Bioimpacts. 15:30804.
doi: 10.34172/bi.30804
Review
Recent innovations in nanomedicine and nano-based techniques for the treatment of breast cancer
Meena Bhandari Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing, 1 
Seema Raj Conceptualization, Data curation, Methodology, Visualization, Writing – original draft, 1 
Md Sabir Alam Investigation, Resources, Supervision, Validation, Visualization, Writing – review & editing, 2, * 
Author information:
1Department of Chemistry, School of Basic and Applied Sciences, K.R. Mangalam University, Sohna, Gurugram, India
2SGT College of Pharmacy, SGT University, Gurgaon-Badli Road Chandu, Budhera, Gurugram, Haryana-122505, India
Abstract
Breast cancer (BC) is a persistent global health challenge, necessitating innovative therapeutic strategies. Recently, nanotechnology has appeared as a transformative methodology to treat BC, suggesting precise targeting, controlled drug delivery, and improved imaging capabilities. This review offers a current overview of the latest innovations around nanotechnology for BC therapy in the field of new nanomedicines and nano-based drug delivery methods by carefully examining the utilization of nanoparticles to enhance the effectiveness of both new and old medications and to enable targeted evaluation using disease markers. Key topics include early detection, targeted drug delivery, multimodal imaging, and combination therapies. The paper underscores the probability of using nanotechnology to reshape BC management landscape and outlines potential future directions.
Keywords: Breast cancer, Drug delivery, Nanotechnology, Immunotherapy, Nanoparticles
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
The authors confirm that no funding, grants, or financial support were received for the preparation of this manuscript.
Introduction
Breast cancer (BC) is a complicated disorder having diverse subtypes exhibiting variable responses to the treatment. It stands second after lung cancer. In 2020, over 2 million new cases including around sixty-five fatalities have been mentioned in literature and it is projected that there will be over 3 million new cases of breast carcinoma and one million fatalities in 2040.1 BC is a proliferative form of cancer that initiates from breast tissues which is strongly influenced by both hereditary and environmental variables.2 Amounts of oestrogen and androgen are accountable for the development of BC.3 Additionally, it is well known that genetic variations in the genes for BRCA1, BRCA2, TP53, RB, PIK3, MDM2, HER2, and TPK53, CDH1, PTEN, STK11, ATM, BRIP1, PALB2, CHEK2, and NBS1 are strongly linked to BC.2,4,5 It is susceptible to numerous other factors also, including a family history of the disease, obesity, a high-calorie diet, not breastfeeding, using oral contraceptives, and ingesting hormones like oestrogen in hormone replacement therapy.6 Depending on specific molecular markers such as the proliferation marker Ki67, human epidermal growth factor receptor 2 (HER2), the oestrogen receptor (ER), and progesterone receptor (PR), BC is categorised as luminal A-like, luminal B-like, HER2 enriched, and triple-negative BC (TNBC).2,7 Luminal A-like subtype shows improved diagnosis than other subtypes.8 Luminal B-like (HER2-) tumours are ER and/or PR positive, HER2 negative, and high Ki67 index or low expression of PR. Luminal B-like (HER2+) tumours are ER-positive while HER2 are positive including any PR level and any Ki67 level. HER2-positive, ER and PR-negative tumours are referred to as HER2-enriched tumours.9 ER, PR, and HER2 are all negative in the basal-like triple-negative subtype of BC.10 The diagnosis of TNBC patients is frequently poorer than people affected with other kinds of BC due to the absence of established therapeutic molecular targets. In addition, younger women are frequently affected by the TNBC.11 Cancer treatment involves the use of radiation, chemotherapy, hormonal therapy, and removal of cancer through surgery. These treatments are not specific as they may kill normal cells or incomplete removal of tumour occurs which causes toxicity. Traditional therapies often lack specificity, leading to systemic toxicity and reduced efficacy. Now, anthracyclines (doxorubicin, epirubicin), taxanes (paclitaxel [PTX], docetaxel), platinum drugs (cisplatin, carboplatin), cyclophosphamide are frequently employed in BC chemotherapeutic treatment.12 Hormonal treatment aims to inhibit the action of oestrogen or reduce oestrogen levels, which may promote the development of BC cells. The primary medications used in endocrine therapy are aromatase inhibition drugs (Ais: letrozole, anastrazole, and exemestane), in addition to selective oestrogen receptor modulators (SERMs: tamoxifen and toremifene) and selective oestrogen receptor degraders (SERDs: Fulvestrant).13 Currently, tyrosine kinase inhibitors (neratinib, lapatinib, etc.), anti-HER2 monoclonal antibodies (trastuzumab & pertuzumab), and antibody-drug conjugates (trastuzumab-emtansine, or T-DM1) are the primary medications used in HER2 selective treatment.14
Furthermore, immunotherapeutic drugs may also be employed to not solely cure the originally diagnosed tumour but also lower the risk of relapse and stop metastatic progression.15 To treat localised or advanced TNBC patients whose tumours express PD-L1, atezolizumab, a PD-L1 inhibitor, is administered in conjunction with nab-PTX.16 Chemotherapeutic drugs don't seem to be tumour specific. Other side effects include alopecia, leucopenia, anaemia, easy bruising or bleeding, motion sickness, vomiting, diarrhoea, ulcers of the mouth, exhaustion, heightened sensitivity to contaminations, and myelosuppression.17 The second challenge facing traditional chemotherapeutic treatments is drug resistance, which diminishes the efficacy of drugs. Thirdly, the curative impact of chemo is further gets decreased by the substantial noxiousness and inadequate solubility of the chemotherapeutic medications. Cardiotoxicity has been linked to the anthracycline family, notably doxorubicin (DOX) formulations. Additionally, taxanes (PTX and DOX) can cause several adverse responses, such as cutaneous reactions, bone marrow restraint, hypersensitivity responses, and dose-limiting neurotoxicity.18 The therapeutic efficacy of chemotherapeutic medications is further compromised by short half-lives and low chemical stability, which might hamper the dose-effect and interfere with the transport and absorption speed in the tumour location.19 Moreover, maximum chemotherapeutic medications are unable to penetrate the blood brain barrier (BBB), hence restricting the beneficial impact of brain metastases from BC.20 Regretfully, metastases account for most of the recurrent disease in early-stage BC cases (30%).21
Cancer nanomedicine synthesis involves multidisciplinary approach focussing on the molecular design, and medical applicability of nanomaterials and nanotechnology to boost the precision of therapy and enhance patient outcomes.22 Many toxoids, or anticancer medications, such as PTX and docetaxel, are not absorbed in the gastrointestinal tract (GIT). When treating advanced BC, PTX is recommended as both a palliative treatment and first-line therapy.23 It has been established that PTX-loaded NPs offer a great deal of promise for chemotherapeutic oral delivery agent, and they can have a much faster drug release than traditional methods.24 Numerous possible benefits of nanoparticulate-based delivery methods incorporate amended biocompatibility, multifunctional encapsulation of active constituents, reduced blood flow, passive or active directing, effectual administration, and moderated adverse impact.25 NPs offer several advantages: i) similar size as that of biomolecules. ii) derivatization from biomolecules which can enter tissues and can be used for therapeutic treatment of cancerous cells. iii) Incorporating additional concoction to nanostructures or altering their surfaces can often alleviate dissolution and stability issues. (iv) Nanostructures have distinctive physical features, like optical qualities via quantum dots, that are successfully applied to bioimaging. (v) Due to their tiny dimensions, NPs can carry or encapsulate a larger pharmacological payload (such as radioactive isotopes or chemotherapy drugs) because they typically have a high surface area. Once the high-dose therapeutic load is delivered and recognised by a receptor, it can trigger even further destruction of tumour cells at the pointed location. (vi) By using passive or active targeting, nanoparticle formulations can repeatedly deliver drugs at tumour spots, significantly reducing indiscriminate cytotoxicity.26 Tumour microenvironment (TME) components include extracellular matrix, stromal cells, neuroendocrine cells, blood vessels, signalling molecules, and immune and inflammatory cells.27,28 New antigens are produced during each malignancy because of changes in cancer cells, mostly brought by genetic anomalies. Apoptosis or tumour necrosis that releases these antigens causes antigen-presenting cells (APCs) to become activated. Following their migration to the lymph nodes, activated dendritic cells (DCs) release antigens through the tumour-derived peptide MHC I complex, which helps the T cell receptors to recognize. This process stimulates and matures B cells and cytotoxic T lymphocytes (CTL). After that, CTLs relocate to the TME, where they kill cancerous tissues and produce more cancer antigens to strengthen the body's defences against cancer.27,28
Immunotherapy
Immunotherapy, a promising form of therapy for cancer, uses a variety of techniques including checkpoint inhibitors, cytokines that stimulate lymphocytes, T cells that have been genetically altered, and cancer vaccines. However, since these treatments can induce serious side effects like autoimmunity and nonspecific inflammation, precise regulation of the absolved process is an essential hurdle for the broad use of cancer immunotherapy. Gaining insight into how it works between the tumour and the host immune system is essential to increase effectiveness and reduce side effects. Tumour and immune cells are being assessed by using new technologies for molecular and functional analysis of individual cells, that checks molecular markers and functional immune responses to treatment. To attain this objective, nano-enabled tools and materials are carefully used to effectively sort, image, and thoroughly characterise immune cells.
The cGAS-STING signalling pathway has become a vital modulator of the natural immune system response by enhancing anti-tumour immunity through immunological effects or reactions. Administration of STING agonist encapsulated nanoparticles (STING-NPs) offers an alluring approach to antitumor immunotherapy.29 Nanomaterials having distinct surface modifications help to specifically distribute and encapsulate STING agonists to specific sites, like tumor microenvironments TMEs and Lymph nodes (LNs). As compared to small molecules, NPs that transfer STING agonists provide increased intracellular delivery and better biocompatibility and lead to increased STING-mediated immune reaction (Fig. 1).
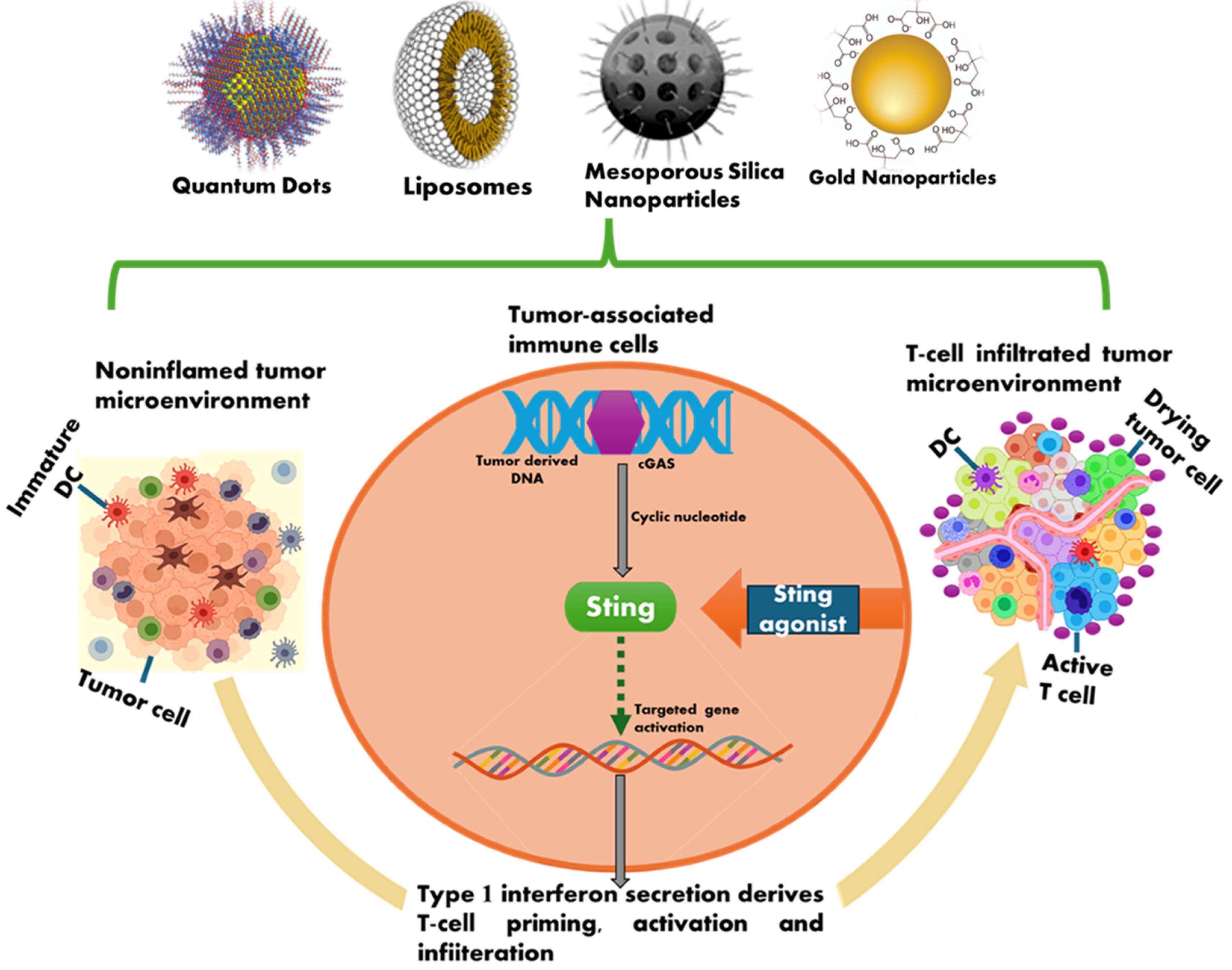
Fig. 1.
Overview of STING agonists and benefits of administering STING-NP.
.
Overview of STING agonists and benefits of administering STING-NP.
Poly (ethylene glycol)-block- [(2-diethylaminoethyl methacrylate)-co-(butyl methacrylate)-co-(pyridyl disulfide ethyl methacrylate)]) (PEG-DBP) is an amphiphilic diblock copolymer that has been engineered to create a polymersome to load STING agonists.30-33 The breakdown of the PEG-DBP vesicle envelope due to change in pH further facilitated endosomal escape and STING agonist cytosolic distribution. Tests conducted both in vitro and in vivo demonstrated that cGAMP-loaded PEG-DBP may produce widespread dispersion in tumor-draining lymph nodes (TDLNs) and extremely effective absorption by APCs and NK cells. On the other hand, unencapsulated cGAMP hardly caused a reaction above baseline, cGAMP-loaded PEG-DBP increased inflammatory STING-driven expression.34
For the simultaneous administration of DMXAA and the prodrug of the chemotherapeutic agent SN-38, Liang et al created a polymeric nanoparticle (NP) using triblock copolymers (poly (ethylene glycol)-block-poly-(DTMASN38)-block-poly[2-(diethylamino)-ethyl methacrylate]) (PEG-PSN38-PDEA).35 During self-assemblage, the structural block of PEG-PSN38-PDEA produced a hydrophobic interior core, however it also contained a cleavable prodrug (PSN38) to initiate redox stimuli in malignancies. This nano-encapsulation triggered immune-stimulation of the type I IFN pathway in murine melanoma, which further enhanced the cellular concentration of DMXAA in APCs. Synergistic antitumor responses may result from the innovative design concept of these block copolymers for a co-delivery nanoplatform.35 STING Agonist dimeric amidobenzimidazole (diABZI)-encapsulated liposomes (dLNPs), having an average particle size of 99.76 ± 0.230 nm and an encapsulation efficiency of 58.29 ± 0.53%, were synthesized. Mice that received dLNPs showed increased IFN-β and IFN-γ expression. The dLNPs were found to possess anticancer properties and are capable of drawing CD8 + T lymphocytes to tumor tissue. The dLNPs-treated mice exhibited the greatest efficacy, with an average tumor volume of 231.46 mm3, which dropped by 78.16% and 54.47%, respectively, as compared to the phosphate buffer saline group and diABZI group.36
STING agonists offer unique possibilities for anticancer immunotherapy in different phases of medical trials. There have been about 20 trials conducted so far to evaluate the clinical use of STING agonists, including phase I and II trials.37 For instance, a phase I research that demonstrated systemic immune activation (NCT02675439) assessed the safety, pharmacokinetics, and effectiveness of synthesized CDN (MIW815) in patients suffering from advanced or metastatic malignancies.38 The safety, pharmacological characteristics, and dose escalation of exoSTING (CDK-002) in patients with advanced metastatic, recurring, solid tumours are being examined in phase 1/2 open-label research (NCT04592484). Additionally, a STING agonist-delivering microparticle is being studied in patients with multiple sclerosis and autoimmune encephalomyelitis (NCT05705986).
Breast tumour subtypes affect the immune reaction and the spread of immune cells in the tissues of the tumour, both quantitatively and qualitatively. Patients with TNBC have the highest levels of T cell penetration and PD-L1 expression, whereas affected patients show positive tests for hormone receptors and have the lowest levels of infiltration. Regardless of the size or subtype of the tumour, higher grades of malignancy can show the intrusion of regulatory T (Treg) cells, and advanced breast tumours are linked to increased CTLA-4 expression.39 For a subset of patients with advanced TNBC, immunotherapy is currently approved; however, there are still significant questions about the safety and effectiveness of current treatment approaches. The main peptide found in European bee venom, melittin, exhibits immunoregulatory and antitumor properties.40 Recently, guanosine monophosphate-adenosine monophosphate (cGAMP) was loaded into a polymerosome and was delivered to mice in vivo, significantly inhibiting the growth of melanoma, upgraded the tumour’s immunological response.41
Nguyen and colleagues created a dual-scale cancer vaccine.42 It consists of mesoporous silica microrods which have been loaded with granulocyte-macrophage colony-stimulating factor (GM-CSF), a chemokine that recruits cells, ovalbumin- and CpG ODN-loaded mesoporous silica NPs. Research indicates that this hybrid vaccine exhibited a significant inhibitory effect on melanoma growth. Additionally, a synergistic effect against tumour development was detected when the vaccine was combined with an immune checkpoint inhibitor.42 Furthermore, the BBB and other biological barriers can be breached by NPs due to their compact size, opening new therapy options for BC patients with brain metastases.43 Table 1 outlines the treatment options for cancer.
Table 1.
Therapeutic options for treatment of cancer
|
Type of treatment
|
Description
|
References
|
| Chemotherapeutic drugs |
-
Overview of commonly used chemotherapeutic drugs: anthracyclines (doxorubicin, epirubicin), taxanes (PTX, docetaxel), platinum drugs (cisplatin, carboplatin), cyclophosphamide.
-
Challenges, side effects, and drug endurance in traditional chemotherapeutical treatments.
|
44-47
|
| Hormonal treatment |
|
13
|
| HER2 Selective treatment |
|
14
|
| Immunotherapeutic drugs |
-
Introduction to immunotherapeutic drugs and their potential in BC treatment.
-
Discussion on atezolizumab, a PD-L1 inhibitor, in conjunction using nab-PTX care for TNBC patients.
|
15,16
|
| Challenges in chemotherapy |
-
Discussion of challenges in traditional chemotherapeutic treatments, including side effects, drug resistance, and limitations in reaching specific locations such as the brain.
|
20
|
| NPs in cancer treatment |
-
Explanation of the advantages of NPs in cancer treatment, including their size, properties, and directed release of medicines.
|
25
|
| Tumor microenvironment and immune response |
-
Discussion on TME components and their role in the immune response.
