/jci.insight.148135 [Crossref] [ Google Scholar]
- Eke I, Zscheppang K, Dickreuter E, Hickmann L, Mazzeo E, Unger K, et al. Simultaneous β1 integrin-EGFR targeting and radiosensitization of human head and neck cancer. J Natl Cancer Inst 2015; 107. doi: 10.1093/jnci/dju419.
- Mardjuadi FI, Carrasco J, Coche JC, Sempoux C, Jouret-Mourin A, Scalliet P. Panitumumab as a radiosensitizing agent in KRAS wild-type locally advanced rectal cancer. Target Oncol 2015; 10:375-83. doi: 10.1007/s11523-014-0342-9 [Crossref] [ Google Scholar]
- Nagasaka M, Zaki M, Kim H, Raza SN, Yoo G, Lin HS. PD1/PD-L1 inhibition as a potential radiosensitizer in head and neck squamous cell carcinoma: a case report. J Immunother Cancer 2016; 4:83. doi: 10.1186/s40425-016-0187-0 [Crossref] [ Google Scholar]
- Kim EG, Kim KM. Strategies and advancement in antibody-drug conjugate optimization for targeted cancer therapeutics. Biomol Ther (Seoul) 2015; 23:493-509. doi: 10.4062/biomolther.2015.116 [Crossref] [ Google Scholar]
- Lewis CD, Singh AK, Hsu FF, Thotala D, Hallahan DE, Kapoor V. Targeting a radiosensitizing antibody-drug conjugate to a radiation-inducible antigen. Clin Cancer Res 2021; 27:3224-33. doi: 10.1158/1078-0432.Ccr-20-1725 [Crossref] [ Google Scholar]
- Zhu Q, Gao Y, Hu Q, Hu D, Wu X. A study on the factors influencing the intention to receive booster shots of the COVID-19 vaccine in China based on the information frame effect. Front Public Health 2024; 12:1258188. doi: 10.3389/fpubh.2024.1258188 [Crossref] [ Google Scholar]
- Wang W, Zheng Y, Wu Z, Wu M, Chen Y, Zhang Y. Antibody targeting of anaerobic bacteria warms cold tumors and improves the abscopal effect of radiotherapy. J Transl Med 2024; 22:657. doi: 10.1186/s12967-024-05469-0 [Crossref] [ Google Scholar]
- Yin Y, Wang J, Yi J, Zhang K, Yin Z, Jin S. AZD1775 and anti-PD-1 antibody synergistically sensitize hepatoma to radiotherapy. Chin Med J (Engl) 2024; 137:222-31. doi: 10.1097/cm9.0000000000002988 [Crossref] [ Google Scholar]
- Tsai YC, Wang TY, Hsu CL, Lin WC, Chen JY, Li JH. Selective inhibition of HDAC6 promotes bladder cancer radiosensitization and mitigates the radiation-induced CXCL1 signalling. Br J Cancer 2023; 128:1753-64. doi: 10.1038/s41416-023-02195-0 [Crossref] [ Google Scholar]
- Babaye Abdollahi B, Ghorbani M, Hamishehkar H, Malekzadeh R, Farajollahi A. Synthesis and characterization of actively HER-2 targeted Fe3O4@Au nanoparticles for molecular radiosensitization of breast cancer. Bioimpacts 2023; 13:17-29. doi: 10.34172/bi.2022.23682 [Crossref] [ Google Scholar]
- Gal O, Betzer O, Rousso-Noori L, Sadan T, Motiei M, Nikitin M. Antibody delivery into the brain by radiosensitizer nanoparticles for targeted glioblastoma therapy. J Nanotheranostics 2022; 3:177-88. doi: 10.3390/jnt3040012 [Crossref] [ Google Scholar]
- Hingorani DV, Allevato MM, Camargo MF, Lesperance J, Quraishi MA, Aguilera J. Monomethyl auristatin antibody and peptide drug conjugates for trimodal cancer chemo-radio-immunotherapy. Nat Commun 2022; 13:3869. doi: 10.1038/s41467-022-31601-z [Crossref] [ Google Scholar]
- Du C, Jiang J, Wan C, Pan G, Kong F, Zhai R. AntiPD-L1 antibody conjugated Au-SPIOs nanoplatform for enhancing radiosensitivity and triggering anti-tumor immune response. Sci Rep 2022; 12:19542. doi: 10.1038/s41598-022-23434-z [Crossref] [ Google Scholar]
