BioImpacts. 9(1):37-43.
doi: 10.15171/bi.2019.05
Original Research
Preparation and characterization of biocomposite films of carrageenan/locust bean gum/montmorrillonite for transdermal delivery of curcumin
Ramanjot Kaur , Ameya Sharma , Vivek Puri , Inderbir Singh *
Author information:
Department of Pharmaceutics, Chitkara College of Pharmacy, Chitkara University, Rajpura-140401, Patiala, Punjab, India
Abstract
Introduction:
Skin can be used as a site for local and systemic drug administration. Diffusion of drugs through the skin has led to the development of different transdermal drug delivery systems. Curcumin is a wound healing and anti-inflammatory agent. Curcumin was incorporated into biocomposite films of carrageenan (κC)/locust bean gum (LBG)/ montmorillonite (MMT) prepared by a solvent casting method.
Methods:
Film-forming solutions were prepared by adding and 2.5% v/v of propylene glycol and MMT (30% w/w). The curcumin loaded polymer composite transdermal films were characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR) spectroscopy and X-ray diffraction (XRD) analysis. Mechanical properties in terms of tensile strength and extensibility were studied. Films were also evaluated for moisture content, moisture uptake, thickness, folding endurance, swelling ratio and water vapor transmission rate (WVTR).
Results:
κC and κC/L40 showed the highest percent cumulative release of 80.42±1.61% and 69.38±1.26% among all of the polymer composite transdermal films in 8 hours and 24 hours respectively.
Conclusion:
In vitro release profiles showed that increasing concentration of LBG and MMT sustained the release of the drug from the polymer composite transdermal films. Decreased percent cumulative release as the concentration of LBG and MMT increases in polymer composite transdermal film.
Keywords: Biocomposite films, Curcumin, Transdermal delivery
Copyright and License Information
© 2019 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Novel bio-based materials have been used for different drug delivery applications. Biopolymers have the advantages of utilizing the renewable sources, biodegradability and potential edibility as compared to synthetic polymers. However, biopolymers have a poor barrier and mechanical properties. Physicochemical properties of biopolymers could be enhanced by preparing biocomposites and reinforcing the polymer matrix with mineral clays Intercalation of clay particles within the biopolymers possibly results in improvement in certain properties like mechanical properties, barrier properties, thermal stability, drug loading efficiency and controlled release behavior.
1
Martins et al prepared biocomposite films of kappa carrageenan and locust bean gum (LBG) with Cloisite 30B gum resulting in an enhanced barrier, mechanical and antibacterial properties for food packaging application. Exfoliation of the clay in the polymer matrix was proposed to be the possible mechanism for enhancement if physicochemical properties of biopolymer composites.
2
Sousa and Goncalves exhibited the enhancement in water resistance and mechanical properties of agar films with the addition of LBG for food packaging application.
3
Slavutsky et al formulated nanocomposite films of brea gum and montmorillonite (MMT). Water and gas permeability of composite film was found to decrease with improvement in Young's module and tensile strength.
4
Similarly, improvement in physicochemical properties of starch/carboxymethyl cellulose/ MMT bionanocomposite films, chitosan/ MMT nanocomposites, cassava starch MMT composite films and gliadin biocomposite filmshave been reported in the literature.
5-8
Carrageenan is a sulfated polysaccharide, composed of galactose and anhydro galactose units and connected by glycosidic bonds. Carrageenan is available as kappa (κ), iota and lambda depending upon the method of extraction and type of algae. All grades of carrageenan produce a thermo-reversible sol-gel phase. Carrageenans are commonly used in the food technology and pharmaceutical industry for their gelling, thickening and stabilizing properties.
9
MMT is aluminosilicate clay. Between two tetrahedral layers of silica, its crystalline structure presents an alumina octahedral. MMT is the most commonly used layered silicate possessing high aspect ratio and surface area. MMT consists of positive counter-ions in the interlamellar space of negatively charged silicate layers.
10
In the present study biocomposite films of κ-carrageenan (κC), LBG and MMT were prepared for transdermal delivery of curcumin. The transdermal films were evaluated for thickness, weight variation, tensile strength, extensibility, surface pH, moisture content, percent moisture absorption, drug content, folding endurance, swelling ratio, water vapor transmission rate, in vitro dissolution and in vitro skin permeation studies. The transdermal films were characterized by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), scanning electron microscopy (SEM) and X-ray diffraction (XRD). A suitable animal model was employed for studying wound healing property of the formulation.
Materials and Methods
Materials
Gift sample of curcumin was kindly supplied by Sanat Products Ltd., New Delhi, India. LBG was kindly gifted by Lucid Colloids Ltd., Mumbai, India. Montmorillonite K 10 (MMT) and kappa carrageenan (κC) were purchased from Sigma Aldrich, USA. Propylene glycol (PG) was purchased from Loba Chemicals Pvt. Ltd. Mumbai, India. All other reagents and chemicals were of analytical grade.