-
Activation of immune cells, incorporating DCs and T cells, and their migration to the TME.
|
27,28,39
|
| Immunotherapy techniques and challenges |
-
Overview of immunotherapy techniques (checkpoint inhibitors, genetically altered T cells, cancer vaccines) and challenges associated with potential adverse reactions and precise regulation of the immune system.
|
40,41,42,48
|
| Breast tumor subtypes and immune response |
-
Exploration of how breast tumour subtypes affect the immune reaction and their distribution.
-
TNBC associated with higher T cell infiltration, while hormone receptor-positive tumours have lower infiltration.
|
39
|
| Innovative immunotherapeutic approaches |
|
40,41
|
| Dual-scale cancer vaccine |
-
Description of a dual-scale cancer vaccine involving mesoporous silica microrods loaded with GM-CSF and mesoporous silica NPs loaded with ovalbumin and CpG ODN, showing inhibitory effects on melanoma growth.
-
Combined with an immune checkpoint inhibitor for synergistic effect.
|
42
|
Early detection and diagnosis
Recognition of cancer cells in the initial stages may pave the way for effective disease management and may significantly lower cancer-related mortality.49 For instance, individuals diagnosed early show about 90% of 5-year relative survival rate, whereas those diagnosed later have been found to exhibit 27% survival rate.50 Lymph node, lung, liver, bone, and brain metastases are frequent causes of BC mortality.51 Because of this, individuals with BC must receive more effective treatment, especially in advanced stages. Currently, histopathology, cytology, and imaging tools assist with the diagnosis of cancer at the early stage. The most common imaging tools, like X-rays, MRIs, CT scans, endoscopies, and ultrasounds, which detect cancerous tissue with noticeable modification. Furthermore, it is impossible to differentiate between benign and malignant lesions by applying current imaging methods. Furthermore, it is impossible to use cytology and histology alone to effectively identify cancer at the earliest.52 Therefore, a significant solution in the prevention of metastasis is the development of early cancer detection technology.
The ability and effectiveness of various imaging techniques for capturing breast tissue varies. For example, mammography is unsuitable for dense breasts, while ultrasound and dynamic contrast-enhanced breast magnetic resonance imaging (DCE-MRI) are appropriate. The DCE-MRI method is being found to be appropriate for analysing axillary lesions and the margins of digital breast tomosynthesis (DBT) tumours. However, before postoperative surveillance and therapy, Ultrasound helps assess lymph nodes, inflammatory/malignant lesions, deep lesions, broad breast, lesions in concealed parts of breasts and the stage of cancer. Employing NPs to identify cancers of the breast with several imaging modalities, particularly magnetic resonance imaging (MRI) may enhance the signal-to-noise ratio as well as contrast of breast images.53
Since 2015, nanotechnology has advanced for early BC detection through the development of novel imaging agents and techniques to detect cancer biomarkers, incorporating exosomes, circulating tumour DNA (ctDNA), and proteins associated with cancer.54 The high surface area as compared to volume of NPs offers a significant advantage by enhancing their sensitivity to fulfil the requirements of specified biomolecular analysis kits.55-56 This feature permits the compact covering of the exterior of NPs with antibodies, small molecules, peptides, aptamers etc. to fix and identify cancer cells (Fig. 2). They can also be designed by using a variety of binding ligands with desired specificity and sensitivity.57
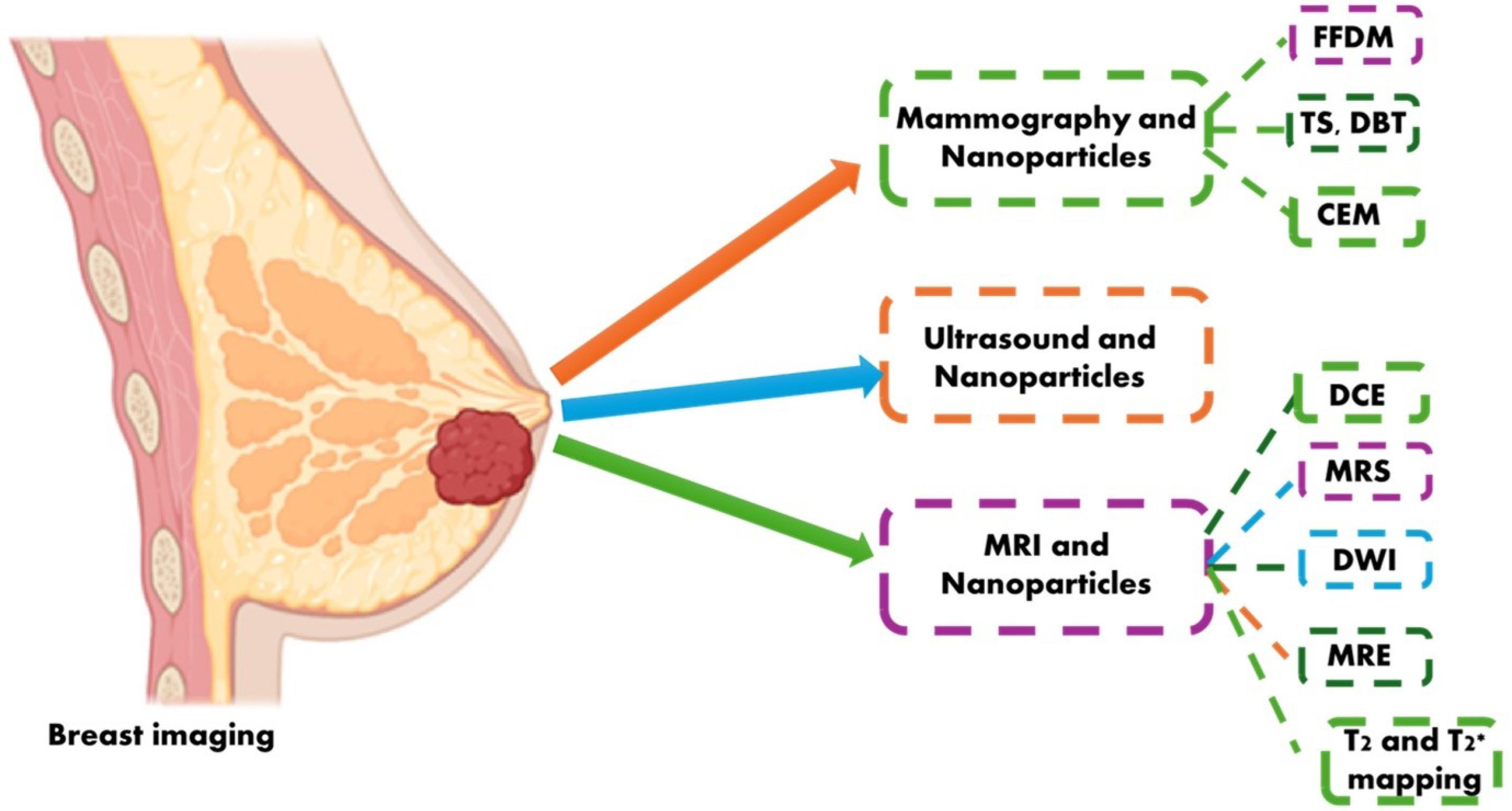
Fig. 2.
Common imaging modalities for BC diagnosis. Abbreviation: FFDM: Full-field digital mammography; TS: Tomosynthesis; DM: Digital mammography; CEM: Contrast-enhanced mammography; DCE-MRI: Dynamic contrast-enhanced breast MRI; MRS: Magnetic resonance spectroscopy; DWI: Diffusion-weighted imaging; MRE: Magnetic resonance elastography.
.
Common imaging modalities for BC diagnosis. Abbreviation: FFDM: Full-field digital mammography; TS: Tomosynthesis; DM: Digital mammography; CEM: Contrast-enhanced mammography; DCE-MRI: Dynamic contrast-enhanced breast MRI; MRS: Magnetic resonance spectroscopy; DWI: Diffusion-weighted imaging; MRE: Magnetic resonance elastography.
PTX, a chemotherapy drug, shows non-target harmful impact because of its solubility in water, when administered alone but in a NP form, it resulted in increased permeability and retention in the cancer cells.58 Gold NPs is the most promising contrast agents due to less toxicity, good X-ray absorption and superior contrast in different imaging techniques. Gold-coated Fe3O4 NPs exhibited improved tumour optical contrast. Chemically non-reactive, and powerful near-infrared (NIR) absorber gold NPs have a genuine chance of being used as an efficient contrasting agent as well as for other biomedical applications. Furthermore, gold NPs’ (AuNPs') small size makes it possible for them to pass through capillaries and circulate easily inside blood vessels. Gold-embedded tumour cells can be seen in vivo using two-photon-induced photoluminescence (TPIP) because of a photophysical characteristic of the gold nanoshells that makes them light up brightly.59 Mobile devices with biochemical sensors can track those suffering from BC quickly, accurately, and non-invasively, transforming the course of therapy. By identifying BC at the early stages, such devices could lower treatment-related mortality and late detection.60
However, several problems such as a small number, variability in biomarker amounts in bodily fluids, timing etc. hamper the application of biomarkers.61 Nevertheless NPs can be used to enhance biosensors response and can enable accurate targeting.62 Tumour-derived strands of DNA, present in blood having approximately 100–200 base pairs are identified as circulating tumour DNA (ctDNA).63 Through cancer-specific genetic mutations, circulating tumour cells (CTCs) or the primary tumour may release ctDNA, which may help to diagnose cancer. To identify a single exon in the BRCA1 gene in BC, a DNA silver nanocluster (NC) fluorescent moiety has been created.64 Recent analysis shows that many cancer types share the genome methylation landscape suggesting it to be common biomarker for cancer detection. Based on differences in DNA-gold affinity and DNA solvation between cancerous and normal genomes, straightforward, fast, accurate, and colorimetric one-step assays for recognition of cancer have been developed.65 Super paramagnetic iron oxide NPs (SPIONs) have gained importance owing to their capability to improve distinction in magnetic resonance imaging (MRI), enabling more accurate tumour localization.66 Additionally, quantum dots and gold NPs have shown potential for highly sensitive and specific detection using optical imaging methods.67
Jia et al discovered that the human BC cells MDA, MB 231 and ZR 75 1 showed increased chemosensitivity and radiosensitivity when single-walled an oxygen-carrying tombarthite-modified folic acid conjugated chitosan (RO2 FACHI) SWCNT nanocarrier, were administered in combination with chemotherapy or radiotherapy.68
Multimodal imaging
Innovations in nanotechnology have steered to the advancement of multimodal imaging agents that combine various imaging modalities. These agents, often integrated with therapeutic payloads, allow real-time monitoring of medication response and amended anatomical and functional insights.69 The integration of MRI, positron emission tomography (PET), and fluorescence imaging within a single nanoparticle has enabled more accurate disease characterization. Superparamagnetic contrast agents, having ferromagnetic superparamagnetic iron oxide NPs (SPIONs), and paramagnetic contrast agents, which are made of gadolinium70,71 and manganese72 exhibit low signal intensities on T2-weighted images, are the two types of nanoprobes that are frequently used.73-76 NP-Neu, a SPION triggered by anti-Neu receptor antibodies, was created.77
When NP-Neu was administered to animals, the T2 relaxation time of the breast tumours have been decreased dramatically due to the precise bonding of NP-Neu to breast cancerous cells, which greatly improved the MRI contrast. A range of studies have explored the use of multi-modal imaging for BC detection. An AI system designed for BC detection has been found to be robust and accurate, regardless of the mammography unit manufacturer or patient demographics.78 Yang developed a digital platform using stimulated Raman scattering microscopy and multi-instance learning for quick and programmed identification of tumours on unprocessed breast core-needle biopsies, achieving a 95% diagnostic accuracy.79 Tower reported a case of mixed metaplastic breast carcinoma, highlighting the importance of accurate diagnosis and treatment.80 Bobowicz proposed a deep learning system for the classification of BC, achieving high accuracy and potential for generalizability.81 Thus recent research validates the potential of multi-modal imaging in improving BC detection and diagnosis.
Targeted drug delivery
Recent years observed remarkable improvements in nanomaterials' design for drug transfer to directed cancerous cells. Antibody-conjugated liposomes, polymer-based nanoparticles, and exosome-derived nanovesicles have been engineered to distribute chemotherapeutic agents and targeted therapies directly to the swelling cancer locations.82,83 For an efficient nano drug delivery system, NPs should be innocuous and biocompatible having size between 10-200 nm. The medications ( > 50 mol%) ought to be enclosed in the NPs and shielded against unintended breakdown or removal while in the bloodstream. These medications or active chemicals under extrinsic or biological control are released in the required dose. Surface functionalization of the nanomaterial should be resilient to conglomeration brought on by changes in temperature, macromolecular interactions, ionic strength, and pH in the normal physiological setting to prevent accumulation in organs or membranes. Good circulation time and maximum therapeutic absorption intensity is required within the targeted areas to minimise negative consequences on normal tissues. The nanoparticle vehicle must have clearance pathways after performing its purpose. This is essential to prevent progressive and/or permanent systemic adverse reactions, such as possible disruption with biological activities. These nanocarriers enhance drug bioavailability, minimise off-target effects, and overcome drug endurance.84
NPs can impact BC through active, passive or stimuli-responsive targeting. NP accumulation in tumour tissues due to augmented penetrability and holding effects result of vascular leaking in the TME, which causes passive targeting.85 Typically, rapidly expanding tumour cells neovascularize in response to hypoxic conditions. The new blood channels frequently have larger holes when compared to normal vessels, which worsens the penetrability and selective nature of tumour vessels.86 When tumour cells expand swiftly, the exterior of vascular endothelial cells typically releases certain antigens or receptors abnormally. The alteration in the surface of NPs with appropriate antibodies or ligands boosts their concentration into the tumour tissues and provide them the potential to deliver medications at specific locations.87 Alteration of ligands leads to the active targeting at tumour vasculatures, tumour stroma, tumour cells, immune cells, and the subcellular level.88
Nanoparticle's dimensions, form, strength, and charge on the surface are responsible for passive targeting and the addition of a chemical or biological component to a nanoparticle's surface that bonds specifically to receptors or other cellular characteristics that are extensively expressed by cells in a target organ results in active targeting.
While passive targeting makes use of NPs' intrinsic qualities, such as increased permeability and retention (nanosizing PEGylation), active targeting NPs use antibodies, peptides, ligands, or carriers (Fig. 3). Passive targeting involves non-specific delivery, which may result in drug buildup in other tissues and cause effects that are not intended. The EPR effect is not always successful in all tumours. Decreased performance may result from drug release into the extracellular matrix rather than cancer cells.89
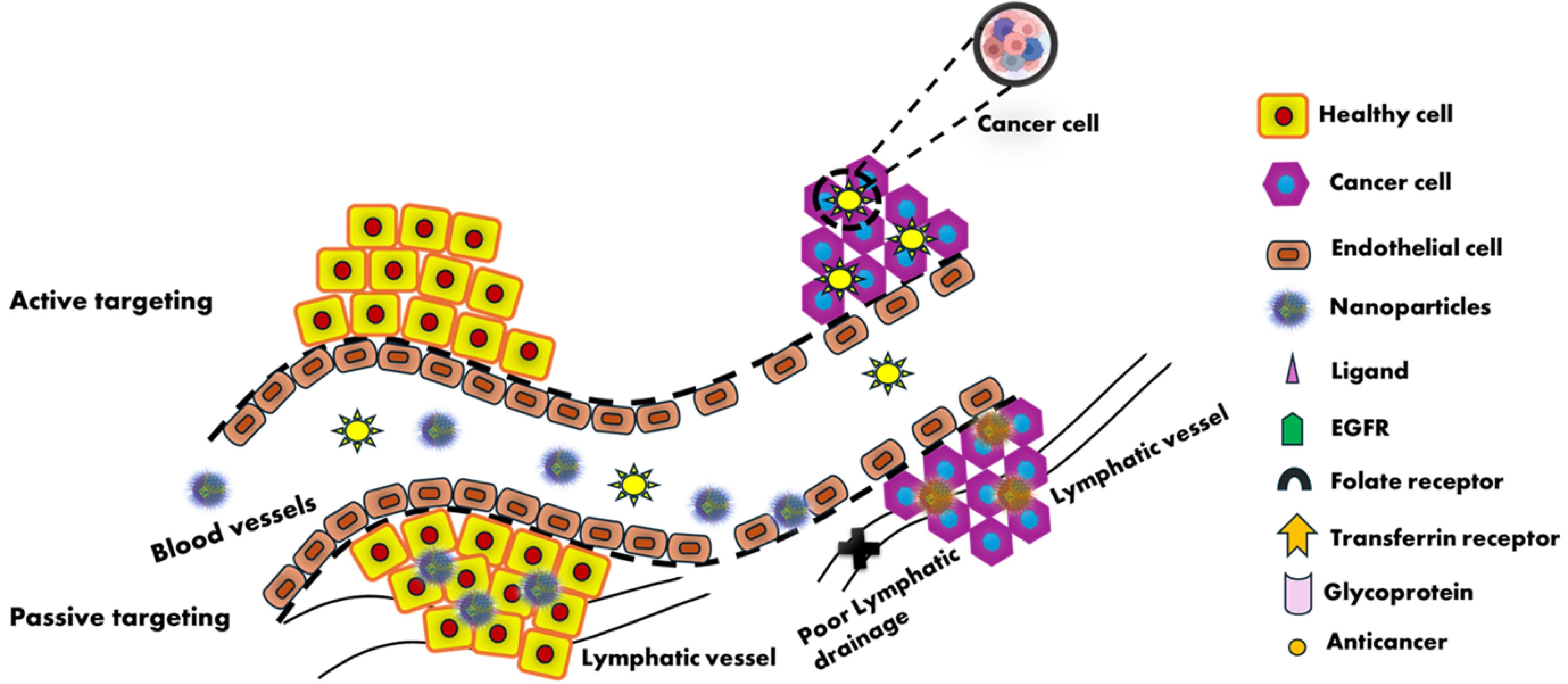
Fig. 3.
Mechanism of Active and Passive targeting by nanoparticles
.