- Schmidt RM, Hara D, Vega JD, Abuhaija MB, Tao W, Dogan N. Quantifying radiosensitization of PSMA-targeted gold nanoparticles on prostate cancer cells at megavoltage radiation energies by Monte Carlo simulation and local effect model. Pharmaceutics 2022; 14:2205. doi: 10.3390/pharmaceutics14102205 [Crossref] [ Google Scholar]
- Görte J, Danen E, Cordes N. Therapy-naive and radioresistant 3-dimensional pancreatic cancer cell cultures are effectively radiosensitized by β1 integrin targeting. Int J Radiat Oncol Biol Phys 2022; 112:487-98. doi: 10.1016/j.ijrobp.2021.08.035 [Crossref] [ Google Scholar]
- Yang J, Wu Z, Chen Y, Hu C, Li D, Chen Y. Pre-treatment with Bifidobacterium infantis and its specific antibodies enhance targeted radiosensitization in a murine model for lung cancer. J Cancer Res Clin Oncol 2021; 147:411-22. doi: 10.1007/s00432-020-03434-0 [Crossref] [ Google Scholar]
- Mignot F, Kirova Y, Verrelle P, Teulade-Fichou MP, Megnin-Chanet F. In vitro effects of trastuzumab emtansine (T-DM1) and concurrent irradiation on HER2-positive breast cancer cells. Cancer Radiother 2021; 25:126-34. doi: 10.1016/j.canrad.2020.06.028 [Crossref] [ Google Scholar]
- Zhou R, Yan L, Dong X, Zhu S, Chen K, Wu Y. Fractionated regimen-suitable immunoradiotherapy sensitizer based on ultrasmall Fe4Se2W18 nanoclusters enable tumor-specific radiosensitization augment and antitumor immunity boost. Nano Today 2021; 36:101003. doi: 10.1016/j.nantod.2020.101003 [Crossref] [ Google Scholar]
- Hingorani DV, Doan MK, Camargo MF, Aguilera J, Song SM, Pizzo D. Precision chemoradiotherapy for HER2 tumors using antibody conjugates of an auristatin derivative with reduced cell permeability. Mol Cancer Ther 2020; 19:157-67. doi: 10.1158/1535-7163.Mct-18-1302 [Crossref] [ Google Scholar]
- Bourillon L, Demontoy S, Lenglet A, Zampieri A, Fraisse J, Jarlier M. Higher anti-tumor efficacy of the dual HER3-EGFR antibody MEHD7945a combined with ionizing irradiation in cervical cancer cells. Int J Radiat Oncol Biol Phys 2020; 106:1039-51. doi: 10.1016/j.ijrobp.2019.12.020 [Crossref] [ Google Scholar]
- Hingorani DV, Crisp JL, Doan MK, Camargo MF, Quraishi MA, Aguilera J. Redirecting extracellular proteases to molecularly guide radiosensitizing drugs to tumors. Biomaterials 2020; 248:120032. doi: 10.1016/j.biomaterials.2020.120032 [Crossref] [ Google Scholar]
- Li S, Bouchy S, Penninckx S, Marega R, Fichera O, Gallez B. Antibody-functionalized gold nanoparticles as tumor-targeting radiosensitizers for proton therapy. Nanomedicine (Lond) 2019; 14:317-33. doi: 10.2217/nnm-2018-0161 [Crossref] [ Google Scholar]
- Hatoyama K, Kitamura N, Takano-Kasuya M, Tokunaga M, Oikawa T, Ohta M. Quantitative analyses of amount and localization of radiosensitizer gold nanoparticles interacting with cancer cells to optimize radiation therapy. Biochem Biophys Res Commun 2019; 508:1093-100. doi: 10.1016/j.bbrc.2018.12.016 [Crossref] [ Google Scholar]
- Song EJ, Ashcraft KA, Lowery CD, Mowery YM, Luo L, Ma Y. Investigating a chimeric anti-mouse PDGFRα antibody as a radiosensitizer in primary mouse sarcomas. EBioMedicine 2019; 40:224-30. doi: 10.1016/j.ebiom.2019.01.046 [Crossref] [ Google Scholar]
- Klapproth E, Dickreuter E, Zakrzewski F, Seifert M, Petzold A, Dahl A. Whole exome sequencing identifies mTOR and KEAP1 as potential targets for radiosensitization of HNSCC cells refractory to EGFR and β1 integrin inhibition. Oncotarget 2018; 9:18099-114. doi: 10.18632/oncotarget.24266 [Crossref] [ Google Scholar]