Preparation of transdermal films
κC/LBG films were prepared by suspending κC (1% w/v) and LBG (0.4% w/v) in distilled water under agitation for 1 h at 25°C. The resulting solutions were further homogenized at 80°C±2°C and stirred for 30 minutes with the addition of 2.5% w/v propylene glycol (PG) as a plasticizer. Curcumin 25% w/w (w.r.t κC) was added in each solution of κC/LBG films.
For preparing κC/LBG/MMT composite films, MMT clay powder was dispersed in distilled water and stirred for 24 hours. The remaining components were dissolved in the clay solution after the addition of propylene glycol (2.5% w/v). Curcumin 25% w/w (w.r.t κC) was then added in each solution of κC/LBG/MMT composite films.
Film forming solutions (with and without clays) were then degassed under vacuum for removing the dissolved air. The film solution 10 mL was then poured in polystyrene Petri plates and dried at 35°C for 16 hours. The prepared films were stored in desiccator until further use. The composition of curcumin loaded κC/LBG films and κC/LBG/MMT composite films are given in Table 1.
Table 1.
Composition of curcumin loaded transdermal films
Formulation
Code
|
Curcumin
(mg)
|
κC
(% w/v)
|
LBG
(%w/v)
|
MMT
(mg)
|
PG
(% w/v)
|
| κC |
25 |
1 |
0 |
0 |
2.5 |
| κC/L40 |
25 |
1 |
0.4 |
0 |
2.5 |
| κC/L100 |
25 |
1 |
1.0 |
0 |
2.5 |
| κC/L40/M |
25 |
1 |
0.4 |
40 |
2.5 |
Physicochemical evaluation of transdermal films
Thickness
The digital micrometer (Mitutoyo, Japan) was used to estimate the thickness of the transdermal films from 3 different points. The average and standard deviation (SD) of the 3 readings were recorded.
Drug content
The transdermal films with the specific area were placed in 100 mL solution containing 50 ml phosphate buffer (pH 7.4) and 50 ml ethanol followed by agitation on a mechanical shaker for 24 hours. Solution was then filtered, diluted suitably and analyzed at 422 nm using UV/Visible spectrophotometer (2202, Systronics, India). The experiment was performed in triplicate and the results were reported as mean ± SD.
Weight variation
Composite films of each batch were weighed individually. Each batch result was reported as mean ± SD.
Percent moisture content
The composite films were weighed individually and placed in a desiccator (37°C for 24 hours) containing silica gel as the desiccant. The final weight was measured when there was no further change in the weight of the film. The percent moisture content was calculated by using equation (Eq) 1.
Percentage moisture absorption
The transdermal composite films were accurately weighed and placed (for 72 hours) in a desiccator maintained at 75% RH condition using a saturated solution of sodium chloride. The percent moisture absorption was calculated by Eq. 2:
Folding endurance test
Folding endurance of the films was evaluated by repeatedly folding the film at the same place and the number of times the film could be folded without breaking represents its folding endurance value.
Mechanical properties
The mechanical properties of transdermal films were assessed by texture analyzer (TA XT plus, Stable Microsystems, UK) equipped with 5 kg of a load cell. Filmstrip (10 x 50 mm) was held between two clamps positioned at a distance of 10 mm and pulled apart at a rate of 50 mm/min. The tensile strength and extensibility were measured for 3 replicates of each batch of transdermal film. Tensile strength and extensibility were expressed in N/mm2 and percentage respectively.
Swelling ratio
The completely dried, pre-weighed films were equilibrated in 250 mL of phosphate buffer (pH 7.4) at 25°C. The swelling ratio (Q) of the film is calculated using Eq. 3:
Q=W
s
/W
d Eq. 3
Where, Ws is the weight of the swollen film at different time intervals and Wd is the weight of dry film.
Water vapor transmission rate
The sample film was set on the top of glass polytope (144 mm2) containing 10 ml of phosphate buffer (pH 7.4). This setup was pre-weighed and placed in an oven at 35ºC for 24 hours. WVTR was determined using following equation:
WVTR= W
i
-W
t
/A × 10
6
g/m
2
day-1 Eq. 4
Where, WVTR is expressed in g2h, A=area of the polytop opening (mm2), Wi and Wt = weight of the polytope before and after placing in the oven, respectively.
Characterization of transdermal films
Scanning electron microscopy
A Nova NanoSEM 200 (Netherlands) instrument was used for SEM analysis. A double adhesive tape was used for mounting the composite films on SEM stubs for observing the surface morphologies of the film at an accelerating voltage from 10 to 15 kV.
X-ray diffraction
Samples were set up by slicing the films to fit the square tiles of the holder, was mounted on the sample cell and scanned between 2 θ of 0-60º with a counting time of 0.1 seconds step size. X-ray patterns of the films were obtained with XPERT-PRO equipment (PANalytical, Netherland).