Mechanism of Active and Passive targeting by nanoparticles
Active targeting is more difficult to execute, necessitating the development of appropriate ligands and surface modification of the drug carrier. Certain malignancies may not respond well to ligand-receptor interactions, which can be influenced by variables such as receptor density and expression. If the ligand attaches to receptors on different tissues, it may lead to off-target consequences.90
Genexol®-PM, also known as PTX, doped with poly (D, L-lactic acid), is an antimitotic chemotherapeutical medication that averts microtubule depolymerization, triggering a cell cycle stoppage and mitotic suppression.91 Clinical research has shown that Genexol-PM can provide PTX at larger doses than typical chemotherapy without producing significant dose-limiting effects.92 Similar observations have been reported with nab PTX (Abraxane).93 In adjuvant care, Doxorubicin-doped human serum albumin NPs (DOX-NP) having surface functionalization by trastuzumab through covalent bonds (DOX–NP–trastuzumab) have been synthesized which is frequently used solely or in combination with other drugs to lower the probability of malignancy relapse and enhance the general survival rate of those diagnosed with HER2-positive BC.94 PEGylated liposome containing DOX hydrochloride, is a potential therapy for metastatic BC that lowers systemic toxicity, preserves DOX's antitumour effects, and lengthens its duration in flow to prevent early ejection.95 Moreover, liposomes can be employed as co-delivery platforms for inhibitors and chemotherapy agents, thereby increasing the susceptibility of cancerous cells to antineoplastic medications. It was discovered that co-condensed DOX and verapamil liposomes might lessen the noxiousness of non-target organs significantly in addition to helping BC cells escape P-gp-mediated multidrug resistance.96 To combat HER2-positive BC, nanocrystal micelles made up of Herceptin-conjugated PTX stacked with PCL-PEG have been designed.97 The study demonstrated that the PTXs have a particular focus on HER2 -positive tumour cells and stay stable in TME while protecting healthy cells from their harmful impacts. AuNPs can transform the energy of NIR radiations into heat energy, which kills cancerous tissues due to their outstanding effectiveness of photothermal transformation.98 Surface-functionalized groups for instance biotin, PEG, and rhodamine B linked β-cyclodextrin can modify PTX attached with AuNPs, which can be advantageous for treating BC without killing non-cancerous cells and can be employed as theragnostic agents.99 When two or more chemotherapeutic drugs are combined, such as when trastuzumab is added to PTX NPs for specific chemotherapeutic treatment of malignant breast cells, multifunctional polymeric NPs are created. These kinds of NPs have several benefits, including the ability to formulate anticancer medications without the need of hazardous adjuvants, produce synergistic therapeutic effects, lessen anticancer medication adverse effects, and deliver cancer medications precisely where it is needed.100 Enhanced radiosensitivity and chemosensitivity of human BC cell lines were detected, when unique functionalized single-walled carbon nanotubes loaded with DOX were used which resulted in to enhanced programmed cell death, DNA destruction, and oxidative stress.101 One significant advancement is the use of PTX. Abraxane represents an additional instance of precisely aimed drug administration to breast tumors; referred to as nab-PTX.102 Human albumin proteins were loaded to create a single-walled carbon nanotubes (SWNT-HAS) as drug transfer transporters. In MCF-7 BC cells, this combination demonstrated 80% intracellular drug delivery along with suppression of growth of tumour, demonstrating better efficacy.103 For BC patients, the use of poly (d,1-lactide-co-glycolide) (PLGA) having cisplatin has established notable effectiveness.104 To co-deliver DOX and cisplatin, poly amido amine (PAMAM) dendrimer NPs tailored with hyaluronic acid (HA) (HA@PAMAM-Pt-Dox) have been synthesized which has established that HA@PAMAM-Pt-Dox is highly potent in enhancing the cisplatin and DOX chemotherapy's ability to destroy BC cells.105 Management through monoclonal antibodies opposed to the HER2 receptor offer an effective targeted remedy.106 Antibody-drug conjugates that actively target the protein tyrosine kinase 7 (PTK7) receptor, ephrin receptor-4, zinc transporter LIV-1, trophoblast cell-surface antigen 2 (Trop-2) and NMB glycoproteins in TNBC have also been researched. The term "stimuli-responsive tumour targeting" signifies the careful release of the medicine at cancer sites, which boosts its effectiveness and lowers its systemic noxiousness.106 Payload is released by activating NPs at cancer tissues because of alterations in redox potential, enzyme activity, pH, temperature, ultrasound, electric field, photodynamic treatment etc.107,108 HA modified carbon dots prepared by self-aggregation of DOX@HA-CD, is a size-shrinkable, pH-responsive drug delivery system.109 In neutral pH, it may show self-aggregation to form a raspberry-like structure but due to fluctuations in charge, hydrophilic, and hydrophobic behaviour at simulated pH 6.5, it quickly crumbles to shotgun-like CD monomer encumbered with DOX, improving cellular uptake and infiltration in the BC cells, and significantly increasing the effectiveness of chemotherapeutic treatment.110 Chen et al developed bimetallic Prussian blue analogues for DOX delivery at the intracellular level, demonstrating superior drug loading capability and exceptional therapeutic efficacy.111 Zhang et al engineered hollow mesoporous silica NPs for dual-responsive delivery of methylene blue and DOX, achieving accurate targeting and exhibiting the remarkable ability to kill tumour cell.96,112 Maddiboyina et al optimized poly (lactic-co-glycolic acid) nanomaterials packed with raloxifene, showing high entrapment efficiency and stability.113 Subhan and Torchilin discussed the potential of biopolymer-based nano systems for siRNA-targeted drug administration to solid BC cells, highlighting their stimuli-responsive properties and controlled-release capabilities.114 These studies collectively underscore the promise of NPs in targeted drug transfer to BC cells.
Combination therapies
Recent years have witnessed the exploration of nanotechnology-enabled combination therapies for BC. Co-delivery of chemotherapy agents, targeted therapies, and immunomodulators using multifunctional NPs has demonstrated synergistic effects, leading to enhanced therapeutic outcomes.115,116 NPs can be designed to release different agents at specific time points, optimizing treatment sequences.
Co-delivery nano systems
Advanced drug delivery platforms called co-delivery nano systems for BC are made to deliver several therapeutic medicines to the tumour site at once. These systems seek to address issues such as medication resistance, diminution systemic toxicity, and advance the therapeutic efficacy.
An outline of co-delivery nano systems for the treatment of BC is provided below:
(a) liposomes: Amphiphilic lipid bilayer-based spherical vesicles having high biocompatibility and their competence to capture hydrophilic and hydrophobic medications e.g. DOX, a chemotherapy drug, to prevent tumour development and metastasis. They can readily fuse with the cell membrane because of their structural resemblance. Liposomal anthracyclines like liposomal daunorubicin and pegylated liposomal DOX offer effectual drug encapsulation, substantial anticancer behaviour, lesser cardiotoxicity, with greatly prolonged circulation. Pegylated liposomal DOX has demonstrated significant effectiveness in treating BC when used alone or in conjunction with conventional therapeutics.117 Liposome-based methods designed to attack BC cells encompass enzymes, receptors on the outside, inside, and across membranes, for dual-targeting of these cancers.118 Clinically available liposome-based treatments for manging breast carcinoma include Doxil, Myocet, Lipodox, Lipusu.118 The liposomes also present chances for developing site-specific treatment by altering the liposome exterior with targeting ligands, reducing the non-specific impact of conventional chemotherapeutic medications. The treatment results are significantly improved by the latest generation liposomes incorporating activating properties, which even offer exact control over payload discharge.119
(b) Polymeric nanoparticles: Biodegradable and biocompatible NPs having polymers for instance poly (lactic-co-glycolic acid) (PLGA) are co-loaded with rapamycin (a mTOR inhibitor) and PTX (a chemotherapeutic drug) to target the propagation of cancer cells. They exhibit high stability and controlled medication release. DOX-loaded PNPs exhibit a favourable interaction with healthy cells and show strong effectiveness towards estrogen-dependent MCF-7 cells, even at modest DOX application levels.120
(c) Micelles: A very efficient drug transporter, polymeric micelles are created by the self-assembly of hydrophilic shells and hydrophobic cores. These micelles can improve the invasion of the malignant vascular system. Amphiphilic molecules having core-shell structures by self-assembling encapsulate the antioxidant quercetin and the chemotherapeutic medication docetaxel to improve therapeutic efficacy and lower drug resistance. They show easy functionalization and a high drug loading capacity. Lipids, polymers, inorganic, and peptide-based nanomedicines with a range of functions have been employed to create an array of nanoformulations. Therefore, it is crucial to have a thorough understanding of such smart nanomedicines for highly intriguing drug delivery methods. Since polymeric micelles (PMs) are often simple to make and have high solubility, they seem like a desirable substitute for other nanosystems.121
Nevertheless, there are other strategies to enhance medication delivery using micelles. Micelles may have targeted ligands added to them that precisely identify and adhere to receptors that are overexpressed in tumour cells. Micelles can also be tracked in vivo for biodistribution investigations by chelating or incorporating imaging molecules. Furthermore, regulated micelle breakdown and controlled drug release are made possible by block copolymers, which show sensitivity to pH, temperature, ultrasound, or light.122,123
(d) Dendrimers: Branched, tree-like nanostructures with several functional groups can be co-loaded with DOX and tamoxifen to target BC that has hormone receptors. They exhibit accurate control over drug targeting and release. These NPs are made up of three components: an outer surface functional group, repeated branching units, and a centre core. Drugs, targeting ligands, and imaging agents can all be complexed with chemically altered outer surface functional group. Poly (L-lysine) (PLL) dendrimers, polypropylene imine (PPI) dendrimers, polyamidoamine (PAMAM) dendrimers, and PAMAM-organosilicon dendrimers (PAMAMOS) are among the dendrimers that are frequently employed for cancer therapy.124
Drug-delivery dendrimer-based nanosystems are generally synthesised from PAMAM dendrimers, which are loaded with medicines and contrast agents. Furthermore, dendrimers are effective conjugates that transport many drugs at once. The generation of new molecular frameworks for medical diagnosis and therapy has spurred the prospect of producing dendrimers with many roles.125 Because of their great loading ability and capacity to distribute medications precisely, dendrimers produce fewer negative consequences.126
(e) Hybrid nanoparticles: To maximise delivery, several components (such as lipids and polymers) are combined. For instance, lipid-polymer hybrid NPs that deliver docetaxel and trastuzumab (an antibody that targets HER2) simultaneously. Hybrid nanocarriers may encapsulate a variety of cargos, and augment encapsulation, stability, circulation time, and structural fragmentation. Hybrid nanocarriers are been used in breast carcinoma for scanning platforms, chemotherapy, gene therapy, photodynamic and photothermal treatment (PTT), and combinational therapy.127 To improve the packaging effectiveness, anti-cancer efficacy of caffeic acid (Caff), and folic acid (FA) for the treatment of BC, salicylic acid (SA) was doped into poly(3-hydroxybutyrate) (PHB), and nanoparticles were synthesised.128
(f) Exosomes: Cell-derived vesicles that occur naturally help in the co-delivery of PTX and siRNA via exosomes for chemotherapeutics and the suppression of genes. The benefits include the capacity to transcend biological constraints and inherent biological compatibility. Exosomes generated from tumours have been investigated to be key regulators in the setting off malignant tumours, influencing the behaviour of immune cells and accelerating the disease's progression.129,130
(g) Nanostructured lipid carriers (NLCs): Lipid mixtures, both liquid and solid, make up NLCs. Because of their many components, NLCs have an unstructured matrix.131 Due to their unstructured characteristics and crystallisation flaws, NLCs in the liquid state offer additional room for drug disintegration and delivery.132 Several NLC loaded with methotrexate/ chlorambucil/ellagic acid have been synthesised, which are found to be effective against BC cells.133-135 NLC encumbered with either Ribociclib or curcumin have been found to exhibit better bioavailability and enhanced cytotoxic effects, respectively.136,137 Tetrahydro curcumin- chitosan-coated NLC, Dexibuprofen loaded NLC, Cabazitaxel loaded NLC have been found to show better skin permeation, prolonged drug release.138-140 NLC having Luteolin/Imatinib/Raloxifene/Docetaxel have also been prepared, which demonstrate better efficacy for management of BC.141-144 Psoralen-loaded polymeric lipid nanoparticles enhance PTX's antimetastatic and curative effects on both in vitro and in vivo TNBC.145
(h) Carbon nanotubes (CNTs): Carbon nanotubes are cylindrical structures having multiple coaxial layers of graphite with dimensions in the nanometre range.146 Medications are confined in carbon NPs through π—π stacking due to their intrinsic hydrophobicity.147 Because of its huge surface area and special optical, electrical, and mechanical properties, CNTs can carry heavy loads.148 As a result, carbon nanotubes can be utilised as biosensors, contrast materials for breast tumour diagnostics and detection, and tools for the delivery and discharge of targeted medications.149
Carbon nanomaterials such as graphene and CNTs are perfect for PTT as they possess high electrical conductivity, enormous surface area, and biocompatibility. By absorbing near-infrared light, carbon nanomaterials allow for deep tissue penetration and precise heating of tumour tissues, which causes necrosis and apoptosis. Additionally, carbon nanomaterials enhance therapeutic results by increasing tumour vascular permeability, which encourages the buildup of chemotherapy drugs.150
Carbon dots (CDs) functionalised with doxorubicin (CDs-DOX), showed superior anti-tumour effectiveness on MCF-7 cells and a higher cellular absorption than free DOX.151
To enhance the targeted medication absorption by the cancerous cell, click chemistry was used to modify the CNPs' surface with folate. Tamoxifen (TAM) discharge at the designated region has been effectively triggered by the pH variation among cancer and normal cells. Approximately 74% of the TAM medication was released by CNPs in an acidic pH after six hours of incubation.152 carbon nanotubes are also used for the early detection of cancer.153
(i) AuNPs: AuNPs show several uses such as high photothermal conversion effectiveness, imaging contrast ratio, biocompatibility, versatility, and the ease of surface alteration.154,155 AuNPs are therefore effective agents for PTT.156 AuNPs can destroy cancer cells by converting light energy in the NIR into heat energy due to their high photothermal conversion efficiency.157
AuNPs show promise as a therapeutic option when used in conjunction with radiation therapy.158 The study emphasizes how the PEG chain contributes to AuNPs' effectiveness in sensitizing cells to ionizing radiation.
A gold atom in the inner core of AuNPs is adorned with a negative charge on the surface. The site-specific localization of AuNPs with targeted therapeutic payload distribution is ensured by designing particle with various biomolecules (proteins, enzymes, or DNA). Nanospheres, nanoclusters, nanorods, nanocubes, nanocages, nanostars, and nanoshells are some of the many morphologies of AuNPs. They ensure pharmacological response in targeted delivery, photodermal and photothermal therapy, SERS-based imaging, photo-electronics, clinical management of cancer, microbial infections, contrast agents and field enhancers, chemical and biochemical sensors, and radiosensitizers.159
(j) Mesoporous silica NPs (MSNs): Due to their huge surface area, customizable pore size and release qualities, high drug content capability, zero premature release, and diverse competencies, MSNs have evolved into devices for drug delivery.160,161 Because of their functionalization and size-shape-driven versatility, MSNPs have recently attracted attention as carriers of drugs.162 MSNs loaded with chemotherapeutic drugs such as docetaxel, DOX, and PTX have demonstrated potential in preclinical investigations. These medications can be encased in the tiny openings of MSNs or placed on their outer layer, enabling regulated delivery patterns and supporting various pharmacological characteristics. Targeted molecules, including folic acid, HER2/neu antibodies, and aptamers, may be affixed to the MSNs to improve selectivity. MSNPs can increase medication release and absorption, which boosts anticancer activity.163 MSN-Res, or resveratrol-modified mesoporous silica nanoparticles, have been produced by chemical methods, which are more successful than Res therapy alone as they block the NF-κB signalling pathway, thereby, slowing the advancement of breast carcinoma.164
Gingerol (Gin) and Letrozole (Let), anticancer medications were loaded on mesoporous silica nanoparticles (MSNs) functionalized with zinc, amine, and graphene oxide (GO) (MZNG). Let and Gin-loaded MZNGs are spherical in shape having mean diameter of 210 nm. Utilizing a pH-dependent extended-release characteristics, the MZNGs offered excellent Let and Gin retention rates. The suppression of tumour cell growth and cell detention in the G0/G1 stage was triggered by controlling gene expression, thereby greatly improving their efficiency.165
After DOX had been introduced into the tiny holes of mesoporous silica nanoparticles (MSN-COOH), polyethyleneimine (PEI) and anisamide (AA) were added to the mesoporous silica surface to create DOX@MSN-PEI-AA (DMPA). Through the process of AA-mediated receptor endocytosis, DMPA particularly got into the cancer cells. PEI was protonated, which caused it to split away from the MSN surface in the acidic atmosphere of cellular lysosomes/endosomes. This led to a consistent discharge of the encased DOX from the MSN pores in the intended target cells' cytoplasm exhibiting targeted and efficient delivery.166
(k) Quantum dots (QDs): Theseare semiconductors at the nanoscale, measuring from 2 and 10 nm.167 To increase the luminescence performance of single quantum dots, core-shell quantum dots have been developed. Their use in sensors is made possible by extracting them into water-based solutions and covering their exteriors with organic ligand.168 QDs can be employed for biosensing, biolabeling, and bioimaging because of their unique optical characteristics, broad excitation spectrum, and extremely small symmetrical intensity distribution.169
A flexible ultrasmall Ag2Te QDs for photonic tumour heating have been developed for guided by high-performance computed tomography (CT) imaging. Furthermore, in xenograft animal models, these Ag2TeQDs with little toxicity and outstanding biocompatibility have demonstrated a significant tumour suppression rate (94.3%) on 4T1 cells due to their high photo-thermal conversion efficacy (50.5%).170
The nano systems show synergistic therapeutic effects by following specific pathways, better targeting, decreased side effects, overcoming drug resistance, controlled release of medication to achieve long-lasting therapeutic benefits. However, formulation, scalability, biocompatibility, and regulatory approvals are the issues that need to be tackled.
The Food and Drug Administration considers a few nano drug delivery systems for BC treatment, including Genexol-PM (PTX micellar formula), Myocet (DOX liposomes), Lipusu (PTX liposomes), and Doxil (pegylated DOX liposomes). Table 2 and Fig. 4 illustrate several potential approaches considering the various kinds of NPs that have been investigated thus far.
Table 2.