- Azad A, Yin Lim S, D'Costa Z, Jones K, Diana A, Sansom OJ. PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol Med 2017; 9:167-80. doi: 10.15252/emmm.201606674 [Crossref] [ Google Scholar]
- Dadey DY, Kapoor V, Hoye K, Khudanyan A, Collins A, Thotala D. Antibody targeting GRP78 enhances the efficacy of radiation therapy in human glioblastoma and non-small cell lung cancer cell lines and tumor models. Clin Cancer Res 2017; 23:2556-64. doi: 10.1158/1078-0432.Ccr-16-1935 [Crossref] [ Google Scholar]
- Dickreuter E, Eke I, Krause M, Borgmann K, van Vugt MA, Cordes N. Targeting of β1 integrins impairs DNA repair for radiosensitization of head and neck cancer cells. Oncogene 2016; 35:1353-62. doi: 10.1038/onc.2015.212 [Crossref] [ Google Scholar]
- Adams SR, Yang HC, Savariar EN, Aguilera J, Crisp JL, Jones KA. Anti-tubulin drugs conjugated to anti-ErbB antibodies selectively radiosensitize. Nat Commun 2016; 7:13019. doi: 10.1038/ncomms13019 [Crossref] [ Google Scholar]
- Sharma A, Bender S, Zimmermann M, Riesterer O, Broggini-Tenzer A, Pruschy MN. Secretome signature identifies ADAM17 as novel target for radiosensitization of non-small cell lung cancer. Clin Cancer Res 2016; 22:4428-39. doi: 10.1158/1078-0432.Ccr-15-2449 [Crossref] [ Google Scholar]
- Popovtzer A, Mizrachi A, Motiei M, Bragilovski D, Lubimov L, Levi M. Actively targeted gold nanoparticles as novel radiosensitizer agents: an in vivo head and neck cancer model. Nanoscale 2016; 8:2678-85. doi: 10.1039/c5nr07496g [Crossref] [ Google Scholar]
- Matzinger O, Viertl D, Tsoutsou P, Kadi L, Rigotti S, Zanna C. The radiosensitizing activity of the SMAC-mimetic, Debio 1143, is TNFα-mediated in head and neck squamous cell carcinoma. Radiother Oncol 2015; 116:495-503. doi: 10.1016/j.radonc.2015.05.017 [Crossref] [ Google Scholar]
- Kriegs M, Gurtner K, Can Y, Brammer I, Rieckmann T, Oertel R. Radiosensitization of NSCLC cells by EGFR inhibition is the result of an enhanced p53-dependent G1 arrest. Radiother Oncol 2015; 115:120-7. doi: 10.1016/j.radonc.2015.02.018 [Crossref] [ Google Scholar]
- Bouras A, Kaluzova M, Hadjipanayis CG. Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibody-conjugated iron-oxide nanoparticles. J Neurooncol 2015; 124:13-22. doi: 10.1007/s11060-015-1807-0 [Crossref] [ Google Scholar]
- Zhuang HQ, Zhuang H, Bo Q, Guo Y, Wang J, Zhao LJ. Experimental study on the regulation of erlotinib-induced radiosensitization with an anti-c-MET monoclonal antibody. Cancer Cell Int 2014; 14:109. doi: 10.1186/s12935-014-0109-5 [Crossref] [ Google Scholar]
- Güster JD, Weissleder SV, Busch CJ, Kriegs M, Petersen C, Knecht R. The inhibition of PARP but not EGFR results in the radiosensitization of HPV/p16-positive HNSCC cell lines. Radiother Oncol 2014; 113:345-51. doi: 10.1016/j.radonc.2014.10.011 [Crossref] [ Google Scholar]
- Dong Q, Jiang Z. Platinum–iron nanoparticles for oxygen-enhanced sonodynamic tumor cell suppression. Inorganics 2024; 12:331. doi: 10.3390/inorganics12120331 [Crossref] [ Google Scholar]
- Zhao Q, Feng J, Liu F, Liang Q, Xie M, Dong J. Rhizoma Drynariae-derived nanovesicles reverse osteoporosis by potentiating osteogenic differentiation of human bone marrow mesenchymal stem cells via targeting ERα signaling. Acta Pharm Sin B 2024; 14:2210-27. doi: 10.1016/j.apsb.2024.02.005 [Crossref] [ Google Scholar]
- Li WQ, Wu JY, Xiang DX, Luo SL, Hu XB, Tang TT. Micelles loaded with puerarin and modified with triphenylphosphonium cation possess mitochondrial targeting and demonstrate enhanced protective effect against isoprenaline-induced H9c2 cells apoptosis. Int J Nanomedicine 2019; 14:8345-60. doi: 10.2147/ijn.S219670 [Crossref] [ Google Scholar]