ATR-FTIR
ATR-FTIR spectra of the transdermal films were carried out ATR-FTIR spectrophotometer (Bruker Alpha, Germany) in the transmittance mode with the wave number region 4000 – 500 cm-1.
In vitro drug release
Transdermal films containing 25 mg equivalent of curcumin were applied to a glass slide and secured with a stainless-steel mesh screen. The assembly was placed at the bottom of the paddle time dissolution test apparatus (Lab India DS 8000, India). A 500 mL volume of phosphate buffer (pH 7.4) and ethanol (1:1) was employed as dissolution media under 32˚C and 50 rpm test conditions. Aliquots of 5 ml were withdrawn at specified intervals and were analyzed at 422 nm using UV/Visible spectrophotometer (2202, Systronics, India). In vitro drug release data were fitted to Zero order, First order, Higuchi, Hixson-Crowell and Korsmeyer-Peppas models for understanding the mechanism of drug release from film formulations.
In vitro skin permeation
In vitro drug permeation studies were carried out using Franz diffusion cell apparatus having 20 ml volume capacity of the receptor compartment. Full thickness rat skin from the dorsal surface was used for permeation studies. The animal skin was applied over the receptor compartment. Aliquots of 5 mL were withdrawn at specified intervals and were analyzed at 422 nm using UV/Visible spectrophotometer (2202, Systronics, India).
Wound healing study
The animal study was conducted in accordance with the protocol approved by the animal ethics committee of Chitkara College of Pharmacy, Chitkara University, India (118/PO/ab/08/CPCSEA). Healthy male rats weighing 240-300 g were anesthetized using ketamine (80 mg/kg). The specific skin area was shaved for creating wound of approximately 200 mm2. Twelve animals were partitioned into 3 groups, Group 1: (Control), Group 2: (κC/L40), Group 3: (κC/L40/M). Transdermal film formulation was applied on the wound and wound area was measured by tracing on a millimeter scale graph paper on predetermined days i.e., 0, 2, 4, 6, 8, 10, 12 and 14 days post wound treatment. Percent of wound contraction was figured using Eq. 5:
Results
Physicochemical evaluation of films
Results of different tests for physicochemical characterization of transdermal films are depicted in Table 2. The thickness of transdermal films was found to be ranging from 0.039±0.004 mm to 0.061±0.008 mm. Drug content of different batches of transdermal films was found to be 95.81±0.75% to 98.08±0.88% affirming the reproducibility of the manufacturing process. The weight of the different film batches was observed to be ranging from 0.389±0.01 g to 0.474±0.01 g, which shows the different film weights were generally similar.
Table 2.
Physicochemical evaluation of biocomposite transdermal films
|
Batch
|
Thickness
(mm)
|
Drug
Content(%)
|
Weight
Variation (g)
|
Moisture
Content (%)
|
Moisture
Uptake (%)
|
Folding
endurance
|
| κC |
0.052±0.007 |
97.72±0.45 |
0.389±0.04 |
19.28±0.58 |
11.52±0.86 |
296±8 |
| κC/L40 |
0.039±0.004 |
95.81±0.75 |
0.462±0.07 |
26.77±1.37 |
12.31±0.76 |
306±12 |
| κC/L100 |
0.043±0.006 |
98.08±0.88 |
0.434±0.05 |
24.81±1.02 |
14.28±0.71 |
312±10 |
| κC/L40/M |
0.061±0.008 |
96.61±0.62 |
0.474±0.09 |
13.25±0.81 |
13.73±0.98 |
234±15 |
The moisture content of films is an indicator of water content within the films. The moisture content increases from 19.28±0.58% to 26.77±1.37% as the concentration of LBG is increased. The percent of moisture uptake of the transdermal films should be low so as to protect the formulations from microbial contamination and also reduce bulkiness. The percentage moisture uptake values ranged from 11.52±0.86% to 14.28 ± 0.71%.
The folding endurance measures the ability of patch to withstand rupture. The folding endurance for the composite films varied in the values ranging between 234±15 to 312±10 folds. The formulations consisting of a higher concentration of LBG showed higher values as compared to the other transdermal films.
Mechanical properties
Mechanical properties are the indication of strength and resistance of films to external stress. Mechanical properties of the transdermal films were assessed in terms of the tensile strength and percent elongation to break (extensibility). Pure κC films showed the tensile strength of 1.39±0.21 N/mm2 and extensibility of 14.4±0.95%. The tensile strength and extensibility of κC/L40 and κC/L100 were found to be 11.30±0.92 N/mm2& 17.14±1.54% and 11.98±0.32 N/mm2 & 25.42±1.26% respectively (Table 3). The increase in mechanical properties was attributed to the interaction between the H bond forming groups of κC and unbranched segments of D-mannose backbone of LBG. The tensile strength and extensibility of transdermal composite film (κC/L40/M) were found to be 14.22±1.01 N/mm2 and 30.12±1.16% respectively. Incorporation of MMT in κC/LBG further increased the mechanical properties. This enhancement in mechanical properties could be attributed to interfacial affinity, interaction and formation of physically crosslinked networks between the MMT clay particles are the κC/LBG polymeric matrix. Similar results were reported by Martin et al
2
and Mirzataheri et al.