Types of nanoparticles used for breast cancer treatment
|
NDDS
|
Size (nm)
|
Drug
|
Cell line
|
Reference
|
| Multi-functionalized iron oxide magnetic NPs (MNPs) |
93 |
AntiCD44 antibody and gemcitabine (GEM) |
CD44-positive cancer cells |
171
|
| PEGylated magnetic NPs |
50 |
DOX and monoclonal antibodies to VEGF |
Murine breast adenocarcinoma 4T1 |
172
|
| Collagen -Gold NPs (Col-AuNP) |
227 |
Berberine (BB) |
Her−2 BC cell lines |
173
|
| Liposomes and HA combine in a phospholipid–polysaccharide NPs (PHYN) |
35 ± 6 |
DOX |
TNBC cell lines (MDA-MB−231 and MDA-MB−468). |
174
|
| Liposomal nanozymes (MP@H-MnO2-Col NPs) |
220 |
DOX |
4T1 cells |
175
|
| Polymeric nanocarrier with chitosan (Meth-Cs-NPs) |
∼143 |
Methotrexate (MTX) |
TNBC (MDA-MB−231) |
176
|
| Polymeric micelles with HA |
200 |
Harmine (HM) |
CD44-positive MDA-MB−231 cells and CD44-negative MCF−7 cells |
177
|
| Collagen-Binding Nanoparticles (nanostructured lipid carrier core with a poly(N-isopropylacrylamide) (pNIPAM) shell) |
140 |
Paclitaxel (PTX) |
MCF−7 cells and T−47D cells |
178
|
| Dendrimer |
< 2 μm |
Trastuzumab (TZ) and neratinib |
SKBR−3 (HER2-positive) BC cell line |
179
|
| Dendrimer (fluorinated dendrimers) |
220–280 |
TZ |
Her−2 positive cells (MCF−7) |
180
|
| PEGylated N-(2 hydroxypropyl) meth acrylamide polymeric micelles |
20–70 |
DOX |
4T1, MCF−7 and TNBC cell lines |
181
|
| Polymeric micelles (Folic acid grafted mixed polymeric micelles) |
35.01 ± 1.20 |
Tamoxifen citrate (TMXC) |
MCF−7 cell line |
182
|
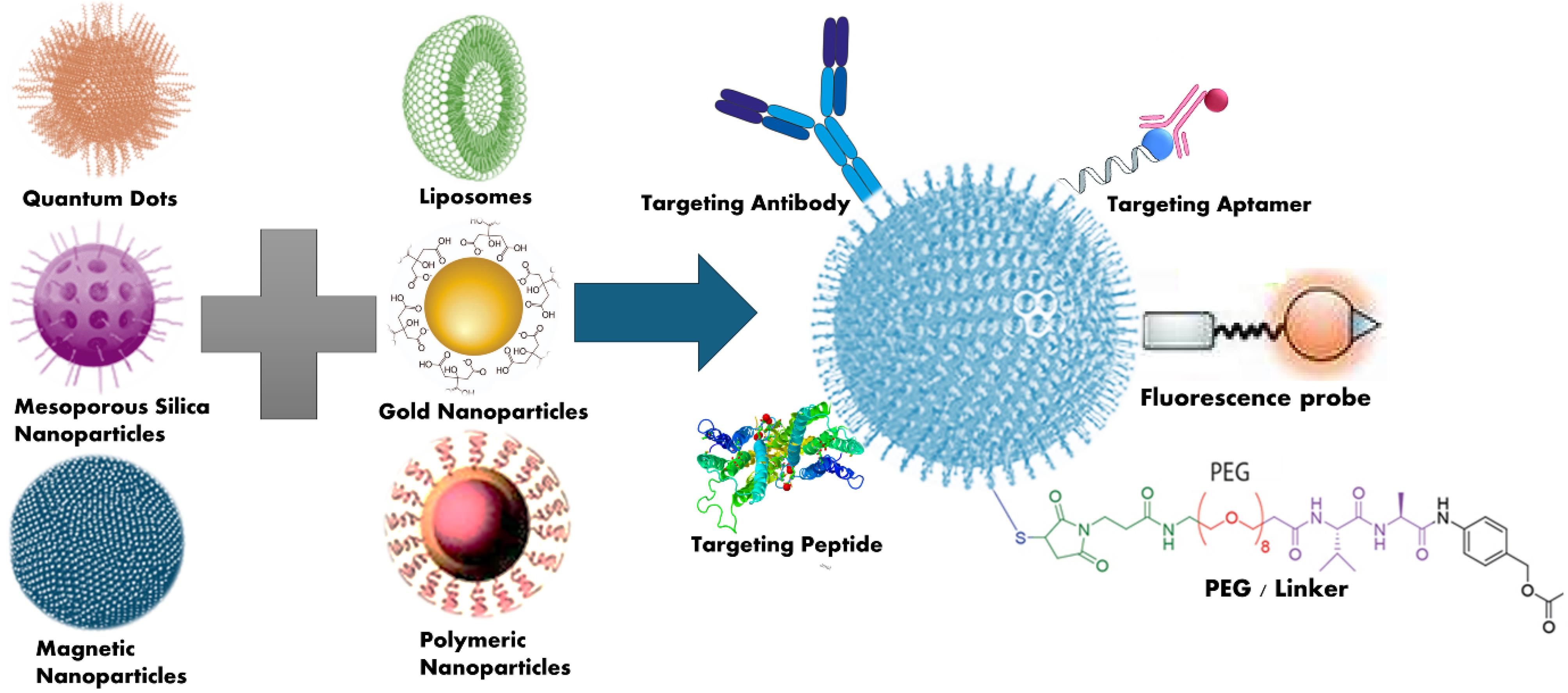
Fig. 4.
Target of various nanocarriers via conjugation to treat breast cancer
.
Target of various nanocarriers via conjugation to treat breast cancer
Combination with PTT
Three therapeutic drugs—DOX, CpG, and indocyanine green as a photothermal agent—were loaded on layered double hydroxide NPs.183 In the 4T1 BC mouse model, this multifunctional nanoparticle exhibited a highly efficient response in eliminating the tumour and averting tumour relapse and metastasis.184 Analogous outcomes were observed with a nanoparticle that incorporates glycol chitosan as an immune stimulatory agent and IR 820 as a photothermal agent.185 An outstanding antitumor effect against BC was demonstrated by gold NPs having anti-PD-1 peptide.186 In the 4T1 mouse model, Fe3O4 NPs combined with reduced-graphene oxide NPs activate immunogenic cell death (ICD) and DCs to eradicate the tumour.187 Furthermore, an improved copper sulphide nanoparticle capable of inducing photothermal effects was prepared.188 The induction of systematic immune responses led to a suppression of primary and distant tumours in the 4T1 tumour model after the in vivo transfer of copper-based NPs in combination with an anti-PD-L1.
Combination with photodynamic therapy (PDT)
PDT encompasses the usage of a photosensitizer (PS) which is either administered locally or the targeted tissues are subjected to electromagnetic rays at a certain wavelength that is compatible with the PS.189 The popular way to strengthen the anti-cancer efficacy is to combine chemotherapy with PDT. For instance, a liposomal system called nano-Pt/VP@MLipo containing verteporfin (VP), a clinical Photosensitizer, and platinum NPs (nano-Pt) in the lipid bilayer was designed to provide photodynamic therapeutic action.190 A cancer-targeted nano-platform (PFTT@CM) has also been synthesized using Fe3+ tetrakis (4-carboxyphenyl) porphyrin (TCPP), and the hypoxia-activable prodrug tirapazamine (TPZ).191 A PLGA-based therapeutic nanoplatforms (IDPNs) was created to distribute near IR dye indocyanine green (ICG) and the chemotherapy medication DOX simultaneously.192 The IDPNs displayed excellent on-demand drug release behaviour, photothermal effect, stability, and biocompatibility. The chemo-photothermal combination therapy effectually inhibited tumour progression in mice bearing BC cells with no systemic toxicity and produced a preferential in vitro chemical-photothermal combination management.
A substantial efficacy for BC control by delivering zinc phthalocyanine photosensitizer to cancer cells via mitochondria-targeted NPs was observed.193 In a related study, photochlor, a different photosensitizer, was also loaded into a nanoparticle to strongly stimulate host antitumor immunity, which in turn inhibited tumour development and metastasis in the 4T1 murine BC model.194 Additionally, it was noted that the 4T1 tumour model's primary and distant tumours are inhibited when an antigen-capturing agent called maleimide is added to photothermal/phototherapeutic-based nanoparticles. This improvement occurs after ICD is induced by the nanoplatforms, greatly increasing antigen presentation to DCs.195
Chemodynamic therapy (CDT)
CDT is an effective technique to cure cancer. It can generate extremely cytotoxic hydroxyl radicals, causing significant oxidative impairment and cell killing. The CDT relied on the Fenton catalysts rather than oxygen or external energy input.196 To create hydroxyl free radicals for CDT, a nanoparticle DMH NPs that could undergo a Fenton-like reaction in the presence of H2O2 was designed.197 According to research, chemotherapy and CDT together could effectively cause the death of MCF-7 cells, improve anticancer activity, and lower cytotoxicity.197 The R848 (an immune-regulator) and DOX (a chemotherapeutic) nanoparticle systems were created as a twofold pH-reactive multifunctional structure. HA-DOX/PHIS/R848 NPs dramatically suppressed cancer expansion by modulating tumour resistance and destroying tumour cells.198 SWCNTs conjugated with PEG and thioaptamer were synthesized,199 and their impact on targeted PTT for BC was studied both in vitro and in vivo. The results demonstrated that SWCNT-PEG-TA dramatically reduced the viability of the cells. Another study investigated MDA-MB-231 cell death induced by SWCNTs-Dox.200 NIR irradiation sped up the release of DOX from SWCNTs. The release of DOX from SWNTs-DOX was found to be efficiently localized into the nucleus of MDA-MB-231 cells, accumulate within the cells at high concentrations, and encourage programmed cell death through mitochondrial interruption and production of reactive oxygen species (ROS) (Fig. 4, Table 3).
Table 3.
Therapeutic Approaches for the use of nanoparticles in cancer treatment
|
Therapeutic approach
|
Nanoparticle/Plam
|
Loaded agents
|
In vitro/in vivo model
|
Outcome
|
Reference
|
| Combination with photothermal therapy (PTT) |
Layered Double Hydroxide Nanoparticle |
Doxorubicin, CpG, Indocyanine Green (Photothermal Agent) |
4T1 BC Mouse Model |
Elimination of primary tumor, avert tumor relapse and metastasis |
201
|
| Nanoparticle with Glycol Chitosan and IR 820 |
Glycol Chitosan, IR 820 (Photothermal Agent) |
- |
Similar antitumor effects |
202
|
| LTZ-BBR@AA-AuNPs. |
Entrapment of both LTZ and BBR |
MDA-MB-231 |
MDA-MB-231 cytotoxicity against cells. |
203
|
| Fe-rGO/DOX |
DOX |
MDA-MB-231 |
Fe-rGO/DOX effectiveness for PTT |
204
|
| (IR780/1-methyl-tryptophan loaded PAMAM dendrimer |
(IR780/1-methyl-tryptophan |
4T1 Tumor Model |
The (PDT)/(PTT) activity of IR780 and IDO which inhibit the pathway |
205
|
| Combination with PDT |
Bi2Se3-RSL3/diABZi (DP-HBN/RA) |
FITC-labeled DP-HBN/RA |
TNBC |
nanomedicine enhances antitumor therapy by inducing X-ray radiation |
206
|
| FCSP@DOX MOFs |
DOX |
4T1 and MC3T3-E1 cells |
TME-activated MOFs for non-apoptotic ferroptosis therapy |
207
|
| Ce6/PTX-H/P NPs |
chlorin e6 (Ce6) and PTX. |
B16F10, FaDu, and HEK-293 cell lines |
Ce6/PTX-H/P NPs exhibited photodynamic therapeutic effects in solid tumors. |
208
|
| Zinc Phthalocyanine Photosensitizer via Mitochondria-Targeted Nanoparticles |
Zinc Phthalocyanine |
- |
Significant improvement in BC treatment |
209,210
|
| GO-PEG-HPPH |
64Cu labeling of HPPH |
4T1 Murine BC Model and Athymic nude mice |
Nanoformulation Stimulate the tumor immunity and inhibits growth and metastasis |
211
|
| Chemo dynamic therapy (CDT) |
SNPs@ZrMOF@RB |
Soft X-ray ligh |
4T1 |
Tumor growth
inhibition |
212
|
| DOX@FA-RHPs-SA |
RHPs and DOX |
- |
chemo-immunotherapy for TNBC to enhance tumor targeting |
213
|
| Other approaches |
SWCNTs Conjugated with PEG and Thioaptamer |
- |
In Vitro and In Vivo |
Targeted PTT for human BC |
214
|
| SWCNTs-DOX-HA |
Doxorubicin |
MDA-MB-231 Cell Death |
Nanomaterials effect to inhibit the growth of cancer cells |
215
|
Potential toxicity of co-delivery system
A few nanocarriers may have the potential to be harmful to healthy tissues, resulting in their inflammation and other unintentional side effects. They may cause an immunological reaction, due to the formation of antibodies, thereby reducing their efficacy. When several medications are administered together, there may be unanticipated interactions that change the toxicity or effectiveness of the medications. To ensure the safety of the nanocarrier and build up in the body, their ability to degrade and eliminate must be meticulously considered. The biological distribution, cellular absorption, and possible cytotoxicity of nanoparticles can all be influenced by their size and form. When nanoparticles' surfaces are altered by adding targeted ligands, for example, new hazards such as toxicity and immunogenicity may arise. Moreover, Co-delivery nano system design and manufacturing offer a challenge in scaling the production and maintaining the quality.216,217
Strategies to reduce toxicity
Selecting biocompatible and biodegradable materials
Biologically compatible and biodegradable nanocarriers can mitigate the toxicity and drug resistance by covalently binding with the targeting moieties. They can also be used in conjunction with synthesized chemotherapeutic drugs and an herbal anticancer bioactive chemical.218
Optimized formulation
Nanoparticles' size, shape, and surface characteristics impact the ability of the nanoparticle to functioning. Developments in AI and machine learning may forecast how changes in NP formulations' size, shape, and surface alterations would impact their behavior in biological systems.219
Targeted and controlled delivery
Targeting cancer cells with ligands or antibodies can increase therapy effectiveness and lessen the time duration to which medications are exposed to healthy tissues. As an extra layer of protection, the nanocarrier guarantees that the dual drug may be enveloped in the ideal amount, enhances the stability and solubility of hydrophobic pharmaceuticals, and lowers the drug removal rate in vivo to increase bioavailability. Through increased infiltration and retention (EPR) effects, delivery nanosystems can be passively tailored to tumor tissue, facilitating the penetration and build-up of anticancer medicines in specific locations. By altering ligands on nanoparticles to recognize specific cell surface receptors, active targeting is an additional technique to enhance absorption capability and lessen injury to healthy tissues during chemotherapy, hence increasing delivery efficiency.181 Nanoparticles, drug delivery systems with controlled medicine distribution in the body, drop the possibility of damage and improve therapeutic outcome.220
Extensive testing
Extensive pre-clinical and clinical testing is required to gauge the safety and efficiency of treatments based on nanoparticles. These nanoparticles can be engineered to deliver the therapeutic elements in a controlled manner to guarantee an effective and long-lasting treatment option.221
Personalized medicine
By customizing treatments to each patient's unique profile, machine learning models can optimize NP development for cancer subtypes or pathways of resistance. This will boost results and cut down on complications.222
Nanomedicine target to inflammatory signalling pathways in breast cancer
The incapacity of chemotherapy to distinguish between malignant and normal cells has led to a new focus on developing targeted medications that are more effective, less toxic, and more precisely tailored to target malignant cells. A variety of strategies are being studied to reduce the inflammatory response in cancer treatment, including small-molecule inhibitors, organic biomolecules, composite cytokines, local radiation exposure, counterbalancing antibodies, oncolytic viruses, Toll-like receptor agonists, and specialized pro-resolving lipid mediators.
Preclinical research has revealed that tackling inflammatory responses can successfully inhibit carcinogenesis and metastasis thus making it a beneficial approach for treatment for people suffering from BC. Furthermore, genetic alterations in proteins that control cell proliferation and stop the TGF-β pathway in inflammatory BC. More studies are required to determine its biological activity and therapeutic benefits in chronic BC. In terms of clinical use, therapies that affect the JAK2 and EGFR pathways are perhaps preferred the most.223
NPs are perfect for targeting BC cells because they can bind selectively to appropriate molecular indicators on the exterior of individual BC cells by binding ligands on their interface. The association between ligand and NP engaged with BC receptors, such as HER2, EGFR, VEGFR, and IGF-IR causes NP absorption through endocytosis and delivers the associated proteins to the tumour cells' active regions through proteolytic degradation.224 The main objective of nanomedicine is to target tumours with medications and phytopharmaceuticals using different nanosized carriers such as liposomes, NPs, polymers, micelles, and conjugates of NPs.188 There are two mechanisms by which nanomedicine functions. One method, known as active targeting, involves covalently attaching drugs to the targeted receptor via linkers. This process is necessary for the drugs to be identified by tumour cells. The other uses the greater penetration and retention effect and is known as passive targeting.225 Stability and medication release rate are the key variables for the targeted tumour cell. A variety of imaging methods, including fluorescence, PET, NIR luminescence, MRI, gamma cameras, and fluorescence, are used to quantify the absorption and uptake of medications in cellular tissues and fluids.
The capability to modify the biodistribution of diverse agents has resulted in improvement of therapeutic indices, primarily through the formulation of liposome medicines. They are flexible containers for drugs that may regulate vesicle residence in the body's systemic circulation or other compartments, manage the persistence of encapsulated pharmaceuticals in biological fluids, and improve vesicle uptake by target cells. Liposomes continue to be the most effective nanomedicine vehicle for anticancer drugs. The basic example of theranostics is the mixing of medications and imaging agents for diagnostic purposes.226 Conjugating anti-EGFR mAb cetuximab (C225, ErbituxTM) to the outer surface of DOX-loaded liposomes produced C225-ILs-DOX, an EGFR-targeted immunoliposome (IL). When EGFR-overexpressing cells were exposed to C225-ILs-DOX in vitro, they internalized and bound to the cells more effectively than non-targeted liposomes. Furthermore, in EGFR-overexpressing MDA-MB-468 BC cells, C225-ILs-DOX demonstrated twenty-nine times more cytotoxicity compared to the similar nontargeted liposomal DOX.227 With both EGFR and EGFRv III overexpressed in the U87/EGFRv III tumor model, C225-ILs-DOX exhibited a six-fold increase in cellular accumulation in comparison to non-targeted liposomes. Furthermore, EGFR-overexpressing tumour xenograft models, C225-ILs-DOX demonstrated a markedly improved antitumor activity in comparison to the nontargeted liposomal DOX.228 By overturning the NO-NOS system, SiO2NP inhibits the PI3K-AKT-mTOR signaling pathway and causes malignant cells to undergo an allergic reaction that ends in apoptosis.229 Novel theranostic principles depending on the medium-sensitive plasmonic absorption that causes infrared light and visible light-induced collective oscillation of the electrons on the gold surface when the NP dimension is much smaller compared to the wavelength of light have introduced gold NPs (GNPs).230,231 Photo-thermal therapy is one of the ways through which GNP plasmons can be helpful to nanomedicine. GNPs offer adaptable scaffolding for identification and array-based "chemical nose" methods of cell surface detection. Clinical studies have been initiated for both passive cancer targeting using PEG and active cancer targeting using covalent bonds to rhTNFα (CYT-6091).232 When it comes to plasmonic properties, the GNP is thought to be more secure and technologically excellent than group 11 elements.233 The ability of Au NPs loaded with quercetin has been reported to successfully limit the epithelial-mesenchymal transition by hindering transcription-repressing agents, such as Snail, Slug, and Twist, as well as angiogenesis. They impact the widespread distribution by inhibiting the EGFR/VEGFR2-assisted pathway and cycle arrest by hampering the EGFR/PI3K/AKT-assisted pathways in BC cell lines.234 Superparamagnetic iron oxide NPs are frequently utilized with magnets for cancer eradication by overheating and MRI, ignoring safety issues.235 Drug molecules are encapsulated by silica and other oxide NPs for delivery.236
Numerous drug molecules comprising of polymers, copolymers, antibodies, aptamers, proteins, and dendrimers, have been thoroughly investigated in the field of nanomedicine.237,238 Clinical trials employ a variety of biodegradable polymers.202 Poor drug loading, burst release, and poor miscibility with the medicines are among the issues with these kinds of polymers.239 Dendrimers possess a great ability to capsule medications and clinical research was done on the micro biocide VivaGel.
In preliminary research and clinical investigations, EGFR mAb-decorated NPs demonstrated improved anticancer activity as well as enhanced tumor-targeting capability. For example, rapamycin-loaded polymeric poly(lactide-co-glycolide) NPs that have anti-EGFR monoclonal antibodies attached to their surface were taken up by MCF-7 cells by over thirteen times greater as compared to unconjugated NPs.240 Researchers created cationic lipid-aided polymeric NPs that are encased with siRNA for the treatment of breast carcinoma stem cells (BCSCs) targeting the oncogene Plk1.240 After that, the BCSCs were successfully. By inhibiting the TGF-β type I receptor and TGF-β signalling, they made it easier for NPs to enter cancer cells.241
Challenges and future directions
Targeted chemotherapy is entering a new era due to cutting-edge treatments and methods in innovative drug delivery systems. Nanotechnology research is becoming more widespread in the pharmaceutical and medical device industries. In recent years, new systems—targeted NPs in particular—have helped to lower the incidence of BC as well as the rate of morbidity and mortality related to the disease. Encasing numerous powerful anticancer medications in NPs can enhance their therapeutic index in BC.242
However, the development of protein corona, rapid renal and hepatic clearance, existence of tumour-associated macrophages, poor tumour blood perfusion are significant physiological obstacles faced by nanostructure-based drug delivery compounds that can drastically change the action and targeting capacity of NPs shortly after they are administered in the blood. BC cells frequently have leaky blood arteries with wide spaces amongst endothelial cells which enable NPs to escape into the tumour tissue. Elevated interstitial fluid pressure inside the tumour may prevent more permeation. The tumour’s intricate extracellular matrix network may make it more difficult for NPs to diffuse and migrate toward cancer cells. This influences the effectiveness of nanomedicines by initiating premature removal from the body, impeding their competence to impact the intended target tissues. Size optimization, surface alteration, selection of biocompatible materials, stimuli responsive designs, active targeting through ligand conjugation are the approaches that can be effectively used to reduce these barriers.243,244 Despite the significant progress, challenges such as long-term safety, regulatory approvals, and scalability of production persist. To overcome these challenges, collaboration amongst researchers, clinicians, and regulatory bodies are crucial.