- Salandari Rabori M, Noroozi Karimabad M, Hajizadeh MR. Facile, low-cost and rapid phytosynthesis of stable and eco-friendly gold nanoparticles using green walnut shell and study of their anticancer potential. World Cancer Res J 2021; 8:e2037. [ Google Scholar]
- Jebali A, Noroozi Karimabad M, Ahmadi Z, Khorramdel H, Kaeidi A, Mirzaei M. Attenuation of inflammatory response in the EAE model by PEGlated nanoliposome of pistachio oils. J Neuroimmunol 2020; 347:577352. doi: 10.1016/j.jneuroim.2020.577352 [Crossref] [ Google Scholar]
- Zhang D, Song J, Jing Z, Qin H, Wu Y, Zhou J. Stimulus responsive nanocarrier for enhanced antitumor responses against hepatocellular carcinoma. Int J Nanomedicine 2024; 19:13339-55. doi: 10.2147/ijn.S486465 [Crossref] [ Google Scholar]
- Colombo I, Overchuk M, Chen J, Reilly RM, Zheng G, Lheureux S. Molecular imaging in drug development: Update and challenges for radiolabeled antibodies and nanotechnology. Methods 2017; 130:23-35. doi: 10.1016/j.ymeth.2017.07.018 [Crossref] [ Google Scholar]
- Zhang Z, Wang L, Guo Z, Sun Y, Yan J. A pH-sensitive imidazole grafted polymeric micelles nanoplatform based on ROS amplification for ferroptosis-enhanced chemodynamic therapy. Colloids Surf B Biointerfaces 2024; 237:113871. doi: 10.1016/j.colsurfb.2024.113871 [Crossref] [ Google Scholar]
- Zhao C, Song W, Wang J, Tang X, Jiang Z. Immunoadjuvant-functionalized metal-organic frameworks: synthesis and applications in tumor immune modulation. Chem Commun (Camb) 2025; 61:1962-77. doi: 10.1039/d4cc06510g [Crossref] [ Google Scholar]
- Sun D, Li X, Nie S, Liu J, Wang S. Disorders of cancer metabolism: the therapeutic potential of cannabinoids. Biomed Pharmacother 2023; 157:113993. doi: 10.1016/j.biopha.2022.113993 [Crossref] [ Google Scholar]
- Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 2017; 9:52. doi: 10.3390/cancers9050052 [Crossref] [ Google Scholar]
- Yang H, He C, Bi Y, Zhu X, Deng D, Ran T. Synergistic effect of VEGF and SDF-1α in endothelial progenitor cells and vascular smooth muscle cells. Front Pharmacol 2022; 13:914347. doi: 10.3389/fphar.2022.914347 [Crossref] [ Google Scholar]
- Rutkowska A, Stoczyńska-Fidelus E, Janik K, Włodarczyk A, Rieske P. EGFR(vIII): an oncogene with ambiguous role. J Oncol 2019; 2019:1092587. doi: 10.1155/2019/1092587 [Crossref] [ Google Scholar]
- Ouellette MM, Zhou S, Yan Y. Cell signaling pathways that promote radioresistance of cancer cells. Diagnostics (Basel) 2022; 12:656. doi: 10.3390/diagnostics12030656 [Crossref] [ Google Scholar]
- Liu YP, Zheng CC, Huang YN, He ML, Xu WW, Li B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm (2020) 2021; 2:315-40. doi: 10.1002/mco2.55 [Crossref] [ Google Scholar]
- Rajaram P, Chandra P, Ticku S, Pallavi BK, Rudresh KB, Mansabdar P. Epidermal growth factor receptor: role in human cancer. Indian J Dent Res 2017; 28:687-94. doi: 10.4103/ijdr.IJDR_534_16 [Crossref] [ Google Scholar]
- Buckley AM, Lynam-Lennon N, O'Neill H, O'Sullivan J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol Hepatol 2020; 17:298-313. doi: 10.1038/s41575-019-0247-2 [Crossref] [ Google Scholar]
- Cheng Y, Wang L, Zhang S, Jian W, Zeng B, Liang L, et al. The investigation of Nfκb inhibitors to block cell proliferation in OSCC cells lines. Curr Med Chem 2024. doi: 10.2174/0109298673309489240816063313.