11
Table 3.
Mechanical properties and swelling ratio of biocomposite transdermal films
|
Batch
|
Tensile strength (N/mm
2
)
|
Extensibility (%)
|
Swelling ratio (%)
|
WVTR (g/m
2
per day)
|
| κC |
1.39±0.21 |
14.4±0.95 |
2.85±0.28 |
614.86±3.42 |
| κC/L40 |
11.30±0.92 |
17.14±1.54 |
4.71±0.54 |
8342.10±10.68 |
| κC/L100 |
10.98±0.32 |
25.42±1.26 |
2.50±0.31 |
734.86±2.48 |
| κC/L40/M |
14.22±1.01 |
30.12±1.16 |
1.90±0.44 |
602.99±2.10 |
Swelling ratio
The swelling ratio of κC and κC/L40 was found to be 2.85±0.28 and 4.51±0.54% respectively. Increase in swelling ratio with the addition of LBG could be due to an increase in the hydrophilic groups in the composite films. Moreover, the physical entanglement of LBG and κC polymeric chains leads to the formation of a hydrogel network. Furthermore, the addition of MMT leads to a decrease in the swelling ratio. This could be due to the physical entanglement of clay particles in the polymer matrix leading to closures of pores/channels, thereby, reducing the swelling ratio.
Water vapor transmission rate
The WVTR was found to be increase with an increase (614.86±3.42 to 8342.10±10.68 g/m2 per day) in the concentration of LBG in the films. This may be due to an increase in the hydrophilic groups (with an increase in LBG content) providing more affinity for the transmission of water vapors. Further increase in the concentration of LBG led to decrease in the WVTR, which could be due to an excessive gelling property imparted by the LBG which led to narrow or closure of pores/channels responsible for the transmission of water vapors. Furthermore, the addition of MMT particles led to the closure of pores/ channels which led to a drastic reduction in WVTR of transdermal composite films.
Characterization of curcumin loaded transdermal films
Scanning electron microscopy
The SEM images of different batches of transdermal films are shown in Fig. 1. Presence of rough surface topology and small rod-like shapes confirm uniform distribution of curcumin in the polymer matrix of transdermal films. The SEM images further indicate the presence of κC/LBG rendered intercalated lamellar structures due to entrapment of MMT particles within the polymer matrix. Martin et al suggested that CloisiteTM 30B forms ex-foliated structure within the polymer matrix of κC/LBG, which is responsible for the enhancement of mechanical property water barrier property and antibacterial activity of κC/LBG composite films.
2
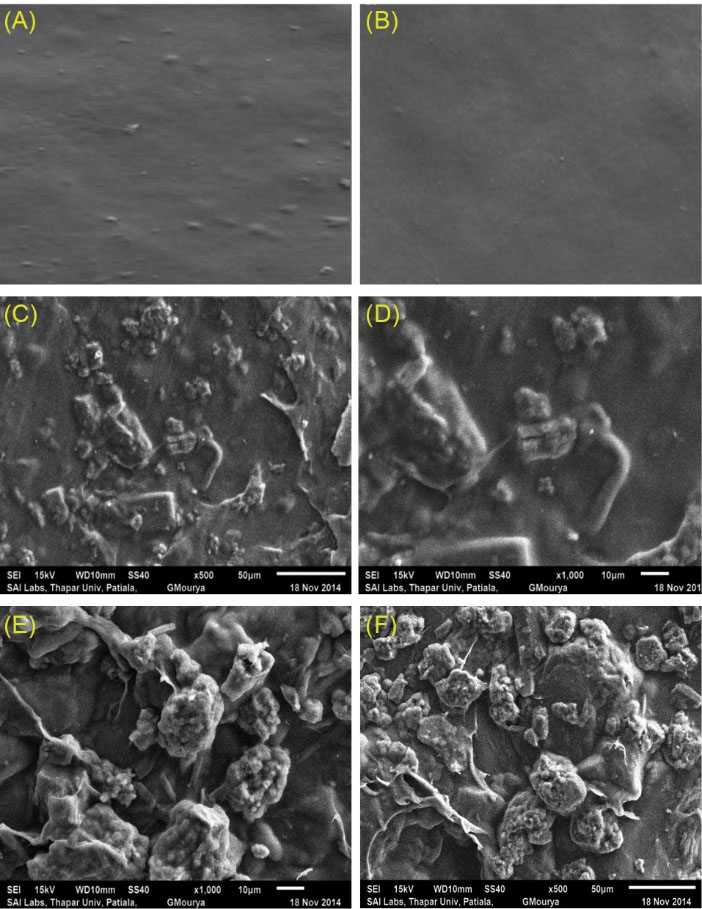
Fig. 1.