Customized and targeted drug delivery by polymeric nanoparticles, aims to overcome the challenges of managing TNBC. Enhanced tumour penetration to gather at specific areas and ligand-mediated active targeting to increase selectivity were employed. Encapsulated chemotherapeutics, such PTX and curcumin, exhibit better anticancer effect if administered intratumorally in controlled, and extended settings. By employing novel copolymers or drug conjugates, the polymer range can be expanded to improve drug encapsulation, stability, and tumor penetration. By integrating gene therapies, imaging agents, or triggering stimuli responsiveness into polymeric nanoparticles, it is also conceivable to address inherent characteristic and acquired medicine resistance in TNBC while monitoring results.245
Using cyclic dinucleotide (CDN), stimulator of interferon genes (STING) agonists and Mn2+, the researchers created a novel self-assembling nanoparticle that can activate the body's immune system. There are certain limitations to the concept of development of nanoparticle-based diagnostic imaging precision as well as real-time effectiveness of treatment.246 Strict safety assessments are necessary to avoid the potential hazards such as systemic toxicity and immunological responses resulting from nanoparticle contact with biological systems.247 The prolonged toxicity of nanomaterials, their influence on immune functioning, pharmaceutical stability issues, reproducing uniform NPs batches, and various other issues need to be tackled prior to the implementation of nanomedicines in clinical practice for management of BC illness. Future directions include the development of personalized nanomedicine approaches through the assimilation of artificial intelligence for treatment optimization and exploring the potential of immunotherapies in combination with nanotechnology.
Conclusion
In the last few years, nanotechnology has become a game-changer in dealing with BC. When it is used alone or in conjunction with additional therapeutic modalities, nanomedicine can be a helpful tool in the treatment of BC. To achieve focused drug delivery, a diverse range of NPs including liposomes, polymeric nanoparticles, polymeric micelles, dendrimers, carbon nanotubes, etc have been studied. In patients having advanced or metastatic TNBC who test positive for PD-L1, a phase I clinical trial has started to assess the combination therapy of atezolizumab (an anti-PD-L1 monoclonal antibody) and nanoparticle albumin-bound PTX. A phase II trial using a similar combination therapy was conducted before surgery in patients with TNBC. In patients having advanced or metastatic TNBC, a phase I/Ib study is being conducted to assess the combination treatment of Etrumadenant, an A2a and A2b adenosine receptor antagonist, having pegylated liposomal DOX or albumin-bound PTX.185 Nanomedicines conjugates such as Kadcyla® (also named T-DM1 or Ado-Trastuzumab emtansine), Enhertu®, (Trastuzumab-duocarmycin), RC48, and HT19-MMAF are being administered to attack on HER-2 receptors and they include maytansinoid, deruxtecan, duocarmicyn, or auristatins as antineoplastic molecules. The conjugates like Trodelvy® (named Sacituzumab), Glembatumumab-Vedotin, Ladiratuzumab-vedotin, Cofetuzumab-pelidotin, and PF-06647263 are being used to aim for Trop-2 glycoprotein, NMB glycoprotein, Zinc transporter LIV-1, and Ephrin receptor-4 etc. These medications are used to target TNBC and incorporate camptothecins, calicheamicins, or auristatins drug molecules. Besides antibody–drug conjugates, other active targeted nano systems such as Abraxane® and Nab-rapamycin, which are composed of albumin NPs having paclitaxel and rapamycin, respectively, and a few liposomes (MM-302, C225-ILS-Dox, and MM-310) loaded with DOX or docetaxel and covered by ligands that aim at the Ephrin A2, EPGF, or HER-2 receptors are administered.
This paper reviewed the present difficulties facing traditional BC therapies as well as the possible benefits of using nanotherapeutics in BC treatment. Nanotechnology offers a variety of ways to increase therapeutic efficacy and precision, from improving early detection to facilitating targeted medication administration and developing multimodal imaging. Even though nanomedicines have demonstrated a promising future for the cure of BC, a few challenges continue to be resolved beforehand using nanomedicines in clinical applications. These include apprehensions about the long-standing toxicological consequences of nanomaterials and the impact they have on the body's defenses, complications with medicine’s stability, the reproducibility of uniform batches of NPs etc. In summary, nanomedicine is a useful tool for treating BC either on its own or in conjunction with other therapeutic approaches, and more research and development of more safe and successful nanotechnology-based cancer treatments is required. Sustained investigation and creativity provide the potential to transform treatment for BC and eventually enhance results.
In summary, nanomedicine is a promising approach for managing BC alone or in combination with other therapeutic approaches, but further investigation and development of safer and better therapeutics based on nanotechnology to completely understand their long-term impacts on the body is required. Research is underway to create more specific and biocompatible nanoparticles. Additionally, the use of nanoparticles in combination with other treatment modalities, like immunotherapy and gene therapy, is also being investigated. Another exciting field of study is the creation of customized nanoparticle treatments that are suited to the distinct molecular subtypes of BC.
Review Highlights
What is the current knowledge?
-
Progress in nanomedicine has positioned nanotechnology-based approaches in the forefront of cancer research.
-
Novel drug delivery systems are made to function as carrier systems for drug delivery to targets, improving targeting, decreasing tumor resistance, extending medication circulation time in vivo, and opening novel therapy and preventative options for a variety of illnesses.
-
The therapeutic effectiveness of chemotherapeutics is hindered by chemoresistance and toxicity.
-
Current treatments include surgery, chemotherapy, phototherapy, biological therapy, and Radiation therapy, but with limited efficacy.
What is new here?
-
This review emphasizes on the most recent developments in nanotechnology-driven BC therapy. The article addresses the most recent advancements in drug formulations based on nanoparticles, such as new materials, surface alterations, and functionalization techniques that enhance therapeutic efficacy and bioavailability.
-
The study investigates new strategies for targeted drug delivery, including biomimetic nanoplatforms, stimulus-responsive nanocarriers, and ligand-functionalized nanoparticles, which maximize drug absorption at tumour locations while reducing systemic toxicity.
-
Therapeutic approaches like Combination with PTT, Combination with PDT, CDT for use of NPs in cancer treatment are also being discussed.
-
In contrast to traditional reviews, this work explores how nanotechnology is improving early diagnosis and detection by integrating NPs for real-time monitoring and simultaneous therapy through multimodal imaging approaches.
-
In addition to reviewing basic developments, the article addresses the translational potential, regulatory issues, and opportunities for clinical use of BC treatments based on nanotechnology.
Competing Interests
The authors declare that there is no conflict of interest, financial or otherwise.
Ethical Approval
Not applicable.
Acknowledgements
The authors gratefully acknowledge the contributions of collaborators and co-workers mentioned in the cited references. MSA are thankful to the SGT School of Pharmacy, and SGT University for their support. MB and SR thanks K R Mangalam University Gurgaon, for their support.
References
- Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast 2022; 66:15-23. doi: 10.1016/j.breast.2022.08.010 [Crossref] [ Google Scholar]
- Gupta P, Neupane YR, Parvez S, Kohli K. Recent advances in targeted nanotherapeutic approaches for breast cancer management. Nanomedicine (Lond) 2021; 16:2605-31. doi: 10.2217/nnm-2021-0281 [Crossref] [ Google Scholar]
- Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 2013; 14:1009-19. doi: 10.1016/s1470-2045(13)70301-2 [Crossref] [ Google Scholar]
- Amjad A, Khan IK, Kausar Z, Saeed F, Azhar L, Anwar P. Risk factors in breast cancer progression and current advances in therapeutic approaches to knockdown breast cancer. Clin Med Biochem 2018; 4:137. doi: 10.4172/2471-2663.1000137 [Crossref] [ Google Scholar]
- Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers (Basel) 2021; 13:4287. doi: 10.3390/cancers13174287 [Crossref] [ Google Scholar]
- La Vecchia C, Carioli G. The epidemiology of breast cancer, a summary overview. Epidemiol Biostat Public Health 2018; 15:e12853-2. doi: 10.2427/12853 [Crossref] [ Google Scholar]
- Bredin P, Walshe JM, Denduluri N. Systemic therapy for metastatic HER2-positive breast cancer. Semin Oncol 2020; 47:259-69. doi: 10.1053/j.seminoncol.2020.07.008 [Crossref] [ Google Scholar]
- Gao JJ, Swain SM. Luminal a breast cancer and molecular assays: a review. Oncologist 2018; 23:556-65. doi: 10.1634/theoncologist.2017-0535 [Crossref] [ Google Scholar]
- Schettini F, Prat A. Dissecting the biological heterogeneity of HER2-positive breast cancer. Breast 2021; 59:339-50. doi: 10.1016/j.breast.2021.07.019 [Crossref] [ Google Scholar]
- Derakhshan F, Reis-Filho JS. Pathogenesis of triple-negative breast cancer. Annu Rev Pathol 2022; 17:181-204. doi: 10.1146/annurev-pathol-042420-093238 [Crossref] [ Google Scholar]
- Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res 2020; 22:61. doi: 10.1186/s13058-020-01296-5 [Crossref] [ Google Scholar]
- Garutti M, Pelizzari G, Bartoletti M, Malfatti MC, Gerratana L, Tell G. Platinum salts in patients with breast cancer: a focus on predictive factors. Int J Mol Sci 2019; 20:3390. doi: 10.3390/ijms20143390 [Crossref] [ Google Scholar]
- Reinbolt RE, Mangini N, Hill JL, Levine LB, Dempsey JL, Singaravelu J. Endocrine therapy in breast cancer: the neoadjuvant, adjuvant, and metastatic approach. Semin Oncol Nurs 2015; 31:146-55. doi: 10.1016/j.soncn.2015.02.002 [Crossref] [ Google Scholar]
- Sirhan Z, Thyagarajan A, Sahu RP. The efficacy of tucatinib-based therapeutic approaches for HER2-positive breast cancer. Mil Med Res 2022; 9:39. doi: 10.1186/s40779-022-00401-3 [Crossref] [ Google Scholar]
- Gavas S, Quazi S, Karpiński TM. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res Lett 2021; 16:173. doi: 10.1186/s11671-021-03628-6 [Crossref] [ Google Scholar]
- Kwapisz D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol Immunother 2021; 70:607-17. doi: 10.1007/s00262-020-02736-z [Crossref] [ Google Scholar]
- Sancho-Garnier H, Colonna M. [Breast cancer epidemiology]. Presse Med 2019; 48:1076-84. doi: 10.1016/j.lpm.2019.09.022.[French] [Crossref] [ Google Scholar]
- Tang X, Loc WS, Dong C, Matters GL, Butler PJ, Kester M. The use of nanoparticulates to treat breast cancer. Nanomedicine (Lond) 2017; 12:2367-88. doi: 10.2217/nnm-2017-0202 [Crossref] [ Google Scholar]
- Malik JA, Ahmed S, Jan B, Bender O, Al Hagbani T, Alqarni A. Drugs repurposed: an advanced step towards the treatment of breast cancer and associated challenges. Biomed Pharmacother 2022; 145:112375. doi: 10.1016/j.biopha.2021.112375 [Crossref] [ Google Scholar]
- Mills MN, Figura NB, Arrington JA, Yu HM, Etame AB, Vogelbaum MA. Management of brain metastases in breast cancer: a review of current practices and emerging treatments. Breast Cancer Res Treat 2020; 180:279-300. doi: 10.1007/s10549-020-05552-2 [Crossref] [ Google Scholar]
- Burguin A, Diorio C, Durocher F. Breast cancer treatments: updates and new challenges. J Pers Med 2021; 11:808. doi: 10.3390/jpm11080808 [Crossref] [ Google Scholar]
- Wang R, Billone PS, Mullett WM. Nanomedicine in action: an overview of cancer nanomedicine on the market and in clinical trials. J Nanomater 2013; 2013:629681. doi: 10.1155/2013/629681 [Crossref] [ Google Scholar]
- Feng SS, Mei L, Anitha P, Gan CW, Zhou W. Poly(lactide)-vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of docetaxel. Biomaterials 2009; 30:3297-306. doi: 10.1016/j.biomaterials.2009.02.045 [Crossref] [ Google Scholar]
- Lee SH, Zhang Z, Feng SS. Nanoparticles of poly(lactide)-tocopheryl polyethylene glycol succinate (PLA-TPGS) copolymers for protein drug delivery. Biomaterials 2007; 28:2041-50. doi: 10.1016/j.biomaterials.2007.01.003 [Crossref] [ Google Scholar]
- Banthia P, Gambhir L, Sharma A, Daga D, Kapoor N, Chaudhary R. Nano to rescue: repository of nanocarriers for targeted drug delivery to curb breast cancer. 3 Biotech 2022; 12:70. doi: 10.1007/s13205-022-03121-6 [Crossref] [ Google Scholar]
- Fan D, Cao Y, Cao M, Wang Y, Cao Y, Gong T. Nanomedicine in cancer therapy. Signal Transduct Target Ther 2023; 8:293. doi: 10.1038/s41392-023-01536-y [Crossref] [ Google Scholar]
- Barrueto L, Caminero F, Cash L, Makris C, Lamichhane P, Deshmukh RR. Resistance to checkpoint inhibition in cancer immunotherapy. Transl Oncol 2020; 13:100738. doi: 10.1016/j.tranon.2019.12.010 [Crossref] [ Google Scholar]
- Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P. Harnessing the immune system to improve cancer therapy. Ann Transl Med 2016; 4:261. doi: 10.21037/atm.2016.04.01 [Crossref] [ Google Scholar]
- Pan J, Fei CJ, Hu Y, Wu XY, Nie L, Chen J. Current understanding of the cGAS-STING signaling pathway: structure, regulatory mechanisms, and related diseases. Zool Res 2023; 44:183-218. doi: 10.24272/j.issn.2095-8137.2022.464 [Crossref] [ Google Scholar]
- Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AK, Sevimli S. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol 2019; 14:269-78. doi: 10.1038/s41565-018-0342-5 [Crossref] [ Google Scholar]
- Wang-Bishop L, Wehbe M, Shae D, James J, Hacker BC, Garland K. Potent STING activation stimulates immunogenic cell death to enhance antitumor immunity in neuroblastoma. J Immunother Cancer 2020; 8:e000282. doi: 10.1136/jitc-2019-000282 [Crossref] [ Google Scholar]
- Wehbe M, Wang-Bishop L, Becker KW, Shae D, Baljon JJ, He X. Nanoparticle delivery improves the pharmacokinetic properties of cyclic dinucleotide STING agonists to open a therapeutic window for intravenous administration. J Control Release 2021; 330:1118-29. doi: 10.1016/j.jconrel.2020.11.017 [Crossref] [ Google Scholar]
- Nguyen DC, Shae D, Pagendarm HM, Becker KW, Wehbe M, Kilchrist KV. Amphiphilic polyelectrolyte graft copolymers enhance the activity of cyclic dinucleotide STING agonists. Adv Healthc Mater 2021; 10:e2001056. doi: 10.1002/adhm.202001056 [Crossref] [ Google Scholar]
- Liang J, Wang H, Ding W, Huang J, Zhou X, Wang H. Nanoparticle-enhanced chemo-immunotherapy to trigger robust antitumor immunity. Sci Adv 2020; 6:eabc3646. doi: 10.1126/sciadv.abc3646 [Crossref] [ Google Scholar]
- Huang J, Yang B, Peng Y, Huang J, Wong SH, Bian L, Zhu K, Shuai X, Han S. Nanomedicine‐boosting tumor immunogenicity for enhanced immunotherapy. Adv Funct Mater 2021; 31:2011171. doi: 10.1002/adfm.202011171 [Crossref] [ Google Scholar]
- Zhang J, Cui X, Huang Y, Xu X, Feng C, Li J. Anticancer effect of STING agonist-encapsulated liposomes on breast cancer. Molecules 2023; 28:3740. doi: 10.3390/molecules28093740 [Crossref] [ Google Scholar]
- Le Naour J, Zitvogel L, Galluzzi L, Vacchelli E, Kroemer G. Trial watch: STING agonists in cancer therapy. Oncoimmunology 2020; 9:1777624. doi: 10.1080/2162402x.2020.1777624 [Crossref] [ Google Scholar]
- Meric-Bernstam F, Sweis RF, Hodi FS, Messersmith WA, Andtbacka RHI, Ingham M. Phase I dose-escalation trial of MIW815 (ADU-S100), an intratumoral STING agonist, in patients with advanced/metastatic solid tumors or lymphomas. Clin Cancer Res 2022; 28:677-88. doi: 10.1158/1078-0432.Ccr-21-1963 [Crossref] [ Google Scholar]
- Gil Del Alcazar CR, Alečković M, Polyak K. Immune escape during breast tumor progression. Cancer Immunol Res 2020; 8:422-7. doi: 10.1158/2326-6066.Cir-19-0786 [Crossref] [ Google Scholar]
- Liu CC, Hao DJ, Zhang Q, An J, Zhao JJ, Chen B. Application of bee venom and its main constituent melittin for cancer treatment. Cancer Chemother Pharmacol 2016; 78:1113-30. doi: 10.1007/s00280-016-3160-1 [Crossref] [ Google Scholar]
- Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, Kim YJ. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine 2018; 14:237-246. doi: 10.1016/j.nano.2017.10.013 [Crossref] [ Google Scholar]
- Nguyen TL, Cha BG, Choi Y, Im J, Kim J. Injectable dual-scale mesoporous silica cancer vaccine enabling efficient delivery of antigen/adjuvant-loaded nanoparticles to dendritic cells recruited in local macroporous scaffold. Biomaterials 2020; 239:119859. doi: 10.1016/j.biomaterials.2020.119859 [Crossref] [ Google Scholar]
- Tagde P, Najda A, Nagpal K, Kulkarni GT, Shah M, Ullah O. Nanomedicine-based delivery strategies for breast cancer treatment and management. Int J Mol Sci 2022; 23:2856. doi: 10.3390/ijms23052856 [Crossref] [ Google Scholar]
- Torrisi R, Zuradelli M, Agostinetto E, Masci G, Losurdo A, De Sanctis R, Santoro A. Platinum salts in the treatment of BRCA-associated breast cancer: A true targeted chemotherapy?. Crit Rev Oncol Hematol 2019; 135:66-75. doi: 10.1016/j.critrevonc.2019.01.016 [Crossref] [ Google Scholar]
- Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast cancer: epidemiology and etiology. Cell Biochem Biophys 2015; 72:333-8. doi: 10.1007/s12013-014-0459-6 [Crossref] [ Google Scholar]
- Tanaka T, Decuzzi P, Cristofanilli M, Sakamoto JH, Tasciotti E, Robertson FM. Nanotechnology for breast cancer therapy. Biomed Microdevices 2009; 11:49-63. doi: 10.1007/s10544-008-9209-0 [Crossref] [ Google Scholar]
- Ávalos-Moreno M, López-Tejada A, Blaya-Cánovas JL, Cara-Lupiañez FE, González-González A, Lorente JA. Drug repurposing for triple-negative breast cancer. J Pers Med 2020; 10:200. doi: 10.3390/jpm10040200 [Crossref] [ Google Scholar]
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 2002; 54:631-51. doi: 10.1016/s0169-409x(02)00044-3 [Crossref] [ Google Scholar]
- Howlander N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF. SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute; 2014.