- Sisakht M, Darabian M, Mahmoodzadeh A, Bazi A, Shafiee SM, Mokarram P. The role of radiation induced oxidative stress as a regulator of radio-adaptive responses. Int J Radiat Biol 2020; 96:561-76. doi: 10.1080/09553002.2020.1721597 [Crossref] [ Google Scholar]
- Noroozi Karimabad M, Niknia S, Bemani Golnabadi M, Fattah Poor S, Hajizadeh MR, Mahmoodi M. Effect of Citrullus colocynthis extract on glycated hemoglobin formation (in vitro). Eurasian J Med 2020; 52:47-51. doi: 10.5152/eurasianjmed.2020.19223 [Crossref] [ Google Scholar]
- Yousefi H, Vatanmakanian M, Mahdiannasser M, Mashouri L, Alahari NV, Rafiee Monjezi M. Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene 2021; 40:1043-63. doi: 10.1038/s41388-020-01588-2 [Crossref] [ Google Scholar]
- Wang NH, Lei Z, Yang HN, Tang Z, Yang MQ, Wang Y. Radiation-induced PD-L1 expression in tumor and its microenvironment facilitates cancer-immune escape: a narrative review. Ann Transl Med 2022; 10:1406. doi: 10.21037/atm-22-6049 [Crossref] [ Google Scholar]
- Ai L, Xu A, Xu J. Roles of PD-1/PD-L1 pathway: signaling, cancer, and beyond. Adv Exp Med Biol 2020; 1248:33-59. doi: 10.1007/978-981-15-3266-5_3 [Crossref] [ Google Scholar]
- Nie Y, Li D, Peng Y, Wang S, Hu S, Liu M. Metal organic framework coated MnO2 nanosheets delivering doxorubicin and self-activated DNAzyme for chemo-gene combinatorial treatment of cancer. Int J Pharm 2020; 585:119513. doi: 10.1016/j.ijpharm.2020.119513 [Crossref] [ Google Scholar]
- Alexander E, Leong KW. Discovery of nanobodies: a comprehensive review of their applications and potential over the past five years. J Nanobiotechnology 2024; 22:661. doi: 10.1186/s12951-024-02900-y [Crossref] [ Google Scholar]
- Li Z, Fan J, Xiao Y, Wang W, Zhen C, Pan J. Essential role of Dhx16-mediated ribosome assembly in maintenance of hematopoietic stem cells. Leukemia 2024; 38:2699-708. doi: 10.1038/s41375-024-02423-3 [Crossref] [ Google Scholar]
- Wu X, Fu M, Ge C, Zhou H, Huang H, Zhong M. m6A-mediated upregulation of lncRNA CHASERR promotes the progression of glioma by modulating the miR-6893-3p/TRIM14 axis. Mol Neurobiol 2024; 61:5418-40. doi: 10.1007/s12035-023-03911-w [Crossref] [ Google Scholar]
- Lyu Z, Xin M, Oyston DR, Xue T, Kang H, Wang X. Cause and consequence of heterogeneity in human mesenchymal stem cells: challenges in clinical application. Pathol Res Pract 2024; 260:155354. doi: 10.1016/j.prp.2024.155354 [Crossref] [ Google Scholar]
- Singh R, Chandley P, Rohatgi S. Recent advances in the development of monoclonal antibodies and next-generation antibodies. Immunohorizons 2023; 7:886-97. doi: 10.4049/immunohorizons.2300102 [Crossref] [ Google Scholar]
- Bemidinezhad A, Zojaji SA, Taraz Jamshidi S, Mohammadi M, Alavi MS, Ghorbani A. Evaluation of acute, subacute, and subchronic toxicity of a hepatoprotective herbal formulation. Toxicol Rep 2023; 11:452-9. doi: 10.1016/j.toxrep.2023.11.002 [Crossref] [ Google Scholar]
- Bemidinezhad A, Abolhassani Y, Sarabian Tabrizi A, Noroozi Karimabad M, Parsa-Kondelaji M, Roshani R. Aptamers in combination therapies for enhanced radiosensitization in cancer. Iran J Biotechnol 2025; 23:e4032. doi: 10.30498/ijb.2025.491856.4032 [Crossref] [ Google Scholar]
- Annell A, Ardemalm H, Kok M, Nilsson S, Sandberg-Wilén A, Östberg A. Replacing Antibodies in Future Medical Applications: An Overview of Non-Antibody Proteins and Peptide Scaffolds. Uppsala Universitet; 2024.