SEM images of transdermal films (A) κC; (B) κCL/40; (C), (D) Curcumin loaded κC/L40 composite films; (E), (F) Curcumin loaded κC/LBG/MMT.
.
SEM images of transdermal films (A) κC; (B) κCL/40; (C), (D) Curcumin loaded κC/L40 composite films; (E), (F) Curcumin loaded κC/LBG/MMT.
X-ray diffraction
As per the XRD pattern (Fig. 2) results, transdermal composite films of curcumin prepared using κC/L40 were found to have peaks corresponding to 2θ of 9.11(d spacing 9.7Å) and 2θ of 17.59 (d spacing 5.04 Å). Whereas κC/L40/M films exhibited diffraction peaks corresponding to 2θ of 5.12 (d spacing 17.26 Å) and 2θ of 9.39(d spacing 9.4 Å). The results suggested intercalation and possible bonding between the clay particles and the polymeric chains of κC/LBG. The results are in agreement with those reported by Aouada et al
12
and Lim et al.
13
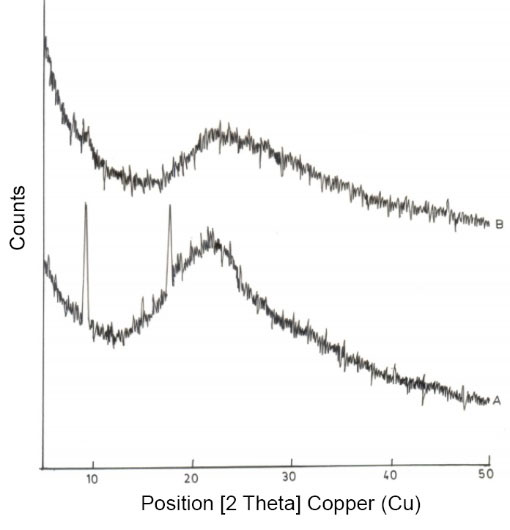
Fig. 2.
X-ray Diffraction pattern of (A) κC/L40; (B) κC/L40/M batches of transdermal films.
.
X-ray Diffraction pattern of (A) κC/L40; (B) κC/L40/M batches of transdermal films.
ATR-FTIR
FTIR analysis was used to evaluate the chemical interactions in the transdermal composite films of κC/LBG/MMT (Fig. 3). Pure κC showed a characteristic peak at about 3406 cm-1 of strong O-H stretching representing the involvement of hydroxyl groups in hydrogen bonding. Strong bands at 1216 cm-1 representing sulfates stretching and at 1121 cm-1 and 927 cm-1 for C-O-C glycosidic linkages for pure κC were also observed. Pure LBG showed characteristic peaks at about 3300 cm-1 (O-H stretching), 2900 cm-1 (C-H stretching), 1370 cm-1 (C-H bending) and 1123 cm-1 (O-H bending). The broad peak at 1000-1100 cm-1attributed to C-O-H stretching/bending vibrations. Pure MMT shows a peak at 3449 cm-1 representing interlayer and intralayer H-bonding. However, peaks at 3619 cm-1 and 1633 cm-1 indicates O-H stretching and O-H bending respectively. The bands around 520 – 560 cm-1 are due to Si-O bending vibrations while 1134 cm-1 band represents Si-O stretching vibrations. In κC/LBG, κC may interact with D-mannose backbone of LBG through hydrogen bonding. The observed shift and marked an increase in the intensity of the peak corresponding to C-O stretching band of C-O-H group band from 1129 cm-1(κC) to 1156 cm-1(κC/LBG) could be due to the hydrogen bridging interaction between the OH group of LBG with the κC structure. In κC/LBG/MMT films, the addition of MMT clay to κC/LBG polymer matrix, leads to the formation of H-bonding between hydroxylated edge groups of MMT and OH groups of κC and LBG. Results are in agreement with those reported by Martin et al.
2
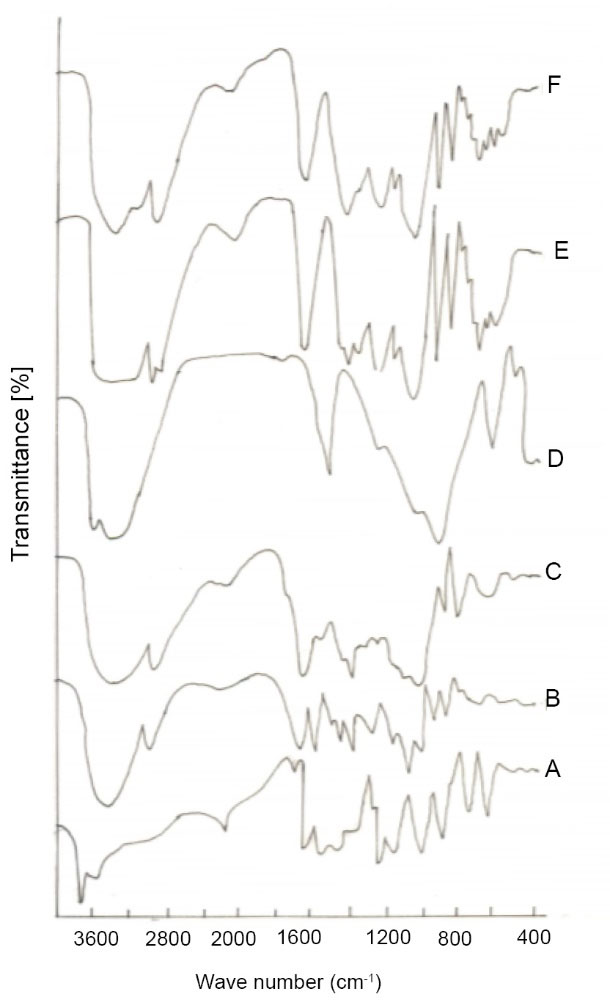
Fig. 3.