- Rezaianzadeh A, Jalali M, Maghsoudi A, Mokhtari AM, Hassanipour Azgomi S, Dehghani SL. The overall 5-year survival rate of breast cancer among Iranian women: a systematic review and meta-analysis of published studies. Breast Dis 2017; 37:63-8. doi: 10.3233/bd-160244 [Crossref] [ Google Scholar]
- Mu Q, Wang H, Zhang M. Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin Drug Deliv 2017; 14:123-36. doi: 10.1080/17425247.2016.1208650 [Crossref] [ Google Scholar]
- Chinen AB, Guan CM, Ferrer JR, Barnaby SN, Merkel TJ, Mirkin CA. Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem Rev 2015; 115:10530-74. doi: 10.1021/acs.chemrev.5b00321 [Crossref] [ Google Scholar]
- Aminolroayaei F, Shahbazi-Gahrouei S, Khorasani A, Shahbazi-Gahrouei D. A review of imaging methods and recent nanoparticles for breast cancer diagnosis. Information 2023; 15(1):10. doi: 10.3390/info15010010 [Crossref] [ Google Scholar]
- Jia S, Zhang R, Li Z, Li J. Clinical and biological significance of circulating tumor cells, circulating tumor DNA, and exosomes as biomarkers in colorectal cancer. Oncotarget 2017; 8:55632-45. doi: 10.18632/oncotarget.17184 [Crossref] [ Google Scholar]
- Song S, Qin Y, He Y, Huang Q, Fan C, Chen HY. Functional nanoprobes for ultrasensitive detection of biomolecules. Chem Soc Rev 2010; 39:4234-43. doi: 10.1039/c000682n [Crossref] [ Google Scholar]
- Doria G, Conde J, Veigas B, Giestas L, Almeida C, Assunção M. Noble metal nanoparticles for biosensing applications. Sensors (Basel) 2012; 12:1657-87. doi: 10.3390/s120201657 [Crossref] [ Google Scholar]
- Kumar B, Singh S, Skvortsova I, Kumar V. Promising targets in anti-cancer drug development: recent updates. Curr Med Chem 2017; 24:4729-52. doi: 10.2174/0929867324666170331123648 [Crossref] [ Google Scholar]
- He Z, Zhang Y, Feng N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: a review. Mater Sci Eng C Mater Biol Appl 2020; 106:110298. doi: 10.1016/j.msec.2019.110298 [Crossref] [ Google Scholar]
- Waller J, DeStefano K, Chiu B, Jang I, Cole Y, Agyemang C. An update on nanoparticle usage in breast cancer imaging. Nano Sel 2022; 3:1103-11. doi: 10.1002/nano.202100320 [Crossref] [ Google Scholar]
- Elaibi HK, Mutlag FF, Halvaci E, Aygun A, Sen F. Review: comparison of traditional and modern diagnostic methods in breast cancer. Measurement 2025; 242:116258. doi: 10.1016/j.measurement.2024.116258 [Crossref] [ Google Scholar]
- Hull LC, Farrell D, Grodzinski P. Highlights of recent developments and trends in cancer nanotechnology research--view from NCI Alliance for Nanotechnology in Cancer. Biotechnol Adv 2014; 32:666-78. doi: 10.1016/j.biotechadv.2013.08.003 [Crossref] [ Google Scholar]
- Sharifi M, Avadi MR, Attar F, Dashtestani F, Ghorchian H, Rezayat SM. Cancer diagnosis using nanomaterials based electrochemical nanobiosensors. Biosens Bioelectron 2019; 126:773-84. doi: 10.1016/j.bios.2018.11.026 [Crossref] [ Google Scholar]
- Schwaederlé MC, Patel SP, Husain H, Ikeda M, Lanman RB, Banks KC. Utility of genomic assessment of blood-derived circulating tumor DNA (ctDNA) in patients with advanced lung adenocarcinoma. Clin Cancer Res 2017; 23:5101-11. doi: 10.1158/1078-0432.Ccr-16-2497 [Crossref] [ Google Scholar]
- Borghei YS, Hosseini M, Ganjali MR, Hosseinkhani S. A novel BRCA1 gene deletion detection in human breast carcinoma MCF-7 cells through FRET between quantum dots and silver nanoclusters. J Pharm Biomed Anal 2018; 152:81-8. doi: 10.1016/j.jpba.2018.01.014 [Crossref] [ Google Scholar]
- Ibn Sina AA, Carrascosa LG, Liang Z, Grewal YS, Wardiana A, Shiddiky MJ. Epigenetically reprogrammed methylation landscape drives the DNA self-assembly and serves as a universal cancer biomarker. Nat Commun 2018; 9:4915. doi: 10.1038/s41467-018-07214-w [Crossref] [ Google Scholar]
- Chen Y, Chen H, Shi J. In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv Mater 2013; 25:3144-76. doi: 10.1002/adma.201205292 [Crossref] [ Google Scholar]
- Han X, Xu K, Taratula O, Farsad K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019; 11:799-819. doi: 10.1039/C8NR07769J [Crossref] [ Google Scholar]
- Jia Y, Weng Z, Wang C, Zhu M, Lu Y, Ding L. Increased chemosensitivity and radiosensitivity of human breast cancer cell lines treated with novel functionalized single-walled carbon nanotubes. Oncol Lett 2017; 13:206-14. doi: 10.3892/ol.2016.5402 [Crossref] [ Google Scholar]
- Barui S, Cauda V. Multimodal decorations of mesoporous silica nanoparticles for improved cancer therapy. Pharmaceutics 2020; 12:527. doi: 10.3390/pharmaceutics12060527 [Crossref] [ Google Scholar]
- Jiang Y, Jiang Z, Wang M, Ma L. Current understandings and clinical translation of nanomedicines for breast cancer therapy. Adv Drug Deliv Rev 2022; 180:114034. doi: 10.1016/j.addr.2021.114034 [Crossref] [ Google Scholar]
- Gao Z, Ma T, Zhao E, Docter D, Yang W, Stauber RH. Small is smarter: nano MRI contrast agents - advantages and recent achievements. Small 2016; 12:556-76. doi: 10.1002/smll.201502309 [Crossref] [ Google Scholar]
- Tullio C, Salvioni L, Bellini M, Degrassi A, Fiandra L, D'Arienzo M. Development of an effective tumor-targeted contrast agent for magnetic resonance imaging based on Mn/H-ferritin nanocomplexes. ACS Appl Bio Mater 2021; 4:7800-10. doi: 10.1021/acsabm.1c00724 [Crossref] [ Google Scholar]
- Schemberg J, Abbassi AE, Lindenbauer A, Chen LY, Grodrian A, Nakos X. Synthesis of biocompatible superparamagnetic iron oxide nanoparticles (SPION) under different microfluidic regimes. ACS Appl Mater Interfaces 2022; 14:48011-28. doi: 10.1021/acsami.2c13156 [Crossref] [ Google Scholar]
- Yang L, Afshari MJ, Ge J, Kou D, Chen L, Zhou D, et al. Functionalized ultrasmall iron oxide nanoparticles for T1-weighted magnetic resonance imaging of tumor hypoxia. Molecules 2022; 27. doi: 10.3390/molecules27206929.
- Li Y, Zhao X, Liu X, Cheng K, Han X, Zhang Y. A bioinspired nanoprobe with multilevel responsive T1-weighted MR signal-amplification illuminates ultrasmall metastases. Adv Mater 2020; 32:e1906799. doi: 10.1002/adma.201906799 [Crossref] [ Google Scholar]
- Chen L, Gao Y, Ge J, Zhou Y, Yang Z, Li C. A clinically translatable kit for MRI/NMI dual-modality nanoprobes based on anchoring group-mediated radiolabeling. Nanoscale 2023; 15:3991-9. doi: 10.1039/d2nr05988f [Crossref] [ Google Scholar]
- Kievit FM, Stephen ZR, Veiseh O, Arami H, Wang T, Lai VP. Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional SPIONs. ACS Nano 2012; 6:2591-601. doi: 10.1021/nn205070h [Crossref] [ Google Scholar]
- Riveira-Martin M, Rodríguez-Ruiz A, Martí R, Chevalier M. Multi-vendor robustness analysis of a commercial artificial intelligence system for breast cancer detection. J Med Imaging (Bellingham) 2023; 10:051807. doi: 10.1117/1.Jmi.10.5.051807 [Crossref] [ Google Scholar]
- Yang Y, Liu Z, Huang J, Sun X, Ao J, Zheng B. Histological diagnosis of unprocessed breast core-needle biopsy via stimulated Raman scattering microscopy and multi-instance learning. Theranostics 2023; 13:1342-54. doi: 10.7150/thno.81784 [Crossref] [ Google Scholar]
- Tower A, Hughes J, Moore L, Srivastava K. Mixed metaplastic carcinoma of the breast: a case report. J Surg Case Rep 2023; 2023:rjad144. doi: 10.1093/jscr/rjad144 [Crossref] [ Google Scholar]
- Bobowicz M, Rygusik M, Buler J, Buler R, Ferlin M, Kwasigroch A. Attention-based deep learning system for classification of breast lesions-multimodal, weakly supervised approach. Cancers (Basel) 2023; 15:2704. doi: 10.3390/cancers15102704 [Crossref] [ Google Scholar]
- Li Y, Wang J, Wientjes MG, Au JL. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv Drug Deliv Rev 2012; 64:29-39. doi: 10.1016/j.addr.2011.04.006 [Crossref] [ Google Scholar]
- Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, Zhou QA. Exosomes nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano 2022; 16:17802-17846. doi: 10.1021/acsnano.2c08774 [Crossref] [ Google Scholar]
- Adair JH, Parette MP, Altinoğlu EI, Kester M. Nanoparticulate alternatives for drug delivery. ACS Nano 2010; 4:4967-70. doi: 10.1021/nn102324e [Crossref] [ Google Scholar]
- Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res 2013; 73:2412-7. doi: 10.1158/0008-5472.Can-12-4561 [Crossref] [ Google Scholar]
- Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev 2011; 63:131-5. doi: 10.1016/j.addr.2010.03.011 [Crossref] [ Google Scholar]
- Wei G, Wang Y, Yang G, Wang Y, Ju R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics 2021; 11:6370-92. doi: 10.7150/thno.57828 [Crossref] [ Google Scholar]
- Abdul Moti LA, Hussain Z, Thu HE, Khan S, Sohail M, Sarfraz RM. Multi-functionalization, a promising adaptation to overcome challenges to clinical translation of nanomedicines as nano-diagnostics and nano-therapeutics for breast cancer. Curr Pharm Des 2021; 27:4356-75. doi: 10.2174/1381612827666210830092539 [Crossref] [ Google Scholar]
- Fraguas-Sánchez AI, Lozza I, Torres-Suárez AI. Actively targeted nanomedicines in breast cancer: from pre-clinal investigation to clinic. Cancers (Basel) 2022; 14:1198. doi: 10.3390/cancers14051198 [Crossref] [ Google Scholar]
- Dilliard SA, Siegwart DJ. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat Rev Mater 2023; 8:282-300. doi: 10.1038/s41578-022-00529-7 [Crossref] [ Google Scholar]
- Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res 2004; 10:3708-16. doi: 10.1158/1078-0432.Ccr-03-0655 [Crossref] [ Google Scholar]
- Chu E. Physicians' Cancer Chemotherapy Drug Manual 2017. Jones & Bartlett Learning; 2016.
- Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol). Clin Cancer Res 2005; 11:4136-43. doi: 10.1158/1078-0432.Ccr-04-2291 [Crossref] [ Google Scholar]
- Anhorn MG, Wagner S, Kreuter J, Langer K, von Briesen H. Specific targeting of HER2 overexpressing breast cancer cells with doxorubicin-loaded trastuzumab-modified human serum albumin nanoparticles. Bioconjug Chem 2008; 19:2321-31. doi: 10.1021/bc8002452 [Crossref] [ Google Scholar]
- Barenholz Y. Doxil®--the first FDA-approved nano-drug: lessons learned. J Control Release 2012; 160:117-34. doi: 10.1016/j.jconrel.2012.03.020 [Crossref] [ Google Scholar]
- Tang J, Zhang L, Gao H, Liu Y, Zhang Q, Ran R. Co-delivery of doxorubicin and P-gp inhibitor by a reduction-sensitive liposome to overcome multidrug resistance, enhance anti-tumor efficiency and reduce toxicity. Drug Deliv 2016; 23:1130-43. doi: 10.3109/10717544.2014.990651 [Crossref] [ Google Scholar]
- Peng J, Chen J, Xie F, Bao W, Xu H, Wang H. Herceptin-conjugated paclitaxel loaded PCL-PEG worm-like nanocrystal micelles for the combinatorial treatment of HER2-positive breast cancer. Biomaterials 2019; 222:119420. doi: 10.1016/j.biomaterials.2019.119420 [Crossref] [ Google Scholar]
- Li Z, Huang H, Tang S, Li Y, Yu XF, Wang H. Small gold nanorods laden macrophages for enhanced tumor coverage in photothermal therapy. Biomaterials 2016; 74:144-54. doi: 10.1016/j.biomaterials.2015.09.038 [Crossref] [ Google Scholar]
- Heo DN, Yang DH, Moon HJ, Lee JB, Bae MS, Lee SC. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials 2012; 33:856-66. doi: 10.1016/j.biomaterials.2011.09.064 [Crossref] [ Google Scholar]
- Sun B, Ranganathan B, Feng SS. Multifunctional poly(D,L-lactide-co-glycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by trastuzumab for targeted chemotherapy of breast cancer. Biomaterials 2008; 29:475-86. doi: 10.1016/j.biomaterials.2007.09.038 [Crossref] [ Google Scholar]
- Al Faraj A, Shaik AP, Shaik AS. Magnetic single-walled carbon nanotubes as efficient drug delivery nanocarriers in breast cancer murine model: noninvasive monitoring using diffusion-weighted magnetic resonance imaging as sensitive imaging biomarker. Int J Nanomedicine 2015; 10:157-68. doi: 10.2147/ijn.S75074 [Crossref] [ Google Scholar]
- Taherian A, Esfandiari N. Nanomedicine, a new therapeutic strategy in breast cancer treatment. Arch Breast Cancer 2019; 6:67-78. doi: 10.32768/abc.20196267-78 [Crossref] [ Google Scholar]
- Shao W, Paul A, Rodes L, Prakash S. A new carbon nanotube-based breast cancer drug delivery system: preparation and in vitro analysis using paclitaxel. Cell Biochem Biophys 2015; 71:1405-14. doi: 10.1007/s12013-014-0363-0 [Crossref] [ Google Scholar]
- Singh SK, Singh S, Lillard JW Jr, Singh R. Drug delivery approaches for breast cancer. Int J Nanomedicine 2017; 12:6205-18. doi: 10.2147/ijn.S140325 [Crossref] [ Google Scholar]
- Guo XL, Kang XX, Wang YQ, Zhang XJ, Li CJ, Liu Y. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater 2019; 84:367-77. doi: 10.1016/j.actbio.2018.12.007 [Crossref] [ Google Scholar]
- Fraguas-Sánchez AI, Martín-Sabroso C, Fernández-Carballido A, Torres-Suárez AI. Current status of nanomedicine in the chemotherapy of breast cancer. Cancer Chemother Pharmacol 2019; 84:689-706. doi: 10.1007/s00280-019-03910-6 [Crossref] [ Google Scholar]
- Oshiro-Júnior JA, Rodero C, Hanck-Silva G, Sato MR, Alves RC, Eloy JO. Stimuli-responsive drug delivery nanocarriers in the treatment of breast cancer. Curr Med Chem 2020; 27:2494-513. doi: 10.2174/0929867325666181009120610 [Crossref] [ Google Scholar]
- Gote V, Nookala AR, Bolla PK, Pal D. Drug resistance in metastatic breast cancer: tumor targeted nanomedicine to the rescue. Int J Mol Sci 2021; 22:4673. doi: 10.3390/ijms22094673 [Crossref] [ Google Scholar]
- Li J, Wang Y, Xu C, Yu Q, Wang X, Xie H. Rapid pH-responsive self-disintegrating nanoassemblies balance tumor accumulation and penetration for enhanced anti-breast cancer therapy. Acta Biomater 2021; 134:546-58. doi: 10.1016/j.actbio.2021.04.022 [Crossref] [ Google Scholar]