- Liu SF, Li MJ, Liang B, Sun W, Shao Y, Hu X. Breaking the barrier: nanoparticle-enhanced radiotherapy as the new vanguard in brain tumor treatment. Front Pharmacol 2024; 15:1394816. doi: 10.3389/fphar.2024.1394816 [Crossref] [ Google Scholar]
- Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 2015; 14:642-62. doi: 10.1038/nrd4663 [Crossref] [ Google Scholar]
- Zhou LY, Qin Z, Zhu YH, He ZY, Xu T. Current RNA-based therapeutics in clinical trials. Curr Gene Ther 2019; 19:172-96. doi: 10.2174/1566523219666190719100526 [Crossref] [ Google Scholar]
- Li S, Ling S, Wang D, Wang X, Hao F, Yin L, et al. Modified lentiviral globin gene therapy for pediatric β0/β0 transfusion-dependent β-thalassemia: a single-center, single-arm pilot trial. Cell Stem Cell 2024; 31: 961-73.e8. doi: 10.1016/j.stem.2024.04.021.
- Zeng X, Yuan X, Liao H, Wei Y, Wu Q, Zhu X. The miR-665/SOST axis regulates the phenotypes of bone marrow mesenchymal stem cells and osteoporotic symptoms in female mice. Am J Pathol 2024; 194:2059-75. doi: 10.1016/j.ajpath.2024.07.022 [Crossref] [ Google Scholar]
- Du F, Ye Z, He A, Yuan J, Su M, Jia Q. An engineered α1β1 integrin-mediated FcγRI signaling component to control enhanced CAR macrophage activation and phagocytosis. J Control Release 2025; 377:689-703. doi: 10.1016/j.jconrel.2024.11.064 [Crossref] [ Google Scholar]
- Patel MM, Patel BM. Crossing the blood-brain barrier: recent advances in drug delivery to the brain. CNS Drugs 2017; 31:109-33. doi: 10.1007/s40263-016-0405-9 [Crossref] [ Google Scholar]
- Xu S, Cui F, Huang D, Zhang D, Zhu A, Sun X. PD-L1 monoclonal antibody-conjugated nanoparticles enhance drug delivery level and chemotherapy efficacy in gastric cancer cells. Int J Nanomedicine 2019; 14:17-32. doi: 10.2147/ijn.S175340 [Crossref] [ Google Scholar]
- Sibuyi NR, Moabelo KL, Fadaka AO, Meyer S, Onani MO, Madiehe AM. Multifunctional gold nanoparticles for improved diagnostic and therapeutic applications: a review. Nanoscale Res Lett 2021; 16:174. doi: 10.1186/s11671-021-03632-w [Crossref] [ Google Scholar]
- Luo L, Keyomarsi K. PARP inhibitors as single agents and in combination therapy: the most promising treatment strategies in clinical trials for BRCA-mutant ovarian and triple-negative breast cancers. Expert Opin Investig Drugs 2022; 31:607-31. doi: 10.1080/13543784.2022.2067527 [Crossref] [ Google Scholar]
- Bemidinezhad A, Radmehr S, Moosaei N, Efati Z, Kesharwani P, Sahebkar A. Enhancing radiotherapy for melanoma: the promise of high-Z metal nanoparticles in radiosensitization. Nanomedicine (Lond) 2024; 19:2391-411. doi: 10.1080/17435889.2024.2403325 [Crossref] [ Google Scholar]
- Lim ZF, Ma PC. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol 2019; 12:134. doi: 10.1186/s13045-019-0818-2 [Crossref] [ Google Scholar]
- Zhang C, Ge H, Zhang S, Liu D, Jiang Z, Lan C. Hematoma evacuation via image-guided para-corticospinal tract approach in patients with spontaneous intracerebral hemorrhage. Neurol Ther 2021; 10:1001-13. doi: 10.1007/s40120-021-00279-8 [Crossref] [ Google Scholar]