ATR-FTIR of various biocomposite transdermal films (A) Curcumin; (B) Pure κC; (C) Pure LBG; (D) Pure MMT; (E) κC/L40; (F) κC/L40/M.
.
ATR-FTIR of various biocomposite transdermal films (A) Curcumin; (B) Pure κC; (C) Pure LBG; (D) Pure MMT; (E) κC/L40; (F) κC/L40/M.
In vitro release study
The results of in vitro drug release study revealed that the cumulative percent drug release decreases with an increase in the concentration of LBG in the transdermal film formulation. The drug release from κC, κC/L40 and κC/L100 was found to be 80.42±4.12%, 69.38±3.28% and 63.94±3.1% respectively after 500 minutes as shown in Fig. 4. Formation of the polymer matrix by LBG is the possible cause of retardation of drug release from the films. The drug release from κC/L40/M was found to be 44.94±1.26% and 56.39±1.74% after 300 to 500 minutes respectively as shown in Fig. 4. The addition of MMT further retards drug release from transdermal films. This may be due to the interpenetration of the clay particles in the polymeric chains of κC and LBG. The interference of the clay particles with polymeric chain relaxation (up to hydration) could also be a cause of the reduction in drug release by MMT. The drug release data obtained after in vitro release study was fitted into various kinetic models. i.e. zero order, first order, Higuchi, Hixon Crowell and Korsmeyer Peppas model. The value of n was found to be ranging from 0.54 to 0.75 for the selected formulations as shown in Table 4, indicating anomalous non-Fickian drug release behavior from the formulation. Further, diffusion and erosion controlled mechanism could be responsible for the release of drug from the transdermal composite films.
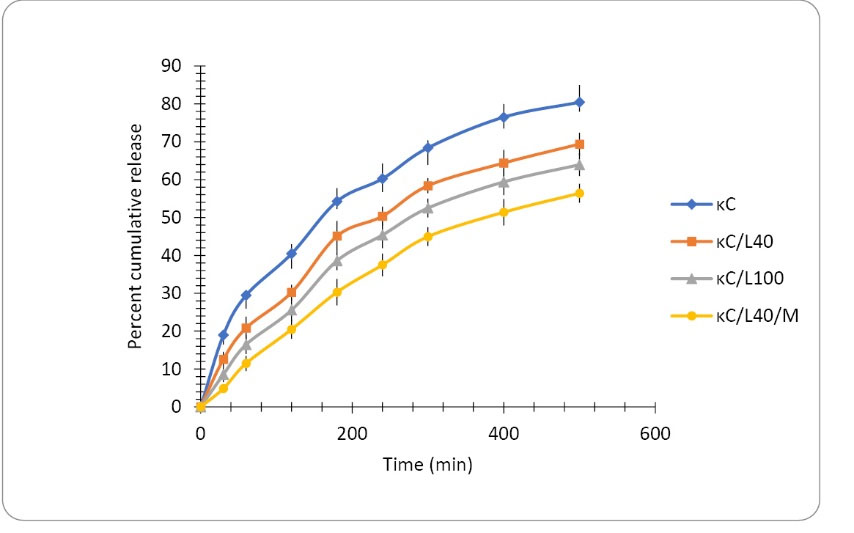
Fig. 4.
In vitro release of curcumin from transdermal biocomposite films of κC, κC/L40, κC/L100, κC/L40/M.
.
In vitro release of curcumin from transdermal biocomposite films of κC, κC/L40, κC/L100, κC/L40/M.
Table 4.