- Yao Y, Saw PE, Nie Y, Wong PP, Jiang L, Ye X. Multifunctional sharp pH-responsive nanoparticles for targeted drug delivery and effective breast cancer therapy. J Mater Chem B 2019; 7:576-85. doi: 10.1039/c8tb02600a [Crossref] [ Google Scholar]
- Chen Q, Huang X, Zhang G, Li J, Liu Y, Yan X. Novel targeted pH-responsive drug delivery systems based on PEGMA-modified bimetallic Prussian blue analogs for breast cancer chemotherapy. RSC Adv 2023; 13:1684-700. doi: 10.1039/d2ra06631a [Crossref] [ Google Scholar]
- Zhang Y, Ye Z, He R, Li Y, Xiong B, Yi M. Bovine serum albumin-based and dual-responsive targeted hollow mesoporous silica nanoparticles for breast cancer therapy. Colloids Surf B Biointerfaces 2023; 224:113201. doi: 10.1016/j.colsurfb.2023.113201 [Crossref] [ Google Scholar]
- Maddiboyina B, Roy H, Nakkala RK, Gandhi S, Kavisri M, Moovendhan M. Formulation, optimization and characterization of raloxifene hydrochloride loaded PLGA nanoparticles by using Taguchi design for breast cancer application. Chem Biol Drug Des 2023; 102:457-70. doi: 10.1111/cbdd.14222 [Crossref] [ Google Scholar]
- Subhan MA, Torchilin VP. Biopolymer-based nanosystems for siRNA drug delivery to solid tumors including breast cancer. Pharmaceutics 2023; 15:153. doi: 10.3390/pharmaceutics15010153 [Crossref] [ Google Scholar]
- Galvan Morales MA, Barrera Rodriguez R, Santiago Cruz JR, Teran LM. Overview of new treatments with immunotherapy for breast cancer and a proposal of a combination therapy. Molecules 2020; 25:5686. doi: 10.3390/molecules25235686 [Crossref] [ Google Scholar]
- Peng Y, Nie J, Cheng W, Liu G, Zhu D, Zhang L. A multifunctional nanoplatform for cancer chemo-photothermal synergistic therapy and overcoming multidrug resistance. Biomater Sci 2018; 6:1084-1098. doi: 10.1039/C7BM01206C [Crossref] [ Google Scholar]
- Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res 2002; 4:95-9. doi: 10.1186/bcr432 [Crossref] [ Google Scholar]
- Nel J, Elkhoury K, Velot É, Bianchi A, Acherar S, Francius G. Functionalized liposomes for targeted breast cancer drug delivery. Bioact Mater 2023; 24:401-37. doi: 10.1016/j.bioactmat.2022.12.027 [Crossref] [ Google Scholar]
- Yang B, Song BP, Shankar S, Guller A, Deng W. Recent advances in liposome formulations for breast cancer therapeutics. Cell Mol Life Sci 2021; 78:5225-43. doi: 10.1007/s00018-021-03850-6 [Crossref] [ Google Scholar]
- Misiak P, Niemirowicz-Laskowska K, Markiewicz KH, Wielgat P, Kurowska I, Czarnomysy R. Doxorubicin-loaded polymeric nanoparticles containing ketoester-based block and cholesterol moiety as specific vehicles to fight estrogen-dependent breast cancer. Cancer Nanotechnol 2023; 14:23. doi: 10.1186/s12645-023-00176-9 [Crossref] [ Google Scholar]
- Junnuthula V, Kolimi P, Nyavanandi D, Sampathi S, Vora LK, Dyawanapelly S. Polymeric micelles for breast cancer therapy: recent updates, clinical translation and regulatory considerations. Pharmaceutics 2022; 14:1860. doi: 10.3390/pharmaceutics14091860 [Crossref] [ Google Scholar]
- Oerlemans C, Bult W, Bos M, Storm G, Nijsen JF, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res 2010; 27:2569-89. doi: 10.1007/s11095-010-0233-4 [Crossref] [ Google Scholar]
- Chaudhuri A, Ramesh K, Kumar DN, Dehari D, Singh S, Kumar D. Polymeric micelles: a novel drug delivery system for the treatment of breast cancer. J Drug Deliv Sci Technol 2022; 77:103886. doi: 10.1016/j.jddst.2022.103886 [Crossref] [ Google Scholar]
- Kesharwani P, Chadar R, Shukla R, Jain GK, Aggarwal G, Abourehab MAS. Recent advances in multifunctional dendrimer-based nanoprobes for breast cancer theranostics. J Biomater Sci Polym Ed 2022; 33:2433-71. doi: 10.1080/09205063.2022.2103627 [Crossref] [ Google Scholar]
- Bober Z, Bartusik-Aebisher D, Aebisher D. Application of dendrimers in anticancer diagnostics and therapy. Molecules 2022; 27:3237. doi: 10.3390/molecules27103237 [Crossref] [ Google Scholar]
- Dubey SK, Kali M, Hejmady S, Saha RN, Alexander A, Kesharwani P. Recent advances of dendrimers as multifunctional nano-carriers to combat breast cancer. Eur J Pharm Sci 2021; 164:105890. doi: 10.1016/j.ejps.2021.105890 [Crossref] [ Google Scholar]
- Rajana N, Mounika A, Chary PS, Bhavana V, Urati A, Khatri D. Multifunctional hybrid nanoparticles in diagnosis and therapy of breast cancer. J Control Release 2022; 352:1024-47. doi: 10.1016/j.jconrel.2022.11.009 [Crossref] [ Google Scholar]
- Kilicay E, Erdal E, Elci P, Hazer B, Denkbas EB. Tumour-specific hybrid nanoparticles in therapy of breast cancer. J Microencapsul 2024; 41:45-65. doi: 10.1080/02652048.2023.2292226 [Crossref] [ Google Scholar]
- Safaei S, Fadaee M, Rahbar Farzam O, Yari A, Poursaei E, Aslan C. Exploring the dynamic interplay between exosomes and the immune tumor microenvironment: implications for breast cancer progression and therapeutic strategies. Breast Cancer Res 2024; 26:57. doi: 10.1186/s13058-024-01810-z [Crossref] [ Google Scholar]
- Bondhopadhyay B, Sisodiya S, Alzahrani FA, Bakhrebah MA, Chikara A, Kasherwal V. Exosomes: a forthcoming era of breast cancer therapeutics. Cancers (Basel) 2021; 13:4672. doi: 10.3390/cancers13184672 [Crossref] [ Google Scholar]
- Haider M, Abdin SM, Kamal L, Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics 2020; 12:288. doi: 10.3390/pharmaceutics12030288 [Crossref] [ Google Scholar]
- Rahman M, Afzal O, Ullah SN, Alshahrani MY, Alkhathami AG, Altamimi AS. Nanomedicine-based drug-targeting in breast cancer: pharmacokinetics, clinical progress, and challenges. ACS Omega 2023; 8:48625-48649. doi: 10.1021/acsomega.3c07345 [Crossref] [ Google Scholar]
- Rathee J, Kanwar R, Kumari L, Pawar SV, Sharma S, Ali ME. Development of nanostructured lipid carriers as a promising tool for methotrexate delivery: physicochemical and in vitro evaluation. J Biomol Struct Dyn 2023; 41:2747-58. doi: 10.1080/07391102.2022.2037465 [Crossref] [ Google Scholar]
- Sahib AS, Wennas ON, Mahdi BW, Al Abood RM. In vivo antitumor activity study of targeted chlorambucil-loaded nanolipid carrier for breast cancer. Pharmacia 2022; 69:631-6. doi: 10.3897/pharmacia.69.e85390 [Crossref] [ Google Scholar]
- Badr-Eldin SM, Aldawsari HM, Fahmy UA, Ahmed OA, Alhakamy NA, Al-Hejaili OD. Optimized apamin-mediated nano-lipidic carrier potentially enhances the cytotoxicity of ellagic acid against human breast cancer cells. Int J Mol Sci 2022; 23:9440. doi: 10.3390/ijms23169440 [Crossref] [ Google Scholar]
- Sartaj A, Annu Annu, Biswas L, Verma AK, Sahoo PK, Baboota S. Ribociclib nanostructured lipid carrier aimed for breast cancer: formulation optimization, attenuating in vitro specification, and in vivo scrutinization. Biomed Res Int 2022; 2022:6009309. doi: 10.1155/2022/6009309 [Crossref] [ Google Scholar]
- Kamel AE, Fadel M, Louis D. Curcumin-loaded nanostructured lipid carriers prepared using PeceolTM and olive oil in photodynamic therapy: development and application in breast cancer cell line. Int J Nanomedicine 2019; 14:5073-85. doi: 10.2147/ijn.S210484 [Crossref] [ Google Scholar]
- Truong TH, Alcantara KP, Bulatao BPI, Sorasitthiyanukarn FN, Muangnoi C, Nalinratana N. Chitosan-coated nanostructured lipid carriers for transdermal delivery of tetrahydrocurcumin for breast cancer therapy. Carbohydr Polym 2022; 288:119401. doi: 10.1016/j.carbpol.2022.119401 [Crossref] [ Google Scholar]
- Thiruchenthooran V, Świtalska M, Bonilla L, Espina M, García ML, Wietrzyk J. Novel strategies against cancer: dexibuprofen-loaded nanostructured lipid carriers. Int J Mol Sci 2022; 23:11310. doi: 10.3390/ijms231911310 [Crossref] [ Google Scholar]
- Chand P, Kumar H, Badduri N, Gupta NV, Bettada VG, Madhunapantula SV. Design and evaluation of cabazitaxel loaded NLCs against breast cancer cell lines. Colloids Surf B Biointerfaces 2021; 199:111535. doi: 10.1016/j.colsurfb.2020.111535 [Crossref] [ Google Scholar]
- Makeen HA, Mohan S, Al-Kasim MA, Sultan MH, Albarraq AA, Ahmed RA. Preparation, characterization, and anti-cancer activity of nanostructured lipid carriers containing imatinib. Pharmaceutics 2021; 13:1086. doi: 10.3390/pharmaceutics13071086 [Crossref] [ Google Scholar]
- Gilani SJ, Bin-Jumah M, Rizwanullah M, Imam SS, Imtiyaz K, Alshehri S. Chitosan coated luteolin nanostructured lipid carriers: optimization, in vitro-ex vivo assessments and cytotoxicity study in breast cancer cells. Coatings 2021; 11:158. doi: 10.3390/coatings11020158 [Crossref] [ Google Scholar]
- Varshosaz J, Davoudi MA, Rasoul-Amini S. Docetaxel-loaded nanostructured lipid carriers functionalized with trastuzumab (Herceptin) for HER2-positive breast cancer cells. J Liposome Res 2018; 28:285-95. doi: 10.1080/08982104.2017.1370471 [Crossref] [ Google Scholar]
- Soni NK, Sonali LJ, Singh A, Mangla B, Neupane YR, Kohli K. Nanostructured lipid carrier potentiated oral delivery of raloxifene for breast cancer treatment. Nanotechnology 2020; 31:475101. doi: 10.1088/1361-6528/abaf81 [Crossref] [ Google Scholar]
- Liu F, Li L, Lan M, Zou T, Kong Z, Cai T. Psoralen-loaded polymeric lipid nanoparticles combined with paclitaxel for the treatment of triple-negative breast cancer. Nanomedicine (Lond) 2021; 16:2411-30. doi: 10.2217/nnm-2021-0241 [Crossref] [ Google Scholar]
- Sargent LM, Shvedova AA, Hubbs AF, Salisbury JL, Benkovic SA, Kashon ML. Induction of aneuploidy by single-walled carbon nanotubes. Environ Mol Mutagen 2009; 50:708-17. doi: 10.1002/em.20529 [Crossref] [ Google Scholar]
- Ou L, Song B, Liang H, Liu J, Feng X, Deng B. Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Part Fibre Toxicol 2016; 13:57. doi: 10.1186/s12989-016-0168-y [Crossref] [ Google Scholar]
- Afzal M, Ameeduzzafar Ameeduzzafar, Alharbi KS, Alruwaili NK, Al-Abassi FA, Al-Malki AAL. Nanomedicine in treatment of breast cancer - a challenge to conventional therapy. Semin Cancer Biol 2021; 69:279-92. doi: 10.1016/j.semcancer.2019.12.016 [Crossref] [ Google Scholar]
- Hashemi M, Yadegari A, Yazdanpanah G, Omidi M, Jabbehdari S, Haghiralsadat F. Normalization of doxorubicin release from graphene oxide: New approach for optimization of effective parameters on drug loading. Biotechnol Appl Biochem 2017; 64:433-42. doi: 10.1002/bab.1487 [Crossref] [ Google Scholar]
- Wei LX. Utilizing carbon nanomaterials for enhanced photothermal therapy in breast cancer treatment. Theor Nat Sci 2024; 50:42-7. doi: 10.54254/2753-8818/50/2024au0122 [Crossref] [ Google Scholar]
- Kong T, Hao L, Wei Y, Cai X, Zhu B. Doxorubicin conjugated carbon dots as a drug delivery system for human breast cancer therapy. Cell Prolif 2018; 51:e12488. doi: 10.1111/cpr.12488 [Crossref] [ Google Scholar]
- Puvvada N, Shaik MA, Samanta D, Shaw M, Mondal I, Basu R. Biocompatible fluorescent carbon nanoparticles as nanocarriers for targeted delivery of tamoxifen for regression of breast carcinoma. Spectrochim Acta A Mol Biomol Spectrosc 2024; 321:124721. doi: 10.1016/j.saa.2024.124721 [Crossref] [ Google Scholar]
- Bura C, Mocan T, Grapa C, Mocan L. Carbon nanotubes-based assays for cancer detection and screening. Pharmaceutics 2022; 14:781. doi: 10.3390/pharmaceutics14040781 [Crossref] [ Google Scholar]
- Alam MS, Garg A, Pottoo FH, Saifullah MK, Tareq AI, Manzoor O. Gum ghatti mediated, one pot green synthesis of optimized gold nanoparticles: Investigation of process-variables impact using Box-Behnken based statistical design. Int J Biol Macromol 2017; 104:758-67. doi: 10.1016/j.ijbiomac.2017.05.129 [Crossref] [ Google Scholar]
- Alam MS, Javed MN, Pottoo FH, Waziri A, Almalki FA, Hasnain MS. QbD approached comparison of reaction mechanism in microwave synthesized gold nanoparticles and their superior catalytic role against hazardous nitro-dye. Appl Organomet Chem 2019; 33:e5071. doi: 10.1002/aoc.5071 [Crossref] [ Google Scholar]
- Granja A, Pinheiro M, Sousa CT, Reis S. Gold nanostructures as mediators of hyperthermia therapies in breast cancer. Biochem Pharmacol 2021; 190:114639. doi: 10.1016/j.bcp.2021.114639 [Crossref] [ Google Scholar]
- Faid AH, Shouman SA, Badr YA, Sharaky M. Enhanced photothermal heating and combination therapy of gold nanoparticles on a breast cell model. BMC Chem 2022; 16:66. doi: 10.1186/s13065-022-00859-1 [Crossref] [ Google Scholar]
- Musielak M, Boś-Liedke A, Piwocka O, Kowalska K, Markiewicz R, Szymkowiak B. The role of functionalization and size of gold nanoparticles in the response of MCF-7 breast cancer cells to ionizing radiation comparing 2D and 3D in vitro models. Pharmaceutics 2023; 15:862. doi: 10.3390/pharmaceutics15030862 [Crossref] [ Google Scholar]
- Mal S, Chakraborty S, Mahapatra M, Pakeeraiah K, Das S, Paidesetty SK. Tackling breast cancer with gold nanoparticles: twinning synthesis and particle engineering with efficacy. Nanoscale Adv 2024; 6:2766-812. doi: 10.1039/d3na00988b [Crossref] [ Google Scholar]
- Poonia N, Lather V, Pandita D. Mesoporous silica nanoparticles: a smart nanosystem for management of breast cancer. Drug Discov Today 2018; 23:315-32. doi: 10.1016/j.drudis.2017.10.022 [Crossref] [ Google Scholar]
- Bharti C, Alam MS, Javed MN, Saifullah MK, Almalki FA, Manchanda R. Silica based nanomaterial for drug delivery. In: Nanomaterials: Evolution and Advancement Towards Therapeutic Drug Delivery (Part II). Bentham Science Publishers. 2021. p. 57-89. doi: 10.2174/9781681088235121010005.