- Mohammad Sadeghipour M, Torabizadeh SA, Noroozi Karimabad M. The Glucose-Regulated Protein78 (GRP78) in the unfolded protein response (UPR) pathway: a potential therapeutic target for breast cancer. Anticancer Agents Med Chem 2023; 23:505-24. doi: 10.2174/1871520622666220823094350 [Crossref] [ Google Scholar]
- Akpa PA, Peter IE, Onwuka AM, Obi BC, Akunne MO, Nworu CS. Nanotheranostics: platforms, current applications, and mechanisms of targeting in breast and prostate cancers. J Nanotheranostics 2023; 4:346-83. doi: 10.3390/jnt4030016 [Crossref] [ Google Scholar]
- Heidarian F, Alavizadeh SH, Kalantari MR, Hoseini SJ, Kaboli Farshchi H, Jaafari MR. Ellagic acid nanoliposomes potentiate therapeutic effects of PEGylated liposomal doxorubicin in melanoma: An in vitro and in vivo study. J Drug Deliv Sci Technol 2024; 93:105396. doi: 10.1016/j.jddst.2024.105396 [Crossref] [ Google Scholar]
- Wei C, Lan X, Qiu M, Cui R, Fu Q, Shinge SAU. Expanding the role of combined immunochemotherapy and immunoradiotherapy in the management of head and neck cancer (review). Oncol Lett 2023; 26:372. doi: 10.3892/ol.2023.13958 [Crossref] [ Google Scholar]
- Jing R, Jiang Z, Tang X. Advances in millimeter-wave treatment and its biological effects development. Int J Mol Sci 2024; 25:8638. doi: 10.3390/ijms25168638 [Crossref] [ Google Scholar]
- Nam J, Son S, Park KS, Zou W, Shea LD, Moon JJ. Cancer nanomedicine for combination cancer immunotherapy. Nat Rev Mater 2019; 4:398-414. doi: 10.1038/s41578-019-0108-1 [Crossref] [ Google Scholar]
- Bemidinezhad A, Mirzavi F, Gholamhosseinian H, Gheybi F, Soukhtanloo M. Green synthesis of glucose-coated gold nanoparticles for improving radiosensitivity in human U87 glioblastoma cell line. Nanomed J 2022; 9:328-33. doi: 10.22038/nmj.2022.67425.1714 [Crossref] [ Google Scholar]
- Zhou C, Kuang M, Tao Y, Wang J, Luo Y, Fu Y, et al. Nynrin preserves hematopoietic stem cell function by inhibiting the mitochondrial permeability transition pore opening. Cell Stem Cell 2024; 31: 1359-75.e8. doi: 10.1016/j.stem.2024.06.007.
- Kaur R, Bhardwaj A, Gupta S. Cancer treatment therapies: traditional to modern approaches to combat cancers. Mol Biol Rep 2023; 50:9663-76. doi: 10.1007/s11033-023-08809-3 [Crossref] [ Google Scholar]
- Kemp JA, Kwon YJ. Cancer nanotechnology: current status and perspectives. Nano Converg 2021; 8:34. doi: 10.1186/s40580-021-00282-7 [Crossref] [ Google Scholar]
- Kumari S, Mukherjee S, Sinha D, Abdisalaam S, Krishnan S, Asaithamby A. Immunomodulatory effects of radiotherapy. Int J Mol Sci 2020; 21:8151. doi: 10.3390/ijms21218151 [Crossref] [ Google Scholar]
- Khot S, Krishnaveni A, Gharat S, Momin M, Bhavsar C, Omri A. Innovative drug delivery strategies for targeting glioblastoma: overcoming the challenges of the tumor microenvironment. Expert Opin Drug Deliv 2024; 21:1837-57. doi: 10.1080/17425247.2024.2429702 [Crossref] [ Google Scholar]
- Kejamurthy P, Devi KTR. Immune checkpoint inhibitors and cancer immunotherapy by aptamers: an overview. Med Oncol 2023; 41:40. doi: 10.1007/s12032-023-02267-4 [Crossref] [ Google Scholar]