Release Kinetic Study of different formulations
| Batch |
Zero Order |
First Order |
Higuchi |
Hixon-Crowell |
Korsmeyer-Pepas |
|
r2
|
k0
|
r2
|
k1
|
r2
|
kH
|
r2
|
kHC
|
r2
|
k |
n |
| κC |
0.984 |
0.21 |
0.992 |
-0.001 |
0.863 |
4.32 |
0.972 |
-0.004 |
0.889 |
2.57 |
0.75 |
| κC /L40 |
0.971 |
0.191 |
0.981 |
-0.001 |
0.902 |
4.02 |
0.951 |
-0.003 |
0.897 |
2.46 |
0.54 |
| κC /L100 |
0.960 |
0.173 |
0.995 |
-0.001 |
0.943 |
3.75 |
0.983 |
-0.003 |
0.920 |
2.40 |
0.58 |
| κC /L40/M |
0.983 |
0.163 |
0.993 |
-0.001 |
0.915 |
3.63 |
0.964 |
-0.003 |
0.881 |
2.37 |
0.65 |
In vitro skin permeation
The results of in vitro permeation from animal skin were from diffusion cells are in line with the in vitro drug release results of the films (Fig. 5). κC and LBG produced a polymer matrix which has less affinity for water; this results in a decrease in the thermodynamic activity of the drug in the membrane thereby leading to a decrease in the drug release from the film. The addition of MMT further retarded the drug release from the formulation.
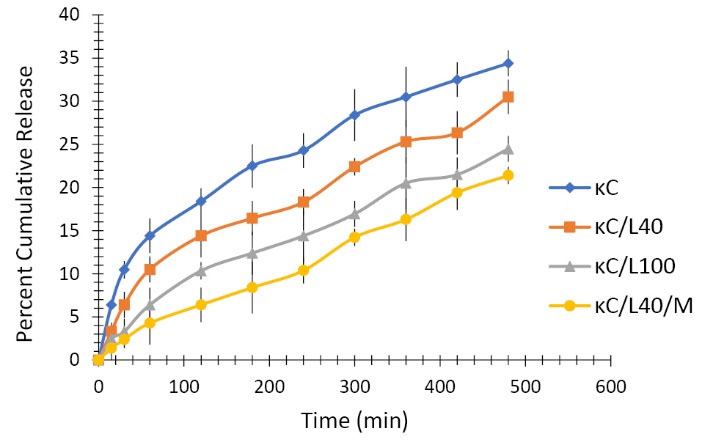
Fig. 5.
In vitro permeation of curcumin from transdermal biocomposite films of κC, κC/L40, κC/L100, κC/L40/M.
.
In vitro permeation of curcumin from transdermal biocomposite films of κC, κC/L40, κC/L100, κC/L40/M.
Wound healing
Percent degree of wound contraction is an indication of wound healing property. As compared to control κC film, κC/L40 and κC/L40/M exhibited faster wound healing property (Table 5). The accelerated wound contraction from transdermal composite films could be attributed to the controlled release of curcumin from the formulations.
Table 5.
Degree of contraction data of various transdermal films
|
Transdermal film
|
Degree of contraction (%)
|
|
Day 2
|
Day 4
|
Day 6
|
Day 8
|
Day 10
|
Day 12
|
Day 14
|
| Control |
9.21±4.09 |
21.39±3.56 |
46.82±2.15 |
62.54±1.43 |
80.54±0.83 |
90.83±0.84 |
96.92±0.13 |
| κC/L40 |
22.36±5.32 |
32.64±3.87 |
53.36±3.34 |
75.24±0.82 |
89.66±0.67 |
96.56±0.14 |
100 |
| κC/L40/M |
34.65±4.65 |
42.53±2.65 |
63.23±2.17 |
86.67±1.12 |
98.57±0.14 |
100 |
100 |
Discussion
Composite materials are a combination of two or more materials, exhibiting enhanced physicochemical properties compared to the individual materials. In the present research, novel composite combination of biopolymers carrageenan and LBG were used along with montmorillonite for developing transdermal films loaded with curcumin. Physicochemical evaluation of biocomposite transdermal films included thickness, drug content, weight variation, moisture content, moisture absorption, swelling, WVTR and mechanical properties. Addition of montmorrillonite clay leads to significant enhancement in tensile strength and extensibility (mechanical properties) of the biocomposite films. The transdermal films were further characterized by SEM, XRD and ATR-FTIR analysis studies. SEM images confirm uniform distribution of curcumin and presence of intercalated lamellar structures due to entrapment of montmorillonite particles in the biopolymer composite matrix of transdermal films. The FTIR and XRD analysis further consolidated the concept of interaction of various groups and intercalation of clay particles in the biopolymer matrix. In vitro drug release and skin permeation studies revealed the mechanism of drug release from the biopolymer composite matrix of transdermal films. Wound healing study of transdermal films exhibited the therapeutic potential of the formulation.
Conclusion
Biocomposite films of Carrageenan/ LBG /Montmorillonite were prepared for transdermal delivery of curcumin. The films were evaluated for different physicochemical parameters. The addition of montmorillonite clay resulted in significant enhancement in mechanical properties of the biocomposite films. WVTR of the films was found to decrease with the addition of LBG and Montmorillonite to Carrageenan in formulating biocomposite films. SEM, XRD and FTIR characterization of the films was also performed. In vitro drug release from the films was retarded with the addition of LBG and montmorillonite to Carrageenan. Wound healing application of the biocomposite films was evaluated employing a suitable animal model.