- Rani R, Malik P, Dhania S, Mukherjee TK. Recent advances in mesoporous silica nanoparticle-mediated drug delivery for breast cancer treatment. Pharmaceutics 2023; 15:227. doi: 10.3390/pharmaceutics15010227 [Crossref] [ Google Scholar]
- Thapa R, Ali H, Afzal O, Bhat AA, Almalki WH, Alzarea SI. Unlocking the potential of mesoporous silica nanoparticles in breast cancer treatment. J Nanopart Res 2023; 25:169. doi: 10.1007/s11051-023-05813-3 [Crossref] [ Google Scholar]
- Gu Y, Fei Z. Mesoporous silica nanoparticles loaded with resveratrol are used for targeted breast cancer therapy. J Oncol 2022; 2022:8471331. doi: 10.1155/2022/8471331 [Crossref] [ Google Scholar]
- Akbarzadeh I, Saremi Poor A, Khodarahmi M, Abdihaji M, Moammeri A, Jafari S. Gingerol/letrozole-loaded mesoporous silica nanoparticles for breast cancer therapy: in-silico and in-vitro studies. Microporous Mesoporous Mater 2022; 337:111919. doi: 10.1016/j.micromeso.2022.111919 [Crossref] [ Google Scholar]
- Chang J, Mo L, Song J, Wang X, Liu H, Meng C. A pH-responsive mesoporous silica nanoparticle-based drug delivery system for targeted breast cancer therapy. J Mater Chem B 2022; 10:3375-85. doi: 10.1039/d1tb02828f [Crossref] [ Google Scholar]
- Nitheesh Y, Pradhan R, Hejmady S, Taliyan R, Singhvi G, Alexander A. Surface engineered nanocarriers for the management of breast cancer. Mater Sci Eng C Mater Biol Appl 2021; 130:112441. doi: 10.1016/j.msec.2021.112441 [Crossref] [ Google Scholar]
- Vasudevan D, Gaddam RR, Trinchi A, Cole I. Core–shell quantum dots: properties and applications. J Alloys Compd 2015; 636:395-404. doi: 10.1016/j.jallcom.2015.02.102 [Crossref] [ Google Scholar]
- Fatima I, Rahdar A, Sargazi S, Barani M, Hassanisaadi M, Thakur VK. Quantum dots: synthesis, antibody conjugation, and HER2-receptor targeting for breast cancer therapy. J Funct Biomater 2021; 12:75. doi: 10.3390/jfb12040075 [Crossref] [ Google Scholar]
- Dong L, Li W, Yu L, Sun L, Chen Y, Hong G. Ultrasmall Ag2Te quantum dots with rapid clearance for amplified computed tomography imaging and augmented photonic tumor hyperthermia. ACS Appl Mater Interfaces 2020; 12:42558-66. doi: 10.1021/acsami.0c12948 [Crossref] [ Google Scholar]
- Aires A, Ocampo SM, Simões BM, Josefa Rodríguez M, Cadenas JF, Couleaud P. Multifunctionalized iron oxide nanoparticles for selective drug delivery to CD44-positive cancer cells. Nanotechnology 2016; 27:065103. doi: 10.1088/0957-4484/27/6/065103 [Crossref] [ Google Scholar]
- Semkina AS, Abakumov MA, Skorikov AS, Abakumova TO, Melnikov PA, Grinenko NF. Multimodal doxorubicin loaded magnetic nanoparticles for VEGF targeted theranostics of breast cancer. Nanomedicine 2018; 14:1733-42. doi: 10.1016/j.nano.2018.04.019 [Crossref] [ Google Scholar]
- Chiu CF, Fu RH, Hsu SH, Yu YA, Yang SF, Tsao TC. Delivery capacity and anticancer ability of the berberine-loaded gold nanoparticles to promote the apoptosis effect in breast cancer. Cancers (Basel) 2021; 13:5317. doi: 10.3390/cancers13215317 [Crossref] [ Google Scholar]
- Curcio M, Brindisi M, Cirillo G, Frattaruolo L, Leggio A, Rago V. Smart lipid-polysaccharide nanoparticles for targeted delivery of doxorubicin to breast cancer cells. Int J Mol Sci 2022; 23:2386. doi: 10.3390/ijms23042386 [Crossref] [ Google Scholar]
- Li J, Gong C, Chen X, Guo H, Tai Z, Ding N. Biomimetic liposomal nanozymes improve breast cancer chemotherapy with enhanced penetration and alleviated hypoxia. J Nanobiotechnology 2023; 21:123. doi: 10.1186/s12951-023-01874-7 [Crossref] [ Google Scholar]
- Verma R, Rani V, Kumar M. In-vivo anticancer efficacy of self-targeted methotrexate-loaded polymeric nanoparticles in solid tumor-bearing rat. Int Immunopharmacol 2023; 119:110147. doi: 10.1016/j.intimp.2023.110147 [Crossref] [ Google Scholar]
- Tang X, Kurban M, Hafiz I, Shen Q, Wang M. Preparation of hyaluronic acid-loaded Harmine polymeric micelles and in vitro effect anti-breast cancer. Eur J Pharm Sci 2023; 183:106388. doi: 10.1016/j.ejps.2023.106388 [Crossref] [ Google Scholar]
- Passos JS, Lopes LB, Panitch A. Collagen-binding nanoparticles for paclitaxel encapsulation and breast cancer treatment. ACS Biomater Sci Eng 2023; 9:6805-20. doi: 10.1021/acsbiomaterials.3c01332 [Crossref] [ Google Scholar]
- Aleanizy FS, Alqahtani FY, Seto S, Al Khalil N, Aleshaiwi L, Alghamdi M. Trastuzumab targeted neratinib loaded poly-amidoamine dendrimer nanocapsules for breast cancer therapy. Int J Nanomedicine 2020; 15:5433-43. doi: 10.2147/ijn.S256898 [Crossref] [ Google Scholar]
- Bartusik-Aebisher D, Chrzanowski G, Bober Z, Aebisher D. An analytical study of Trastuzumab-dendrimer-fluorine drug delivery system in breast cancer therapy in vitro. Biomed Pharmacother 2021; 133:111053. doi: 10.1016/j.biopha.2020.111053 [Crossref] [ Google Scholar]
- Bobde Y, Patel T, Paul M, Biswas S, Ghosh B. PEGylated N-(2 hydroxypropyl) methacrylamide polymeric micelles as nanocarriers for the delivery of doxorubicin in breast cancer. Colloids Surf B Biointerfaces 2021; 204:111833. doi: 10.1016/j.colsurfb.2021.111833 [Crossref] [ Google Scholar]
- Nasr M, Hashem F, Teiama M, Tantawy N, Abdelmoniem R. Folic acid grafted mixed polymeric micelles as a targeted delivery strategy for tamoxifen citrate in treatment of breast cancer. Drug Deliv Transl Res 2024; 14:945-58. doi: 10.1007/s13346-023-01443-3 [Crossref] [ Google Scholar]
- Zhang LX, Sun XM, Xu ZP, Liu RT. Development of multifunctional clay-based nanomedicine for elimination of primary invasive breast cancer and prevention of its lung metastasis and distant inoculation. ACS Appl Mater Interfaces 2019; 11:35566-76. doi: 10.1021/acsami.9b11746 [Crossref] [ Google Scholar]
- Kumar P, Srivastava R. IR 820 dye encapsulated in polycaprolactone glycol chitosan: poloxamer blend nanoparticles for photo immunotherapy for breast cancer. Mater Sci Eng C Mater Biol Appl 2015; 57:321-7. doi: 10.1016/j.msec.2015.08.006 [Crossref] [ Google Scholar]
- Luo L, Zhu C, Yin H, Jiang M, Zhang J, Qin B. Laser immunotherapy in combination with perdurable PD-1 blocking for the treatment of metastatic tumors. ACS Nano 2018; 12:7647-62. doi: 10.1021/acsnano.8b00204 [Crossref] [ Google Scholar]
- Wang L, Wang M, Zhou B, Zhou F, Murray C, Towner RA. PEGylated reduced-graphene oxide hybridized with Fe3O4 nanoparticles for cancer photothermal-immunotherapy. J Mater Chem B 2019; 7:7406-14. doi: 10.1039/c9tb00630c [Crossref] [ Google Scholar]
- Wang M, Song J, Zhou F, Hoover AR, Murray C, Zhou B. NIR-triggered phototherapy and immunotherapy via an antigen-capturing nanoplatform for metastatic cancer treatment. Adv Sci (Weinh) 2019; 6:1802157. doi: 10.1002/advs.201802157 [Crossref] [ Google Scholar]
- Aghajanzadeh M, Zamani M, Rajabi Kouchi F, Eixenberger J, Shirini D, Estrada D. Synergic antitumor effect of photodynamic therapy and chemotherapy mediated by nano drug delivery systems. Pharmaceutics 2022; 14:322. doi: 10.3390/pharmaceutics14020322 [Crossref] [ Google Scholar]
- Liu W, Chen B, Zheng H, Xing Y, Chen G, Zhou P. Advances of nanomedicine in radiotherapy. Pharmaceutics 2021; 13:1757. doi: 10.3390/pharmaceutics13111757 [Crossref] [ Google Scholar]
- Pan WL, Tan Y, Meng W, Huang NH, Zhao YB, Yu ZQ. Microenvironment-driven sequential ferroptosis, photodynamic therapy, and chemotherapy for targeted breast cancer therapy by a cancer-cell-membrane-coated nanoscale metal-organic framework. Biomaterials 2022; 283:121449. doi: 10.1016/j.biomaterials.2022.121449 [Crossref] [ Google Scholar]
- Shen X, Li T, Chen Z, Xie X, Zhang H, Feng Y. NIR-light-triggered anticancer strategy for dual-modality imaging-guided combination therapy via a bioinspired hybrid PLGA nanoplatform. Mol Pharm 2019; 16:1367-84. doi: 10.1021/acs.molpharmaceut.8b01321 [Crossref] [ Google Scholar]
- Marrache S, Tundup S, Harn DA, Dhar S. Ex vivo programming of dendritic cells by mitochondria-targeted nanoparticles to produce interferon-gamma for cancer immunotherapy. ACS Nano 2013; 7:7392-402. doi: 10.1021/nn403158n [Crossref] [ Google Scholar]
- Yu X, Gao D, Gao L, Lai J, Zhang C, Zhao Y. Inhibiting metastasis and preventing tumor relapse by triggering host immunity with tumor-targeted photodynamic therapy using photosensitizer-loaded functional nanographenes. ACS Nano 2017; 11:10147-58. doi: 10.1021/acsnano.7b04736 [Crossref] [ Google Scholar]
- Wang R, He Z, Cai P, Zhao Y, Gao L, Yang W. Surface-functionalized modified copper sulfide nanoparticles enhance checkpoint blockade tumor immunotherapy by photothermal therapy and antigen capturing. ACS Appl Mater Interfaces 2019; 11:13964-72. doi: 10.1021/acsami.9b01107 [Crossref] [ Google Scholar]
- Xin Z, Shen Y, Hao H, Zhang L, Hu X, Wang J. Hyaluronic acid coated mesoporous carbon-copper peroxide for H2O2 self-supplying and near-infrared responsive multi-mode breast cancer oncotherapy. Colloids Surf B Biointerfaces 2022; 218:112776. doi: 10.1016/j.colsurfb.2022.112776 [Crossref] [ Google Scholar]
- Xue T, Xu C, Wang Y, Wang Y, Tian H, Zhang Y. Doxorubicin-loaded nanoscale metal-organic framework for tumor-targeting combined chemotherapy and chemodynamic therapy. Biomater Sci 2019; 7:4615-23. doi: 10.1039/c9bm01044k [Crossref] [ Google Scholar]
- Liu Y, Qiao L, Zhang S, Wan G, Chen B, Zhou P. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater 2018; 66:310-24. doi: 10.1016/j.actbio.2017.11.010 [Crossref] [ Google Scholar]
- Han Z, Han X, Wang Z, Wu S, Zheng R. Thioaptamer conjugated single-wall carbon nanotubes in human breast cancer targeted photothermal therapy in-vivo and in-vitro. Int J Clin Exp Med 2016; 9:58-64. [ Google Scholar]
- Oh Y, Jin JO, Oh J. Photothermal-triggered control of sub-cellular drug accumulation using doxorubicin-loaded single-walled carbon nanotubes for the effective killing of human breast cancer cells. Nanotechnology 2017; 28:125101. doi: 10.1088/1361-6528/aa5d7d [Crossref] [ Google Scholar]
- Persano F, Leporatti S. Nano-clays for cancer therapy: state-of-the art and future perspectives. J Pers Med 2022; 12:1736. doi: 10.3390/jpm12101736 [Crossref] [ Google Scholar]
- Ranjan AP, Fudala R, Shah S, Gdowski A, Kumar P, Grycznski I. MMP9 responsive molecular beacon-loaded nanovesicles for imaging and therapy for triple negative breast cancer. Cancer Res 2018; 78:3721. doi: 10.1158/1538-7445.am2018-3721 [Crossref] [ Google Scholar]
- Foudah AI, Alam A, Salkini MA, Ross SA, Kumar P, Aldawsari MF. Synergistic combination of letrozole and berberine in ascorbic acid-stabilized AuNPs: a promising solution for breast cancer. Pharmaceuticals (Basel) 2023; 16:1099. doi: 10.3390/ph16081099 [Crossref] [ Google Scholar]
- Donoso-González O, Riveros AL, Marco JF, Venegas-Yazigi D, Paredes-García V, Olguín CF. Iron-reduced graphene oxide core-shell micromotors designed for magnetic guidance and photothermal therapy under second near-infrared light. Pharmaceutics 2024; 16:856. doi: 10.3390/pharmaceutics16070856 [Crossref] [ Google Scholar]
- Zhang Y, Du X, Liu S, Yan H, Ji J, Xi Y. NIR-triggerable ROS-responsive cluster-bomb-like nanoplatform for enhanced tumor penetration, phototherapy efficiency and antitumor immunity. Biomaterials 2021; 278:121135. doi: 10.1016/j.biomaterials.2021.121135 [Crossref] [ Google Scholar]
- Aishajiang R, Liu Z, Liang Y, Du P, Wei Y, Zhuo X. Concurrent amplification of ferroptosis and immune system activation via nanomedicine-mediated radiosensitization for triple-negative breast cancer therapy. Adv Sci (Weinh) 2025; 12:e2407833. doi: 10.1002/advs.202407833 [Crossref] [ Google Scholar]
- Liang Y, Zhang L, Peng C, Zhang S, Chen S, Qian X. Tumor microenvironments self-activated nanoscale metal-organic frameworks for ferroptosis based cancer chemodynamic/photothermal/chemo therapy. Acta Pharm Sin B 2021; 11:3231-43. doi: 10.1016/j.apsb.2021.01.016 [Crossref] [ Google Scholar]
- Paul M, Bhatt H, Kumbham S, Ghosh B, Biswas S. Concurrent delivery of paclitaxel and chlorin e6 to tumors using albumin/PLGA nanoparticles for NIR light-triggered chemo/photodynamic therapy. ACS Appl Nano Mater 2023; 6:13385-99. doi: 10.1021/acsanm.3c02056 [Crossref] [ Google Scholar]
- Marrache S, Tundup S, Harn DA, Dhar S. Ex vivo generation of functional immune cells by mitochondria-targeted photosensitization of cancer cells. Methods Mol Biol 2015; 1265:113-22. doi: 10.1007/978-1-4939-2288-8_9 [Crossref] [ Google Scholar]
- Rong P, Yang K, Srivastan A, Kiesewetter DO, Yue X, Wang F. Photosensitizer loaded nano-graphene for multimodality imaging guided tumor photodynamic therapy. Theranostics 2014; 4:229-39. doi: 10.7150/thno.8070 [Crossref] [ Google Scholar]
- Zhao X, Li Y, Du L, Deng Z, Jiang M, Zeng S. Soft X-ray stimulated lanthanide@ MOF nanoprobe for amplifying deep tissue synergistic photodynamic and antitumor immunotherapy. Adv Healthc Mater 2021; 10:e2101174. doi: 10.1002/adhm.202101174 [Crossref] [ Google Scholar]
- Xiong YX, Li N, Han MM, Ye F, Liu T, Ye HY. Rhodiola rosea polysaccharides-based nanoparticles loaded with DOX boosts chemo-immunotherapy for triple-negative breast cancer by re-educating tumor-associated macrophages. Int J Biol Macromol 2023; 239:124110. doi: 10.1016/j.ijbiomac.2023.124110 [Crossref] [ Google Scholar]
- Makki MM, Hashem AR, Dhiyab AA. Carbon nanotubes for thermal therapy CNTs: effect of nanotube structure and doping on photothermal properties, anticancer efficacy of CNT-enhanced PTT and systemic delivery and biocompatibility of CNTs for PTT. Curr Clin Med Educ 2024; 2:29-47. [ Google Scholar]
- Liu D, Zhang Q, Wang J, Fan L, Zhu W, Cai D. Hyaluronic acid-coated single-walled carbon nanotubes loaded with doxorubicin for the treatment of breast cancer. Pharmazie 2019; 74:83-90. doi: 10.1691/ph.2019.8152 [Crossref] [ Google Scholar]
- Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020; 395:1078-88. doi: 10.1016/s0140-6736(20)30164-1 [Crossref] [ Google Scholar]
- Arshad R, Kiani MH, Rahdar A, Sargazi S, Barani M. Nano-based theranostic platforms for breast cancer: a review of latest advancements. Bioengineering 2022; 9:320. doi: 10.3390/bioengineering9070320 [Crossref] [ Google Scholar]
- Abolhassani H, Eskandari A, Saremi Poor A, Zarrabi A, Khodadadi B, Karimifard S. Nanobiotechnological approaches for breast cancer management: drug delivery systems and 3D In-Vitro models. Coord Chem Rev 2024; 508:215754. doi: 10.1016/j.ccr.2024.215754 [Crossref] [ Google Scholar]
- Liu Y, Zhang Y, Li H, Hu TY. Recent advances in the bench-to-bedside translation of cancer nanomedicines. Acta Pharm Sin B 2025; 15:97-122. doi: 10.1016/j.apsb.2024.12.007 [Crossref] [ Google Scholar]
- Gong J, Shi T, Liu J, Pei Z, Liu J, Ren X. Dual-drug codelivery nanosystems: an emerging approach for overcoming cancer multidrug resistance. Biomed Pharmacother 2023; 161:114505. doi: 10.1016/j.biopha.2023.114505 [Crossref] [ Google Scholar]
- Ulldemolins A, Seras-Franzoso J, Andrade F, Rafael D, Abasolo I, Gener P. Perspectives of nano-carrier drug delivery systems to overcome cancer drug resistance in the clinics. Cancer Drug Resist 2021; 4:44-68. doi: 10.20517/cdr.2020.59 [Crossref] [ Google Scholar]
- Elumalai K, Srinivasan S, Shanmugam A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed Technol 2024; 5:109-22. doi: 10.1016/j.bmt.2023.09.001 [Crossref] [ Google Scholar]
- Bazzazan MA, Fattollazadeh P, Keshavarz Shahbaz S, Rezaei N. Polymeric nanoparticles as a promising platform for treating triple-negative breast cancer: current status and future perspectives. Int J Pharm 2024; 664:124639. doi: 10.1016/j.ijpharm.2024.124639 [Crossref] [ Google Scholar]
- Yang F, He Q, Dai X, Zhang X, Song D. The potential role of nanomedicine in the treatment of breast cancer to overcome the obstacles of current therapies. Front Pharmacol 2023; 14:1143102. doi: 10.3389/fphar.2023.1143102 [Crossref] [ Google Scholar]
- Man V, Suen D, Kwong A. Use of superparamagnetic iron oxide (SPIO) versus conventional technique in sentinel lymph node detection for breast cancer: a randomised controlled trial. Ann Surg Oncol 2023; 30:3237-44. doi: 10.1245/s10434-023-13252-6 [Crossref] [ Google Scholar]
- Kim YK, Choi Y, Nam GH, Kim IS. Functionalized exosome harboring bioactive molecules for cancer therapy. Cancer Lett 2020; 489:155-62. doi: 10.1016/j.canlet.2020.05.036 [Crossref] [ Google Scholar]
- Al-Hatamleh MA, Ahmad S, Boer JC, Lim J, Chen X, Plebanski M. A perspective review on the role of nanomedicine in the modulation of TNF-TNFR2 axis in breast cancer immunotherapy. J Oncol 2019; 2019:6313242. doi: 10.1155/2019/6313242 [Crossref] [ Google Scholar]
- Farrell D, Ptak K, Panaro NJ, Grodzinski P. Nanotechnology-based cancer therapeutics--promise and challenge--lessons learned through the NCI Alliance for Nanotechnology in Cancer. Pharm Res 2011; 28:273-8. doi: 10.1007/s11095-010-0214-7 [Crossref] [ Google Scholar]
- Chehelgerdi M, Chehelgerdi M, Allela OQ, Pecho RD, Jayasankar N, Rao DP. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer 2023; 22:169. doi: 10.1186/s12943-023-01865-0 [Crossref] [ Google Scholar]
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 2013; 65:36-48. doi: 10.1016/j.addr.2012.09.037 [Crossref] [ Google Scholar]
- Mamot C, Drummond DC, Greiser U, Hong K, Kirpotin DB, Marks JD. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res 2003; 63:3154-61. [ Google Scholar]
- Mamot C, Drummond DC, Noble CO, Kallab V, Guo Z, Hong K. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res 2005; 65:11631-8. doi: 10.1158/0008-5472.Can-05-1093 [Crossref] [ Google Scholar]
- Duan J, Yu Y, Yu Y, Li Y, Wang J, Geng W. Silica nanoparticles induce autophagy and endothelial dysfunction via the PI3K/Akt/mTOR signaling pathway. Int J Nanomedicine 2014; 9:5131-41. doi: 10.2147/ijn.S71074 [Crossref] [ Google Scholar]
- Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev 2012; 41:2740-79. doi: 10.1039/c1cs15237h [Crossref] [ Google Scholar]
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 2004; 104:293-346. doi: 10.1021/cr030698+ [Crossref] [ Google Scholar]
- Libutti SK, Paciotti GF, Byrnes AA, Alexander HR Jr, Gannon WE, Walker M. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res 2010; 16:6139-49. doi: 10.1158/1078-0432.Ccr-10-0978 [Crossref] [ Google Scholar]
- Astruc D. Introduction to nanomedicine. Molecules 2015; 21:E4. doi: 10.3390/molecules21010004 [Crossref] [ Google Scholar]
- Balakrishnan S, Bhat FA, Raja Singh P, Mukherjee S, Elumalai P, Das S. Gold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif 2016; 49:678-97. doi: 10.1111/cpr.12296 [Crossref] [ Google Scholar]
- Mahmoudi M, Simchi A, Imani M, Shokrgozar MA, Sadeghzadeh Milani A, Häfeli UO. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf B Biointerfaces 2010; 75:300-9. doi: 10.1016/j.colsurfb.2009.08.044 [Crossref] [ Google Scholar]
- Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008; 2:889-96. doi: 10.1021/nn800072t [Crossref] [ Google Scholar]
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov 2003; 2:347-60. doi: 10.1038/nrd1088 [Crossref] [ Google Scholar]
- Cho K, Wang XU, Nie S, Chen Z, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 2008; 14:1310-1316. doi: 10.1158/1078-0432.CCR-07-1441 [Crossref] [ Google Scholar]
- Lammers T. Nanomedicine tumor targeting. Adv Mater 2024; 36:2312169. doi: 10.1002/adma.202312169 [Crossref] [ Google Scholar]
- Song M, Liu T, Shi C, Zhang X, Chen X. Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano 2016; 10:633-47. doi: 10.1021/acsnano.5b06779 [Crossref] [ Google Scholar]
- Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol 2006; 24:1211-7. doi: 10.1038/nbt1006-1211 [Crossref] [ Google Scholar]
- Peng C, Huang Y, Zheng J. Renal clearable nanocarriers: overcoming the physiological barriers for precise drug delivery and clearance. J Control Release 2020; 322:64-80. doi: 10.1016/j.jconrel.2020.03.020 [Crossref] [ Google Scholar]
- Ashique S, Garg A, Hussain A, Farid A, Kumar P, Taghizadeh-Hesary F. Nanodelivery systems: an efficient and target-specific approach for drug-resistant cancers. Cancer Med 2023; 12:18797-18825. doi: 10.1002/cam4.6502 [Crossref] [ Google Scholar]
- Liu J, Li M, Luo Z, Dai L, Guo X, Cai K. Design of nanocarriers based on complex biological barriers in vivo for tumor therapy. Nano Today 2017; 15:56-90. doi: 10.1016/j.nantod.2017.06.010 [Crossref] [ Google Scholar]
- Bahreyni A, Mohamud Y, Luo H. Emerging nanomedicines for effective breast cancer immunotherapy. J Nanobiotechnology 2020; 18:180. doi: 10.1186/s12951-020-00741-z [Crossref] [ Google Scholar]