Acknowledgments
The authors gratefully acknowledge Dr. Madhu Chitkara, Vice-Chancellor, Chitkara University, Rajpura, Punjab, India, and Dr. Sandeep Arora, Dean, Chitkara College of Pharmacy, Chitkara University, Rajpura, Punjab, India for the support and institutional facilities.
Funding sources
None to be declared.
Ethical statement
No ethical issues to be declared.
Competing interests
The authors declare no conflict of interests.
Authors contribution
RJK and IBS: Conceptualization, data handling, experiments design, data analysis, study validation, data presentation and project administration. AS and VP: Draft preparation, draft writing, draft reviewing.
Research Highlights
What is the current knowledge?
simple
-
√ In the present work, biocomposite films of carrageenan/
LBG /montmorillonite for the transdermal delivery of
curcumin were prepared by a casting method and were
characterized and evaluated for various parameters.
-
√ The films were found to possess good mechanical properties
and were effective in wound care management.
What is new here?
simple
-
√ Novel combination of biopolymers (carrageenan and LBG)
along with clay material (montmorillonite) was used for
developing transdermal films of curcumin.
-
√ Biocomposite films were effective in terms of
physicochemical properties and therapeutic application.
References
- Khurana IS, Kaur S,
Kaur
H, Khurana RK. Multifaceted role of clay minerals in pharmaceuticals. Future Sci OA 2015; 1:FSO6. doi: 10.4155/fso.15.6 [Crossref] [ Google Scholar]
- Martins JT, Souza WS, Vicente AA. Biocomposite films based on κ-carrageenan/locust bean gum blends and clays: Physical and antimicrobial properties. Food Bioprocess Tech 2013; 6:2081-92. doi: 10.1007/s11947-012-0851-4 [Crossref] [ Google Scholar]
- Sousa AM, Goncalves MP. Strategies to improve the mechanical strength and water resistance of agar films for food packaging applications. Carbohydr Polym 2015; 132:196-204. doi: 10.1016/j.carbpol.2015.06.022 [Crossref] [ Google Scholar]
- Slavutsky AM, Bertuzzi MA, Armada M, Ochoa NA. Preparation and characterization of montmorillonite/brea gum nanocomposites films. Food Hydrocoll 2014; 35:270-78. doi: 10.1016/j.foodhyd.2013.06.008 [Crossref] [ Google Scholar]
- Ghanbarzadeh B, Almasi H, Oleyaei SA.
A novel modified starch/
carboxy methyl cellulose/montmorillonite bionanocomposite film:
Structural and physical properties
. J Food Eng 2014; 10:121-30. doi: 10.1515/ijfe-2012-0197 [Crossref] [ Google Scholar]
- Wang SF, Shen L, Tong YJ, Liu T.
Biopolymer chitosan/
montmorillonite nanocomposites: Preparation and
characterization
. Polym Degrad Stab 2005; 90:123-31. doi: 10.1016/j.polymdegradstab.2005.03.001 [Crossref] [ Google Scholar]
- Kampeerapappun P, Aht-ong D, Pentrakoon D, Srikulkit K. Preparation of cassava starch/montmorillonite composite film. Carbohydr Polym 2007; 67:155-63. doi: 10.1016/j.carbpol.2006.05.012 [Crossref] [ Google Scholar]
- Huang D, Ma Z, Zhang Z, Quan Q. Reducing water sensitivity of chitosan biocomposite films using gliadin particles made by in situ method. Polymers 2017; 9:583. doi: 10.3390/polym9110583 [Crossref] [ Google Scholar]
- Necas J, Bartosikova L. Carrageenan: A review. Veterinarni Medicina 2013; 58:187-205. doi: 10.17221/6758-VETMED [Crossref] [ Google Scholar]
- Hartwell JM. The diverse uses of Montmorillonite. Clay Miner 1965; 6:111-118. [ Google Scholar]
- Mirzataheri M, Atai M, Mahdavian AR. Physical and mechanical properties of nanocomposite barrier film containing encapsulated nanoclay. J Appl Polym Sci 2010; 118:3284-91. doi: 10.1002/app.32711 [Crossref] [ Google Scholar]
- Aouada FA, Mattoso LHC, Longo E. New strategies in the preparation of exfoliated thermoplastic starch–montmorillonite nanocomposites. Ind Crop Prod 2011; 34:1502-08. doi: 10.1016/j.indcrop.2011.05.003 [Crossref] [ Google Scholar]
- Lim GO, Jang SA, Song KB. Physical and antimicrobial properties of gelidium corneum/nano-clay composite film containing grapefruit seed extract or thymol. J Food Engg 2010; 98:415-20. doi: 10.1016/j.jfoodeng.2010.01.021 [Crossref] [ Google Scholar]